Abstract
This study was conducted to examine if modulating transporters like transient receptor potential cation channels, subfamily M, member 7 (TRPM7) underlies the hippocampal neuroprotection afforded by melatonin (Mel) in rats exposed to cerebral hypoperfusion (CHP). Experimental groups included control, Mel-treated (1.87 g/kg), CHP, and CHP + Mel (1.87 g/kg)-treated rats. CHP was induced by the permanent bilateral occlusion of the common carotid arteries (2VO) method and treatments were conducted for 7 days, orally. Mel prevented the damage of the dental gyrus and memory loss in CHP rats and inhibited the hippocampal reactive oxygen species (ROS), lipid peroxidation levels of tumor necrosis factor-α (TNF-α), interleukine-6 (IL-6), interleukine-1 beta (IL-1β), and prostaglandin E2 (PGE2). It also reduced the hippocampal transcription of the TRPM7 channels and lowered levels of calcium (Ca2+) and zinc (Zn2+). Mel Also enhanced the levels of total glutathione (GSH) and superoxide dismutase (SOD) in the hippocampus of the control and CHP-treated rats. In conclusion, downregulation of TRPM7 seems to be one mechanism underlying the neuroprotective effect of Mel against global ischemia and is triggered by its antioxidant potential.
Keywords: Melatonin, Ischemia, Cerebral hypoperfusion, TRPM7, Calcium, Oxidative stress, Hippocampus, Memory
1. Introduction
Stroke is a major vascular disease that affects the brain cells due to a reduction in the blood and is associated with increased mortality and disability (Kuriakose and Xiao, 2020). Based on the etiology, stroke can be ischemic (80–87%) or hemorrhagic (<15%) (Deb et al., 2010). The ischemic results from the vascular occlusion of any of the cerebral arteries due to thrombosis, cardio-embolism, or atherosclerosis, and platelets plug (focal) or complete reduction in blood to the brain (e.g. in cardiac arrest) (global) (Kuriakose and Xiao, 2020). Typical clinical manifestations of stroke are motor, visual, and speech abnormalities including numbness, non-orthostatic vertigo; aphasia and altered speech, diplopia, and sudden unilateral weakness that is contralateral to the affected brain hemisphere (Hankey, 2017).
Cerebral hypoperfusion (CHP) due to cardiac arrest and other vascular abnormalities is the major trigger of ischemia global stroke (Auer, 2016). The increment in the intracellular Ca2+ levels within the neurons is the major mechanism that leads to neurodegeneration and cognitive deficits in patients or animals after CHP (Aoyagi et al., 2012, Auer, 2016). At the molecular levels, CHP increases glutamate accumulation which leads to sustained lethal levels of intracellular Ca2+ levels within the neurons (McBean and Kelly, 1998). Within this view, glutamate facilitates direct Ca2+ entry in the neurons through activating N-methyl-D-aspartate (NMDA) receptors and indirectly from the endoplasmic reticulum (ER) stored and through L-type-dependent Ca2+ channels (Lai et al., 2014, Suvanish Kumar et al., 2014). Consequently, glutamate-induced high Ca2+ levels activate various signaling pathways in the neural cortex, hippocampus, and other areas which leads to oxidative and inflammatory-induced apoptosis and cognitive impairment (McBean and Kelly, 1998, Lai et al., 2014, Lewerenz and Maher, 2015).
However, until now, there is a big debate if CHP-mediated neurodegeneration occurs only through the voltage-gated calcium channels (VGCCs) and findings regarding this matter are still controversial. Although many preclinical studies have shown the benefits of VGCCs and NMDA antagonists (Wu and Tymianski, 2018), results were disappointing in several clinical studies (Cataldi, 2013). This raised the question about the precise molecular mechanisms responsible for the neural damage after blood loss and led to a conclusion that other glutamate-independent mechanisms are involved (Cataldi, 2013, Annunziato et al., 2007). As a result, much research is now focusing on targeting non-glutamate-related Ca + 2 channels as a therapeutic protective strategy against CHP-induced brain damage and memory loss (Li et al., 2011).
Independently of glutamate, transporters like transient receptor potential cation channels, subfamily M, member 7 (TRPM7) were shown to have an indispensable role in the mechanism of neurons apoptosis during the early phases of brains ischemia (Lipski et al., 2006, Lin and Xiong, 2017). TRP channels include eight members (i.e. TRPM1-8) in which TRPM7 are widely found in the neonatal and adult brains, as well as other peripheral tissues including the heart, intestine, smooth muscles, kidney, and liver (Bae and Sun, 2011). In the brain, immunostaining of TRPM7 was detected in the plasma membranes, processes, and cell bodies of the hippocampal neurons, and cholinergic vesicles (Pepperberg and Nakayama, 2016, Bae and Sun, 2011). In general, TRPM7 channels are activated, mainly by free radicals such as reactive oxygen species (ROS) and peroxynitrite (NOO—) (Lipski et al., 2006, Lin and Xiong, 2017). Under normal physiological conditions, TRPM7 channels are important for cell proliferation, survival, and adhesion due to their high permeability of essential metals such as Zn2+, Mg2+, and Ca2+ (Lin and Xiong, 2017). However, several studies have confirmed the damaging and apoptotic roles of TRPM7 during brain ischemia (Visser et al., 2014). In this regard, accumulating data have shown that CHP-induced activation of TRPM7 channels is associated with higher neural levels of Ca2+ and Zn2+ (Lin and Xiong, 2017). Indeed, TRPM7 channels have been identified as a unique pathway for the entry of both Ca2+ and Zn2+ in the neurons with a preference to Zn2+ permeability (Monteilh-Zoller et al., 2003, Clapham, 2003, Aarts et al., 2003a, Aarts et al., 2003b). The inhibition or deletion of TRPM7 rescued neurons from ischemic injury by suppressing the Ca2+ and Zn2+ influx (Choi et al., 2011, Aarts et al., 2003a, Aarts et al., 2003b, Inoue et al., 2010, Bae and Sun, 2011, Chen et al., 2015). Therefore, targeting TRPM7 could be a novel mechanism to protect neural damage and memory deficits after cerebral hypoperfusion.
On the other hand, melatonin (Mel) is an indoleamine released mainly from the pineal gland to regulate circadian rhythms (Reiter et al., 2005). In addition, Mel has many neuroprotective properties and can alleviate the neural oxidative injury and improve cognitive function (Reiter et al., 2003, Reiter et al., 2010, Wang, 2009, Asayama et al., 2003, Kiedrowski et al., 1992). Recently, our laboratory has revealed that Mel could protect against hippocampal damage and defects in memory function in CHP rats by downregulating the SK channels (Al Dera et al., 2019). Until now, it is still largely unknown if such protection also involves modulating the TRMP7 channels.
Therefore, in this study, we aimed to investigate if the protective effect of Mel against CPH-induced hippocampal damage and memory deficits in rats is medaited by supresssing/downregulating TRPM7.
2. Materials and methods
2.1. Animals
Adult male rats of the Wistar type (9 weeks/250 g) were used. All rats were provided by the animal unit at the College of Medicine, Taif University, Saudi Arabia, and were maintained there during the whole period of the study. Housing conditions were always ambient (22 ℃, 60–63% humidity). Normal chow and water were provided ed libetum. All animal protocols were approved by the institutional ethical board at Taif University (IRB #.HAO-02-T-105).
2.2. Induction of global ischemic (CHP)
The induction of the CHP was performed as per our established method using the 2-VO method (Al Dera et al., 2019). In brief, anesthesia was induced by ketamine hydrochloride/ Xylazin hydrochloride solution (50/5 mg/kg). Briefly, the neck of each animal was opened and both carotid arteries were permanently ligated. The incision was then sutured and closed in three-layer. During the surgery, blood temperature was continuously monitored using an anal probe and eye dryness was prevented by applying an ointment. A similar procedure without carotid arteries ligation was also followed in the Sham-operated rats.
