Abstract
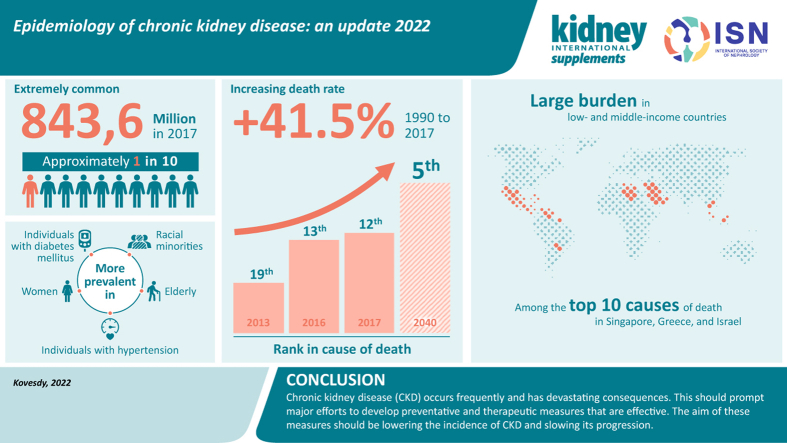
Chronic kidney disease is a progressive condition that affects >10% of the general population worldwide, amounting to >800 million individuals. Chronic kidney disease is more prevalent in older individuals, women, racial minorities, and in people experiencing diabetes mellitus and hypertension. Chronic kidney disease represents an especially large burden in low- and middle-income countries, which are least equipped to deal with its consequences. Chronic kidney disease has emerged as one of the leading causes of mortality worldwide, and it is one of a small number of non-communicable diseases that have shown an increase in associated deaths over the past 2 decades. The high number of affected individuals and the significant adverse impact of chronic kidney disease should prompt enhanced efforts for better prevention and treatment.
Keywords: chronic kidney disease, mortality, prevalence, risk factors
Graphical abstract
Chronic kidney disease (CKD) has emerged as one of the most prominent causes of death and suffering in the 21st century. Due in part to the rise in risk factors, such as obesity and diabetes mellitus, the number of patients affected by CKD has also been increasing, affecting an estimated 843.6 million individuals worldwide in 2017.1 Although mortality has declined in patients with end-stage kidney disease (ESKD),2 the Global Burden of Disease (GBD) studies have shown that CKD has emerged as a leading cause of worldwide mortality.3,4 It is, therefore, paramount that CKD is identified, monitored, and treated, and that preventative and therapeutic measures addressing CKD are systematically implemented worldwide. This narrative review summarizes information about global CKD prevalence, its trends over time, its various determinants, and its associated mortality. Other aspects of kidney disease epidemiology, such as CKD in pediatric patients, CKD incidence, progression to ESKD, or various clinical (e.g., cardiovascular disease) and patient-reported outcomes caused by CKD, are mentioned briefly or not discussed.
Definitions of CKD and its pitfalls in epidemiologic studies
The diagnosis of CKD is made by laboratory testing, most often by estimating glomerular filtration rate (GFR) from a filtration marker, such as serum creatinine or cystatin C, using various formulas, or by testing urine for the presence of albumin or protein (or a combination of these).5 The classification schemas advocated by various professional organizations in the past 2 decades5 have laid the groundwork for the systematic detection and monitoring of CKD worldwide, resulting in an improved understanding of its prevalence and the resulting impact on outcomes, such as mortality. Most studies have used estimated GFR (eGFR) to determine the presence of CKD (and, therefore, report on the prevalence of CKD stages 3–5), whereas other studies have combined albuminuria (typically defined as an albumin-to-creatinine ratio of >30 mg/g) and decreased eGFR to report on CKD stages 1–5. Finally, to differentiate CKD (which is considered to be a chronic progressive disease) from conditions such as acute kidney injury or from transient fluctuations in kidney function unrelated to kidney damage, the standard definition of CKD includes a so-called “chronicity criterion” (i.e., that the low eGFR or elevated urine albumin should be detectable for at least 90 days, requiring the presence of repeated measurements over time).5 There is currently no consensus on the length of time used in the assessment of CKD when applying the chronicity criterion, with epidemiologic studies applying various algorithms, from single measurements to any repeated measurements past 90 days, or limiting the repeated measurement(s) to 90 to 365 days, and from requiring consecutive repeated markers of CKD to accepting CKD markers interspersed with markers not conforming to CKD criteria. The potential impact of using 6 different definition algorithms (5 laboratory measurement based and one based on International Classification of Diseases [ICD] diagnostic codes) to ascertain the prevalence of CKD was recently examined in a population-based cohort from Northern Denmark.6 The prevalence of CKD varied considerably between the various laboratory-based definitions, ranging from 8327 cases per 100,000 population when using a single eGFR value to 4637 cases per 100,000 population when using a time-limited repeated eGFR-based definition. Furthermore, when using an ICD diagnostic code-based definition, the prevalence of CKD was markedly lower, at 775 cases per 100,000 population. Studies assessing the prevalence of CKD have applied a variety of definitions of CKD, and thus their results (and especially the results of studies aggregating their findings, as described below) must be interpreted with caution.
