Abstract
The problem of antibiotic resistance considers one of the most dangerous challenges facing the medical field. So, it is necessary to find substitutions to conventional antibiotics. Antimicrobial peptides (AMPs) are a bio-functional derivative that have been observed as one of the important solutions to such upcoming crisis. Owing to their role as the first line of defense against bacteria, fungi, and viruses. This study was conducted to induce the immune response of Spodoptera littoralis larvae by inoculation of sub lethal doses of Staphylococcus aureus and its enterotoxin. Since Staphylococcal enterotoxin A (SEA) considers the major causative agents of Staphylococcal food poisoning, our study oriented to purify and characterize this toxin to provoke its role in yielding AMPs with broad spectrum antimicrobial activity. A great fluctuation was recorded in the biochemical properties of immunized hemolymph not only in the total protein content but also protein banding pattern. Protein bands of ∼22 kDa (attacin-like) and ∼15 kDa (lysozyme-like) were found to be common between the AMPs induced as a result of both treatments. While protein bands of molecular weight ∼70 kDa (phenoloxidase-like) and ∼14 kDa (gloverin-like) were found specific for SEA treatment. Chromatographic analysis using HPLC for the induced AMPs showed different types of amino acids appeared with differences in their quantities and velocities. These peptides exhibited noticeable antimicrobial activity against certain Gram-positive and Gram-negative bacteria. In conclusion, the antimicrobial potential of the antimicrobial peptides (AMP) induced in the larval hemolymph of S. littoralis will be a promising molecule for the development of new therapeutic alternatives.
Keywords: Antimicrobial peptides (AMPs), Staphylococcus aureus, Staphylococcal enterotoxin A (SEA)
1. Introduction
Insects have evolved and prospered in environments full of potentially pathogenic and parasitic contributors. Defensive weapons of insects rely on humoral and cellular innate mechanisms. It has been approved that innate immune systems of insects and mammals share resemblance in the function and structure. So many studies have been established using insects as an alternative model host for examining virulence factors of human pathogenic bacteria (Junqueira and Mylonakis, 2019). Staphylococcus aureus is considered a dangerous and versatile human pathogen that causes a lot of various infections, ranging from mild food poisoning to life-threatening diseases (Abulreesh et al., 2017). Also, it causes skin infections and respiratory tract infections (Saluzzo et al., 2018). Moreover, S. aureus is considered the causative agent of clinical and subclinical bovine mastitis worldwide (Tonacini et al., 2019). Staphylococcal enterotoxins (SEs) are exoproteins produced by certain strains in culture media and in foods; these toxins are the causative agents of Staphylococcal food poisoning (SFP). More than 20 SEs are being identified (Loncarevic et al., 2005). The most common SEs encountered in food poisoning outbreaks is Staphylococcal enterotoxin A (SEA) (Clarisse et al., 2013). The SEA is resistant to many denaturing conditions, like low pH and heat treatment that destroys the bacterial enterotoxin producing them easily (Asao et al., 2003), and to proteolytic enzymes, thus retaining their activity in the digestive tract after ingestion (Regenthal et al., 2017).
Antimicrobial peptides (AMPs) are protein molecules of small size show a critical role in host inborn immune outline. One of their specific properties are often considered as a barrier against various pathogenic particles (Borah et al., 2021). In insects the induced AMPs mediates a humoral immune response which persists more than the initial cellular responses, and function as a back-up against persistent infections (Makarova et al., 2016). Advanced studies have been directed to recognize many types of host defense peptides, including cecropins (Boman, 2000), defensins (Lehrer, 2004), and others with different structures and bioactivity profiles (Wang, 2017). Owing to their low toxicity to eukaryotic cells and their wide spectrum of action against antimicrobial and antitumor activity (Hu et al., 2013). As a result of their great effect against antibiotic resistant bacteria, the induced AMPs represent a novel field of antibiotics and as a new therapeutic choice for many infections caused by multidrug-resistant bacteria (Giuliani et al., 2007, Kendurkar and Sengupta, 2018). Because of insect's biodiversity, they considered one of the richest and most advanced sources for these molecules. Many studies have been established using insects AMPs and studied its bactericidal and bacteriostatic effect towards different bacteria (Radwan et al., 2019).
Insect AMPs are classified into three major structural classes; linear α-helical peptides without any cysteine residues, peptides with a β-sheet globular structure stabilized by intramolecular disulfide bridges necessary for AMP activity (Yi et al., 2014), and peptides containing high numbers of specific amino acid residues, such as glycine or proline (Wiesner and Vilcinskas, 2010). They are cationic and contain up to 50% hydrophobic residues. This is the reason for the interaction of those AMPs with the lipophilic, negatively charged membranes of bacterial cells. So, AMPs are attached to bacterial cell membranes electrostatically, and just contact is established the hydrophobic residues enhance integration, causing the membrane outer leaflet to extend and become thinner, afterward forming pores or even causing lysis (Brown and Hancock, 2006). Data base of antimicrobial Peptide shows an interface to predict antimicrobial activity of any submitted sequence, based on a count method and a simple residue analysis and some valuable statistical information on peptides sequence, structure and function (Bhadra et al., 2018, Tucker et al., 2018).
The present study was oriented to isolate, purify and characterize the staphylococcal enterotoxin A (SEA). As well as, to clarify the efficiency of S. aureus and SEA in the stimulation of Spodoptera littoralis larvae immune response to induce the production of several antimicrobial peptides. Insect induced AMPs are a promising alternative to traditionally used antibiotics against serious pathogenic bacteria.
2. Materials and methods
2.1. Experimental insect
Spodoptera littoralis (cotton leaf worm) used in this study was maintained from the Cotton Leaf-worm Research Department, Plant Protection Research Institute, Agricultural Research Center, Egypt. Preserved and reared according to the method reported by Rivnay and Meisner (1966). Using the fifth-instar larvae in all the subsequent experiments and performed with a group of 20 larvae.
2.2. DNA extraction and SEA gene isolation
DNA was extracted according to gene jet genomic DNA (Thermo Fisher Scientific) from S. aureus culture two specific primers for Ent A gene according to Chen et al. (2012). All used primers were manufactured by Biosearch Technologies (US & Canada). All PCRs were directed in the following conditions: one cycle of 4 min at 94 °C, 30 cycles of 94 °C for 35 s, 55 °C for 45 s and 45 s at 72 °C and one cycle at 72 °C for 7 min. DNA amplified fragments were cloned into pGEM-T easy vector (A high-efficiency TA cloning vector which contain multiple cloning sites) as described by Hanahan and Meselson (1983) and then transformed into E. coli GC5 competent cells. Blue-white selection was conducted, and recombinant plasmids were isolated from overnight grown E. coli using Wizard® plasmid mini-preparation (Promega). Isolated plasmids were confirmed as recombinant through restriction digestion. Target DNA fragments were subcloned into PGEX-4T-1plasmid by BamH I and XhoI restriction enzymes and transformed E. coli BL21.
2.3. Enterotoxin A (Ent A) gene expression and protein purification
E. coli BL21 cells contain the construct of Ent A gene were grown at 37 °C and was induced by 0.1 mM Isopropyl ß-D-1-thiogalactopyranoside (IPTG), when the growth optical density (O.D600) reached 0.6 at 28 °C. Cells were harvested and re-suspended in lysis buffer by ice sonication. Recombinant GST-tagged proteins were purified by affinity-based chromatography method under native conditions using glutathione resin from GE Healthcare. Ent A protein were eluted, and their purity was visualized using 12% SDS-PAGE. Recombinant proteins were liberated from GST-moiety by Thrombin Protease. Protein concentration was determined using Bradford assay according to Bradford (1976).
2.4. Western blot
Western blot was performed according to Sambrook and Russel (2001). Purified Ent A protein is electroblotted onto polyvinylidene difluoride membrane (PVDF; Thermo Scientific). Standard polyclonal antibodies (Sigma-Aldrich) against recombinant toxin were used as the primary antibody. Universal anti-mouse, conjugated with alkaline phosphatase (Sigma Aldrich), was used as the secondary antibody and detection was conducted using Nitro-Blue Tetrazolium chloride (NBT) and 5-bromo-4-chloro-3′-indolyphosphate p-toluidine salt (BCIP) substrates.
2.5. Insect susceptibility level
A stock suspension of S. aureus bacteria was adjusted to a concentration of 1 × 108 cells/ml by using the pour plate count technique according to Sutton (2011). From this stock, serial concentrations; 1 × 103, 1 × 104, 1 × 105, 1 × 106, 1 × 107 and 1 × 108 cells/ml were prepared and 5 μl of each were injected into each of 20 S. littoralis larval groups. Control insects were injected with equivalent volumes of distilled water only. Larval injection was carried out using a 10 μl Hamilton micro-syringe fitted with a 26-gauge needle according to Meylaers et al. (2007). To determine the sub-lethal dose of SEA, 0.4 μg, 0.5 μg, 0.6 μg, 0.7 μg, 0.8 μg, 0.9 μg and 1 μg of the toxin were injected into each individual larva (Saad et al., 2021). The treated larvae were maintained in separate cages at 30 °C. Final mortality percentages were recorded 48 h post-injection. For bioassay test, the LC50 value was estimated according to Finney (1971).
A suspension of S. aureus and SEA that produces 20% larval mortality was prepared to be injected into the hemocoel of the experimental larvae.