2.2.1. Experimental groups
Four groups of rats were included in this study (n = 10 rats/each) as follows: (1) Control rats: sham-operated rats and treated with normal saline as a vehicle, (2) Mel-treated rats: sham-operated rats which were treated with Mel (1.87 g/kg) (# M5250, Sigma Aldrich, MO, USA) (prepared in 96% ethanol which was then diluted with isotonic solution) at a final dose of 1.87 g/kg) (Al Dera et al., 2019), (3) CHP-induced rats: rats with CHP and treated with normal saline, and (4) CHP + Mel-treated rats: rats with CHP and receive Mel (1.87 g/kg) 10 min before exposure to CHP and continue on Mel treatment (1.87 g/kg) for 7 days. All treatments were given by gavage, daily, and orally as 0.5 ml. The selected dose of Mel and treatment regimen were adopted from our previous study which has shown a potent ability of Mel at this dose to alleviate hippocampal degeneration and memory loss, 7 days after induction of CHP (Al Dera et al., 2019).
2.3. Memory function evaluation
The Morris water maze (MWM) test was conducted to evaluate the memory function in all tested rats (Bromley-Brits et al., 2011). Briefly, each rat was trained to find a fixed rescue platform that is submerged in a large swimming container (60 cm m × 1.6 m). This training procedure was conducted daily (three trails/each of 90 s) for 4 days, each by releasing the rats from different 4 hypothetical quadrants with the hidden (submerged) rescue base is always fixed in the one quadrant (northern). The time required for each rat to localize and stand over the submerged rescue base (escape time) was recorded. Finally, a probe trial, with the removal of the escape platform was conducted on day 5 and the number of trials each rat crosses above this area was recorded.
2.4. Collection of the hippocampi and processing
After the behavioral analysis, all rats were anesthetized again as mentioned above and authenticated by the neck dislocation. The brains were removed and each hippocampus was isolated (Al Dera et al., 2019). Four hippocampi were preserved in 10% buffered formalin and the other 6 hippocampi were directly frozen at −70 °C until use.
2.5. Analysis in the hippocampal homogenates
To prepare total hippocampal homogenates, 40 mg of each hippocampus were homogenized in phosphate-buffered saline (PBS/pH = 7.4), centrifuged (11,000×, g/10 min, 4 ℃), and stored −80 ℃. Levels of tumor necrosis factor-alpha (TNF-α), prostaglandin-E2 (PGE2) malondialdehyde (MDA), manganese superoxide dismutase (MnSOD), total reduced glutathione, and interleukin-6 (IL-6) were measured in the homogenates using specific rat’s ELISA kit (# MBS2507393, MBS262150, # MBS268427, # MBS2881838, # MBS265966, # MBS175908 MyBiosources, CA, USA). Homogenate levels of interleukin-1β (IL-1β) were measured by ELISA (# 100768, Abcam, MA, USA). The content of ROS was analyzed using a special fluorometric kit (#186027). The neural levels of Zn+2 and Ca + 2 were assessed using special kits (# 102505, Caymen Chemical, MI, USA and # ab102507, Abcam, MA, USA). All protocols were conducted as instructed by each kit and for a total of 6 samples/group.
2.6. Real-time PCR (qPCR)
Primers were designed and purchased from ThermoFisher. The primer sequences for TRPM7 (acc. # NM_021450) were F: AACCAACACTCTGGAAGAGATCA R: TCAGTCAAGTTTTCTCCCACAC (128 bp) whereas the primer sequences for β-actin (acc. # NM_031144) were: F: ATCTGGCACCACACCTTC and R: AGCCAGGTCCAGACGCA (291 bp). Extraction of RNA and cDNA synthesis was achieved using commercially available kits and as per the provider’s instructions (# 12183018A and # K1621, ThermoFisher, Germany). qPCR was conducted in a CFX96 thermal cycler (BioRad, USA) using the Ssofast Evergreen Supermix (# 172-5200, BioRad, USA) and in steps recommended by the manufactures. The mRNA levels of TRPM7 were normalized against β-actin. cDNA was be removed in two samples/plate as control. The analysis was done in duplicate for n = 6 samples/group.
2.7. Western blot
A small fraction of each hippocampus (35 mg) was homogenized in the radioimmunoprecipitation (RIPA) buffer lysis buffer (# MBS842826, MyBioSource, CA, USA) plus protease inhibitors. The supernatants were isolated (11,200× g/10 min/4 ℃) and protein levels were determined using an assay reagent (# 23225, ThermoFIsher, USA). Equal protein concentrations were separated using the SDS-PAGE. After successful transfer, the nitrocellulose membranes were incubated with the target antibodies (i.e TRPM7 and the loading control, β-actin) (# OST00031W, 240 kDa, 1:2000, TherromFisher Scientific, USA and # 24042, 45 kDa, 1:2000, Cell Signaling technology, MI, USA, respectively). Membranes were incubated with the horseradish peroxidase (HPR)-corresponding antibodies. The ECL Plus Western Blotting Substrate (# 32106, ThermoFisher Scientific, USA) was used as a developing agent and all images were captured and analyzed using the C-Di Git scanner and its available software.
2.8. Histological analysis
Freshly collected hippocampi were in 10% buffered formalin overnight. Tissues were deparaffinized and rehydrated using xylene and descending alcohol concentrations (100%, 90%, and 70%) and then embedded in wax. Using a microtome, all tissues were cut (5 µM) and then stained with hematoxylin/glacial acetic acid solution. All tissues were then rinsed with deionized water, destained with 1:400 v/v HCL/70% ethanol solution. All tissues were then stained with Eosin, dehydrated with 95% and 100% ethanol. Slides were mounted using special media and covered with a coverslip. All images were photographed under a light microscope at a magnification of 200×
2.9. Statistical analysis
All analysis and graphing were performed on the GraphPad prism analysis software by the 1-way ANOVA (version 8, Australia). The normality of the data was evaluated by the Kolmogorov-Smirnov test. The post hoc test was Tukey's test. Values were considered statistically varied at p < 0.05.
3. Results
3.1. Memory
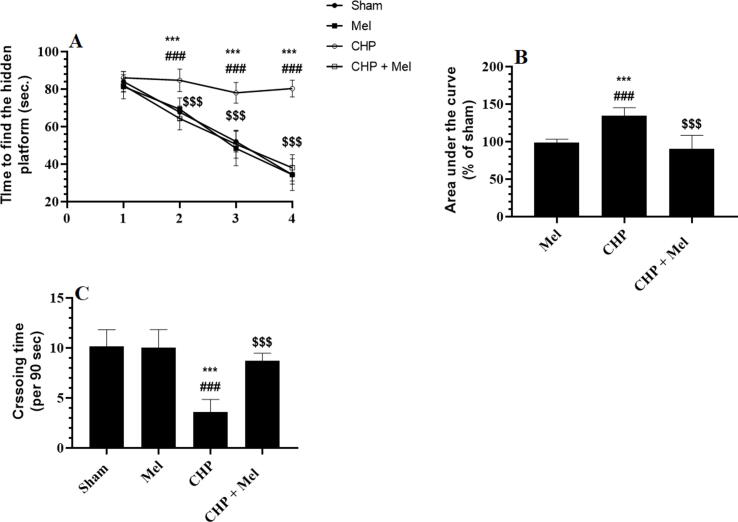
Data obtained from the MWM is shown in Fig. 1A-C. The escape time was significantly and progressively declined in the sham and Mel-treated rats among days 2, 3, and 4 as compared to the start time recorded on day 1 (Fig. 1A&B). Besides, the escape time measured on all these days in the Mel-treated rats was not significantly different as compared to their corresponding time intervals measured on the same data in the sham rats (Fig. 1A&B). Also, the measured crossing number of trials over the removed rescue base was not significantly varied between Mel-treated and sham rats (Fig. 1C). This progressive decline in the escape time (over days 1–4) was not seen in CHP rats where all measured time intervals, on all days of the study, were not significantly varied with each other and were significantly higher than their corresponding daily levels measured in the control and Mel-treated animals (Fig. 1A&B). The CHP rats also had a reduction in the number of crossing trials of the probe trial as compared to sham rats (Fig. 1C). The changes in the 4 days’ escape time, as well as crossing trials, were significantly reversed in CHP + Mel-treated rats when studies against the CHP rats, values which were almost similar and not significantly different to those recorded in sham rats (Fig. 1A–C).
