Abstract
A large body of evidence implicates the renin–angiotensin system in the pathogenesis of cardiovascular disease. However, not everyone understands that the magnitude of the risk reduction achieved in clinical trials with angiotensin-converting enzyme inhibitors and angiotensin receptor blockers is only a fraction of the residual risk for cardiovascular events and death. This paper addresses limitations of current therapeutic approaches based on renin–angiotensin system blockade for hypertension and cardiovascular disease by illustrating the complex biochemical physiology and mechanism of classical and alternate angiotensin peptide formation. Emerging evidence of alternate mechanisms that bypass both renin and angiotensin-converting enzyme to produce the angiotensins in tissues and cells is not currently universally recognized. Currently available treatment would benefit from further insights to help fully meet the aims of patient care, and the challenge is to delve more deeply into the renin–angiotensin system cascade, with the aim of enhancing therapeutics for renin–angiotensin system inhibition. This article provides a reappraisal of the renin–angiotensin–aldosterone cascade, highlighting newly elucidated intermediary components and interplay, and their consequent implications and relevance for understanding the long-term contribution of angiotensin II in cardiovascular diseases and their therapy.
Keywords: angiotensin II, angiotensin-(1-7), chronic kidney disease, chymase, monoclonal antibodies, primary hypertension
Graphical abstract
The aim of science is not to open a door to endless wisdom, but to put a limit to endless error.
— Bertolt Brecht, Life of Galileo1
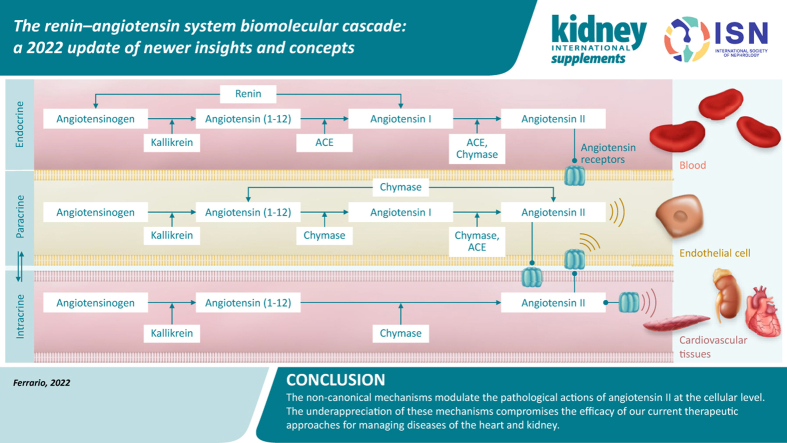
This review provides a reappraisal of the renin–angiotensin system (RAS) cascade, highlighting newly elucidated intermediary components and their consequent implications and relevance for understanding cardiovascular (CV) disease progression. This topic continues to attract greater attention2 because of the current overriding importance of angiotensin-converting enzyme (ACE) 2 (ACE2) as the entry vector for coronavirus disease 2019 (COVID-19) infection (see also the article by Hollenberg and Epstein in this supplement3). Despite the extensive literature examining the homeostatic regulation performed by the angiotensins, this topic remains fresh and vibrant. Alongside established clinical evidence for the benefit of preventing the production of angiotensin II (Ang II) or blocking access of the hormone to its receptors, evidence is also emerging for alternate mechanisms that bypass both renin and ACE to produce angiotensins. This latter evidence is still not universally accepted,4 which highlights the fact that more remains to be learned about RAS. In addressing this issue, we acknowledge the benefits that can be achieved with a multifactorial therapeutic approach that includes non-RAS medicines, as exemplified by the enhanced reduction in CV risk reported in the Steno-2 trial5,6 by combining RAS inhibitors with tight glucose control, aspirin, and and lipid-lowering agents. The limitations of restricting therapy with pharmacologic RAS inhibition in non-albuminuric diabetic kidney disease (DKD) is underscored in a recently published review.6
Although the proven therapeutic benefits attained with chemical inhibitors targeting renin, ACE, and Ang II type 1 receptors (ARBs) are generally accepted, clinicians’ awareness of the existence of a residual risk of CV events7, 8, 9, 10 remains low. Given the impressive genetic, molecular, physiological, and clinical evidence for a role of Ang II in the pathogenesis of CV disease,11,12 the long-term effects of RAS blockade, using direct renin inhibitors, ACE inhibitors, and ARBs, fall short of expectations. In reexamining the magnitude of the risk reduction attained in clinical trials with ACE inhibitors and ARBs, the residual risk of CV events is shown to be several orders of magnitude greater than the relative risk reduction of myocardial infarction, heart failure, or CV death.13, 14, 15, 16, 17, 18, 19 These data are disconcerting, given the strength of the research implicating the RAS in the pathogenesis of hypertension. The non-superiority of RAS inhibitors over other antihypertensive agents remains a source of confusion, as reflected in the recommendations provided by major professional organizations endorsing the benefits of blood pressure lowering per se over the range of available antihypertensive agents.20, 21, 22 Yet, a century of rigorous experimental research reveals the diverse nature of the biochemical processes that, within the RAS, lead to the formation of the angiotensins, and the fundamental importance of Ang II as a critical participant in hypertension pathogenicity, adverse cardiac and vascular remodeling, atherogenesis, diabetes, and acute and chronic kidney disease. In the authors’ view, the explanation of this dilemma lies in the potential inability of RAS drugs to reach the intracellular sites where Ang II influences CV function,23, 24, 25 owing to poor intracellular penetrability of these inhibitors, activation of alternate Ang II–forming serine endopeptidases with an affinity similar to or even higher than that for ACE,26,27 or both of these factors combined. We address these issues by providing a detailed new look at the biochemical physiology of the classical and alternate angiotensin peptide–forming mechanisms.
RAS Current Biochemical Pathways
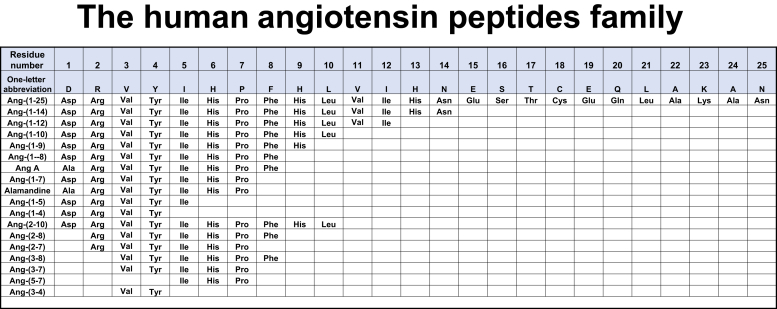
The cascade of peptides derived from the angiotensinogen (AGT) protein is illustrated in Figure 1. As stressed by Ryan,28 linear polypeptides without repeating amino acids have multiple homologs. Ang II has 35 possible lower homologs, and 54 possible homologs can be derived from angiotensin I (Ang I). Hence, viewing the RAS as a linear system in which renin and ACE are the 2 enzymatic steps in the biotransformation of AGT into Ang II is without fundamentals. Over the years, we have been engaged in revising the tenet that the RAS is constituted of a linear biochemical processing path in which the terminal product Ang II is the final biologically active compound.29, 30, 31
Figure 1.
Amino acid sequences of the family of angiotensin peptides identified as possessing biological activity. Three-letter abbreviations are given for amino acids. Alamandine is Des[Asp1]-[Ala1]-Ang-(1-7). Ala, alanine; Ang, angiotensin; Arg, arginine; Asn, asparagine; Asp, aspartic acid; Cys, cysteine; Gln, glutamine; Glu, glutamic acid; His, histidine; lle, isoleucine; Leu, leucine; Lys, lysine; Phe, phenylalanine; Pro, proline; Ser, serine; Thr, threonine; Tyr, tyrosine; Val, valine. Created with BioRender.com.
The old and the not so new
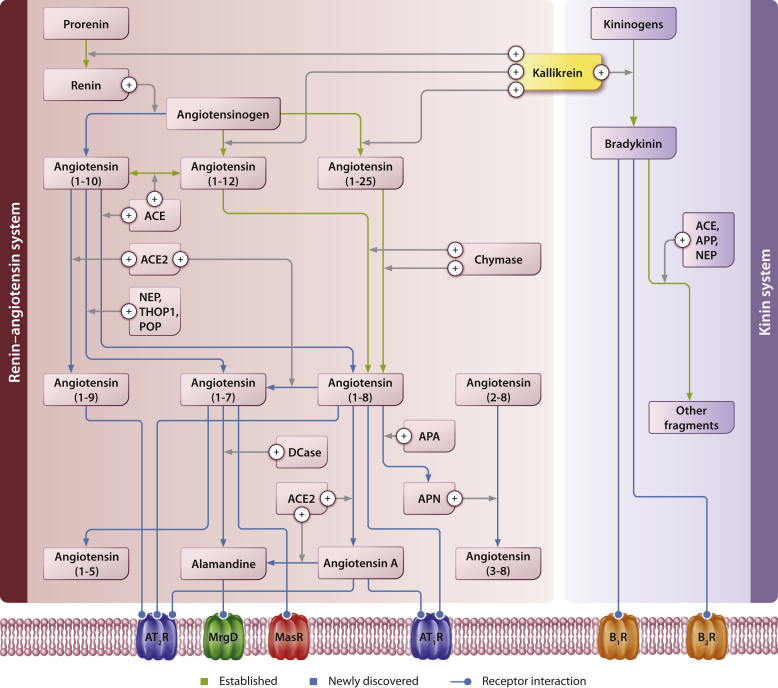
The biotransformation mechanisms participating in the generation and metabolism of biologically active angiotensins are illustrated in Figure 2.32,33 Although the reaction of kidney renin with the plasma AGT remains the crucial step in the initiation of the biochemical cascade, proteolytic enzymes not belonging to the aspartyl protease renin and elaborated by digestive glands were first reported to hydrolyze AGT by Croxatto and Croxatto.34 From those earlier attempts to identify the nature of non-renin enzymes, only cathepsin G,35 kallikrein,36 and tonin37 remain as enzymes known to belong to the RAS cascade.
