Abstract
Purpose
New vertebral compression fractures(NVCFs) after minimally invasive surgery in patients with osteoporotic vertebral compression fracture (OVCF) is a challenging issue worldwide. Predicting the occurrence of NVCFs is key to addressing such questions. Therefore, we aimed to investigate the risk factors for patients who developed NVCFs after undergoing surgical treatment and establish a nomogram model to reduce the occurrence of NVCFs.
Methods
This study is a retrospective analysis that collected the general characteristics and surgical features of patients who underwent surgical treatment at 2 central institutions between January 2017 and December 2020. Patients were divided into training and testing sets based on the presence or absence of NVCFs. Independent risk factors for NVCFs were obtained in the training set of patients, and then a nomogram model was constructed. Internal and external validation of the nomogram model was performed using the consistency index (C index), receiver operating characteristic curve(ROC), calibration curves, and decision curve analysis (DCA).
Results
A total of 562 patients were included in this study. Patients from the first center were used for nomogram construction and internal validation, and patients from the second center were used as an external validation population. Multivariate regression analysis showed that age, Hounsfield unit (Hu) value, cement leakage, and thoracolumbar (TL) junction fracture were independent risk factors for NVCFs after minimally invasive surgery. The C index was .85, and the validation of internal and external validation shows that the predicted values of the established model is in good agreement with the actual values.
Conclusions
In this study, 4 independent risk factors were obtained by regression analysis, and a nomogram model was constructed to guide clinical work. The application of this model can help surgeons to make more accurate judgments to prevent the occurrence of NVCFs.
Keywords: osteoporotic vertebral compression fracture, new vertebral compression fracture, risk factor, nomogram
Background
One of the most common complications of osteoporosis is osteoporotic vertebral compression fractures. Osteoporosis may be caused by bone structure abnormalities and progressive dicalcium. This may cause compression fractures of the vertebral body during low-energy trauma (e.g., falls, violent coughing, or rolling over).1,2 Patients with OVCFs often suffer from acute or chronic lower back pain, progressive deformity, and even increased mortality.3,4 Previously, conservative treatment was the usual treatment for OVCFs, such as bed rest, opioid analgesia, and bracing. 5 However, it was ineffective and did not relieve the patient’s pain.
Minimally invasive surgery(vertebroplasty and kyphoplasty) has made significant progress in treating OVCFs due to continuous innovations in surgical techniques. The percutaneous kyphoplasty(PKP) technique is a relatively safe and definitive procedure that is widely recognized worldwide.6-8 However, after long-term follow-up, cement leakage and vertebral refracture were common complications in patients receiving PKP for OVCFs.9,10 In a current retrospective study of 403 patients, Li et al. 11 found that the probability of NVCFs was 12.16% in patients who underwent minimally invasive surgery for OVCFs and suggested that bone cement dispersion, bone cement leakage, and anti-osteoporotic treatment were independent risk factors for the development of NVCFs. However, their sample size was small, and there was a lack of external verification, so the practicability of the results cannot be confirmed.
This study is different from previous studies. We developed a nomogram model, that was drawn from independent risk factors obtained by regression analysis. It can improve the readability of the results and the convenience of use. Internal and external validation were also set up in this study, which allows verification of the value of the established model. A scientific and systematic approach was used to evaluate the probability of NVCFs in patients after surgery. The nomogram model could be applied postoperatively to guide surgeons to perform postoperative interventions in high-risk populations, reduce the occurrence of NVCFs, and avoid wasting of medical resources.
Materials and Methods
Study Subjects
The study reviewed 562 patients who underwent PKP surgery for OVCFs from September 2016 to February 2020. The study was approved by the ethics committees of our hospitals. The inclusion criteria were as follows: 1. Low-energy injuries of the elderly individual caused a single-level VCF; 2. The patient has definite back pain, and the visual analog pain score is greater than or equal to 6; 3. Sagittal X-ray showed a decrease in the height of the vertebral body. MRI suggested a hypointense T1 signal and a hyperintense T2 signal or T2 signal, showing significant bone edema in the fractured vertebral body; 4. The patient is a long-term local resident; 5. The posterior wall of the vertebral body is intact with no fractures.
The exclusion criteria were as follows: (1). obvious neural symptoms and spinal cord compression(e.g., numbness, muscle weakness)due to vertebral burst fractures. (2). tumours, infections, and serious medical systemic diseases; (3). Long-term steroid or hormone use; (4). Incomplete follow-up data and presence of language disorders or psychiatric abnormalities.
Our follow-up for all patients was greater than or equal to 2 years. All patients in this study were divided into 2 populations, the modeling population (413 cases) and the validation population (149 cases), who came from 2 different regions and hospitals. The modeling population was divided into a training set (70%) and a testing set (30%). The nomogram model was built with a training set, a testing set was used for internal validation, and a validation population was used for external validation.
Through our review of the previous literature, we collected and analysed risk factors associated with the possible development of NVCFs. We collected general characteristics (e.g., age, sex, BMI), radiological characteristics (e.g., Hu value, kyphotic angle, the volume of bone cement, leakage of cement, fracture level), and previous history (e.g., smoking, alcohol, fracture history, and bisphosphonate therapy).