2.6. Hemolymph collection
Hemolymph samples from normal, control and immune challenged larvae were collected 48 h following injection. 5th instar larvae were chilled for 15 min on ice, to slow down hemolymph coagulation and reduce the larval activity, and then dried on a piece of absorbent paper. Chilled insects were surface sterilized with 70% ethanol. The hemolymph was collected by suction with a fine-tipped calibrated glass capillary by piercing the cuticle on the first proleg with a fine sterile dissecting needle and transferred into sterile and chilled Eppendorf tubes containing 1 mg of phenylthiourea (Sigma chemical) to prevent melanization, which was kept at −20 °C until further analyses.
2.6.1. Preparation of cell-free hemolymph
Hemocytes were collected from hemolymph by Centrifugation (Human Centrifuge, TGL-16XYJ-2, 16,000 rpm, Korea) at 6000 rpm for 20 min at 4 °C. The supernatant (plasma) was taken from the hemocyte pellet and immediately transferred to sterile and refrigerated Eppendorf tubes, which were then stored at 18oC until needed. For several days at room temperature, the pure plasma showed no signs of coagulation under these conditions.
2.7. Antimicrobial susceptibility test
For the estimation of the antibacterial activity of the induced antimicrobial peptides from the hemolymph of immunized larvae against different bacteria we apply the disc diffusion technique according to Matuschek et al., (2014) and Saad et al., 2021a, Saad et al., 2021b) using Muller Hinton agar (oxoid). Several morphologically analogous colonies were selected from an overnight bacterial growth with a sterile cotton swab and were suspended in sterile saline (0.85% NaCl w/v in water) to prepare the inoculum suspension (El-Saadony et al., 2021a, El-Saadony et al., 2021b). Positive control was made by Tetracycline antibiotic disks (30 μg/ml) (SIGMA). A distinct zone clear of bacterial growth was seen surrounding the disks that contained immune plasma. The actual zone width was measured as the following:
2.8. Quantification of total hemolymph proteins
The total protein content was assessed as mg/ml using the equation derived from the standard calibration curve of the Bovine serum albumin solution (BSA) according to the method described by Bradford (1976) and Saad et al., (2015) using spectrophotometer at 595 nm (UNICO Spectrophotometer, SP2100 UV, China)
2.9. Electrophoretic analysis of the hemolymph proteins
SDS polyacrylamide gel electrophoresis was performed according to Laemmli (1970).
2.10. Analysis of protein amino acids
The common protein bands of both treatments and the bands specific for SEA injection were eluted byside-strip technique and hydrolyzed to their constituent amino acids using High-performance liquid chromatography and amino acid analyzer (LC3000 Eppendorf, Germany) as described by Cooper et al. (2001).
3. Results
3.1. Expression and purification of Ent a recombinant protein
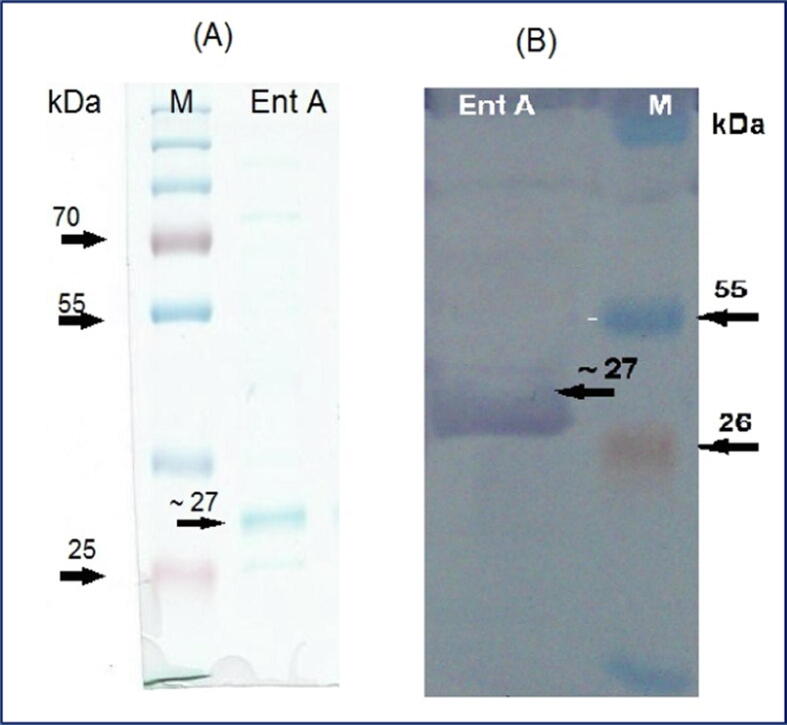
Amplified Ent A gene was sub-cloned into PGEX-4T-1expression vector in a frame fused with GST coding genes to facilitate the downstream-purification of expressed proteins. The Ent A protein was determined using SDS-page analysis. Under native conditions, affinity chromatography was used to purify the expressed protein from the lysate of E. coli BL21 bacteria. Protein purification with affinity tags such as glutathione S-transferase (GST). GST-column purified Ent A protein band fused to the GST protein appears at ∼53 kDa (Fig. 1). The expressed GST-tagged protein was cleaved by thrombin protease, and a pure single band was visible at ∼27 kDa on the SDS-page stained by Commassie Fig. 1, which was confirmed by Western blot Fig. 2.
Fig. 1.
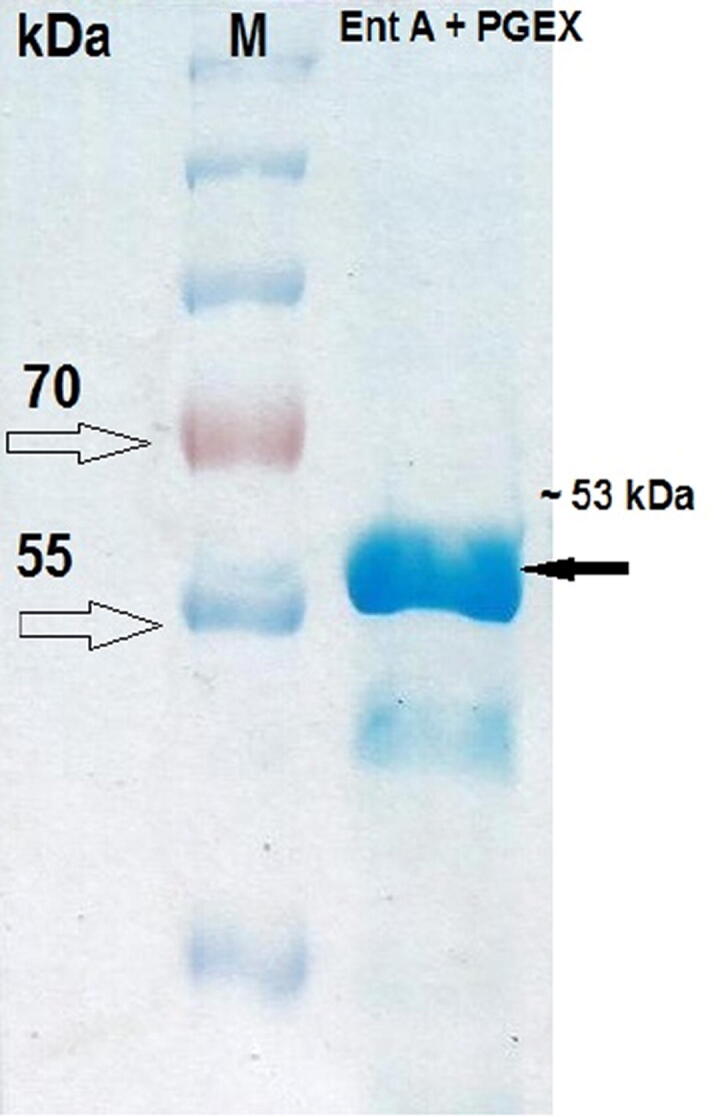
Purification of Ent A - GST fused protein. M: protein ladder (Thermo Scientific). Lane 1: Purified fusion protein.
Fig. 2.
Purified expressed Ent A toxin protein of E. coli BL21 transformed with PGEX-4T-1 recombinant with Ent A gene after their liberation from GST-tag. (A); SDS-Page, (B); Western blotting, Lane 1(M): protein ladder, Lane 2 (Ent A): purified Ent A (∼27 kDa).
3.2. Susceptibility of S. Littoralis to bacterial pathogens and SEA toxin
Data obtained from the susceptibility tests of larvae to the intra-hemocoelic injection of S. aureus and SEA were shown in Table 1, Table 2 respectively. For S. aureus, the estimated LC50 and LC20 values, at 95% probability, were 4.8 × 105and 1.1 × 104cell/ml, respectively, while the LD50 and LD20 for SEA were 0.73 and 0.59 μg/ larva, respectively.
Table 1.
Susceptibility of 5th instar larvae of S. littoralis to S. aureus.
| Concentration (CFU/ml) | Dead/ total | Observed mortality (%) | Expected mortality (%) |
|---|---|---|---|
| 1 × 103 | 1.5 / 20 | 7.50 | 7.76 |
| 1 × 104 | 4.5 / 20 | 22.50 | 19.23 |
| 1 × 105 | 7.0 / 20 | 35.00 | 37.56 |
| 1 × 106 | 10.5 / 20 | 52.50 | 59.30 |
| 1 × 107 | 15.6 / 20 | 78.00 | 78.46 |
| 1 × 108 | 17.6 / 20 | 88.00 | 91.00 |
| Control | 0 / 20 | – | – |
| Chi2 | 0.383 | ||
| Slope | 0.5136 ± 0.0854 | ||
| LC50 | 4.8 × 105 cells/ml | ||
| LC20 | 1.1 × 104 cells/ml | ||
Table 2.
