Abstract
Bacterial wilt is one of the main diseases of Solanum spp., which caused by Ralstonia solanacearum (RS), formerly known as Pseudomonas solanacearum. Different concentrations of chitosan nanoparticles have been evaluated as one of the alternative methods of disease management in vitro and in vivo to reduce the risks of pesticide residues. Results in vitro experiment indicated that RS5 isolate was the most virulence one compared to RS1 and RS3. Increasing concentration of nano-chitosan, lead to increase inhibition zone, and this was observed at higher concentrations (100 and 200 µg/ml). In vivo results showed the highest concentration of spraying chitosan nanoparticles increase percentage reduction of disease incidence and severity, in effected potato and tomato plants. Recorded data of disease incidence and severity in treated potato plants were 78.93% and 71.85%, while on tomato plants were 81.64% and 77.63%, respectively compared to untreated infected potato plants were recorded 15.38%, 20.87%, and tomato plants were 20.98% and 28.64%. Results also revealed that 100 µg/ml of chitosan nanoparticles the lowest treatments used as soil amended curative treatments led to incease percentage reduction of disease incidence and severity, respectively on potato and tomato plants, but less than preventive treatment. The results registered that on potato plant were 54.93% and 52.65%, whilst recorded on tomato plants were 59.93% and 56.74%. Transmission electron microscopy (TEM) micrpgraphs illustrated that morphological of healthy R. solanacearum cells were undesirably stained with uranyl. The electron-dense uranyl acetate dye was limited to the cell surface slightly than the cytoplasm, which designated the integrity of the cell film of healthy cells. While bacterial cells treated with nano-chitosan, showed modification in the external shape, such as lysis of the cell wall and loss of cell flagella. Also, the result of using Random amplified polymorphic DNA (RAPD)-PCR observed that differences in treated Ralstonia solanancearum genotype by nano-chitosan compared to the genotype of the same untreated isolate.
Keywords: Potato, Tomato, Ralstonia solanacearum, Nano-Chitosan, TEM- and DNA (RAPD)-PCR
1. Introduction
Over the past two decades, the number of undernourished people on the African continent has increased, demonstrating the increasing importance of ensuring food security (Gitari et al., 2018). In order to meet the growing demand for food, potato crops, as a food source, have a good balance of essential amino acids and proteins, and produce more energy per unit of land than any other crop (Ahuja et al., 2013). Furthermore, tomato (Solanum lycopersicum L.) is one of the main vegetable crops in the world and is grown throughout the world for the consumption or processing of fresh vegetables. Most of the tomato plants in the world have been grown for many years (Gatahi, 2020).
Therefore, obtaining high-quality planting materials is a major limitation for the successful production of potatoes and tomatoes. Additionally, high-quality potatoes and tomatoes can only be successfully grown in areas and fields where there are no serious soil-borne pathogens such as warts, cyst nematodes, bacterial wilt, black scale, and common scabs (Buckseth et al., 2016). One of the most important diseases of potatoes bacterial wilt (Yabuuchi et al., 1995), which was reported in Egypt many years ago (Briton-Jones, 1925). During the export of edible potato tubers to Europe, the disease caused many quarantine problems in Egypt (Sabet, 1961).
Bacterial wilt can infect more than 200 plant species representing more than 50 plant families (Janse, 2012). This disease is known to occur in the humid tropics, subtropics, and some temperate regions of the world. It is estimated that around 3.75 million acres of land in about 80 countries around the world will be affected, and the global loss is currently estimated to exceed 950 M dollar per year (Charkowski et al., 2020). Therefore, the use of new methods such as chitosan and other nanotechnologies to reduce the negative impact of pesticides on the environment is crucial, as some bacterial strains have been used to produce antibacterial agents due to their continuous application in various parts around the world, especially on farmland drug resistance. The increased use of pesticides will affect non-target species, in addition polluting water bodies, affecting the environment and human health (Manjunatha et al., 2016).
For nanotechnology, it is considered an important technique with economic, social and ecological significance (El-Saadony et al., 2021a, El-Saadony et al., 2021b). The Nanotechnology field is one of the most active areas of research of modern data (El-Saadony et al., 2021c, El-Saadony et al., 2021d). Nanoparticles represent a completely new or enhanced feature based on specific properties such as size, distribution and shape (Saad et al., 2021, El-Saadony et al., 2021e). A new application of nanoparticles and nano materials is quickly injured. Nanotechnology is used as a tool to explore the darkest road of the current antibacterial (Elizabath et al., 2019).
Chitosan (CS) is a natural cationic biopolymer composed of N-acetyl-D-glucosamine and Dglucosamine units connected by β-1,4-glycosidic linkages (Elieh-Ali-Komi and Hamblin, 2016). Previous studies have explored the antibacterial activity of CS, and more recently different types of CS derivates have been synthesized to enhance its natural antibacterial activity (Abd El-Hack et al., 2020).
Additionally, chitosan treatment regulates several genes in plants, especially the activation of plant defense signaling pathways, including the acquisition of plant antitoxin and pathogenesis- related (PR) proteins (Pichyangkura and Chadchawan, 2015). Due to the high environmental risks of chemical pesticides, the use of biological pesticides for biological control of plant diseases is strongly encouraged and recommended. Chitosan and chitosan oligosaccharides have become well-known biological control agents due to their non-toxic, biodegradable and biocompatible properties (Singh and Chaudhary, 2010). Chitosan is considered to be the most abundant natural polymer with dual functions: it capable to control pathogenic microorganisms by preventing growth, sporulation, spore viability, germination and cell destruction, inducing different defense responses in host plants, and inhibiting various biochemical activities during the phytopathogenic interaction. Chitosan has been tested to control multiple diseases in many crops before and after harvest and has a positive effect on enriching rhizosphere biodiversity (Hassan and Chang, 2017). The aim of the research is to use alternative methods to combat bacterial wilt in some crops of the solanaceae family, the most important of which is the use of modern inducer compounds such as nanochitosan to reduce the use of chemical compounds to achieve the goal of sustainable agriculture.
2. Materials and methods
2.1. Preparation of chitosan nanoparticles solution
Low molecular weight chitosan (>85% deacetylated) was purchased from Sigma- Aldrich (USA). Chitosan solution was prepared by dispersing it in 0.25% acetic acid solution (Merck, Germany). Nano-chitosan was dissolved in 1% acetic acid and for complete dissolution of its particles kept overnight under magnetic stirring then with distilled water to obtain the desired volume. The concentration of chitosan nanoparticles were adjusted to 50, 75, 100 and 200 µg/ml, according to Borines et al. (2015).
The three types of R. solanacearum bacteria (RS1, RS3 and RS5) were detected, isolated and identified by traditional and serological methods in previous studies (khairy et al., 2021).
