Abstract
Sordarin derivatives constitute a new group of synthetic antifungal agents that selectively inhibit fungal protein synthesis. They have demonstrated in vitro activity against the most important fungal pathogens, both yeast and filamentous. This new family of compounds has also shown in vivo activity against murine Candida albicans, Histoplasma capsulatum, and Coccidioides immitis experimental infections, as well as against Pneumocystis carinii pneumonia in rats. After intravenous dosing in animals, both the area under the concentration-time curve and the elimination half-life were highest in Cynomolgus monkeys, followed by those in rats, mice, and rabbits. The volume of distribution at steady state for sordarin derivatives was similar in all species tested. The clearance in rats and mice was higher than for other species. GM 237354, a sordarin derivative, was characterized by high serum protein binding in mouse, rat, and monkey serum (unbound fraction, ≤5%). An indirect evaluation of the effect of liver function upon the metabolism of this class of compounds has been made in animals with impaired liver function such as Gunn rats, as well as in allometric studies that showed better correlations of half-life to liver blood flow than to animal body weight. Linearity of the main pharmacokinetic parameters was demonstrated after intravenous dosing of the representative compound GM 193663 at 10 and 20 mg/kg of body weight in rats. Allometry was used to determine whether human pharmacokinetic parameters can be predicted from animal data by regression analysis against body weight and liver blood flow. All these results have demonstrated that the human pharmacokinetics of sordarin derivatives can be forecast from animal data.
Opportunistic fungal pathogens remain an important cause of morbidity and mortality in immunocompromised individuals, such as patients with AIDS and those receiving chemotherapy or immunosuppressive therapy for oncological malignancies or other pathological conditions (11, 16, 24).
Sordarin derivatives are a new group of synthetic antifungal agents that selectively inhibit fungal protein synthesis by targeting the yeast protein elongation cycle (8, 9). Sordarins have demonstrated in vitro activity against a wide range of pathogenic fungi (14). In addition, sordarin derivatives have shown in vivo activity against systemic and local experimental infections involving Candida albicans and Pneumocystis carinii (1, 2, 19), as well as murine histoplasmosis (13) and coccidioidomycosis (5).
It is well known that anti-infective treatment outcome is a function of several variables, including intrinsic microbiological activity and pharmacokinetic (PK) behavior (10). In this way, PK properties have been shown to be very useful for understanding the in vivo activities of structurally related compounds with similar in vitro potencies (12, 17, 22, 23). Recently, close relationships between PK properties and the efficacy of sordarin derivatives in a murine model of systemic candidiasis have been described (1). In addition, PK parameters derived in animals can be scaled using allometric relationships, whereby the human PK can be forecast or predicted (4). Information obtained by integrating human forecast PK and PK predictors of in vivo efficacy in animal models can be invaluable when clinical trials are being designed. However, when sordarin derivatives are considered, PK results for this class of compounds in humans are not available, and prospective scaling is thus of great importance.
The aim of the present study was to define the PK behavior of a new class of systemic antifungal agents in animals and to determine whether the PK of sordarin derivatives in humans can be predicted. To this effect, PK studies have been performed in different species of animals. Serum protein binding, PK linearity versus increasing doses, in vivo metabolic inhibition, and allometric interspecies scaling have also been investigated. In summary, the PK properties of a new class of systemic antifungal agents have been described.
(Part of this work was presented at the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy, Toronto, Ontario, Canada, 1997 [P. Aviles, A. Pateman, R. San Roman, D. Gargallo, Abstr. 37th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-66, 1997], and at the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Diego, Calif., 24 to 27 September 1998 [P. Aviles, A. Pateman, R. San Roman, F. Gomez de las Heras, D. Gargallo-Viola, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. J-071, 1998].)
MATERIALS AND METHODS
Drugs and reagents.
GM 222712, GM 193663, and GM 237354 were synthesized at GlaxoWellcome S.A. (Tres Cantos, Madrid, Spain). All other reagents were of analytical grade unless specified otherwise.
Animals.