Susceptibility of 5th instar larvae of S. littoralis to SEA.
| Injection dose | Dead/ total | Observed mortality (%) | Expected mortality (%) |
|---|---|---|---|
| 0.4 μg | 0.1/20 | 0.50 | 0.90 |
| 0.5 μg | 2.0/20 | 10.00 | 6.84 |
| 0.6 μg | 4.0/20 | 20.00 | 22.00 |
| 0.7 μg | 8.5/20 | 42.50 | 43.36 |
| 0.8 μg | 12.3/20 | 61.50 | 63.94 |
| 0.9 μg | 15.4/20 | 77.00 | 79.36 |
| 1 μg | 18.6/20 | 93.00 | 89.11 |
| Control | 0.0/20 | – | – |
| Chi2 | 0.835 | ||
| Slope | 9.04 | ||
| LD50 | 0.73 μg/larva | ||
| LD20 | 0.59 μg/larva | ||
3.3. Antimicrobial susceptibility test
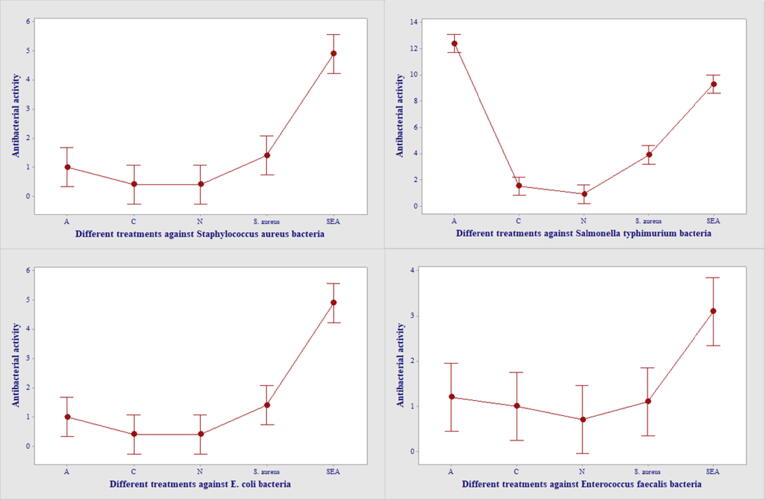
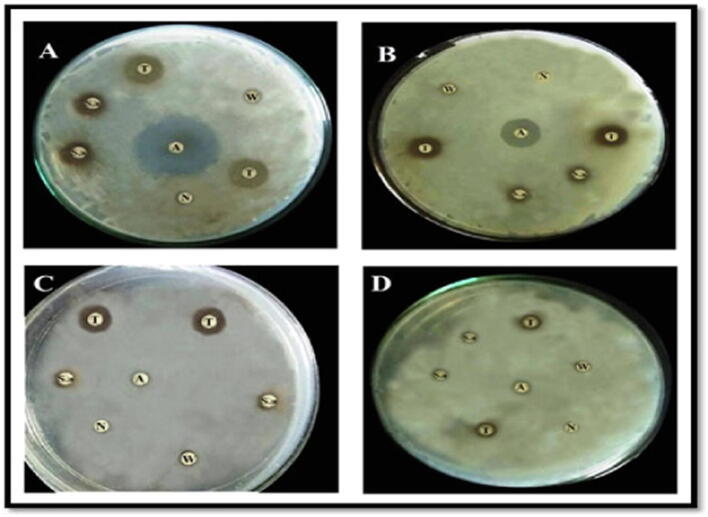
The bactericidal activity of the cotton leaf worm plasma was performed against S. aureus, S. typhimurium, E. coli and E. faecalis bacteria. Results were demonstrated graphically in Fig. 3 and photographically in Plate 1. A weak antibacterial activity was observed in the hemolymph of the 5th instar larvae, against the tested bacteria. The inhibition zone in water-injected larvae did not show any significant difference with the normal larval serum. In addition, a significant induction of antimicrobial activity in the larval serum injected with SEA against S. aureus and S. typhimurium as compared with other treatments (10.00 mm ± 1.06 and 9.30 mm ± 1.20), respectively. At the same time, it has been proven to have the greatest bactericidal activity against E. coli and E. faecalis (4.90 mm ± 1.29 and 3.10 mm ± 0.89), respectively.
Fig. 3.
Antimicrobial activity test of immune plasma against S. aureus, Salmonella typhimurium, E. coli, Enterococcus faecalis, (A) antibiotic disc; (C) Control; (N) normal plasma; (S. aureus); plasma of S. aureus injected larvae; (SEA); plasma of SEA injected larvae.
Plate 1.
Photomicrograph of inhibition zone of antibacterial activity test of different treatments against different bacteria; (A) S. aureus; (B) S. typhimurium; (C) E. coli and (D) E. faecalis. A (antibiotic disc; tetracycline); N (normal plasma); W (plasma of water-injected larvae); S. aureus (plasma of S. aureus injected larvae); and T (plasma of larvae injected with SEA toxin).
3.4. Effect of microbial injection on S. Littolaris total hemolymph proteins
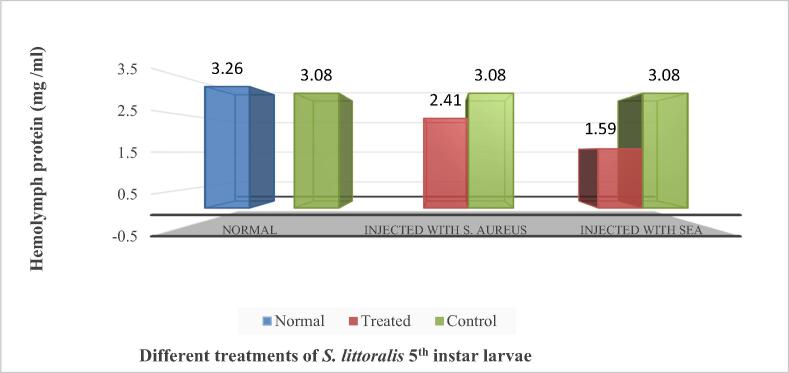
Results of the total hemolymph protein content of the 5th larval instar of the cotton leaf worm [normal, water-injected, S. aureus-injected and SEA-injected larvae (48 h post-injection)] was graphically assembled in Fig. 4; no significant change was recorded in the hemolymph protein content of water-injected insects as compared with normal insects, while the hemolymph protein content of S. aureus injected larvae and SEA injected larvae was decreased significantly (P = 0.004 and P = 0.0008), respectively than that of control.
Fig. 4.
Total protein content (mg/ml) of the hemolymph of S. littoralis5th in star larvae determined at 48 h post-injection with S. aureus and SEA.
3.5. Electrophoretic analysis of S. Littoralis hemolymph proteins
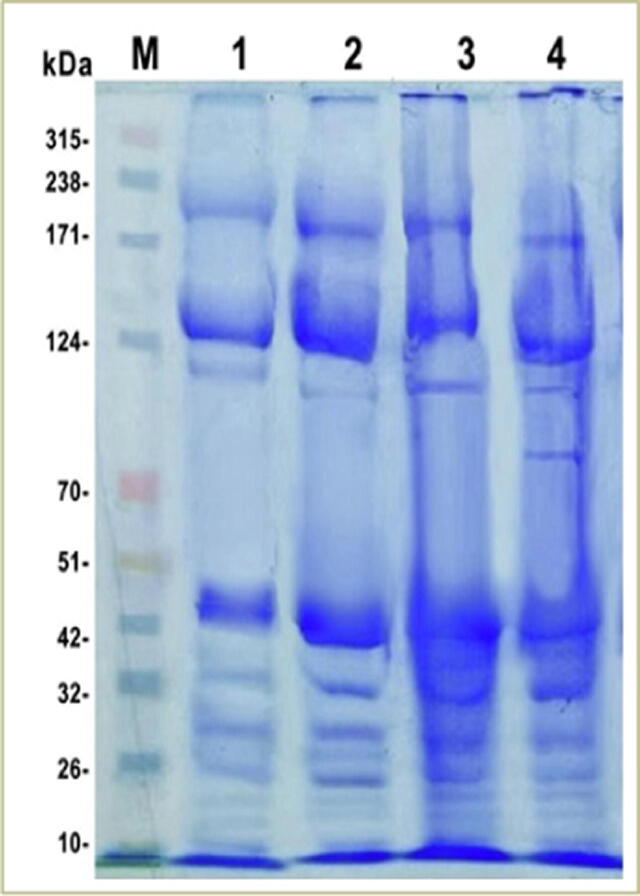
Protein profile of hemolymph plasma of the 5th in star larvae was performed for normal larvae as well as control and treated ones. Data are represented in Fig. 5. The hemolymph of the un-injected larvae was separated into eleven protein bands. Some proteins were disappeared or at least had different band percentage post injection with water, bacteria or toxin. Moreover, new bands were induced to synthesize, where seven new protein bands were detected as a response of water injection and five new synthesized protein bands were found to be specific for S. aureus injection and SEA injection. Plasma proteins from larvae injected with S. aureus and SEA were separated into 12 bands with MW ranging from 350 to 15 kDa, and 350 to 14 kDa, respectively. Results also showed that there are three major bands were common between normal, control and treated larvae. Three bands were common between control and treated larvae, appeared to be specific for injection; two protein bands with MWs 22 and 15 kDa were found to be common between treated larvae; and two bands with MWs 70 and 14 kDa were specific for SEA injection.