Figure 2.
Schematic representation of angiotensinogen cleaved derivatives by renin and alternate processing by kallikrein or a kallikrein-like enzyme.32,33 The renin–angiotensin system, along with multiple overlapping systems (the kinin system, the endothelin system, and the natriuretic peptides system) plays a role in the regulation of blood pressure and cardiovascular function through the enzyme-catalyzed formation and degradation of vasoactive peptides and hormones. ACE, angiotensin-converting enzyme; ACE2, angiotensin-converting enzyme 2; ANP, atrial natriuretic peptide; APA, aminopeptidase A; APN, aminopeptidase N; APP, aminopeptidase P; BNP, B-type natriuretic peptide; B1R, Bradykinin receptor B1; B2R, Bradykinin receptor B2; CNP, C-type natriuretic peptide; ETAR, endothelin A receptor; ETBR, endothelin B receptor; NEP, neprilysin; NPR-A, natriuretic peptide receptor-A; NPR-B, natriuretic peptide receptor-B; NPR-C, natriuretic peptide receptor-C; POP, prolyl oligopeptidase; PRCP, prolyl carboxypeptidase; THOP1, thimet oligopeptidase.
Conversion of Ang I
Following the catalytic generation of Ang I by renin, the decapeptide is processed by the somatic active carboxy terminal site of ACE (EC 3.4.15.1) to yield Ang II, and by ACE2 (E.C. 3.4.17.23) to generate angiotensin-(1-9) [Ang-(1-9)].38 As a dipeptidyl carboxypeptidase, the 2 penultimate amino acids His9-Leu10 of Ang I are cleaved from the C-terminus of Ang I to generate Ang II, and the endopeptidase neprilysin (E.C. 3.4.24.11) cleaves the tripeptide Phe8-His9-Leu10 of Ang I to yield angiotensin-(1-7) [Ang-(1-7)].39 Of current significant importance, owing to its participation as the entry vehicle for infection by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2),40, 41, 42 the mono-carboxypeptidase ACE2 cleaves with high affinity the Pro7-Phe8 bond of Ang II to generate Ang-(1-7).43 The first demonstrations of Ang-(1-7) vasodilator effects,44 the presence of low Ang-(1-7) urinary concentrations in primary untreated hypertensive patients,45 and the report that captopril treatment augmented plasma Ang-(1-7) concentrations in parallel with an antihypertensive effect in hypertensive adults46 led Ferrario et al.47 to conclude that Ang-(1-7) may be acting as an endogenous inhibitor of Ang II pathologic actions and that its deficit may facilitate or directly account for the development of primary hypertension. Other investigators now subscribe to this hypothesis.47
Release of angiotensin III and IV
Further metabolism of the C-terminus residues of Ang II by aminopeptidases releases the biologically active peptides angiotensin III [Ang-(2-8)] and angiotensin IV [Ang-(3-8)], and ACE further catalyzes Ang-(1-7) into the less-understood functional role of the pentapeptide Asp1-Arg2-Val3-Tyr4-Ile5- [Ang-(1-5)]. A dipeptide angiotensin-(3-4) [Ang-(3-4)] is reported to possess strong antihypertensive activity in humans and rats, in part by acting as an allosteric enhancer of renal Ang II type 2 receptor.48,49 The inclusion of this di-peptide (Val3-Ty4) as a genuine member of the angiotensin family underscores the economy of function that is an inherent property of neuropeptidergic systems.50 The processing of Ang I and Ang II into the smaller terminally critical active hormones seems to be dictated by tissue-specific compartmentalization of the enzymes,51 rate of permeability, and diffusion of AGT and Ang I into the organs’ interstitial spaces,52 and the cellular uptake of peptides based on receptor internalization and processing.23,24,53
The role of Ang-(1-7)
In exploring the role of Ang-(1-7) as an Ang II counterregulatory peptide, Lautner and colleagues54 isolated a decarboxylated product in which alanine substituted for aspartic acid in position 1 of the Ang-(1-7) sequence. Their study extended the previous discovery of angiotensin A [Des[Asp1]-[Ala1]-Ang II] (Figure 1) in human plasma.55 The decarboxylation process appears to engage a mononuclear leukocyte-derived aspartate decarboxylase.55 The importance of this parallel processing of Ang II into Des[Asp1]-[Ala1]-Ang II and Des[Asp1]-[Ala1]-Ang-(1-7) (alamandine) remains to be established, although the decarboxylation of the parent peptides seems to modify the biological actions through decreased dependency of Des[Asp1]-[Ala1]-Ang II on Ang II type 1 receptor (AT1-R) signaling56 and coupling of alamandine to Mas-related G protein–coupled receptors (MrgD), a subset of relatively promiscuous receptors signaling pruriceptive sensations.57
Returning to Ang I, 2 enzymes, other than neprilysin, generate Ang-(1-7). The tissue endopeptidases thimet oligopeptidase (E.C. 3.4.24.15) and prolyl oligopeptidase (E.C. 3.4.21.26)58, 59, 60 generate Ang-(1-7) from Ang I. Thimet oligopeptidase, a thiol-sensitive metalol endopeptidase, engaged in neuropeptide metabolism and inflammation,61 metabolizes Ang I into Ang-(1-7), and prolyl oligopeptidase can form Ang-(1-7) directly from either Ang I or Ang II.59,60
New Biochemical Pathways for The Generation of Angiotensins
In the preface of a mostly forgotten 1973 book addressing the heterogeneity of renin and renin substrate, Professor Mohinder P. Sambhi stated the following: “The discovery of multiple forms of renin and renin substrate has generated new directions in research on the biochemistry of the renin-angiotensin system and its functional significance.”62 Almost 50 years later, Sambhi’s comments remain true. Renewed interest in the heterogeneity of AGT substrate in mediating acute or chronic changes in RAS activity remains unappreciated, even though the detection of low and high molecular forms of the substrate in the plasma of pregnant women were construed as evidence of the existence of nonhepatic sources for AGT synthesis.63,64 The potential functionality of heterogenic forms of the AGT protein, first reported by Gordon and Sachin,65 remained dormant for decades until the identification of proangiotensin-12.66
Understanding the role of angiotensin-(1–12)
Proangiotensin-12 was renamed, by us, to the established international nomenclature as angiotensin-(1–12) (Ang-[1–12]; Figure 2). The original studies by Nagata et al.66 were expanded on by pilot observations showing that kallikrein or a kallikrein-like enzyme cleaved Ang-(1-12) from AGT.42 Renin, on the other hand, shows no catalytic action in the metabolism of AGT into Ang-(1-12) or its degradation into the cleavage of downstream angiotensins.67 Studies of the fate of Ang-(1-12) revealed that ACE is the primary Ang-(1-12) catalytic enzyme in the circulation of rats,68 and chymase hydrolyzes Ang-(1-12) in both human and rodent hearts.69, 70, 71 Ang-(1-12)’s functional contribution to blood pressure regulation was shown by blood pressure normalization during cerebroventricular administration of an Ang-(1-12) polyclonal antibody in transgenic rats expressing the Ren-2 gene,72 and in studies assessing the effects of local delivery of the substrate in the brainstem region associated with the control of baroreflexes.73,74 Accumulating evidence indicates that Ang-(1-12) may source Ang II contribution to adverse cardiac and vascular remodeling in hypertension, as increased immunoreactive Ang-(1-12) content was found in the heart of spontaneously hypertensive rats75 and the blood and heart of transgenic rats expressing the human AGT gene.76,77 Ang-(1-12) participation as a source of Ang II modulation of cardiac function was revealed in studies in which the protein increased the contractile function of WKY cardiomyocytes via augmented intracellular K(+) currents78 and mobilization of L-type Ca(2+) channels.79 Consistent with their participation as major sources for tissue generation of angiotensins, high chymase expression and Ang-(1-12) in rat bone marrow CD68(+) myeloid lineage cells were recently demonstrated.80
Angiotensin-(1–12): translation of basic science to human studies
Basic research studies were consistent in revealing broad actions of Ang-(1-12) as an Ang II-forming substrate. The successful development of a sensitive radioimmunoassay by Ahmad et al.81 for measurements of human Ang-(1-12) in blood and urine allowed the demonstration of increased plasma Ang-(1-12) in primary hypertensive patients who were naïve82 or not naïve2 to antihypertensive therapy. The demonstration of augmented circulating Ang-(1-12) in hypertensive patients suggests a participatory role of this substrate as a hypertension-biomarker with a discriminative value greater than that of circulating AGT and even Ang II.82
The range of shorter amino acid sequences of AGT with an apparent high affinity in generating Ang II by chymase (E.C. 3.4.21.39) and ACE was expanded with the additional demonstration of Big angiotensin 25,83 a 25 amino acid N-terminal protein that is resistant to renin and generates Ang II through the catalytic activity of chymase (Figures 1 and 2). This alternate substrate present in multiple human organs appears to localize specifically to glomerular podocytes and was detected in the urine of patients with diabetes mellitus.84
Interwoven with the biochemical physiology of the RAS, the detection of these intermediate sequences of AGT capable of generating Ang II by non-renin and non-ACE-dependent pathways gives new impetus to unveil the mechanisms by which these substrates may have a preeminent role in tissue production of Ang II, particularly their possible role as an intracellular source for functionally active angiotensins. This interpretation is consistent with a selective increase in chymase activity in cardiac myocytes from ovariectomized spontaneously hypertensive rats.85 A critical role of chymase as an Ang II–forming pathway is strengthened by a critical role of cardiac chymase in the evolution of experimental volume overload and mitral regurgitation in humans.86,87 Chymase inhibition augments plasma Ang II concentrations and worsens hypertension in spontaneously hypertensive rats88 and ameliorates intrarenal renin–angiotensin activity in salt-dependent hypertension.89
Biochemical physiology of the RAS: relevance to clinical practice
Pioneer efforts to determine the impact of preventing the actions of Ang II in the control of high blood pressure led to the isolation and synthesis of peptide inhibitors of ACE,90 half a century ago. The evolution of this proof-of-concept clinical finding to the modern pharmacotherapy of CV and renal diseases, and diabetes mellitus, is enthroned as the basic underpinning of contemporary therapeutic regimens and international medical guidelines. The use of ACE inhibitors and ARBs, and to a certain extent the direct renin inhibitor aliskiren fumarate,91,92 is fundamental to any antihypertensive therapy.93 Despite their proven benefits, their therapeutic usefulness has several limitations related to limited efficacy, the need of polypharmacy, medication adherence, and side effects. Dzau and Balatbat94 have underscored the current apathy in identifying new targets for hypertension drug development using molecular and cell-based therapies. Older efforts to explore the use of nonchemical agents in CV disease treatment were abandoned prematurely,95 owing to the successful results obtained with ACE inhibitors. The status of immunologic approaches for the treatment of high blood pressure is documented below.