Fig. 1.
Melatonin improves spatial memory function in CHP-model rats. A and B: Represent the escape time intervals and their area under the curve to find the hidden base. C: Represents the average number of crossing times during the probe trial. All data are presented as means ± SD (n = 10 rats/group). ***: p < 0.0001 vs. sham-operated rats. ###: p < 0.0001 vs. Mel-treated rats. $$$: p < 0.0001 vs. rats with cerebral hypoperfusion (CHP).
3.2. Changes in markers of oxidative stress and inflammatory mediators
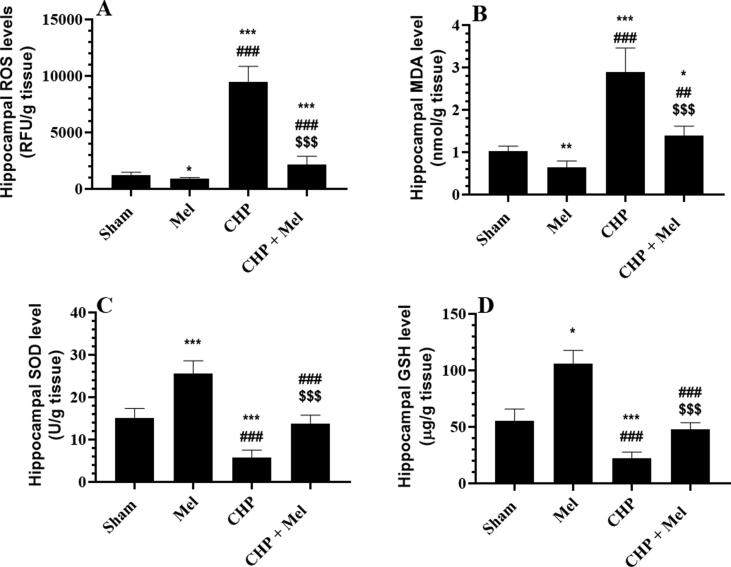
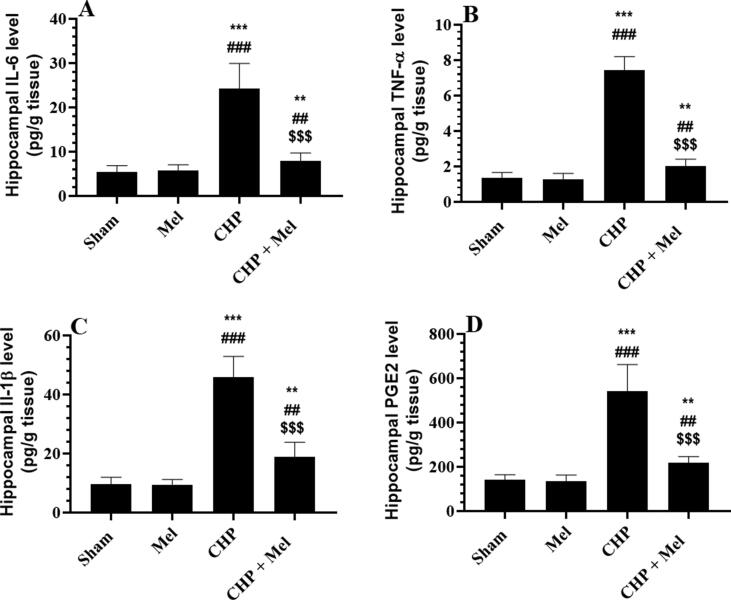
Mel-treated rats showed a reduction in the hippocampal content of MDA and ROS with a parallel increase in the content of GSH and SOD as compared to sham rats (Fig. 2A–D). a significant increment in the content of MDA, ROS, TNF-α, IL-6, IL-1β, and PGE2 with reduced content of SOD and GSH were observed in the decreased in the hippocampi of CHP-induced rats versus the sham rats, all of which were reversed in the CHP + Mel-treated rats (Figs. 2A–D and 3A–D).
Fig. 2.
Melatonin (Mel) suppresses oxidative stress and enhances antioxidant levels in the hippocampi of CHP rats. All data are presented as means ± SD (n = 10 rats/group) *,**,***: p < 0.05, 0.01, and 0.0001, respectively vs. sham-operated rats. ##,###: p < 0.01 and 0.0001, respectively Vs. Mel-treated rats. $$$: p < 0.0001 vs. CHP-induced rats.
Fig. 3.
Melatonin (Mel) suppresses inflammation in the hippocampi of CHP-induced rats. All data are presented as means ± SD (n = 10 rats/group). **,***: p < 0.01, and 0.0001, respectively vs. sham-operated rats. ##,###: p < 0.01 and 0.0001, respectively Vs. Mel-treated rats. $$$: p < 0.0001 vs. CHP-induced rats.
3.3. Hippocampal content of Ca2+ and Zn2+
Sham and Mel-treated rats had similar non-significant hippocampal content of Ca2+ and Zn2+ whereas a significant increment in the levels of both metals was seen in CHP as compared to both groups (Fig. 4A&B). Lower hippocampal content of Ca2+ and Zn2+ were seen in CHP + Mel-treated rats versus the CHP rats (Fig. 4A&B). Interestingly, the hippocampal levels of both Ca2+ and Zn2+ were positively and strongly correlated with the ROS content (Fig. 4C&D).
Fig. 4.
Melatonin (Mel) reduces the elevation in intracellular Zn+2 and Ca + 2 levels in the hippocampi of CHP-induced rats which are positively correlated with the intracellular levels of ROS All data are presented as means ± SD (n = 10 rats/group). *,**,***: p < 0.05, 0.01, and 0.0001, respectively vs. sham-operated rats. ##,###: p < 0.01 and 0.0001, respectively vs. Mel-treated rats. $$$: p < 0.0001 vs. CHP-induced rats.