Five-week-old male CD1 mice weighing 27 to 30 g (Charles River, France Inc., Lyon, France), 6-week-old male Sprague Dawley (Charles River) rats weighing 190 to 220 g, male Gunn rats (Harlan, Indianapolis, Ind.) weighing 230 g, 2- to 3-month-old male New Zealand White rabbits weighing 2 to 2.5 kg, and male Cynomolgus monkeys (HRP Inc., Denver, Pa.) weighing 3.5 to 4 kg were used. The research complied with European legislation and with company policy on the care and use of animals and with related guidelines.
Compound administration.
Immediately before use, each compound was dissolved in sterile deionized water at the desired concentration. Intravenous (i.v.) dosing was by venipuncture of the tail vein in mice. Rats were cannulated in the jugular vein 24 h before PK studies, as previously described (25). A 24G angiocatheter inserted into a marginal ear vein was used for i.v. dosing in rabbits. Administration in monkeys was performed via the cephalic vein.
Dosing and sampling.
Each compound was administered once at a dose of 20 mg/kg of body weight (BW) to mice, rats, rabbits, and monkeys for PK studies. In the case of mice, blood samples were taken by cardiac puncture at 0, 0.25, 0.5, 0.75, 1.5, 2, 2.5, and 3 h postadministration. Three mice were sacrificed by cervical dislocation at each sampling point. Rats (n = 3) were sampled from the end of the tail (15) up to 4 h after drug administration. Rabbits were sampled using an angiocatheter placed in the central artery of the ear contralateral to the dosing ear up to 4 h postdosing. Monkey blood samples were in turn obtained from the hindquarters of the animals by direct venipuncture at 0.08, 0.16, 0.25, 0.5, 1, 2, 4, 6, 8, and 24 h postdosing. The blood samples were allowed to clot for at least 2 h and were then centrifuged to separate the serum. Once obtained, sera were kept at −80°C until analysis.
Sample analysis.
Serum samples were analyzed for compound concentrations by a reversed-phase high-performance liquid chromatography (HPLC) procedure using a method previously described (1). HPLC was performed with a HP1090 chromatograph (Hewlett Packard, Palo Alto, Calif.). The mobile phase was a mixture of acetonitrile (HPLC grade; Panreac Quimica, Barcelona, Spain) and phosphate-octane sulfonic acid solution (Reactivos Scharlau S.L., Barcelona, Spain) buffered at pH 5. The column was a Novapack (Waters Corporation, Milford, Mass.) RP-C18 column (4.5 mm by 25 cm) with a guard column. Chromatography was isocratic at several percentages of acetonitrile. GM 222712, GM 193663, and GM 237354 displayed retention times equal to 10, 8, and 6 min when 55, 65, and 65% acetonitrile was added to mobile phase, respectively. The mobile phases were prepared daily, and the buffer and acetonitrile mixtures were filtered through a glass microfiber filter. The flow rate was 1 ml/min, and UV detection of sordarin-derivative compounds was performed at 215 nm.
Test samples (50 μl) were mixed with 50 μl of acetonitrile, vortexed for 30 s, and centrifuged for 15 min at 9,600 × g. Then supernatants were removed, and 20 μl of deproteinized sample was used for HPLC analysis.
Calibration standards were prepared by adding known amounts of each compound to corresponding blank sera and assayed as described above. Intra-assay precision was determined to be 8.5 and 7.3% for 5 and 25 μg/ml, respectively. The accuracy was 3.6 and 4.3% at 5 and 25 μg/ml, respectively. The peak areas were used for analysis (linearity from 2 to 100 μg/ml).
Serum protein binding.
[3H]GM 237354 was used as a reference compound for serum protein binding studies. Radiochemical purity of GM 237354 was assessed by HPLC-UV analysis. Drug at 1, 2, 10, and 50 μg/ml in 1.0 ml of blank serum was incubated at 37°C for 2 h before the start of the experiment. Protein binding of GM 237354 in serum from mouse, rat, rabbit, and monkey was determined by equilibrium dialysis as described elsewhere (1). The dialysis chambers (Cellusep; Spectrum) have a volume of 250 μl and are separated by a membrane measuring 1 cm2. Serum samples were dialyzed against the same volume of red blood cell buffer (20) placed in the second compartment. The chamber was then placed in a rotator (Dianorm, Munich, Germany), and dialysis was carried out at 16 rpm at 37°C for 24 h. Following incubation, aliquots of both compartments were counted, and the free fraction was calculated.
