Abstract
Aldosterone controls salt–water homeostasis by acting on the mineralocorticoid receptor (MR), a ligand-activated transcription factor, in kidney epithelial cells. However, it is now evident that the MR is expressed in multiple cell types and tissues, acting as a key driver of cardiovascular disease. MR antagonists have proven to be highly efficient in patients with heart failure and reduced ejection fraction, and they are a cornerstone of contemporary therapy. In the past decade, a series of experimental studies using models with cell type–specific MRs uncovered the cellular and molecular mechanisms underlying its detrimental effect on left ventricular remodeling. Based on these findings, the potential of MR antagonists has been evaluated in other cardiovascular diseases, including coronary artery disease, arterial hypertension, heart failure with preserved ejection fraction, pulmonary hypertension, atrial fibrillation, and heart valve disease. The present review summarizes the current knowledge on MR activation and antagonism in cardiovascular disease.
Keywords: aldosterone, arterial hypertension, heart failure, mineralocorticoid receptor, myocardial infarction, pulmonary hypertension
Graphical abstract
Aldosterone, a steroid hormone produced by zona glomerulosa cells of the adrenal cortex, is a central effector hormone of the renin–angiotensin–aldosterone system.1,2 The physiological role of aldosterone is to control salt–water homeostasis by acting on the mineralocorticoid receptor (MR), a ligand-activated transcription factor, in kidney epithelial cells. Aldosterone via the MR leads to an upregulation and activation of the amiloride-sensitive epithelial Na+ channel, thereby increasing Na+ reabsorption and K+ secretion.2 The first MR antagonist (MRA), spironolactone, was developed as an antihypertensive drug, with the intention to prevent Na+ retention and decrease blood volume.3,4 However, because of its activity at the progesterone receptor and other nuclear receptors, spironolactone may cause relevant side effects, such as gynecomastia.3 This effect could be ameliorated by the second-generation compound eplerenone and, more recently, a new class of highly selective, potent nonsteroidal MRAs. such as finerenone and esaxerenone.3,4
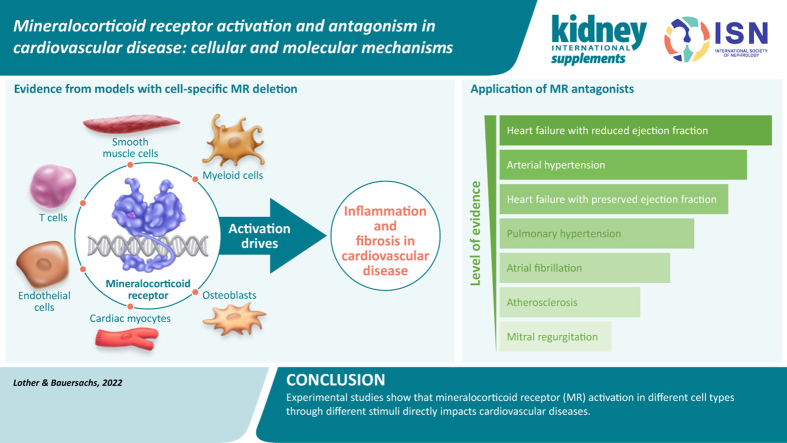
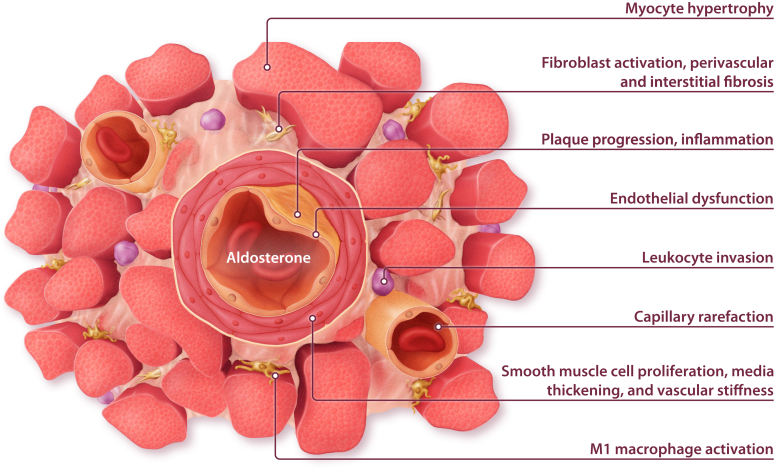
The protective cardiovascular effect of MRAs was first attributed to their effects on diuresis, blood volume, and electrolyte homeostasis.5 However, the MR is expressed in multiple cell types and tissues outside the kidney, and it is now evident that MR in extrarenal tissues is a key driver of disease (Figure 1).6,7 More than 20 years ago, major clinical trials provided evidence that MRA treatment improves mortality and morbidity in patients with heart failure with reduced ejection fraction (HFrEF), leading to a class IA guideline recommendation.8, 9, 10 Since then, a series of experimental studies uncovered the cellular and molecular mechanisms underlying the beneficial effect on left ventricular (LV) remodeling. Based on these findings, the potential of MRAs has been evaluated in other cardiovascular diseases, including coronary artery disease, arterial hypertension, heart failure with preserved ejection fraction (HFpEF), pulmonary hypertension (PH), and heart valve disease. The present review summarizes the current knowledge on MR activation and antagonism in cardiovascular disease.
Figure 1.
Biological effects of mineralocorticoid receptor activation in the cardiovascular system.