Prevalence and global burden of CKD
The prevalence of CKD has been reported in an increasing number of studies worldwide (the individual discussion of which is beyond the scope of this review), which has made it possible to aggregate their findings and to derive information about global CKD prevalence overall, as well as in various patient subgroups and geographic regions. A study assessing the prevalence and burden of CKD in 2010 pooled the results of 33 population-based representative studies from around the world and reported an age-standardized global prevalence of CKD stages 1–5 in individuals aged ≥20 years of 10.4% among men and 11.8% among women.7 The study reported important differences by geographic region classified by income level, with a CKD age-standardized prevalence of 8.6% and 9.6% in men and women, respectively, in high-income countries, and 10.6% and 12.5% in men and women, respectively, in low- and middle-income countries. The age-standardized global prevalence of CKD stages 3–5 in adults aged ≥20 years in the same study was 4.7% in men and 5.8% in women. A more recent study performed a comprehensive systematic review and meta-analysis of 100 studies comprising 6,908,440 patients, and reported a global prevalence of 13.4% for CKD stages 1–5 and 10.6% for CKD stages 3–5.8 The prevalence of the individual CKD stages was 3.5% (stage 1), 3.9% (stage 2), 7.6% (stage 3), 0.4% (stage 4), and 0.1% (stage 5).8 On the basis of the results of studies examining the global prevalence of CKD, the current total number of individuals affected by CKD stages 1–5 worldwide was estimated to be 843.6 million.1
Changes in CKD prevalence over time
There are significantly fewer studies examining changes in CKD prevalence over time, as this requires a reassessment of the same population using similar methods. In the United States, the Centers for Disease Control and Prevention CKD Surveillance System reported that the prevalence of CKD stages 1–4 was 11.8% in 1988 to 1994, and it increased to 14.2% in 2015 to 2016.9 This increase was not linear, as was reported by a study examining data from the National Health and Nutrition Examination Survey; this study showed that although the prevalence of CKD stage 3–4 increased from the 1990s to the 2000s, it has remained largely stable since.10 A similarly stable prevalence of CKD stages 1–5 was reported in Norway for the time period between 1995 and 2008.11 Interestingly, the prevalence of CKD stages 3–5 declined significantly over 7 years in the United Kingdom based on the nationally representative Health Survey for England. In this study, the adjusted odds ratio of an eGFR <60 ml/min per 1.73 m2 comparing 2003 with 2009/2010 was 0.73 (95% confidence interval, 0.57–0.93).12 The reasons for recently reported stabilized or improved CKD prevalence are unclear. These trends have occurred despite a concomitant increase in common risk factors of CKD, such as diabetes and obesity, although hypertension control has improved over this time period.12 It is worth mentioning that, due to population growth, a stable trend in CKD prevalence still represents an increase in the absolute number of patients with CKD. The reason(s) for the observed dynamic changes in CKD prevalence (and the discrepancies observed between the data from different countries) is difficult to determine. Disease prevalence could vary due to changes in disease incidence, but information about CKD incidence is much sparser in the literature, and the results of published studies cannot be interpreted in the context of prevalence estimates performed in different populations and different eras,13, 14, 15, 16, 17 due to the major impact of characteristics, such as age, sex, or race, on incidence values. Prevalence can also change because of changes in survival or longer lifetime duration of diagnosed CKD (e.g., from better screening); it is possible that the aggregate change in CKD prevalence may be the result of a combination of factors.