Evaluation of in vivo glucuronosyltransferase activity.
GM 193663 was chosen as a reference compound to evaluate the impact of in vivo UDP-glucuronosyltransferase activity upon the PK behavior of sordarin derivatives. PK parameters of GM 193663 were evaluated after i.v. administration of single 20-mg/kg doses to congenic male RHA rats with normal (homozygous, RHA +/+), moderately deficient (heterozygous, RHA +/j), and severely deficient (heterozygous, RHA j/j) activities of bilirubin UDP-glucuronosyltransferase (7, 18). After compound analysis, the main PK parameters were calculated and compared between groups.
Linearity of pharmacokinetics.
Double doses of GM 193663 were used to assess the influence of increased dose on PK parameters. Doses of 10 and 20 mg/kg were administered i.v. in male rats, and dosing, sampling, and analysis of the results were performed as described above.
Pharmacokinetic and statistical analysis.
Results are expressed as the means ± standard errors of the means. Data for concentration in serum versus time were modeled to one-compartmental model through 1/C2 (where C is concentration) weighted analysis by using WinNonlin software (Scientific Consulting, Inc., Apex, N.C.). Evaluation of the in vivo glucuronosyltransferase activity was statistically assessed by comparing the serum time curves with the F test. P values of ≤0.05 were considered statistically significant. Analysis of variance and the correlation coefficient evaluated the goodness of the allometric relationships.
Allometric parameters.
The reference compound for allometric scaling studies was GM 237354. Allometric equations describing the mathematical relationships between physiological properties (BW and liver blood flow [LBF]) and several PK parameters were calculated by regression analysis. The interspecies allometric relationships were studied according to a previously described report (4). PK parameter (PK) was described as a function of physiological property as follows: log PK = a · log B + b, where B is the physiological property, a is the allometric coefficient, and b is the allometric exponent. Physiological parameters used for regression studies were taken from the literature (6). PK parameters were fitted versus BW and LBF, taken as representative physiological parameters. The equations were then used to forecast the PK of GM 237354 in humans.
RESULTS
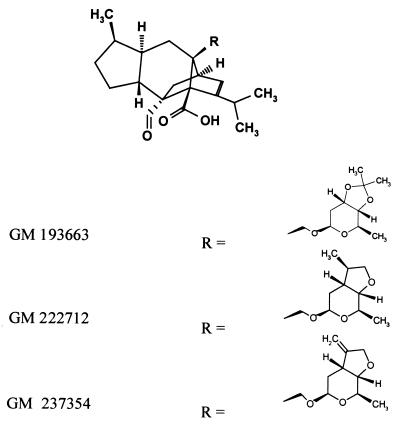
Figure 1 shows the chemical structures of the three sordarin derivatives (GM 193663, GM 222712, and GM 237354) selected as reference compounds to study the PK behavior of a new class of antifungal agents in several animal species and to evaluate whether it is possible to predict the PK of sordarins in humans.
FIG. 1.
Structures of sordarin-derivative compounds.
GM 193663, GM 222712, and GM 237354 are structurally related compounds with different types of fused rings at positions C-3′ and C-4′ of the sugar moiety in the sordarin structure. GM 193663 is chemically characterized by the presence of a 3′,4′-fused dioxolane ring, whereas GM 222712, and GM 237354 contain a 3′,4′-fused tetrahydrofurane ring with a methyl and an exomethylene group, respectively, at position 19 of the sordarin molecule.
Serum PK.