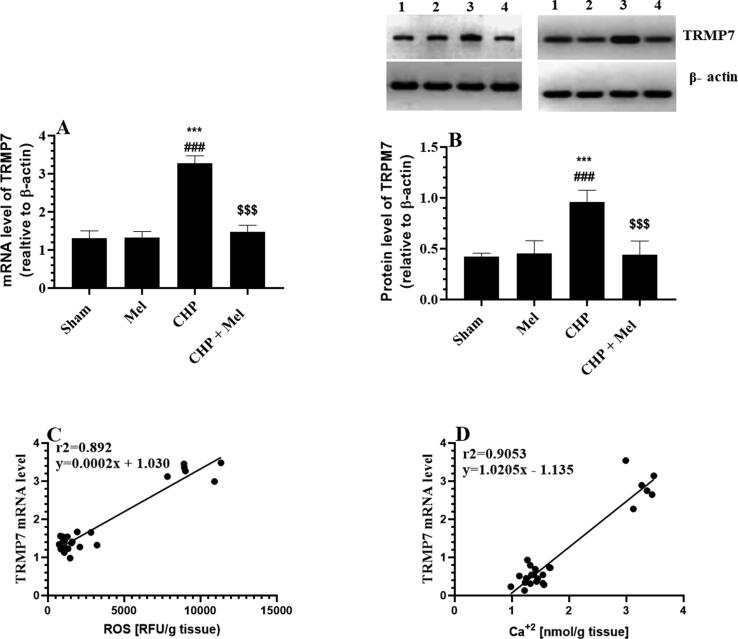
3.4. Expression of TRPM7
Administration of Mel to control rats didn’t alter the transcription or the translation of TRPM7 as compared to sham rats (Fig. 5A&B). As compared to sham rats, significantly upregulated mRNA and protein levels of TRPM7 were seen in the hippocampi of CHP rats which were significantly reduced after administration of Mel (Fig. 5A&B). Also, the mRNA expression of TRPM7 was strongly and positively correlated with the hippocampal content of both ROS and Ca+2 (Fig. 5C&D).
Fig. 5.
Melatonin (Mel) downregulates TRMP7 in the hippocampi of CHP-induced rats which are positively correlated with the intracellular levels of Zn+2 and Ca + 2 levels. All data are presented as means ± SD (n = 10 rats/group). ***: p < 0.0001 vs. to sham-operated rats (lane 1). ###: p < 0.0001, respectively vs. Mel-treated rats (lane 2). $$$: p < 0.0001 vs. CHP-induced rats (lane 3). Lane 4: CHP + Mel.
3.5. Effect of Mel on the hippocampus structure
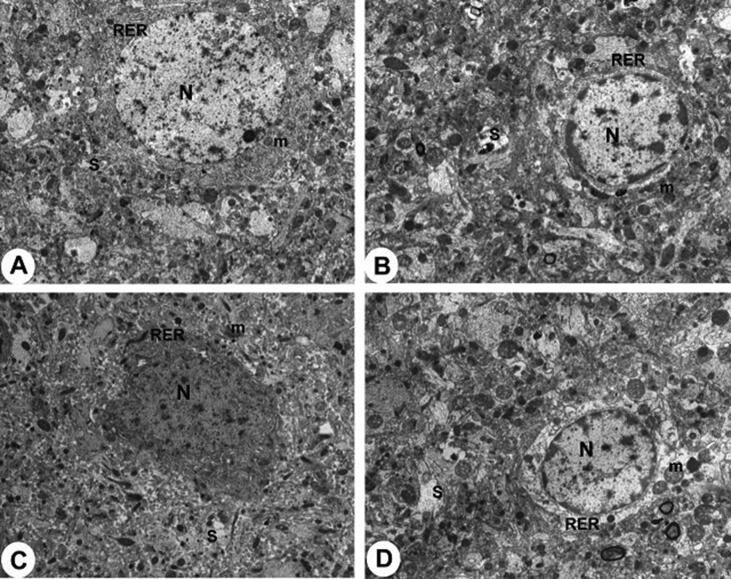
Melatonin administration didn’t affect the morphological features of the dental gyrus (DG) of the treated control rats and this area in both groups showed multiple layers of neural cells that contain intact nuclei (Fig. 6A&B). At the same time, no ultrastructural abnormalities of the DG area were seen between the sham and Mel-treated rats (Fig. 7A&B). An obvious reduction in the number of cells forming the glandular layer of the DG with an increased number of pyknotic cells was observed in CHP rats (Fig. 6C). At the ultrastructural levels, neurons of the DG of CHP-induced rats showed apoptotic cells with pyknotic nuclei, pleomorphic rough endoplasmic reticulum (RER), and damaged mitochondria (Fig. 7C). Normal morphological and ultrastructural features were seen in the DG of the CHP + Mel-treated rats (Fig. 6D& Fig. 7D).
Fig. 6.
Histological micrographs obtained from rat dental gyrus (DG) hippocampus (CA1 region) stained by H&E. 200X. A&B: represents control and Mel-treated rats, demonstrating a normal number of cell layers in the glandular layer of the DG with intact cells containing normally rounded nuclei (Long arrow). C: represents CHP-induced rats and showed an obvious reduction in the number of cells (layers) forming the glandular layer of the DG hippocampal area (short arrow) with many pyknotic apoptotic nuclei (long arrow). D: represents CHP + Mel-treated rats and shows normal DG structure similar to the control rats (long arrow).
Fig. 7.
Transmission electron micrographs (TEM) of the neuron in the hippocampus of rats. (X5000). A & B: represent control and Mel-treated rats, respectively, and show normal hippocampus neurons containing normally rounded nuclei (N), intact mitochondria (m) with double membranes, normal rough endoplasmic reticulum (RER), and. Also, note the presence of intact synapses (S). C: represents CHP-induced rats and showed apoptotic hippocampus neurons with clear damage in the mitochondria (m), apoptotic nuclei (N), and pleomorphic disturbed RER, (X5000). D: represents CHP + Mel-treated rats and showing intact with partially changed hippocampus neuron with normally appeared organelle and clear synapse.
4. Discussion
The exclusive results of this study confirm the role of TRPM7 channels in mediating the hippocampal oxidative and inflammatory damage, as well as memory impairment after CHP in rats which involves increasing Ca2+ and Zn2+ permeability. Besides, they clearly show that Mel is a novel drug that could prevent CHP-induced hippocampal damage by downregulating the expression of these channels mainly by decreasing the generation of ROS.
The brain, and particularly, the hippocampus is very rich in fatty acids which make it vulnerable to oxidative damage (Beaudoin-Chabot et al., 2019). After CHP, glucose, and energy (ATP) deprivation are the major mechanisms leading to neural apoptosis by promoting oxido-inflmmatory damage, (Liu and Zhang, 2012), mechanisms which were well-explained in multiple reviews (Chen et al., 2011, Liu and Zhang, 2012, Shooshtari et al., 2020, Kim et al., 2015). In this context, several authors have shown that ROS and inflammatory cytokines are cross-talked with each other and act in a vicious activation cycle. Within this context, ROS can directly damage the cellular macromolecules, induce lipid peroxidation, scavenge antioxidants, and activate the cellular inflammation by activating the transcription factor, NF-κB (Qu et al., 2014, Moghaddasi et al., 2014, Pirmoradi et al., 2019, Canzoniero et al., 1999, Hafez and El-Kazaz, 2020). Besides, ROS can induce inflammation by upregulating and activating the PGE/EP2 signaling (Liu et al., 2019). Also, ROS and inflammatory cytokines promote mitochondria-mediated (intrinsic) cell apoptosis in the hippocampal neurons after CHP and brain ischemia through upregulating Bax and downregulating Bcl2 (Aboutaleb et al., 2015, Hankey, 2017, Zhao et al., 2018). Furthermore, neural-derived ROS can induce oxidative stress and impair the cerebral blood flow by interacting with nitric oxide (NO) and trigger the generation of peroxynitrite (ONOO—), a vasoconstrictor and potent oxidant molecule (Qu et al., 2014, Gryglewski et al., 1982, Shakil and Saleem, 2014, Shooshtari et al., 2020). In addition, ROS impair the cholinergic function by decreasing the cerebral and hippocampal levels of ACh and AChT (Ferrucci et al., 2018, Wu and Tymianski, 2018).