HFrEF and post–myocardial infarction remodeling
MRAs are established drugs in the treatment of chronic HFrEF, as evidenced in multiple studies.8 The Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure (EMPHASIS-HF) demonstrated a reduction in mortality by 24% in patients with HFrEF and mild symptoms treated with eplerenone versus placebo.11 In the Randomized Aldactone Evaluation Study (RALES), spironolactone had a similar effect in patients with severe heart failure symptoms, in whom mortality was reduced by 30% versus placebo.12 Studies have shown that, like spironolactone and eplerenone, the nonsteroidal MRA finerenone reduced levels of pro-B-type natriuretic peptide (BNP) or N-terminal BNP (NT-proBNP) in phase II trials.13,14 Early initiation of MRA treatment in patients with acute heart failure was found to be safe and well tolerated.15,16 Eplerenone improved outcomes of patients with impaired LV function after myocardial infarction (MI).17 Subsequent studies tested the hypothesis that initiation of MR blockade early after MI might prevent cardiac remodeling and the occurrence of heart failure. When initiated within 72 hours after symptom onset, MRA treatment improved BNP/NT-proBNP levels in patients without preexisting heart failure.18 However, in a later study, a potential benefit of early MRA treatment on clinical outcomes was observed only in the subgroup of high-risk patients with ST-elevation MI.19 An individual patient-level meta-analysis of 3 large randomized controlled trials in patients with HFrEF also demonstrated a 23% reduction in sudden cardiac death with MRA treatment.20 In patients with newly diagnosed HFrEF, treatment with higher MRA dosages was associated with superior amelioration of LV ejection fraction beyond 3 months.21
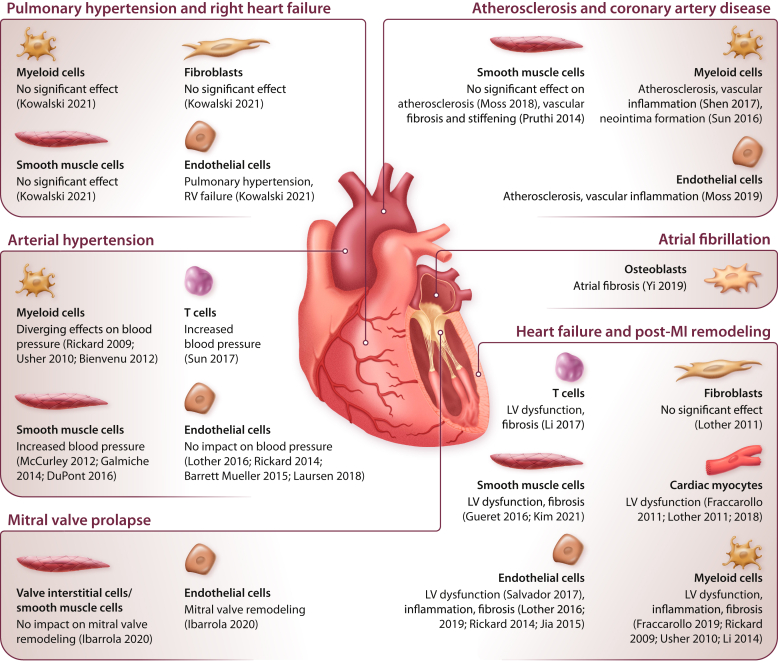
Experimental studies using MRAs in animal models of heart failure and post-MI remodeling demonstrated beneficial effects on cardiac hypertrophy, fibrosis, or both (Figure 1).22, 23, 24, 25 Subsequently, the use of mouse models with cell-specific MR deletion provided evidence that these effects were mediated by MR activation in cardiovascular cells. MR deletion from cardiac myocytes resulted in smaller scar size, less fibrosis of the remote tissue, and improved LV function.26 Reduced fibrosis after ischemic injury was associated with attenuated oxidative stress and myocyte apoptosis, but higher numbers of neutrophils and monocytes were detected in myocardial tissue from MR-deficient mice compared with wild-type mice.26 Notably, MR deletion from myeloid cells likewise improved LV remodeling and induced a shift toward the more-reparative M2 macrophage subtype.27 MR deletion from smooth muscle cells (SMCs) attenuated LV fibrosis but had minor effects on LV function.25 This implies that MRAs have effects on different cell types that synergistically contribute to damage control and healing after MI.
The centrality of inflammation in mediating the deleterious effect of MR activation has been confirmed in models of chronic heart failure (Figure 2).25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54 MR deletion from myeloid cells prevented cardiac remodeling in response to pressure overload or N(G)-nitro-L-arginine methyl ester (L-NAME)/angiotensin II infusion.28,29 Similar effects were observed in mice lacking MRs in T cells.30 Very recently, SMC MR deletion was shown to improve pressure overload–induced LV hypertrophy, inflammation, fibrosis, and dysfunction.31 MR deletion from endothelial cells or cardiac myocytes improved LV function, but in contrast to ischemic injury, it did not regulate fibrosis after pressure overload.32,33 No differences were detected after MR deletion from fibroblasts.32 These findings suggest that the impact of the MR on cardiac remodeling depends on not only the cell type but also the type of injury.
Figure 2.
Cell type–specific function of the mineralocorticoid receptor (MR) in cardiovascular disease.25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54 Function of the MR in different cardiovascular cell types and diseases as revealed by experimental studies using mouse models with cell type-specific MR deletion. LV, left ventricular; MI, myocardial infarction; MR, mineralocorticoid receptor; RV, right ventricular.
Substantial efforts have been made to decipher molecular regulatory mechanisms behind aldosterone/MR-induced LV remodeling. Well-characterized inflammatory and fibrotic effector molecules of the MR in the cardiovascular system include galectin 3 (LGALS3) and lipocalin 2 (NGAL).55, 56, 57, 58 Intriguingly, pharmacologic inhibition by modified citrus pectin or genetic deletion of galectin 3 attenuated aldosterone-induced cardiac remodeling.55,56 Plasma levels of NGAL were positively correlated with circulating aldosterone levels and fibrosis biomarkers in humans.57 Deletion of NGAL from immune cells prevented LV fibrosis in response to aldosterone infusion.58 Likewise, MR deletion from myeloid cells improved cardiac remodeling after myocardial infarction, which was associated with reduced NGAL expression in cardiac macrophages.27 A recent high-throughput screening of microRNAs identified miR-181a as a crucial regulator of MR signaling.59 miR-181a overexpression downregulated NGAL expression in vitro and in vivo and improved cardiac function in a rodent MI model.59
HFpEF
The prevalence of HFpEF is increasing and already accounts for more than 50% of heart failure cases.60 Despite overlapping symptoms, HFpEF is considered to be a separate entity from HFrEF.60 Compared with patients with HFrEF, patients with HFpEF are older, more often female and obese, and have more comorbidities, such as diabetes and kidney disease, that are associated with chronic inflammation.60,61 MR activation increases oxidative stress and impairs nitric oxide (NO) signaling, leading to endothelial dysfunction, inflammation, and perivascular fibrosis.34 Although the ideal preclinical model to study HFpEF remains to be defined, a clear finding is that MR activation is associated with many of the pathophysiological features that characterize HFpEF.62,63 MRAs improved diastolic dysfunction induced by obesity, ovariectomy, nephrectomy, or deoxycorticosterone acetate (DOCA)/salt hypertension in mice.64, 65, 66, 67 Cell type–specific MR deletion from cardiac myocytes attenuated leukocyte invasion and fibrosis after DOCA treatment.68 In line with the paradigm of systemic inflammation in HFpEF, MR deletion from endothelial cells or myeloid cells demonstrated the most striking effect on cardiac remodeling (Figure 2).35, 36, 37, 38,54,69
Early clinical trials suggested beneficial effects of MRAs in patients with HFpEF.70, 71, 72 Thus, it was unexpected that spironolactone failed to improve the composite primary outcome of death from cardiovascular causes, aborted cardiac arrest, or hospitalization for heart failure in the large phase III Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) trial.73 However, serious concerns about study conduct call into question the validity of the study.74,75 In the FInerenone in reducinG cArdiovascular moRtality and mOrbidity in Diabetic Kidney Disease (FIGARO-DKD) trial, the non-steroidal MRA finerenone reduced the incidence of cardiovascular events in patients with diabetic kidney disease,76 a patient group at high risk for developing HFpEF.60 Notably, the beneficial effect of finerenone was predominantly driven by a lower rate of hospitalization for heart failure, although patients with preexisting HFrEF were excluded from the trial.76 Two additional phase III clinical trials comparing spironolactone (Spironolactone in the Treatment of Heart Failure [SPIRIT-HF]; NCT04727073; EudraCT 2017-000697-11) and finerenone (Finerenone Trial to Investigate Efficacy and Safety Superior to Placebo in Patients with Heart Failure [FINEARTS-HF]; NCT04435626) with placebo in patients with heart failure and mid-range or preserved ejection fraction are currently ongoing.