2.2. In vitro evaluation of chitosan nanoparticles on potato and tomato bacterial wilt caused by Ralstonia solanacearum
The growth of the three isolates were adjusted to (108 ml−1 cells) by spectrophotometric measurements. one ml of each bacterial suspension from the three isolates into a sterile Petri dish and put up King's B medium (King et al., 1954). Then use a sterile agar plate to punch 6 mm diameter holes in the agar plate using a Pasteur pipette. Seal the bottom of the well by pouring a drop of molten King's B medium. After that, pour 50 μL of nano chitosan solution (50, 75, 100 and 200 μg / ml) into the wells. The plates were incubated at 28 °C for 28 h. Measurement the diameter of inhibition zone (Islam et al., 2011).
2.3. In vivo evaluation of chitosan nanoparticles on potato and tomato bacterial wilt caused by Ralstonia solanacearum
Assessment of efficacy the preferable of chitosan nanoparticles solution concentrations based on results in vitro were (100 and 200 µg/ml) against the virulence isolate of R. solanacearum (RS5). The sterilized potato tubers cultivar (Spunta) and healthy tomato seedlings cultivar (0 6 6) were used. The experiment was divided into two basic treatments and each treatment also divided into two sub-treatments, which are a preventive treatment and curative treatment for bacterial wilt disease on hosts by spraying treatment on the foliage and soil amended. For preventive treatments, chemicals were sprayed or soil amended before infestation with bacterial suspensions (108 cells ml−1) of RS5 isolate. While in the curative treatments, the chemicals were added after the artificial inoculation and the appearance of infection on the plant. The soil used in the previous experiment was obtained from pest free area and bacteriologically checked to ensure that it is R. solanacearum free. Infested soil created by soaking 240 ml of bacterial suspension per potting soil (30 ml/kg soil), according to Malerba and Cerana (2018). However, the control was divided to two treatments. The first was done with sterile water instead of bacteria. The second was executed using bacterial suspension (108 CFU/ ml−1). The inoculated plants in pots were cover with a polyethylene bag at 28 ± 2° C for three days, then the bag removed and watered daily. Four plastic pots (20 cm in diameter) containing 8 kg sandy-clay soil (1/1, v/v), and each pot containing one seedling of tomato or one potato tuber. Five replicates for each treatment, and pots arranged in a random all-block design. Transplanted potato seedlings were observed for disease assessment.
2.4. Epidemic assessment
Percentage disease incidence on potato plants (DI) recorded 40 days after inoculation. But it was measured on tomato plants after 7 days after inoculation.
The percentage of disease severity (DS) on potato and tomato plants were calculated by using the scale described by Kempe and Sequeira (1983), wilt symptoms were assessed, where, (0 = no symptoms, 1 = up to 25% wilt, 2 = 26–50% wilt, 3 = 51–75% wilt, 4 = 76–99% of the foliage wilted and 5 = dead plants. Each plant was examined, the disease rating was assessed, and the mean value for each treatment was calculated.
The percentage reduction in disease incidence and severity for each potato and tomato plant was calculated using the following formula:
C = control
T = treatment
2.5. Transmission electron microscope (TEM)
The procedure was carried out in the Biotechnology Laboratory in the Research Park of the Faculty of Agriculture Cairo University Research Park (CURP). The cells of R. solanacearum isolate (RS5) were grown in nutrient broth media on scrow caps. Chitosan nanoparticles solution (200 μg / ml) was added in some scrow caps and the other was not treated. Then incubating at 28 °C for two days. The cell of bacteria treated with nano-chitosan and untreated were applied to Formvar-coated Cu (400 mesh) mesh, (polyvinyl formal), film preparation was performed according to the methods described by (Santiago et al., 2019), and the treated cells were observed using TEM and photographed.
2.6. Random amplified polymorphic DNA (RAPD)-PCR
This procedure was conducted at CURP according to Singh et al. (2021), to study the effect of chitosan nanoparticles on the genome of bacterial cells. DNA is extracted by heating a bacterial suspension of RS5 isolate at 95° C for 10 min and then fast refrigeration on ice for 5 min (Table 1).
Table 1.
Primers and their sequences used in RAPD technique.
| No. | Name | Sequences (5…3) |
|---|---|---|
| p1 | A01 | CCCAAGGTCC |
| p2 | A04 | CTTCACCCGA |
| p3 | B07 | AGATCGAGCC |
2.6.1. RAPD-PCR technique
Three Random Primer (A01, A04 and B07) Gene jet genomic DNA purification kit from (Thermo scientific cat No. 0721), Bangalore, India, were evaluated for PCR amplification of treated and un-treated nano-chitosan R. solanacearum RS5 isolate according to Nishat et al. (2015).
2.6.2. Gel analysis
The PCR products were electrophoresed in 1.5% (w / v) agarose at 80 V using 1X TBE buffer (45 Mm tris–borate, 1 Mm EDTA) stained with ethidium bromide, observed in a beam lamp UV-trans, and gel were imaged with a camera.
The different molecular weights of bands were determined against a DNA standard by using thermo scientific gene ruler. The ready-to-use 1 kb DNA ladder is designed for the size determination and approximate quantification of broad-spectrum double-stranded DNA fragments on agarose gels. The ladder is made up of fourteen chromatographically purified individual DNAs (in base pairs): 10,000, 8000, 6000, 5000, 4000, 3500, 3000, 2500, 2000, 1500, 1000, 750, 500 and contains three references. (6000, 3000 and 1000 bp) bands for easy positioning. The ladder can be used out of the box and is premixed with the 6X DNA loading dye for direct loading onto the gel.
2.7. Statically analysis
The data of all experiments were analyzed statistically using analysis of variance according to Gomez and Gomez (1984). Means were compared using the least significant differences (LSD).
3. Results
3.1. In vitro evaluation of chitosan nanoparticles to control bacterial wilt in potato and tomato plants
Data in Fig. 1 and Table 2 show a significant difference between the three isolates (RS1, RS3 and RS5) of R. solanacearum on percentage reduction of inhibition zones (cm) against their growth in response to chitosan nanoparticles, recorded after 48 h incubation period. Isolate (RS5) was the most virulent one compared totwo other isolates (RS1 and RS3), as it showed the highest reduction percentage of inhibition zone, compared to the control treatments. Therefore, it was chosen for application in the greenhouse experiment. As well, increasing the concentration of nano-chitosan, lead to the increase of inhibition zone, and this was observed clear at higher concentrations (100 and 200 µg/ml).
Fig. 1.
in vitro antagonistic activity of chitosan nanoparticles concentration aganist three isolates of R. solanacearum.
Table 2.
