Abstract
Fluoroquinolone antibiotic agents have demonstrated efficacy in the treatment of respiratory tract infections. This analysis was designed to examine the relationship between drug exposure, as measured by the free-drug area under the concentration-time curve at 24 h (AUC24)/MIC ratio, and clinical and microbiological responses in patients with community-acquired respiratory tract infections involving Streptococcus pneumoniae. The study population included 58 adult patients (34 males, 24 females) who were enrolled in either of two phase III, randomized, multicenter, double-blind studies of levofloxacin versus gatifloxacin for the treatment of community-acquired pneumonia or acute exacerbation of chronic bronchitis. Clearance equations from previously published population pharmacokinetic models were used in conjunction with dose and adjusted for protein binding to estimate individual patient free-drug AUC24s. In vitro susceptibility was determined in a central laboratory by broth microdilution in accordance with NCCLS guidelines. Pharmacodynamic analyses were performed on data from all evaluable patients with documented S. pneumoniae infection using univariate and multivariable logistic regression; pharmacodynamic breakpoints were estimated using Classification and Regression Tree analysis. A statistically significant (P = 0.013) relationship between microbiological response and the free-drug AUC24/MIC ratio was detected. At a free-drug AUC24/MIC ratio of <33.7, the probability of a microbiological response was 64%, and at a free-drug AUC24/MIC ratio of >33.7, it was 100% (P < 0.01). These findings may provide a minimum target free-drug AUC24/MIC ratio for the treatment of infections involving S. pneumoniae with fluoroquinolone antibiotics and provide a paradigm for the selection of fluoroquinolones to be brought forward from drug discovery into clinical development and dose selection for clinical trials. Further, when target free-drug AUC24/MIC ratios are used in conjunction with stochastic modeling techniques, these findings may be used to support susceptibility breakpoints for fluoroquinolone antibiotics and S. pneumoniae.
The relationship that exists between drug exposure and the MIC for an infectious organism has been shown to be predictive of microbiological eradication (3, 4, 5, 14). Existing data suggest that, for fluoroquinolones, an area under the concentration-time curve at 24 h (AUC24)/MIC ratio of 100 to 125 correlates with optimal clinical and microbiological outcomes in seriously ill patients infected with gram-negative enteric pathogens and Pseudomonas aeruginosa (7, 8). However, over the past several years there has been considerable controversy as to whether or not this pharmacodynamic target applies to all patient populations and all organisms.
Data from in vitro and animal models of infection have recently emerged and suggest that, for Streptococcus pneumoniae, the optimal free-drug AUC24/MIC ratio is much lower than 100 to 125. For instance, an in vitro model of pneumococcal infection demonstrated that, for levofloxacin and ciprofloxacin, free-drug AUC24/MIC ratios of 30 were associated with a 4-log-unit kill but that ratios less than 30 were associated with significantly reduced extents of bacterial killing and in some instances regrowth (10). Similarly, Lister and Sanders reported that, for levofloxacin and ciprofloxacin, free-drug AUC24/MIC ratios of 32 to 64 were associated with eradication of S. pneumoniae from an in vitro model of infection (11). These observations are supported by data from nonneutropenic animal models of pneumococcal infection, where for ciprofloxacin, gatifloxacin, gemifloxacin, levofloxacin, moxifloxacin, and sitafloxacin survival was associated with a free-drug AUC24/MIC ratio of 25 to 34 (W. A. Craig and D. R. Andes, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 289, 2000). Limited prospective clinical data also suggest that the free-drug AUC24/MIC ratios associated with optimal clinical outcome are significantly less than 125 for community-acquired pathogens (7; G. L. Drusano, unpublished data).
To clarify this issue further, we investigated the pharmacodynamics of levofloxacin and gatifloxacin against S. pneumoniae in patients with community-acquired respiratory tract infections.
MATERIALS AND METHODS
Patient population and clinical data.
Study data were obtained from two double-blind, randomized, multicenter, phase III clinical trials comparing levofloxacin and gatifloxacin for the treatment of either community-acquired pneumonia (CAP) or acute exacerbation of chronic bronchitis (AECB) (15, 17). The studies were performed in accordance with guidelines developed by the Infectious Disease Society of America for evaluation of new anti-infective agents for treatment of respiratory tract infections (2). Moreover, these studies were performed in compliance with Institutional Review Board and Code of Federal Regulations standards and the principles of the Declaration of Helsinki and its amendments.