Supporting these studies, and associated with the obvious decline in the spatial memory function and the damage of the dental gyrus, a significant increment in the hippocampal content of free radicals (ROS/RNS), lipid peroxides (MDA), and inflammatory mediators (PGE2, TNF-α IL-1β, & IL-6) were seen in the CHP-model rats of this study. These data validated our animal model. Similar morphological alterations with reduced learning abilities were also reported in the hippocampi of CHP-induced rats in our previous studies, and effects that and were attributed to increasing levels of ROS (Al Dera et al., 2019). However, antioxidant therapy protected against CHP-induced neural cell death and apoptosis (Hardeland and Pandi-Perumal, 2005, Shirley et al., 2014, Singh et al., 2019). In this study, Mel attenuated all these damaging oxidative, inflammatory, and apoptotic effects, improved the dental gyrus structure, and enhanced spatial memory function in hypoperfused rats. We have also shown previously a similar protective effect in the same animal model, an effect that was also associated with stimulating the hippocampal total antioxidant capacity (Al Dera et al., 2019). However, in this study, we are providing more mechanisms and showed the ability of Mel to also increase levels of intracellular antioxidants in the hippocampi of both the sham and CHP rats. However, since Mel didn’t modulate the hippocampal content of the above-mentioned inflammatory in the sham rats but attenuated them in CHP-rats, these data suggest that scavenging ROS and upregulation of antioxidants is a major mechanism underlying the anti-inflammatory neuroprotective effect of Mel in this animal model.
Similar to our data, Ozacmak et al. (2009) have also shown that chronic administration of Mel prevented ischemia-induced cerebral apoptosis in ovariectomized rats by decreasing MDA levels, restoring contents of GSH and SOD, and suppressing the expression of stress proteins. Many other studies have also reported a similar antioxidant neuroprotective potential of Mel in rodents after induction of ischemia (Tan et al., 2015, Ramos et al., 2017, Qu et al., 2014). Also, the antioxidant protective effect of Mel was shown in other tissue of different animal models of tissue injury (Ji et al., 2019, Johns and Platts, 2014, Anwar et al., 2015, Visser et al., 2014, Wu and Tymianski, 2018). Interestingly, accumulating studies have shown the ability of Mel to cross the BBB and plasma membranes and directly scavenge ROS/RNS (up to 10/1 molecule) as compared to other known classic antioxidants (Anwar et al., 2015, Hacışevki and Baba, 2018). Besides, Mel can alleviate oxidative stress and apoptosis by stimulating GSH and antioxidant enzymes, reducing lipid peroxidation, improving mitochondria function, repairing the DNA after oxidation (Visser et al., 2014, Anwar et al., 2015, Hacışevki and Baba, 2018). The antioxidant potential of Mel was also shown to be largely mediated by generating several antioxidant metabolites like 4OHM; 6OHM, 7HM; and AFMK which all can also BBB and the plasma membranes (Johns and Platts, 2014, Hacışevki and Baba, 2018).
On the other hand, increased neural contents of Ca + 2 and Zn+2 were seen in ischemic cortices and hippocampi and were suggested to be major damaging pathways after ischemia and major mechanisms associated with cognitive impairment (Zhao et al., 2014; Suvanish Kumar et al., 2014; Ji et al., 2019). Blocking Ca2+ current or intracellular chelating of Zn+2 prevented neural cell death in the ischemic brain, cortices, hippocampi after an ischemic episode (Lukic-Panin et al., 2007, Vázquez et al., 2017). While Zn2+ can directly activate apoptotic pathways, intracellular Ca2+ overload induces neural injury and apoptosis by decreasing protein synthesis, promoting mitochondria and plasma membrane damage, stimulating ROS/RNS generation, and activating numerous apoptotic pathways (Li et al., 2011, Morley et al., 1994, Vázquez et al., 2017).
However, glutamate excitotoxicity is a key player that mediates neural apoptosis during brain ischemia through increasing intracellular levels of Ca+2 and ROS (Belov Kirdajova et al., 2020). However, the failure of anti-excitatory drugs to alleviate CHP-induced hippocampal and neural damage in human have led to a conclusion that other glutamate-independent channels are involved (Davis, 2000; Li et al., 2011) Among all non-glutamate Ca2+ channels, particular interest was given to the TRPM7 in mediating global ischemia and neural apoptosis owing to their important roles as pathways for Zn2+ and Ca2+ (Sun et al., 2009, Bae and Sun, 2011, Sun, 2017). Indeed, in vivo and in vitro, hypoxia upregulated mRNA and protein levels and enhanced activities of TRPM7 which were coincided with higher intracellular levels of Zn2+and Ca2+, increased neural and hippocampal cell apoptosis, and impaired memory and behavioral outcomes (Jiang et al., 2008, Bae and Sun, 2011, Chen et al., 2015, Turlova et al., 2016, Sun, 2017).
In the same line with these studies, a significant increment in the transcripts and protein levels of TRPM7 transcription was detected in the hippocampi of the CHP rats of this study. This was also concomitant with higher hippocampal levels of Ca2+ and Zn2+. Although such an increase in Ca2+ levels could be secondary to glutamate excitotoxicity (2010), the concomitant increase in the intracellular Zn2+levels in the CHP-induced rats of this study supports our hypothesis from the involvement of TRPM7 in the obvious hypoxic damage of the hippocampus. Besides, a very strong positive correlation between mRNA levels of TRPM7 and ROS, as well as between Ca2+ and Zn2+ levels and ROS were seen in the hippocampi of rats of this study. This further confirms the role of TRPM7 in CHP-induced hippocampal damage and memory loss and suggests they are mainly triggered by ROS. Yet, the ability of Mel to downregulate mRNA and protein expression of TRPM7, in the hippocampi of CHP-induced rats, but not the control, suggests that the protective effect of Mel involves modulating the expression of these channels. This could be explained by the previously discussed ROS scavenging and antioxidant stimulatory effects of Mel. Interestingly, pharmacological suppression or knocking down TRPM7 prevented neural apoptosis in cultured cortical cells deprived of nutrients and oxygen (Jiang et al., 2008; Sun et al., 2017). It also improved the hippocampus structure and associated behavioral and motor outcomes (i.e. fear response, force, spatial memory, vestibular function, proprioceptive functions, grip test, maladaptive impulsive behavior) and attenuated Bax and caspase-3 in rodents after brain hypoxia (Aarts et al., 2003a, Aarts et al., 2003b, Sun et al., 2009, Bae and Sun, 2011, Chen et al., 2015, Turlova et al., 2016; Sun et al., 2017).
These data indicate that Mel alleviates CHP-mediated hippocampal damage via downregulating TRPM7 and possibly through attenuating oxidative stress by scavenging ROS levels and upregulating antioxidants. Although these data are novel on the expression of TRPM7 channels in the brain of CHP rats, some other existing data may support our findings. Indeed, Mel inhibited Ca2+ overload in the cerebrum of old mice (Molina-Carballo et al., 2007) and reduced protein levels of T-type VGCCs, TRPM-7, and NMDA receptors in rat's brain in epileptic models and exposure to radiation (Reuss et al., 2010, Davis, 2000).
Despite these data, some limitations still exist in this study. Importantly, these data remain observational results and may require more specific animal models and techniques to confirm it. In this context, the effect of Mel on Ca2+ and Zn2+ current should be targeted using more advanced techniques such as patch-clamp studies. Unfortunately, this is not unavailable in our laboratory. In addition, if Mel affords its effect using targeting these pathways could be further examined using transgenic animals or cells lacking TRPM7. In addition, ROS is a major product of glutamate excitotoxicity and given the well-known inhibitory effect of Mel on this pathway (Juan et al., 2014). It could be possible that Mel neuroprotection is also mediated by suppressing this pathway. This needs further future investigation to precisely reveal the proper mechanism of protection.