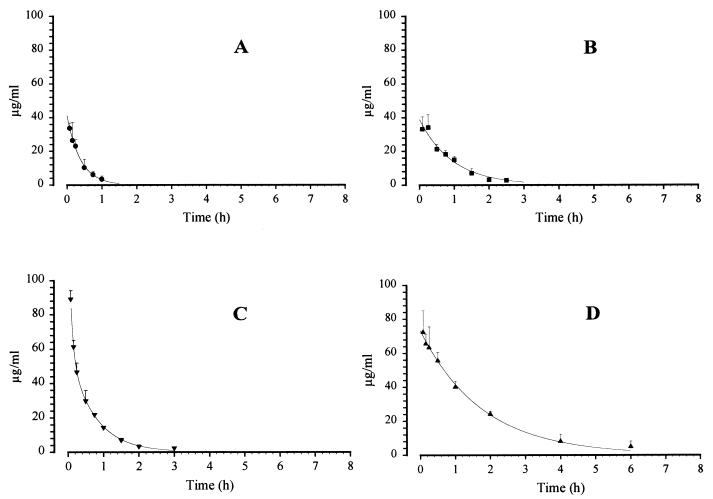
Figure 2 displays the GM 237354 serum concentration-time curve observed in the sera of mice, rats, rabbits, and monkeys given a single intravenous dose of 20 mg/kg. Values for the area under the serum concentration-time curve (AUC), the elimination half-life (t1/2), the volume of distribution at steady state (VSS), and the total clearance (CLp) for each compound are shown in Table 1. For each compound, both the AUC and t1/2 were greatest in monkeys, followed by those in rats, mice, and rabbits. GM 193663 exhibited an AUC in monkeys of 180 μg.h/ml, this figure being 7.5- and 5.5-fold higher than those in mice and rats, respectively. In this way, GM 237354 had an AUC in Cynomolgus monkeys 3.8-, 4.2-, and 9-fold higher than in rabbits, rats, and mice, respectively. The VSS for each compound was similar in all species tested and ranged from 0.2 to 0.6 liter/kg in all species. Small intraspecific differences were also detected, which can be explained by small differences in serum protein binding properties. Slight differences detected in analyses of VSS in the same animal species can be justified by lipophilic dissimilarities of the sordarin derivatives. The CLp for rats and mice was higher than for other species. These findings are in keeping with an allometric interspecies relationship.
FIG. 2.
Concentrations of GM 237354 after i.v. administration of 20 mg/kg in mice (A), rats (B), rabbits (C), and monkeys (D).
TABLE 1.
PK parameters of fungal protein synthesis compounds in experimental animalsa
| Compoundb | Animal (n) | Cmax (μg/ml) | AUC (μg · h/ml) | t1/2 (h) | VSS (liters/kg) | CLp (ml/min/kg) |
|---|---|---|---|---|---|---|
| GM 193663 | Mouse (30) | 38.1 | 24.3 | 0.45 | 0.53 | 20 |
| Rat (3)c | 16.8 | 13.3 | 0.55 | 0.60 | 13 | |
| Rat (3) | 45.4 | 33.7 | 0.51 | 0.44 | 10 | |
| Monkey (3) | 69.3 | 180 | 1.75 | 0.28 | 1.9 | |
| GM 222712 | Mouse (30) | 22.3 | 9.00 | 0.20 | 0.60 | 35 |
| Monkey (3) | 102.9 | 348 | 3.03 | 0.25 | 0.96 | |
| GM 237354 | Mouse (30) | 33.6 | 17.8 | 0.28 | 0.39 | 19 |
| Rat (3) | 33.1 | 38.1 | 0.59 | 0.44 | 8.7 | |
| Rabbit (3) | 89.1 | 42.4 | 0.30 | 0.23 | 8.6 | |
| Monkey (2) | 72.4 | 161 | 1.73 | 0.31 | 2.1 |
PK parameters were calculated from the mean levels in plasma by a one-compartment model.
Compounds were administered i.v. at a dose of 20 mg/kg.
Compound was administered i.v. at a dose of 10 mg/kg.
Large differences between compounds were not detected when sordarin derivatives were analyzed in the same animal species.
Serum protein binding.
The percentage binding of GM 237354 was estimated as 95, 97.7, 97.6, and 98.5% in mouse, rat, rabbit, and Cynomolgus sera, respectively. These values were constant (variation coefficient under 1.1%) over the concentration range studied (1 to 50 μg/ml). The binding of GM 237354 to animal serum is a reversible process at equilibrium.
In vivo glucuronosyltransferase activity evaluation.
