Abstract
Advanced multidimensional NMR techniques have been employed to investigate the intramolecular hydrogen bonds (HBs) in a series of N,N′-([1,1′-binaphthalene]-2,2′-diyl)bis(benzamide) derivatives, with the site-specific substitution of different functional groups. The existence of intramolecular HBs and the elimination of any molecular aggregation and possible intermolecular HBs are ascertained by various experimental NMR techniques, including solvent polarity dependent modifications of HB strengths. In the fluorine substituted derivative, direct evidence for the engagement of organic fluorine in HB is obtained by the detection of heteronuclear through-space correlation and the coupling between two NMR active nuclei where the transmission of spin polarization is mediated through HBs (1hJFH). The extent of reduction in the strength of 1hJFH on dilution with high polarity solvents directly provided the qualitative measure of HB strength. The HB, although becoming weakened, does not get nullified even in pure high polarity solvent, which is attributed to the structural constraints. The rate of exchange of a labile hydrogen atom with the deuterium of the solvent permitted the measurement of their half-lives, that are correlated to the relative strengths of HBs. The experimental NMR findings are further validated by XRD and DFT-based theoretical computations, such as, NCI and QTAIM.
NMR studies reveal very strong hydrogen bond unbreakable even in high polarity solvents.
Introduction
The HB, a weak molecular interaction plays a pivotal role in understanding many chemical and biological phenomena.1–4 Numerous applications through inter- or intra-molecular interactions are found in supramolecular chemistry, biomolecules, such as, proteins, peptides, etc.5–7 The HB has thus been proven to be an important area of research, and is investigated by various analytical techniques8 and many groups are constantly exploring the diverse utility of HB interactions.9,10 The commonly encountered HB motifs are of the type, C–H⋯O, C–H⋯N and N–H⋯π, N–H⋯O, O–H⋯O, and O–H⋯N. These HB interactions could be both intra- and inter and have significant contributions in determining the structures and conformations of the molecules.11 The strength of intramolecular HBs also can control the population of different conformers for a molecule.12
The fluorine is one of the essential atoms presents in majority of the drugs due to its bio-availability and stability. On an average nearly 30% of commercial drugs contain at least one fluorine atom to enhance the metabolic stability, binding affinity and to alter physicochemical properties.13 To highlight the importance of fluorine containing drugs, the three out of the top 10 best-selling drugs, Lipitor, Advair and Crestor contain a fluorine atom, and seven out of 35 approved drugs in 2011 contain a fluorine atom.14 The dynamics and the interaction of these drug molecules with the biomolecules are controlled by HB. Dunitz et al. have reported that organic fluorine hardly ever accepts hydrogen bond.15 Nevertheless over the years the involvement of organic fluorine in HB in large number of molecules including foldamers have been reported.16–22 The studies report that the inter- or intra-molecular HB of the type X–H⋯F–C (X = O, N) plays a significant role in the dynamics as well as the functionalities of the drugs23viz., dipole–dipole interaction, HB and steric hinderance.
In the present work, various 1,1′-binaphthalene-2,2′-diamine (BINAM) derivatives have been synthesized and characterised using several one and two-dimensional multi-nuclear NMR techniques as well as, XRD and ESI-HR mass spectrometry. The procedure for synthesis of all the investigated molecules and their NMR spectra along with the assignment of peaks are reported in ESI.† The structural frameworks of the molecules investigated are given in Scheme 1. The utility of BINAM and its derivatives is multifold, viz., synthesis of [BINAM–FeCl2] complex for the conversion of indole into bisindolylmethane with high yield,24 to derivatize an organocatalyst for the asymmetric synthesis in Henry reaction which yields the high reactivity and enantioselectivity,25 the covalently linked azobenzene and the enantiopure BINAM is able to produce materials that exhibits photoresponsive chiroptical behaviour due to cis–trans isomerism shown by its backbone.26 In many chemical reactions, the proton transfer due to the complex formation through HB is also reported.27 The transfer of hydrogen atom occurs during the rate determining step. The lability of this hydrogen depends on its chemical environment and the strength of HB. It has been previously reported that the rate of H/D exchange depends on both the strength of HB as well as electronic effects of the substituents.28,29 When the deuterated solvent is present in excess, this exchange phenomenon is known to follow the pseudo first order kinetics. This concept has been exploited in the present study for monitoring the rate of exchange of labile hydrogen atom with the deuterium of the solvent to extract information on the relative strengths of HB. The proton transfer due to the complex formation through hydrogen bonding is also reported. In this investigation we have convincingly established the presence of very strong HB in the number of synthesize derivatives of BINAM. This could aid in the architectural design and understanding of the role of BINAM in many other chemical and photochemical reactions.
Scheme 1. The structural framework of (R)-(+)-N,N′-([1,1′-binaphthalene]-2,2′-diyl)bis(benzamide) and its derivatives.
Results and discussion
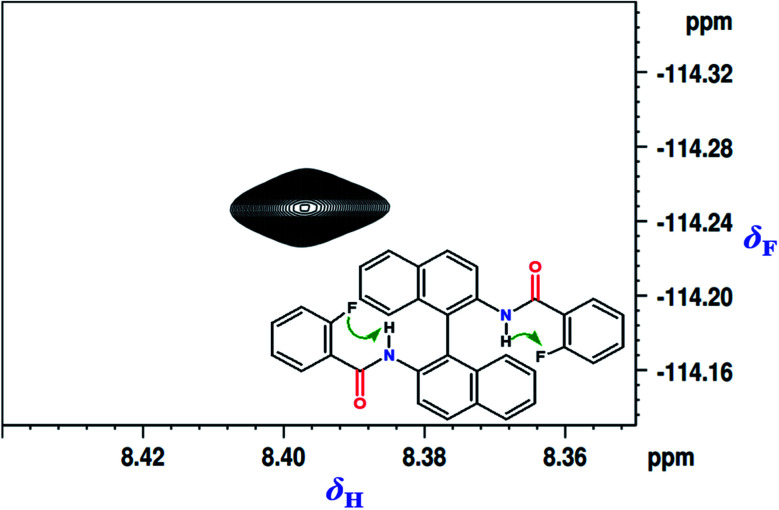
Initially the fluorinated derivative (molecule 1 in Scheme 1) was chosen for in-depth analysis. The spatial proximity being the fundamental requirement for any two nuclei to get involved in HB, the 2D 1H–19F HOESY (Hetero-nOE Spectroscopy)30–32 experiment was carried out and the corresponding spectrum is reported in Fig. 1. The strong through space correlation detected between 19F and the NH proton established their close spatial proximity conducive for the HB interaction.
Fig. 1. 2D 1H–19F HOESY spectrum of molecule 1 in the solvent CDCl3. The chemical structure of the molecule is also given in the inset and the through space correlated spins are identified by arrows.
NMR detection of HB
Number of NMR parameters are available for understanding the presence of HB in a molecule. In the solution state, the chemical shift of the protons involved in HB is usually monitored, which is known to move downfield compared to non-hydrogen bonded proton owing to the depletion of electronic charge distribution around it. The chemical shift of NH proton of the unsubstituted molecule is 7.76 ppm, while those of the substituted molecules are between 7.95 to 9.67 ppm with OCH3 substituted molecule shifted to extreme downfield (9.67 ppm) confirming the presence of HB in all these molecules.
The energy of HB can be calculated by using experimentally measured chemical shifts employing the relation EHB (kcal mol−1) = Δδ + (0.4 ± 0.2), where Δδ pertains to the difference in the chemical shift of proton between the substituted and unsubstituted molecules.12,33 The calculated EHB for the molecules 1–3 are compiled in Table 1.