Overall, our data is still very interesting and show for the first time that Mel neuroprotection against CHP-mediated hippocampal damage involves at least, downregulating TRPM7 channels and subsequent reduction in the intracellular levels of Ca2+ and Zn2+. Mechanism of action involves, at least suppressing ROS and upregulating antioxidants.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement
All authors would like to thank the animal facility unit at Taif University for their contribution to the current study. They would like to thank the technical staff at the core laboratory at the College of Medicine at King Saud Bin AbdulAziz University for Health Sciences for their help in measuring some biochemcial parameters and preparing the histological sections. All authors appeciate Taif University researcher's supporting project (Niumer TURSP-2020/288), Taif University, Saudi Arabia.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Peer review under responsibility of King Saud University.
References
- Aarts E., Aarts E.H., Lenstra J.K. Princeton University Press; 2003. Local Search in Combinatorial Optimization. [Google Scholar]
- Aarts M., Iihara K., Wei W.-L., Xiong Z.-G., Arundine M., Cerwinski W., MacDonald J.F., Tymianski M. A key role for TRPM7 channels in anoxic neuronal death. Cell. 2003;115:863–877. doi: 10.1016/s0092-8674(03)01017-1. [DOI] [PubMed] [Google Scholar]
- Aboutaleb N., Shamsaei N., Khaksari M., Erfani S., Rajabi H., Nikbakht F. Pre-ischemic exercise reduces apoptosis in hippocampal CA3 cells after cerebral ischemia by modulation of the Bax/Bcl-2 proteins ratio and prevention of caspase-3 activation. J. Phsiol. Sci. 2015;65:435–443. doi: 10.1007/s12576-015-0382-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Dera H., Alassiri M., Eleawa S.M., AlKhateeb M.A., Hussein A.M., Dallak M., Sakr H.F., Alqahtani S., Khalil M.A. Melatonin improves memory deficits in rats with cerebral hypoperfusion, possibly, through decreasing the expression of small-conductance Ca(2+)-activated K(+) channels. Neurochem. Res. 2019;44:1851–1868. doi: 10.1007/s11064-019-02820-6. [DOI] [PubMed] [Google Scholar]
- Annunziato F., Cosmi L., Santarlasci V., Maggi L., Liotta F., Mazzinghi B., Parente E., Filì L., Ferri S., Frosali F. Phenotypic and functional features of human Th17 cells. J. Exp. Med. 2007;204:1849–1861. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwar M.J., Muhammad B.Y., Bader A.A., Abdulghani M., Mahmood D., Haider M. An insight into the scientific background and future perspectives for the potential uses of melatonin. Egypt. J. Basic Appl. Sci. 2015;2:139–152. [Google Scholar]
- Aoyagi T., Kusakari Y., Xiao C.Y., Inouye B.T., Takahashi M., Scherrer-Crosbie M., Rosenzweig A., Hara K., Matsui T. Cardiac mTOR protects the heart against ischemia-reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 2012;303:H75–H85. doi: 10.1152/ajpheart.00241.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asayama K., Yamadera H., Ito T., Suzuki H., Kudo Y., Endo S. Double blind study of melatonin effects on the sleep-wake rhythm, cognitive and non-cognitive functions in Alzheimer type dementia. J. Nippon Med. Sch. 2003;70:334–341. doi: 10.1272/jnms.70.334. [DOI] [PubMed] [Google Scholar]
- Auer, R.N., 2016. Histopathology of brain tissue response to stroke and injury. In: Stroke, Elsevier, pp. 47–59.
- Bae C.Y.-J., Sun H.-S. TRPM7 in cerebral ischemia and potential target for drug development in stroke. Acta Pharmacol. Sin. 2011;32:725–733. doi: 10.1038/aps.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaudoin-Chabot C., Wang L., Smarun A.V., Vidović D., Shchepinov M.S., Thibault G. Deuterated polyunsaturated fatty acids reduce oxidative stress and extend the lifespan of C. elegans. Front. Physiol. 2019;10(641) doi: 10.3389/fphys.2019.00641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belov Kirdajova D., Kriska J., Tureckova J., Anderova M. Ischemia-triggered glutamate excitotoxicity from the perspective of glial cells. Front. Cell Neurosci. 2020;14:51. doi: 10.3389/fncel.2020.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromley-Brits K., Deng Y., Song W. Morris water maze test for learning and memory deficits in Alzheimer's disease model mice. J. Vis. Exp. 2011 doi: 10.3791/2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canzoniero L.M., Turetsky D.M., Choi D.W. Measurement of intracellular free zinc concentrations accompanying zinc-induced neuronal death. J. Neurosci. 1999;19:RC31-RC31 doi: 10.1523/JNEUROSCI.19-19-j0005.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cataldi M. The changing landscape of voltage-gated calcium channels in neurovascular disorders and in neurodegenerative diseases. Curr. Neuropharmacol. 2013;11:276–297. doi: 10.2174/1570159X11311030004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Yoshioka H., Kim G.S., Jung J.E., Okami N., Sakata H., Maier C.M., Narasimhan P., Goeders C.E., Chan P.H. Oxidative stress in ischemic brain damage: mechanisms of cell death and potential molecular targets for neuroprotection. Antioxid. Redox Signal. 2011;14:1505–1517. doi: 10.1089/ars.2010.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Xu B., Xiao A., Liu L., Fang X., Liu R., Turlova E., Barszczyk A., Zhong X., Sun C.L. TRPM7 inhibitor carvacrol protects brain from neonatal hypoxic-ischemic injury. Mol. Brain. 2015;8:1–13. doi: 10.1186/s13041-015-0102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi B.R., Kwon K.J., Park S.H., Jeon W.K., Han S.H., Kim H.Y., Han J.S. Alternations of septal-hippocampal system in the adult Wistar rat with spatial memory impairments induced by chronic cerebral hypoperfusion. Exp. Neurobiol. 2011;20:92–99. doi: 10.5607/en.2011.20.2.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham D.E. TRP channels as cellular sensors. Nature. 2003;426:517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- Davis R.J. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- Deb P., Sharma S., Hassan K. Pathophysiologic mechanisms of acute ischemic stroke: An overview with emphasis on therapeutic significance beyond thrombolysis. Pathophysiology. 2010;17:197–218. doi: 10.1016/j.pathophys.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Ferrucci M., Biagioni F., Ryskalin L., Limanaqi F., Gambardella S., Frati A., Fornai F. Ambiguous effects of autophagy activation following hypoperfusion/ischemia. Int. J. Mol. Sci. 2018;19:2756. doi: 10.3390/ijms19092756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryglewski R., Nowak S., Kostka-Trąbka E., Bieroń K., Dembińska-Kieć A., Błaszczyk B., Kuśmiderski J., Markowska E., Szmatoła S. Clinical use of prostacyclin (PGI2) in ischaemic stroke. Pharmacol. Res. Commun. 1982;14:879–908. doi: 10.1016/s0031-6989(82)80012-x. [DOI] [PubMed] [Google Scholar]
- Hacışevki, A., Baba, B., 2018. An overview of melatonin as an antioxidant molecule: a biochemical approach. Melatonin molecular biology, clinical and pharmaceutical approaches, pp. 59–85.