In the Heart ‘OMics’ in AGEing (HOMAGE) trial, spironolactone reduced synthesis and increased degradation of type I collagen, and reduced blood pressure, left atrial volume, and BNP levels in people at risk for HFpEF.77 Whether early MRA treatment is able to delay occurrence of heart failure in such populations remains to be determined.
Atherosclerosis and coronary artery disease
Atherosclerosis and coronary artery disease are considered chronic inflammatory diseases,78 and the strong influence of MR on vascular inflammation described above suggests a role for the MR in their pathophysiology. In the apolipoprotein E knockout mouse model, aldosterone infusion exacerbated atherosclerosis development.79 Conversely, MRAs attenuated inflammation and formation of reactive oxygen species but improved NO bioavailability and vascular function in obesity models.65, 80, 81, 82, 83 Aldosterone effects on monocyte recruitment and plaque inflammation were attenuated in mice lacking placental growth factor79 or intercellular adhesion molecule 1,84 indicating an interaction of endothelial cells and monocytes in the process. In vitro, MR promoted the expression of inflammatory molecules in endothelial cells and SMCs.39,54,85, 86, 87 In vivo, MR deletion from endothelial cells or myeloid cells, but not from SMCs, ameliorated vascular inflammation in mouse models of atherosclerosis.39, 40, 41 In addition, stimulation of monocytes with aldosterone augmented inflammatory cytokine production, depending on an upregulation of the fatty acid synthesis pathway.88 The growing body of literature on the function of myeloid cell MRs in innate immunity and atherosclerosis has been summarized by van der Heijden et al. (2018).89 Aside from their effects in atherosclerosis, MRA treatment and MR deletion from SMC or myeloid cells yielded beneficial effects on vascular remodeling following mechanical injury,42,43,90 indicating a potential benefit of MRAs on postangioplasty restenosis. Despite this compelling experimental evidence, data from clinical trials on MRAs in atherosclerosis are still scarce.91 The recent Finerenone in Reducing Kidney Failure and Disease Progression in Diabetic Kidney Disease (FIDELIO-DKD) trial showed for the first time that treatment with the novel MRA finerenone could reduce the incidence of MI in patients with diabetic kidney disease,92 indicating a potential role of MRAs in the primary prevention of cardiovascular events.
Arterial hypertension
The impact of aldosterone and MR on arterial hypertension has been recognized for decades. For instance, aldosterone infusion substantially increases blood pressure in uninephrectomized rats receiving a high-salt diet.93,94 In addition to its effects on Na+ and fluid retention, aldosterone controls blood pressure via the MR in cells outside the kidney.95 Endothelial MR activation induced the production of reactive oxygen species and impaired endothelium-dependent vascular relaxation.44,80 Notably, these effects were more pronounced in female mice, compared with male mice.96 MR overexpression led to a moderate increase in blood pressure.97 However, MR deletion from endothelial cells did not alter blood pressure at baseline or in response to stimulus,36,44,45,69 indicating that the MR in endothelial cells at physiological expression levels is dispensable for blood pressure control. In contrast, numerous studies point at MRs in SMCs as a key determinant of vascular stiffness and hypertension, particularly in aged mice (Figure 2).46, 47, 48,98 It was suggested that the MR via repression of miR-155 enhances the expression and activity of L-type Ca2+ channels in SMCs, leading to an increase in vascular tone.46,47 Angiotensin II-induced hypertension and subsequent organ injury were markedly suppressed by MR deletion from T cells.49 Regarding the role of MR in myeloid cells, partly contradictory results have been reported on the blood pressure response to DOCA/salt hypertension37 or angiotensin II/L-NAME.29,38 Spironolactone proved effective in patients with resistant hypertension in multiple clinical trials and is now recommended in patients with sustained hypertension despite triple therapy.99,100 The nonsteroidal MRA esaxerenone had effectiveness similar to that of eplerenone in lowering blood pressure and is now approved in Japan for the treatment of essential hypertension.101,102 In a recent phase 2b trial, the nonsteroidal MRA KBP-5074 was able to significantly lower blood pressure in patients with chronic kidney disease and uncontrolled hypertension despite treatment including a renin–angiotensin system inhibitor.103 In contrast, in patients with chronic kidney disease and well controlled hypertension, finerenone had only minor additional effects on blood pressure.76,104
PH and right heart failure
Increased plasma aldosterone levels have been observed in patients with PH and in mice after exposure to chronic hypoxia,50,105,106 hinting at a role for the MR in pulmonary vascular remodeling. In fact, aldosterone stimulation induces PH phenotypes in vivo and in cultured SMCs or endothelial cells in vitro.50,107, 108, 109 MRAs improve vascular remodeling and right ventricular function induced by chronic hypoxia or monocrotaline in mice and rats.50,107,110 However, MRA treatment had no effect in a pulmonary artery banding model, implying that the benefit of MRAs on right ventricular function can be indirectly explained by the reduced afterload.110 Experimental studies using mice with cell type–specific MR deletion revealed that the detrimental effect of aldosterone on the pulmonary vasculature is mediated by the MR in endothelial cells rather than SMCs, fibroblasts, or macrophages (Figure 2).50 Gene expression analyses and in vitro studies point at an interaction between endothelial cells and other cell types in the process, involving the endothelin-1 signaling pathway and paracrine crosstalk via exosomes.50,108,109,111 A post hoc analysis from the Ambrisentan for the Treatment of Pulmonary Arterial Hypertension (ARIES) 1 and 2 trials suggested a beneficial effect of spironolactone when added to the endothelin-1 receptor antagonist ambrisentan in patients with PH.112 A prospective randomized phase 2 clinical trial on MRA use in PH is currently ongoing (NCT01712620).
Potential future directions
Knowledge of MR effects in cardiovascular disease continues to expand, pointing to new potential indications for MRAs. The availability of new, nonsteroidal MRAs may further broaden the spectrum of indications and enable clinical use of MRAs in high-risk patient populations.4 Preclinical and early clinical data suggest that MRAs may be effective in preventing chemotherapy-induced cardiotoxicity, a relevant side effect of anticancer drugs leading to LV failure, in female patients.51,113,114 Additionally, growing evidence indicates that MR activation causes adverse remodeling of not only the ventricles, but also the atria. Patients with primary aldosteronism are at higher risk of developing atrial fibrillation compared with patients with essential hypertension.115 Intriguingly, atrial fibrosis induced by transforming growth factor β was attenuated by MR deletion in osteoblasts (Figure 2).52 In various experimental models, MRAs reduced atrial fibrosis and thus the burden of atrial arrhythmia,116, 117, 118 suggesting a potential benefit of MRAs in patients with atrial fibrillation. In line with this possibility, a meta-analysis of clinical trials revealed a substantial reduction in the occurrence of atrial fibrillation in MRA-treated patients, compared with control groups.119
Mitral regurgitation is a common heart valve disorder often associated with structural deterioration and a disturbed extracellular matrix of the mitral valve leaflets.120 Recent evidence suggests that aldosterone, by activating the MR, drives proteoglycan production by interstitial cells and endothelial-to-mesenchymal transition in mitral valves.53 In mice, MRA treatment or MR deletion in endothelial cells attenuated mitral valve remodeling.53 This effect was accompanied by decreased expression of fibrotic markers in LV tissue in mice treated with spironolactone.121 Although currently limited to interventional or surgical repair, MRAs may thus represent a new treatment option for mitral regurgitation.6,120
Conclusions
Evidence is accumulating from a number of experimental studies demonstrating that MRs in cardiac myocytes, endothelial cells, SMCs, myeloid cells, T cells, and osteoblasts have direct impact on heart failure and other cardiovascular diseases. Depending on the type of disease or stimulus, different cell types have MRs with distinct functions that contribute to the net effect of inflammation and fibrosis following activation. The available insights discussed in this review will provide the basis for further development and the evaluation of classical and novel MRAs for additional cardiovascular indications.