Surgical Procedures
Patients were treated in the prone position under local anesthesia. The fractured vertebral was localized using puncture needles under the guidance of C-arm fluoroscopy. The puncture needle was placed one-third anterior to the fractured vertebral body through the pedicle under fluoroscopy lateral radiographs. Then take out the puncture needle was removed, and the balloon was inflated to restore the vertebral height in the PKP. The “toothpaste-like” polymethylmethacrylate (PMMA) was instilled, filling the fractured bone. The whole process was slowly completed under fluoroscopy to avoid bone cement leakage. After 5-6 hours of bed rest, the patient can move appropriately after wearing the brace.
Postoperative Management and Assessment
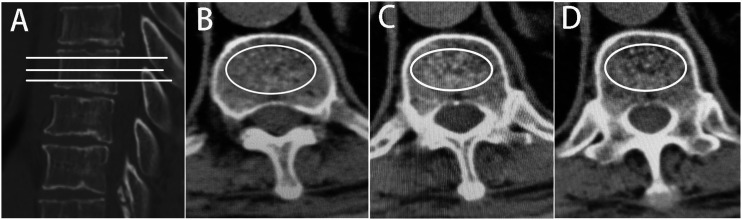
Postoperative patients were recommended to give .25 μg/d calcitriol and D3 600 mg/d calcium. Additionally, after surgery, the patients received zoledronic acid once a year at a dose of 5 mg dissolved in 100 mL saline and were infused intravenously for at least 15 minutes. BMI was defined as the weight (kg) divided by the height (m2) squared. The T12-L1 segment, subject to relatively high spinal activity, is defined as thoracolumbar (TL). Leakage of bone cement was defined as exceeding beyond the superior or inferior endplates. The compression angle of the fractured vertebrae was measured preoperatively and postoperatively. On the lateral radiograph, the angle between the inferior endplate of the superior vertebra of the fractured vertebral and the superior endplate of the inferior vertebra was defined as the kyphotic angle. Change angle restoration was described as a preoperative angle - postoperative angle. Hu values were obtained by analyzing the vertebral body using the picture archiving and communication system (PACS, version 3.6; YLZ Information Technology Co., Ltd; CHINA) system after measurement by our CT equipment. The specific measurements were as follows: the TL junction of the nonfractured vertebral body was selected, and the vertebral body was evenly divided into 3 equal parts in the sagittal position, and the largest elliptical region of interest(ROI) containing only bone trabeculae was drawn in the axial position to obtain the average CT value (Figure 1). Osteoporosis was defined as an HU value less than 100, and osteopenia was defined as an HU value greater than 100 and less than 150.12,13
Figure 1.
CT values were measured by PACS. (A) sagittal image of the lumbar spine with 3 tangents made on the measured vertebrae, corresponding to the 3 levels of (B), (C) and (D) in the axial position. The largest elliptical region of interest(ROI) containing only bone trabeculae was drawn in the axial position to obtain the average Hu value.
Statistical Analysis
In this study, SPSS 23 (IBM Corp., Armonk, NY, USA) and R software (version 3.6.1) were used for statistics and analysis. The continuity variables were statistically analyzed by Student's t test and expressed as the mean values ± standard deviation. The categorical variables were statistically analysed using the chi-square test and reported as percentages. First, the modeling population and the validation population were compared. Then the training and testing sets were compared between groups in the modeling population. Univariate regression analysis was used in the training set to obtain risk factors (P < .05). Furthermore, the final independent risk factors for NVCFs received were used in binary regression analysis. This study used the independent risk factors to develop a predictive nomogram model using R software’s “rms” package.
After the nomogram model was built, we used the testing set and validation population to validate the model. This study used the C index to evaluate the model’s predictive ability. The predictive capacity was gradually increased from 0 to 1. The more widely used ROC curve was plotted, and then the area under the ROC curve was calculated, but the ROC has shortcomings in practical application. Therefore, we supplemented the model validation with calibration curve and decision curve analysis.
Results
Independent Risk Factors to Construct the Nomogram Models
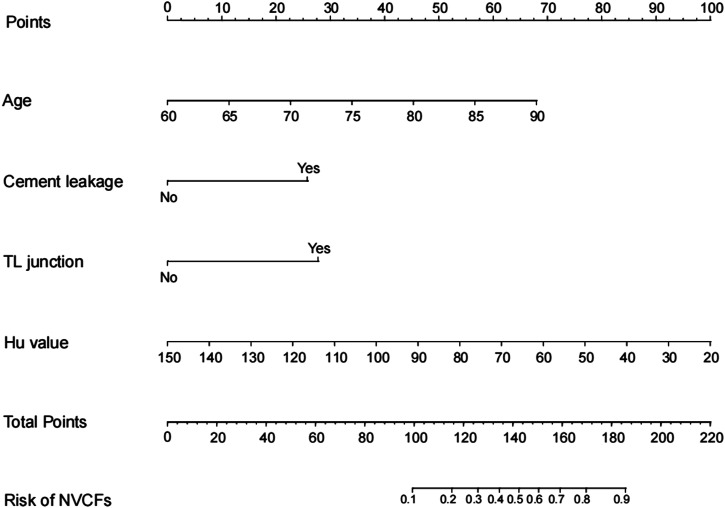
This study included 562 patients, the modeling population was 413 cases, and the validation population was 149 cases. The modeling population included 292 patients in the training set and 121 patients in the testing set. Baseline information on the patients in both groups is shown in Table 1. In the modeling population, the general characteristics of the training set and testing set of patients are shown in Table 2. In the training set, there were significant differences between the NVCFs group and the N-NVCFs group in terms of age, Hu value, cement leakage, and TL junction fracture (Table 3). Multivariate analysis showed that lower age, Hu value, cement leakage, and TL junction fracture were independent risk factors for NVCFs after PKP surgery (Table 4). A novel nomogram model was constructed based on 4 independent risk factors (Figure 2).