Renin inhibition
Early recognition of renin immunogenicity prompted Haber’s96,97 conceptualization of inhibiting renin to prevent Ang II production. Three classes of compounds were visualized—specific antibodies, acid protease inhibitors, and substrate analogs.98 Focus was placed on exploring several renin-specific antibodies, including the generation of Fab fragments that were documented to induce systemic depressor responses in canines exposed to sodium depletion or acute renal artery constriction.99 Research included the description of the specificity of renin monoclonal antibodies (mAbs).99,100 The finding that renin antibodies triggered autoimmune disease in animal models led to cessation of further research into this field.101
Gene silencing to inhibit the production of AGT
Research exploring therapeutic interventions upstream from Ang I using novel molecular biological approaches of gene silencing includes strategies directed at blocking the synthesis of hepatic AGT. Mullick et al.102 tested an N-acetylgalactosamine-conjugated AGT antisense oligonucleotide targeting hepatic production of the AGT, and Dutch investigators103,104 employed small interfering ribonucleic acids (siRNAs) targeting hepatic AGT to achieve similar goals. Dzau and colleagues105 reported preliminary results of Crispr-Cas9–mediated disruption of AGT in a BRL 3A rat liver cell line and the effective reduction in the systolic blood pressure of prehypertensive and hypertensive spontaneously hypertensive rats, at the American Heart Association (AHA) 2020 meeting. Preclinical data obtained with hepatic AGT suppression generated 2 clinical trials (Table 1102,106, 107, 108, 109, 110, 111, 112). Ionis Pharmaceuticals, Inc. (Carlsbad, CA) is investigating the tolerability and antihypertensive action of the AGT antisense ONIS-AGT-LRx (AGT antisense oligonucleotide) given subcutaneously once weekly for 12 weeks to primary hypertensive patients (ClinicalTrials.gov Identifier: NCT04714320). Additionally, Alnylam Pharmaceuticals (Cambridge, MA) evaluated the effect of single subcutaneous doses of their AGT-interfering RNA (ALN-AGT01) in hypertensive patients with or without altered salt intake and concomitant treatment with irbesartan (ClinicalTrials.gov Identifier: NCT03934307). The data presented at the 2020 Scientific Sessions of the AHA showed that a 95% reduction in plasma AGT concentrations at week 8 was associated with mean blood pressure reductions of 11 ± 2/8 ± 1 mm Hg.107 These newer studies build on early original independent basic science studies using antisense oligonucleotide and mAbs to probe the functions of renin and AGT.113,114
Table 1.
Summary of currently registered clinical trials aimed at suppressing renin–angiotensin system (RAS) activity
| ClinicalTrials.gov identifier | Condition, phase, and sponsor | Study title and intervention | Study design and outcome measures |
|---|---|---|---|
| NCT04083222 |
|
Study design
|
|
| NCT03714776 |
|
|
Study design
|
| NCT03934307 |
|
|
Study design
|
| NCT01015703 |
|
|
Study design
|
| NCT00702221 |
|
|
Outcome measures
|
| NCT00500786 |
|
|
Study design
|
| NCT00701649 |
|
Objectives
|
|
| NCT00710372 |
|
|
Outcome measures
|
| ACTRN12617001192370 |
|
|
Study design
|
ABPM, ambulatory blood pressure monitoring; AE, adverse events; AGT, angiotensinogen; ALN, Alnylam Pharmaceuticals; Ang II, angiotensin II; ATV, angiotensin therapeutic vaccine; AUC, area under the concentration-time curve; BTG, BTG International Inc.; Cmax, maximum observed plasma concentration; DBP, diastolic blood pressure; IgG, immunoglobulin G; IgM, immunoglobulin M; KLH, keyhole limpet hemocyanin; PD, pharmacodynamic; PK, pharmacokinetic; SBP, systolic blood pressure; s.c., subcutaneous; SAD, single ascending dose; SD, single dose.
Although the apparent benefits of AGT inhibition to treat hypertension alone or in reinforcing the therapeutic effects of other antihypertensive agents may be obvious, these interventions assume that the function of AGT is restricted to being a protein substrate for generation of angiotensins. Preliminary data obtained with the ALN-AGT RNA interference combined tolerability with effectiveness107; however, targeting AGT gene silencing may lead to unexpected complications over the long term, as AGT silencing assumes that no other biological functions are contained within the protein.115 The human AGT protein is composed of 452 amino acids, of which the N-terminus signal peptide comprises the first 33. The sequence of angiotensins is initiated by Asp1 at position 34 from the N-terminus Met1.116 Thus, the 12 amino acids coding for the current major studied angiotensins constitute 2.65% of the human AGT protein. As recently reported by us,115 this therapeutic approach assumes that the remaining ∼97% of the molecule—des-(Ang I)-AGT117, 118, 119—has no function. However, Corvol and colleagues120, 121, 122, 123 reported the antiangiogenic actions of des-(Ang I)-AGT and, recently, overexpression of AGT was reported to protect mice from sinusoid remodeling and arterialization in cancerous tissue.123 Other studies show that AGT acts as an inhibitor of vascular endothelial growth factor cell migration,120 a modulator of blood–brain barrier permeability,124 diet-induced obesity, and hepatic steatosis.125 AGT expression in the renal tubules and its implicated role in the regulation of renal function may be impaired by suppression of the AGT gene.126, 127, 128 We suggest that AGT gene silencing may have other consequences, as depletion of the substrate causes dramatic increases in circulating renin, and in principle, the loss of both the pressor [ACE/Ang II/AT1-R] and depressor [ACE2/Ang-(1-7)/Mas-R axis] component of the system.129 We think that the potential long-term negative effects of total AGT inhibition are reminiscent of the severe renal dysfunction reported with the combined use of ACE inhibitors and ARBs.130
Ang I and Ang II
The promissory value of vaccines in the treatment of cancer, rheumatoid arthritis, and even Alzheimer’s disease continues to stimulate research in the development of vaccines targeted to Ang II (Table 1). Ang I vaccines show minimal antihypertensive effects, both in normal and hypertensive subjects.131 On the other hand, at least 4 clinical trials registered with the National Institutes of Health evaluate the use of Ang II in hypertension (Table 1). Of the completed trials, administration of CYT—6-AngQb (virus-like particles covalently coupled to Ang II) seems to be promising in that it increases anti-Ang II antibody titers, eliminating the morning blood pressure surge, and reducing ambulatory daytime blood pressure at the highest dose tested.110,132 Unfortunately, additional studies that induced higher anti-Ang II antibody titers failed to duplicate the original findings, and adverse effects related to employed doses led to the termination of another Ang II vaccine incorporating an adjuvant (CoVaccine HT) in a phase 2 randomized, double-blind, placebo-controlled trial (ClinicalTrials.gov Identifier: NCT00702221; Table 1).108,109
Ang II type 1 receptors
Preclinical studies suggest that therapeutic efficacy may be achieved with vaccines derived from the second extracellular loop of AT1-R.133,134 However, detection of AT1-R autoantibodies in preeclamptic patients,135 kidney transplant recipients,136 and those with primary aldosteronism137 raises questions as to their clinical potential.