Disclosure
This article is published as part of a supplement sponsored by Bayer AG.
AL received fees for lectures and/or serving on advisory boards from AstraZeneca and Bayer. JB received honoraria for lectures/consulting from Abiomed, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Cardior, CVRx, Daiichi-Sankyo, Medtronic, MSD, Novartis, Orion, Pfizer, Servier, and Vifor; and research support from Abiomed, CVRx, Vifor, and Zoll, unrelated to this article. JB and AL received no personal funding for this article.
Acknowledgments
This work was supported by the German Research Foundation (Deutsche Forschungsgemeinschaft, Germany, DFG SFB 1425 – ID 422681845, and DFG – ID 470188766). Development of this article was funded by an unrestricted educational grant from Bayer AG. The authors acknowledge Onyx Adesakin, PhD, of Chameleon Communications International, who provided editorial assistance with funding via an unrestricted educational grant from Bayer AG. The authors also acknowledge Alexander Roeder, Ronny Guenther, Katja Marx, Martin Bajcsik, and Josephin Schoenrich, of CAST PHARMA, who designed the figures with funding from Bayer AG, Germany.
Contributor Information
Johann Bauersachs, Email: bauersachs.johann@mh-hannover.de.
Achim Lother, Email: achim.lother@uniklinik-freiburg.de.
References
- 1.Lother A., Moser M., Bode C., et al. Mineralocorticoids in the heart and vasculature: new insights for old hormones. Annu Rev Pharmacol Toxicol. 2015;55:289–312. doi: 10.1146/annurev-pharmtox-010814-124302. [DOI] [PubMed] [Google Scholar]
- 2.Shibata S. 30 years of the mineralocorticoid receptor: mineralocorticoid receptor and NaCl transport mechanisms in the renal distal nephron. J Endocrinol. 2017;234:T35–T47. doi: 10.1530/JOE-16-0669. [DOI] [PubMed] [Google Scholar]
- 3.Kolkhof P., Barfacker L. 30 years of the mineralocorticoid receptor: mineralocorticoid receptor antagonists: 60 years of research and development. J Endocrinol. 2017;234:T125–T140. doi: 10.1530/JOE-16-0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agarwal R., Kolkhof P., Bakris G., et al. Steroidal and non-steroidal mineralocorticoid receptor antagonists in cardiorenal medicine. Eur Heart J. 2021;42:152–161. doi: 10.1093/eurheartj/ehaa736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Struthers A.D. Why does spironolactone improve mortality over and above an ACE inhibitor in chronic heart failure? Br J Clin Pharmacol. 1999;47:479–482. doi: 10.1046/j.1365-2125.1999.00954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lother A. Mineralocorticoid receptors: master regulators of extracellular matrix remodeling. Circ Res. 2020;127:354–356. doi: 10.1161/CIRCRESAHA.120.317424. [DOI] [PubMed] [Google Scholar]
- 7.Bauersachs J., Jaisser F., Toto R. Mineralocorticoid receptor activation and mineralocorticoid receptor antagonist treatment in cardiac and renal diseases. Hypertension. 2015;65:257–263. doi: 10.1161/HYPERTENSIONAHA.114.04488. [DOI] [PubMed] [Google Scholar]
- 8.Ponikowski P., Voors A.A., Anker S.D., et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 9.Berliner D., Hanselmann A., Bauersachs J. The treatment of heart failure with reduced ejection fraction. Dtsch Arztebl Int. 2020;117:376–386. doi: 10.3238/arztebl.2020.0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lother A., Hein L. Pharmacology of heart failure: from basic science to novel therapies. Pharmacol Ther. 2016;166:136–149. doi: 10.1016/j.pharmthera.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 11.Zannad F., McMurray J.J., Krum H., et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364:11–21. doi: 10.1056/NEJMoa1009492. [DOI] [PubMed] [Google Scholar]
- 12.Pitt B., Zannad F., Remme W.J., et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study investigators. N Engl J Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 13.Pitt B., Kober L., Ponikowski P., et al. Safety and tolerability of the novel non-steroidal mineralocorticoid receptor antagonist BAY 94-8862 in patients with chronic heart failure and mild or moderate chronic kidney disease: a randomized, double-blind trial. Eur Heart J. 2013;34:2453–2463. doi: 10.1093/eurheartj/eht187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Filippatos G., Anker S.D., Bohm M., et al. A randomized controlled study of finerenone vs. eplerenone in patients with worsening chronic heart failure and diabetes mellitus and/or chronic kidney disease. Eur Heart J. 2016;37:2105–2114. doi: 10.1093/eurheartj/ehw132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Butler J., Anstrom K.J., Felker G.M., et al. Efficacy and safety of spironolactone in acute heart failure: the ATHENA-HF randomized clinical trial. JAMA Cardiol. 2017;2:950–958. doi: 10.1001/jamacardio.2017.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Asakura M, Ito S, Yamada T, et al. Efficacy and Safety of Early Initiation of Eplerenone Treatment in Patients with Acute Heart Failure (EARLIER trial): a multicenter, randomized, double-blind, placebo-controlled trial. Eur Heart J Cardiovasc Pharmacother. 2022;8:108–117. [DOI] [PubMed]
- 17.Pitt B., Remme W., Zannad F., et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–1321. doi: 10.1056/NEJMoa030207. [DOI] [PubMed] [Google Scholar]
- 18.Montalescot G., Pitt B., Lopez de Sa E., et al. Early eplerenone treatment in patients with acute ST-elevation myocardial infarction without heart failure: the Randomized Double-Blind Reminder Study. Eur Heart J. 2014;35:2295–2302. doi: 10.1093/eurheartj/ehu164. [DOI] [PubMed] [Google Scholar]
- 19.Beygui F., Cayla G., Roule V., et al. Early aldosterone blockade in acute myocardial infarction: the ALBATROSS randomized clinical trial. J Am Coll Cardiol. 2016;67:1917–1927. doi: 10.1016/j.jacc.2016.02.033. [DOI] [PubMed] [Google Scholar]
- 20.Rossello X., Ariti C., Pocock S.J., et al. Impact of mineralocorticoid receptor antagonists on the risk of sudden cardiac death in patients with heart failure and left-ventricular systolic dysfunction: an individual patient-level meta-analysis of three randomized-controlled trials. Clin Res Cardiol. 2019;108:477–486. doi: 10.1007/s00392-018-1378-0. [DOI] [PubMed] [Google Scholar]
- 21.Duncker D., Konig T., Hohmann S., et al. Avoiding untimely implantable cardioverter/defibrillator implantation by intensified heart failure therapy optimization supported by the wearable cardioverter/defibrillator–the PROLONG study. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.116.004512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuster G.M., Kotlyar E., Rude M.K., et al. Mineralocorticoid receptor inhibition ameliorates the transition to myocardial failure and decreases oxidative stress and inflammation in mice with chronic pressure overload. Circulation. 2005;111:420–427. doi: 10.1161/01.CIR.0000153800.09920.40. [DOI] [PubMed] [Google Scholar]