Table 1.
Demographic characteristics of the modeling population and the validation population.
| Characteristics | Modeling population(413) | Validation population(149) | P value |
|---|---|---|---|
| Gender | .76 | ||
| Male | 122(29.5%) | 42(28.2%) | |
| Female | 291(70.5%) | 107(71.8%) | |
| Age(year) | 72.47 ± 6.87 | 73.44 ± 7.10 | .15 |
| BMI(Kg/m2) | 22.96 ± 3.23 | 23.26 ± 3.30 | .34 |
| Hu value | 79.71 ± 25.40 | 83.76 ± 26.14 | 1.00 |
| Cement volume | 4.70 ± .80 | 3.12 ± .77 | .01 |
| Cement leakage | .17 | ||
| No | 291(70.5%) | 96(64.4%) | |
| Yes | 122(29.5%) | 53(35.6%) | |
| TL junction | .81 | ||
| No | 148(35.8%) | 55(36.9%) | |
| Yes | 265(64.2%) | 94(63.1%) | |
| Preoperative angle | 18.33 ± 5.86 | 18.79 ± 6.07 | .41 |
| Postoperative angle | 12.05 ± 4.67 | 13.07 ± 6.45 | .08 |
| Change angle | 6.28 ± 4.00 | 5.72 ± 3.52 | .13 |
| Bisphosphonates | .92 | ||
| No | 136(32.9%) | 48(32.2%) | |
| Yes | 277(67.1%) | 101(67.8%) | |
| Smoking | .60 | ||
| No | 243(58.8%) | 84(56.4%) | |
| Yes | 170(41.2%) | 65(43.6%) | |
| Alcohol | .08 | ||
| No | 230(55.7%) | 95(63.8%) | |
| Yes | 183(44.3%) | 54(36.2%) | |
| Fracture history | .34 | ||
| No | 228(55.2%) | 89(59.7%) | |
| Yes | 185(44.8%) | 60(40.3%) | |
| Re-fracture | 1.00 | ||
| No | 313(75.8%) | 113(75.8%) | |
| Yes | 100(24.2%) | 36(24.2%) |
BMI: body mass index; Hu: Hounsfield unit; TL: thoracolumbar.
Table 2.
Demographic Characteristics of the Training Set and Testing Set.
| Characteristics | Training set(292) | Testing set(121) | P value |
|---|---|---|---|
| Gender | .68 | ||
| Male | 88(30.1%) | 34(28.1%) | |
| Female | 204(69.9%) | 87(71.9%) | |
| Age(year) | 72.46 ± 6.99 | 72.50 ± 6.57 | .96 |
| BMI(Kg/m2) | 23.01 ± 3.24 | 22.73 ± 3.19 | .34 |
| Hu value | 79.67 ± 25.45 | 79.86 ± 25.37 | .94 |
| Cement volume | 4.47 ± .80 | 4.79 ± .79 | .16 |
| Cement leakage | .59 | ||
| No | 208(71.2%) | 83(68.8%) | |
| Yes | 84(28.8%) | 38(31.4%) | |
| TL junction | .60 | ||
| No | 107(36.6%) | 41(33.9%) | |
| Yes | 185(63.4%) | 80(66.1%) | |
| Preoperative angle | 18.60 ± 5.88 | 17.67 ± 5.79 | .14 |
| Postoperative angle | 12.28 ± 4.64 | 11.49 ± 4.69 | .12 |
| Change angle | 6.32 ± 4.05 | 6.17 ± 3.89 | .74 |
| Bisphosphonates | .47 | ||
| No | 93(31.8%) | 43(35.5%) | |
| Yes | 199(68.2%) | 78(64.5%) | |
| Smoking | .40 | ||
| No | 168(57.5%) | 75(62%) | |
| Yes | 124(42.5%) | 46(38%) | |
| Alcohol | .93 | ||
| No | 163(55.8%) | 67(55.4%) | |
| Yes | 129(44.2%) | 54(44.6%) | |
| Fracture history | .70 | ||
| No | 163(55.8%) | 65(53.7%) | |
| Yes | 129(44.2%) | 56(46.3%) | |
| Re-fracture | .67 | ||
| No | 223(76.4%) | 90(74.4%) | |
| Yes | 69(23.6%) | 31(25.6%) |
BMI: body mass index; Hu: Hounsfield unit; TL: thoracolumbar.
Table 3.
