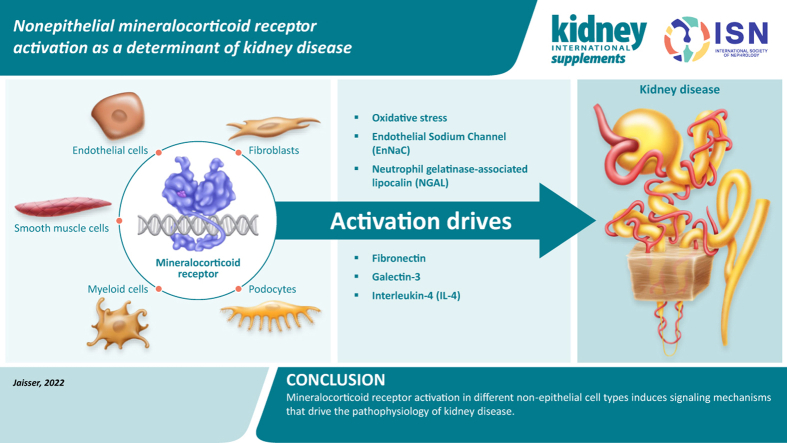
Abstract
Chronic kidney disease is a major global health challenge, and mineralocorticoid receptor (MR) signaling is thought to play a role in disease progression. The classic role of the MR is the regulation of fluid and electrolyte homeostasis via differential gene expression, and recently its role in modulating inflammation and fibrosis has been identified. In addition to expression of the MR in renal epithelial cells, it is also found in nonepithelial cells, such as endothelial cells, vascular smooth muscle cells, podocytes, and fibroblasts. Targeting the MR in these cells may play a role in offering protection against inflammation and fibrosis in the kidneys and the cardiovascular system. Herein, data from preclinical cell-specific MR knockout mouse models and in vitro studies that help uncover the role of the MR in nonepithelial cells are presented. This review also discusses several potential targets that offer opportunities for the targeting of MR signaling in nonepithelial cells.
Keywords: chronic kidney disease, mineralocorticoid receptor, nonepithelial cells
Graphical abstract
The mineralocorticoid receptor (MR), a member of the steroid hormone receptor family, acts as a ligand-dependent transcription factor.1 It is involved in the regulation of inflammation and fibrosis and fluid and electrolyte homeostasis via differential gene expression.1,2 The MR mediates the physiologic activity of the adrenal steroid hormone, aldosterone,3 and it is best known for its role in the control of electrolyte and fluid balance via the MR expressed in aldosterone-sensitive kidney epithelial cells in the distal nephron that express the 11β-hydroxysteroid dehydrogenase type 2 enzyme.2,4 Thus, although the MR binds aldosterone and cortisol with equal affinity, the presence of 11β-hydroxysteroid dehydrogenase type 2 in epithelial cells confers specificity of the MR for aldosterone.1, 2, 3 However, the MR is also expressed in nonepithelial tissues, including the heart, adipocytes, podocytes, inflammatory cells, endothelial cells (ECs), and vascular smooth muscle cells (VSMCs) (Figure 1), where in some cases it is unprotected by 11β-hydroxysteroid dehydrogenase type 2, leading to potential activation by cortisol.3,4 MR overactivation, which contributes to inflammation and fibrosis, is well documented in cardiovascular (CV) disease, as discussed by Bauersachs and Lother,5 and is recognized as a potential treatment target to slow the progression of chronic kidney disease (CKD). The aims of this narrative review are to discuss the evidence regarding the role of nonepithelial MR activation as a determinant of CKD, and to review the latest evidence for MR targets that are relevant for disease management.
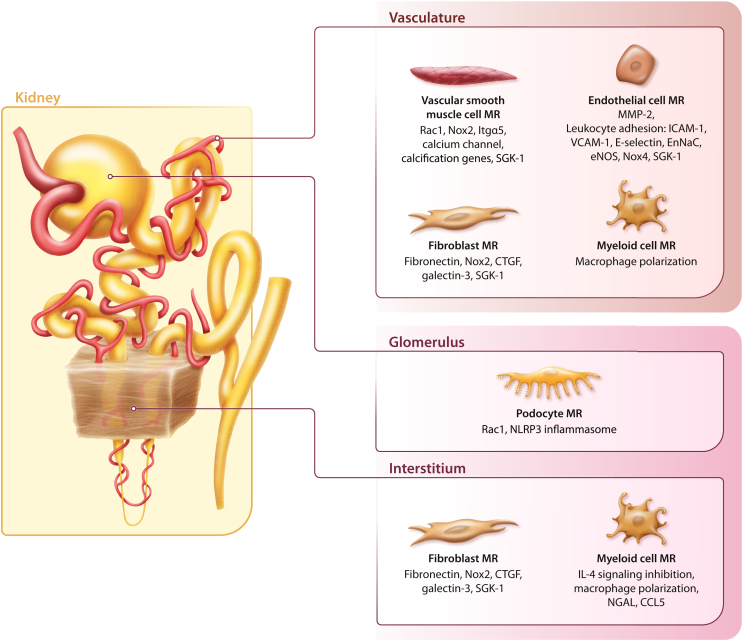
Figure 1.