Calculated EHB employing the difference in chemical shift values of the substituted molecules 1–3 with respect to that of unsubstituted molecule 4.
| Molecule | Substituent (X) | δ NH in the solvent CDCl3 (ppm) | Difference in δNH with respect to molecule 4 | Energy of HB (EHB) (kcal mol−1) |
|---|---|---|---|---|
| 1 | F | 8.34 | 0.59 | 0.99 |
| 2 | Cl | 7.97 | 0.22 | 0.62 |
| 3 | OMe | 9.67 | 1.92 | 2.32 |
| 4 | H | 7.75 | — | — |
Distinguishing between intra- and inter-molecular HB
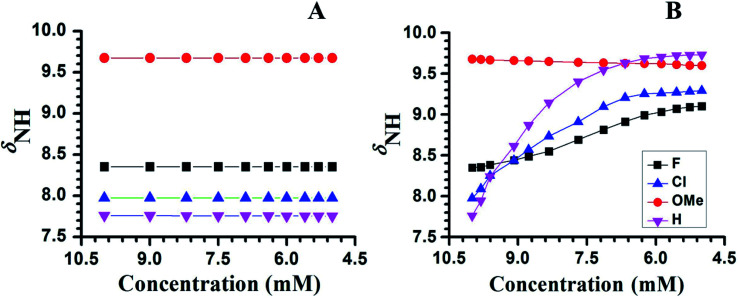
To ascertain whether HB is intra- or inter-molecular, generally the solvent dilution experiments are employed.34–37 This experiment also provides information on the effect of monomeric water absorbed by the solvent from the atmosphere.36,37 The changes in the chemical shift of NH proton as a function of the concentration of CDCl3, in the molecules 1–4, is graphically illustrated in Fig. 2A. The chemical shift of NH proton remained nearly same on dilution, within the experimental error, establishing the absence of intermolecular HB, as well as any possible aggregation and dimerization in all the molecules. This also convincingly establishes the presence of intramolecular HB. The chemical shift of water in CDCl3 at 1.54 ppm remained unchanged, except for marginal change during dilution, further discarding any effect of atmospheric water on the molecular interactions. The subsequent step is to determine the relative strengths of these intramolecular HBs, which is determined by the ease at which they can be ruptured in a high polarity solvent, such as, DMSO.38 The strong interaction between DMSO and the NH proton results in the disruption of intramolecular HBs causing a downfield shift of NH proton chemical shift.38,39 The quantity of DMSO required to rupture a HB is directly proportional to its strength. The changes in the chemical shift of NH proton with the incremental addition of DMSO-d6 to the initial 10 mM solution in CDCl3 is reported in Fig. 2B, for all the molecules. As expected, in the molecules 2 and 4 there is substantial down field shift in the resonance position of NH proton. On the contrary in molecule 3 the NH proton shifted to high field, which is attributed to the comparatively stronger HB between oxygen atom of the methoxy group and NH proton compared to its interaction with DMSO in the two competitive processes.40
Fig. 2. Chemical shifts of NH proton (δNH) of molecules 1–4, as a function of concentration in CDCl3 and DMSO-d6 respectively. The initial concentration taken was 10 mM in the solvent CDCl3 and the temperature was maintained at 298 K. (A) With the incremental addition of CDCl3 to an initial volume of 450 μL. (B) With the incremental addition of DMSO-d6 to an initial volume of 450 μL in CDCl3. The molecules are identified by the symbols given in the inset of (B).
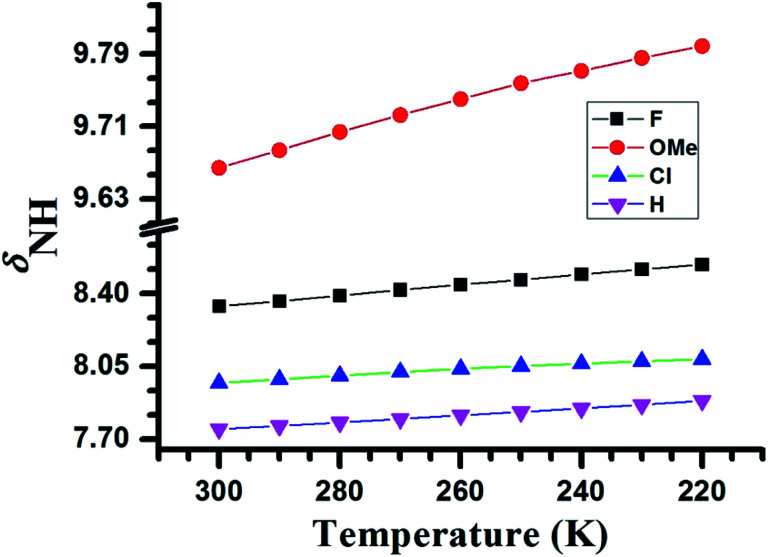
Another physical parameter that provides information on the HB is the temperature. The lowering of the temperature enhances the strength of HB and results in the downfield shift of the proton involved in HB.41,42 When the temperature is varied from 220 K to 300 K the NH proton of molecules 1–4 exhibited the downfield shift, which is evident from Fig. 3. From the magnitude of change in the value of δNH, one can conclude that the relative HB strengths follow the trend, OMe > F > Cl > H.
Fig. 3. Variation in the chemical shifts of NH proton (δNH) as a function of temperature in the range of 300 K to 220 K for the molecules 1–4. The molecules are identified by the symbols given in the inset.
HB mediated coupling between NMR active nuclei
When 19F is involved in HB, often the coupling between 1H and 19F mediated through HB is detected, giving a strong evidence on the existence of HB.33,43
The substantially large values of 3JFH about 4–5 Hz, between ortho fluorine and OH proton, have been measured in the derivatives of 2-fluoro phenol44 which is not uncommon. In general, the observed nJFH values are in the range of 40–60 Hz, 2–15 Hz, 1–5 Hz, for n = 1, 2 and 3 respectively. The 5JFH is usually very small and less than 0.5 Hz. On the other hand, the observation of coupling of 14–18 Hz between 19F and 1H separated by five chemical bonds is unusual and is attributed to the HB mediated coupling.
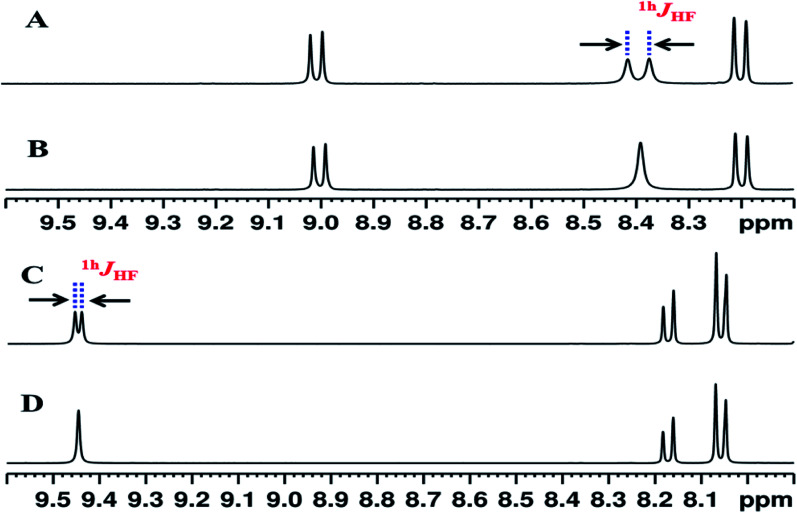
The 1H and 1H{19F} spectra of molecule 1 in the solvent CDCl3 and DMSO-d6 are reported in Fig. 4. The NH proton is a doublet with a separation of 16.3 Hz. The reported values of (5JFH) is usually less than 1 Hz and this large value of coupling is attributed to the one bond HB mediated coupling between 1H and 19F (1hJFH). This is due to the interaction between NH proton and fluorine atom, which is also validated by collapsing of the doublet to a singlet in the 1H{19F} spectrum. Previous studies have shown that such coupling gets completely nullified in a high polarity solvent, such as, DMSO giving another strong evidence for weak interaction.45 Interestingly in the present study the NH proton continues to remain as a doublet even in the solvent DMSO-d6, although coupling strength is substantially reduced. This indicates that the HB interaction in this molecule is significantly stronger and does not get ruptured even in the highly polar solvent medium compared to the previously reported studies.45,46
Fig. 4. Selected regions of the 400 MHz 1H NMR spectra of molecule 1: (A) in the solvent CDCl3; (B) 1H{19F} spectrum in the solvent CDCl3; (C) in the solvent DMSO-d6; and (D) 1H{19F} spectrum in the solvent DMSO-d6. The doublet separation pertaining to 1hJFH in A and C are identified. It may be noted that upon addition of DMSO-d6 the NH peak shifted downfield and the magnitude of 1hJFH is also reduced. The peaks at 9.0 ppm and 8.2 ppm in CDCl3, have moved high field to 8.17 and 8.06 respectively in DMSO-d6 due to rupturing of C O⋯H (ring) HB.