Preoperative Demographic Characteristics in the Training Set.
| Characteristics | N-NVCF(223) | NVCF(69) | P value |
|---|---|---|---|
| Gender | .12 | ||
| Male | 62(27.8%) | 26(37.7%) | |
| Female | 161(72.2%) | 43(62.3%) | |
| Age(year) | 71.26 ± 6.67 | 76.33 ± 6.63 | <.01 |
| BMI(Kg/m2) | 23.21 ± 3.12 | 22.59 ± 3.58 | .17 |
| Hu value | 84.44 ± 23.59 | 64.17 ± 25.24 | <.01 |
| Cement volume | 4.65 ± .79 | 4.73 ± .82 | .44 |
| Cement leakage | <.01 | ||
| No | 176(78.9%) | 32(46.4%) | |
| Yes | 47(21.1%) | 37(53.6%) | |
| TL junction | <.01 | ||
| No | 96(43.0%) | 11(15.9%) | |
| Yes | 127(57.0%) | 58(84.1%) | |
| Preoperative angle | 18.81 ± 5.98 | 17.92 ± 5.53 | .27 |
| Postoperative angle | 12.43 ± 4.55 | 11.80 ± 4.92 | .33 |
| Change angle | 6.38 ± 4.46 | 6.12 ± 2.28 | .64 |
| Bisphosphonates | .76 | ||
| No | 70(31.4%) | 23(33.3%) | |
| Yes | 153(68.8%) | 46(66.7%) | |
| Smoking | .93 | ||
| No | 128(57.4%) | 40(58.0%) | |
| Yes | 95(42.6%) | 29(42.0%) | |
| Alcohol | .89 | ||
| No | 124(55.6%) | 39(56.5%) | |
| Yes | 99(44.4%) | 30(43.5%) | |
| Fracture history | .49 | ||
| No | 127(57.0%) | 36(52.2%) | |
| Yes | 96(43.0%) | 33(47.8%) |
BMI: body mass index; Hu: Hounsfield unit; TL: thoracolumbar; N-NVCFs: non- new vertebral compression fractures.
Table 4.
Univariate and Multivariate Logistic Analysis of Risk Factors of Non- New Vertebral Compression Fractures After Osteoporotic Vertebral Compres`sion Fracture.
| Characteristics | Univariate | Multivariate | ||
|---|---|---|---|---|
| OR(95%CI) | P value | OR(95%CI) | P value | |
| Gender(female) | .64(.36-1.12) | .12 | ||
| Age(year) | 1.12(1.07-1.17) | <.01 | 1.12(1.07-1.18) | <.01 |
| BMI(Kg/m2) | .94(.86-1.03) | .17 | ||
| Hu value | .96(.95-.97) | <.01 | .96(.94-.98) | <.01 |
| Cement volume | 1.15(.81-1.62) | .44 | ||
| Cement leakage(yes) | 4.33(2.44-7.67) | <.01 | 3.71(1.89-7.28) | <.01 |
| TL junction(yes) | 3.99(1.99-8.00) | <.01 | 4.10(1.84-9.12) | .01 |
| Preoperative angle | 9.74(.93-1.02) | .27 | ||
| Postoperative angle | .97(.91-1.03) | .33 | ||
| Change angle | .98(.92-1.05) | .64 | ||
| Bisphosphonates(yes) | .92(.52-1.63) | .76 | ||
| Smoking(yes) | .98(.57-1.69) | .93 | ||
| Alcohol(yes) | .96(.56-1.66) | .89 | ||
| Fracture history(yes) | 1.21(.71-2.08) | .49 | ||
NVCF: new vertebral compression fracture; OVCF: osteoporotic vertebral compression fracture; BMI: body mass index; Hu: Hounsfield unit; TL: thoracolumbar.
Figure 2.
The nomogram for predicting New vertebral compression fractures in patients with osteoporotic vertebral compression fracture after percutaneous kyphoplasty operation. Each risk factor was assigned a point, which was summed to give a total point that corresponded to the probability of the hazard on the bottom row of the figure according to the total point.