- 23.Fraccarollo D., Galuppo P., Schmidt I., et al. Additive amelioration of left ventricular remodeling and molecular alterations by combined aldosterone and angiotensin receptor blockade after myocardial infarction. Cardiovasc Res. 2005;67:97–105. doi: 10.1016/j.cardiores.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 24.Fraccarollo D., Galuppo P., Schraut S., et al. Immediate mineralocorticoid receptor blockade improves myocardial infarct healing by modulation of the inflammatory response. Hypertension. 2008;51:905–914. doi: 10.1161/HYPERTENSIONAHA.107.100941. [DOI] [PubMed] [Google Scholar]
- 25.Gueret A., Harouki N., Favre J., et al. Vascular smooth muscle mineralocorticoid receptor contributes to coronary and left ventricular dysfunction after myocardial infarction. Hypertension. 2016;67:717–723. doi: 10.1161/HYPERTENSIONAHA.115.06709. [DOI] [PubMed] [Google Scholar]
- 26.Fraccarollo D., Berger S., Galuppo P., et al. Deletion of cardiomyocyte mineralocorticoid receptor ameliorates adverse remodeling after myocardial infarction. Circulation. 2011;123:400–408. doi: 10.1161/CIRCULATIONAHA.110.983023. [DOI] [PubMed] [Google Scholar]
- 27.Fraccarollo D., Thomas S., Scholz C.J., et al. Macrophage mineralocorticoid receptor is a pleiotropic modulator of myocardial infarct healing. Hypertension. 2019;73:102–111. doi: 10.1161/HYPERTENSIONAHA.118.12162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li C., Zhang Y.Y., Frieler R.A., et al. Myeloid mineralocorticoid receptor deficiency inhibits aortic constriction-induced cardiac hypertrophy in mice. PLoS One. 2014;9 doi: 10.1371/journal.pone.0110950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Usher M.G., Duan S.Z., Ivaschenko C.Y., et al. Myeloid mineralocorticoid receptor controls macrophage polarization and cardiovascular hypertrophy and remodeling in mice. J Clin Invest. 2010;120:3350–3364. doi: 10.1172/JCI41080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li C., Sun X.N., Zeng M.R., et al. Mineralocorticoid receptor deficiency in T cells attenuates pressure overload-induced cardiac hypertrophy and dysfunction through modulating T-Cell activation. Hypertension. 2017;70:137–147. doi: 10.1161/HYPERTENSIONAHA.117.09070. [DOI] [PubMed] [Google Scholar]
- 31.Kim S.K., Biwer L.A., Moss M.E., et al. Mineralocorticoid receptor in smooth muscle contributes to pressure overload-induced heart failure. Circ Heart Fail. 2021;14 doi: 10.1161/CIRCHEARTFAILURE.120.007279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lother A., Berger S., Gilsbach R., et al. Ablation of mineralocorticoid receptors in myocytes but not in fibroblasts preserves cardiac function. Hypertension. 2011;57:746–754. doi: 10.1161/HYPERTENSIONAHA.110.163287. [DOI] [PubMed] [Google Scholar]
- 33.Salvador A.M., Moss M.E., Aronovitz M., et al. Endothelial mineralocorticoid receptor contributes to systolic dysfunction induced by pressure overload without modulating cardiac hypertrophy or inflammation. Physiol Rep. 2017;5:e13313. doi: 10.14814/phy2.13313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lother A., Hein L. Vascular mineralocorticoid receptors: linking risk factors, hypertension, and heart disease. Hypertension. 2016;68:6–10. doi: 10.1161/HYPERTENSIONAHA.116.07418. [DOI] [PubMed] [Google Scholar]
- 35.Jia G., Habibi J., DeMarco V.G., et al. Endothelial mineralocorticoid receptor deletion prevents diet-induced cardiac diastolic dysfunction in females. Hypertension. 2015;66:1159–1167. doi: 10.1161/HYPERTENSIONAHA.115.06015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rickard A.J., Morgan J., Chrissobolis S., et al. Endothelial cell mineralocorticoid receptors regulate deoxycorticosterone/salt-mediated cardiac remodeling and vascular reactivity but not blood pressure. Hypertension. 2014;63:1033–1040. doi: 10.1161/HYPERTENSIONAHA.113.01803. [DOI] [PubMed] [Google Scholar]
- 37.Rickard A.J., Morgan J., Tesch G., et al. Deletion of mineralocorticoid receptors from macrophages protects against deoxycorticosterone/salt-induced cardiac fibrosis and increased blood pressure. Hypertension. 2009;54:537–543. doi: 10.1161/HYPERTENSIONAHA.109.131110. [DOI] [PubMed] [Google Scholar]
- 38.Bienvenu L.A., Morgan J., Rickard A.J., et al. Macrophage mineralocorticoid receptor signaling plays a key role in aldosterone-independent cardiac fibrosis. Endocrinology. 2012;153:3416–3425. doi: 10.1210/en.2011-2098. [DOI] [PubMed] [Google Scholar]
- 39.Moss M.E., Lu Q., Iyer S.L., et al. Endothelial mineralocorticoid receptors contribute to vascular inflammation in atherosclerosis in a sex-specific manner. Arterioscler Thromb Vasc Biol. 2019;39:1588–1601. doi: 10.1161/ATVBAHA.119.312954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shen Z.X., Chen X.Q., Sun X.N., et al. Mineralocorticoid receptor deficiency in macrophages inhibits atherosclerosis by affecting foam cell formation and efferocytosis. J Biol Chem. 2017;292:925–935. doi: 10.1074/jbc.M116.739243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moss M.E., DuPont J.J., Iyer S.L., et al. No significant role for smooth muscle cell mineralocorticoid receptors in atherosclerosis in the apolipoprotein-E knockout mouse model. Front Cardiovasc Med. 2018;5:81. doi: 10.3389/fcvm.2018.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pruthi D., McCurley A., Aronovitz M., et al. Aldosterone promotes vascular remodeling by direct effects on smooth muscle cell mineralocorticoid receptors. Arterioscler Thromb Vasc Biol. 2014;34:355–364. doi: 10.1161/ATVBAHA.113.302854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun J.Y., Li C., Shen Z.X., et al. Mineralocorticoid receptor deficiency in macrophages inhibits neointimal hyperplasia and suppresses macrophage inflammation through SGK1-AP1/NF-kappaB pathways. Arterioscler Thromb Vasc Biol. 2016;36:874–885. doi: 10.1161/ATVBAHA.115.307031. [DOI] [PubMed] [Google Scholar]