In vitro efficacy of chitosan nanoparticles against three R. solanacearum isolates, measured as inhibition zone (cm) and reduction percentage.
| R. solanacearum isolates | Concentration (µg/ml) | Inhibition zone (cm) | Reduction (%) |
|---|---|---|---|
| RS1 | 50 | 0.41 | 4.55 |
| 75 | 0.97 | 10.66 | |
| 100 | 1.68 | 18.67 | |
| 200 | 2.59 | 28.77 | |
| Control | 00 | 00 | |
| RS3 | 50 | 0.67 | 7.44 |
| 75 | 1.23 | 13.66 | |
| 100 | 2.01 | 22.34 | |
| 200 | 3.10 | 34.44 | |
| Control | 00 | 00 | |
| RS5 | 50 | 0.25 | 2.77 |
| 75 | 0.62 | 6.88 | |
| 100 | 1.49 | 16.56 | |
| 200 | 2.00 | 22.23 | |
| Control | 00 | 00 | |
3.2. In vivo evaluation of chitosan nanoparticles to control bacterial wilt in potato and tomato plants
This work was planned to suppress bacterial wilt disease incidence and severity on potato and tomato plants using chitosan nanoparticles treatments under artificial inoculation conditions. The results indicated in Table 3 and Fig. 2, Fig. 3 revealed that significant differences between treatments when Nano-chitosan particles used as preventive and curative technique in controlling bacterial wilt on potato plants. The results showed that using these particles in a preventive technique was better with bacterial wilt, whether it was used as spraying or soil amended. The results recorded the highest percentage reduction of disease incidence and severity respectively. Spraying 200 µg/ml concentration on potato plants, as preventive technique reduced disease by 78.93% and 71.85%. The same concentration reduced 69.43% and 71.87%, when it used as a curative treatment. On the other hand, both concentrations 100 µg/ml and 200 µg/ml used as curative soil amended reduced the lowest values that led to decrease percentage reduction of disease incidence and severity, respectively. The higher concentration calculated 58.14% and 54.17%, and lower concentration recorded 54.93% and 52.65%, compare to the control treatment.
Table 3.
In vivo efficacy of chitosan nanoparticles on potato bacterial wilt, measured by the percentage reduction in disease incidence and severity.
| Treatment and concentration | Nano-chitosan |
||||||
|---|---|---|---|---|---|---|---|
| spraying |
Average | Soil amended |
Average | ||||
| DI reduction (%) | DS reduction (%) | DI reduction (%) | DS reduction (%) | ||||
| Preventive | 100 µg/ml | 64.70 | 61.94 | 63.32 | 59.95 | 57.94 | 58.94 |
| 200 µg/ml | 78.93 | 71.85 | 75.39 | 64.29 | 60.91 | 62.60 | |
| Healthy cont. | 100 | 100 | 100 | 100 | 100 | 100 | |
| Infected cont. | 15.38 | 20.87 | 18.12 | 13.91 | 15.80 | 14.53 | |
| Average | 64.67 | 63.66 | 64.16 | 59.53 | 51.16 | 55.34 | |
| Curative | 100 µg/ml | 58.72 | 55.92 | 57.32 | 54.93 | 52.65 | 54.15 |
| 200 µg/ml | 69.43 | 71.87 | 70.65 | 58.14 | 54.17 | 56.15 | |
| Healthy cont. | 100 | 100 | 100 | 100 | 100 | 100 | |
| Infected cont. | 13.63 | 21.91 | 17.77 | 14.91 | 12.97 | 13.94 | |
| Average | 60.44 | 62.42 | 61.43 | 56.99 | 45.94 | 51.46 | |
| LSD for 0.05 | 1.754 | 1.928 | – | 1.815 | 1.605 | – | |
Fig. 2.
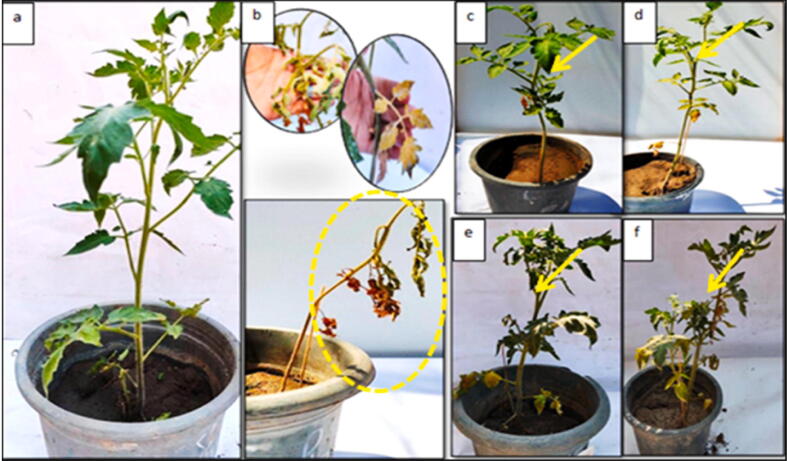
In vivo efficacy of chitosan nanoparticles as curative technique against bacterial wilt of potato, measured as disease severity. a) Healthy Control, b) Infected control, c) Spraying (nano-chitosan 100 µg/ml), d) Soil amended (nano-chitosan 100 µg/ml), e) Spraying (nano-chitosan 200 µg/ml) and f) Soil amended (nano-chitosan 200 µg/ml).
Fig. 3.
In vivo efficacy of chitosan nanoparticles as preventive technique against bacterial wilt of potato, measured as disease severity. a) Healthy Control, b) Infected control, c) Spraying (nano-chitosan 100 µg/ml), d) Soil amended (nano-chitosan 100 µg/ml), e) Spraying (nano-chitosan 200 µg/ml) and f) Soil amended (nano-chitosan 200 µg/ml).
Results in Table 4 and Fig. 4, Fig. 5 illustrate that significant differences between the treatments, when using nano chitosan particles used as preventive and curative technique to control bacterial wilt on tomatoes. Data show that the concentration of 200 µg/ml was the best to increase the percentage reduction of disease incidence and severity, respectively. Spraying it on tomato plants used as preventive treatment recorded 81.64% and 77.63%. The same concentration reduced 73.91% and 71.87%, when it used as a curative treatment. On the other hand, both concentrations 100 µg/ml and 200 µg/ml used as curative soil amended reduced the lowest values that led to decrease percentage reduction of disease incidence and severity, respectively. The higher concentration calculated 63.14% and 61.80%, and the lower concentration recorded 59.93% and 56.74%, compare to the control treatment.
Table 4.
In vivo efficacy of chitosan nanoparticles on tomato bacterial wilt, measured by the percentage reduction in disease incidence and severity.
| Treatment and concentration | Nano-chitosan |
|||||||
|---|---|---|---|---|---|---|---|---|
| spraying |
Average | Soil amended |
Average | |||||
| DI reduction (%) | DS reduction (%) | DI reduction (%) | DS reduction (%) | |||||
| Preventive | 00 µg/ml | 70.63 | 69.71 | 70.17 | 64.29 | 68.48 | 66.38 | |
| 200 µg/ml | 81.64 | 77.63 | 79.63 | 70.09 | 68.93 | 69.51 | ||
| Healthy cont. | 100 | 100 | 100 | 100 | 100 | 100 | ||
| Infected cont. | 20.98 | 28.64 | 24.81 | 23.61 | 27.41 | 25.51 | ||
| Average | 68.31 | 68.99 | 68.65 | 64.49 | 66.20 | 65.35 | ||
| Curative | 100 µg/ml | 64.09 | 62.39 | 63.24 | 59.93 | 56.74 | 58.33 | |
| 200 µg/ml | 73.91 | 71.87 | 72.89 | 63.14 | 61.80 | 62.47 | ||
| Healthy cont. | 100 | 100 | 100 | 100 | 100 | 100 | ||
| Infected cont. | 24.86 | 26.91 | 25.90 | 26.81 | 29.74 | 28.27 | ||
| Average | 65.71 | 65.29 | 65.50 | 62.47 | 62.07 | 62.26 | ||
| LSD for 0.05 | 1.838 | 1.709 | – | 1.576 | 1.686 | – | ||
Fig. 4.