Potential mediators of cellular injury following mineralocorticoid receptor (MR) overactivation in nonepithelial cells, leading to vascular dysfunction, inflammation, and fibrosis. CCL5, chemokine (C-C motif) ligand 5; CTGF, connective tissue growth factor; EnNaC, epithelial sodium channel in the endothelium; eNOS, endothelial nitric oxide synthase; ICAM-1, intercellular adhesion molecule 1; IL-4, interleukin-4; Itgα5, integrin subunit α 5; MMP-2, matrix metalloproteinase-2; NGAL, neutrophil gelatinase-associated lipocalin; NLRP3, nucleotide-binding oligomerization domain–like receptor family, pyrin domain-containing 3; NOX, nicotinamide adenine dinucleotide phosphate oxidase; Rac1, Rac family small GTPase 1; SGK-1, serum and glucocorticoid-regulated kinase 1; VCAM-1, vascular cell adhesion molecule 1.
Models of MR deficiency in nonepithelial cells and kidney disease
Increasing evidence suggests that targeting the MR in nonepithelial cells plays a key role in offering protection against inflammation and fibrosis in the kidneys and the CV system.4 The development of cell-specific MR knockout mouse models as well as in vitro studies has helped elucidate the specific role of the MR in podocytes, vascular cells (ECs and VSMCs), inflammatory cells, and fibroblasts in kidney injury associated with MR overactivation, as discussed below and presented in Table 1.3,4,6, 7, 8, 9, 10, 11, 12, 13, 14 Of note, sodium handling is not affected in mouse models of endothelial, smooth muscle, or myeloid MR deficiency.15, 16, 17
Table 1.
Overview of mediators of cellular injury in nonepithelial cells: evidence from preclinical models of MR deficiency
| Nonepithelial cell type | Mediator of cellular injury | Role of mediator | Impact of mediator | Reference |
|---|---|---|---|---|
| Podocytes | ||||
| Rac1 |
|
|
Shibata et al., 20086 | |
| NLRP3 inflammasome |
|
|
Bai et al., 20177 | |
| Endothelial cells | ||||
| MMP-2 |
|
|
Butler et al., 20198 | |
| Vascular smooth muscle cells | ||||
| Rac1 |
|
|
Barrera-Chimal et al., 20179 | |
| Inflammatory cells | ||||
| MR inhibition of IL-4 receptor signaling |
|
|
Barrera-Chimal et al., 201810 Barrera-Chimal et al., 201911 |
|
| Fibroblasts | ||||
| Fibronectin |
|
|
Chen et al., 201312 Bowers et al., 201913 |
|
eNOS, endothelial nitric oxide synthase; IL-4, interleukin-4; MMP-2, matrix metalloproteinase-2; MR, mineralocorticoid receptor; NLRP3, nucleotide-binding oligomerization domain–like receptor family, pyrin domain-containing 3; NO, nitric oxide; Rac1, Rac family small GTPase 1.
MR activation in podocytes
Podocytes are one of the most important cell types for overall glomerular health, and podocyte injury is considered a critical event in progression of kidney disease in patients with diabetes.18 MR activation in podocytes may occur by aldosterone-dependent and -independent mechanisms. In 2008, Shibata et al. reported that Rac1 (Rac family small GTPase 1) mediates the activation of the MR, given that the presence of a constitutively active form of Rac1 induced MR nuclear translocation and activation in podocytes.6 Furthermore, mice in which Rac1 activity was elevated developed podocyte lesions and severe albuminuria, which were prevented by MR antagonism with eplerenone.6,19 Therefore, MR overactivation in podocytes appears to induce podocyte injury. Autophagy is a process essential for podocyte maintenance and homeostasis and is disrupted in CKD in type 2 diabetes.20 Spironolactone treatment of diabetic rats increased the autophagy of podocytes in vivo21 and restored autophagy in podocytes under mechanical stress.22 Moreover, aldosterone mediates podocyte injury through nucleotide-binding oligomerization domain–like receptor family, pyrin domain-containing 3 inflammasome activation.7 Furthermore, aldosterone addition to podocytes in vitro induces the downregulation of nephrin, podocin, podoplanin, and podocalyxin while activating the Wnt/β-catenin pathway.23
Podocyte-specific MR deletion had no effect in ameliorating antiglomerular basement membrane glomerulonephritis,17 and it is not yet clear to what extent podocyte MRs contribute to other types of renal injury, such as CKD or CKD in type 2 diabetes. The lack of effect of podocyte MR deletion in glomerulonephritis does not exclude that MR inhibition in podocytes might have beneficial effects on other forms of kidney disease, such as CKD in type 2 diabetes, in which the pathologic mechanisms leading to podocyte injury and effacement differ.24
MR knockout in the endothelium
Endothelial dysfunction is reported in patients with CKD and leads to an increased risk of CV disease. It has been suggested that abnormalities in endothelial function may be a linking factor between CKD and CV disease,25,26 which may be mediated by the endothelial MR.27 In endothelial cells, aldosterone increases intercellular adhesion molecule-1, vascular cell adhesion molecule-1, E-selectin, and monocyte chemoattractant protein-1 expression.28,29 Moreover, aldosterone promotes oxidative injury through increasing the expression of the nicotinamide adenine dinucleotide phosphate oxidase subunits nicotinamide adenine dinucleotide phosphate oxidase 2 (Nox2), p47(phox), and p22(phox) and by stimulating Rac1 activation.30,31 The endothelial function is also affected by aldosterone through a reduction in endothelial nitric oxide synthase (eNOS) phosphorylation.29,32
Studies have reported that the endothelial MR has no role in ischemic acute kidney injury (AKI),9 cyclosporine A nephrotoxicity,33 or renal injury induced by chronic deoxycorticosterone acetate administration.34 In agreement with these observations, it was reported that endothelial MR knockout does not modify the contractile properties of the renal artery or afferent arterioles, and the kidney function and morphology remain similar between phenotypes.6 Similarly, MR deletion in ECs did not protect the kidney against angiotensin II–induced contraction in renal arteries, albuminuria, or kidney injury.6 However, endothelial MR knockout in females prevented Western diet-induced kidney artery stiffening, proteinuria, oxidative stress, reduced eNOS activation, and inflammatory M1 macrophage activation.35 It is possible that sex-specific effects of the endothelial MR could account for the differential effects observed. However, side-by-side studies comparing male versus female phenotypes will confirm these sex-specific changes.