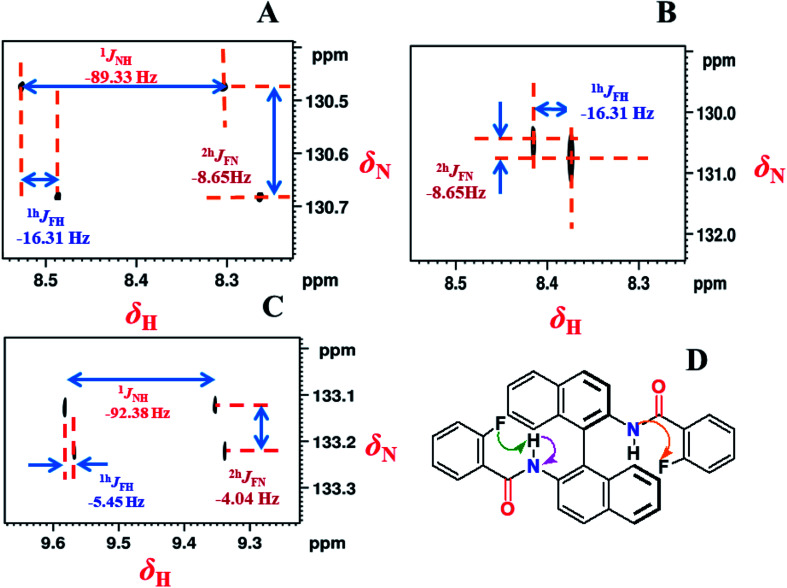
The 2D 1H coupled and decoupled 1H–15N HSQC spectra, where the 15N is in its natural abundance, provide strong evidence on the presence of intramolecular HBs and also helps in the precise determination of the relative signs and magnitudes of couplings.46,47 The corresponding spectra are reported in Fig. 5. The coupled and decoupled 1H–15N HSQC spectra in the solvent CDCl3 are reported in Fig. 5A and B, respectively. In Fig. 5A the couplings 1JNH, 1hJFH and 2hJFN persist while in Fig. 5B the 1JNH has completely disappeared. The coupled 1H–15N HSQC spectrum in the solvent DMSO-d6 (Fig. 5C), revealed all the couplings that were observed in CDCl3 (Fig. 5A). However, there is a significant change in the magnitudes of the couplings. The 1JNH increased by nearly 3 Hz, whereas 1hJFH and 2hJFN decreased significantly in their strengths. The 1H–15N HSQC spectra of all the remaining molecules are reported in the ESI.† Taking advantage of the previous reports, the relative signs of 1hJFH and 2hJFN are assigned to be negative.33,39 The measured magnitudes and relative signs of all the couplings are reported in the figure.
Fig. 5. 800 MHz 1H–15N-HSQC spectra of molecule 1 in the solvent CDCl3; (A) NH-coupled; (B) NH-decoupled; (C) 400 MHz 1H–15N-HSQC spectrum (NH-coupled) in the solvent DMSO-d6; (D) the chemical structure of the molecule. The scalar and HB mediated couplings are identified by arrows in the chemical structure. The separations providing the scalar and HB mediated couplings and the measured values along with their relative signs derived from the slopes of the displacement of cross sections are mentioned in the spectra.
Nature of HB
For covalent and electrostatic HB, there will be a noticeable difference in the angle formed among the hydrogen bonding nuclei. In the directional covalent type HB, the bond angle tends to be linear. On the other hand, in electrostatic nature of HB the bond angle is less leading to non-directional coulombic interaction. In electrostatic nature of HB, due to the Fermi-contact mechanism the 1JNH increases with the shortening of N–H bond length. On the other hand, in covalent type HB due to both Fermi-contact and paramagnetic spin-orbital mechanisms the 1JNH decreases with the lengthening of N–H bond.46 The scalar coupling constant 1JNH is also utilized for understanding the nature of HBs. If the HB is predominantly electrostatic46 the strength of 1JNH increases when compared to unsubstituted molecule, whereas it decreases for covalent type.47 From the 1JNH values given in Table 5, it is evident that the HBs are predominantly of covalent type.
Electron density (ρ(r)) and Laplacian of electron density (Δ2ρ(r)) at different BCPs of type (3, −1) for (X⋯HN) HBs and the energy of particular HBs calculated on the basis of potential energy density (V(r)) are listed. The calculations were done using a default solvation medium of chloroform.
| Molecule | HB type (X⋯HN) | Electron density (ρ(r)) (a.u.) | Laplacian of electron density (Δ2 × ρ(r)) | Energy of HB (EHB) (kcal mol−1) | Theoretically calculated value of δNH (ppm) | Experimental value of δNH (ppm) | 1 J NH in the solvent CDCl3 |
|---|---|---|---|---|---|---|---|
| 1 | F⋯HN | 0.0253 | 0.1074 | −7.0029 | 8.27 | 8.35 | −89.33 |
| CO…HC | 0.0201 | 0.0753 | −4.3731 | 8.46 | 8.96 | ||
| 2 | Cl⋯HN | 0.0407 | 0.1419 | −12.175 | 14.59 | 7.97 | −89.91 |
| CO⋯HC | 0.0307 | 0.1477 | −8.5565 | 8.46 | 8.75 | ||
| 3 | MeO⋯HN | 0.0405 | 0.1594 | −12.584 | 10.05 | 9.68 | −87.52 |
| CO⋯HC | 0.0181 | 0.0721 | −4.0159 | 7.75 | 9.07 | ||
| 4 | H⋯HN | 0.0189 | 0.0634 | −3.5621 | 10.39 | 7.8 | −88.63 |
| CO⋯HC | 0.0301 | 0.1389 | −8.1036 | 8.11 | 8.09 |
Strong and unbreakable HB in highly polar solvent
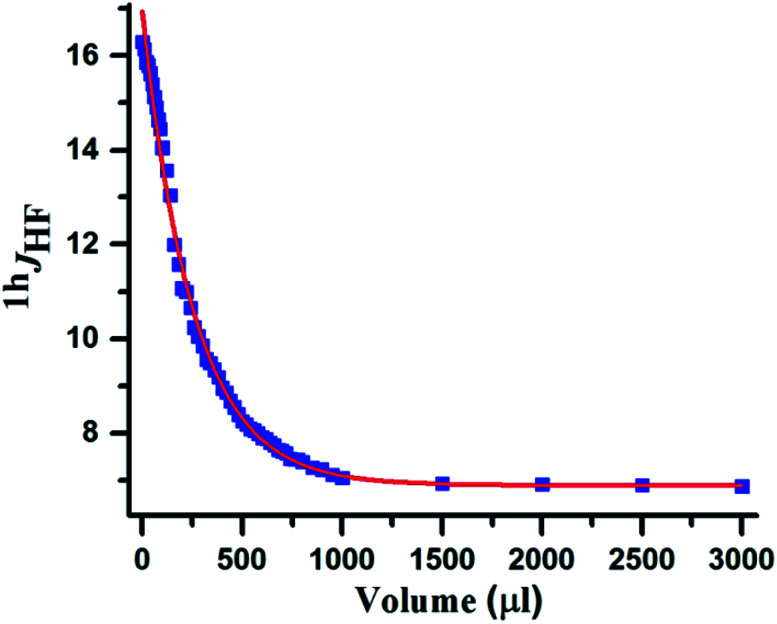
In the number of previous studies reported from our group, the 1hJFH disappeared in a high polarity solvent DMSO-d6.16,29 Interestingly in the present study the strength of N–H⋯F–C HB is so strong that it gets weakened but does not get completely ruptured even in pure solvent DMSO-d6. Such an unusual behaviour has been reported earlier in monosaccharides, inositols and ginkgolides48 where the strength of HB has been correlated to the value of 1hJHF. In fluorinated myo-inositol 1hJHF reduced from 8.8 Hz in CDCl3 to 3.8 Hz in DMSO-d6. The coupling constant did not reduce beyond this value, and it was interpreted due to molecular constraints and the solvation of the F⋯H–O bond was not completely favourable by DMSO-d6. Similar observation is made in the present study, where upon increasing the concentration of DMSO-d6 there is a smooth reduction in 1hJFH consequent to weakening of HB and beyond a particular concentration the coupling strength remained unaltered even with excessive addition of DMSO-d6. This is graphically illustrated in Fig. 6. These observations evidently establish the presence of a very strong HB. This was also observed in the 2D 15N–1H HSQC spectrum (Fig. 5C) where 1hJHF is retained in pure DMSO-d6 solvent with a reduced value compared to that in CDCl3.