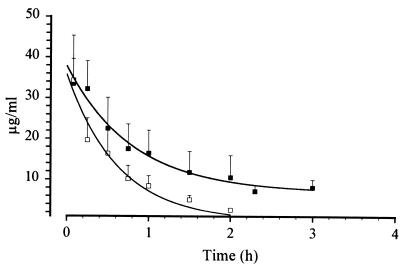
Dosing and sampling were performed as described above. PK parameters were estimated using conventional noncompartmental PK analysis (see Materials and Methods). There were no statistically significant differences between PK profiles of GM 193663 for homozygous (RHA +/+) and heterozygous (RHA +/j) Gunn rats. The AUC and t1/2 of GM 193663 in homozygous (RHA +/+) Gunn rats were 23.9 μg.h/ml and 0.6 h, respectively, and 25.7 μg.h/ml and 0.7 h in heterozygous (RHA +/j) Gunn rats. VSS and CLp values were also very similar in both rat strains. However, there were statistically significant differences between the fitted serum-time curves obtained in normal and homozygous Gunn rats (P < 0.05, F test). GM 193663 had a greater t1/2 and AUC (1.3 h and 56 μg.h/ml, respectively) in homozygous Gunn rats (j/j) than in control rats (+/+ and j/+). Despite Gunn rat (j/j) hyperalbuminemia (18) and the fact that GM 193663 can bind to serum proteins to a degree similar to that described for GM 237354 (97.7%), an increasing VSS was not evident. On the other hand, the lower CLp is compatible with the impaired hepatic metabolic activity found in the homozygous animals. Figure 3 shows levels in serum obtained after compound administration in the two classes of rats. Table 2 summarizes the main PK parameters obtained from RHA +/+, RHA j/+, and RHA j/j rats.
FIG. 3.
Concentrations of GM 193663 after i.v. administration of 20 mg/kg in male Gunn rats (+/+ [▪] and j/j [▫]).
TABLE 2.
PK parameters of GM 193663 in male Gunn ratsa
| Animal (n) | AUC (μg · h/ml) | t1/2 (h) | VSS (liters/kg) | CLp (ml/min/kg) |
|---|---|---|---|---|
| Gunn j/j (3) | 56.0 | 1.3 | 0.68 | 5.9 |
| Gunn +/+ (3) | 23.9 | 0.6 | 0.68 | 14 |
| Gunn j/+ (2) | 25.7 | 0.7 | 0.80 | 12 |
Compound was administered i.v. at a dose of 20 mg/kg. PK parameters were calculated from the mean levels in plasma by a one-compartment model.
Linearity of pharmacokinetics.
Table 1 summarizes the serum PK parameters of GM 193663 obtained after i.v. administration of 10 and 20 mg/kg in male rats. AUCs obtained with 10 and 20 mg/kg were 13.3 and 33.6 μg.h/ml, respectively. Proportionality between doses and maximum concentration of drug in serum (Cmax) was also reasonable (16.8 μg/ml after administration of 10 mg/kg, versus 45.4 after 20 mg/kg). t1/2 and VSS remained constant (0.5 h and 0.44 to 0.6 liter/kg, respectively). These results confirm that reasonably linear PK characteristics are present in this range of doses.
Allometric parameters.
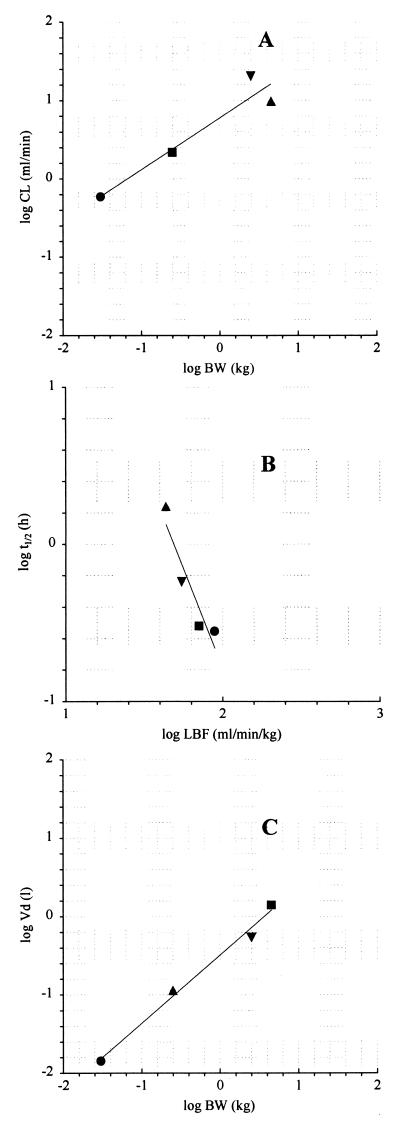
Allometric studies were performed using PK parameters of GM 237354 obtained in four animal species: mouse, rat, rabbit, and Cynomolgus monkey. PK parameters were fitted versus BW and LBF, as displayed in Fig. 4. CLp and VSS scaled with BW (Fig. 4A and C, respectively), whereas t1/2 showed better agreement with LBF (Fig. 4B) than with BW. Table 3 shows allometric equations, correlation coefficients obtained for each relationship, and PK parameters that were forecast.