In the glomerular endothelium, it was reported that aldosterone disrupts the endothelial glycocalyx through increasing glomerular matrix metalloproteinase-2 activity and reducing syndecan 4 expression, thus causing albuminuria8; therefore, it is thought that aldosterone–MR targeting in glomerular ECs could account for the antiproteinuric effects of MR antagonists (MRAs) in several kidney disease conditions.
MR knockout in vascular smooth muscle cells
The MR plays a role in the regulation of vascular tone.9,36 Thus, the MR expressed in VSMCs makes an important contribution in situations in which kidney injury is mediated by, or is associated with, vasoconstriction. For example, in a study with MR deletion in VSMCs, the findings showed blunted kidney dysfunction and tubular injury after an ischemic AKI episode.9 The protection was mediated by a reduction in Rac-1–induced reactive oxygen species production in VSMCs, which prevented a sulfenic acid modification on the endothelin-B receptor that would have led to impaired endothelin-B receptor signaling and eNOS inactivation.9 This is of particular interest because MR antagonists prevent sulfenic acid modification of the endothelin-B receptor occurring in ischemic AKI, and because the protective effect of MR inhibition against AKI is at least partially dependent on endothelin-B receptor activity.37,38 Similarly, in kidney injury associated with vasoconstriction due to cyclosporine A administration, MR deficiency in VSMCs prevented kidney dysfunction and tubular vacuolization and stabilized renal vascular resistance through diminishing the cyclosporine A–induced phosphorylation of contractile proteins.33 MR activation in isolated smooth muscle cells promotes the activation of nicotinamide adenine dinucleotide phosphate oxidase and increases the expression of genes involved in vascular calcification (alkaline phosphatase, parathyroid hormone receptor-2, and bone morphogenetic protein-2), vascular fibrosis (collagen I and III), vascular stiffness, (integrin α-5 subunit), and inflammation (interleukin-16 and cytotoxic T-lymphocyte antigen-4).16,39,40
MR knockout in myeloid cells
It has been reported that renal injury can be reduced by targeting MR signaling in myeloid cells.17 In myeloid cells, aldosterone stimulates the expression of tumor necrosis factor-α, monocyte chemoattractant protein-1, regulated on activation T cell expressed and secreted, and interleukin-12.41 In a mouse model of glomerulonephritis, myeloid-specific MR deletion prevented increases in cystatin C levels and reduced crescent numbers, tubular injury, fibrosis, and inflammatory cytokine levels to a similar extent as the protection observed from treatment with the selective, steroidal MRA eplerenone.17 Similarly, myeloid MR knockout protected against CKD progression after an ischemic AKI episode in the mouse model by favoring M2 repairing macrophage polarization via interleukin-4–enhanced signaling,10 suggesting that the myeloid MR is important and is a target for MRAs to limit CKD progression. Continued infiltration of inflammatory cells and macrophages impacts the repair process after ischemic AKI. Infiltration of M1-proinflammatory macrophages favors maladaptive repair and injury perpetuation, whereas the early switch from M1 to M2 macrophages facilitates an effective repair.42,43 Indeed, infiltrating macrophages display increased M2 polarization markers in mice lacking the MR in the myeloid lineage and subjected to bilateral renal ischemia–reperfusion, and the population of proinflammatory Ly6Chigh macrophages is smaller than that of control littermates.10 This effect was associated with the prevention of kidney fibrosis and the transition from AKI to CKD.10 Thus, MR gene deletion in myeloid cells recapitulates the beneficial effects of MR antagonism with a target treatment, such as the novel, nonsteroidal, selective MRA finerenone, which has been shown to protect against the progression from AKI to CKD in a mouse model.10
MR activation in fibroblasts
Evidence suggests that increased MR activation may lead to fibroblast proliferation and the production of profibrotic molecules, thus inducing kidney fibrosis.11 Aldosterone stimulates fibronectin synthesis in isolated renal fibroblasts.12 In addition, fibroblasts incubated with spironolactone or eplerenone have shown reduced extracellular matrix component production induced by platelet-derived growth factor or connective tissue growth factor,44 suggesting that MR activation in renal fibroblasts may also contribute to kidney remodeling during CKD through mechanisms similar to those reported in cardiac fibroblasts. In isolated renal fibroblasts, aldosterone also stimulates collagen I, III, and IV synthesis and promotes its proliferation.45,46 However, no studies in which the MR has been specifically deleted in fibroblasts have been done to evaluate its consequences on CKD or CKD in type 2 diabetes progression.