Patients 18 years of age and older who had a history of chronic bronchitis were eligible for enrollment into the AECB study. Chronic bronchitis was defined as a productive cough on most days for at least three consecutive months in two consecutive years. Patients were selected for inclusion on the basis of having increases in at least two of the following signs and symptoms: cough and/or dyspnea, sputum volume, and sputum purulence.
Exclusion criteria for the AECB study consisted of pregnancy, lactation, and inability or unwillingness to use effective birth control, radiographically documented pulmonary infiltrates, and recent (within 7 days) or concurrent systemic antibacterial therapy. Patents receiving antiviral agents, antifungal agents, or oral corticosteroids were enrolled if all other inclusion criteria and none of the exclusion criteria were met.
Ambulatory or hospitalized patients 18 years of age and older with a clinical diagnosis of CAP were eligible for enrollment into the CAP study. The clinical diagnosis of CAP required evidence of a new pulmonary infiltrate on chest radiograph and two or more of the following clinical findings: fever (oral temperature of 38°C or higher); leukocytosis (greater than 10,000 white blood cells/μl or greater than 15% bands); cough; chest pain; purulent sputum (more than 25 polymorphonuclear neutrophils and fewer than 10 squamous epithelial cells per low-power optical field); transtracheal aspirate, bronchial brushings, or biopsy material demonstrating neutrophils and a predominate pathogen on Gram staining; a direct lung aspirate with a predominate pathogen on Gram staining; and auscultatory findings.
Patients were excluded from the CAP study for any of the following reasons: if more than one dose of a systemic antibiotic was administered within 7 days prior to enrollment, if the requirement for another systemic antibiotic seemed likely during the study period, if there were clinical reasons necessitating more than 14 days of antibiotic therapy, or if patients were likely to die of intercurrent disease within 3 days.
Also excluded from the CAP study were patients with evidence of the following: a rapidly progressive underlying disease that would preclude evaluation of response to therapy; a preexisting medical condition that mimicked or altered the course of therapy; known or suspected active tuberculosis or other pulmonary infection caused by mycobacteria, fungi, parasites, or viruses; empyema; an immunologic disease; or long-term use (at least 2 weeks) of corticosteroids (equivalent to 10 mg or more of prednisone per day) or other immunosuppressive agents.
Patients with known serious hypersensitivity to quinolone antibiotics, renal insufficiency (serum creatinine level higher than 1.5 mg/dl or renal dialysis), or clinically significant hepatic disease (alanine aminotransferase, aspartate aminotransferase, and/or bilirubin levels three or more times the upper limit of normal) were excluded from both studies. Finally, patients with any malabsorption syndrome or other gastrointestinal condition that would affect drug absorption and pregnant or breast-feeding women were not enrolled in either study. No patient participated in both studies.
Drug dosage and administration.
In the AECB study, patients were randomly assigned to receive either 500 mg of levofloxacin or 400 mg of gatifloxacin every 24 h administered orally for 7 to 10 days. In the CAP study, patients were randomly assigned to receive either 500 mg of levofloxacin or 400 mg of gatifloxacin every 24 h for 7 to 14 days. Gatifloxacin was administered either orally or as an intravenous infusion over 1 h. Levofloxacin was administered either orally or as an intravenous infusion over 1 h. At the investigator's discretion, patients received either oral therapy alone or intravenous therapy followed by oral therapy.
Outcome evaluation.
Clinical response was determined by comparing the patient's baseline signs and symptoms of infection with those after therapy and then categorized as either cure or failure. Cure was defined as resolution or improvement of all signs and symptoms present at study entry at the test-of-cure visit (7 to 14 days after the end of therapy) without need of further antibiotics. Failure was defined as any one or more of the following circumstances: persistent or worsened signs and symptoms after at least 3 days of therapy, new clinical findings consistent with progression of infection, progressive radiological abnormalities, additional antibiotic therapy needed for the study indication, and/or death due to the study indication.