FIG. 4.
Log-log plot of CLp (A), p1/2 (B), and VSS (C) against physiological properties. Lines represent linear regression analysis of the data. Corresponding equations are presented in Table 3. ●, mouse; ■, rat; ▾, rabbit; ▴, monkey.
TABLE 3.
Allometric relationships for the PK parameters of GM 237354 across four species (mouse, rat, rabbit, and monkey)
| Parameter | Forecast value (95% CI) | Allometric equationa | r2 | P value |
|---|---|---|---|---|
| CLp (ml/min) | 1.5 (0.8–2.2) | log(CLp) = 0.78 + [0.66 · log(BW)] | 0.949 | 0.050 |
| t1/2 (h) | 8.9 (7.2–10.6) | log(t1/2) = 4.29 + [−2.54 · log(LBF)] | 0.940 | 0.059 |
| t1/2 (h) | log(t1/2) = −0.21 + [0.21.log(BW)] | 0.585 | 0.413 | |
| VSS (liters) | 0.2 (0.09–0.31) | log(VSS) = −0.49 + [0.87 · log(BW)] | 0.996 | 0.004 |
LBF values (in milliliters per minute per kilogram) used (6): mouse, 90; rat, 55; rabbit, 71; cynomolgus monkey, 44; human, 21.
DISCUSSION
In keeping with allometric expectations, the t1/2 of GM 237354 was shortest (0.28 h) in mice and longest (1.73 h) in monkeys. In general, animals with low body weights eliminated sordarin-derivatives more rapidly than animals with higher body weights (3). This was true except for rabbits, which had an elimination t1/2 for GM 237354 shorter than that observed in rats. As discussed below, this relationship could be associated to the LBF in each species.
The VSS of the sordarin derivatives were similar. The small differences detected between compounds may be explained by dissimilarities in the lipophilic properties of each, as well as by differences in protein binding. The VSS were similar in all species studied and were in the range of 0.2 to 0.6 liter/kg. This might suggest that the compounds distribute out of extracellular fluid, though only to a limited extent. The relatively low volumes should result in high concentrations of the sordarins in extracellular fluids; this is a beneficial property for an antifungal agent, since most infecting microorganisms are confined to the extracellular spaces (21).
Classical studies for the total recovery of sordarins or their metabolites from feces and urine have not been carried out. However, indirect evaluations of the effect of liver function on the metabolism of this class of compounds have been made both in experiments performed in animals with impaired liver function, such as Gunn rats (7), and in allometric studies that showed better correlations to LBF than to animal BW (see below). In fact, there were 218 and 243% increases in AUC and t1/2 for GM 193663 in rats with impaired UDP-glucuronosyltransferase activity (18) compared with the values for normal rats. These results suggest that a significant proportion of the elimination process of GM 193663 is mediated by conjugation to a glucuronide. There are few potential sites for conjugation on these molecules (Fig. 1), though the most likely site of attack is the carboxylic acid group. Further studies involving structural elucidation methods, such as nuclear magnetic resonance and liquid chromatography-tandem mass spectrometry, would be required to definitively confirm this point.
Linearity of the PK properties of GM 193663 is reasonable with i.v. dosing between 10 and 20 mg/kg, since Cmax and AUC are approximately doubled after administration of a twofold increasing dose, without significant changes in either t1/2 or CLp.