In vivo efficacy of chitosan nanoparticles as preventive technique against bacterial wilt of tomato plants, measured as disease severity. a) Healthy Control, b) Infected control, c) Spraying (nano-chitosan 100 µg/ml), d) Soil amended (nano-chitosan 100 µg/ml), e) Spraying (nano-chitosan 200 µg/ml) and f) Soil amended (nano-chitosan 200 µg/ml).
Fig. 5.
In vivo efficacy of chitosan nanoparticles as curative technique against bacterial wilt of tomato plants, measured as disease severity. A) Healthy Control, B) Infected control, C) Spraying (nano-chitosan 100 µg/ml), D) Soil amended (nano-chitosan 100 µg/ml), E) Spraying (nano-chitosan 200 µg/ml) and F) Soil amended (nano-chitosan 200 µg/ml).
3.3. Morphological properties of nano-chitosan on R. solanacearum cells observed by transmission electron microscopy
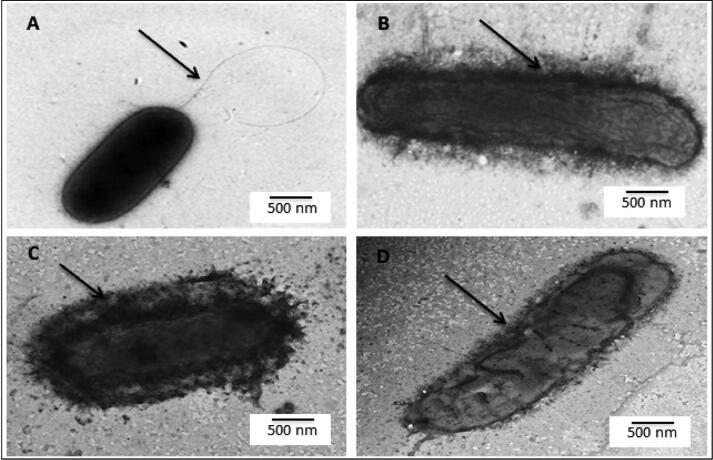
Healthy R. solanacearum cells were negatively stained with uranyl acetate, as observed in TEM micrograph (Fig. 6). Electron dense uranyl acetate staining binds to the cell surface instead of the cytoplasm, indicating the integrity of the cell membrane of healthy cells. While bacterial cell previously treated with nano-chitosan, showed modification in the external shape of the bacteria, such as cell wall lysis and loss of cell flagella, comparing with un- treated bacterial cell.
Fig. 6.
Morphological properties of Ralstonia solanacearum treated with nano-chitosan particles observed after 2 days by transmission electron microscopy. (A) Arrow denote to the cell un-treated and containing flagella, (B) Arrow denote to the cell aggregation of nano particals on cell wall and (C), (D) Arrows denote to the cell aggregation of nano particals on cell lysis of cell wall.
3.4. Random amplified polymorphic DNA (RAPD)-PCR
Results in Fig. 7 indicate the differences in the genotype of R. solanancearum RS5 isolate treated with nano-chitosan compared to the original genotype of the same untreated isolate, using three primers A01, A04 and B07. The different molecular weights of bands were determined against a DNA standard by using thermo scientific gene ruler 1 kb DNA ladder. The ladder consists of fourteen individual DNAs purified by chromatography (in base pairs): 10,000, 8000, 6000, 5000, 4000, 3500, 3000, 2500, 2000, 1500, 1000, 750, 500 and 250. Data showed that using primer (A04), led to the appearance of new bands differ with the genotype of the untreated bacteria. This indicates that nano-chitosan particles not only affect the cell wall of bacteria, but occurring difference of genotype of bacterial cell. While, using the other two primers (A01 and B07), were unable to clarify differences between the genotype of treated and untreated bacterial cell with nano-chitosan.
Fig. 7.
Genetic differences by RAPD amplification profiles between isolate (RS5) of Ralstonia solanacearum with treated and untreated nano-chitosan particles, using three primers A01, A04 and B07. M) Marker or ladder, 1) Un-treated bacterial isolate and 2) Treated bacterial isolate with nano-chitosan particles.
4. Discussion
Potato wilt, caused by the bacterium Ralstonia solanacearum, is considered the second most important disease in most potato-producing countries after late blight caused by Phytophthora infestans fungus (Damtew et al., 2018). Vascular pathogens casuing diseases on a variety of host plants, including many economically important crops in over 40 countries (Álvarez et al., 2010). Control of bacterial wilt in disease-affected areas is difficult: there is no chemical cure, so there is increasing interest in developing alternative plant disease management strategies to reduce dependence on synthetic chemicals. Phytopathogenic bacteria are believed to be the most versatile agents for adapting to the environment and destroying plant growth. Among the many strategies, nanotechnology-powered inventions have produced quantifiable data against fungal diseases in plants primarily through applications of nanoparticles (El-Mohamedy et al., 2019).
Therefore, this study aims to evaluate the control of bacterial wilt disease in potato and tomato plants in vitro and in vivo using chitosan nanoparticles. Nanochitosan, a biopolymer made from glucosamine and N-acetyl residues, is the free product of chitin acetylation. It can be obtained in large quantities from lobster exoskeleton remains and shrimp walls at an inexpensive cost (Sharif et al., 2018). To a large extent, it is suitable for exploration in plants together with other nanomaterials. Increasing the number of scientific papers on chitosan nanoparticles in plant growth and protection increases the enormous potential of nanochitosan relative to chitosan. Because nanochitosan has superior physicochemical properties compared to chitosan, it provides improved plant biological activity (Van et al., 2013). Therefore, it is necessary to explore chitosan biopolymers, not only because of its new antibacterial and plant defense properties, but also because of its role in plant growth and pest control (Kumaraswamy et al., 2018). Chitosan can be used to control these pathogens by inhibiting their growth at different life cycle points (Kong et al., 2010).
The results showed in vitro that applying nano-chitosan particles at 100 and 200 µg/ml concentrations were the best in increasing the inhibition area of the bacterial growth. So these concentrations were used to management disease incidence on plants. The results also showed when applying these concentrations in vivo to potato and tomato plants, whether it was sprayed or soil amended or using it as preventive or curative treatments. The results indicated that use of 200 µg/ml sprays in the preventive strategy was better than other treatments, which led to a significant reduction in disease incidence and severity. This effect due to the fact that nano-chitosan is biological material that is well recognized in agriculture and is applied successfully to control diseases or increase crop production in many crops (Borines et al., 2015, Abd El-Aziz et al., 2019, Esyanti et al., 2020). As we all know, nano-chitosan can enhance the defense response of plants, and also has antibacterial properties (Vanti et al., 2020) as nanotechnology advances.