Microbiologically evaluable patients were those clinically evaluable patients with a susceptible pretreatment pathogen. The microbiological response to therapy was determined 7 to 14 days after the completion of study drug therapy and was classified as either eradicated, presumed eradicated, persistent, or presumed persistent. “Eradicated” was defined as the absence of the pretreatment pathogen from the posttreatment sputum. If a patient's clinical response was classified as a cure and no material was available for culture, the pretreatment pathogen was presumed eradicated. “Persistent” was defined as the presence of the pretreatment pathogen in the posttreatment culture. If a patient's clinical response was classified as a failure and no material was available for culture, the pretreatment pathogen was considered presumed persistent.
Microbiological susceptibility testing and determination of drug exposure.
All isolated pathogens were tested for susceptibility to levofloxacin and gatifloxacin by broth microdilution in accordance with guidelines recommended by the National Committee for Clinical Laboratory Standards (NCCLS) (12)
For the subset of clinically and microbiologically evaluable patients infected with S. pneumoniae (n = 58), estimates of clearance were generated by using each patient's demographic characteristics and a regression equation. For levofloxacin, equation 1, a previously published and validated regression equation, was used; for gatifloxacin, equation 2 was used (13; D. M. Grasela, unpublished data). clearance = constant + race + (age · −0.032) + (creatinine clearance · 0.070) (1)clearance = 8.11 + 0.0629 (creatinine clearance − 75.0) (2)
The clearance estimates were then used in conjunction with dose to obtain a total-drug AUC24 for each patient (equation 3). total-drug AUC24 = dose/clearance (3)
The total-drug AUC24 estimates were subsequently used in combination with protein-binding data to estimate a free-drug AUC24 for each patient (equation 4). free-drug AUC24 = total-drug AUC24 · unbound fraction (4)
Pharmacodynamic analysis.
The evaluation of an antimicrobial agent requires both a measure of drug exposure and a measure of drug potency for a given pathogen. The measure of drug exposure chosen was the free-drug AUC24, and the measure of drug potency was the MIC. The free-drug AUC24/MIC ratio was determined by dividing the free-drug AUC24 for each patient by the MIC for the patient's isolate.
Univariate and multivariable logistic regression analyses were used to evaluate the probability of clinical cure and microbiological eradication and were performed using the Logistic procedure of SAS software, version 6.12 (16). For the purposes of these analyses, free-drug AUC24/MIC ratios were treated as categorical data, and levofloxacin and gatifloxacin free-drug AUC24/MIC ratios were pooled.
Both the clinical and microbiological analyses used stepwise model selection with an entry level of significance of 0.05 and an exit level of significance of 0.05. Demographic variables considered in the analyses included age, gender, weight, and creatinine clearance; pharmacodynamic variables included MIC, free-drug AUC24, and free-drug AUC24/MIC. For the clinical evaluation, patients were classified as cured or the treatment was classified a failure as previously described. For the microbiological evaluation, eradication and presumed eradication of the organism were considered successful outcomes; the persistence and presumed persistence of the organism were considered unsuccessful outcomes.
Classification and Regression Tree (CART) analysis (as implemented in S-Plus 2000 professional release 2) was used to select a breakpoint(s) which partitioned patients' responses on the basis of free-drug AUC24/MIC ratios.
Model bias and precision.
Model bias and precision were evaluated using drug concentration data from the subset of patients from whom actual drug concentration data were obtained. The plasma-sampling schedules included two steady-state time points (peak and trough) and were measured using high-performance liquid chromatographic assay (9). A plot of free-drug AUC24s estimated using these concentrations in plasma in conjunction with demographic data versus free-drug AUC24s estimated using demographic data alone was made to validate the regression equation used in these analyses. The slope, y intercept, r value, median bias, and level of precision for AUC prediction were calculated.
RESULTS
Of the 778 patients randomized to receive either levofloxacin or gatifloxacin for the therapy of CAP or AECB, 635 were clinically evaluable. Most patients categorized as clinically unevaluable were either clinically ineligible (i.e., a new infiltrate on pretreatment radiograph not confirmed by a radiologist was present), failed to undergo a test-of-cure assessment, or received inadequate antibiotic dosing. Of these 635 patients, 376 were microbiologically evaluable. Microbiologically evaluable patients represent the subset of clinically evaluable patients who had an identified microorganism. Of the 376 microbiologically evaluable patients, 58 had CAP or AECB associated with S. pneumoniae. These 58 patients represent the population used in all pharmacodynamic analyses.