Fig. 5.
Plasma protein banding patterns of S. littoralis normal, control, S. aureus injected larvae, and SEA injected larvae M: protein molecular weight marker; 1: plasma from normal larvae; 2: plasma from control larvae; 3: plasma from S. aureus injected larvae;and 4: plasma from SEA injected larvae.
3.6. Amino acid analysis
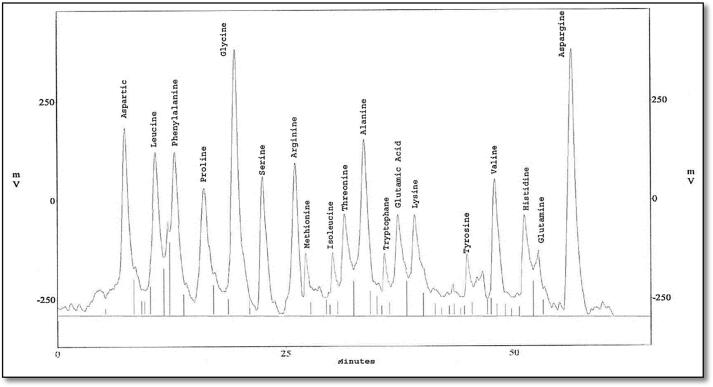
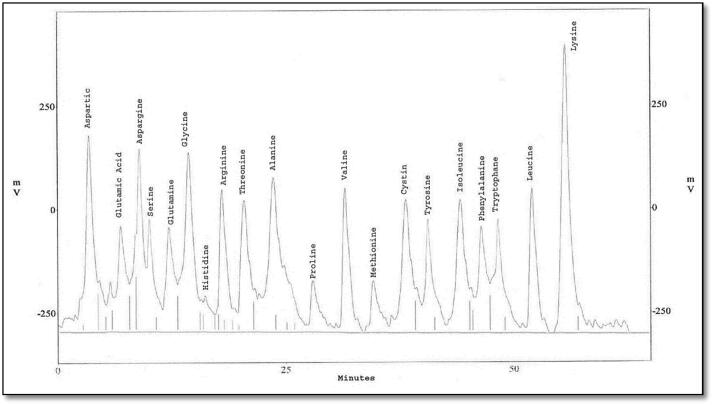
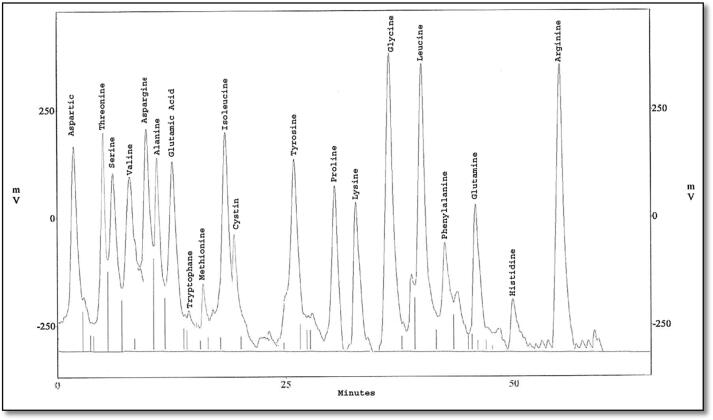
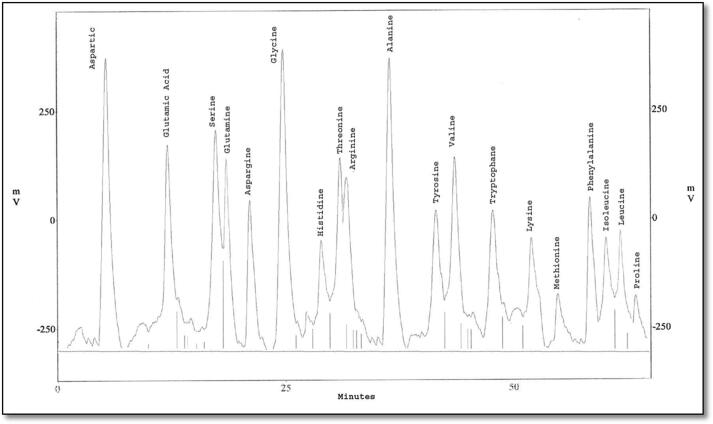
The eluted proteins were analyzed to study their amino acid composition using HPLC. Protein bands which were common between the treated larvae (MW 22 and 13 kDa) were eluted using side-strip technique, and analyzed into nineteen and twenty different amino acids, respectively (Fig. 6, Fig. 7, Fig. 8, Fig. 9). Besides, protein bands specific for SEA injection (MW 70 and 14 kDa) were analyzed into twenty and nineteen amino acids, respectively.
Fig. 6.
HPLC analysis of eluted protein band with molecular weight 22 KDa showing its amino acid composition.
Fig. 7.
HPLC analysis of eluted protein band with molecular weight 15 KDa showing its amino acid composition.
Fig. 8.
HPLC analysis of eluted protein band with molecular weight 70 KDa showing its amino acid composition.
Fig. 9.
HPLC analysis of eluted protein band no. 34 with molecular weight 14 KDa showing its amino acid composition.
These analyzed proteins have shown different amino acids composition with different concentrations and are compared with the amino acid compositions of other lepidopterous insect species Table 3, Table 4, Table 5, Table 6.
Table 3.
Amino acid composition of the eluted protein band (22 kDa) “attacin-like” of S. littoralis 5th instar larvae and its comparison with other attacin AMPs from other lepidopterous insects.
| Amino acid | Band (22 kDa) “attacin-like” | Attacin of Spodoptera exigua | Attacin of Bombyx mori | Attacin of Manduca Sexta |
|---|---|---|---|---|
| Ala (A) | 8.0 | 6.3 | 11.2 | 9.3 |
| Arg (R) | 6.6 | 4.3 | 4.7 | 3.1 |
| Asn (N) | 9.1 | 9.1 | 8.9 | 7.6 |
| Asp (D) | 8.5 | 5.5 | 5.1 | 9.3 |
| Cys (C) | 0.0 | 0.0 | 0.9 | 0.1 |
| Gln (Q) | 3.5 | 3.4 | 1.4 | 0.0 |
| Glu (E) | 3.6 | 3.9 | 0.9 | 0.9 |
| Gly (G) | 10.2 | 10.6 | 10.3 | 9.3 |
| His (H) | 3.9 | 3.2 | 2.8 | 3.1 |
| Ile (I) | 2.7 | 1.2 | 2.3 | 2.2 |
| Leu (L) | 7.9 | 10.2 | 9.8 | 8.4 |
| Lys (K) | 4.0 | 3.9 | 5.6 | 6.7 |
| Met (M) | 1.1 | 3.9 | 0.9 | 3.1 |
| Phe (F) | 7.0 | 6.7 | 7.5 | 7.1 |
| Pro (P) | 4.8 | 6.3 | 4.2 | 4.0 |
| Ser (S) | 6.0 | 6.3 | 12.1 | 7.1 |
| Thr (T) | 4.2 | 4.3 | 3.7 | 4.0 |
| Trp (W) | 1.6 | 1.4 | 0.5 | 7.1 |
| Tyr (Y) | 2.5 | 2.0 | 1.4 | 6.7 |
| Val (V) | 4.8 | 7.5 | 5.6 | 0.9 |
Table 4.
Amino acids composition of the eluted protein band (15 kDa) “lysozyme” of S. littoralis 5th instar larvae and its comparison with other lysozymes from other lepidopterous insects.
| Amino acid | Band (15 kDa) “lysozyme” | Lysozyme of Spodopteralitura | Lysozyme of Bombyx mori | Lysozyme of Manduca Sexta |
|---|---|---|---|---|
| Ala (A) | 5.3 | 5.7 | 5.3 | 6.6 |
| Arg (R) | 7.5 | 5.0 | 3.8 | 9.8 |
| Asn (N) | 6.0 | 5.0 | 4.3 | 4.9 |
| Asp (D) | 6.2 | 6.4 | 4.8 | 4.4 |
| Cys (C) | 6.1 | 7.1 | 4.3 | 4.4 |
| Gln (Q) | 3.0 | 5.0 | 2.4 | 4.9 |
| Glu (E) | 3.9 | 4.3 | 4.3 | 5.5 |
| Gly (G) | 6.8 | 6.4 | 9.1 | 5.5 |
| His (H) | 0.7 | 2.1 | 4.8 | 0.5 |
| Ile (I) | 5.9 | 3.5 | 7.2 | 4.4 |
| Leu (L) | 5.7 | 8.5 | 6.7 | 9.8 |
| Lys (K) | 13.1 | 10.6 | 4.8 | 9.3 |
| Met (M) | 1.7 | 2.1 | 2.9 | 2.2 |
| Phe (F) | 3.3 | 3.5 | 3.8 | 3.3 |
| Pro (P) | 2.6 | 2.1 | 5.8 | 4.4 |
| Ser (S) | 5.0 | 5.0 | 6.2 | 5.5 |
| Thr (T) | 4.5 | 7.1 | 7.2 | 3.8 |
| Trp (W) | 2.6 | 2.8 | 2.4 | 2.7 |
| Tyr (Y) | 2.1 | 2.8 | 3.4 | 2.7 |
| Val (V) | 4.0 | 5.0 | 6.2 | 5.5 |
Table 5.