The in vitro and in vivo results obtained in this study show that chitosan nanoparticles have several advantages over other biofilm-forming agents (cellulose, starch, galactomannans, etc.): they are safe, cheap, and their chemical structure is easy to introduce specific Molecules are designed for polymers. These characteristics make chitosan nanoparticles play an important role in a wide range of potential users from the medical and biotechnology industries to agricultural applications (Asgari-Targhi et al., 2018). Especially in recent years, more and more researchers have studied the effects of compounds based on chitosan nanoparticles on plants. Katiyar et al. (2015) pointed out that chitosan induces systemic acquired resistance and rapidly induces disease-related variable enzymes, such as phenylalanine, ammonia, peroxidase, polyphenoloxidase, catalase, and superoxide substance dismutase, glucanase and chitinase salt. Therefore, chitosan nanoparticles lead to an increase in plant biological activity.
The antibacterial activity of CS-NPs is most likely due to interactions with either the bacterial cell wall or the cell membrane. the electrostatic communication between the positively charged amino groups of glucosamine and the negatively charged cell membranes of bacteria is the most commonly acknowledged CS-NPs paradigm of antibacterial activity (Chandrasekaran et al., 2020). This contact causes widespread changes to the cell's surface, resulting in a change in membrane permeability, which causes osmotic imbalance and intracellular substance efflux, ultimately leading to cell death (Qi et al., 2004). The electrostatic force between the CS and the bacteria cell wall encourages a deeper interaction with charged molecules, allowing CS-NPs to pass through the cell wall (Mohammed et al., 2017). As a result, the likelihood of CS-NPs collecting at the point of interaction rises. Furthermore, CS-NPs have the ability to alter the electron transport chain in bacteria (Garg et al., 2019). Electrostatic interactions, which alter membrane permeability, are the most commonly suggested antibacterial activity of CS. It then binds to DNA, preventing DNA replication and ultimately causing bacterial cell death. Furthermore, lower MW-CS has been proven to penetrate the cell, bind to DNA, and block replication machinery (Chandrasekaran et al., 2020).
Flocculation of electronegative components in the cell by CS disrupts bacterial physiological functions and results in bacterial cell death (Garg et al., 2019).
The results showed that using nano-chitosan as a modern strategy in fertilizing potato and tomato plants, whether it was sprayed or soil treatment, it led to a reduction in the disease rate and severity. This is due to nano- chitosan particles (CNPs) mode of action. This is a bio-fertilizer application that effectively minimizes fungal infections and plant growth (Oh et al., 2019). CS (NP) polymer nanoparticles are biodegradable and used for the controlled production of NPK fertilizers (Guo et al., 2018). CNPs also modulate supplementation of 1-naphthylacetic acid enhanced micronutrients under different pH and temperature conditions; thereby enhancing uptake of plant growth hormones (Oh et al., 2019).
In plants, chitosan is mainly used to simulate biological and abiotic stresses, Allan and Hadwiger (1979) conducted the first study of chitosan as an anti-pathogen in plants, and they proved the fungicidal effect of chitosan on different components of fungal cell walls. The improvement of the defense system after the application of chitin and chitosan in monocotyledon and dicotyledons plants is the focus of the method of this biopolymer in multiple research fields (Khan et al., 2002). Chitosan is a biological fungicide, biological fungicide and bioviral, which can stimulate the defense system of plants against pathogens, thereby inducing the immune system of plants, fruits and vegetables (Sharif et al., 2018). In addition, the increasing demand for food has also stimulated the increase in the use of industrial fertilizers, causing serious environmental imbalances and disastrous effects on human health. Therefore, consider using chitosan as a biological fertilizer. According to reports, chitosan has a positive effect on the growth of rhizosphere bacteria. Among them, chitosan has a symbiotic relationship with the growth-promoting rhizosphere bacteria, triggering germination rate and improving plant nutrient absorption (Kaur and Dhillon, 2014).
Chitosan is considered to be the most abundant natural polymer with dual functions: it prevents growth, sporulation, spore viability, germination and cell changes, as well as inducing different defense responses in host plants, inducing and/or inhibiting different biochemical activities to control pathogenic microorganisms during the interaction between plants and pathogens (Hassan and Chang, 2017). There is some concrete evidence regarding the antibacterial mechanism of chitosan and its ability to trigger plant defense responses. More and more evidences show that chitosan and its derivatives have a dual mechanism of action: they inhibit the growth of pathogens and change the defense response of host plants (Xing et al., 2015). The antibacterial ability of chitosan is due to the deposition and formation of a dense polymer film on the surface of pathogens. This dense polymer membrane inhibits the flow of nutrients and the metabolism necessary for microorganisms to survive in nature (Zhang et al., 2015). Plants protect themselves from pathogens by developing an astonishing series of structural, chemical, and protein defenses designed to identify invading pathogens and stop them before they can cause widespread damage (Freeman and Beattie, 2008). Some reports indicate that chitosan and its derivatives are effective inducers and inducers of systemic acquired resistance (SAR) in plants. Chitosan and oligochitosan induce the production of defense-related proteins (Hadwiger, 2013), enzymes (Lin et al., 2005), and secondary metabolites.
Furthermore, chitosan treatment regulates several genes in plants, especially the activation of phytoprotective signaling pathways (Pichyangkura and Chadchawan, 2015). This includes the activation of phytoalexin and pathogenesis-related (PR) proteins. Also, chitosan has been used as a plant nutrient in the soil, and when combined with other industrial fertilizers, it shows great efficacy without affecting beneficial soil microorganisms. In addition, due to its coating capacity, it helps reduce the loss of fertilizer, which is important to control environmental pollution. Based on such excellent performance, the use of chitosan biopolymers in agricultural systems is surprising (Sharif et al., 2018).
By comparing the morphology of R. solanacearum cells as seen in TEM micrographs, healthy R. solanacearum cells were negatively stained with uranyl acetate. Electron-dense uranyl acetate stain binds to the cell surface rather than the cytoplasm, indicating that the cell membrane of healthy cells is intact. While bacterial cell treated with nano-chitosan, showed modification in the external shape of the bacteria, such as lysis of the cell wall and loss of cell flagella. Moreover, the results are clearly indicated that differences in the genotype of R. solanancearum isolate treated with nano-chitosan compared to the genotype of the same untreated one, by using RAPD-PCR compering with un- treated bacterial wilt pathogen. Changes in the morphological bacterial cell properties might be due to the chitosan antibacterial activity which caused by two mechanisms. The first is that the positively charged chitosan interacts with the negatively charged phospholipids of the plasma membrane altering cell permeability, causing leakage of cellular components and subsequent cell death. The latter chitosan has the property of chelating metal ions, which is a possible cause of its antimicrobial activity. It has also been reported that chitosan can penetrate the cell wall and bind to DNA by inhibiting mRNA synthesis (Rozman et al., 2019).