Effect of patient characteristics and comorbidities on CKD prevalence
The prevalence of CKD is affected by both its definition and its pathophysiology. Because most CKD cases are identified using eGFR, its determinants will impact the estimates of CKD prevalence. Most important, higher age results in lower eGFR independent of the other components of the equation; hence, even with a stable serum creatinine concentration, an individual can develop CKD as a result of advancing age due to the assumption that age-related losses in muscle mass will obscure the decrease in age-associated losses in GFR. Indeed, the aforementioned meta-analysis by Hill et al. assessed the impact of age on CKD prevalence and reported a linearly higher prevalence for CKD stages 1–5 associated with advancing age, ranging from 13.7% in the 30- to 40-year-old group to 27.9% in patients aged >70 to 80 years.8 Similar trends were reported in the United States during 2015 to 2016, where the prevalence of CKD stages 1–4 was 5.6% among individuals aged 20 to 39 years and 44% among those aged >70 years.9 Notwithstanding the biological plausibility of age-associated loss of GFR, the pathologic significance of early-stage (i.e., stage 3a) CKD that is solely a result of advanced age (and characterized by normal urine albumin and serum creatinine values) continues to be debated.18
The prevalence of CKD has been reported to be higher in females than in males. In the United States, the age-adjusted prevalence of CKD stages 1–4 in 2015 to 2016 was 14.9% in females and 12.3% in males,9 similar to the sex-based differences reported in the global studies mentioned above.7,8 The reasons for these differences are unclear and are likely to be complex. Although GFR estimating equations include a correction factor for sex, a single cutoff of <60 ml/min per 1.73 m2 for CKD definition may result in overdiagnosing CKD in women.19 The higher CKD prevalence described in women also contrasts with experimental data showing the protective effects of estrogen and potential deleterious effects of testosterone on nondiabetic CKD,20 as well as data that indicate a higher incidence of kidney failure in men.21,22 A meta-analysis of 30 studies examining sex-stratified data concluded that CKD progression was faster in men compared with women,23 although other studies have cautioned that such differences may be due to nonbiological factors, such as lifestyle, cultural, and socioeconomic factors.24 Better characterization of the effects of sex on CKD incidence, prevalence, and progression requires further examination, including the study of potential development of sex-specific disease markers.19
Racial differences in the incidence and prevalence of CKD and kidney failure are well described in the United States,25, 26, 27 but a global and systematic evaluation of such differences is difficult because variances between countries are complex and represent a combination of risk factors (including differences in race). Furthermore, within-country comparisons may not always be possible due to racial/ethnic homogeneities and/or local restrictions on reporting individuals’ race and ethnicity. An additional challenge is the inaccuracy of GFR estimation formulas in individuals of different races, and an ongoing debate in the United States over the exclusion of the correction factor for self-reported African American race from the existing estimation formulas as a means to alleviate racial disparities.28 In the United States, the age-adjusted prevalence of CKD stages 1–4 among non-Hispanic Whites, non-Hispanic Blacks, and Mexican Americans in 2015 to 2016 was 13%, 16.5%, and 15.3%, respectively.9 The reasons for race-associated differences are complex, and include differences in the prevalence of CKD risk factors (such as diabetes mellitus, hypertension, and obesity), genetic causes, lifestyle and cultural differences, and socioeconomic disparities.29, 30, 31, 32
Diabetes mellitus has emerged as the most important risk factor for CKD in the developed world; this is reflected in studies examining CKD prevalence. In the United States, the prevalence of CKD stages 3–4 among diagnosed diabetics was 24.5% in 2011 to 2014, whereas in prediabetics it was 14.3% and in nondiabetics it was 4.9%.9 The association between diabetes mellitus and the prevalence of CKD was also reported in a meta-analysis that included 82 global studies.8 The effect of diabetes mellitus on kidney function and on the development and progression of CKD is well established.33 Nevertheless, epidemiologic studies examining CKD in diabetics have to contend with the fact that diabetic populations (especially type 2 diabetics) often experience multiple other comorbid conditions, such as hypertension or vascular disease, which are themselves independent risk factors for CKD. A study examining a national cohort of US veterans with newly diagnosed type 2 diabetes mellitus reported a crude prevalence of CKD stages 1–5 of 31.6%, half of whom had CKD stages 3–5.34 Although the timing of incident type 2 diabetes mellitus is difficult to ascertain, the high prevalence of CKD in this study suggests that at least some of the CKD cases diagnosed in diabetics may not be a direct result of diabetes-related mechanisms.