Amino acids composition of the eluted protein band (70 kDa) “phenoloxidase” of S. littoralis 5th instar larvae and its comparison with other phenoloxidase from other lepidopterous insects.
| Amino acid | Band (70 kDa)Phenoloxidase | PO of Spodoptera litura | PO of Bombyx mori | PO of Manduca Sexta |
|---|---|---|---|---|
| Ala (A) | 6.8 | 6.4 | 6.1 | 6.2 |
| Arg (R) | 7.0 | 5.9 | 4.5 | 6.0 |
| Asn (N) | 4.0 | 3.7 | 5.2 | 4.4 |
| Asp (D) | 6.8 | 6.4 | 4.3 | 6.8 |
| Cys (C) | 3.9 | 4.3 | 5.4 | 1.2 |
| Gln (Q) | 5.6 | 5.1 | 5.4 | 2.3 |
| Glu (E) | 5.7 | 3.7 | 3.9 | 6.0 |
| Gly (G) | 8.0 | 9.9 | 8.8 | 9.8 |
| His (H) | 0.9 | 1.3 | 1.6 | 1.5 |
| Ile (I) | 7.0 | 5.3 | 6.1 | 4.1 |
| Leu (L) | 7.4 | 6.1 | 7.3 | 8.5 |
| Lys (K) | 4.4 | 3.2 | 5.2 | 5.8 |
| Met (M) | 1.0 | 0.8 | 1.1 | 2.3 |
| Phe (F) | 3.9 | 2.7 | 2.3 | 4.8 |
| Pro (P) | 4.6 | 5.9 | 7.0 | 5.6 |
| Ser (S) | 5.3 | 7.5 | 5.7 | 5.4 |
| Thr (T) | 7.1 | 7.0 | 6.8 | 5.2 |
| Trp (W) | 0.7 | 1.9 | 1.1 | 3.1 |
| Tyr (Y) | 4.7 | 4.6 | 3.6 | 4.4 |
| Val (V) | 5.2 | 5.0 | 8.4 | 6.6 |
Table 6.
Amino acids composition of the eluted protein band (14 kDa) “Gloverin-like” of S. littoralis 5th instar larvae and its comparison with other gloverin AMPs from other lepidopterous insects.
| Amino acid | Band no. 34“Gloverin-like” | Gloverin of Spodoptera exigua | Gloverin of Bombyx mori | Gloverin of Manduca Sexta |
|---|---|---|---|---|
| Ala (A) | 6.0 | 6.3 | 7.0 | 7.3 |
| Arg (R) | 7.6 | 8.0 | 7.0 | 4.0 |
| Asn (N) | 5.0 | 4.6 | 5.8 | 4.5 |
| Asp (D) | 9.4 | 7.4 | 7.6 | 5.6 |
| Cys (C) | 0.0 | 1.7 | 0.6 | 1.1 |
| Gln (Q) | 4.3 | 4.6 | 3.5 | 7.9 |
| Glu (E) | 5.2 | 2.3 | 4.7 | 0.6 |
| Gly (G) | 14.2 | 14.3 | 15.2 | 15.8 |
| His (H) | 2.2 | 4.6 | 1.8 | 2.8 |
| Ile (I) | 4.5 | 4.0 | 3.5 | 2.8 |
| Leu (L) | 6.1 | 8.6 | 5.8 | 7.9 |
| Lys (K) | 4.8 | 4.0 | 5.3 | 6.2 |
| Met (M) | 1.1 | 1.1 | 1.8 | 1.1 |
| Phe (F) | 5.2 | 5.7 | 4.7 | 5.6 |
| Pro (P) | 2.6 | 3.4 | 2.3 | 3.4 |
| Ser (S) | 5.1 | 5.7 | 5.3 | 5.1 |
| Thr (T) | 5.3 | 4.6 | 5.3 | 5.6 |
| Trp (W) | 3.2 | 1.1 | 2.3 | 2.3 |
| Tyr (Y) | 3.0 | 3.4 | 4.1 | 4.5 |
| Val (V) | 5.2 | 4.6 | 6.4 | 5.6 |
4. Discussion
Referring to the emergence of multidrug resistant bacterial strains are becoming ordinary. The need for innovative and efficient alternatives is essential such as peptides and nanoparticles (Abdel-Moneim et al., 2021, El-Saadony et al., 2020, Saad et al., 2021c). Although the great progress in the information of the resistance mechanisms resulted from the uncontrolled usage of antibiotics, the solution of this problem is still indefinable. Since the discovery of antimicrobial peptides (AMPs) with their pharmacological properties have been regarded as one of the significant solutions to the disaster of antimicrobial resistance. Extra studies are required to discover new antimicrobial peptides and study their applicability.
The current research attempts to approve the ability of S. littoralis larvae to induce the antimicrobial peptides in their hemolymph upon immunization with S. aureus bacteria and its enterotoxins. Also, assessment the efficiency of the induced AMPs as natural antibiotics against different G +ve and G −ve lethal septic bacterial infections. In addition, the characterization of the immunized hemolymph biochemically to determine the functional properties and amino acid analysis of these AMPs. Our findings shown that caterpillars are a good source of novel antimicrobial peptides and compounds that can be screened against multidrug-resistant pathogens.
Staphylococcus aureus strains with the Staphylococcal enterotoxin A gene are a common reason of foodborne disease due to improper handling and storage. A wide variety of foods support the growth of Staphylococcus aureus and are perfect for enterotoxin production including milk, meat, meat products, dairy products, and fast food (Diab et al., 2021). Standard SEs are classified into five types, namely, SEA, SEB, SEC, SED and SEE (Schelin et al., 2011). Until now 23 SE types are known. SEA is viewed to be most associated with SFP and is detected in >50% of relevant outbreaks.
Several experimental studies have been carried out to characterize the diverse SE gene profiles in strains (Bianchi et al., 2014, Chen et al., 2018) we designed two sets of primers according to the highly varied regions of gene. After the Ent A gene was amplified and cloned it was constructed on the PGEX-4T-1 bacterial expression vector.
Several scientists apply the inoculums in direct contact with the hemocoel by injection, to avoid the dose losses and invasion irregularities for the study of insect immunity and the observation of physiological and biochemical changes induced by pathogenic infection. Among the numerous authors who have followed the same technique are: (Pereira et al., 2015, de Viedma and Nelson, 2017, Parthuisot et al., 2018) using different insects and different pathogenic bacteria and (Radwan et al., 2019) tested different pathogenic bacteria on the same insect.
Results clearly approved that the 5th instar larvae of S. littoralis are more susceptible to SEA than S. aureus infection, indicating high toxicity of this virulent factor based on qualitative and qualitative descriptions on the way S. littoralis larvae were infected and the resulting mortality patterns, since it inhibits totally many metabolic functions of the insect. Data of antimicrobial susceptibility test demonstrated that normal insects exhibit a very weak antibacterial activity towards virulent factors. This may be due to the cellular damage formed when collecting the larval hemolymph by centrifuging after incising their bodies. This result agrees with Yoon et al. (2018) who found a weak antibacterial activity in the studied insects as the fatty acids, lipids of sterol type and monoglycerides are known to induce the antibacterial activity in vitro. The substances which are liberated from destructed cells or any modification in the natural environment of infected cells are indicators of disease severity (Swelum et al., 2020, Yoon et al., 2018).
We recorded immune induction is the control injection signifying to the appearance of antibacterial activity and synthesis of new immune proteins are not only induced by bacteria, but also through the injection of distilled water or sterile saline solutions as reported by Meshrif (2008).
Larval injection with SEA mediates a higher activation of Spodoptera immune response over the other injected bacteria and induced the strongest antimicrobial activity in larval hemolymph. This comes from the fact that this toxin acts as a danger signal for the insect, leading to the activation of many proteolytic cascades in the insect hemolymph (An et al., 2010). This suggested that the SEA treatment induced a broader spectrum of AMPs and proteins in comparison with the live bacteria.
Hemolymph of S. aureus-injected and SEA-injected larvae recorded drastic changes in both the total protein content and the protein banding patterns after induction of larval immune response. Where, the total hemolymph proteins (THPs) decreased significantly at 48 h after larval microbial immune challenge, this can be attributed to the intensive consumption of plasma proteins during multiplication and growth of bacteria. Also, involvement of some hemolymph sticky proteins and soluble proteins in the attachment of the injected pathogen to the hemocytes or conversion of some native into glycoproteins or lipoproteins following injection. The same explanation was also reported by An et al., 2010, Radwan et al., 2019.
Electrophoretic analysis of protein fractions of S. littoralis larval plasma showed great variations in the number, kinds and percentage, where they totally lie within MW ranged between 14 and 350 kDa. Water, bacterial and toxin injection into the larval hemocoel change the hemolymph proteins profile qualitatively through the induction of new proteins and the disappearance of others simultaneously, which may be attributed to their incorporation in the immune reactions.
