Abstract
Use of chimeric antigen receptors (CARs), as an immune cell therapy, has generated excellent clinical outcomes against hematologic tumors in recent years. Among them, the CAR-NK (natural killer) therapy has shown better efficacy, and less toxicity, than chimeric antigen receptor T-cell (CAR-T) therapy. In our phase II clinical trials, administering chimeric costimulatory converting receptor (CCCR)-NK92 cells on advanced non-small cell lung cancer patients proved efficacious in cell and animal experiments. However, we observed occurrence of cytokine release syndrome (CRS), a rare and unexpected side effect, never reported before during CAR-NK therapy. Here, we provide a detailed report of the patient’s case, emphasize on the need to pay attention to CRS in NK cell therapy, and suggest improvements that will minimize potential toxicity.
Keywords: immune cell therapy, chimeric antigen receptor, cytokine release syndrome, CAR-NK
Introduction
Immune cell therapy, in which immune cells are used to fight tumors, is a novel approach for treating cancer 1 . Chimeric antigen receptor T-cell (CAR-T) therapy is one of the most commonly used cell therapies that uses CARs-engineered T cells for cancer therapy 2 . Its targeting, lethality, and persistence makes it the most convincing breakthrough in immune cell therapy. According to the 2019 New York Cancer Institute (CRI) analysis of global cancer cell treatment results, there were 1011 cell therapies worldwide, as of May 2019, of which 568 (56.2%) were CAR-T (the global landscape of cancer cell therapy) 3 . However, the CAR-T technology has been associated with numerous limitations, including difficulty to collect enough lymphocytes, especially from heavily pretreated or lymphopenic patients, as well as time-consuming and costly manufacture of CAR-T cells 4 . In addition, CAR-T therapy has been shown to cause substantial morbidities, which are mainly associated with a serious side effect called cytokine release syndrome (CRS) caused by abundant cytokine release 5 .
Natural killer (NK) cells are innate immune effector cells that are capable of killing tumor cells and producing cytokines without previous stimulation 6 . The CAR-NK therapy does not cause CRS, due to a different spectrum of the secreted cytokines, and is therefore considered safer than CAR-T7,8. The NK92, a cell line established from non-Hodgkin’s lymphoma, has potential therapeutic effects due to its characteristics of activated NK cells 9 . Consequently, it has been extensively studied in the management of hematologic and solid tumors 10 . In our research group, we sought to produce an efficient, industrially produced NK cell therapy technology. Therefore, we designed a novel chimeric costimulatory converting receptor (CCCR), comprising mainly the extracellular domain of PD1, transmembrane and cytoplasmic domains of NKG2D, and the cytoplasmic domain of 41BB. This NK-tailored CCCR was able to switch the negative PD1 signal to an activating signal and hence reversed the immune suppressive effects of PD1. We first adopted quantitative real-time polymerase chain reaction (PCR) (qPCR) technology to synthesize an artificially synthesized chimeric costimulatory convert receptor coding gene PD1-NKG2D-41BB; we then obtained recombinant vector PD1-NKG2D-41BB-pCDH by using lentivirus as vector; and then, the lentivirus-packaged recombinant vector was transfected into NK-92 cells to obtain stably expressed CCCR-NK92 cell. Next, we detected the expression of PD-1 and the cell proliferation rate in CCCR-NK92 cells, and verified the killing effect of CCCR-NK92 cells on lung cancer cell lines in vitro. Finally, animal experiments were carried out in the human lung cancer NOG mouse subcutaneous xenografts model. The tumor volumes of the mice treated with CCCR-NK92 cells were significantly smaller than those of the control, and no significant toxic or side effects were observed in the experimental mouse 11 . Thereafter, we designed a phase II clinical trial to evaluate efficacy and safety of the CCCR-NK92 cell therapy. Two patients were enrolled into the clinical trial; one patient developed CRS after CCCR-NK92 cell therapy. Here, we provide a detailed report of this case and emphasize on the need to pay attention to CRS during NK cell therapy. To our knowledge, this is the first report describing CRS after NK cell therapy.