Validation of the Nomogram Model
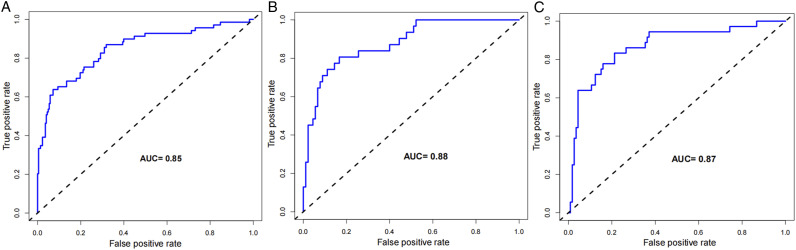
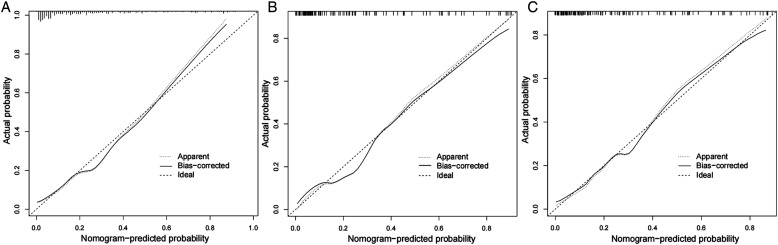
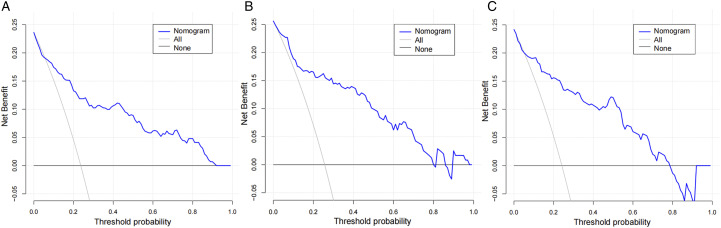
The constructed nomogram model was validated by the populations of internal and external populations. In the modeling population, the C-index of this model was .85 in the training set, which shows that this model has high accuracy. In the testing set and the validation population, the C-index were .88 and .87, respectively. ROC curves were plotted in this study, and the area under the ROC curve (AUC) was calculated for the training set, testing sets, and validation population (Figure 3). The calibration curve results show excellent agreement between the predicted probability and the actual probability of occurrence of NVCFs (Figure 4). The DCA results show that if its threshold is maintained at the 1%-91% range, the nomogram model we constructed is an excellent tool for predicting the occurrence of NVCFs (Figure 5).
Figure 3.
Comparison of the area under the receiver operating characteristic curve between nomogram independent predictors in the training set (A), testing set (B), and validation population(C)
Figure 4.
Comparison of calibration curves between the training set (A), testing set (B), and validation population(C).
Figure 5.
Comparison of decision curve analyses between the training set (A), testing set (B), and validation population(C).
A good model is easy to use in primary care, and the nomogram model is superior to other models in terms of accuracy and convenience. This model was performed by plotting a predictor’s associated independent risk factors high and low with scaled line segments. The prediction scores corresponding to each risk factor were then summed to obtain the total score. Finally, the probability of occurrence of NVCFs was calculated. For example, the Hu value was 50 in an 80-year-old patient. The fractured segment was the TL junction, there was cement leakage, the age was 45, the Hu score was 77, the TL junction score was 28, the cement leakage score was 26, and the total score was 45+77+28+26 = 176, which was equivalent to 82% of the risk of NVCFs after the PKP operation.
Discussion
Minimally invasive surgery (PKP/PVP) is 1 of the best ways to treat patients with OVCF and has effectively relieved patients' low back pain. 14 Despite the many advantages of this technique, postoperative complications are common. 15 Some studies reported 6.5%-34.8% recurrence of NVCFs in patients who underwent PKP surgery.16-19 There are various risk factors for NVCFs, and articles have been published that point to cement leakage, thoracolumbar junction, age, low bone density, and sagittal imbalances as independent risk factors for NVCFs.20-22 In this study, we found that a low Hu value, bone cement leakage, and TL junction are independent risk factors by multivariate regression analysis for the occurrence of NVCFs after surgery. By obtaining clinically available risk factors, this study is the first to construct a nomogram model of postoperative refracture in PKP, which can help surgeons to estimate the probability of postoperative refracture and develop individualized treatment plans for patients.
In a previous study, bone mineral density (BMD) was used to indicate the degree of osteoporosis, and dual-energy X-ray absorptiometry (DXA) was considered as the tool of choice for measuring BMD. 23 Osteoporosis occurs when the BMD (T value) is less than or equal to −2.5 in affected individuals compared to healthy young adults. 24 However, these thresholds were initially used to assess the prevalence of osteoporosis rather than guide treatment thresholds. 25 Related studies have suggested that BMD often overestimates T values when evaluating patients with spinal deformities or severe osteophytes. 12 Previous research suggested that structural changes in the spine will result in a 20% error rate in the T value obtained by DXA. 26 Therefore, it is necessary to find a new method of measuring BMD to improve the accuracy. Quantitative computerized tomography has received much attention in recent years. CT is more widely used in clinical practice and has a more significant advantage in diagnosing vertebral fractures. Therefore, we transformed the CT images through PACS software to obtain the Hu value and used the Hu value to assess the degree of osteoporosis in patients. With the progressive use of the Hu value in clinical practice, some scholars consider the Hu value as an essential complement to BMD.27,28 Hendrickson et al. performed a retrospective analysis by comparing Hu and T values and showed that even though the lumbar Hu value was less than the T value, high sensitivity and specificity of the results could be achieved by adjusting the threshold. 29 In the present study, we included the Hu value as a factor for the occurrence of NVCFs, and the results of multifactorial regression analysis showed that the Hu value was an independent risk factor. Ji et al in 2020, retrospectively analyzed a study of 317 patients with OVCFs. They showed that the Hu value less than 50 was an independent risk factor for the vertebral body in collapse fracture, which remained consistent with our findings. 27
The age in the training set NVCFs vs N-NVCFs groups was (76.33 ± 6.63) vs (71.26 ± 6.67), respectively, with a significant difference between the 2 groups of patients. After a binary retrospective analysis, advanced age was an independent risk factor for the development of NVCFs. Takahara et al 21 retrospectively analyzed 61 female patients with a mean age of 78.9 years. 14 patients had vertebral refractures within 1 month after undergoing PVP, and regression analysis revealed that older age was an independent risk factor for vertebral refractures. A current study by Cui et al. showed that less outdoor exercise increased the risk of osteoporotic fractures as patients aged and that increased exercise reduced this risk. 30 In a meta-analysis conducted by Mao et al. 31 in 2021, 1882 patients were included in 9 articles, of which 340 had a diagnosis of NVCFs, and their results showed that older age and cement leakage were independent risk factors for vertebral refracture.