- Hafez M.H., El-Kazaz S.E. The impact of phosphodiesterase-5 inhibitor (sildenafil citrate) on some hippocampal neurotransmitters, oxidative stress status, minerals, and anxiety-like behavior in rats. J. Adv. Vet. Anim. Res. 2020;7:281. doi: 10.5455/javar.2020.g419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankey G.J. Stroke. Lancet. 2017;389:641–654. doi: 10.1016/S0140-6736(16)30962-X. [DOI] [PubMed] [Google Scholar]
- Hardeland R., Pandi-Perumal S. Melatonin, a potent agent in antioxidative defense: actions as a natural food constituent, gastrointestinal factor, drug and prodrug. Nutr. Metab. 2005;2:1–15. doi: 10.1186/1743-7075-2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K., Branigan D., Xiong Z.-G. Zinc-induced neurotoxicity mediated by transient receptor potential melastatin 7 channels. J. Biol. Chem. 2010;285:7430–7439. doi: 10.1074/jbc.M109.040485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter J. L.C.D.-X.Neurotoxins: free radical mechanisms and melatonin protection. Curr. Neuropharmacol. 2010;8:194–210. doi: 10.2174/157015910792246236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen S.L., Forbes D.A., Duncan V., Morgan D.G. Melatonin for cognitive impairment. Cochrane Database Syst. 2006 doi: 10.1002/14651858.CD003802.pub3. Rev:Cd003802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji S.G., Medvedeva Y.V., Wang H.-L., Yin H.Z., Weiss J.H. Mitochondrial Zn2+ accumulation: a potential trigger of hippocampal ischemic injury. The Neuroscientist. 2019;25:126–138. doi: 10.1177/1073858418772548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H., Tian S.-L., Zeng Y., Li L.-L., Shi J. TrkA pathway(s) is involved in regulation of TRPM7 expression in hippocampal neurons subjected to ischemic-reperfusion and oxygen-glucose deprivation. Brain Res. Bull. 2008;76:124–130. doi: 10.1016/j.brainresbull.2008.01.013. [DOI] [PubMed] [Google Scholar]
- Johns J.R., Platts J.A. Theoretical insight into the antioxidant properties of melatonin and derivatives. Org. Biomol. Chem. 2014;12:7820–7827. doi: 10.1039/c4ob01396d. [DOI] [PubMed] [Google Scholar]
- Juan W.S., Huang S.Y., Chang C.C., Hung Y.C., Lin Y.W., Chen T.Y., Lee A.H., Lee A.C., Wu T.S., Lee E.J. Melatonin improves neuroplasticity by upregulating the growth-associated protein-43 (GAP-43) and NMDAR postsynaptic density-95 (PSD-95) proteins in cultured neurons exposed to glutamate excitotoxicity and in rats subjected to transient focal cerebral ischemia even during a long-term recovery period. J. Pineal Res. 2014;56:213–223. doi: 10.1111/jpi.12114. [DOI] [PubMed] [Google Scholar]
- Kiedrowski L., Costa E., Wroblewski J.T. Glutamate receptor agonists stimulate nitric oxide synthase in primary cultures of cerebellar granule cells. J. Neurochem. 1992;58:335–341. doi: 10.1111/j.1471-4159.1992.tb09315.x. [DOI] [PubMed] [Google Scholar]
- Kim G.H., Kim J.E., Rhie S.J., Yoon S. The role of oxidative stress in neurodegenerative diseases. Exp. Neurobiol. 2015;24:325. doi: 10.5607/en.2015.24.4.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriakose D., Xiao Z. Pathophysiology and treatment of stroke: present status and future perspectives. Int. J. Mol. Sci. 2020;21:7609. doi: 10.3390/ijms21207609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai T.W., Zhang S., Wang Y.T. Excitotoxicity and stroke: identifying novel targets for neuroprotection. Prog. Neurobiol. 2014;115:157–188. doi: 10.1016/j.pneurobio.2013.11.006. [DOI] [PubMed] [Google Scholar]
- Lewerenz J., Maher P. Chronic glutamate toxicity in neurodegenerative diseases—what is the evidence? Front. Neurol. 2015;9:469. doi: 10.3389/fnins.2015.00469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M.-H., Inoue K., Si H.-F., Xiong Z.-G. Calcium-permeable ion channels involved in glutamate receptor-independent ischemic brain injury. Acta Pharmacol. Sin. 2011;32:734–740. doi: 10.1038/aps.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J., Xiong Z.-G. TRPM7 is a unique target for therapeutic intervention of stroke. Int. J. Physiol. Pathophysiol. 2017;9:211. [PMC free article] [PubMed] [Google Scholar]
- Lipski J., Park T.I., Li D., Lee S.C., Trevarton A.J., Chung K.K., Freestone P.S., Bai J.-Z. Involvement of TRP-like channels in the acute ischemic response of hippocampal CA1 neurons in brain slices. Brain Res. 2006;1077:187–199. doi: 10.1016/j.brainres.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Liu H., Zhang J. Cerebral hypoperfusion and cognitive impairment: the pathogenic role of vascular oxidative stress. Int. J. Neurosci. 2012;122:494–499. doi: 10.3109/00207454.2012.686543. [DOI] [PubMed] [Google Scholar]
- Liu Q., Liang X., Wang Q., Wilson E.N., Lam R., Wang J., Kong W., Tsai C., Pan T., Larkin P.B. PGE2 signaling via the neuronal EP2 receptor increases injury in a model of cerebral ischemia. Proc. Natl. Acad. Sci. 2019;116:10019–10024. doi: 10.1073/pnas.1818544116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukic-Panin V., Kamiya T., Zhang H., Hayashi T., Tsuchiya A., Sehara Y., Deguchi K., Yamashita T., Abe K. Prevention of neuronal damage by calcium channel blockers with antioxidative effects after transient focal ischemia in rats. Brain Res. 2007;1176:143–150. doi: 10.1016/j.brainres.2007.07.038. [DOI] [PubMed] [Google Scholar]
- McBean D.E., Kelly P.A. Rodent models of global cerebral ischemia: a comparison of two-vessel occlusion and four-vessel occlusion. Gen. Pharmacol. 1998;30:431–434. doi: 10.1016/s0306-3623(97)00284-x. [DOI] [PubMed] [Google Scholar]
- Moghaddasi M., Javanmard S.H., Reisi P., Tajadini M., Taati M. The effect of regular exercise on antioxidant enzyme activities and lipid peroxidation levels in both hippocampi after occluding one carotid in rat. J. Physiol. Sci. 2014;64:325–332. doi: 10.1007/s12576-014-0322-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina-Carballo A., Munoz-Hoyos A., Sanchez-Forte M., Uberos-Fernandez J., Moreno-Madrid F., Acuna-Castroviejo D. Melatonin increases following convulsive seizures may be related to its anticonvulsant properties at physiological concentrations. Neuropediatrics. 2007;38:122–125. doi: 10.1055/s-2007-985138. [DOI] [PubMed] [Google Scholar]
- Monteilh-Zoller M.K., Hermosura M.C., Nadler M.J., Scharenberg A.M., Penner R., Fleig A. TRPM7 provides an ion channel mechanism for cellular entry of trace metal ions. J. Gen. Physiol. 2003;121:49–60. doi: 10.1085/jgp.20028740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley P., Hogan M.J., Hakim A.M. Calcium-mediated mechanisms of ischemic injury and protection. Brain Path. 1994;4:37–47. doi: 10.1111/j.1750-3639.1994.tb00809.x. [DOI] [PubMed] [Google Scholar]
- Ozacmak V.H., Barut F., Ozacmak H.S. Melatonin provides neuroprotection by reducing oxidative stress and HSP70 expression during chronic cerebral hypoperfusion in ovariectomized rats. J. Pineal Res. 2009;47:156–163. doi: 10.1111/j.1600-079X.2009.00695.x. [DOI] [PubMed] [Google Scholar]