Case Presentation
A 69-year-old female patient was histopathologically diagnosed with non-small cell lung cancer (NSCLC) on February 2, 2017, in the First Affiliated Hospital of Xinxiang Medical University. Image results showed intracranial metastases, whereas genetic tests revealed Exon-19 19-del mutant and T790 mutation, as well as PD-L1 expression ≥1% in the lung tissue. She was first given gefitinib for 3 months. However, she was switched to Osimertinib for 10 months due to disease progression. After that, she received head radiotherapy on July 13, 2018, due to intracranial metastases. She was administered with targeted therapeutic drugs and local radiation after 3 months, and then evaluated on September 27, 2018. She refused chemotherapy for fear of side effects. Considering that her condition was suitable, she was enrolled in the clinical trial comprising CCCR-NK92 cells for NSCLC. However, multistage treatment of late recurrence and metastasis failed (NCT03656705) in November 2018. The CCCR-NK92 cells were intravenously injected, twice a week (d0/d3), for a 7-day treatment cycle. The first cycle comprised 1 × 107 CCCR-NK92 cells, as the beginning dose, which was increased to 2 × 107 at the second injection. The dose was increased during the second and third cycles, with 5 × 107 and 1 × 108 cells per injection, administered, respectively, according to her tolerance levels. During those treatment cycles, the patient experienced high fever, which was subsequently controlled to a normal range after symptomatic treatment. A chest computed tomography (CT), performed after this three-cycle treatment, revealed that the lesion was stable.
After the third injection, of the second cycle, we observed a series of unexpected symptoms. Specifically, the patient developed hypotensive shock, coupled with high fever, hemoptysis, myalgia, liver damage, and abnormal blood coagulation (Fig. 1 and Table 1). We found neither evidence of infection in blood cultures nor signs of tumor lysis. Multidisciplinary consultations suggested occurrence of CRS. Consequently, we immediately performed anti-infection, continuous pumping of norepinephrine to maintain blood pressure, methylprednisolone shock, transfusion of clotting factor with cryoprecipitation, and symptomatic treatment. We also reported the severe adverse effect (SAE) to the ethics committee at our hospital. We exposed the patient to an initial dose of 80 mg/d methylprednisolone on January 4, 2019; gradually increased this dose to a maximum 180 mg/d on January 9, 2019; and then gradually reduced the dose. Finally, we administered a low-dose oral prednisone tapering and then stopped the medication on March 16, 2019. Telephone follow-up was terminated SAE.
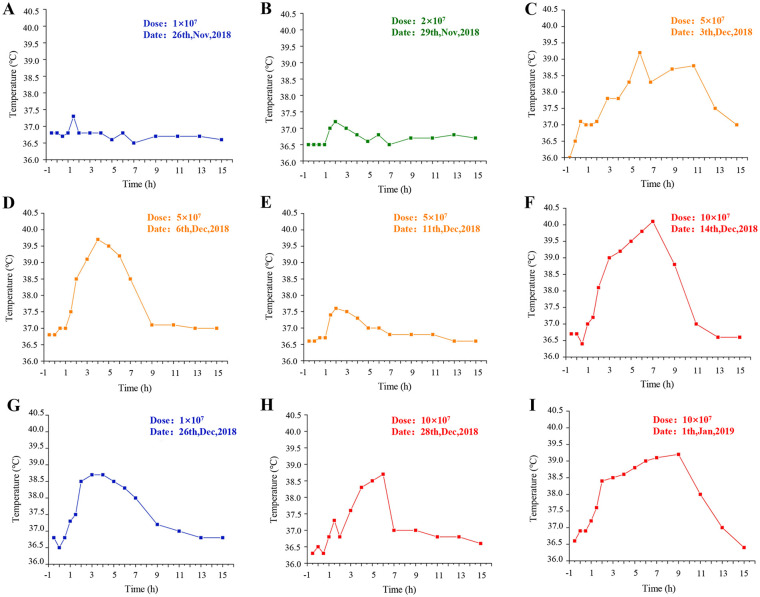
Figure 1.