Bone cement is the most commonly used in PKP and provides rapid pain relief to patients when injected into the fractured vertebral body. However, cement leakage is prone to occur during cement injection, and most patients are asymptomatic. 32 Baek et al. showed that leakage of bone cement did not increase the risk of refracture. 22 In contrast, Mao et al. 31 performed a meta-analysis in 2021, and the results showed a strong correlation between bone cement leakage and vertebral refracture. A study on cement leakage showed that 14.7% of patients with vertebral fractures underwent minimally invasive treatment-experienced intraoperative cement leakage. 33 Therefore, some studies have also found a correlation between vertebral refracture and cement leakage,28,31 consistent with the results of the present study. The intervertebral disc is a cushioning device between 2 vertebral bodies. When the bone cement leaks through the upper and lower vertebral plates into the disc, the cushioning capacity of the disc decreases, increasing the stress on the adjacent vertebral body and, therefore, refracture of the adjacent vertebral body is likely to occur.31,34 Ahn et al. 35 insisted that the cause of refracture of non-adjacent vertebrae is the dynamic hammer effect due to the different mobilities of different segments of the spine. Therefore, when injecting bone cement into fractured vertebrae, it should be injected slowly under fluoroscopy to minimize cement leakage and reduce postoperative complications.
TL junction fractures are a common site for vertebral compression fractures. In this study, 63.9% of patients had a TL segment fracture. In a recent study meta-analysis by Yu et al. 36 , nine articles were included, and after analysis, 5 independent risk factors for vertebral refracture were identified, including fractures of the TL segment, which is in line with our study. Holmes et al. 37 studied 260 fractured vertebrae in 152 patients. Since the TL segment makes up the biomechanical anatomy of the transition zone and is significantly more mobile than other non-TL segments, their results showed that the TL segment is the most common site of fracture in the spine.
The nomogram model has been widely used in clinical settings. A large sample identifies risk factors for the occurrence of certain disease and constructs a nomogram model. Therefore, we developed a nomogram model and applied it to provide more accurate and individualized guidance and advice to patients with recurrent vertebral collapse fractures.
There are some shortcomings in this study. First, this was a retrospective study, and there was some bias in the selection of patients. However, we reduce this deficiency by using a relatively large sample size; Second, the nomogram model has been validated by internal and external tests, but there is a lack of other data from different hospitals and regions for validation. Third, the risk factors included in this study are readily available in the clinical setting. We believe that the model applies to any area and hospital; Thirdly, The risk factors included in this study were the Hu value rather than T values, thus improving the accuracy of this study. However, when measuring these 2 indicators, different hospitals cannot guarantee the use of the same type of equipment, so some errors exist.
Conclusion
The age, Hu value, cement leakage, and TL junction fracture were risk factors for NVCFs after undergoing surgical treatment according to multifactorial regression analysis. A nomogram model was constructed using these factors. This model can help surgeons in the aggressive postoperative management of vertebral fractures in elderly patients with osteoporosis, thereby reducing the incidence of NVCFs and avoiding the waste of health care resources.
Footnotes
Authors' Contributions: Author FC B, YS A, and JH F designed the study. Author GY B and YS A collected the clinical data and conducted the statistical analysis. Author FC B and DY W wrote the manuscript. Author FC B, YS A, DY W, and JH F revised the manuscript; All authors critically read the manuscript to improve intellectual content. All authors read and approved the final manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics Approval: This research was approved by the ethics committee of Daqing Oilfield General Hospital and Chengde Medical University Affiliated Hospital.
Consent to participate: Written informed consent was obtained from all participants. All participants agreed with the data and publication of the manuscript.
Consent for publication: All participants agreed with the data and publication of the manuscript.