The electrostatic force between CS and the bacterial cell wall supports a closer interaction with charged molecules, resulting in the penetration of CS-NP through the bacterial cell wall (Cava et al., 2010). Therefore, the capacity of CS-NP accumulate at the site of interaction increases. Furthermore, CS-NPs can regulate bacterial electron transport chains (Birsoy et al., 2015). The most significant antibacterial activity of CS is electrostatic interaction, which changes the permeability of the membrane. It then binds to DNA and disrupts DNA replication, causing bacterial cell death. In addition, it has been shown that lower MW-CS can enter cells, bind to DNA and inhibit the replication mechanism (Birsoy et al., 2015). CS flocculates negatively charged elements in cells, changes the physiological activities of bacteria and causes bacterial cell death.
5. Conclusions
In this study, four different concentrations of nano-chitosan were used in vitro on R. solanacearum, and the two highest concentrations (100 μg/ml and 200 μg/ml) were selected, and they were applied as a preventive and curative in vivo on potato and tomato plants. They were used as spraying and soil amended. The results showed that the highest concentration (200 µg/ml) of chitosan nanoparticles sprayed increased the incidence and severity of the disease in infected potato and tomato plants, compared to other treatments. Moreover, the results showed a difference in the morphological shape of the bacteria cell treated with nano-chitosan and the untreated, and it was clarified using Transmission electron microscopy (TEM).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgment
All thanks to God and then to my professors and everyone who supported this research. I would like to thank the Head of the Plant Pathology Laboratory at the Faculty of Agriculture at Zagazig University and the director of Potato Brown Rot Project (PBRC), Agric. Res. Center, Giza- Egypt, as well as the laboratory Biotechnology Lab at Cairo University Research Park, Faculty of Agriculture.
Funding: Taif University Researchers Supporting Project number (TURSP-2020/138), Taif University. Taif Saudi Arabia.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abd El-Aziz M.E., Morsi S.M.M., Salama D.M., Abdel-Aziz M.S., Abd Elwahed M.S., Shaaban E.A., Youssef A.M. Preparation and characterization of chitosan/polyacrylic acid/copper nanocomposites and their impact on onion production. Int. J. Biol. Macromol. 2019;123:856–865. doi: 10.1016/j.ijbiomac.2018.11.155. [DOI] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Shafi M.E., Zabermawi N.M., Arif M., Batiha G.E., Khafaga A.F., Abd El-Hakim Y.M., Al-Sagheer A.A. Antimicrobial and antioxidant properties of chitosan and its derivatives and their applications: A review. Int. J. Biol. Macromol. 2020;164:2726–2744. doi: 10.1016/j.ijbiomac.2020.08.153. [DOI] [PubMed] [Google Scholar]
- Ahuja J.K.C., Haytowitz D.B., Pehrsson P.R., Roseland J., Exler J., Khan M., et al. Composition of foods raw, processed, prepared usda national nutrient database for standard reference, release 27 documentation and user guide. U.S. Dept. Agric. Res. Serv., Beltsv. Hum. Nutr. Res. Cent., Nutr. Data Lab. 2013;2(11):1–136. [Google Scholar]
- Allan C.R., Hadwiger L.A. The fungicidal effect of chitosan on fungi of varying cell wall composition. Exp. Mycol. 1979;3(3):285–287. doi: 10.1016/S0147-5975(79)80054-7. [DOI] [Google Scholar]
- Álvarez B., Biosca E.G., López M.M. On the life of Ralstonia solanacearum, a destructive bacterial plant pathogen. Current research, technology and education. Top. Appl. Microbiol. Microbial Biotechnol. 2010;1:267–279. https://www.researchgate.net/publication/267772811 [Google Scholar]
- Asgari-Targhi G., Iranbakhsh A., Ardebili Z.O. Potential benefits and phytotoxicity of bulk and nano-chitosan on the growth, morphogenesis, physiology, and micropropagation of Capsicum annuum. Plant Physiol. Biochem. 2018;127:393–402. doi: 10.1016/j.plaphy.2018.04.013. [DOI] [PubMed] [Google Scholar]
- Birsoy K., Wang T.C., Chen W.W., Freinkman E., Abu-Remaileh M., Sabatini D.M. An essential role of the mitochondrial electron transport chain in cell proliferation is to enable aspartate synthesis. Cell Press J. 2015;162(3):540–551. doi: 10.1016/j.cell.2015.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borines L.M., Sagarino R.M., Calamba R.B., Contioso M.A.A., Jansalin J.G.F., Calibo C.L. Potential of chitosan for the control of tomato bacterial wilt caused by Ralstonia solanacearum (Smith) Yabuuchi et al. Ann. Tropical Res. 2015;37(2):57–69. [Google Scholar]
- Briton-Jones H.R. Government Press; Cairo, Egypt: 1925. Mycological work in Egypt during the period 1920–1922; pp. 49–129. [Google Scholar]
- Buckseth T., Sharma A.K., Pandey K.K., Singh B.P., Muthuraj R. Methods of pre-basic seed potato production with special reference to aeroponics—Areview. Scientia Horticulturae. 2016;204:79–87. doi: 10.1016/j.scienta.2016.03.041. [DOI] [Google Scholar]
- Cava F., Lam H., De Pedro M.A., Waldor M.K. Emerging knowledge of regulatory roles of d-amino acids in bacteria. Cell. Mol. Life Sci. 2010;68(5):817–831. doi: 10.1007/s00018-010-0571-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekaran M., Kim K.D., Chun S.C. Antibacterial activity of chitosan nanoparticles: A review. Processes. 2020;8(9):1173–1196. doi: 10.3390/pr8091173. [DOI] [Google Scholar]
- Charkowski A., Sharma K., Parker M.L., Secor G.A., Elphinstone J. Springer; 2020. Bacterial diseases of potato; pp. 351–388. [Google Scholar]
- Damtew E., Tafesse S., Lie R., van Mierlo B., Lemaga B., Sharma K., Struik P.C., Leeuwis C. Diagnosis of management of bacterial wilt and late blight in potato in Ethiopia: A systems thinking perspective. NJAS - Wagening. J. Life Sci. 2018;86-87:12–24. doi: 10.1016/j.njas.2018.03.003. [DOI] [Google Scholar]
- Elieh-Ali-Komi D., Hamblin M.R. Chitin and chitosan: production and application of versatile biomedical nanomaterials. Int. J. Adv. Res. 2016;4(3):411–427. [PMC free article] [PubMed] [Google Scholar]