- Pei Z., Pang S.F., Cheung R.T.F. Administration of melatonin after onset of ischemia reduces the volume of cerebral infarction in a rat middle cerebral artery occlusion stroke model. Stroke. 2003;34:770–775. doi: 10.1161/01.STR.0000057460.14810.3E. [DOI] [PubMed] [Google Scholar]
- Pepperberg I.M., Nakayama K. Robust representation of shape in a Grey parrot (Psittacus erithacus) Cognition. 2016;153:146–160. doi: 10.1016/j.cognition.2016.04.014. [DOI] [PubMed] [Google Scholar]
- Pirmoradi Z., Yadegari M., Moradi A., Khojasteh F., Mehrjerdi F.Z. Effect of berberine chloride on caspase-3 dependent apoptosis and antioxidant capacity in the hippocampus of the chronic cerebral hypoperfusion rat model. Iran J. Basic Med. Sci. 2019;22:154. doi: 10.22038/ijbms.2018.31225.7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu J., Zhou Q., Du Y., Zhang W., Bai M., Zhang Z., Xi Y., Li Z., Miao J. Rutin protects against cognitive deficits and brain damage in rats with chronic cerebral hypoperfusion. Brit. J. Pharmacol. 2014;171:3702–3715. doi: 10.1111/bph.12725. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ramos E., Patiño P., Reiter R.J., Gil-Martín E., Marco-Contelles J., Parada E., de Los Rios C., Romero A., Egea J. Ischemic brain injury: new insights on the protective role of melatonin. Free Radic. Biol. Med. 2017;104:32–53. doi: 10.1016/j.freeradbiomed.2017.01.005. [DOI] [PubMed] [Google Scholar]
- Reiter R.J., Tan D.-X., Mayo J.C., Sainz R.M., Leon J., Czarnocki Z. Melatonin as an antioxidant: biochemical mechanisms and pathophysiological implications in humans. Acta Biochim. Pol. 2003;50:1129–1146. [PubMed] [Google Scholar]
- Reiter R.J., Tan D.X., Leon J., Kilic U., Kilic E. When melatonin gets on your nerves: its beneficial actions in experimental models of stroke. Exp. Biol. Med. 2005;230:104–117. doi: 10.1177/153537020523000205. [DOI] [PubMed] [Google Scholar]
- Reuss S., Disque-Kaiser U., Binzen U., Greffrath W., Peschke E. 'TRPing' synaptic ribbon function in the rat pineal gland: neuroendocrine regulation involves the capsaicin receptor TRPV1. Neuroendocrinology. 2010;92:133–142. doi: 10.1159/000289765. [DOI] [PubMed] [Google Scholar]
- Shakil H., Saleem S. Prostaglandin i2 ip receptor agonist, beraprost, prevents transient global cerebral ischemia induced hippocampal ca1 injury in aging mice. J. Neurol. Disord. 2014;2 doi: 10.4172/2329-6895.1000174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirley R., Ord E.N., Work L.M. Oxidative stress and the use of antioxidants in stroke. Antioxidants. 2014;3:472–501. doi: 10.3390/antiox3030472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shooshtari M.K., Sarkaki A., Mansouri S.M.T., Badavi M., Khorsandi L., Dehcheshmeh M.G., Farbood Y. Protective effects of Chrysin against memory impairment, cerebral hyperemia and oxidative stress after cerebral hypoperfusion and reperfusion in rats. Metab. Brain Dis. 2020;35:401–412. doi: 10.1007/s11011-019-00527-9. [DOI] [PubMed] [Google Scholar]
- Singh V., Mishra V.N., Chaurasia R.N., Joshi D., Pandey V. Modes of calcium regulation in ischemic neuron. Indian J. Clin. Biochem. 2019;34:246–253. doi: 10.1007/s12291-019-00838-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H.-S., Jackson M.F., Martin L.J., Jansen K., Teves L., Cui H., Kiyonaka S., Mori Y., Jones M., Forder J.P. Suppression of hippocampal TRPM7 protein prevents delayed neuronal death in brain ischemia. Nat. Neurosci. 2009;12:1300–1307. doi: 10.1038/nn.2395. [DOI] [PubMed] [Google Scholar]
- Sun H.S. Role of TRPM7 in cerebral is chaemia and hypoxia. J. Physiol. 2017;595:3077–3083. doi: 10.1113/JP273709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suvanish Kumar V., Gopalakrishnan A., Naziroglu M., Rajanikant G. Calcium ion–the key player in cerebral ischemia. Curr. Med. Chem. 2014;21:2065–2075. doi: 10.2174/0929867321666131228204246. [DOI] [PubMed] [Google Scholar]
- Tan D.X., Manchester L.C., Esteban-Zubero E., Zhou Z., Reiter R.J. Melatonin as a potent and inducible endogenous antioxidant: synthesis and metabolism. Molecules. 2015;20:18886–18906. doi: 10.3390/molecules201018886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turlova E., Bae C.Y., Deurloo M., Chen W., Barszczyk A., Horgen F.D., Fleig A., Feng Z.-P., Sun H.-S. TRPM7 regulates axonal outgrowth and maturation of primary hippocampal neurons. Mol. Neurobiol. 2016;53:595–610. doi: 10.1007/s12035-014-9032-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vázquez J., González B., Sempere V., Mas A., Torija M.J., Beltran G. Melatonin reduces oxidative stress damage induced by hydrogen peroxide in Saccharomyces cerevisiae. Front. Microbiol. 2017;8:1066. doi: 10.3389/fmicb.2017.01066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser D., Middelbeek J., van Leeuwen F.N., Jalink K. Function and regulation of the channel-kinase TRPM7 in health and disease. Eur. J. Cell Biol. 2014;93:455–465. doi: 10.1016/j.ejcb.2014.07.001. [DOI] [PubMed] [Google Scholar]
- Wang X. The antiapoptotic activity of melatonin in neurodegenerative diseases. CNS Neurosci. Ther. 2009;15:345–357. doi: 10.1111/j.1755-5949.2009.00105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q.J., Tymianski M. Targeting NMDA receptors in stroke: new hope in neuroprotection. Mol. Brain. 2018;11:1–14. doi: 10.1186/s13041-018-0357-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao T., Fu Y., Sun H., Liu X. Ligustrazine suppresses neuron apoptosis via the Bax/Bcl-2 and caspase-3 pathway in PC12 cells and in rats with vascular dementia. IUBMB Life. 2018;70:60–70. doi: 10.1002/iub.1704. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Pan R., Li S., Luo Y., Yan F., Yin J., Qi Z., Yan Y., Ji X., Liu K.J. Chelating intracellularly accumulated zinc decreased ischemic brain injury through reducing neuronal apoptotic death. Stroke. 2014;45:1139–1147. doi: 10.1161/STROKEAHA.113.004296. [DOI] [PubMed] [Google Scholar]
Further Reading
- Krajewski S., Mai J.K., Krajewska M., Sikorska M., Mossakowski M.J., Reed J.C. Upregulation of bax protein levels in neurons following cerebral ischemia. J. Neurosci. 1995;15:6364–6376. doi: 10.1523/JNEUROSCI.15-10-06364.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaran D., Udayabanu M., Kumar M., Aneja R., Katyal A. Involvement of angiotensin converting enzyme in cerebral hypoperfusion induced anterograde memory impairment and cholinergic dysfunction in rats. Neuroscience. 2008;155:626–639. doi: 10.1016/j.neuroscience.2008.06.023. [DOI] [PubMed] [Google Scholar]
- Reiter R.J., ACUÑA-CASTROVIEJO D., TAN D.X., Burkhardt S. Free radical-mediated molecular damage: mechanisms for the protective actions of melatonin in the central nervous system. Ann. N.Y. Acad. Sci. 2001;939:200–215. [PubMed] [Google Scholar]