Hypertension is the strongest cardiovascular risk factor worldwide and is also closely associated with CKD.35 The prevalence of CKD among hypertensive US adults was 35.8% in 2011 to 2014, compared with a prevalence of 14.4% in prehypertensives and 10.2% among nonhypertensive individuals.9 A significant association between hypertension and the prevalence of CKD was also reported in a meta-analysis that included 75 global studies.8
Mortality associated with CKD
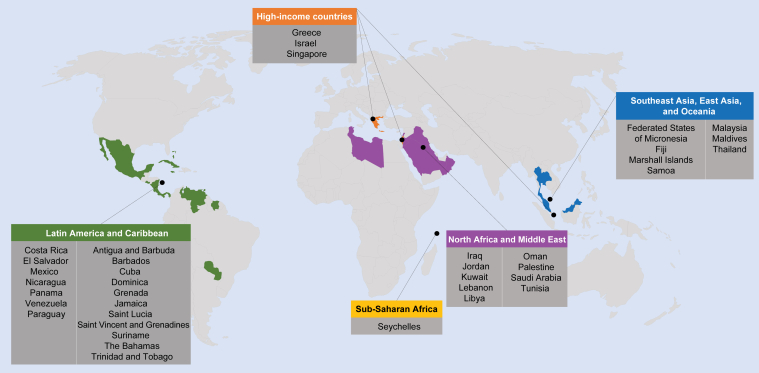
CKD is now widely recognized as one of the leading causes of death worldwide. The GBD reports have been tracking causes of death across the globe for the past decade. The 2013 GBD report indicated that although relative death rates decreased for most communicable and noncommunicable diseases, CKD (defined as all stages, including patients on dialysis) was one of only a handful of conditions to show an increase since 1990.3,4 The global all-age mortality rate attributed to CKD increased by 41.5% between 1990 and 2017.36 Besides being one of the leading causes of death, CKD also became the 19th leading cause of years of life lost (which is calculated from the number of deaths attributable to CKD and the life expectancy of individuals in various age groups at the time of their death from CKD3) in 2013, compared with being the 36th leading cause in 1990.3 Subsequent GBD reports indicate that the rise of CKD among the list of causes of death has continued, occupying the 13th place in 201637 and 12th place in 2017,36 with predictions suggesting that it will become the fifth highest cause of years of life lost globally by 2040.38 The GBD reports also shed light on the disproportionate nature of the burden imparted by CKD-associated death in different world regions, with Latin America, the Caribbean region, Southeast Asia and East Asia, Oceania, North Africa, and the Middle East being especially affected. Among high-income nations, CKD was among the top 10 causes of death in Singapore, Greece, and Israel (Figure 1).3,4 These reports are especially noteworthy when considering that they did not include deaths that were caused indirectly by CKD, such as those related to acute kidney injury or to various cardiovascular causes, both of which can be caused or potentiated by CKD.4
Figure 1.
Regions and countries where chronic kidney disease is in the top 10 causes of years of life lost in 2013. On the basis of data from the Global Burden of Disease Study 2013.3
Conclusions
CKD is extremely common and has emerged as one of the leading noncommunicable causes of death worldwide. It is projected to affect an increasing number of individuals over time and to further rise in importance among the various global causes of death. CKD affects populations in different regions of the world unequally, likely as a result of differences in population demographic characteristics, their comorbidities, and access to health care resources. The common nature and devastating effects of CKD should prompt major efforts to develop and implement effective preventative and therapeutic efforts aimed at lowering the development of CKD and slowing its progression.
Disclosure
This article is published as part of a supplement sponsored by Bayer AG.
CPK is a consultant for Abbott, Akebia, AstraZeneca, Bayer, Cara Therapeutics, CSL Behring, Dr. Schar, Reata, Rockwell, Takeda, Tricida and Vifor. CPK received no personal funding for this article.
Acknowledgments
Development of this article was funded by an unrestricted educational grant from Bayer AG. CPK would like to acknowledge Jo Luscombe, PhD, of Chameleon Communications International, who provided editorial assistance with funding via an unrestricted educational grant from Bayer AG.