A conventional allometric approach was used for forecasting human PK parameters from animal data, which employed conventional allometric correlations to relate PK parameters (CLp, t1/2, and VSS) to physiological properties such as BW or LBF. VSS and CLp fitted well when BW was used, whereas LBF was chosen as the physiological parameter that correlates to t1/2. The regression fit to the line was better with LBF than with BW (Table 3). The resulting prediction for human t1/2 is 8.9 h.
At present, there are no data on sordarin derivatives dosing in humans. However, several representatives of this new class of antifungal agents have demonstrated in vivo efficacy against P. carinii pneumonia (2), systemic murine candidiasis (1), histoplasmosis (13), and coccidioidomycosis (5), and most such reports include animal PK information. Sordarin derivatives have also displayed slow fungicidal activity related to their PK behavior in an in vitro PK-simulating model (P. Aviles, C. Falcoz, M. J. Guillén, R. San Román, and D. Gargallo-Viola, Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 2000, 1999). By integrating all of the above-mentioned findings, a prediction of PK behavior in humans can greatly contribute to the study design of initial clinical trials with sordarin derivatives. This is in accordance with the growing interest of regulatory authorities in approving clinical trials that have been designed with a rational background.
ACKNOWLEDGMENTS
We gratefully acknowledge members of the GlaxoWellcome Spain Organic Chemistry Laboratories for providing sordarin derivative compounds and thank the Structural Chemistry Department for advice in the HPLC analytical work.
REFERENCES
- 1.Aviles P, Falcoz C, San Román R, Gargallo-Viola D. Pharmacokinetics-pharmacodynamics of a sordarin derivative (GM 237354) in a murine model of lethal candidiasis. Antimicrob Agents Chemother. 2000;44:2333–2340. doi: 10.1128/aac.44.9.2333-2340.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aviles P, Aliouat E M, Martinez A, Dei-Cas E, Herreros E, Dujardin L, Gargallo-Viola D. In vitro pharmacodynamic parameters of sordarin derivatives in comparison with those of marketed compounds against Pneumocystis carinii isolated from rats. Antimicrob Agents Chemother. 2000;44:1284–1290. doi: 10.1128/aac.44.5.1284-1290.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boxenbaum H. Interspecies scaling, allometry, physiological time and the ground plan of pharmacokinetics. J Pharmacokinet Biopharm. 1982;10:201–227. doi: 10.1007/BF01062336. [DOI] [PubMed] [Google Scholar]
- 4.Boxenbaum H. Interspecies pharmacokinetic scaling and the evolutionary-comparative paradigm. Drug Metab Rev. 1984;15:1071–1121. doi: 10.3109/03602538409033558. [DOI] [PubMed] [Google Scholar]
- 5.Clemons K V, Stevens D A. Efficacies of sordarin derivatives GM193663, GM211676, and GM237354 in a murine model of systemic coccidioidomycosis. Antimicrob Agents Chemother. 2000;44:1874–1877. doi: 10.1128/aac.44.7.1874-1877.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davies B I, Morris T H. Physiological parameters in laboratory animals and humans. Pharm Res. 1993;10:1093–1095. doi: 10.1023/a:1018943613122. [DOI] [PubMed] [Google Scholar]
- 7.De Morais SMF, Chow S Y M, Wells P G. Biotransformation and toxicity of acetaminophen in congenic RHA rats with or without a hereditary deficiency in bilirubin UDP-glucuronosyltransferase. Toxicol Appl Pharmacol. 1992;117:81–87. doi: 10.1016/0041-008x(92)90220-m. [DOI] [PubMed] [Google Scholar]
- 8.Dominguez J M, Martin J J. Identification of elongation factor 2 as the essential protein targeted by sordarins in Candida albicans. Antimicrob Agents Chemother. 1998;42:2279–2283. doi: 10.1128/aac.42.9.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dominguez J M, Kelly V A, Kinsman O S, Marriott M S, Gomez de las Heras F, Martin J J. Sordarins: a new class of antifungals with selective inhibition of the protein synthesis elongation cycle in yeasts. Antimicrob Agents Chemother. 1998;42:2274–2278. doi: 10.1128/aac.42.9.2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drusano G L. Role of pharmacokinetics in the outcome of infections. Antimicrob Agents Chemother. 1988;32:289–297. doi: 10.1128/aac.32.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edwards J E., Jr International conference for the development of a consensus on the management and prevention of severe candidal infections. Clin Infect Dis. 1997;25:43–59. doi: 10.1086/514504. [DOI] [PubMed] [Google Scholar]