Fig. 6. The plot of HB mediated coupling (1hJHF (Hz)) vs. volume of DMSO-d6 (μL) for molecule 1.
Solvent polarities and strengths of HB
The 1hJFH was also measured in solvents of different polarities and are assimilated in Table 2. From the table it is evident that with the increase in the solvent polarity there is a substantial decrease in the coupling constant. Although the decrease is not monotonic, the change is drastic in the solvents of high polarity. This is attributed to the structural constraint. The XRD structure of this molecule (Fig. 8, discussed in the later part of the article) revealed the constrained structure for binaphthyl derivative, and the HB between F⋯HN is facing towards one of the naphthyl rings. Thus, the complete solvation of this hydrogen bond is restricted by this constrained structure, thereby preventing the complete nullification of 1hJFH (Table 2).
Measured magnitudes of 1hJHF for molecule 1 in solvents of different polarities. The polarity increases on going from top to bottom of the table.
| Solvent | 1h J HF (Hz) |
|---|---|
| Toluene-d8 (C7D8) | 17.45 |
| Benzene-d6 (C6D6) | 17.39 |
| Chloroform (CDCl3) | 16.34 |
| Pyridine-d5 | 8.99 |
| DMSO-d6 | 5.45 |
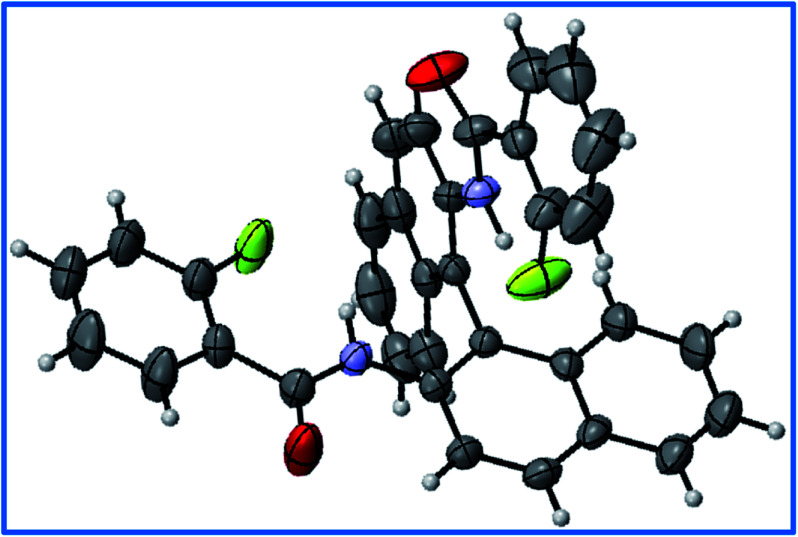
Fig. 8. The structure of molecule 1 determined from a single-crystal XRD study with ORTEP view.
H/D exchange
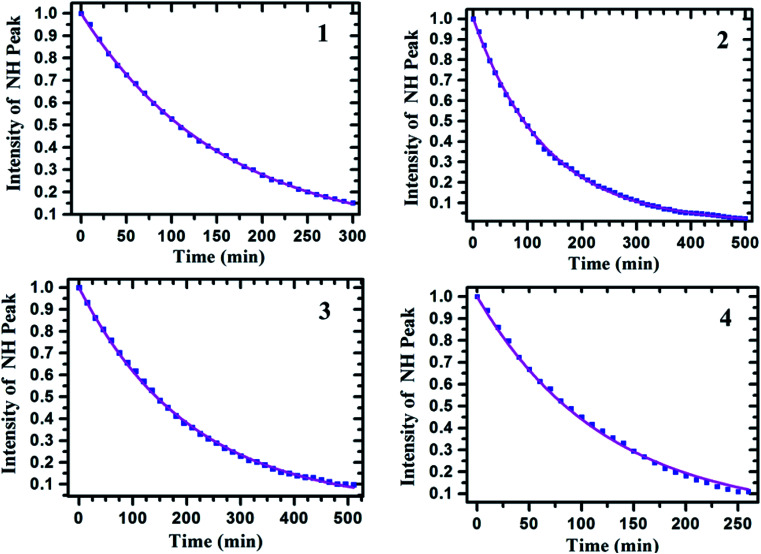
The H/D exchange studies were carried out for all the samples to determine the exchange rate constants and the half-lives, which were subsequently utilized to determine the relative strengths of HBs. For such a purpose usually OD group of the D2O is used. Since in the present organic molecules the solubility in water is an issue, the deuterated solvent CD3OD was added to the solution of each sample. The series of 1H NMR spectra were then obtained at the concentration of about 5 mM, with 450 μL of CDCl3 solvent, at two minutes intervals till the intensity of NH proton drops to nearly zero. At this concentration the possibility for any kind of aggregation and intermolecular interaction are negligible. To minimize the effect of atmospheric water on H/D exchange, the fresh ampules of CD3OD and CDCl3 were used. The final methanol concentration was kept at nearly 2.47 M, in 10% methanol : chloroform solution, in order to ensure that the exchange phenomenon follows pseudo-first order kinetics. A constant baseline correction was applied with the line broadening window function of 1 Hz. The intensities of NH peaks were measured relative to the integral intensity of a non-exchanging and distinct signal of aromatic proton. The integral intensity of the NH peak was normalized to 1 at zero time and the intensities of the peak at different times were calibrated relative to this intensity. The change in the integral intensity of NH peak as a function of time is graphically represented in Fig. 7. From the graph, the slope of a nonlinear least squares fit on the first order kinetics equation A(t) = A(0)exp(−kt), the rate constants and their corresponding half-lives were measured. The similar procedure was adopted for all the molecules 1–4 (Table 3).