Of the 58 patients, 34 were male and 24 were female; their ages were between 20 and 83 years. The numbers of patients treated for CAP and AECB were essentially equal. The four microbiological responses classified as unsuccessful were evenly distributed between the CAP and AECB studies. Of the seven clinical responses classified as unsuccessful, two were from the CAP study and five were from the AECB study. Approximately 69% of patients were white, 28% were black, and 3% were classified as other. Patient demographics stratified by study drug are presented in Table 1. Overall, there were no significant differences in demographic variables between those patients treated with levofloxacin and those treated with gatifloxacin. The median MIC of levofloxacin for baseline pneumococcal isolates was 1.0, and the MIC range was between 0.015 and 1.0 μg/ml. The median MIC of gatifloxacin for baseline pneumococcal isolates was 0.25, and the MIC range was between 0.03 and 0.25 μg/ml.
TABLE 1.
Demographic data of the study population
| Parameter | Value for group
|
||
|---|---|---|---|
| Gatifloxacin | Levofloxacin | All patients | |
| No. of subjects | 25 | 33 | 58 |
| Mean age (yr) (SD) | 49 (17.5) | 53 (17.0) | 51 (17.2) |
| % Male | 56 | 61 | 59 |
| Mean height (cm) (SD) | 172.6 (10.9) | 173.8 (9.3) | 173.2 (10.0) |
| Mean weight (kg) (SD) | 76.9 (22.3) | 74.7 (14.6) | 75.7 (18.2) |
| Race (%) | |||
| White | 60 | 76 | 69 |
| Black | 32 | 24 | 28 |
| Other | 8 | 0 | 3 |
| Mean level of creatinine (mg/dl) (SD) | 0.88 (0.31) | 0.80 (0.24) | 0.84 (0.27) |
| Mean CLCRa (ml/min) (SD) | 97 (41) | 106 (42) | 102 (42) |
| Disease (%) | |||
| CAP | 48 | 48 | 48 |
| AECB | 52 | 52 | 52 |
CLCR, creatinine clearance.
Model bias and precision.
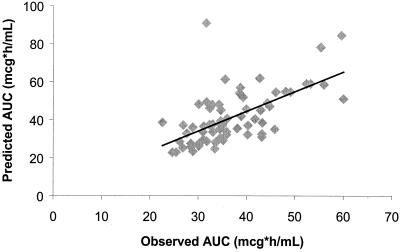
Of the 778 patients randomized to receive study drug for the therapy of CAP or AECB, 67 had measured plasma gatifloxacin concentrations. A plot of free-drug AUC24/MIC ratios estimated using concentrations in plasma in conjunction with demographic data versus free-drug AUC24/MIC ratios estimated using demographic data alone is presented in Fig. 1. The slope, y intercept, and r value were 1.03, 3.01, and 0.62, respectively, and for the prediction of free-drug AUC24 the median bias was 0.05 and the level of precision was 17.6%.
FIG. 1.
Observed versus predicted gatifloxacin free-drug AUC24s for 67 patients. The slope is 1.03, and the y intercept is 3.01. For the prediction of free-drug AUC24, the median bias and median level of precision were 0.05 and 17.6%, respectively. The correlation coefficient (r) was 0.62.
Pharmacodynamic analysis.
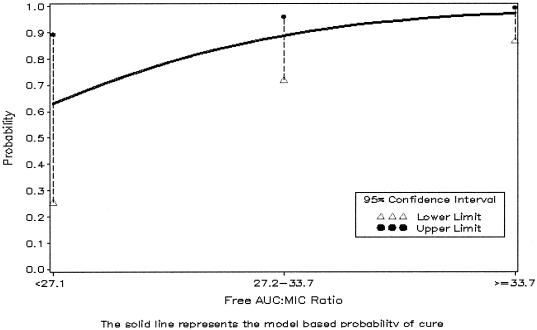
The covariates tested for association with microbiological response were the same for both the univariate and multivariable analyses. In the univariate analysis, a free-drug AUC24/MIC ratio below 33.7 was inferior for microbiological response compared with ratios greater than 33.7 (64% versus 100%, P < 0.01). Because the free-drug AUC24/MIC ratio was the only covariate that reached a statistical significance level of 0.05 (Table 2), the results of the multivariable analysis were identical. The probability plot with the CART-determined breakpoint (i.e., 33.7) for microbiological response is illustrated in Fig. 2. The percentage of patients with a positive microbiological response are stratified by free-drug AUC24/MIC ratios in Table 3. The percentages of microbiological cures for patients whose free-drug AUC24/MIC ratios were 21 to 30 were similar to those for patients whose ratios were 31 to 40. Further, as the free-drug AUC24/MIC ratios increased beyond 40, there was no possible improvement in microbiological response.