Ang-(1-12)
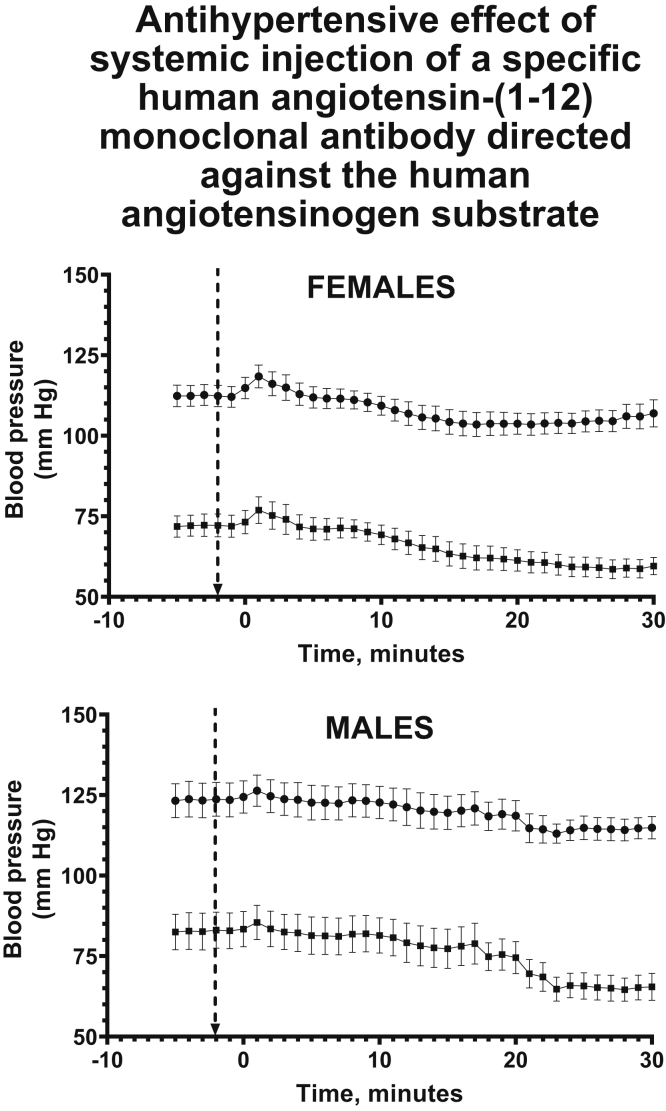
Monoclonal antibody–based treatments of malignancies, familial dyslipidemia, and autoimmune diseases are yielding extraordinary results as new therapeutic strategies.138,139 Building on these findings, we are examining the efficacy of using the human sequence of Ang-(1-12) [h-Ang-(1-12)] to generate mAbs for the treatment of hypertension and hypertension-related target organ damage. A highly selective mAb has been generated with essentially no cross-reactivity against human AGT or angiotensin peptides. This h-Ang-(1-12)–directed mAb has been injected into the circulation of transgenic hypertensive rats engineered to express the human AGT gene.77 As illustrated in Figure 3,76,77 systemic delivery of a single intravenous injection of the h-Ang-(1-12) mAb induced a significant antihypertensive response that was sustained for up to 90 minutes following its administration. These proof-of-concept results are consistent with the demonstration of higher Ang-(1-12) plasma levels in primary hypertensive patients who are naïve or not naïve to antihypertensive medications.81,82 The new data confirm a real need for reexamining the utility of mAbs for use in treatment strategies against primary hypertension and hypertension-related target organ damage.
Figure 3.
Time course of the changes in systolic and diastolic blood pressure of anesthetized transgenic hypertensive rats (n = 12) expressing the human angiotensinogen gene in its genome [TGR(hAGT)L1623}]76,77before and after the intravenous injection of a h-Ang-(1-12) mAb at a dose of 30 mg/kg. Data graphed in Prism version 8 and formatted with BioRender.com. mAb, monoclonal antibody.
The translation of both preclinical and clinical research on vaccines targeting Ang II in the treatment of high blood pressure and hypertension-related target organ damage remains to be crystalized. Of the various approaches described above, AGT gene silencing shows significant therapeutic promise, although the concomitant suppression of des-(Ang I)-AGT functions is a serious concern. These limitations would not influence an approach in which Ang-(1-12) immunoneutralization is targeted, because it will only neutralize the 12–amino acid string of the AGT protein that is the substrate for the generation of the angiotensins.
Commentary
Increased acceptance of the multifaceted pathways through which the AGT substrate can be metabolized into the active angiotensins within the distinct extracellular and intracellular compartments of the human body is likely to yield advances in the treatment of human diseases. Randomized clinical trials have cemented the clinical utility of suppressing Ang II formation or binding to AT1-R receptors in the treatment of heart disease, type 2 diabetes,15,140 and chronic kidney disease.141,142 Inconsistencies in the results of randomized controlled trials using RAS, in terms of the presence of a residual risk several orders of magnitude greater than the benefit, need to be more rigorously investigated.12,13,143 The limitations of current therapeutic approaches to the treatment of hypertensive vascular disease must be appreciated, while alternate mechanisms by which the angiotensins induce pathology are explored. Engineering molecular approaches to increase the anti-Ang II immunogenicity, expanding on our ongoing work using mAbs directed against the human sequence of Ang-(1-12) or even Ang-(1-25) provides a more selective way to suppress Ang II biological actions and avoid the consequences of concomitant suppression of des-(Ang I)-AGT elimination.115 Further engineering of mAbs into single-domain antibodies (nanobodies)144,145 will permit intracellular neutralization of angiotensins. Given that these treatments would not require daily administration, the challenge of adherence to medications could also be addressed. We suggest that the approach will yield more specific methods to prevent Ang II from exerting pathologic actions, avoiding the expression or activation of alternate enzymes with catalytic activity against AGT or the intermediate peptides from which Ang II and Ang-(1-7) are generated. Issues related to physician inertia and patients’ adherence to therapy will be obviated in large part, as these molecular treatments do not require daily use of medicines. We suggest that such an approach will yield more specific methods to prevent Ang II from exerting pathologic actions, avoiding the activation of alternate enzymes with catalytic activity against AGT or the intermediate peptides from which Ang II and Ang-(1–7) are generated.
Conclusions
Patient care involves the effective management of both hypertension and cardiometabolic risk factors. Currently available treatment would benefit from further insights to help fully meet the aims of patient care. The challenge is to delve deeper into the RAS cascade, with the aim of enhancing therapeutics for RAS inhibition through recognition of the existence of intermediate alternate mechanisms to produce angiotensins. The presence of renin-independent noncanonical pathways for Ang II production is largely unaffected by agents inhibiting RAS activity. Therefore, it is recommended that future efforts be directed toward development of treatments that can effectively block the synthesis or action of intracellular Ang II through inhibition of the primary intracellular enzymes accounting for Ang II production. Improved drug penetration into cardiac or renal sites of disease, inhibiting chymase, the primary Ang II–forming enzyme in the human heart, or inhibiting Ang-(1-12) as a source for Ang II production may all be more effective strategies to inhibit the system.
Disclosure
This article is published as part of a supplement sponsored by Bayer AG.
The work described here is supported by the National Institutes of Health grant HL-051952 from the National Heart, Lung and Blood Institute and grant AG070371 from the Aging Institute. All the authors declare no financial conflict of interest and received no personal funding for this article.
Acknowledgments
Development of this article was funded by an unrestricted educational grant from Bayer AG. The authors would like to acknowledge Jo Luscombe, PhD, of Chameleon Communications International, who provided editorial assistance with funding via an unrestricted educational grant from Bayer AG. The authors also acknowledge Alexander Roeder, Ronny Guenther, Sven Goepfert, and Josephin Schoenrich, of CAST PHARMA, who designed the figures with funding from Bayer AG.