In the plasma of SEA-injected larvae protein bands of MWs70and 14 kDa were found to be characteristic bands from the literature review, we deduced that they may be a phenol oxidase and a glycine-rich AMP (Gloverin), respectively. This confirmed by the results obtained in many lepidopterous species, including S. litura (Rajagopal et al., 2005), Ephestia kuehniella (Delkash-Roudsari et al., 2015), S. exigua (Valadez-Lira et al., 2012), Hyphantria cunea (Ajamhassani et al., 2012), Helicoverpa armigera (Goudru et al., 2013), and Plodia interpunctella (Hartzer et al., 2005). All these studies detected PO at MW of ∼70 kDa. Also, many studies on lepidopterous insects detected Gloverin AMP at MW of ∼14 kDa including; B. mori (Mrinal and Nagaraju, 2008), M. sexta (Xu et al., 2012), Plutella xylostella (Etebari et al., 2011), and S. exigua (Hwang and Kim, 2011).
Furthermore, protein bands of MWs22 and 15 kDa were found to be common in the plasma of both treated larvae. From the literature data, we deduced that they may be a glycine-rich AMP (Attacin,) and a bacteriolytic enzyme (Lysozyme), respectively. This confirmed by the results obtained in many lepidopterous species, including, Trichoplusiani (Tamez-Guerra et al., 2008), Manduca sexta (Rao and Yu, 2010), Hyphantria cunea (Kwon et al., 2008), Helicoverpa armigera (Wang et al., 2010) and S. exigua (Bang et al., 2012). which detected attacin at ∼22 kDa. On the other hand, other studies including B. mori (Abraham et al., 1995) and H. virescens (Lockey and Ourth, 1996) found that lysozyme has a MW of ∼15 kDa, these results are in agreement with those of (Yu et al., 2002) who stated that lepidopteran lysozyme increased drastically in the hemolymph after bacterial injection since it is a basic anti-bacterial protein (Lockey and Ourth, 1996).
Changes occurred in electrophoretic pattern of hemolymph proteins in response of S. Littoralis larvae to bacterial and toxin injections encouraged us to analyze these proteins, the variations in amino acids composition and the changes occurred in their concentrations.
Data obtained by amino acid analysis can be used to compare between the amino acid composition of an unknown protein sample with other protein compositions in databases (compositional search). Compared to protein sequencing, amino acid analysis is much cheaper, faster, and allows higher sample throughput. Thus, the method may replace protein sequencing as a first attempt in identification, provided a homolog can be found in the database (Hobohm et al., 1994).
The present study indicated the presence of twenty types of amino acids using HPLC in both bands with MWs ∼70 kDa phenoloxidase-like and ∼15 kDa lysozyme-like. They are listed as follow; Aspartatic acid (D), Serine (S), Threonine (T), Glutamatic acid (E), Glutamine (Q), Glycine (G), Tryptophan (W), Alanine (A), Cysteine (C), Methionine (M), Valine (V), Isoleucine (I), Leucine (L), Phenylalanine (F), Tyrosine (Y), Proline (P), Lysine (K), Histidine (H), Asparagine (N) and Arginine (R) with differences in their quantities and velocities. For PO, this amino acid composition was similar to this obtained in the studies on B. mori (Ashida, 1971) and M. sexta (Aso et al., 1985). Results of lysozyme amino acid analysis are like (Jollès et al., 1979) working on G. mellonella, B. mori and S. littoralis and also agree with the results obtained from Zhang et al. (2013) on Ostrinia sp.
On the other hand, cysteine amino acid disappeared in both bands with MWs ∼22, attacin-like AMP, and ∼14 kDa; gloverin-like AMP. These results in agreement with the study of Kim et al. (2011) on Papilio Xuthus and Cheng et al. (2006) on B. mori with the comparison of amino acids composition of the eluted proteins with proteins having approximately the same molecular weight, a high degree of similarity appeared. This similarity confirms that the eluted proteins of MWs ∼22, ∼15, ∼70 and ∼14 are attacin-like, lysozyme-like, PO-like and gloverin-like, respectively.
5. Conclusion
The alarming phenomenon of infectious diseases resistant to conventional treatments requires crucial global actions. Antimicrobial peptides (AMPs) represent natural potential alternatives in the handling of multi-drug resistant. Here, we focused on describing their characterization and antimicrobial activity against selected Gram-positive and Gram-negative pathogens. We reported several data suggesting that the enhancement of insect immune response induces the production of AMPs with a promising antibacterial effect.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abdel-Moneim A.M.E., El-Saadony M.T., Shehata A.M., Saad A.M., Aldhumri S.A., Ouda S.M., Mesalam N.M. Antioxidant and antimicrobial activities of Spirulina platensis extracts and biogenic selenium nanoparticles against selected pathogenic bacteria and fungi. Saudi J. Biol. Sci. 2021 doi: 10.1016/j.sjbs.2021.09.046. assessed online 17 September 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham E.G., Nagaraju J., Salunke D., Gupta H.M., Datta R.K. Purification and partial characterization of an induced antibacterial protein in the silkworm, Bombyx mori. J. Invertebr. Pathol. 1995;65(1):17–24. doi: 10.1006/jipa.1995.1003. [DOI] [PubMed] [Google Scholar]
- Abulreesh, H.H., Organji, S.R., Osman, G.E.H., El banna, K., Almalki, M.H.K. Ahmed, I., 2017. Prevalence of antibiotic resistant and virulence factors encoding genes in clinical Staphylococcus aureus in Saudi Arabia. Clin. Epidem. Glob. H. 5, 196–202.
- Ajamhassani M., Sendi J.J., Farsi M.J., Zibaee A. Purification and characterization of phenoloxidase from the hemolymph of Hyphantriacunea (Lepidoptera: Arctiidae) Invertebr. Surviv. J. 2012;9(1):64–71. [Google Scholar]
- An C., Jiang H., Kanost M.R. Proteolytic activation and function of the cytokine Spätzle in the innate immune response of a lepidopteran insect, Manduca sexta. FEBS J. 2010;277(1):148–162. doi: 10.1111/j.1742-4658.2009.07465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asao T., Kumeda Y., Kawai T., Shibata T., Oda H., Haruki K., Nakazawa H., Kozaki S. An extensive outbreak of staphylococcal food poisoning due to low-fat milk in Japan: estimation of enterotoxin A in the incriminated milk and powdered skim milk. Epidemiol. Infect. 2003;130(1):33–40. doi: 10.1017/s0950268802007951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashida M. Purification and characterization of pre-phenoloxidase from hemolymph of the silkworm Bombyx mori. Arch. Biochem. Biophys. 1971;144(2):749–762. doi: 10.1016/0003-9861(71)90383-3. [DOI] [PubMed] [Google Scholar]
- Aso Y., Kramer K.J., Hopkins T.L., Lookhart G.L. Characterization of hemolymph protyrosinase and a cuticular activator from Manduca sexta L. Insect Biochem. 1985;15(1):9–17. [Google Scholar]
- Bang K., Park S., Yoo J.Y., Cho S. Characterization and expression of attacin, an antibacterial protein-encoding gene, from the beet armyworm, Spodoptera exigua (Hübner) (Insecta: Lepidoptera: Noctuidae) Mol. Biol. Rep. 2012;39(5):5151–5159. doi: 10.1007/s11033-011-1311-3. [DOI] [PubMed] [Google Scholar]
- Bhadra P., Yan J., Li J., Fong S., Siu S.W. AmPEP: Sequence-based prediction of antimicrobial peptides using distribution patterns of amino acid properties and random forest. Sci. Rep. 2018;8(1):1697. doi: 10.1038/s41598-018-19752-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi D.M., Gallina S., Bellio A., Chiesa F., Civera T., Decastelli L. Enterotoxin gene profiles of Staphylococcus aureus isolated from milk and dairy products in Italy. Lett. Appl. Microbiol. 2014;58(2):190–196. doi: 10.1111/lam.12182. [DOI] [PubMed] [Google Scholar]
- Boman H.G. Innate immunity and the normal microflora. Immunol. Rev. 2000;173(1):5–16. doi: 10.1034/j.1600-065x.2000.917301.x. [DOI] [PubMed] [Google Scholar]
- Borah A., Deb B., Chakraborty S. A Crosstalk on Antimicrobial Peptides. Int. J. Pept. Res. Ther. 2021;27(1):229–244. [Google Scholar]
- Bradford M.M. A rapid and sensitive for the quantitation of microgram quantitites of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brown K.L., Hancock R.E. Cationic host defense (antimicrobial) peptides. Curr. Opin. Immunol. 2006;18(1):24–30. doi: 10.1016/j.coi.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Chen L., Li S., Wang Z., Chang R., Su J., Han B. Protective effect of recombinant staphylococcal enterotoxin A entrapped in polylactic-co-glycolic acid microspheres against S. aureus infection. Vet. Res. 2012;43(1):20. doi: 10.1186/1297-9716-43-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D., Song Q., Xu Z., Zhang D. Characterization of enterotoxin A-producing Staphylococcus aureus. Infect. Drug Resist. 2018;11:531–538. doi: 10.2147/IDR.S164278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng T., Zhao P., Liu C., Xu P., Gao Z., Xia Q., Xiang Z. Structures, regulatory regions, and inductive expression patterns of antimicrobial peptide genes in the silkworm Bombyx mori. Genomics. 2006;87(3):356–365. doi: 10.1016/j.ygeno.2005.11.018. [DOI] [PubMed] [Google Scholar]
- Clarisse T., Michèle S., Olivier T., Valérie E., Vincent L.M., Jacques-Antoine H., Michel G., Florence V. Detection and quantification of staphylococcal enterotoxin A in foods with specific and sensitive polyclonal antibodies. Food Control. 2013;32(1):255–261. [Google Scholar]
- Cooper, C., Packer, N., Williams, K. (Eds.). 2001. Amino acid analysis protocols, vol. 159. Springer Science & Business Media.