MR targets relevant for kidney disease
Upregulation of the MR stimulates fibrosis and inflammation, resulting in progression of CKD.4 Common polymorphisms also influence MR protein expression and modify the phenotypes (e.g., the common MR polymorphism c.-2G>C [rs2070951] affects blood pressure, renin, and aldosterone levels).47 Therefore, it is plausible that polymorphisms might also lead to differential fibrotic and inflammatory phenotypes elicited by the MR. Given the current lack of an approved treatment that primarily targets inflammation and fibrosis, this is an area of ongoing interest, and research has focused on identifying potential targets that may be suitable in kidney disease. The latest evidence of potential interest regarding downstream targets of the MR is discussed herein.
Epithelial sodium channel in the endothelium
Overactivation of the MR in ECs may contribute to kidney injury by modulating the expression of the epithelial sodium channel in the endothelium (EnNaC). Indeed, it has been shown that increased EnNaC expression, induced by aldosterone stimulation, is associated with stiff ECs and reduced nitric oxide generation.48,49 The decrease in nitric oxide levels after MR activation may be at least partially mediated by the effect of the MR on endothelial stiffness, through its influence on epithelial sodium channel expression in the endothelium. In addition, the action of the MR on eNOS activation may be at least partially mediated by upregulation of the EnNaC on MR activation. Indeed, specific EnNaC deletion in the endothelium showed a similar beneficial effect to endothelial MR deletion in Western diet-induced obesity on aortic stiffness and fibrosis.50 Similarly, EnNaC deletion prevented Western diet-induced renal artery and renal EC stiffening, effects that were linked to prevention in eNOS inactivation, reduced oxidative stress, and renal perivascular fibrosis.51
We recently showed that endothelial EnNaC knockout ameliorates kidney lesions induced by renal ischemia and leads to faster recovery of kidney oxygenation.52 The effect of endothelial EnNaC deletion in promoting better recovery from hypoxia may be related to the increased generation of nitric oxide, thus preventing sustained vasoconstriction and allowing better kidney perfusion. Indeed, pharmacologic endothelial sodium channel inhibition in human ECs decreased eNOS phosphorylation of threonine 495, thus activating eNOS, as shown by the increased dimer versus monomer ratio.52 Further studies are required to identify whether this protective effect could play a role in the prevention of the transition from AKI to CKD.
Neutrophil gelatinase-associated lipocalin
Neutrophil gelatinase-associated lipocalin (NGAL), a small circulating protein, has shown enhanced levels in a range of pathologic conditions and has been suggested as an early biomarker of renal injury or inflammation.53 NGAL is now identified as an MR target in the CV system, which contributes to the aldosterone-MR–mediated profibrotic effects, through the induction of collagen-I, galectin-3, and cardiotrophin-1.54,55 In particular, NGAL deficiency in inflammatory cells and especially from dendritic cells prevented CV fibrosis induced by a nephrectomy-aldosterone-salt challenge.56,57 In the context of CKD, it was shown that NGAL is not only a marker of the disease but also plays a causative role in kidney damage because following severe nephron reduction, mice lacking NGAL had reduced kidney lesions compared with controls.58 Similarly, NGAL gene deletion reduced tubulointerstitial fibrosis and tubular cell death induced by endoplasmic reticulum stress in proteinuric mice.59 In an antibody-mediated nephritis model, NGAL-deficient mice showed amelioration of histology lesions and reduced proteinuria, whereas exogenous NGAL administration exacerbated kidney injury, proteinuria, and mortality through the promotion of inflammation and apoptosis.60 Furthermore, we reported recently that a newly characterized pharmacologic NGAL inhibitor prevents renal tubulointerstitial fibrosis and reduced profibrotic marker expression in a mouse model of CKD, mimicking the benefit of NGAL gene deletion.53
Nicotinamide adenine dinucleotide phosphate oxidase
Activation of the MR in nonepithelial tissues often leads to oxidative injury. Many studies have shown that MR activation in ECs increases the activity and expression of nicotinamide adenine dinucleotide phosphate oxidase, thus promoting reactive oxygen species generation and oxidative stress-induced injury.29, 30, 31 Similar to ECs, one of the consequences of MR activation in VSMCs is increased oxidative stress mediated by the activation and increased expression of nicotinamide adenine dinucleotide phosphate oxidases.39,61,62
Galectin-3
One of the profibrotic molecules induced by aldosterone-MR is galectin-3. It has been demonstrated that galectin-3 pharmacologic inhibition or knockout protects against renal fibrosis and renal epithelial-to-mesenchymal transition induced by aldosterone–salt treatment.63 Therefore, galectin-3 is thought to be a key molecule that mediates the profibrotic effects of aldosterone and the MR in the kidney. Whether inhibition of galectin-3 prevents aldosterone-MR–mediated CKD progression remains to be explored.