References
- 1.Brecht B., Willett J., Manheim R. Arcade Publications; New York, NY: 1994. Life of Galileo. [Google Scholar]
- 2.Ferrario C.M., Groban L., Iyer S.R., et al. Human angiotensin-(1-12) [Ang-(1-12)] is a hypertension and cardiac disease biomarker. FASEB J. 2020;34:(S1):1. [Google Scholar]
- 3.Hollenberg MD, Epstein M. The innate immune response, microenvironment proteinases, and the COVID-19 pandemic: pathophysiologic mechanisms and emerging therapeutic targets. Kidney Int Suppl. 2022;12:48–62. [DOI] [PMC free article] [PubMed]
- 4.Ferrario C.M., Ahmad S., Nagata S., et al. An evolving story of angiotensin-II-forming pathways in rodents and humans. Clin Sci (Lond) 2014;126:461–469. doi: 10.1042/CS20130400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaede P., Vedel P., Larsen N., et al. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348:383–393. doi: 10.1056/NEJMoa021778. [DOI] [PubMed] [Google Scholar]
- 6.Leoncini G., Viazzi F., De Cosmo S., et al. Blood pressure reduction and RAAS inhibition in diabetic kidney disease: therapeutic potentials and limitations. J Nephrol. 2020;33:949–963. doi: 10.1007/s40620-020-00803-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomopoulos C., Parati G., Zanchetti A. Effects of blood pressure lowering on outcome incidence in hypertension: 3. Effects in patients at different levels of cardiovascular risk—overview and meta-analyses of randomized trials. J Hypertens. 2014;32:2305–2314. doi: 10.1097/HJH.0000000000000380. [DOI] [PubMed] [Google Scholar]
- 8.Vanuzzo D. The epidemiological concept of residual risk. Intern Emerg Med. 2011;6(suppl 1):45–51. doi: 10.1007/s11739-011-0669-5. [DOI] [PubMed] [Google Scholar]
- 9.Zanchetti A., Hansson L., Dahlof B., et al. Effects of individual risk factors on the incidence of cardiovascular events in the treated hypertensive patients of the Hypertension Optimal Treatment Study. HOT Study Group. J Hypertens. 2001;19:1149–1159. doi: 10.1097/00004872-200106000-00021. [DOI] [PubMed] [Google Scholar]
- 10.Zanchetti A., Thomopoulos C., Parati G. Randomized controlled trials of blood pressure lowering in hypertension: a critical reappraisal. Circ Res. 2015;116:1058–1073. doi: 10.1161/CIRCRESAHA.116.303641. [DOI] [PubMed] [Google Scholar]
- 11.Carretero O.A., Oparil S. Essential hypertension : part II: treatment. Circulation. 2000;101:446–453. doi: 10.1161/01.cir.101.4.446. [DOI] [PubMed] [Google Scholar]
- 12.Reyes S., Varagic J., Ahmad S., et al. Novel cardiac intracrine mechanisms based on Ang-(1-12)/chymase axis require a revision of therapeutic approaches in human heart disease. Curr Hypertens Rep. 2017;19:16. doi: 10.1007/s11906-017-0708-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brugts J.J., van Vark L., Akkerhuis M., et al. Impact of renin-angiotensin system inhibitors on mortality and major cardiovascular endpoints in hypertension: a number-needed-to-treat analysis. Int J Cardiol. 2015;181:425–429. doi: 10.1016/j.ijcard.2014.11.179. [DOI] [PubMed] [Google Scholar]
- 14.Dusing R. Mega clinical trials which have shaped the RAS intervention clinical practice. Ther Adv Cardiovasc Dis. 2016;10:133–150. doi: 10.1177/1753944716644131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dusing R. Pharmacological interventions into the renin-angiotensin system with ACE inhibitors and angiotensin II receptor antagonists: effects beyond blood pressure lowering. Ther Adv Cardiovasc Dis. 2016;10:151–161. doi: 10.1177/1753944716644130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferrario C.M., Leanne G., Wang H., et al. The angiotensin-(1-12)/chymase axis as an alternate component of the tissue renin angiotensin system. Mol Cell Endocrinol. 2021;529:111119. doi: 10.1016/j.mce.2020.111119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trask A.J.F., Ferrario C.M. Singh AK and Williams GH, eds. Textbook of Nephro-Endocrinology. 2nd ed. Elsevier/Academic Press; London: 2018. The renin angiotensin system and the heart; pp. 179–188. [Google Scholar]
- 18.Turnbull F. Blood Pressure Lowering Treatment Trialists’ Collaboration. Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. Lancet. 2003;362:1527–1535. doi: 10.1016/s0140-6736(03)14739-3. [DOI] [PubMed] [Google Scholar]
- 19.Turnbull F., Woodward M., Neal B., et al. Do men and women respond differently to blood pressure-lowering treatment? Results of prospectively designed overviews of randomized trials. Eur Heart J. 2008;29:2669–2680. doi: 10.1093/eurheartj/ehn427. [DOI] [PubMed] [Google Scholar]
- 20.Unger T., Borghi C., Charchar F., et al. 2020 International Society of Hypertension global hypertension practice guidelines. J Hypertens. 2020;38:982–1004. doi: 10.1097/HJH.0000000000002453. [DOI] [PubMed] [Google Scholar]
- 21.Whelton P.K., Carey R.M., Aronow W.S., et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:e13–e115. doi: 10.1161/HYP.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 22.Williams B., Mancia G., Spiering W., et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021–3104. doi: 10.1093/eurheartj/ehy339. [DOI] [PubMed] [Google Scholar]
- 23.Abadir P.M., Walston J.D., Carey R.M. Subcellular characteristics of functional intracellular renin-angiotensin systems. Peptides. 2012;38:437–445. doi: 10.1016/j.peptides.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baker K.M., Kumar R. Intracellular angiotensin II induces cell proliferation independent of AT1 receptor. Am J Physiol Cell Physiol. 2006;291:C995–C1001. doi: 10.1152/ajpcell.00238.2006. [DOI] [PubMed] [Google Scholar]
- 25.Re R.N. Role of intracellular angiotensin II. Am J Physiol Heart Circ Physiol. 2018;314:H766–H771. doi: 10.1152/ajpheart.00632.2017. [DOI] [PubMed] [Google Scholar]
- 26.Dell'Italia L.J., Collawn J.F., Ferrario C.M. Multifunctional role of chymase in acute and chronic tissue injury and remodeling. Circ Res. 2018;122:319–336. doi: 10.1161/CIRCRESAHA.117.310978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yokosawa N., Takahashi N., Inagami T., et al. Isolation of completely inactive plasma prorenin and its activation by kallikreins. A possible new link between renin and kallikrein. Biochim Biophys Acta. 1979;569:211–219. doi: 10.1016/0005-2744(79)90056-1. [DOI] [PubMed] [Google Scholar]
- 28.Ryan JW. The fate of angiotensin II. In: Page IH, Bumpus FM (eds). Angiotensin. Handbuch der experimentellen Pharmakologie / Handbook of Experimental Pharmacology, vol 37. Springer, Berlin, Heidelberg. 10.1007/978-3-642-65600-2_4 [DOI]
- 29.Ferrario C.M. Angiotensin-converting enzyme 2 and angiotensin-(1-7): an evolving story in cardiovascular regulation. Hypertension. 2006;47:515–521. doi: 10.1161/01.HYP.0000196268.08909.fb. [DOI] [PubMed] [Google Scholar]
- 30.Ferrario C.M. New physiological concepts of the renin-angiotensin system from the investigation of precursors and products of angiotensin I metabolism. Hypertension. 2010;55:445–452. doi: 10.1161/HYPERTENSIONAHA.109.145839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferrario C.M. ACE2: more of Ang-(1-7) or less Ang II? Curr Opin Nephrol Hypertens. 2011;20:1–6. doi: 10.1097/MNH.0b013e3283406f57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uehara Y., Miura S., Yahiro E., et al. Non-ACE pathway-induced angiotensin II production. Curr Pharm Des. 2013;19:3054–3059. doi: 10.2174/1381612811319170012. [DOI] [PubMed] [Google Scholar]
- 33.Schmaier A.H. The kallikrein-kinin and the renin-angiotensin systems have a multilayered interaction. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1–R13. doi: 10.1152/ajpregu.00535.2002. [DOI] [PubMed] [Google Scholar]
- 34.Croxatto H., Croxatto R. “Pepsitensin”—a hypertensinlike substance produced by peptic digestion of proteins. Science. 1942;95:101–102. doi: 10.1126/science.95.2456.101. [DOI] [PubMed] [Google Scholar]
- 35.Rykl J., Thiemann J., Kurzawski S., et al. Renal cathepsin G and angiotensin II generation. J Hypertens. 2006;24:1797–1807. doi: 10.1097/01.hjh.0000242404.91332.be. [DOI] [PubMed] [Google Scholar]
- 36.Sealey J.E., Atlas S.A., Laragh J.H. Glandular kallikrein. N Engl J Med. 1979;301:729–730. [PubMed] [Google Scholar]
- 37.Boucher R., Demassieux S., Garcia R., et al. Tonin, angiotensin II system. A review. Circ Res. 1977;41:26–29. doi: 10.1161/01.res.41.4.26. [DOI] [PubMed] [Google Scholar]
- 38.Ocaranza M.P., Michea L., Chiong M., et al. Recent insights and therapeutic perspectives of angiotensin-(1-9) in the cardiovascular system. Clin Sci (Lond) 2014;127:549–557. doi: 10.1042/CS20130449. [DOI] [PubMed] [Google Scholar]