Fig. 7. The plot of integral intensity of the NH peak versus time (in min). The number in the inset refers to molecule; 1 for fluoro derivative, 2 for chloro derivative, 3 for methoxy derivative and 4 for unsubstituted molecule.
H/D exchange rate constants and half-lives of approximately 5 mM solution of molecules 1–4 in the solvent CDCl3. The values were calculated using the integral area of NH peaks in the 1H NMR spectrum of 10% CD3OD/CDCl3 samples.
| Molecule | Substituent (X) | H/D exchange rate constant (k) (min−1) | Half-life (t1/2) (min) |
|---|---|---|---|
| 1 | F | 6.4 × 10−3 | 108.28 |
| 2 | Cl | 7.46 × 10−3 | 92.89 |
| 3 | OMe | 4.82 × 10−3 | 143.77 |
| 4 | H | 8.19 × 10−3 | 84.61 |
From the measured rate constants and the half-lives, it is observed that the relative strengths of HB in the molecules follow the order; OMe > F > Cl > H. This is also in agreement with the temperature dependent change in chemical shift of NH proton discussed in the earlier part of the manuscript.
Single crystal X-ray diffraction (XRD) analysis
One of the criteria set by IUPAC49 for the existence of HB in a molecule is the linearity of the (180°) Y–H⋯X bond angle. The bond angle closer to this value causes shortening of the H⋯X distance which ensures stronger HB. For measuring the bond angles and bond lengths, the single crystal XRD structure was obtained for the molecule 1 and is given in Fig. 8. The bond angle and bond lengths obtained from XRD data are given in Table 4 and compared with those obtained from DFT based computations. The detailed discussion on the DFT calculations is provided in the later part of the article. The structural parameters derived from DFT are also reported in Table 4. The bond angles and bond lengths thus derived agree with the criteria for the existence of HB in the molecule.
Structural parameters obtained from the DFT calculations and single crystal XRD studies. All the bond distances are in Å and bond angles are in degree.
| Parameter | DFT | XRD |
|---|---|---|
| NH⋯F | 1.909 | 1.887 |
| N–H | 1.011 | 0.857 |
| N⋯F | 2.732 | 1.991 |
| <N–H⋯F | 136.27 | 138.84 |
| <C–F⋯H | 101.32 | 99.89 |
| O⋯H | 2.154 | 2.226 |
| <C O⋯H | 121.31 | 103.01 |
Theoretical studies
The information obtained from NMR experiments was further confirmed by DFT based theoretical computations. All the molecular structures were optimised by Gaussian 09 suite,50 with a B3LYP/6-311+G(d,p) level of theory and default chloroform for solvation where integral equation formalism model (IEFPCM) is the solvent model. The lowest energy molecular structures were justified based on the positive harmonic vibrational frequency values. From the coordination of the minimum energy structures, the wave function files were generated and used for Non-Covalent Interaction (NCI)51 and Quantum Theory of Atoms In Molecules (QTAIM)52 studies.
The non-covalent interaction
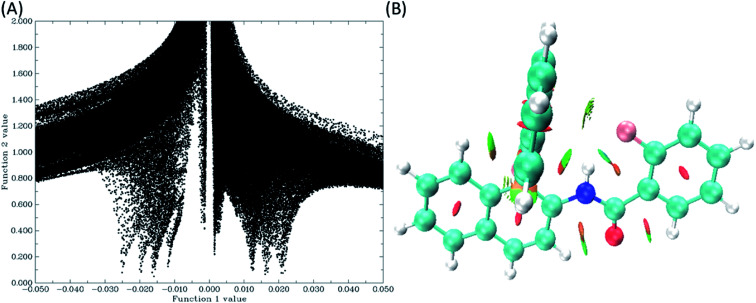
The NCI is a powerful theoretical technique used for the visualization of weak non-covalent interactions in the molecules, such as steric repulsions, van der Waals interactions and the HBs.53 This approach is based on the electron density and its derivatives. The condition for the covalent and non-covalent bonding regions is that there must be very large positive gradient of the reduced density gradient (RDG), and the RDG values have to be small, approaching to zero in the density tail, respectively. There is always a correlation between the electron density and the weak interactions in the corresponding regions. The correlation values are negative for the HBs, positive for steric interactions, whereas for van der Waals interactions they are close to zero. RDGs are significantly important while extracting the information on the weak molecular interactions, viz. the presence of dual HB in 1,4-bis-(3-hydroxy-4-oxo-4H-chromen-2-yl)-benzene (bisflavonol) system is confirmed by RDGs which was observed experimentally.54 The calculated grid points are plotted for a defined real space function, with sin(λ2(r))ρ(r) as function 1 and the reduced density gradient (RDG) as function 2 using the Multiwfn55 program for all the molecules. Using these grid points, coloured filled isosurfaces have also been plotted by using VMD program.56 The plot of sin(λ2(r))ρ(r) vs. RDG, and the coloured isosurfaces are given in Fig. 9(B).
Fig. 9. (A) The plot of sin(λ2(r))ρ(r) as function 1 vs. the RDG as function 2, and (B) coloured isosurface plot (green colour denotes weak HB and red colour stands for steric effect) for molecule 1. The plots for the remaining molecules, 2–4, are given in the ESI.†.
QTAIM calculations
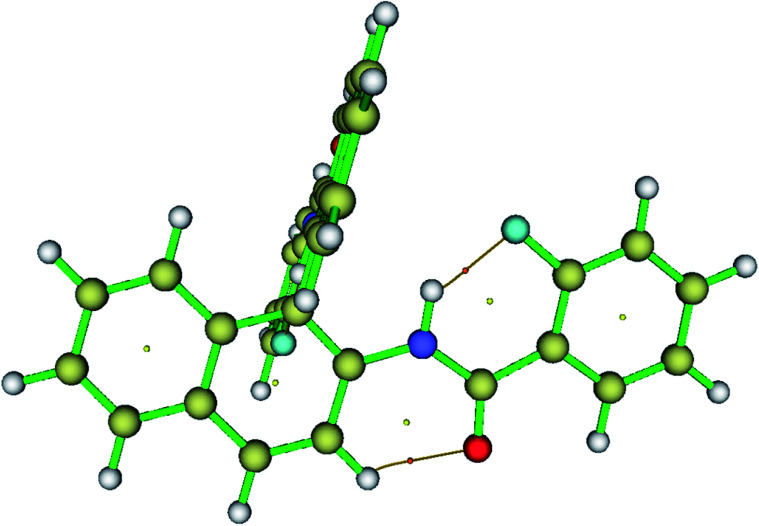
In order to study the electron density of the system, QTAIM can be used as a powerful tool. In topology analysis, critical points (CPs) are defined as the points at which the gradient norm of electron density goes to zero (except at infinity).57–60 Among all four CPs, the electron density at (3, −1) is known as the bond critical point (BCP). BCP arises whenever there is an attractive interaction between two atoms. Bond path (BP), which passes through the BCP, indicates the presence of bond between atoms. The molecular models depicting the BCP and BP are reported in Fig. 10. From the information on the sign of the Laplacian of electron density (Δ2 × ρ(r)) values and the electron density (ρ(r)), the bond type and bond strength are determined. Therefore, for all the investigated molecules, the magnitude of electron density and the Laplacian electron density at the (3, −1) BCPs were determined and compiled in Table 5. It is clear from the table that the Laplacian of electron density is positive, which provides the evidence for the HB type of interactions. The theoretically calculated energies of HB (EHB) show that the HBs in the present study are relatively stronger compared to the previously studied molecules. This is also reflected in the experimental data during titration study and the spectra in the solvent DMSO-d6.
Fig. 10. The visualization of BCPs and bond paths of HB for molecule 1 plotted using Multiwfn software. Dots represent the CPs and thin bars represent the HB interactions.