Serum and glucocorticoid-induced kinase 1
Serum and glucocorticoid-induced kinase 1 stimulates aldosterone-dependent sodium resorption and modulates blood pressure. A transgenic mouse model with overexpression of serum and glucocorticoid-induced kinase 1 showed exacerbated albuminuria, glomerular hypertrophy, and renal fibrosis associated with altered fibrotic and inflammatory molecules.64 Findings suggest that enhanced serum and glucocorticoid-induced kinase 1 activity is a risk factor for the development of mineralocorticoid-dependent kidney injury, and the authors concluded that serum and glucocorticoid-induced kinase 1 may offer a therapeutic target to delay CKD development.
Conclusions
CKD is a major global health challenge, and the evidence presented herein highlights the central role of MR signaling in disease progression, providing insights into the role of the MR in nonepithelial cells in the pathogenesis of CKD. Data from preclinical MR knockout models in nonepithelial cells, including podocytes, ECs, VSMCs, and myeloid cells, highlight the opportunities for targeting the effects of MR signaling as a treatment option. There is growing interest in the use of MRAs for the management of patients with CKD, and the preclinical and clinical evidence to date is discussed in accompanying articles by Luther and Fogo65 and Rossing.66 In summary, the literature shows that this is an active area of research, and the identification of several potential targets shows promise for targeting the MR signaling in nonepithelial cells.
Disclosure
This article is published as part of a supplement sponsored by Bayer AG.
FJ received research grants from AstraZeneca and Bayer SAS and honoraria from Bayer SAS and KBP Biosciences. All the other authors declared no competing interests. The authors received no personal funding for this article.
Acknowledgements
Development of this article was funded by an unrestricted educational grant from Bayer AG. The authors would like to acknowledge Jo Luscombe, PhD, of Chameleon Communications International, who provided editorial assistance with funding via an unrestricted educational grant from Bayer AG. The authors would like to also acknowledge Alexander Roeder, Ronny Guenther, Katja Marx, and Josephin Schoenrich, of CAST PHARMA, who designed the figure with funding from Bayer AG. This work was supported by the Fight-HF Avenir Investment Program (ANR-15-RHUS-0004), the Fondation de Recherche sur l’Hypertension Artérielle (REIN/NgalPA-2017/2018), and the ANR NGAL-HT (ANR-19-CE14-0013). TN was supported by a fellowship from the Uehara Memorial Foundation.
References
- 1.Ong G.S., Young M.J. Mineralocorticoid regulation of cell function: the role of rapid signalling and gene transcription pathways. J Mol Endocrinol. 2017;58:R33–R57. doi: 10.1530/JME-15-0318. [DOI] [PubMed] [Google Scholar]
- 2.Agarwal R., Kolkhof P., Bakris G., et al. Steroidal and non-steroidal mineralocorticoid receptor antagonists in cardiorenal medicine. Eur Heart J. 2021;42:152–161. doi: 10.1093/eurheartj/ehaa736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cole T.J., Young M.J. 30 Years of the mineralocorticoid receptor: mineralocorticoid receptor null mice: informing cell-type-specific roles. J Endocrinol. 2017;234:T83–T92. doi: 10.1530/JOE-17-0155. [DOI] [PubMed] [Google Scholar]
- 4.Tesch G.H., Young M.J. Mineralocorticoid receptor signaling as a therapeutic target for renal and cardiac fibrosis. Front Pharmacol. 2017;8:313. doi: 10.3389/fphar.2017.00313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bauersachs J., Lother A. Mineralocorticoid receptor activation and antagonism in cardiovascular disease: cellular and molecular mechanisms. Kidney Int Suppl. 2022;12:19–26. doi: 10.1016/j.kisu.2021.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shibata S., Nagase M., Yoshida S., et al. Modification of mineralocorticoid receptor function by Rac1 GTPase: implication in proteinuric kidney disease. Nat Med. 2008;14:1370–1376. doi: 10.1038/nm.1879. [DOI] [PubMed] [Google Scholar]
- 7.Bai M., Chen Y., Zhao M., et al. NLRP3 inflammasome activation contributes to aldosterone-induced podocyte injury. Am J Physiol Renal Physiol. 2017;312:F556–F564. doi: 10.1152/ajprenal.00332.2016. [DOI] [PubMed] [Google Scholar]
- 8.Butler M.J., Ramnath R., Kadoya H., et al. Aldosterone induces albuminuria via matrix metalloproteinase-dependent damage of the endothelial glycocalyx. Kidney Int. 2019;95:94–107. doi: 10.1016/j.kint.2018.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barrera-Chimal J., Andre-Gregoire G., Nguyen Dinh Cat A., et al. Benefit of mineralocorticoid receptor antagonism in AKI: role of vascular smooth muscle Rac1. J Am Soc Nephrol. 2017;28:1216–1226. doi: 10.1681/ASN.2016040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barrera-Chimal J., Estrela G.R., Lechner S.M., et al. The myeloid mineralocorticoid receptor controls inflammatory and fibrotic responses after renal injury via macrophage interleukin-4 receptor signaling. Kidney Int. 2018;93:1344–1355. doi: 10.1016/j.kint.2017.12.016. [DOI] [PubMed] [Google Scholar]