- 39.Yamamoto K., Ohishi M., Katsuya T., et al. Deletion of angiotensin-converting enzyme 2 accelerates pressure overload-induced cardiac dysfunction by increasing local angiotensin II. Hypertension. 2006;47:718–726. doi: 10.1161/01.HYP.0000205833.89478.5b. [DOI] [PubMed] [Google Scholar]
- 40.Batlle D., Wysocki J., Satchell K. Soluble angiotensin-converting enzyme 2: a potential approach for coronavirus infection therapy? Clin Sci (Lond) 2020;134:543–545. doi: 10.1042/CS20200163. [DOI] [PubMed] [Google Scholar]
- 41.Davidson A.M., Wysocki J., Batlle D. Interaction of SARS-CoV-2 and other coronavirus with ACE (angiotensin-converting enzyme)-2 as their main receptor: therapeutic implications. Hypertension. 2020;76:1339–1349. doi: 10.1161/HYPERTENSIONAHA.120.15256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ferrario C.M., Ahmad S., Groban L. Twenty years of progress in angiotensin converting enzyme 2 and its link to SARS-CoV-2 disease. Clin Sci (Lond) 2020;134:2645–2664. doi: 10.1042/CS20200901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rice G.I., Thomas D.A., Grant P.J., et al. Evaluation of angiotensin-converting enzyme (ACE), its homologue ACE2 and neprilysin in angiotensin peptide metabolism. Biochem J. 2004;383:45–51. doi: 10.1042/BJ20040634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Benter I.F., Diz D.I., Ferrario C.M. Cardiovascular actions of angiotensin(1-7) Peptides. 1993;14:679–684. doi: 10.1016/0196-9781(93)90097-z. [DOI] [PubMed] [Google Scholar]
- 45.Ferrario C.M., Martell N., Yunis C., et al. Characterization of angiotensin-(1-7) in the urine of normal and essential hypertensive subjects. Am J Hypertens. 1998;11:137–146. doi: 10.1016/s0895-7061(97)00400-7. [DOI] [PubMed] [Google Scholar]
- 46.Luque M., Martin P., Martell N., et al. Effects of captopril related to increased levels of prostacyclin and angiotensin-(1-7) in essential hypertension. J Hypertens. 1996;14:799–805. doi: 10.1097/00004872-199606000-00017. [DOI] [PubMed] [Google Scholar]
- 47.Ferrario C.M., Chappell M.C., Tallant E.A., et al. Counterregulatory actions of angiotensin-(1-7) Hypertension. 1997;30:535–541. doi: 10.1161/01.hyp.30.3.535. [DOI] [PubMed] [Google Scholar]
- 48.Axelband F., Dias J., Miranda F., et al. Angiotensin-(3-4) counteracts the angiotensin II inhibitory action on renal Ca2+-ATPase through a cAMP/PKA pathway. Regul Pept. 2012;177:27–34. doi: 10.1016/j.regpep.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 49.Dias J., Axelband F., Lara L.S., et al. Is angiotensin-(3-4) (Val-Tyr), the shortest angiotensin II-derived peptide, opening new vistas on the renin-angiotensin system? J Renin Angiotensin Aldosterone Syst. 2017;18 doi: 10.1177/1470320316689338. 1470320316689338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nicoll R.A., Schenker C., Leeman S.E. Substance P as a transmitter candidate. Annu Rev Neurosci. 1980;3:227–268. doi: 10.1146/annurev.ne.03.030180.001303. [DOI] [PubMed] [Google Scholar]
- 51.Balcells E., Meng Q.C., Johnson W.H., Jr., et al. Angiotensin II formation from ACE and chymase in human and animal hearts: methods and species considerations. Am J Physiol. 1997;273:H1769–H1774. doi: 10.1152/ajpheart.1997.273.4.H1769. [DOI] [PubMed] [Google Scholar]
- 52.Baker K.M., Chernin M.I., Schreiber T., et al. Evidence of a novel intracrine mechanism in angiotensin II-induced cardiac hypertrophy. Regul Pept. 2004;120:5–13. doi: 10.1016/j.regpep.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 53.Kumar R., Thomas C.M., Yong Q.C., et al. The intracrine renin-angiotensin system. Clin Sci (Lond) 2012;123:273–284. doi: 10.1042/CS20120089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lautner R.Q., Villela D.C., Fraga-Silva R.A., et al. Discovery and characterization of alamandine: a novel component of the renin-angiotensin system. Circ Res. 2013;112:1104–1111. doi: 10.1161/CIRCRESAHA.113.301077. [DOI] [PubMed] [Google Scholar]
- 55.Jankowski V., Vanholder R., van der Giet M., et al. Mass-spectrometric identification of a novel angiotensin peptide in human plasma. Arterioscler Thromb Vasc Biol. 2007;27:297–302. doi: 10.1161/01.ATV.0000253889.09765.5f. [DOI] [PubMed] [Google Scholar]
- 56.Forrester S.J., Booz G.W., Sigmund C.D., et al. Angiotensin II signal transduction: an update on mechanisms of physiology and pathophysiology. Physiol Rev. 2018;98:1627–1738. doi: 10.1152/physrev.00038.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bader M., Alenina N., Andrade-Navarro M.A., et al. MAS and its related G protein-coupled receptors. Mrgprs. Pharmacol Rev. 2014;66:1080–1105. doi: 10.1124/pr.113.008136. [DOI] [PubMed] [Google Scholar]
- 58.Chappell M.C. Biochemical evaluation of the renin-angiotensin system: the good, bad, and absolute? Am J Physiol Heart Circ Physiol. 2016;310:H137–H152. doi: 10.1152/ajpheart.00618.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Welches W.R., Brosnihan K.B., Ferrario C.M. A comparison of the properties and enzymatic activities of three angiotensin processing enzymes: angiotensin converting enzyme, prolyl endopeptidase and neutral endopeptidase 24.11. Life Sci. 1993;52:1461–1480. doi: 10.1016/0024-3205(93)90108-f. [DOI] [PubMed] [Google Scholar]
- 60.Welches W.R., Santos R.A., Chappell M.C., et al. Evidence that prolyl endopeptidase participates in the processing of brain angiotensin. J Hypertens. 1991;9:631–638. doi: 10.1097/00004872-199107000-00008. [DOI] [PubMed] [Google Scholar]
- 61.Santos N.B.D., Franco R.D., Camarini R., et al. Thimet oligopeptidase (EC 3.4.24.15) key functions suggested by knockout mice phenotype characterization. Biomolecules. 2019;9:382. doi: 10.3390/biom9080382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sambhi M.P. Proceedings of a Satellite to the Seventh Scientific Meeting of the International Society of Hypertension. Elsevier/North-Holland; New York, NY: 1981. Heterogeneity of renin and renin-substrate. [Google Scholar]
- 63.Tewksbury D.A., Tryon E.S. Immunochemical comparison of high molecular weight angiotensinogen from amniotic fluid, plasma of men, and plasma of pregnant women. Am J Hypertens. 1989;2:411–413. doi: 10.1093/ajh/2.5.411. [DOI] [PubMed] [Google Scholar]
- 64.Tewksbury D.A., Tryon E.S., Burrill R.E., et al. High molecular weight angiotensinogen: a pregnancy associated protein. Clin Chim Acta. 1986;158:7–12. doi: 10.1016/0009-8981(86)90110-5. [DOI] [PubMed] [Google Scholar]
- 65.Gordon D.B., Sachin I.N., Dodd V.N. Heterogeneity of renin substrate in human plasma: effect of pregnancy and oral contraceptives. Proc Soc Exp Biol Med. 1976;153:314–318. doi: 10.3181/00379727-153-39536. [DOI] [PubMed] [Google Scholar]
- 66.Nagata S., Kato J., Sasaki K., et al. Isolation and identification of proangiotensin-12, a possible component of the renin-angiotensin system. Biochem Biophys Res Commun. 2006;350:1026–1031. doi: 10.1016/j.bbrc.2006.09.146. [DOI] [PubMed] [Google Scholar]
- 67.Trask A.J., Jessup J.A., Chappell M.C., et al. Angiotensin-(1-12) is an alternate substrate for angiotensin peptide production in the heart. Am J Physiol Heart Circ Physiol. 2008;294:H2242–H2247. doi: 10.1152/ajpheart.00175.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moniwa N., Varagic J., Simington S.W., et al. Primacy of angiotensin converting enzyme in angiotensin-(1-12) metabolism. Am J Physiol Heart Circ Physiol. 2013;305:H644–H650. doi: 10.1152/ajpheart.00210.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ahmad S., Ferrario C.M. Chymase inhibitors for the treatment of cardiac diseases: a patent review (2010-2018) Expert Opin Ther Pat. 2018;28:755–764. doi: 10.1080/13543776.2018.1531848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ahmad S., Simmons T., Varagic J., et al. Chymase-dependent generation of angiotensin II from angiotensin-(1-12) in human atrial tissue. PLoS One. 2011;6 doi: 10.1371/journal.pone.0028501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ahmad S., Varagic J., Groban L., et al. Angiotensin-(1-12): a chymase-mediated cellular angiotensin II substrate. Curr Hypertens Rep. 2014;16:429. doi: 10.1007/s11906-014-0429-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Isa K., Garcia-Espinosa M.A., Arnold A.C., et al. Chronic immunoneutralization of brain angiotensin-(1-12) lowers blood pressure in transgenic (mRen2)27 hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2009;297:R111–R115. doi: 10.1152/ajpregu.90588.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Arnold A.C., Isa K., Shaltout H.A., et al. Angiotensin-(1-12) requires angiotensin converting enzyme and AT1 receptors for cardiovascular actions within the solitary tract nucleus. Am J Physiol Heart Circ Physiol. 2010;299:H763–H771. doi: 10.1152/ajpheart.00345.