Profiles of the patient’s body temperature after chimeric costimulatory converting receptor-NK92 cell injection. (A–C). The body temperature curve at first cycle of cell therapy. (D–F). The body temperature curve at second cycle of cell therapy. (G–I). The body temperature curve at third cycle of cell therapy.
Table 1.
Patient Blood Test Results After CCCR-NK92 Cell Therapy.
| Date | LDH (U/l) (109–245 U/l) | ALT (U/l) (7–40 U/l) | AST (U/l) (13–35 U/l) | FBG (mg/dl) (200–400 mg/dl) | D-D E-(μg/ml) (0–l μg/ml) | PLT (109/l) (125–350 × 109/l) | WBC (109/l) (3.5–9.5 × 109/l) |
|---|---|---|---|---|---|---|---|
| 2018/12/25 | 407 | 8 | 13 | 426 | 11.1 | 306 | 6.6 |
| 2019/01/03 | 1155 | — | — | 308 | 27 | 122 | 3.9 |
| 2019/01/05 | 321 | 82 | 95 | 97.9 | 43.7 | 118 | 6 |
| 2019/01/07 | 287 | 124 | 254 | 53.8 | 3.3 | 78 | 8.2 |
| 2019/01/11 | 241 | 59 | 53 | 122.l | 3 | 314 | 7.6 |
ALT: alanine aminotransferase; AST: aspartate aminotransferase; CCCR: chimeric costimulatory converting receptor; D-D: D-Dimer; FBG: fibrinogen; LDH: lactate dehydrogenase; PLT: platelets; WBC: white blood cells.
Results
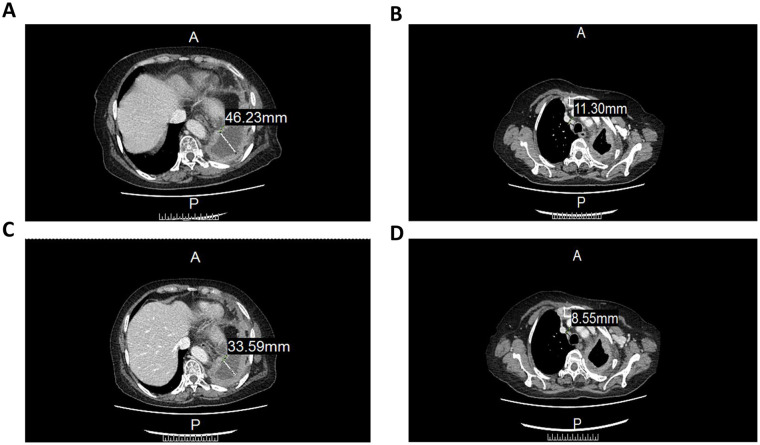
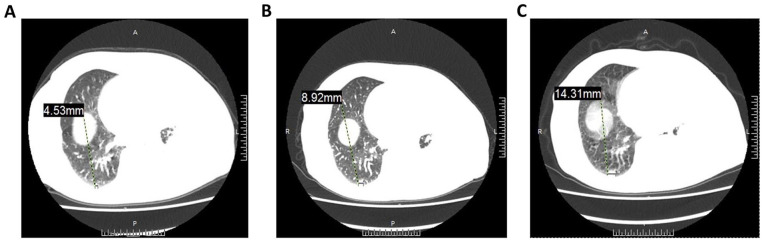
Analysis of serum cytokine levels, following occurrence of severe symptoms, showed that the expression of interferon-γ and tumor necrosis factor α (TNF-α) reached a peak at eighth treatment (Fig. 1). The CT images showed that cell therapy had some efficacy in treating the tumor. Specifically, the left lung encapsulated intrathoracic fluid reduced from 46.22 to 33.59 mm (Fig. 2A, C), whereas lymph nodes of the anterior tracheal-posterior vena cava interstitial area decreased from 11.3 to 8.55 mm (Fig. 2B, D). The CT image revealed that the right pulmonary nodule was significantly larger than the baseline one (Fig. 3). Although the patient’s condition gradually stabilized, after a series of symptomatic treatments, she opted out of this clinical trial considering the possibility of serious side effects (evaluation reference Recist 1.1).