- 11.Barrera-Chimal J., Rocha L., Amador-Martinez I., et al. Delayed spironolactone administration prevents the transition from acute kidney injury to chronic kidney disease through improving renal inflammation. Nephrol Dial Transplant. 2019;34:794–801. doi: 10.1093/ndt/gfy246. [DOI] [PubMed] [Google Scholar]
- 12.Chen D., Chen Z., Park C., et al. Aldosterone stimulates fibronectin synthesis in renal fibroblasts through mineralocorticoid receptor-dependent and independent mechanisms. Gene. 2013;531:23–30. doi: 10.1016/j.gene.2013.08.047. [DOI] [PubMed] [Google Scholar]
- 13.Bowers S.L.K., Davis-Rodriguez S., Thomas Z.M., et al. Inhibition of fibronectin polymerization alleviates kidney injury due to ischemia-reperfusion. Am J Physiol Renal Physiol. 2019;316:F1293–F1298. doi: 10.1152/ajprenal.00117.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nishiyama A. Pathophysiological mechanisms of mineralocorticoid receptor-dependent cardiovascular and chronic kidney disease. Hypertens Res. 2019;42:293–300. doi: 10.1038/s41440-018-0158-6. [DOI] [PubMed] [Google Scholar]
- 15.Laursen S.B., Finsen S., Marcussen N., et al. Endothelial mineralocorticoid receptor ablation does not alter blood pressure, kidney function or renal vessel contractility. PLoS One. 2018;13 doi: 10.1371/journal.pone.0193032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galmiche G., Pizard A., Gueret A., et al. Smooth muscle cell mineralocorticoid receptors are mandatory for aldosterone-salt to induce vascular stiffness. Hypertension. 2014;63:520–526. doi: 10.1161/HYPERTENSIONAHA.113.01967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang L.L., Nikolic-Paterson D.J., Han Y., et al. Myeloid mineralocorticoid receptor activation contributes to progressive kidney disease. J Am Soc Nephrol. 2014;25:2231–2240. doi: 10.1681/ASN.2012111094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin J.S., Susztak K. Podocytes: the weakest link in diabetic kidney disease? Curr Diab Rep. 2016;16:45. doi: 10.1007/s11892-016-0735-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shibata S., Ishizawa K., Uchida S. Mineralocorticoid receptor as a therapeutic target in chronic kidney disease and hypertension. Hypertens Res. 2017;40:221–225. doi: 10.1038/hr.2016.137. [DOI] [PubMed] [Google Scholar]
- 20.Tang C., Livingston M.J., Liu Z., et al. Autophagy in kidney homeostasis and disease. Nat Rev Nephrol. 2020;16:489–508. doi: 10.1038/s41581-020-0309-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dong D., Fan T.T., Ji Y.S., et al. Spironolactone alleviates diabetic nephropathy through promoting autophagy in podocytes. Int Urol Nephrol. 2019;51:755–764. doi: 10.1007/s11255-019-02074-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li D., Lu Z., Xu Z., et al. Spironolactone promotes autophagy via inhibiting PI3K/AKT/mTOR signalling pathway and reduce adhesive capacity damage in podocytes under mechanical stress. Biosci Rep. 2016;36 doi: 10.1042/BSR20160086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu J.J., Chen Y.P., Yang M., et al. Aldosterone is involved in the pathogenesis of obesity-related glomerulopathy through activation of Wnt/beta-catenin signaling in podocytes. Mol Med Rep. 2018;17:4589–4598. doi: 10.3892/mmr.2018.8386. [DOI] [PubMed] [Google Scholar]
- 24.Anders H.J., Huber T.B., Isermann B., et al. CKD in diabetes: diabetic kidney disease versus nondiabetic kidney disease. Nat Rev Nephrol. 2018;14:361–377. doi: 10.1038/s41581-018-0001-y. [DOI] [PubMed] [Google Scholar]
- 25.Martens C.R., Kirkman D.L., Edwards D.G. The vascular endothelium in chronic kidney disease: a novel target for aerobic exercise. Exerc Sport Sci Rev. 2016;44:12–19. doi: 10.1249/JES.0000000000000065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amador-Martinez I., Perez-Villalva R., Uribe N., et al. Reduced endothelial nitric oxide synthase activation contributes to cardiovascular injury during chronic kidney disease progression. Am J Physiol Renal Physiol. 2019;317:F275–F285. doi: 10.1152/ajprenal.00020.2019. [DOI] [PubMed] [Google Scholar]
- 27.Davel A.P., Anwar I.J., Jaffe I.Z. The endothelial mineralocorticoid receptor: mediator of the switch from vascular health to disease. Curr Opin Nephrol Hypertens. 2017;26:97–104. doi: 10.1097/MNH.0000000000000306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caprio M., Newfell B.G., la Sala A., et al. Functional mineralocorticoid receptors in human vascular endothelial cells regulate intercellular adhesion molecule-1 expression and promote leukocyte adhesion. Circ Res. 2008;102:1359–1367. doi: 10.1161/CIRCRESAHA.108.174235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hashikabe Y., Suzuki K., Jojima T., et al. Aldosterone impairs vascular endothelial cell function. J Cardiovasc Pharmacol. 2006;47:609–613. doi: 10.1097/01.fjc.0000211738.63207.c3. [DOI] [PubMed] [Google Scholar]