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sapru H.N. Role of the hypothalamic arcuate nucleus in cardiovascular regulation. Auton Neurosci. 2013;175:38–50. doi: 10.1016/j.autneu.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jessup J.A., Trask A.J., Chappell M.C., et al. Localization of the novel angiotensin peptide, angiotensin-(1-12), in heart and kidney of hypertensive and normotensive rats. Am J Physiol Heart Circ Physiol. 2008;294:H2614–H2618. doi: 10.1152/ajpheart.91521.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ferrario C.M., VonCannon J., Ahmad S., et al. Activation of the human angiotensin-(1-12)-chymase pathway in rats with human angiotensinogen gene transcripts. Front Cardiovasc Med. 2019;6:163. doi: 10.3389/fcvm.2019.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ferrario C.M., VonCannon J., Jiao Y., et al. Cardiac angiotensin-(1-12) expression and systemic hypertension in rats expressing the human angiotensinogen gene. Am J Physiol Heart Circ Physiol. 2016;310:H995–H1002. doi: 10.1152/ajpheart.00833.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.De Mello W.C., Dell'Itallia L.J., Varagic J., et al. Intracellular angiotensin-(1-12) changes the electrical properties of intact cardiac muscle. Mol Cell Biochem. 2016;422:31–40. doi: 10.1007/s11010-016-2801-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Reyes S., Cheng C.P., Roberts D.J., et al. Angiotensin-(1-12)/chymase axis modulates cardiomyocyte L-type calcium currents in rats expressing human angiotensinogen. Int J Cardiol. 2019;297:104–110. doi: 10.1016/j.ijcard.2019.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yamashita T., Ahmad S., Wright K.N., et al. Noncanonical mechanisms for direct bone marrow generating Ang II (angiotensin II) predominate in CD68 positive myeloid lineage cells. Hypertension. 2020;75:500–509. doi: 10.1161/HYPERTENSIONAHA.119.13754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ahmad S., Punzi H.A., Wright K.N., et al. Newly developed radioimmunoassay for human angiotensin-(1-12) measurements in plasma and urine. Mol Cell Endocrinol. 2021;529:111256. doi: 10.1016/j.mce.2021.111256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ferrario C.M., Iyer S.R., Burnett J.C., Jr., et al. Angiotensin (1-12) in humans with normal blood pressure and primary hypertension. Hypertension. 2021;77:882–890. doi: 10.1161/HYPERTENSIONAHA.120.16514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nagata S., Hatakeyama K., Asami M., et al. Big angiotensin-25: a novel glycosylated angiotensin-related peptide isolated from human urine. Biochem Biophys Res Commun. 2013;441:757–762. doi: 10.1016/j.bbrc.2013.10.124. [DOI] [PubMed] [Google Scholar]
- 84.Nagata S., Fukuda A., Kikuchi M., et al. Development of a novel AlphaLISA immunoassay for big angiotensin-25. Nephrology (Carlton) 2021;26:479–484. doi: 10.1111/nep.13845. [DOI] [PubMed] [Google Scholar]
- 85.Ahmad S., Sun X., Lin M., et al. Blunting of estrogen modulation of cardiac cellular chymase/RAS activity and function in SHR. J Cell Physiol. 2018;233:3330–3342. doi: 10.1002/jcp.26179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Butts B., Calhoun D.A., Denney T.S., Jr., et al. Plasma xanthine oxidase activity is related to increased sodium and left ventricular hypertrophy in resistant hypertension. Free Radic Biol Med. 2019;134:343–349. doi: 10.1016/j.freeradbiomed.2019.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Powell P.C., Wei C.C., Fu L., et al. Chymase uptake by cardiomyocytes results in myosin degradation in cardiac volume overload. Heliyon. 2019;5 doi: 10.1016/j.heliyon.2019.e01397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Roszkowska-Chojecka M.M., Baranowska I., Gawrys O., et al. Role of chymase in blood pressure control, plasma and tissue angiotensin II, renal haemodynamics, and excretion in spontaneously hypertensive rats. Clin Exp Hypertens. 2021;43:392–401. doi: 10.1080/10641963.2021.1890762. [DOI] [PubMed] [Google Scholar]
- 89.Ansary T.M., Urushihara M., Fujisawa Y., et al. Effects of the selective chymase inhibitor TEI-F00806 on the intrarenal renin-angiotensin system in salt-treated angiotensin I-infused hypertensive mice. Exp Physiol. 2018;103:1524–1531. doi: 10.1113/EP087209. [DOI] [PubMed] [Google Scholar]
- 90.Ondetti M.A., Williams N.J., Sabo E.F., et al. Angiotensin-converting enzyme inhibitors from the venom of Bothrops jararaca. Isolation, elucidation of structure, and synthesis. Biochemistry. 1971;10:4033–4039. doi: 10.1021/bi00798a004. [DOI] [PubMed] [Google Scholar]
- 91.Bakris G.L., Oparil S., Purkayastha D., et al. Randomized study of antihypertensive efficacy and safety of combination aliskiren/valsartan vs valsartan monotherapy in hypertensive participants with type 2 diabetes mellitus. J Clin Hypertens (Greenwich) 2013;15:92–100. doi: 10.1111/jch.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pimenta E., Oparil S. Role of aliskiren in cardio-renal protection and use in hypertensives with multiple risk factors. Ther Clin Risk Manag. 2009;5:459–464. doi: 10.2147/tcrm.s5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Epstein M., Brunner H.R. Hanley & Belfus; Philadelphia, PA: 2001. Angiotensin II Receptor Antagonists. [Google Scholar]
- 94.Dzau V.J., Balatbat C.A. Future of hypertension. Hypertension. 2019;74:450–457. doi: 10.1161/HYPERTENSIONAHA.119.13437. [DOI] [PubMed] [Google Scholar]
- 95.Gyurko R., Wielbo D., Phillips M.I. Antisense inhibition of AT1 receptor mRNA and angiotensinogen mRNA in the brain of spontaneously hypertensive rats reduces hypertension of neurogenic origin. Regul Pept. 1993;49:167–174. doi: 10.1016/0167-0115(93)90438-e. [DOI] [PubMed] [Google Scholar]
- 96.Haber E. Immunologic approaches to the inhibition of renin and the adrenergic system. Fed Proc. 1983;42:2661–2666. [PubMed] [Google Scholar]
- 97.Haber E. Inhibitors of renin: present and future. Clin Exp Hypertens A. 1983;5:1193–1205. doi: 10.3109/10641968309048851. [DOI] [PubMed] [Google Scholar]
- 98.Haber E. Peptide inhibitors of renin in cardiovascular studies. Fed Proc. 1983;42:3155–3161. [PubMed] [Google Scholar]
- 99.Dzau V.J., Devine D., Mudgett-Hunter M., et al. Antibodies as specific renin inhibitors: studies with polyclonal and monoclonal antibodies and Fab fragments. Clin Exp Hypertens A. 1983;5:1207–1220. doi: 10.3109/10641968309048852. [DOI] [PubMed] [Google Scholar]
- 100.Dzau V.J., Ellison K.E., Brody T., et al. A comparative study of the distributions of renin and angiotensinogen messenger ribonucleic acids in rat and mouse tissues. Endocrinology. 1987;120:2334–2338. doi: 10.1210/endo-120-6-2334. [DOI] [PubMed] [Google Scholar]
- 101.Michel J.B., Sayah S., Guettier C., et al. Physiological and immunopathological consequences of active immunization of spontaneously hypertensive and normotensive rats against murine renin. Circulation. 1990;81:1899–1910. doi: 10.1161/01.cir.81.6.1899. [DOI] [PubMed] [Google Scholar]
- 102.Mullick A.E., Yeh S.T., Graham M.J., et al. Blood pressure lowering and safety improvements with liver angiotensinogen inhibition in models of hypertension and kidney injury. Hypertension. 2017;70:566–576. doi: 10.1161/HYPERTENSIONAHA.117.09755. [DOI] [PubMed] [Google Scholar]
- 103.Uijl E., Mirabito Colafella K.M., Sun Y., et al. Strong and sustained antihypertensive effect of small interfering RNA targeting liver angiotensinogen. Hypertension. 2019;73:1249–1257. doi: 10.1161/HYPERTENSIONAHA.119.12703. [DOI] [PubMed] [Google Scholar]
- 104.Uijl E., Ren L., Mirabito Colafella K.M., et al. No evidence for brain renin-angiotensin system activation during DOCA-salt hypertension. Clin Sci (Lond) 2021;135:259–274. doi: 10.1042/CS20201239. [DOI] [PubMed] [Google Scholar]
- 105.Sun H., Hodgkinson C., Pratt R.E., et al. Abstract 15555: potential cure for hypertension? The effect of Crispr genome editing. Circulation. 2020;142:A15555. [Google Scholar]
- 106.Wu C.H., Wang Y., Ma M., et al. Antisense oligonucleotides targeting angiotensinogen: insights from animal studies. Biosci Rep. 2019;39 doi: 10.1042/BSR20180201. BSR20180201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Huang S.A., Taubel J., Fiore G., et al. Abstract 14387: Dose-related reductions in blood pressure with a RNA interference (RNAi) therapeutic targeting angiotensinogen in hypertensive patients: interim results from a first-in-human phase 1 study of ALN-AGT01. Circulation. 2020;142:A14387. [Google Scholar]