Figure 2.
A CT image of mediastinal window before and after CCCR-NK92 cell therapy. (A) CT image before CCCR-NK92 cell therapy. (B) CT images after one cycle of CCCR-NK92 cell treatment. (C) CT image after one cycle of CCCR-NK92 cell treatment. (D) CT images after two cycles of cell treatment. CCCR: chimeric costimulatory converting receptor; CT: computed tomography.
Figure 3.
A CT image of lung window before and after CCCR-NK92 cell therapy. (A) CT image before CCCR-NK92 cell therapy. (B) CT images after one cycle of CCCR-NK92 cell treatment. (C) CT images after two cycles of CCCR-NK92 cell treatment. CCCR: chimeric costimulatory converting receptor; CT: computed tomography.
Ethics and Knowledge
With an institutional review board (IRB) approval from the First Affiliated Hospital of Xinxiang Medical University (Xinxiang, China), a phase II clinical trial (NCT03656705) was initiated to evaluate the safety and effectiveness of CCCR-NK92 cell therapy with NSCLC. The clinical study was performed in accordance with the principles of the Declaration of Helsinki. Each patient had received formal informed consent before entering the study.
Discussion
The CRS, caused by cytokine release from target and immune effector cells, is one of the most common life-threatening toxicities associated with cellular immunotherapy 12 . To date, the exact mechanism of CRS is not fully understood. The NK cells reportedly exert nonspecific tumor cell killing effects that are not restricted by human leukocyte antigen (HLA). This mainly occurs through the surface cytotoxic receptors that trigger release of perforin and granzyme. To date, a series of CAR-modified NK and CAR-expressing NK-92 cells that target a wide range of cancer antigens have been used in cancer therapy 13 . Functionally, NK cells produce cytokines, like interferon (IFN)-γ, interleukin (IL)-3, and TNF-α, that differ from the proinflammatory cytokines produced by T cells that initiate CRS 14 . Theoretically, CRS rarely occurs during CAR-NK cell therapy 4 . However, our patient manifested the CRS side effect on the 13th day of treatment. Specifically, she showed a 10-fold upregulation of IFN-γ and TNF-α (Fig. 4) and this was accompanied by a series of side effects such as persistent fever, persistent hypotension, increased inflammatory indexes, electrolyte disturbance, and abnormal blood coagulation function. Generally, an interaction between immune effector cells with tumor cells causes a release of inflammatory factors that subsequently activate the immune cells and exacerbate the anti-tumor effect. However, the high level of cytokines triggers a simultaneous occurrence of a series of side effects.
Figure 4.
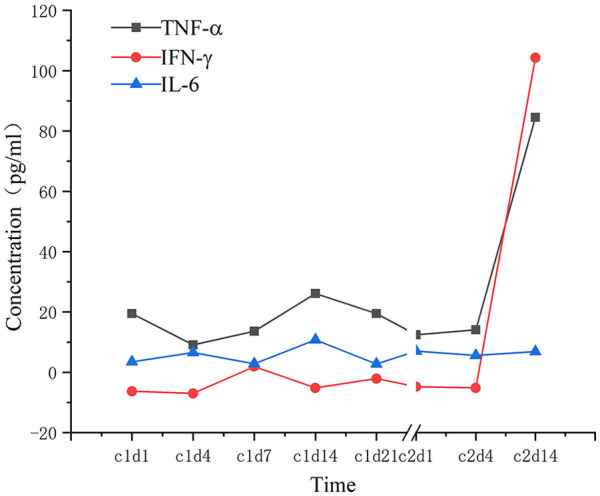
Cytokine levels in the patient’s serum after chimeric costimulatory converting receptor-NK92 cell therapy. IL-6, IFN-γ, and TNF-α were tested on 1, 4, 7, 14, and 21 day of each treatment cycle. IFN-γ: interferon gamma; IL-6: interleukin 6; TNF-α: tumor necrosis factor α.