Availability of Data and Material: The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
ORCID iD
FuCheng Bian https://orcid.org/0000-0003-4277-3039
References
- 1.Yang H, Pan J, Wang G. A review of osteoporotic vertebral fracture nonunion management. Spine (Phila Pa 1976). 2014;39:B4-B6. doi: 10.1097/BRS.0000000000000538. [DOI] [PubMed] [Google Scholar]
- 2.Longo UG, Loppini M, Denaro L, Maffulli N, Denaro V. Conservative management of patients with an osteoporotic vertebral fracture: a review of the literature. J Bone Joint Surg Br. 2012;94:152-157. doi: 10.1302/0301-620X.94B2.26894. [DOI] [PubMed] [Google Scholar]
- 3.Silverman S. The clinical consequences of vertebral compression fracture. Bone. 1992;13(suppl 2):S27-S31. doi: 10.1016/8756-3282(92)90193-z. [DOI] [PubMed] [Google Scholar]
- 4.Korovessis P, Vardakastanis K, Repantis T, Vitsas V. Balloon kyphoplasty versus KIVA vertebral augmentation--comparison of 2 techniques for osteoporotic vertebral body fractures: a prospective randomized study. Spine (Phila Pa 1976). 2013;38:292-299. doi: 10.1097/BRS.0b013e31826b3aef. [DOI] [PubMed] [Google Scholar]
- 5.Yang W, Yang J, Liang M. Percutaneous vertebroplasty does not increase the incidence of new fractures in adjacent and nonadjacent vertebral bodies. Clin Spine Surg. 2019;32:E99-E106. doi: 10.1097/BSD.0000000000000734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li YX, Guo DQ, Zhang SC, et al. Risk factor analysis for re-collapse of cemented vertebrae after percutaneous vertebroplasty (PVP) or percutaneous kyphoplasty (PKP). Int Orthop. 2018;42:2131-2139. doi: 10.1007/s00264-018-3838-6. [DOI] [PubMed] [Google Scholar]
- 7.Buchbinder R, Osborne RH, Ebeling PR, et al. A randomized trial of vertebroplasty for painful osteoporotic vertebral fractures. N Engl J Med. 2009;361:557-568. doi: 10.1056/NEJMoa0900429. [DOI] [PubMed] [Google Scholar]
- 8.Kaliya-Perumal AK, Lin TY. Clinical outcomes of percutaneous vertebroplasty for selective single segment dorsolumbar vertebral compression fractures. J Clin Orthop Trauma. 2018;9:S140-S144. doi: 10.1016/j.jcot.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang C, Fan S, Liu J, Suyou L, Shan Z, Zhao F. Basivertebral foramen could be connected with intravertebral cleft: a potential risk factor of cement leakage in percutaneous kyphoplasty. Spine J. 2014;14:1551-1558. doi: 10.1016/j.spinee.2013.09.025. [DOI] [PubMed] [Google Scholar]
- 10.Rho YJ, Choe WJ, Chun YI. Risk factors predicting the new symptomatic vertebral compression fractures after percutaneous vertebroplasty or kyphoplasty. Eur Spine J. 2012;21:905-911. doi: 10.1007/s00586-011-2099-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Q, Long X, Wang Y, et al. Development and validation of a nomogram for predicting the probability of new vertebral compression fractures after vertebral augmentation of osteoporotic vertebral compression fractures. BMC Musculoskelet Disord. 2021;22:957. doi: 10.1186/s12891-021-04845-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gausden EB, Nwachukwu BU, Schreiber JJ, Lorich DG, Lane JM. Opportunistic use of CT imaging for osteoporosis screening and bone density assessment: A qualitative systematic review. J Bone Joint Surg Am. 2017;99:1580-1590. doi: 10.2106/JBJS.16.00749. [DOI] [PubMed] [Google Scholar]
- 13.Pickhardt PJ, Pooler BD, Lauder T, del Rio AM, Bruce RJ, Binkley N. Opportunistic screening for osteoporosis using abdominal computed tomography scans obtained for other indications. Ann Intern Med. 2013;158:588-595. doi: 10.7326/0003-4819-158-8-201304160-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Voormolen MH, Mali WP, Lohle PN, et al. Percutaneous vertebroplasty compared with optimal pain medication treatment: short-term clinical outcome of patients with subacute or chronic painful osteoporotic vertebral compression fractures. The VERTOS study. AJNR Am J Neuroradiol. 2007;28:555-560. [PMC free article] [PubMed] [Google Scholar]
- 15.Lee MJ, Dumonski M, Cahill P, Stanley T, Park D, Singh K. Percutaneous treatment of vertebral compression fractures: a meta-analysis of complications. Spine (Phila Pa 1976). 2009;34:1228-1232. doi: 10.1097/BRS.0b013e3181a3c742. [DOI] [PubMed] [Google Scholar]
- 16.Lee BG, Choi JH, Kim DY, Choi WR, Lee SG, Kang CN. Risk factors for newly developed osteoporotic vertebral compression fractures following treatment for osteoporotic vertebral compression fractures. Spine J. 2019;19:301-305. doi: 10.1016/j.spinee.2018.06.347. [DOI] [PubMed] [Google Scholar]
- 17.Movrin I, Vengust R, Komadina R. Adjacent vertebral fractures after percutaneous vertebral augmentation of osteoporotic vertebral compression fracture: a comparison of balloon kyphoplasty and vertebroplasty. Arch Orthop Trauma Surg. 2010;130:1157-1166. doi: 10.1007/s00402-010-1106-3. [DOI] [PubMed] [Google Scholar]