- Elizabath A., Babychan M., Mathew A.M., Syriac G.M. Application of nanotechnology in agriculture. Int. J. Pure Appl. Biosci. 2019;7(2):131–139. doi: 10.18782/2320-7051.6493. [DOI] [Google Scholar]
- El-Mohamedy R.S., El-Aziz M.E.A., Kamel S. Antifungal activity of chitosan nanoparticles against some plant pathogenic fungi in vitro. Agric. Eng. Int. 2019;21(4):201–209. [Google Scholar]
- El-Saadony M.T., Alkhatib F.M., Alzahrani S.O., Shafi M.E., El. Abdel-Hamid S., Taha T.F., Aboelenin S.M., Soliman M.M., Ahmed N.H. Impact of mycogenic zinc nanoparticles on performance, behavior, immune response, and microbial load in Oreochromis niloticus. Saudi J. Biol. Sci. 2021;28(8):4592–4604. doi: 10.1016/j.sjbs.2021.04.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony M.T., Desoky E.S.M., Saad A.M., Eid R.S., Selem E., Elrys A.S. Biological silicon nanoparticles improve Phaseolus vulgaris L. yield and minimize its contaminant contents on a heavy metals-contaminated saline soil. J. Environ. Sci. 2021;106:1–14. doi: 10.1016/j.jes.2021.01.012. [DOI] [PubMed] [Google Scholar]
- El-Saadony M.T., Saad A.M., Najjar A.A., Alzahrani S.O., Alkhatib F.M., Shafi M.E., Selem E., Desoky E.-S., Fouda S.E.E., El-Tahan A.M., Hassan M.A.A. The use of biological selenium nanoparticles to suppress Triticum aestivum L. crown and root rot diseases induced by Fusarium species and improve yield under drought and heat stress. Saudi. J. Biol. Sci. 2021;28(8):4461–4471. doi: 10.1016/j.sjbs.2021.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony M.T., Saad A.M., Taha T.F., Najjar A.A., Zabermawi N.M., Nader M.M., et al. Selenium nanoparticles from Lactobacillus paracasei HM1 capable of antagonizing animal pathogenic fungi as a new source from human breast milk. Saudi J. Biol. Sci. 2021;28(12):6782–6794. doi: 10.1016/j.sjbs.2021.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony M.T., Sitohy M.Z., Ramadan M.F., Saad A.M. Green nanotechnology for preserving and enriching yogurt with biologically available iron (II) Innov. Food Sci. Emerg. Technol. 2021;69:102645. doi: 10.1016/j.ifset.2021.102645. [DOI] [Google Scholar]
- Esyanti R.R., Farah N., Bajra B.D., Nofitasari D., Martien R., Sunardi S., Safitri R. Comparative study of nano-chitosan and synthetic bactericide application on chili pepper (Capsicum annuum L.) infected by Xanthomonas campestris. Agrivita. 2020;42(1):13–23. [Google Scholar]
- Freeman B.C., Beattie G.A. An overview of plant defenses against pathogens and herbivores. Plant Pathol. Microbiol. 2008 doi: 10.1094/PHI-I-2008-0226-01. [DOI] [Google Scholar]
- Garg U., Chauhan S., Nagaich U., Jain N. Current advances in chitosan nanoparticles based drug delivery and targeting. Adv. Pharm. Bull. 2019;9(2):195–204. doi: 10.15171/apb.2019.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatahi D.M. Challenges and opportunities in tomato production Chain and sustainable standards. Int. J. Hortic. Sci. Technol. 2020;7(3):235–262. doi: 10.22059/IJHST.2020.300818.361. [DOI] [Google Scholar]
- Gitari H.I., Karanja N.N., Gachene C.K.K., Kamau S., Sharma K., Schulte-Geldermann E. Nitrogen and phosphorous uptake by potato (Solanum tuberosum L.) and their use efficiency under potato-legume intercropping systems. F. Crop. Res. 2018;222:78–84. doi: 10.1016/j.fcr.2018.03.019. [DOI] [Google Scholar]
- Gomez K.A., Gomez A.A. 2nd ed. Wiley and Sons; New York, USA: 1984. Statistical Procedures for Agricultural Research. [Google Scholar]
- Guo H., White J.C., Wang Z., Xing B. Nano-enabled fertilizers to control the release and use efficiency of nutrients. Curr. Opin. Environ. Sci. Health. 2018;6(41):77–83. doi: 10.1016/j.coesh.2018.07.009. [DOI] [Google Scholar]
- Hadwiger L.A. Multiple effects of chitosan on plant systems: Solid science or hype. Plant Sci. 2013;208:42–49. doi: 10.1016/j.plantsci.2013.03.007. [DOI] [PubMed] [Google Scholar]
- Hassan O., Chang T. Chitosan for eco-friendly control of plant disease. Asian J. Plant Pathol. 2017;11(2):53–70. doi: 10.3923/ajppaj.2017.53.70. [DOI] [Google Scholar]
- Islam M., Masum S., Mahbub K.R., Haque Z. Antibacterial activity of crab-chitosan against Staphylococcus aureus and Escherichia coli. J. Adv. Scient. Res. 2011;2(4):63–66. [Google Scholar]
- Janse J.D. Review on brown rot (Ralstonia solanacearum race 3, biovar 2, phylotype IIB) epidemiology and control in the Netherlands since 1995: a success story of integrated pest management. J. Plant Pathol. 2012;94(2):257–272. https://www.jstor.org/stable/45156035 [Google Scholar]
- Katiyar D., Hemantaranjan A., Singh B. Chitosan as a promising natural compound to enhance potential physiological responses in plant: a review. Indian J. Plant Physiol. 2015;20(1):1–9. doi: 10.1007/s40502-015-0139-6. [DOI] [Google Scholar]
- Kaur S., Dhillon G.S. The versatile biopolymer chitosan: potential sources, evaluation of extraction methods and applications. Crit. Rev. Microbiol. 2014;40(2):155–175. doi: 10.3109/1040841X.2013.770385. [DOI] [PubMed] [Google Scholar]
- Kempe J., Sequeira L. Biological control of bacterial wilt of potatoes: Attempts to induce resistance by treating tubers with bacteria. Plant Dis. 1983;67:499–503. doi: 10.1094/PD-67-499. [DOI] [Google Scholar]
- Khairy A.M., Tohamy M.R.A., Zayed M.A., Ali M.A.S. Detecting pathogenic bacterial wilt disease of potato using biochemical markers and evaluate resistant in some cultivars. Saudi J. Biol. Sci. 2021;28(9):5193–5203. doi: 10.1016/j.sjbs.2021.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan W.M., Prithiviraj B., Smith D.L. Effect of foliar application of chitin and chitosan oligosaccharides on photosynthesis of maize and soybean. Photosynthetica. 2002;40(4):621–624. [Google Scholar]
- King E.O., Ward M.K., Raney D.E. Two simple media for the demonstration of pyocyanin and fluorescin. J. Lab. Clin. Med. 1954;44(2):301–307. doi: 10.5555/uri:pii:002221435490222X. [DOI] [PubMed] [Google Scholar]