- 30.Taye A., Morawietz H. Spironolactone inhibits NADPH oxidase-induced oxidative stress and enhances eNOS in human endothelial cells. Iran J Pharm Res. 2011;10:329–337. [PMC free article] [PubMed] [Google Scholar]
- 31.Iwashima F., Yoshimoto T., Minami I., et al. Aldosterone induces superoxide generation via Rac1 activation in endothelial cells. Endocrinology. 2008;149:1009–1014. doi: 10.1210/en.2007-0864. [DOI] [PubMed] [Google Scholar]
- 32.Nagata D., Takahashi M., Sawai K., et al. Molecular mechanism of the inhibitory effect of aldosterone on endothelial NO synthase activity. Hypertension. 2006;48:165–171. doi: 10.1161/01.HYP.0000226054.53527.bb. [DOI] [PubMed] [Google Scholar]
- 33.Amador C.A., Bertocchio J.P., Andre-Gregoire G., et al. Deletion of mineralocorticoid receptors in smooth muscle cells blunts renal vascular resistance following acute cyclosporine administration. Kidney Int. 2016;89:354–362. doi: 10.1038/ki.2015.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lother A., Fürst D., Bergemann S., et al. Deoxycorticosterone acetate/salt-induced cardiac but not renal injury is mediated by endothelial mineralocorticoid receptors independently from blood pressure. Hypertension. 2016;67:130–138. doi: 10.1161/HYPERTENSIONAHA.115.06530. [DOI] [PubMed] [Google Scholar]
- 35.Aroor A.R., Habibi J., Nistala R., et al. Diet-induced obesity promotes kidney endothelial stiffening and fibrosis dependent on the endothelial mineralocorticoid receptor. Hypertension. 2019;73:849–858. doi: 10.1161/HYPERTENSIONAHA.118.12198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCurley A., Pires P.W., Bender S.B., et al. Direct regulation of blood pressure by smooth muscle cell mineralocorticoid receptors. Nat Med. 2012;18:1429–1433. doi: 10.1038/nm.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lattenist L., Lechner S.M., Messaoudi S., et al. Nonsteroidal mineralocorticoid receptor antagonist finerenone protects against acute kidney injury-mediated chronic kidney disease: role of oxidative stress. Hypertension. 2017;69:870–878. doi: 10.1161/HYPERTENSIONAHA.116.08526. [DOI] [PubMed] [Google Scholar]
- 38.Barrera-Chimal J., Prince S., Fadel F., et al. Sulfenic acid modification of endothelin B receptor is responsible for the benefit of a nonsteroidal mineralocorticoid receptor antagonist in renal ischemia. J Am Soc Nephrol. 2016;27:398–404. doi: 10.1681/ASN.2014121216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Callera G.E., Touyz R.M., Tostes R.C., et al. Aldosterone activates vascular p38MAP kinase and NADPH oxidase via c-Src. Hypertension. 2005;45:773–779. doi: 10.1161/01.HYP.0000154365.30593.d3. [DOI] [PubMed] [Google Scholar]
- 40.Jaffe I.Z., Tintut Y., Newfell B.G., et al. Mineralocorticoid receptor activation promotes vascular cell calcification. Arterioscler Thromb Vasc Biol. 2007;27:799–805. doi: 10.1161/01.ATV.0000258414.59393.89. [DOI] [PubMed] [Google Scholar]
- 41.Usher M.G., Duan S.Z., Ivaschenko C.Y., et al. Myeloid mineralocorticoid receptor controls macrophage polarization and cardiovascular hypertrophy and remodeling in mice. J Clin Invest. 2010;120:3350–3364. doi: 10.1172/JCI41080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jo S.K., Sung S.A., Cho W.Y., et al. Macrophages contribute to the initiation of ischaemic acute renal failure in rats. Nephrol Dial Transplant. 2006;21:1231–1239. doi: 10.1093/ndt/gfk047. [DOI] [PubMed] [Google Scholar]
- 43.Vinuesa E., Hotter G., Jung M., et al. Macrophage involvement in the kidney repair phase after ischaemia/reperfusion injury. J Pathol. 2008;214:104–113. doi: 10.1002/path.2259. [DOI] [PubMed] [Google Scholar]
- 44.Koszegi S., Molnar A., Lenart L., et al. RAAS inhibitors directly reduce diabetes-induced renal fibrosis via growth factor inhibition. J Physiol. 2019;597:193–209. doi: 10.1113/JP277002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nagai Y., Miyata K., Sun G.P., et al. Aldosterone stimulates collagen gene expression and synthesis via activation of ERK1/2 in rat renal fibroblasts. Hypertension. 2005;46:1039–1045. doi: 10.1161/01.HYP.0000174593.88899.68. [DOI] [PubMed] [Google Scholar]
- 46.Huang L.L., Nikolic-Paterson D.J., Ma F.Y., et al. Aldosterone induces kidney fibroblast proliferation via activation of growth factor receptors and PI3K/MAPK signalling. Nephron Exp Nephrol. 2012;120:e115–e122. doi: 10.1159/000339500. [DOI] [PubMed] [Google Scholar]
- 47.van Leeuwen N., Caprio M., Blaya C., et al. The functional c.-2G>C variant of the mineralocorticoid receptor modulates blood pressure, renin, and aldosterone levels. Hypertension. 2010;56:995–1002. doi: 10.1161/HYPERTENSIONAHA.110.155630. [DOI] [PubMed] [Google Scholar]