- 44.Barrett Mueller K., Bender S.B., Hong K., et al. Endothelial mineralocorticoid receptors differentially contribute to coronary and mesenteric vascular function without modulating blood pressure. Hypertension. 2015;66:988–997. doi: 10.1161/HYPERTENSIONAHA.115.06172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Laursen S.B., Finsen S., Marcussen N., et al. Endothelial mineralocorticoid receptor ablation does not alter blood pressure, kidney function or renal vessel contractility. PLoS One. 2018;13 doi: 10.1371/journal.pone.0193032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McCurley A., Pires P.W., Bender S.B., et al. Direct regulation of blood pressure by smooth muscle cell mineralocorticoid receptors. Nat Med. 2012;18:1429–1433. doi: 10.1038/nm.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.DuPont J.J., McCurley A., Davel A.P., et al. Vascular mineralocorticoid receptor regulates microRNA-155 to promote vasoconstriction and rising blood pressure with aging. JCI Insight. 2016;1:e88942. doi: 10.1172/jci.insight.88942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Galmiche G., Pizard A., Gueret A., et al. Smooth muscle cell mineralocorticoid receptors are mandatory for aldosterone-salt to induce vascular stiffness. Hypertension. 2014;63:520–526. doi: 10.1161/HYPERTENSIONAHA.113.01967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun X.N., Li C., Liu Y., et al. T-cell mineralocorticoid receptor controls blood pressure by regulating interferon-gamma. Circ Res. 2017;120:1584–1597. doi: 10.1161/CIRCRESAHA.116.310480. [DOI] [PubMed] [Google Scholar]
- 50.Kowalski J., Deng L., Suennen C., et al. Eplerenone improves pulmonary vascular remodeling and hypertension by inhibition of the mineralocorticoid receptor in endothelial cells. Hypertension. 2021;78:456–465. doi: 10.1161/HYPERTENSIONAHA.120.16196. [DOI] [PubMed] [Google Scholar]
- 51.Lother A., Bergemann S., Kowalski J., et al. Inhibition of the cardiac myocyte mineralocorticoid receptor ameliorates doxorubicin-induced cardiotoxicity. Cardiovasc Res. 2018;114:282–290. doi: 10.1093/cvr/cvx078. [DOI] [PubMed] [Google Scholar]
- 52.Yi Y., Du L., Qin M., et al. Regulation of atrial fibrosis by the bone. Hypertension. 2019;73:379–389. doi: 10.1161/HYPERTENSIONAHA.118.11544. [DOI] [PubMed] [Google Scholar]
- 53.Ibarrola J., Garcia-Pena A., Matilla L., et al. A new role for the aldosterone/mineralocorticoid receptor pathway in the development of mitral valve prolapse. Circ Res. 2020;127:e80–e93. doi: 10.1161/CIRCRESAHA.119.316427. [DOI] [PubMed] [Google Scholar]
- 54.Lother A., Deng L., Huck M., et al. Endothelial cell mineralocorticoid receptors oppose VEGF-induced gene expression and angiogenesis. J Endocrinol. 2019;240:15–26. doi: 10.1530/JOE-18-0494. [DOI] [PubMed] [Google Scholar]
- 55.Calvier L., Martinez-Martinez E., Miana M., et al. The impact of galectin-3 inhibition on aldosterone-induced cardiac and renal injuries. JACC Heart Fail. 2015;3:59–67. doi: 10.1016/j.jchf.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 56.Martinez-Martinez E., Calvier L., Fernandez-Celis A., et al. Galectin-3 blockade inhibits cardiac inflammation and fibrosis in experimental hyperaldosteronism and hypertension. Hypertension. 2015;66:767–775. doi: 10.1161/HYPERTENSIONAHA.115.05876. [DOI] [PubMed] [Google Scholar]
- 57.Tarjus A., Martinez-Martinez E., Amador C., et al. Neutrophil gelatinase-associated lipocalin, a novel mineralocorticoid biotarget, mediates vascular profibrotic effects of mineralocorticoids. Hypertension. 2015;66:158–166. doi: 10.1161/HYPERTENSIONAHA.115.05431. [DOI] [PubMed] [Google Scholar]
- 58.Buonafine M., Martinez-Martinez E., Amador C., et al. Neutrophil gelatinase-associated lipocalin from immune cells is mandatory for aldosterone-induced cardiac remodeling and inflammation. J Mol Cell Cardiol. 2018;115:32–38. doi: 10.1016/j.yjmcc.2017.12.011. [DOI] [PubMed] [Google Scholar]
- 59.Garg A., Foinquinos A., Jung M., et al. MiRNA-181a is a novel regulator of aldosterone-mineralocorticoid receptor-mediated cardiac remodelling. Eur J Heart Fail. 2020;22:1366–1377. doi: 10.1002/ejhf.1813. [DOI] [PubMed] [Google Scholar]
- 60.Gladden J.D., Chaanine A.H., Redfield M.M. Heart failure with preserved ejection fraction. Annu Rev Med. 2018;69:65–79. doi: 10.1146/annurev-med-041316-090654. [DOI] [PubMed] [Google Scholar]
- 61.Paulus W.J., Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–271. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 62.Valero-Munoz M., Backman W., Sam F. Murine models of heart failure with preserved ejection fraction: a "fishing expedition.". JACC Basic Transl Sci. 2017;2:770–789. doi: 10.1016/j.jacbts.2017.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Riehle C., Bauersachs J. Small animal models of heart failure. Cardiovasc Res. 2019;115:1838–1849. doi: 10.1093/cvr/cvz161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bender S.B., DeMarco V.G., Padilla J., et al. Mineralocorticoid receptor antagonism treats obesity-associated cardiac diastolic dysfunction. Hypertension. 2015;65:1082–1088. doi: 10.1161/HYPERTENSIONAHA.114.04912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pieronne-Deperrois M., Gueret A., Djerada Z., et al. Mineralocorticoid receptor blockade with finerenone improves heart function and exercise capacity in ovariectomized mice. ESC Heart Fail. 2021;8:1933–1943. doi: 10.1002/ehf2.13219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bonnard B., Pieronne-Deperrois M., Djerada Z., et al. Mineralocorticoid receptor antagonism improves diastolic dysfunction in chronic kidney disease in mice. J Mol Cell Cardiol. 2018;121:124–133. doi: 10.1016/j.yjmcc.2018.06.008. [DOI] [PubMed] [Google Scholar]
- 67.Brown L., Duce B., Miric G., et al. Reversal of cardiac fibrosis in deoxycorticosterone acetate-salt hypertensive rats by inhibition of the renin-angiotensin system. J Am Soc Nephrol. 1999;10(suppl 11):S143–S148. [PubMed] [Google Scholar]
- 68.Rickard A.J., Morgan J., Bienvenu L.A., et al. Cardiomyocyte mineralocorticoid receptors are essential for deoxycorticosterone/salt-mediated inflammation and cardiac fibrosis. Hypertension. 2012;60:1443–1450. doi: 10.1161/HYPERTENSIONAHA.112.203158. [DOI] [PubMed] [Google Scholar]
- 69.Lother A., Fürst D., Bergemann S., et al. Deoxycorticosterone acetate/salt-induced cardiac but not renal injury is mediated by endothelial mineralocorticoid receptors independently from blood pressure. Hypertension. 2016;67:130–138. doi: 10.1161/HYPERTENSIONAHA.115.06530. [DOI] [PubMed] [Google Scholar]
- 70.Kurrelmeyer K.M., Ashton Y., Xu J., et al. Effects of spironolactone treatment in elderly women with heart failure and preserved left ventricular ejection fraction. J Card Fail. 2014;20:560–568. doi: 10.1016/j.cardfail.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 71.Kosmala W., Rojek A., Przewlocka-Kosmala M., et al. Effect of aldosterone antagonism on exercise tolerance in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2016;68:1823–1834. doi: 10.1016/j.jacc.2016.07.763. [DOI] [PubMed] [Google Scholar]