There are two possible causes for the release of large amounts of cytokines. First, NK cells have nonspecific tumor cell killing effects that are not restricted by human leukocyte antigen (HLA), It mainly through the surface cytotoxic receptors to trigger the release of perforin and granzyme, producing cytotoxic factors and tumor necrosis factor and other cytokines, but not IL-2, IL-6 and other cytokines which could cause cytokine storms. However, based on the strategic consideration of constructing CCCR-NK92 cells, the construct of CCCR includes PD-1-NKG2D-41BB, which is expected to convert the inhibitory signaling from PD-1 into activating signaling when killing tumor cells; this design may lead to NK cell overactivation, resulting in overproduction of cytokines, such as IFN-γ and TNF-α.
In addition, the immune system in patient may recognize CCCR-NK92 cells as foreign substances and produce an immune response to it, and the release of inflammatory factors is related to the activation of the body’s immune system. We will test this hypothesis in future clinical experiments. We can reinfuse CCCR-NK92 cells after cell therapy to observe whether the infused cells are quickly cleared by the body’s immune system. If the CCCR-NK92 cells are quickly cleared, it is confirmed that the body has a rejection reaction to foreign cells. The release of cytokines can be considered to be caused by the body’s autoimmune reaction. In addition, we can also extract the serum of patients after cell treatment and detect the presence of antibodies to CCCRNK-92 cells in the serum by flow cytometry, which can also prove the hypothesis if there are antibodies in the serum.
Managing CRS-related toxicity is a critical consideration during application of CAR-NK therapy. First, it is important to monitor biomarkers used for predicting the risk of CRS, such as cytokines, and their changes during the CAR-NK therapy. Early intervention, associated with close monitoring of these biomarkers, can prevent the patient from developing a deadly syndrome. Second, clinicians should strive to reduce the tumor burden prior CAR-NK cell infusion. Previous studies have associated higher levels of tumor burden with elevated side effects in immune cell therapy 15 . Therefore, applying chemotherapy prior to immune cell therapy can increase its efficacy and reduce side effects such as CRS 16 . Third, it is important to reduce CAR-NK cell dosage in patients, owing to the fact that it plays a key role in determining the intensity and kinetics of CRS17,18. In this case, clinicians should ensure that a patient’s condition is stable before increasing cell dose. This will minimize the chances of CRS.
Overall, it is important to consider potential occurrence of toxicity during CAR-NK treatment. Consequently, clinicians should pay more attention to CRS in CAR-NK therapy and other immune therapies.
Acknowledgments
We thank Fei Lin for the management of our laboratory; we thank the life and science research center of the Xinxiang Medical University first Affiliated Hospital for providing experimental venues for our study.
Footnotes
Ethical Approval: Ethical approval to report this case was obtained from the Ethics Committee of the First Affiliated Hospital of Xinxiang Medical University.
Statement of Human and Animal Rights: All procedures in this study were conducted in accordance with the protocol approved by the Ethics Committee of the First Affiliated Hospital of Xinxiang Medical University. This article does not contain any studies with animal subjects.
Statement of Informed Consent: Written informed consent was obtained from the patient(s) for their anonymized information to be published in this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by the National Key R&D Program of China (Grant No.2019YFA0906000) and the First Clinical College of Xinxiang Medical College Graduate Research and Innovation Support Fund (YJSCX201915Z).