Experimental
The 1H{13C} NMR spectra for all the compounds and the DMSO titration experiments were obtained on the 500 MHz NMR spectrometer. All the 1H{19F}, CDCl3 titration, variable temperature studies and 1H–19F-HOESY experiments were carried out on 400 MHz NMR spectrometer. The 2D 1H-13C and 1H–15N HSQC correlation experiments were performed on the 800 MHz NMR spectrometer. For all the synthesized molecules, analysis of molecular mass has been carried out by using electrospray ionization mass spectrometry (ESI-HRMS). XRD data were collected on an oil coated crystal using an Oxford Xcalibur diffractometer with an Eos detector and a Mova microsource (Mo-Kα radiation, λ = 0.71073 Å) equipped with an Oxford Cobra open stream non-liquid nitrogen cooling device in the ω-scan mode at 100(2) K. The structure was solved by direct methods in Shelx-2014 61 and refined by full-matrix least squares methods against F2 (XD2015).62
General procedure for synthesis
The molecules were synthesized using (R)-(+)-1,1′-binaphthyl-2,2′-diamine and benzoyl chlorides of interest with desired substitution on ortho position. The (R)-(+)-1,1′-binaphthyl-2,2′-diamine and benzoyl chlorides of high purity were purchased and used as received. The AR grade solvents n-hexane (C6H14), chloroform (CHCl3), n-pentane (C5H12), dichloromethane (CH2Cl2) and HPLC grade methanol (CH3OH) were used for the purification.
200 mg of (R)-(+)-1,1′-binaphthyl-2,2′-diamine and the benzoyl chloride of interest were taken in a 1 : 2.2 molar ratio in a silica crucible and mixed using a spatula. The resulting mixture was exposed to microwave irradiation at 450 W for 5 min. The reaction mixture was monitored using TLC after completion. For further purification the obtained product was passed through a column loaded with neutral alumina (Al2O3) using a mixture of the solvents n-hexane and chloroform. The gradient of the solvent was varied from 5% to 30% chloroform with the gradual increments. The obtained product was crystallized using methanol (CH3OH) and dichloromethane (CH2Cl2) in a 2 : 1 ratio.
Conclusions
The various one- and two-dimensional NMR experiments provided unambiguous evidence for the presence of intramolecular HBs in all the molecules and their relative strengths of substituted molecules follow the trend OMe > F > Cl > H. The studies also discarded the possibility of intermolecular HB and aggregation. In fluorinated derivative the two dimensional 1H–19F HOESY experiment established the spatial proximity and provided direct and explicit evidence for the participation of organic fluorine in intramolecular HBs. Interestingly the HB in this molecule is very strong and is unbreakable even in high polarity solvent like DMSO, which is attributed to the structural constraint preventing the solvation of HB. The H/D exchange rates and the half-lives provided the qualitative information on the relative strengths of HBs. The NMR findings were ratified by DFT based computations. The single crystal-XRD structure provided insight into the structure and geometry of molecules and concurred with the NMR findings about the conditions for existence of intramolecular HBs. We envisage that this type of unusual behaviour of HB in highly polar solvent like DMSO-d6 might help in understanding the structural constraints in biomolecules, supramolecules, and foldamers.
Conflicts of interest
Authors declare no conflicts of interest.
Supplementary Material
Acknowledgments
AKP would like to thank Indian Institute of Science (IISc) for Research Fellowship. NS gratefully acknowledges the generous financial support by the Science and Engineering Research Board (SERB), New Delhi (Grant Number: CRG/2018/002006).
Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra07299c
References
- Kojić-Prodić B. Molčanov K. The nature of hydrogen bond: new insights into old theories. Acta Chim. Slov. 2008;55:692–708. [Google Scholar]
- Schuster P. Wolschann P. Hydrogen Bonding: From Small Clusters to Biopolymers. Annu. Rev. Biochem. 1999;960:947–960. [Google Scholar]
- Desiraju G. R. and Steiner T., The Weak Hydrogen Bond, Oxford University Press, 2001 [Google Scholar]
- Nagy P. I. Competing intramolecular vs. intermolecular hydrogen bonds in solution. Int. J. Mol. Sci. 2014;15:19562–19633. doi: 10.3390/ijms151119562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessler C. D. Rahm M. Becker S. Goldberg J. M. Wang F. Lippard S. J. CF2H, A Hydrogen Bond Donor. J. Am. Chem. Soc. 2017;139:9325–9332. doi: 10.1021/jacs.7b04457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. Sun B. Feng D. Hu H. Chu M. Qu Q. Tarrasch J. T. Li S. Sun Kobilka T. Kobilka B. K. Skiniotis G. Cryo-EM structure of the activated GLP-1 receptor in complex with a G protein. Nature. 2017;546:248–253. doi: 10.1038/nature22394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra S. Roy Chowdhury D. Addicoat M. Heine T. Paul A. Banerjee R. Molecular Level Control of the Capacitance of Two-Dimensional Covalent Organic Frameworks: Role of Hydrogen Bonding in Energy Storage Materials. Chem. Mater. 2017;29:2074–2080. doi: 10.1021/acs.chemmater.6b04178. [DOI] [Google Scholar]
- Jeffrey G. A., An Introduction to Hydrogen Bonding, Oxford University Press, 1997 [Google Scholar]
- Piskorz K. Dust J. M. Buncel E. Lebel O. Nunzi J. M. Deceleration of thermal ring closure in a glass-forming mexylaminotriazine-substituted merocyanine (MC) linked to intramolecular hydrogen bonding. New J. Chem. 2017;41:940–947. doi: 10.1039/C6NJ03368G. [DOI] [Google Scholar]
- Prabhakar C. Promila Tripathi A. Bhanuprakash K. Jayathirtharao V. Visible absorbing croconium dyes with intramolecular hydrogen bonding: a combined experimental and computational study. J. Mol. Struct. 2017;1146:684–691. doi: 10.1016/j.molstruc.2017.06.005. [DOI] [Google Scholar]
- Guo X. Tian Y. Zhang M. Li Y. Wen R. Li X. Li X. Xue Y. Ma L. Xia C. Li S. Mechanistic Insight into Hydrogen-Bond-Controlled Crystallinity and Adsorption Property of Covalent Organic Frameworks from Flexible Building Blocks. Chem. Mater. 2018;30:2299–2308. doi: 10.1021/acs.chemmater.7b05121. [DOI] [Google Scholar]
- Arya N. Mishra S. K. Suryaprakash N. Intramolecular hydrogen bond directed distribution of conformational populations in the derivatives of N′-benzylidenebenzohydrazide. New J. Chem. 2019;43:13134–13142. doi: 10.1039/C9NJ03071A. [DOI] [Google Scholar]