- 108.Bodewes R., Kreijtz J.H., van Amerongen G., et al. A single immunization with CoVaccine HT-adjuvanted H5N1 influenza virus vaccine induces protective cellular and humoral immune responses in ferrets. J Virol. 2010;84:7943–7952. doi: 10.1128/JVI.00549-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lakhan N., Stevens N.E., Diener K.R., et al. CoVaccine HT adjuvant is superior to Freund's adjuvants in eliciting antibodies against the endogenous alarmin HMGB1. J Immunol Methods. 2016;439:37–43. doi: 10.1016/j.jim.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 110.Tissot A.C., Maurer P., Nussberger J., et al. Effect of immunisation against angiotensin II with CYT006-AngQb on ambulatory blood pressure: a double-blind, randomised, placebo-controlled phase IIa study. Lancet. 2008;371:821–827. doi: 10.1016/S0140-6736(08)60381-5. [DOI] [PubMed] [Google Scholar]
- 111.Maurer P., Bachmann M.F. Immunization against angiotensins for the treatment of hypertension. Clin Immunol. 2010;134:89–95. doi: 10.1016/j.clim.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 112.Phisitkul S. CYT-006-AngQb, a vaccine against angiotensin II for the potential treatment of hypertension. Curr Opin Investig Drugs. 2009;10:269–275. [PubMed] [Google Scholar]
- 113.Phillips M.I., Gyurko R. In vivo applications of antisense oligonucleotides for peptide research. Regul Pept. 1995;59:131–141. doi: 10.1016/0167-0115(95)00104-j. [DOI] [PubMed] [Google Scholar]
- 114.Phillips M.I., Kimura B. Antisense therapeutics for hypertension: targeting the renin-angiotensin system. Methods Mol Med. 2005;106:51–68. [PubMed] [Google Scholar]
- 115.Ferrario C.M., Groban L., Wang H., Ahmad S. Letter to the editor: brain renin-angiotensin system and liver-directed siRNA targeted to angiotensinogen. Clin Sci (Lond) 2021;135:907–910. doi: 10.1042/CS20210163. [DOI] [PubMed] [Google Scholar]
- 116.Ohkubo H., Kageyama R., Ujihara M., et al. Cloning and sequence analysis of cDNA for rat angiotensinogen. Proc Natl Acad Sci U S A. 1983;80:2196–2200. doi: 10.1073/pnas.80.8.2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lu H., Cassis L.A., Vander Kooi C.W., Daugherty A. Structure and functions of angiotensinogen. Hypertens Res. 2016;39:492–500. doi: 10.1038/hr.2016.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lu H., Cassis L.A., Vander Kooi C.W., Daugherty A. Corrigendum: structure and functions of angiotensinogen. Hypertens Res. 2016;39:827. doi: 10.1038/hr.2016.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lu H., Wu C., Howatt D.A., et al. Angiotensinogen exerts effects independent of angiotensin II. Arterioscler Thromb Vasc Biol. 2016;36:256–265. doi: 10.1161/ATVBAHA.115.306740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bouquet C., Lamande N., Brand M., et al. Suppression of angiogenesis, tumor growth, and metastasis by adenovirus-mediated gene transfer of human angiotensinogen. Mol Ther. 2006;14:175–182. doi: 10.1016/j.ymthe.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 121.Celerier J., Cruz A., Lamande N., et al. Angiotensinogen and its cleaved derivatives inhibit angiogenesis. Hypertension. 2002;39:224–228. doi: 10.1161/hy0202.103441. [DOI] [PubMed] [Google Scholar]
- 122.Corvol P., Lamande N., Cruz A., et al. Inhibition of angiogenesis: a new function for angiotensinogen and des(angiotensin I)angiotensinogen. Curr Hypertens Rep. 2003;5:149–154. doi: 10.1007/s11906-003-0072-3. [DOI] [PubMed] [Google Scholar]
- 123.Vincent F., Bonnin P., Clemessy M., et al. Angiotensinogen delays angiogenesis and tumor growth of hepatocarcinoma in transgenic mice. Cancer Res. 2009;69:2853–2860. doi: 10.1158/0008-5472.CAN-08-2484. [DOI] [PubMed] [Google Scholar]
- 124.Kakinuma Y., Hama H., Sugiyama F., et al. Impaired blood-brain barrier function in angiotensinogen-deficient mice. Nat Med. 1998;4:1078–1080. doi: 10.1038/2070. [DOI] [PubMed] [Google Scholar]
- 125.Massiera F., Seydoux J., Geloen A., et al. Angiotensinogen-deficient mice exhibit impairment of diet-induced weight gain with alteration in adipose tissue development and increased locomotor activity. Endocrinology. 2001;142:5220–5225. doi: 10.1210/endo.142.12.8556. [DOI] [PubMed] [Google Scholar]
- 126.Kobori H., Kamiyama M., Harrison-Bernard L.M., et al. Cardinal role of the intrarenal renin-angiotensin system in the pathogenesis of diabetic nephropathy. J Investig Med. 2013;61:256–264. doi: 10.231/JIM.0b013e31827c28bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kobori H., Urushihara M., Xu J.H., et al. Urinary angiotensinogen is correlated with blood pressure in men (Bogalusa Heart Study) J Hypertens. 2010;28:1422–1428. doi: 10.1097/HJH.0b013e3283392673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Navar L.G. Intrarenal renin-angiotensin system in regulation of glomerular function. Curr Opin Nephrol Hypertens. 2014;23:38–45. doi: 10.1097/01.mnh.0000436544.86508.f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ferrario C.M., Jessup J., Chappell M.C., et al. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111:2605–2610. doi: 10.1161/CIRCULATIONAHA.104.510461. [DOI] [PubMed] [Google Scholar]
- 130.Misra S., Stevermer J.J. ACE inhibitors and ARBs: one or the other—not both—for high-risk patients. J Fam Pract. 2009;58:24–27. [PMC free article] [PubMed] [Google Scholar]
- 131.Downham M.R., Auton T.R., Rosul A., et al. Evaluation of two carrier protein-angiotensin I conjugate vaccines to assess their future potential to control high blood pressure (hypertension) in man. Br J Clin Pharmacol. 2003;56:505–512. doi: 10.1046/j.1365-2125.2003.01926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ambuhl P.M., Tissot A.C., Fulurija A., et al. A vaccine for hypertension based on virus-like particles: preclinical efficacy and phase I safety and immunogenicity. J Hypertens. 2007;25:63–72. doi: 10.1097/HJH.0b013e32800ff5d6. [DOI] [PubMed] [Google Scholar]
- 133.Nakamaru R., Nakagami H., Hayashi H., et al. A novel angiotensin II peptide vaccine without an adjuvant in mice. J Hypertens. 2021;39:181–189. doi: 10.1097/HJH.0000000000002597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Nakamaru R., Nakagami H., Rakugi H., et al. Future directions of therapeutic vaccines for chronic diseases. Circ J. 2020;84:1895–1902. doi: 10.1253/circj.CJ-20-0703. [DOI] [PubMed] [Google Scholar]
- 135.Wallukat G., Schimke I. Agonistic autoantibodies directed against G-protein-coupled receptors and their relationship to cardiovascular diseases. Semin Immunopathol. 2014;36:351–363. doi: 10.1007/s00281-014-0425-9. [DOI] [PubMed] [Google Scholar]
- 136.Dragun D., Muller D.N., Brasen J.H., et al. Angiotensin II type 1-receptor activating antibodies in renal-allograft rejection. N Engl J Med. 2005;352:558–569. doi: 10.1056/NEJMoa035717. [DOI] [PubMed] [Google Scholar]
- 137.Rossitto G., Regolisti G., Rossi E., et al. Elevation of angiotensin-II type-1-receptor autoantibodies titer in primary aldosteronism as a result of aldosterone-producing adenoma. Hypertension. 2013;61:526–533. doi: 10.1161/HYPERTENSIONAHA.112.202945. [DOI] [PubMed] [Google Scholar]
- 138.Adams G.P., Weiner L.M. Monoclonal antibody therapy of cancer. Nat Biotechnol. 2005;23:1147–1157. doi: 10.1038/nbt1137. [DOI] [PubMed] [Google Scholar]
- 139.Lu R.M., Hwang Y.C., Liu I.J., et al. Development of therapeutic antibodies for the treatment of diseases. J Biomed Sci. 2020;27:1. doi: 10.1186/s12929-019-0592-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Scheen A.J. Prevention of type 2 diabetes mellitus through inhibition of the renin-angiotensin system. Drugs. 2004;64:2537–2565. doi: 10.2165/00003495-200464220-00004. [DOI] [PubMed] [Google Scholar]
- 141.Epstein M. Effects of ACE inhibitors and calcium antagonists on progression of chronic renal disease. Blood Press Suppl. 1995;2:108–112. [PubMed] [Google Scholar]
- 142.Epstein M. Re-examining RAS-blocking treatment regimens for abrogating progression of chronic kidney disease. Nat Clin Pract Nephrol. 2009;5:12–13. doi: 10.1038/ncpneph0980. [DOI] [PubMed] [Google Scholar]
- 143.Roig E., Perez-Villa F., Morales M., et al. Clinical implications of increased plasma angiotensin II despite ACE inhibitor therapy in patients with congestive heart failure. Eur Heart J. 2000;21:53–57. doi: 10.1053/euhj.1999.1740. [DOI] [PubMed] [Google Scholar]
- 144.Dupre E., Danis C., Arrial A., et al. Single domain antibody fragments as new tools for the detection of neuronal Tau protein in cells and in mice studies. ACS Chem Neurosci. 2019;10:3997–4006. doi: 10.1021/acschemneuro.9b00217. [DOI] [PubMed] [Google Scholar]
- 145.Schumacher D., Helma J., Schneider A.F.L., et al. Nanobodies: chemical functionalization strategies and intracellular applications. Angew Chem Int Ed Engl. 2018;57:2314–2333. doi: 10.1002/anie.201708459. [DOI] [PMC free article] [PubMed] [Google Scholar]