- 72.Edelmann F., Wachter R., Schmidt A.G., et al. Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: the Aldo-DHF randomized controlled trial. JAMA. 2013;309:781–791. doi: 10.1001/jama.2013.905. [DOI] [PubMed] [Google Scholar]
- 73.Pitt B., Pfeffer M.A., Assmann S.F., et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370:1383–1392. doi: 10.1056/NEJMoa1313731. [DOI] [PubMed] [Google Scholar]
- 74.de Denus S., O'Meara E., Desai A.S., et al. Spironolactone metabolites in TOPCAT— new insights into regional variation. N Engl J Med. 2017;376:1690–1692. doi: 10.1056/NEJMc1612601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pfeffer M.A., Claggett B., Assmann S.F., et al. Regional variation in patients and outcomes in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial. Circulation. 2015;131:34–42. doi: 10.1161/CIRCULATIONAHA.114.013255. [DOI] [PubMed] [Google Scholar]
- 76.Pitt B., Filippatos G., Agarwal R., et al. Cardiovascular events with finerenone in kidney disease and type 2 diabetes. N Engl J Med. 2021;385:2252–2263. doi: 10.1056/NEJMoa2110956. [DOI] [PubMed] [Google Scholar]
- 77.Cleland J.G.F., Ferreira J.P., Mariottoni B., et al. The effect of spironolactone on cardiovascular function and markers of fibrosis in people at increased risk of developing heart failure: the heart 'OMics' in AGEing (HOMAGE) randomized clinical trial. Eur Heart J. 2021;42:684–696. doi: 10.1093/eurheartj/ehaa758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Soehnlein O., Libby P. Targeting inflammation in atherosclerosis—from experimental insights to the clinic. Nat Rev Drug Discov. 2021;20:589–610. doi: 10.1038/s41573-021-00198-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McGraw A.P., Bagley J., Chen W.S., et al. Aldosterone increases early atherosclerosis and promotes plaque inflammation through a placental growth factor-dependent mechanism. J Am Heart Assoc. 2013;2 doi: 10.1161/JAHA.112.000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schafer N., Lohmann C., Winnik S., et al. Endothelial mineralocorticoid receptor activation mediates endothelial dysfunction in diet-induced obesity. Eur Heart J. 2013;34:3515–3524. doi: 10.1093/eurheartj/eht095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.DeMarco V.G., Habibi J., Jia G., et al. Low-dose mineralocorticoid receptor blockade prevents western diet-induced arterial stiffening in female mice. Hypertension. 2015;66:99–107. doi: 10.1161/HYPERTENSIONAHA.115.05674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li W., Chen X., Riley A.M., et al. Long-term spironolactone treatment reduces coronary TRPC expression, vasoconstriction, and atherosclerosis in metabolic syndrome pigs. Basic Res Cardiol. 2017;112:54. doi: 10.1007/s00395-017-0643-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Suzuki J., Iwai M., Mogi M., et al. Eplerenone with valsartan effectively reduces atherosclerotic lesion by attenuation of oxidative stress and inflammation. Arterioscler Thromb Vasc Biol. 2006;26:917–921. doi: 10.1161/01.ATV.0000204635.75748.0f. [DOI] [PubMed] [Google Scholar]
- 84.Marzolla V., Armani A., Mammi C., et al. Essential role of ICAM-1 in aldosterone-induced atherosclerosis. Int J Cardiol. 2017;232:233–242. doi: 10.1016/j.ijcard.2017.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Caprio M., Newfell B.G., la Sala A., et al. Functional mineralocorticoid receptors in human vascular endothelial cells regulate intercellular adhesion molecule-1 expression and promote leukocyte adhesion. Circ Res. 2008;102:1359–1367. doi: 10.1161/CIRCRESAHA.108.174235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jaffe I.Z., Mendelsohn M.E. Angiotensin II and aldosterone regulate gene transcription via functional mineralocortocoid receptors in human coronary artery smooth muscle cells. Circ Res. 2005;96:643–650. doi: 10.1161/01.RES.0000159937.05502.d1. [DOI] [PubMed] [Google Scholar]
- 87.Newfell B.G., Iyer L.K., Mohammad N.N., et al. Aldosterone regulates vascular gene transcription via oxidative stress-dependent and -independent pathways. Arterioscler Thromb Vasc Biol. 2011;31:1871–1880. doi: 10.1161/ATVBAHA.111.229070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.van der Heijden C., Keating S.T., Groh L., et al. Aldosterone induces trained immunity: the role of fatty acid synthesis. Cardiovasc Res. 2020;116:317–328. doi: 10.1093/cvr/cvz137. [DOI] [PubMed] [Google Scholar]
- 89.van der Heijden C., Deinum J., Joosten L.A.B., et al. The mineralocorticoid receptor as a modulator of innate immunity and atherosclerosis. Cardiovasc Res. 2018;114:944–953. doi: 10.1093/cvr/cvy092. [DOI] [PubMed] [Google Scholar]
- 90.Dutzmann J., Musmann R.J., Haertle M., et al. The novel mineralocorticoid receptor antagonist finerenone attenuates neointima formation after vascular injury. PLoS One. 2017;12 doi: 10.1371/journal.pone.0184888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dutzmann J., Bauersachs J., Sedding D.G. Evidence for the use of mineralocorticoid receptor antagonists in the treatment of coronary artery disease and post-angioplasty restenosis. Vascul Pharmacol. 2018;107:20–26. doi: 10.1016/j.vph.2017.12.065. [DOI] [PubMed] [Google Scholar]
- 92.Filippatos G., Anker S.D., Agarwal R., et al. Finerenone and cardiovascular outcomes in patients with chronic kidney disease and type 2 diabetes. Circulation. 2021;143:540–552. doi: 10.1161/CIRCULATIONAHA.120.051898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Brilla C.G., Matsubara L.S., Weber K.T. Anti-aldosterone treatment and the prevention of myocardial fibrosis in primary and secondary hyperaldosteronism. J Mol Cell Cardiol. 1993;25:563–575. doi: 10.1006/jmcc.1993.1066. [DOI] [PubMed] [Google Scholar]
- 94.Young M., Fullerton M., Dilley R., et al. Mineralocorticoids, hypertension, and cardiac fibrosis. J Clin Invest. 1994;93:2578–2583. doi: 10.1172/JCI117269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.DuPont J.J., Jaffe I.Z. 30 years of the mineralocorticoid receptor: the role of the mineralocorticoid receptor in the vasculature. J Endocrinol. 2017;234:T67–T82. doi: 10.1530/JOE-17-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Faulkner J.L., Lluch E., Kennard S., et al. Selective deletion of endothelial mineralocorticoid receptor protects from vascular dysfunction in sodium-restricted female mice. Biol Sex Differ. 2020;11:64. doi: 10.1186/s13293-020-00340-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nguyen Dinh Cat A., Griol-Charhbili V., Loufrani L., et al. The endothelial mineralocorticoid receptor regulates vasoconstrictor tone and blood pressure. FASEB J. 2010;24:2454–2463. doi: 10.1096/fj.09-147926. [DOI] [PubMed] [Google Scholar]