- Shah P. Westwell A. D. The role of fluorine in medicinal chemistry. J. Enzyme Inhib. Med. Chem. 2007;22:527–540. doi: 10.1080/14756360701425014. [DOI] [PubMed] [Google Scholar]
- Zhang H. Li Y. Jiang Z. Fluorine is Flourishing in Pharmaceuticals. J. Biomol. Res. Ther. 2012;72:6905–6917. [Google Scholar]
- Dunitz J. D. Taylor R. Organic Fluorine Hardly Ever Accepts Hydrogen Bonds. Chem.–Eur. J. 1997;3:89–98. doi: 10.1002/chem.19970030115. [DOI] [Google Scholar]
- Lakshmipriya A. Suryaprakash N. Two- and Three-Centered Hydrogen Bonds Involving Organic Fluorine Stabilize Conformations of Hydrazide Halo Derivatives: NMR, IR, QTAIM, NCI, and Theoretical Evidence. J. Phys. Chem. A. 2016;120:7810–7816. doi: 10.1021/acs.jpca.6b06362. [DOI] [PubMed] [Google Scholar]
- Mishra S. K. Suryaprakash N. Intramolecular hydrogen bonds involving organic fluorine in the derivatives of hydrazides: an NMR investigation substantiated by DFT based theoretical calculations. Phys. Chem. Chem. Phys. 2015;17:15226–15235. doi: 10.1039/C5CP01505G. [DOI] [PubMed] [Google Scholar]
- Lakshmipriya A. Rama Chaudhari S. Shahi A. Arunan E. Suryaprakash N. Three centered hydrogen bonds of the type CO⋯H(N)⋯X-C in diphenyloxamide derivatives involving halogens and a rotating CF3 group: NMR, QTAIM, NCI and NBO studies. Phys. Chem. Chem. Phys. 2015;17:7528–7536. doi: 10.1039/C4CP05917D. [DOI] [PubMed] [Google Scholar]
- Mishra S. K. Suryaprakash N. Intramolecular Hydrogen Bonding Involving Organic Fluorine: NMR Evidence corroborated by DFT based theoretical calculations. Molecules. 2017;22:423–466. doi: 10.3390/molecules22030423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari D. Hebbar S. Suryaprakash N. Intra-molecular hydrogen bonding with organic fluorine in the solution state: deriving evidence by a two dimensional NMR experiment. Chem. Phys. Lett. 2012;525–526:129–133. doi: 10.1016/j.cplett.2011.12.051. [DOI] [Google Scholar]
- Kareev I. E. Quiñones G. S. Kuvychko I. V. Khavrel P. A. Ioffe I. N. Goldt I. V. Lebedkin S. F. Seppelt K. Strauss S. H. Boltalina O. V. Variable-temperature 19F NMR and theoretical study of 1,9- and 1,7-C60F(CF3) and Cs− and C1-C60F17(CF3): hindered CF3 rotation and through-space JFF coupling. J. Am. Chem. Soc. 2005;127:11497–11504. doi: 10.1021/ja051954y. [DOI] [PubMed] [Google Scholar]
- Bécart D. Diemer V. Salaün A. Oiarbide M. Nelli Y. R. Kauffmann B. Fischer L. Palomo C. Guichard G. Helical Oligourea Foldamers as Powerful Hydrogen Bonding Catalysts for Enantioselective C-C Bond-Forming Reactions. J. Am. Chem. Soc. 2017;139:12524–12532. doi: 10.1021/jacs.7b05802. [DOI] [PubMed] [Google Scholar]
- Paul B. K. Ray D. Guchhait N. Spectral deciphering of the interaction between an intramolecular hydrogen bonded ESIPT drug, 3,5-dichlorosalicylic acid, and a model transport protein. Phys. Chem. Chem. Phys. 2012;14:8892–8902. doi: 10.1039/C2CP23496C. [DOI] [PubMed] [Google Scholar]
- Badigenchala S. Ganapathy D. Das A. Singh R. Sekar G. Iron(II) Chloride–1,1′-Binaphthyl-2,2′-Diamine (FeCl2–BINAM) Complex Catalyzed Domino Synthesis of Bisindolylmethanes from Indoles and Primary Alcohols. Synthesis. 2014;46:101–109. doi: 10.1055/s-0034-1378339. [DOI] [Google Scholar]
- Kitagaki S. Uedab T. Mukaib C. Planar chiral[2.2]paracyclophane-based bis(thiourea) catalyst: application to asymmetric Henry reaction. Chem. Commun. 2013;49:4030–4032. doi: 10.1039/C3CC41789A. [DOI] [PubMed] [Google Scholar]
- Gary D. Jaycox, Stimuli-Responsive Polymers. VII. Photomodulated Chiroptical Switches: Periodic Copolyaramides Containing Azobenzene, Phenylene, and Chiral Binaphthylene Main-Chain Linkages. J. Polym. Sci., Part A: Polym. Chem. 2003;42:566–577. [Google Scholar]
- Zhao J. Dong H. Zheng Y. Elaborating the excited state multiple proton transfer mechanism for 9H pyrido [3,4-b]indole. J. Lumin. 2018;195:228–233. doi: 10.1016/j.jlumin.2017.11.026. [DOI] [Google Scholar]
- Perrin C. L. Mechanism of Hydrogen Exchange in Amides. J. Am. Chem. Soc. 1974;96:5628–5631. doi: 10.1021/ja00824a083. [DOI] [Google Scholar]
- Mishra S. K. Suryaprakash N. Study of H/D exchange rates to derive the strength of intramolecular hydrogen bonds in halo substituted organic building blocks: an NMR spectroscopic investigation. Chem. Phys. Lett. 2015;639:254–260. doi: 10.1016/j.cplett.2015.09.038. [DOI] [Google Scholar]
- Rinaldi P. L. Heteronuclear 2D-NOE Spectroscopy. J. Am. Chem. Soc. 1983;105:5167–5168. doi: 10.1021/ja00353a071. [DOI] [Google Scholar]
- Yu C. Levy G. C. Solvent and Intramolecular Proton Dipolar Relaxation of the Three Phosphates of ATP: A Heteronuclear 2D NOE Study. J. Am. Chem. Soc. 1983;105:6994–6996. doi: 10.1021/ja00361a059. [DOI] [Google Scholar]
- Yu C. Levy G. C. Two-Dimensional Heteronuclear NOE (HOESY) Experiments: Investigation of Dipolar Interactions between Heteronuclei and Nearby Protons. J. Am. Chem. Soc. 1984;106:6533–6537. doi: 10.1021/ja00334a013. [DOI] [Google Scholar]
- Dingley A. J. Masse J. E. Peterson R. D. Barfield M. Feigon J. Grzesiek S. Internucleotide scalar couplings across hydrogen bonds in Watson-Crick and Hoogsteen base pairs of a DNA triplex. J. Am. Chem. Soc. 1999;121:6019–6027. doi: 10.1021/ja9908321. [DOI] [Google Scholar]
- Bartolomé C. Espinet P. Martín-Alvarez J. M. Is there any bona fide example of O-H⋯F-C bond in solution? The cases of HOC(CF3)2(4-X-2,6-C6H2(CF3)2) (X = Si(i-Pr)3, CF3) Chem. Commun. 2007;2:4384–4386. doi: 10.1039/B710304B. [DOI] [PubMed] [Google Scholar]
- Chaudhari S. R. Mogurampelly S. Suryaprakash N. Engagement of CF3 group in N-H⋯F-C hydrogen bond in the solution state: NMR spectroscopy and MD simulation studies. J. Phys. Chem. B. 2013;117:1123–1129. doi: 10.1021/jp310798d. [DOI] [PubMed] [Google Scholar]
- Nakahara M. Wakai C. Monomeric and Cluster States of Water Molecules in Organic Solvent. Chem. Lett. 1992;21:809–812. doi: 10.1246/cl.1992.809. [DOI] [Google Scholar]
- Dhanishta P. Mishra S. K. Suryaprakash N. Intramolecular HB Interactions Evidenced in Dibenzoyl Oxalamide Derivatives: NMR, QTAIM, and NCI Studies. J. Phys. Chem. A. 2018;122:199–208. doi: 10.1021/acs.jpca.7b10598. [DOI] [PubMed] [Google Scholar]
- Manjunatha Reddy G. N. Vasantha Kumar M. V. Guru Row T. N. Suryaprakash N. N-H⋯F hydrogen bonds in fluorinated benzanilides: NMR and DFT study. Phys. Chem. Chem. Phys. 2010;12:13232–13237. doi: 10.1039/C0CP00492H. [DOI] [PubMed] [Google Scholar]