- Kong M., Chen X.G., Xing K., Park H.J. Antimicrobial properties of chitosan and mode of action: a state of the art review. Int. J. Food Microbiol. 2010;144(1):51–63. doi: 10.1016/j.ijfoodmicro.2010.09.012. [DOI] [PubMed] [Google Scholar]
- Kumaraswamy R.V., Kumari S., Choudhary R.C., Pal A., Raliya R., Biswas P., Saharan V. Engineered chitosan based nanomaterials: bioactivities, mechanisms and perspectives in plant protection and growth. Int. J. Biol. Macromol. 2018;113:494–506. doi: 10.1016/j.ijbiomac.2018.02.130. [DOI] [PubMed] [Google Scholar]
- Lin W., Hu X., Zhang W., Rogers W.J., Cai W. Hydrogen peroxide mediates defense responses induced by chitosans of different molecular weights in rice. J. Plant Physiol. 2005;162:937–944. doi: 10.1016/j.jplph.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Malerba M., Cerana R. Recent advances of chitosan applications in plants. Polymers. 2018;10(2):118–128. doi: 10.3390/polym10020118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manjunatha S.B., Biradar D.P., Aladakatti Y.R. Nanotechnology and its applications in agriculture: A review. J. farm Sci. 2016;29(1):1–13. [Google Scholar]
- Mohammed M.A., Syeda J., Wasan K.M., Wasan E.K. An overview of chitosan nanoparticles and its application in non-parenteral drug delivery. Pharmaceutics. 2017;9(4):53–79. doi: 10.3390/pharmaceutics9040053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishat S., Hamim I., Khalil M.I., Ali M.A., Hossain M.A., Meah M.B., Islam M.R. Genetic diversity of the bacterial wilt pathogen Ralstonia solanacearum using a RAPD marker. Comptes Rendus Biologies. 2015;338(11):757–767. doi: 10.1016/j.crvi.2015.06.009. [DOI] [PubMed] [Google Scholar]
- Oh J.W., Chun S.C., Chandrasekaran M. Preparation and in vitro characterization of chitosan nanoparticles and their broad-spectrum antifungal action compared to antibacterial activities against phytopathogens of tomato. Agronomy. 2019;9(1):21–33. doi: 10.3390/agronomy9010021. [DOI] [Google Scholar]
- Pichyangkura R., Chadchawan S. Biostimulant activity of chitosan in horticulture. Scientia Horticulturae. 2015;196:49–65. doi: 10.1016/j.scienta.2015.09.031. [DOI] [Google Scholar]
- Qi L., Xu Z., Jiang X., Hu C., Zou X. Preparation and antibacterial activity of chitosan nanoparticles. Carbohydrate Res. 2004;339(16):2693–2700. doi: 10.1016/j.carres.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Rozman N.A.S., Tong W.Y., Leong C.R., Tan W.N., Hasanolbasori M.A., Abdullah S.Z. Potential antimicrobial applications of chitosan nanoparticles (ChNP) J. Microbiol. Biotechnol. 2019;29(7):1009–1013. doi: 10.4014/jmb.1904.04065. [DOI] [PubMed] [Google Scholar]
- Saad A.M., El-Saadony M.T., El-Tahan A.M., Sayed S., Moustafa M.A., Taha A.E., Ramadan M.M. Polyphenolic extracts from pomegranate and watermelon wastes as substrate to fabricate sustainable silver nanoparticles with larvicidal effect against Spodoptera littoralis. Saudi J. Biol. Sci. 2021;28(10):5674–5683. doi: 10.1016/j.sjbs.2021.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabet K.A. The occurrence of bacterial wilt of potatoes caused by Pseudomonas solanacearum (EF Smith) EF. Sm. in Egypt. Min. of Agric., Extension Dept. Tech. Bull. 1961;112:116–119. [Google Scholar]
- Santiago T.R., Bonatto C.C., Rossato M., Lopes C.A., Mizubuti G.E., Silva L.P. Green synthesis of silver nanoparticles using tomato leaf extract and their entrapment in chitosan nanoparticles to control bacterial wilt. J. Sci. Food Agric. 2019;99(9):4248–4259. doi: 10.1002/jsfa.9656. [DOI] [PubMed] [Google Scholar]
- Sharif R., Mujtaba M., Ur Rahman M., Shalmani A., Ahmad H., Anwar T., Wang X. The multifunctional role of chitosan in horticultural crops; a review. Molecules. 2018;23(4):872–891. doi: 10.3390/molecules23040872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A., Chaudhary B. Preliminary phyco-chemical analysis and in vitro antibacterial screening of Pithophora oedogonia (Mont.) Wittrock: A freshwater green alga forming mats in the water bodies. J. Algal Biomass Util. 2010;1(2):33–41. [Google Scholar]
- Singh D., Chaudhary G., Yadav D.K. Characterization and diversity of Indian isolates of Ralstonia solanacearum inciting bacterial wilt of tomato. Indian Phytopathology. 2021;74:425–429. doi: 10.1007/s42360-021-00365-9. [DOI] [Google Scholar]
- Van N.S., Minh D.H., Anh N.D. Study on chitosan nanoparticles on biophysical characteristics and growth of Robusta coffee in greenhouse. Biocatal. Agric. Biotechnol. 2013;2(4):289–294. doi: 10.1016/j.bcab.2013.06.001. [DOI] [Google Scholar]
- Vanti G.L., Masaphy S., Kurjogi M., Chakrasali S., Nargund V.B. Synthesis and application of chitosan-copper nanoparticles on damping off causing plant pathogenic fungi. Int. J. Biolo. Macromol. 2020;156:1387–1395. doi: 10.1016/j.ijbiomac.2019.11.179. [DOI] [PubMed] [Google Scholar]
- Xing K., Zhu X., Peng X., Qin S. Chitosan antimicrobial and eliciting properties for pest control in agriculture: A review. Agron. Sustain. Dev. 2015;35:569–588. doi: 10.1007/s13593-014-0252-3. [DOI] [Google Scholar]
- Yabuuchi E., Kosako Y., Yano I., Hotta H., Nishiuchi Y. ransfer of two Burkholderia and an Alcaligenes species to Ralstonia Gen. Nov.: Proposal of Ralstonia pickettii (Ralston, Palleroni and Doudoroff 1973) Comb. Nov., Ralstonia solanacearum (Smith 1896) Comb. Nov. and Ralstonia eutropha (Davis 1969) Comb. Nov. Microbiol. Immunol. 1995;39(11):897–904. doi: 10.1111/j.1348-0421.1995.tb03275.x. [DOI] [PubMed] [Google Scholar]
- Zhang D., Wang H., Hu Y., Liu Y. Chitosan controls postharvest decay on cherry tomato fruit possibly via the mitogen-activated protein kinase signaling pathway. J. Agric. Food Chem. 2015;63:7399–7404. doi: 10.1021/acs.jafc.5b01566. [DOI] [PubMed] [Google Scholar]
Further Reading
- Winstead N.N., Kelman A. Inoculation techniques for evaluating resistance to Pseudomonas solanacearum. Phytopathology. 1952;42(11):628–634. [Google Scholar]