- 98.Kim S.K., McCurley A.T., DuPont J.J., et al. Smooth muscle cell-mineralocorticoid receptor as a mediator of cardiovascular stiffness with aging. Hypertension. 2018;71:609–621. doi: 10.1161/HYPERTENSIONAHA.117.10437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chen C., Zhu X.Y., Li D., et al. Clinical efficacy and safety of spironolactone in patients with resistant hypertension: a systematic review and meta-analysis. Med (Balt) 2020;99 doi: 10.1097/MD.0000000000021694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Unger T., Borghi C., Charchar F., et al. 2020 International Society of Hypertension global hypertension practice guidelines. J Hypertens. 2020;38:982–1004. doi: 10.1097/HJH.0000000000002453. [DOI] [PubMed] [Google Scholar]
- 101.Ito S., Itoh H., Rakugi H., et al. Double-blind randomized phase 3 study comparing esaxerenone (CS-3150) and eplerenone in patients with essential hypertension (ESAX-HTN Study) Hypertension. 2020;75:51–58. doi: 10.1161/HYPERTENSIONAHA.119.13569. [DOI] [PubMed] [Google Scholar]
- 102.Duggan S. Esaxerenone: first global approval. Drugs. 2019;79:477–481. doi: 10.1007/s40265-019-01073-5. [DOI] [PubMed] [Google Scholar]
- 103.Bakris G., Pergola P.E., Delgado B., et al. Effect of KBP-5074 on blood pressure in advanced chronic kidney disease: results of the BLOCK-CKD Study. Hypertension. 2021;78:74–81. doi: 10.1161/HYPERTENSIONAHA.121.17073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bakris G.L., Agarwal R., Anker S.D., et al. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med. 2020;383:2219–2229. doi: 10.1056/NEJMoa2025845. [DOI] [PubMed] [Google Scholar]
- 105.Maron B.A., Opotowsky A.R., Landzberg M.J., et al. Plasma aldosterone levels are elevated in patients with pulmonary arterial hypertension in the absence of left ventricular heart failure: a pilot study. Eur J Heart Fail. 2013;15:277–283. doi: 10.1093/eurjhf/hfs173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Calvier L., Legchenko E., Grimm L., et al. Galectin-3 and aldosterone as potential tandem biomarkers in pulmonary arterial hypertension. Heart. 2016;102:390–396. doi: 10.1136/heartjnl-2015-308365. [DOI] [PubMed] [Google Scholar]
- 107.Preston I.R., Sagliani K.D., Warburton R.R., et al. Mineralocorticoid receptor antagonism attenuates experimental pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2013;304:L678–L688. doi: 10.1152/ajplung.00300.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Samokhin A.O., Stephens T., Wertheim B.M., et al. NEDD9 targets COL3A1 to promote endothelial fibrosis and pulmonary arterial hypertension. Sci Transl Med. 2018;10 doi: 10.1126/scitranslmed.aap7294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Maron B.A., Zhang Y.Y., White K., et al. Aldosterone inactivates the endothelin-B receptor via a cysteinyl thiol redox switch to decrease pulmonary endothelial nitric oxide levels and modulate pulmonary arterial hypertension. Circulation. 2012;126:963–974. doi: 10.1161/CIRCULATIONAHA.112.094722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Boehm M., Arnold N., Braithwaite A., et al. Eplerenone attenuates pathological pulmonary vascular rather than right ventricular remodeling in pulmonary arterial hypertension. BMC Pulm Med. 2018;18:41. doi: 10.1186/s12890-018-0604-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Maron B.A., Oldham W.M., Chan S.Y., et al. Upregulation of steroidogenic acute regulatory protein by hypoxia stimulates aldosterone synthesis in pulmonary artery endothelial cells to promote pulmonary vascular fibrosis. Circulation. 2014;130:168–179. doi: 10.1161/CIRCULATIONAHA.113.007690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Maron B.A., Waxman A.B., Opotowsky A.R., et al. Effectiveness of spironolactone plus ambrisentan for treatment of pulmonary arterial hypertension (from the [ARIES] study 1 and 2 trials) Am J Cardiol. 2013;112:720–725. doi: 10.1016/j.amjcard.2013.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hullin R., Metrich M., Sarre A., et al. Diverging effects of enalapril or eplerenone in primary prevention against doxorubicin-induced cardiotoxicity. Cardiovasc Res. 2018;114:272–281. doi: 10.1093/cvr/cvx162. [DOI] [PubMed] [Google Scholar]
- 114.Akpek M., Ozdogru I., Sahin O., et al. Protective effects of spironolactone against anthracycline-induced cardiomyopathy. Eur J Heart Fail. 2015;17:81–89. doi: 10.1002/ejhf.196. [DOI] [PubMed] [Google Scholar]
- 115.Hundemer G.L., Curhan G.C., Yozamp N., et al. Incidence of atrial fibrillation and mineralocorticoid receptor activity in patients with medically and surgically treated primary aldosteronism. JAMA Cardiol. 2018;3:768–774. doi: 10.1001/jamacardio.2018.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Takemoto Y., Ramirez R.J., Kaur K., et al. Eplerenone reduces atrial fibrillation burden without preventing atrial electrical remodeling. J Am Coll Cardiol. 2017;70:2893–2905. doi: 10.1016/j.jacc.2017.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Du L., Qin M., Yi Y., et al. Eplerenone prevents atrial fibrosis via the TGF-beta signaling pathway. Cardiology. 2017;138:55–62. doi: 10.1159/000471918. [DOI] [PubMed] [Google Scholar]
- 118.Kimura S., Ito M., Tomita M., et al. Role of mineralocorticoid receptor on atrial structural remodeling and inducibility of atrial fibrillation in hypertensive rats. Hypertens Res. 2011;34:584–591. doi: 10.1038/hr.2010.277. [DOI] [PubMed] [Google Scholar]
- 119.Alexandre J., Dolladille C., Douesnel L., et al. Effects of mineralocorticoid receptor antagonists on atrial fibrillation occurrence: a systematic review, meta-analysis, and meta-regression to identify modifying factors. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.119.013267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nishimura R.A., Vahanian A., Eleid M.F., et al. Mitral valve disease—current management and future challenges. Lancet. 2016;387:1324–1334. doi: 10.1016/S0140-6736(16)00558-4. [DOI] [PubMed] [Google Scholar]
- 121.Ibarrola J., Garaikoetxea M., Garcia-Pena A., et al. Beneficial effects of mineralocorticoid receptor antagonism on myocardial fibrosis in an experimental model of the myxomatous degeneration of the mitral valve. Int J Mol Sci. 2020;21:5372. doi: 10.3390/ijms21155372. [DOI] [PMC free article] [PubMed] [Google Scholar]