- 48.Schierke F., Wyrwoll M.J., Wisdorf M., et al. Nanomechanics of the endothelial glycocalyx contribute to Na(+)-induced vascular inflammation. Sci Rep. 2017;7:46476. doi: 10.1038/srep46476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fels J., Oberleithner H., Kusche-Vihrog K. Menage a trois: aldosterone, sodium and nitric oxide in vascular endothelium. Biochim Biophys Acta. 2010;1802:1193–1202. doi: 10.1016/j.bbadis.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 50.Jia G., Habibi J., Aroor A.R., et al. Epithelial sodium channel in aldosterone-induced endothelium stiffness and aortic dysfunction. Hypertension. 2018;72:731–738. doi: 10.1161/HYPERTENSIONAHA.118.11339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xiong Y., Aroor A.R., Ramirez-Perez F.I., et al. Western diet induces renal artery endothelial stiffening that is dependent on the epithelial Na(+) channel. Am J Physiol Renal Physiol. 2020;318:F1220–F1228. doi: 10.1152/ajprenal.00517.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tarjus A., Gonzalez-Rivas C., Amador-Martinez I., et al. The absence of endothelial sodium channel alpha (αENaC) reduces renal ischemia/reperfusion injury. Int J Mol Sci. 2019;20:3132. doi: 10.3390/ijms20133132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bonnard B., Martinez-Martinez E., Fernandez-Celis A., et al. Antifibrotic effect of novel neutrophil gelatinase-associated lipocalin inhibitors in cardiac and renal disease models. Sci Rep. 2021;11:2591. doi: 10.1038/s41598-021-82279-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Latouche C., El Moghrabi S., Messaoudi S., et al. Neutrophil gelatinase-associated lipocalin is a novel mineralocorticoid target in the cardiovascular system. Hypertension. 2012;59:966–972. doi: 10.1161/HYPERTENSIONAHA.111.187872. [DOI] [PubMed] [Google Scholar]
- 55.Tarjus A., Martínez-Martínez E., Amador C., et al. Neutrophil gelatinase-associated lipocalin, a novel mineralocorticoid biotarget, mediates vascular profibrotic effects of mineralocorticoids. Hypertension. 2015;66:158–166. doi: 10.1161/HYPERTENSIONAHA.115.05431. [DOI] [PubMed] [Google Scholar]
- 56.Buonafine M., Martinez-Martinez E., Amador C., et al. Neutrophil gelatinase-associated lipocalin from immune cells is mandatory for aldosterone-induced cardiac remodeling and inflammation. J Mol Cell Cardiol. 2018;115:32–38. doi: 10.1016/j.yjmcc.2017.12.011. [DOI] [PubMed] [Google Scholar]
- 57.Araos P., Prado C., Lozano M., et al. Dendritic cells are crucial for cardiovascular remodeling and modulate neutrophil gelatinase-associated lipocalin expression upon mineralocorticoid receptor activation. J Hypertens. 2019;37:1482–1492. doi: 10.1097/HJH.0000000000002067. [DOI] [PubMed] [Google Scholar]
- 58.Viau A., El Karoui K., Laouari D., et al. Lipocalin 2 is essential for chronic kidney disease progression in mice and humans. J Clin Invest. 2010;120:4065–4076. doi: 10.1172/JCI42004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.El Karoui K., Viau A., Dellis O., et al. Endoplasmic reticulum stress drives proteinuria-induced kidney lesions via lipocalin 2. Nat Commun. 2016;7:10330. doi: 10.1038/ncomms10330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pawar R.D., Pitashny M., Gindea S., et al. Neutrophil gelatinase-associated lipocalin is instrumental in the pathogenesis of antibody-mediated nephritis in mice. Arthritis Rheum. 2012;64:1620–1631. doi: 10.1002/art.33485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maron B.A., Zhang Y.Y., Handy D.E., et al. Aldosterone increases oxidant stress to impair guanylyl cyclase activity by cysteinyl thiol oxidation in vascular smooth muscle cells. J Biol Chem. 2009;284:766572. doi: 10.1074/jbc.M809460200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Callera G.E., Montezano A.C., Yogi A., et al. c-Src-dependent nongenomic signaling responses to aldosterone are increased in vascular myocytes from spontaneously hypertensive rats. Hypertension. 2005;46:1032–1038. doi: 10.1161/01.HYP.0000176588.51027.35. [DOI] [PubMed] [Google Scholar]
- 63.Calvier L., Martinez-Martinez E., Miana M., et al. The impact of galectin-3 inhibition on aldosterone-induced cardiac and renal injuries. JACC Heart Fail. 2015;3:59–67. doi: 10.1016/j.jchf.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 64.Sierra-Ramos C., Velazquez-Garcia S., Keskus A.G., et al. Increased SGK1 activity potentiates mineralocorticoid/NaCl-induced kidney injury. Am J Physiol Renal Physiol. 2021;320:F628–F643. doi: 10.1152/ajprenal.00505.2020. [DOI] [PubMed] [Google Scholar]
- 65.Luther J.M., Fogo A.B. The role of mineralocorticoid receptor activation in kidney inflammation and fibrosis. Kidney Int Suppl. 2022;12:63–68. doi: 10.1016/j.kisu.2021.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rossing P. Clinical perspective—evolving evidence of mineralocorticoid receptor antagonists in patients with chronic kidney disease and type 2 diabetes. Kidney Int Suppl. 2022;12:27–35. doi: 10.1016/j.kisu.2021.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]