ORCID iDs: Xiaodi Zhang  https://orcid.org/0000-0002-3831-6188
https://orcid.org/0000-0002-3831-6188
Yanting Liu  https://orcid.org/0000-0001-6795-8545
https://orcid.org/0000-0001-6795-8545
References
- 1. Fesnak AD, June CH, Levine BL. Engineered T cells: the promise and challenges of cancer immunotherapy. Nat Rev Cancer. 2016;16(9):566–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Watanabe K, Kuramitsu S, Posey AD, Jr, June CH. Expanding the therapeutic window for CAR T cell therapy in solid tumors: the knowns and unknowns of CAR T cell biology. Front Immunol. 2018;9:2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Xin Yu J, Hubbard-Lucey VM, Tang J. The global pipeline of cell therapies for cancer. Nat Rev Drug Discov. 2019;18(11):821–2. www.cancerresearch.org/io-cell-therapy [DOI] [PubMed] [Google Scholar]
- 4. Rezvani K, Rouce R, Liu E, Shpall E. Engineering natural killer cells for cancer immunotherapy. Mol Ther. 2017;25(8): 1769–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Santomasso B, Bachier C, Westin J, Rezvani K, Shpall EJ. The other side of CAR T-cell therapy: cytokine release syndrome, neurologic toxicity, and financial burden. Am Soc Clin Oncol Educ Book. 2019;39:433–44. [DOI] [PubMed] [Google Scholar]
- 6. Pietra G, Vitale C, Pende D, Bertaina A, Moretta F, Falco M, Vacca P, Montaldo E, Cantoni C, Mingari MC, Moretta A, et al. Human natural killer cells: news in the therapy of solid tumors and high-risk leukemias. Cancer Immunol Immunother. 2016;65(4):465–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu E, Marin D, Banerjee P, Macapinlac HA, Thompson P, Basar R, Nassif Kerbauy L, Overman B, Thall P, Kaplan M, Nandivada V, et al. Use of CAR-transduced natural killer cells in CD19-positive lymphoid tumors. N Engl J Med. 2020;382(6):545–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Klingemann H. Are natural killer cells superior CAR drivers? Oncoimmunology. 2014;3:e28147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gong JH, Maki G, Klingemann HG. Characterization of a human cell line (NK-92) with phenotypical and functional characteristics of activated natural killer cells. Leukemia. 1994;8(4):652–58. [PubMed] [Google Scholar]
- 10. Geller MA, Miller JS. Use of allogeneic NK cells for cancer immunotherapy. Immunotherapy. 2011;3(12):1445–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lu C, Guo C, Chen H, Zhang H, Zhi L, Lv T, Li M, Niu Z, Lu P, Zhu W. A novel chimeric PD1-NKG2D-41BB receptor enhances antitumor activity of NK92 cells against human lung cancer H1299 cells by triggering pyroptosis. Mol Immunol. 2020;122:200–206. [DOI] [PubMed] [Google Scholar]
- 12. Shimabukuro-Vornhagen A, Godel P, Subklewe M, Stemmler HJ, Schlosser HA, Schlaak M, Kochanek M, Boll B, von Bergwelt-Baildon MS. Cytokine release syndrome. J Immunother Cancer. 2018;6(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Glienke W, Esser R, Priesner C, Suerth JD, Schambach A, Wels WS, Grez M, Kloess S, Arseniev L, Koehl U. Advantages and applications of CAR-expressing natural killer cells. Front Pharmacol. 2015;6:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Habib S, Tariq SM, Tariq M. Chimeric antigen receptor-natural killer cells: the future of cancer immunotherapy. Ochsner J. 2019;19(3):186–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brown CE, Mackall CL. CAR T cell therapy: inroads to response and resistance. Nat Rev Immunol. 2019;19(2):73–74. [DOI] [PubMed] [Google Scholar]
- 16. Mahadeo KM, Khazal SJ, Abdel-Azim H, Fitzgerald JC, Taraseviciute A, Bollard CM, Tewari P, Duncan C, Traube C, McCall D, Steiner M, et al. Management guidelines for paediatric patients receiving chimeric antigen receptor T cell therapy. Nat Rev Clin Oncol. 2019;16(1):45–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hay KA, Hanafi LA, Li D, Gust J, Liles WC, Wurfel MM, Lopez JA, Chen J, Chung D, Harju-Baker S, Cherian S, et al. Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor-modified T-cell therapy. Blood. 2017;130(21):2295–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gust J, Hay KA, Hanafi LA, Li D, Myerson D, Gonzalez-Cuyar LF, Yeung C, Liles WC, Wurfel M, Lopez JA, Chen J, et al. Endothelial activation and blood-brain barrier disruption in neurotoxicity after adoptive immunotherapy with CD19 CAR-T cells. Cancer Discov. 2017;7(12):1404–19. [DOI] [PMC free article] [PubMed] [Google Scholar]