- 12.Fantin B, Leggett J E, Ebert S, Craig W A. Correlation between in vitro and in vivo activity of antimicrobial agents against gram-negative bacilli in a murine infection model. Antimicrob Agents Chemother. 1991;35:1413–1422. doi: 10.1128/aac.35.7.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graybill J R, Najvar L K, Fothergill A, Bocanegra R, Gomez de las Heras F. Activities of sordarins in murine histoplasmosis. Antimicrob Agents Chemother. 1999;43:1716–1718. doi: 10.1128/aac.43.7.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herreros E, Martinez C M, Almela M J, Marriott M S, Gomez de las Heras F, Gargallo-Viola D. Sordarins: in vitro activities of new antifungal derivatives against pathogenic yeasts, Pneumocystis carinii, and filamentous fungi. Antimicrob Agents Chemother. 1998;42:2863–2869. doi: 10.1128/aac.42.11.2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu J Y, Vadgama J V, Diaz III T G, Henry J P. Tail sectioning: a rapid and simple method for repeated blood sampling of the rat for cortisone determination. Lab Anim Sci. 1996;46:243–245. [PubMed] [Google Scholar]
- 16.Lortholary O, Dupont B. Antifungal prophylaxis during neutropenia and immunodeficiency. Clin Microbiol Rev. 1997;10:477–504. doi: 10.1128/cmr.10.3.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Louie A, Drusano G L, Barnejee P, Liu Q F, Liu W, Kaw P, Shayegani M, Taber H, Miller M H. Pharmacodynamics of fluconazole in a murine model of systemic candidiasis. Antimicrob Agents Chemother. 1998;42:1105–1109. doi: 10.1128/aac.42.5.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mackenzie P L, Owens L S. Differences in UDP-glucuronosyl transferase activities in congenic inbred rats homozygous and heterozygous for the jaundice locus. Biochem Pharmacol. 1984;32:3777–3781. doi: 10.1016/0006-2952(83)90149-1. [DOI] [PubMed] [Google Scholar]
- 19.Martinez A, Aviles P, Jimenez E, Caballero J, Gargallo-Viola D. Activities of sordarins in experimental models of candidiasis, aspergillosis, and pneumocystosis. Antimicrob Agents Chemother. 2000;44:3389–3394. doi: 10.1128/aac.44.12.3389-3394.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pradotta J, Pruitt A W. Furosemide binding to human albumin and plasma of nephrotic children. Clin Pharmacol Ther. 1975;17:159–166. doi: 10.1002/cpt1975172159. [DOI] [PubMed] [Google Scholar]
- 21.Schentag J J. The significance of the relationship between tissue:serum ratios, tissue concentrations and the location of microorganisms. Res Clin Forums. 1990;12:23–27. [Google Scholar]
- 22.Soriano F, García-Corbeira P, Ponte C, Fernández-Roblas R, Gadea I. Correlation of pharmacodynamic parameters of five beta-lactam antibiotics with therapeutic efficacies in an animal model. Antimicrob Agents Chemother. 1996;40:2686–2690. doi: 10.1128/aac.40.12.2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Ogtrop M L, Mattie H, Guiot H F L, van Strijen E, Hazekamp-van Dokkum A M, van Furth R. Comparative study of the effects of four cephalosporins against Escherichia coli in vitro and in vivo. Antimicrob Agents Chemother. 1990;34:1932–1937. doi: 10.1128/aac.34.10.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walsh T J, Hiemenz J W, Anaissie E J. Recent progress and current problems in treatment of invasive fungal infections in neutropenic patients. Infect Dis Clin N Am. 1996;10:365–400. doi: 10.1016/s0891-5520(05)70303-2. [DOI] [PubMed] [Google Scholar]
- 25.Waynforth H B, Flecknell P A. Catheterisation of the jugular vein. In: Waynforth H B, Flecknell P A, editors. Experimental and surgical technique in the rat. San Diego, Calif: Academic Press Inc.; 1992. pp. 215–222. [Google Scholar]