References
- 1.Jager K.J., Kovesdy C., Langham R., et al. A single number for advocacy and communication-worldwide more than 850 million individuals have kidney diseases. Kidney Int. 2019;96:1048–1050. doi: 10.1016/j.kint.2019.07.012. [DOI] [PubMed] [Google Scholar]
- 2.Saran R., Robinson B., Abbott K.C., et al. US Renal Data System 2019 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2020;75:A6–A7. doi: 10.1053/j.ajkd.2019.09.003. [DOI] [PubMed] [Google Scholar]
- 3.GBD Mortality Causes of Death Collaborators Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117–171. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rhee C.M., Kovesdy C.P. Epidemiology: spotlight on CKD deaths-increasing mortality worldwide. Nat Rev Nephrol. 2015;11:199–200. doi: 10.1038/nrneph.2015.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150. [Google Scholar]
- 6.Vestergaard S.V., Christiansen C.F., Thomsen R.W., et al. Identification of patients with CKD in medical databases: a comparison of different algorithms. Clin J Am Soc Nephrol. 2021;16:543–551. doi: 10.2215/CJN.15691020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mills K.T., Xu Y., Zhang W., et al. A systematic analysis of worldwide population-based data on the global burden of chronic kidney disease in 2010. Kidney Int. 2015;88:950–957. doi: 10.1038/ki.2015.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hill N.R., Fatoba S.T., Oke J.L., et al. Global prevalence of chronic kidney disease - a systematic review and meta-analysis. PLoS One. 2016;11 doi: 10.1371/journal.pone.0158765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention Chronic kidney disease (CKD) surveillance system: 2021. https://nccd.cdc.gov/ckd/default.aspx Accessed September 30, 2021.
- 10.Murphy D., McCulloch C.E., Lin F., et al. Trends in prevalence of chronic kidney disease in the United States. Ann Intern Med. 2016;165:473–481. doi: 10.7326/M16-0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hallan S.I., Ovrehus M.A., Romundstad S., et al. Long-term trends in the prevalence of chronic kidney disease and the influence of cardiovascular risk factors in Norway. Kidney Int. 2016;90:665–673. doi: 10.1016/j.kint.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 12.Aitken G.R., Roderick P.J., Fraser S., et al. Change in prevalence of chronic kidney disease in England over time: comparison of nationally representative cross-sectional surveys from 2003 to 2010. BMJ Open. 2014;4 doi: 10.1136/bmjopen-2014-005480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grams M.E., Chow E.K., Segev D.L., et al. Lifetime incidence of CKD stages 3-5 in the United States. Am J Kidney Dis. 2013;62:245–252. doi: 10.1053/j.ajkd.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grams M.E., Rebholz C.M., McMahon B., et al. Identification of incident CKD stage 3 in research studies. Am J Kidney Dis. 2014;64:214–221. doi: 10.1053/j.ajkd.2014.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shlipak M.G., Katz R., Kestenbaum B., et al. Rate of kidney function decline in older adults: a comparison using creatinine and cystatin C. Am J Nephrol. 2009;30:171–178. doi: 10.1159/000212381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drey N., Roderick P., Mullee M., et al. A population-based study of the incidence and outcomes of diagnosed chronic kidney disease. Am J Kidney Dis. 2003;42:677–684. doi: 10.1016/s0272-6386(03)00916-8. [DOI] [PubMed] [Google Scholar]
- 17.Bash L.D., Coresh J., Kottgen A., et al. Defining incident chronic kidney disease in the research setting: the ARIC study. Am J Epidemiol. 2009;170:414–424. doi: 10.1093/aje/kwp151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Delanaye P., El Nahas M., Glassock R.J. The myth of the future burden of CKD in United States. Am J Kidney Dis. 2015;66:171–172. doi: 10.1053/j.ajkd.2015.01.035. [DOI] [PubMed] [Google Scholar]
- 19.Bairey Merz C.N., Dember L.M., Ingelfinger J.R., et al. Sex and the kidneys: current understanding and research opportunities. Nat Rev Nephrol. 2019;15:776–783. doi: 10.1038/s41581-019-0208-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silbiger S.R., Neugarten J. The impact of gender on the progression of chronic renal disease. Am J Kidney Dis. 1995;25:515–533. doi: 10.1016/0272-6386(95)90119-1. [DOI] [PubMed] [Google Scholar]