- Dunger A. Limbach H. H. Weisz K. Geometry and strength of hydrogen bonds in complexes of 2'-deoxyadenosine with 2'-deoxyuridine. J. Am. Chem. Soc. 2000;122:10109–10114. doi: 10.1021/ja000718e. [DOI] [Google Scholar]
- Gellman S. H. Dado G. P. Liang G. B. Adams B. R. Conformation-Directing Effects of a Single Intramolecular Amide-Amide Hydrogen Bond: Variable-Temperature NMR and IR Studies on a Homologous Diamide Series. J. Am. Chem. Soc. 1991;113:1164–1173. doi: 10.1021/ja00004a016. [DOI] [Google Scholar]
- Gellman S. H. Adams B. R. Dado G. P. Temperature-Dependent Changes in the Folding Pattern of a Simple Triamide. J. Am. Chem. Soc. 1990;112:460–461. doi: 10.1021/ja00157a077. [DOI] [Google Scholar]
- Martínez-Martínez F. J. Ariza-Castolo A. Tlahuext H. Tlahuextl M. Contreras R. 1H, 13C, 15N, 2D and variable temperature NMR study of the role of hydrogen bonding in the structure and conformation of oxamide derivatives. J. Chem. Soc., Perkin Trans. 2. 1993:1481–1485. doi: 10.1039/P29930001481. [DOI] [Google Scholar]
- Grzesiek S. Cordier F. Jaravine V. Barfield M. Insights into biomolecular hydrogen bonds from hydrogen bond scalar couplings. Prog. Nucl. Magn. Reson. Spectrosc. 2004;45:275–300. doi: 10.1016/j.pnmrs.2004.08.001. [DOI] [Google Scholar]
- Cormanich R. A. Moreira M. A. Freitas M. P. Ramalho T. C. Anconi C. P. A. Rittner R. Contreras R. H. Tormena C. F. 1h J FH coupling in 2-fluorophenol revisited: is intramolecular hydrogen bond responsible for this long-range coupling? Magn. Reson. Chem. 2011;49:763–767. doi: 10.1002/mrc.2838. [DOI] [PubMed] [Google Scholar]
- Mishra S. K. Suryaprakash N. Organic fluorine involved intramolecular hydrogen bonds in the derivatives of imides: NMR evidence corroborated by DFT based theoretical calculations. RSC Adv. 2015;5:86013–86022. doi: 10.1039/C5RA19537C. [DOI] [Google Scholar]
- Afonin A. V. Ushakov I. A. Sobenina L. N. Stepanova Z. V. Petrova O. V. Trofimov B. A. Different types of hydrogen bonds in 2-substituted pyrroles and 1-vinyl pyrroles as monitored by 1H, 13C and 15N NMR spectroscopy and ab initio calculations. Magn. Reson. Chem. 2006;44:59–65. doi: 10.1002/mrc.1727. [DOI] [PubMed] [Google Scholar]
- King M. M. Yeh H. J. C. Dudek G. O. Nitrogen NMR spectroscopy: application to some substituted pyrroles. Org. Magn. Reson. 1976;8:208–212. doi: 10.1002/mrc.1270080411. [DOI] [Google Scholar]
- Bernet B. Vasella A. 1H-NMR analysis of intra- and intermolecular H-bonds of alcohols in DMSO: chemical shift of hydroxy groups and aspects of conformational analysis of selected monosaccharides, inositols, and ginkgolides. Helv. Chim. Acta. 2000;83:995–1021. doi: 10.1002/(SICI)1522-2675(20000510)83:5<995::AID-HLCA995>3.0.CO;2-Q. [DOI] [Google Scholar]
- Arunan E. Desiraju G. R. Klein R. A. Sadlej J. Scheiner S. Alkorta I. Clary D. C. Crabtree R. H. Dannenberg J. J. Hobza P. Kjaergaard H. G. Legon A. C. Mennucci B. Nesbitt D. J. Defining the hydrogen bond: an account (IUPAC Technical Report) Pure Appl. Chem. 2011;83:1619–1636. [Google Scholar]
- Frisch M. J., Trucks G. W., Schlegel H. B., Scuseria G. E., Robb M. A., Cheeseman J. R., Scalmani G., Barone V., Mennucci B., Petersson G. A., Nakatsuji H., Caricato M., Li X., Hratchian H. P., Izmaylov A. F., Bloino J., Zheng G., Sonnenberg J. L., Hada M., Ehara M., Toyota K., Fukuda R., Hasegawa J., Ishida M., Nakajima T., Honda Y., Kitao O., Nakai H., Vreven T., Montgomery Jr J. A., Peralta J. E., Ogliaro F., Bearpark M. J., Heyd J., Brothers E. N., Kudin K. N., Staroverov V. N., Kobayashi R., Normand J., Raghavachari K., Rendell A. P., Burant J. C., Iyengar S. S., Tomasi J., Cossi M., Rega N., Millam N. J., Klene M., Knox J. E., Cross J. B., Bakken V., Adamo C., Jaramillo J., Gomperts R., Stratmann R. E., Yazyev O., Austin A. J., Cammi R., Pomelli C., Ochterski J. W., Martin R. L., Morokuma K., Zakrzewski V. G., Voth G. A., Salvador P., Dannenberg J. J., Dapprich S., Daniels A. D., Farkas Ö., Foresman J. B., Ortiz J. V., Cioslowski J. and Fox D. J., Gaussian, Inc., Wallingford, CT, USA, 2009 [Google Scholar]
- Contreras-García J. Yang W. Johnson E. R. Analysis of hydrogen-bond interaction potentials from the electron density: integration of noncovalent interaction regions. J. Phys. Chem. A. 2011;115:12983–12990. doi: 10.1021/jp204278k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader R. F. W., Atoms in Molecules: A Quantum Theory, Oxford University Press, Oxford, 1994 [Google Scholar]
- Johnson E. R. Keinan S. Mori-sánchez P. Contreras-garcía J. Aron J. Yang W. Revealing Noncovalent Interactions. J. Am. Chem. Soc. 2011;132:6498–6506. doi: 10.1021/ja100936w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J. Dong H. Yang H. Zheng Y. Exploring and elaborating the novel excited state dynamical behavior of a bisflavonol system. Org. Chem. Front. 2018;5:2710–2718. doi: 10.1039/C8QO00688A. [DOI] [Google Scholar]
- Lu T. Chen F. Multiwfn: a multifunctional wavefunction analyzer. J. Comput. Chem. 2012;33:580–592. doi: 10.1002/jcc.22885. [DOI] [PubMed] [Google Scholar]
- Humphrey W. Dalke A. Schulten K. VMD: Visual Molecular Dynamics. J. Mol. Graphics. 1996;14:33–38. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- Bader R. F. W. Anderson S. G. Duke A. J. Quantum Topology of Molecular Charge Distributions. J. Am. Chem. Soc. 1979;101:1389–1395. doi: 10.1021/ja00500a006. [DOI] [Google Scholar]
- Bader R. F. W. Essén H. The characterization of atomic interactions. J. Chem. Phys. 1984;80:1943–1960. doi: 10.1063/1.446956. [DOI] [Google Scholar]
- Bader R. F. W. Nguyen-Dang T. T. Tal Y. A topological theory of molecular structure. Rep. Prog. Phys. 1981;44:893–948. doi: 10.1088/0034-4885/44/8/002. [DOI] [Google Scholar]
- Runtz G. R. Bader R. F. W. Messer R. R. Definition of bond paths and bond directions in terms of the molecular charge distribution. Can. J. Chem. 1977;55:3040–3045. doi: 10.1139/v77-422. [DOI] [Google Scholar]
- Sheldrick G. M. Crystal structure refinement with SHELXL. Acta Crystallogr., Sect. C: Struct. Chem. 2015;71:3–8. doi: 10.1107/S2053229614024218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Refinement M., Manual Version 2015 Program Version 6.03, February 2015
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.