- de Viedma M.G., Nelson M.L. Notes on insect injection, anesthetization, and bleeding. Great Lakes Entomol. 2017;10(4):12. [Google Scholar]
- Delkash-Roudsari S., Zibaee A., Bigham Z. Purification and characterization of a phenoloxidase in the hemocytes of Ephestia kuehniella Zeller (Lepidoptera: Pyralidae): effects of insect growth regulators and endogenous inhibitors. J. Enzyme Inhib. Med. Chem. 2015;30(4):569–574. doi: 10.3109/14756366.2014.954107. [DOI] [PubMed] [Google Scholar]
- Diab M.S., Ibrahim N.A., Elnaker Y.F., Zidan S.A., Saad M.A. Molecular detection of Staphylococcus aureus enterotoxin genes isolated from mastitic milk and humans in El-Behira. Egypt. Int. J. One Health. 2021;7:70–77. [Google Scholar]
- El-Saadony M.T., Elsadek M.F., Mohamed A.S., Taha A.E., Ahmed B.M., Saad A.M. Effects of chemical and natural additives on cucumber juice’s quality, shelf life, and safety. Foods. 2020;9(5):639. doi: 10.3390/foods9050639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony M.T., Saad A.M., Taha T.F., Najjar A.A., Zabermawi N.M., Nader M.M., Salama A. Selenium nanoparticles from Lactobacillus paracasei HM1 capable of antagonizing animal pathogenic fungi as a new source from human breast milk. Saudi J. Biol. Sci. 2021;28(12):6782–6794. doi: 10.1016/j.sjbs.2021.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony M.T., Sitohy M.Z., Ramadan M.F., Saad A.M. Green nanotechnology for preserving and enriching yogurt with biologically available iron (II) Innov. Food Sci. Emerg. Technol. 2021;69:102645. doi: 10.1016/j.ifset.2021.102645. [DOI] [Google Scholar]
- Etebari K., Palfreyman R.W., Schlipalius D., Nielsen L.K., Glatz R.V., Asgari S. Deep sequencing-based transcriptome analysis of Plutella xylostella larvae parasitized by Diadegma semiclausum. BMC Genom. 2011;12(1):446. doi: 10.1186/1471-2164-12-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finney, D.J. 1971. Profit Analysis. Cambridge University Press, Cambridge, 333.
- Giuliani A., Pirri G., Nicoletto S. Antimicrobial peptides: an overview of a promising class of therapeutics. Open Life Sci. 2007;2(1):1–33. [Google Scholar]
- Goudru H.G., Kumar S., Jayalakshmi S.K., Ballal C.R., Sharma H.C., Sreeramulu K. Purification and characterization of prophenoloxidase from cotton bollworm, Helicoverpaar migera. Entomol. Res. 2013;43(1):55–62. [Google Scholar]
- Hanahan, D., Meselson, M., 1983. In: Methods in Enzymology, vol. 100. Acad. Press. NY, p. 333. [DOI] [PubMed]
- Hartzer K.L., Zhu K.Y., Baker J.E. Phenoloxidase in larvae of Plodia interpunctella (Lepidoptera: Pyralidae): molecular cloning of the proenzyme cDNA and enzyme activity in larvae paralyzed and parasitized by Habrobracon hebetor (Hymenoptera: Braconidae) Arch. Insect Biochem. Physiol.: Published in Collaboration with the ESA. 2005;59(2):67–79. doi: 10.1002/arch.20056. [DOI] [PubMed] [Google Scholar]
- Hobohm U., Houthaeve T., Sander C. Amino acid analysis and protein database compositional search as a rapid and inexpensive method to identify proteins. Anal. Biochem. 1994;222(1):202–209. doi: 10.1006/abio.1994.1474. [DOI] [PubMed] [Google Scholar]
- Hu H., Wang C., Guo X., Li W., Wang Y., He Q. Broad activity against porcine bacterial pathogens displayed by two insect antimicrobial peptides moricin and cecropin B. Mol. Cells. 2013;35(2):106–114. doi: 10.1007/s10059-013-2132-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang J., Kim Y. RNA interference of an antimicrobial peptide, gloverin, of the beet armyworm, Spodoptera exigua, enhances susceptibility to Bacillus thuringiensis. J. Invertebr. Pathol. 2011;108(3):194–200. doi: 10.1016/j.jip.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Jollès J., Schoentgen F., Croizier G., Croizier L., Jollès P. Insect lysozymes from three species of Lepidoptera: their structural relatedness to the C (chicken) type lysozyme. J. Mol. Evol. 1979;14(4):267–271. doi: 10.1007/BF01732494. [DOI] [PubMed] [Google Scholar]
- Junqueira J.C., Mylonakis E. Current Status and Trends in Alternative Models to Study Fungal Pathogens. J. Fungi. 2019;5(1):12. doi: 10.3390/jof5010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendurkar, S.V., Sengupta, D., 2018. U.S. Patent Application No. 15/503,899.
- Kim S.-R., Hwang J.-S., Park S.-W., Goo T.-W., Kim I.-S., Kang S.-W. Molecular Cloning and Characterization of Attacin from the Swallowtail Butterfly, Papilioxuthus. Int. J. Indust. Entomol. 2011;23(2):231–238. [Google Scholar]
- Kwon Y.M., Kim H.J., Kim Y.I., Kang Y.J., Lee I.H., Jin B.R., Han Y.S., Cheon H.M., Ha N.G., Seo S.J. Comparative analysis of two attacin genes from Hyphantr iacunea. Comparative Biochemistry and Physiology Part B. Biochem. Mol. Biol. Educ. 2008;151(2):213–220. doi: 10.1016/j.cbpb.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Laemmli U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lehrer R.I. Primate defensins. Nat. Rev. Microbiol. 2004;2(9):727–738. doi: 10.1038/nrmicro976. [DOI] [PubMed] [Google Scholar]
- Lockey T.D., Ourth D.D. Purification and characterization of lysozyme from hemolymph of Heliothis virescenslarvae. Biochem. Biophys. Res. Commun. 1996;220(3):502–508. doi: 10.1006/bbrc.1996.0434. [DOI] [PubMed] [Google Scholar]
- Loncarevic S., Jorgensen H.J., Lovseth A., Mathisen T., Rorvik L.M. Diversity of Staphylococcus aureus enterotoxin types within single samples of raw milk and raw milk products. J. Appl. Microbiol. 2005;98(2):344–350. doi: 10.1111/j.1365-2672.2004.02467.x. [DOI] [PubMed] [Google Scholar]
- Makarova O., Rodriguez-Rojas A., Eravci M., Weise C., Dobson A., Johnston P., Rolff J. Antimicrobial defence and persistent infection in insects revisited. Phil. Trans. R. Soc. B. 2016;371(1695):20150296. doi: 10.1098/rstb.2015.0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matuschek E., Brown D.F.J., Kahlmeter G. Development of the EU cast disk diffusion antimicrobial susceptibility testing method and its implementation in routine microbiology laboratories. Clin. Microbiol Infect. 2014;20(4):O255–O266. doi: 10.1111/1469-0691.12373. [DOI] [PubMed] [Google Scholar]
- Meshrif, W. S., 2008. Defense reactions and biochemical changes in the hemolymph of Spodoptera littoralis (Boisduval) (Lepidoptera: Noctuidae) following infection with entomopathogenic hyphomycete fungi Ph. D Thesis, Sci. Fac., Tanta Univ.