- 21.Hecking M., Bieber B.A., Ethier J., et al. Sex-specific differences in hemodialysis prevalence and practices and the male-to-female mortality rate: the Dialysis Outcomes and Practice Patterns Study (DOPPS) PLoS Med. 2014;11 doi: 10.1371/journal.pmed.1001750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iseki K., Nakai S., Shinzato T., et al. Increasing gender difference in the incidence of chronic dialysis therapy in Japan. Ther Apher Dial. 2005;9:407–411. doi: 10.1111/j.1744-9987.2005.00318.x. [DOI] [PubMed] [Google Scholar]
- 23.Neugarten J., Golestaneh L. Influence of sex on the progression of chronic kidney disease. Mayo Clin Proc. 2019;94:1339–1356. doi: 10.1016/j.mayocp.2018.12.024. [DOI] [PubMed] [Google Scholar]
- 24.Ricardo A.C., Yang W., Sha D., et al. Sex-related disparities in CKD progression. J Am Soc Nephrol. 2019;30:137–146. doi: 10.1681/ASN.2018030296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Easterling R.E. Racial factors in the incidence and causation of end-stage renal disease (ESRD) Trans Am Soc Artif Intern Organs. 1977;23:28–33. doi: 10.1097/00002480-197700230-00008. [DOI] [PubMed] [Google Scholar]
- 26.Hsu C.Y., Lin F., Vittinghoff E., et al. Racial differences in the progression from chronic renal insufficiency to end-stage renal disease in the United States. J Am Soc Nephrol. 2003;14:2902–2907. doi: 10.1097/01.asn.0000091586.46532.b4. [DOI] [PubMed] [Google Scholar]
- 27.Rostand S.G., Kirk K.A., Rutsky E.A., et al. Racial differences in the incidence of treatment for end-stage renal disease. N Engl J Med. 1982;306:1276–1279. doi: 10.1056/NEJM198205273062106. [DOI] [PubMed] [Google Scholar]
- 28.Levey A.S., Titan S.M., Powe N.R., et al. Kidney disease, race, and GFR estimation. Clin J Am Soc Nephrol. 2020;15:1203–1212. doi: 10.2215/CJN.12791019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nicholas S.B., Kalantar-Zadeh K., Norris K.C. Socioeconomic disparities in chronic kidney disease. Adv Chronic Kidney Dis. 2015;22:6–15. doi: 10.1053/j.ackd.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nicholas S.B., Kalantar-Zadeh K., Norris K.C. Racial disparities in kidney disease outcomes. Semin Nephrol. 2013;33:409–415. doi: 10.1016/j.semnephrol.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Norris K.C., Mensah G.A., Boulware L.E., et al. Age, race and cardiovascular outcomes in African American veterans. Ethn Dis. 2016;26:305–314. doi: 10.18865/ed.26.3.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Norris K.C., Williams S.F., Rhee C.M., et al. Hemodialysis disparities in African Americans: the deeply integrated concept of race in the social fabric of our society. Semin Dial. 2017;30:213–223. doi: 10.1111/sdi.12589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Boer I.H., Caramori M.L., Chan J.C.N., et al. Executive summary of the 2020 KDIGO diabetes management in CKD guideline: evidence-based advances in monitoring and treatment. Kidney Int. 2020;98:839–848. doi: 10.1016/j.kint.2020.06.024. [DOI] [PubMed] [Google Scholar]
- 34.Gatwood J., Chisholm-Burns M., Davis R., et al. Evidence of chronic kidney disease in veterans with incident diabetes mellitus. PLoS One. 2018;13 doi: 10.1371/journal.pone.0192712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kjeldsen S.E. Hypertension and cardiovascular risk: general aspects. Pharmacol Res. 2018;129:95–99. doi: 10.1016/j.phrs.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 36.GBD Chronic Kidney Disease Collaboration Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395:709–733. doi: 10.1016/S0140-6736(20)30045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.GBD Causes of Death Collaborators Global, regional, and national age-sex specific mortality for 264 causes of death, 1980-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1151–1210. doi: 10.1016/S0140-6736(17)32152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Foreman K.J., Marquez N., Dolgert A., et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: reference and alternative scenarios for 2016-40 for 195 countries and territories. Lancet. 2018;392:2052–2090. doi: 10.1016/S0140-6736(18)31694-5. [DOI] [PMC free article] [PubMed] [Google Scholar]