- 18.Ko BS, Cho KJ, Park JW. Early adjacent vertebral fractures after balloon kyphoplasty for osteoporotic vertebral compression fractures. Asian Spine J. 2019;13:210-215. doi: 10.31616/asj.2018.0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harrop JS, Prpa B, Reinhardt MK, Lieberman I. Primary and secondary osteoporosis' incidence of subsequent vertebral compression fractures after kyphoplasty. Spine (Phila Pa 1976). 2004;29:2120-2125. doi: 10.1097/01.brs.0000141176.63158.8e. [DOI] [PubMed] [Google Scholar]
- 20.Park JS, Park YS. Survival analysis and risk factors of new vertebral fracture after vertebroplasty for osteoporotic vertebral compression fracture. Spine J. 2021;21:1355-1361. doi: 10.1016/j.spinee.2021.04.022. [DOI] [PubMed] [Google Scholar]
- 21.Takahara K, Kamimura M, Moriya H, et al. Risk factors of adjacent vertebral collapse after percutaneous vertebroplasty for osteoporotic vertebral fracture in postmenopausal women. BMC Musculoskelet Disord. 2016;17:12. doi: 10.1186/s12891-016-0887-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baek SW, Kim C, Chang H. The relationship between the spinopelvic balance and the incidence of adjacent vertebral fractures following percutaneous vertebroplasty. Osteoporos Int. 2015;26:1507-1513. doi: 10.1007/s00198-014-3021-x. [DOI] [PubMed] [Google Scholar]
- 23.Kanis JA. Diagnosis of osteoporosis and assessment of fracture risk. Lancet. 2002;359:1929-1936. doi: 10.1016/S0140-6736(02)08761-5. [DOI] [PubMed] [Google Scholar]
- 24.Kanis JA. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: synopsis of a WHO report. WHO Study Group. Osteoporos Int. 1994;4:368-381. doi: 10.1007/BF01622200. [DOI] [PubMed] [Google Scholar]
- 25.Schuit SC, van der Klift M, Weel AE, et al. Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam Study. Bone. 2004;34:195-202. doi: 10.1016/j.bone.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 26.Bolotin HH. DXA in vivo BMD methodology: an erroneous and misleading research and clinical gauge of bone mineral status, bone fragility, and bone remodelling. Bone. 2007;41:138-154. doi: 10.1016/j.bone.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 27.Ji C, Rong Y, Wang J, et al. Risk factors for refracture following primary osteoporotic vertebral compression fractures. Pain Physician. 2021;24:E335-E340. [PubMed] [Google Scholar]
- 28.Zhong BY, He SC, Zhu HD, et al. Risk prediction of new adjacent vertebral fractures after pvp for patients with vertebral compression fractures: Development of a prediction model. Cardiovasc Intervent Radiol. 2017;40:277-284. doi: 10.1007/s00270-016-1492-1. [DOI] [PubMed] [Google Scholar]
- 29.Hendrickson NR, Pickhardt PJ, Del Rio AM, Rosas HG, Anderson PA. Bone mineral density T-scores derived from CT attenuation numbers (Hounsfield Units): Clinical utility and correlation with dual-energy X-ray absorptiometry. Iowa Orthop J. 2018;38:25-31. [PMC free article] [PubMed] [Google Scholar]
- 30.Cui L, Chen L, Xia W, et al. Vertebral fracture in postmenopausal Chinese women: a population-based study. Osteoporos Int. 2017;28:2583-2590. doi: 10.1007/s00198-017-4085-1. [DOI] [PubMed] [Google Scholar]
- 31.Mao W, Dong F, Huang G, et al. Risk factors for secondary fractures to percutaneous vertebroplasty for osteoporotic vertebral compression fractures: a systematic review. J Orthop Surg Res. 2021;16:644. doi: 10.1186/s13018-021-02722-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martikos K, Greggi T, Faldini C, Vommaro F, Scarale A. Osteoporotic thoracolumbar compression fractures: long-term retrospective comparison between vertebroplasty and conservative treatment. Eur Spine J. 2018;27:244-247. doi: 10.1007/s00586-018-5605-1. [DOI] [PubMed] [Google Scholar]
- 33.Chen C, Fan P, Xie X, Wang Y. Risk factors for cement leakage and adjacent vertebral fractures in kyphoplasty for osteoporotic vertebral fractures. Clin Spine Surg. 2020;33:E251-E255. doi: 10.1097/BSD.0000000000000928. [DOI] [PubMed] [Google Scholar]
- 34.Sun YC, Teng MM, Yuan WS, et al. Risk of post-vertebroplasty fracture in adjacent vertebral bodies appears correlated with the morphologic extent of bone cement. J Chin Med Assoc. 2011;74:357-362. doi: 10.1016/j.jcma.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 35.Ahn Y, Lee JH, Lee HY, Lee SH, Keem SH. Predictive factors for subsequent vertebral fracture after percutaneous vertebroplasty. J Neurosurg Spine. 2008;9:129-136. doi: 10.3171/SPI/2008/9/8/129. [DOI] [PubMed] [Google Scholar]
- 36.Yu W, Xu W, Jiang X, Liang D, Jian W. Risk factors for recollapse of the augmented vertebrae after percutaneous vertebral augmentation: a systematic review and meta-analysis. World Neurosurg. 2018;111:119-129. doi: 10.1016/j.wneu.2017.12.019. [DOI] [PubMed] [Google Scholar]
- 37.Holmes JF, Miller PQ, Panacek EA, Lin S, Horne NS, Mower WR. Epidemiology of thoracolumbar spine injury in blunt trauma. Acad Emerg Med. 2001;8:866-872. doi: 10.1111/j.1553-2712.2001.tb01146.x. [DOI] [PubMed] [Google Scholar]