- Meylaers K., Freitak D., Schoofs L. Immuno-competence of Galleria mellonella: sex-and stage-specific differences and the physiological cost of mounting an immune response during metamorphosis. J. Insect Physiol. 2007;53(2):146–156. doi: 10.1016/j.jinsphys.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Mrinal N., Nagaraju J. Intron loss is associated with gain of function in the evolution of the gloverin family of antibacterial genes in Bombyx mori. J Biol. Chem. 2008;283(34):23376–23387. doi: 10.1074/jbc.M801080200. [DOI] [PubMed] [Google Scholar]
- Parthuisot N., Rouquette J., Ferdy J.-B. A high-throughput technique to quantify bacterial pathogens' virulence on the insect model Galleria mellonella. J. Microbiol. Methods. 2018;152:69–72. doi: 10.1016/j.mimet.2018.07.013. [DOI] [PubMed] [Google Scholar]
- Pereira M.F., Rossi C.C., Vieira de Queiroz M., Martins G.F., Isaac C., Bossé J.T., Li Y., Wren B.W., Terra V.S., Cuccui J., Langford P.R., Bazzolli D.M.S. Galleria mellonella is an effective model to study Actino bacillus pleuro pneumoniae infection. Microbiology. 2015;161(2):387–400. doi: 10.1099/mic.0.083923-0. [DOI] [PubMed] [Google Scholar]
- Radwan M., Momen S., Abdou M., El-Gohary E.-G., ElShafe A., Barakat E. Induction of antimicrobial peptides in the hemolymph of Spodoptera littoralis larvae following treatment with Salmonella typhimurium. Egypt. Acad. J. Biol. Sci. 2019;12(4):9–21. [Google Scholar]
- Rajagopal R., Thamilarasi K., Venkatesh G.R., Srinivas P., Bhatnagar R.K. Immune cascade of Spodoptera litura: cloning, expression, and characterization of inducible prophenoloxidase. Biochem. Biophys. Res. Commun. 2005;337(1):394–400. doi: 10.1016/j.bbrc.2005.09.057. [DOI] [PubMed] [Google Scholar]
- Rao X.-J., Yu X.-Q. Lipoteichoic acid and lipopolysaccharide can activate antimicrobial peptide expression in the tobacco hornworm Manduca sexta. Dev. Comp. Immunol. 2010;34(10):1119–1128. doi: 10.1016/j.dci.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regenthal P., Hansen J.S., André I., Lindkvist-Petersson K., Permyakov E.A. Thermal stability and structural changes in bacterial toxins responsible for food poisoning. PloS one. 2017;12(2):e0172445. doi: 10.1371/journal.pone.0172445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivnay E., Meisner J. The effects of rearing conditions on the immature stages and adults of Spodoptera littoralis (Boisd.) Bull. Entomol. Res. 1966;56(4):623–634. [Google Scholar]
- Saad A.M., Elmassry R.A., Wahdan K.M., Ramadan F.M. Chickpea (Cicer arietinum) steep liquor as a leavening agent: effect on dough rheology and sensory properties of bread. . Acta Period. Technol. 2015;46:91–102. [Google Scholar]
- Saad A.M., El-Saadony M.T., El-Tahan A.M., Sayed S., Moustafa M.A., Taha A.E., Ramadan M.M. Polyphenolic extracts from pomegranate and watermelon wastes as substrate to fabricate sustainable silver nanoparticles with larvicidal effect against Spodoptera littoralis. Saudi J. Biol. Sci. 2021;28(10):5674–5683. doi: 10.1016/j.sjbs.2021.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad A.M., El‐Saadony M.T., Mohamed A.S., Ahmed A.I., Sitohy M.Z. Impact of cucumber pomace fortification on the nutritional, sensorial and technological quality of soft wheat flour-based noodles. Int. J. Food Sci. Technol. 2021;56(7):3255–3268. [Google Scholar]
- Saad A.M., Mohamed A.S., El-Saadony M.T., Sitohy M.Z. Palatable functional cucumber juices supplemented with polyphenols-rich herbal extracts. LWT - Food Sci. Technol. 2021;148:111668. doi: 10.1016/j.lwt.2021.111668. [DOI] [Google Scholar]
- Saad A.M., Sitohy M.Z., Ahmed A.I., Rabie N.A., Amin S.A., Aboelenin S.M., El-Saadony M.T. Biochemical and functional characterization of kidney bean protein alcalase-hydrolysates and their preservative action on stored chicken meat. Molecules. 2021;26(15):4690. doi: 10.3390/molecules26154690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saluzzo S., Layer F., Stingl G., Stary G. Staphylococcal scalded skin syndrome caused by a rare variant of exfoliative-toxin-A+ S. aureus in an adult immunocompromised woman. Acta Derm. Venereol. 2018;98(1):138–139. doi: 10.2340/00015555-2778. [DOI] [PubMed] [Google Scholar]
- Sambrook, J., Russel, D.W.,2001. Molecular Cloning: A Laboratory Manual, third ed. 1, 5.26–5.28.
- Schelin J., Wallin-Carlquist N., Cohn M.T., Lindqvist R., Barker G.C., Radstrom P. The formation of Staphylococcus aureus enterotoxin in food environments and advances in risk assessment. Virulence. 2011;2(6):580–592. doi: 10.4161/viru.2.6.18122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton S. Accuracy of plate counts. J. Valid. Technol. 2011;17(3):42–46. [Google Scholar]
- Swelum A.A., Shafi M.E., Albaqami N.M., El-Saadony M.T., Elsify A., Abdo M., Mohamed E. COVID-19 in human, animal, and environment: a review. Front. Vet. Sci. 2020;7:578. doi: 10.3389/fvets.2020.00578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamez-Guerra P., Valadez-Lira J.A., Alcocer-González J.M., Oppert B., Gomez-Flores R., Tamez-Guerra R., Rodríguez-Padilla C. Detection of genes encoding antimicrobial peptides in Mexican strains of Trichoplusiani (Hübner) exposed to Bacillus thuringiensis. J. Invertebr. Pathol. 2008;98(2):218–227. doi: 10.1016/j.jip.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Tonacini J., Stephan D., Vogel G., Avondet M., Kalman F., Crovadore J., Lefort F., Schnyder B. Intact Staphylococcus Enterotoxin SEB from culture supernatant detected by MALDI-TOF Mass Spectrometry. Toxins. 2019;11:101. doi: 10.3390/toxins11020101. https://doi:10.3390/toxins11020101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker A.T., Leonard S.P., DuBois C.D., Knauf G.A., Cunningham A.L., Wilke C.O., Trent M.S., Davies B.W. Discovery of next-generation antimicrobials through bacterial self-screening of surface-displayed peptide libraries. Cell. 2018;172(3):618–628.e13. doi: 10.1016/j.cell.2017.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valadez-Lira J.A., Alcocer-Gonzalez J.M., Damas G., Nuñez-Mejía G., Oppert B., Rodriguez-Padilla C., Tamez-Guerra P. Comparative evaluation of phenoloxidase activity in different larval stages of four lepidopteran pests after exposure to Bacillus thuringiensis. J. Insect Sci. 2012;12(80):1–11. doi: 10.1673/031.012.8001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Liu Y., He H.-J., Zhao X.-F., Wang J.-X. Immune responses of Helicoverpaar migera to different kinds of pathogens. BMC Immunol. 2010;11(1):9. doi: 10.1186/1471-2172-11-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, G. (Ed.). 2017. Antimicrobial peptides: discovery, design and novel therapeutic strategies. Cabi.
- Wiesner J., Vilcinskas A. Antimicrobial peptides: the ancient arm of the human immune system. Virulence. 2010;1(5):440–464. doi: 10.4161/viru.1.5.12983. [DOI] [PubMed] [Google Scholar]
- Xu X.-X., Zhong X., Yi H.-Y., Yu X.-Q. Manduca sexta gloverin binds microbial components and is active against bacteria and fungi. Dev. Comp. Immunol. 2012;38(2):275–284. doi: 10.1016/j.dci.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi H.-Y., Chowdhury M., Huang Y.-D., Yu X.-Q. Insect antimicrobial peptides and their applications. Appl. Microbiol. Biotechnol. 2014;98(13):5807–5822. doi: 10.1007/s00253-014-5792-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon B., Jackman J., Valle-González E., Cho N.J. Antibacterial free fatty acids and monoglycerides: biological activities, experimental testing, and therapeutic applications. Int. J. Mol. Sci. 2018;19(4):1114. doi: 10.3390/ijms19041114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu K.H., Kim K.N., Lee J.H., Lee H.S., Kim S.H., Cho K.Y., Nam M.H., Lee I.H. Comparative study on characteristics of lysozymes from the hemolymph of three lepidopteran larvae, Galleria mellonella, Bombyx mori, Agrius convolvuli. Develop. Comp. Immunol. 2002;26(8):707–713. doi: 10.1016/s0145-305x(02)00027-7. [DOI] [PubMed] [Google Scholar]
- Zhang M., Zhou F., Chu Y., Zhao Z., An C. Identification and expression profile analysis of antimicrobial peptide/protein in Asian corn borer, Ostrinia furnacalis (Guenée) Int. J. Biol. Sci. 2013;9(9):1004–1012. doi: 10.7150/ijbs.6813. [DOI] [PMC free article] [PubMed] [Google Scholar]
Further Reading
- Aronson A.I. The two faces of Bacillus thuringiensis: insecticidal proteins and post-exponential survival. Mol. Microbiol. 1993;7(4):489–496. doi: 10.1111/j.1365-2958.1993.tb01139.x. [DOI] [PubMed] [Google Scholar]
- Hammer T.J., Janzen D.H., Hallwachs W., Jaffe S.P., Fierer N. Caterpillars lack a resident gut microbiome. Proc. Natl. Acad. Sci. USA. 2017;114(36):9641–9646. doi: 10.1073/pnas.1707186114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei J., Sun L.C., Huang S., Zhu C., Li P., He J., Mackey V., Coy D.H., He Q.Y. The antimicrobial peptides and their potential clinical applications. Am. J. Transl. Res. 2019;11:3919–3931. [PMC free article] [PubMed] [Google Scholar]
- Yahya Ahmed M., Abdalbagi Ali H., Mohammed Taher Gorish B., Omer Ali S., Saif Aldein Abdalrhim E., Hamza Mergani M., Abass Abd Elgadir A., Khalid Mohammed S., Omer Ahmed S., Alsaeid Musa N., Saeed Ahmed A., Mohammed Abdalla W., Fadlallah Hamedelnil Y., Ibrahim Hashim A., Altayb H.N., Comi G. Molecular detection of Staphylococcal enterotoxins and mecA genes products in selected food samples collected from different areas in khartoum State. Int. J. Microbiol. 2021;2021:1–9. doi: 10.1155/2021/5520573. [DOI] [PMC free article] [PubMed] [Google Scholar]