TABLE 2.
Logistic regression analysis results (microbiological response)a
| Covariate | Significance | Covariate | P value |
|---|---|---|---|
| Age | 0.3835 | Free-drug AUC | 0.2165 |
| Gender | 0.5003 | MIC | 0.999c |
| CLCRb | 0.2551 | Free-drug AUC/MICd | 0.0131 |
| Weight | 0.3801 |
The number of organisms was 58.
CLCR, creatinine clearance.
Data convergence.
AUC/MIC was treated as a categorical variable; two breakpoints were identified: 27.1 and 33.7.
FIG. 2.
Probability of microbiological eradication (n = 58 organisms; four patients experienced persistent infection). Two breakpoints, 27.1 and 33.7, were identified by CART analysis. Overall, the higher breakpoint of 33.7 represents the free-drug AUC24/MIC ratio above which there is a significantly increased probability of bacterial eradication.
TABLE 3.
Clinical and microbiological responses stratified by free-drug AUC24/MIC ratios
| AUC/MIC ratio range | No. of patients | Clinical cure
|
Microbiological cure
|
||
|---|---|---|---|---|---|
| No. of patients | % | No. of patients | % | ||
| 21–30 | 8 | 6 | 75 | 6 | 75 |
| 31–40 | 6 | 4 | 66 | 4 | 66 |
| 41–100 | 13 | 13 | 100 | 13 | 100 |
| 101–150 | 9 | 8 | 89 | 9 | 100 |
| 151–200 | 4 | 4 | 100 | 4 | 100 |
| 201–250 | 4 | 3 | 75 | 4 | 100 |
| 251–300 | 4 | 4 | 100 | 4 | 100 |
| 301–350 | 3 | 3 | 100 | 3 | 100 |
| >350 | 7 | 6 | 86 | 7 | 100 |
In the univariate analysis of clinical response, the results for no variable were significant and therefore multivariable analysis was not necessary. The percentages of patients with a positive clinical response are stratified by free-drug AUC24/MIC ratios in Table 3.
DISCUSSION
The pharmacodynamics of fluoroquinolone antimicrobial agents have been well elucidated. These agents have concentration-dependent bactericidal effects against gram-positive and gram-negative bacteria, and their free-drug AUC24/MIC ratios generally have the strongest correlation with outcome in animal and in vitro models of infection and in human infections (6, 7, 10; W. A. Craig and D. R. Andes, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr 289, 2000). Current data indicate that the pharmacodynamic goal of therapy for gram-positive microorganisms appears to be different from that for gram-negative microorganisms. For gram-negative microorganisms, an AUC24/MIC ratio of at least 125 has been associated with optimal clinical and microbiological outcomes in patients with serious infections (8). Unfortunately, this target has been inappropriately applied to all other microorganisms and all other patient populations. The data indicate that in stochastic models, in animal and in vitro models of infection, and in less severely ill patients with gram-positive bacterial infections, lower free-drug AUC24/MIC ratios (i.e., 25 to 34) are appropriate (1, 7, 10, 11; Craig and Andes, 40th ICAAC; N. L. Jumbe, A. Louie, W. Liu, M. H. Miller, and G. L. Drusano, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr 291, 2000; G. L. Drusano, unpublished data). The microbiological response rate and CART analysis determined that a free-drug AUC24/MIC ratio breakpoint of 33.7 in the present study is consistent with the aforementioned observations from animal and in vitro models of infections, stochastic models, and the limited clinical data that is available in the literature. One limitation of the present analysis, as well other similar analyses (7, 8, 14), is that there were few failures in the data set, which limits the precision in setting target exposure breakpoints. However, pharmacodynamic modeling can be utilized to minimize the risk of obtaining suboptimal drug exposures in clinical trials.
When one compares the patients classified as having successful microbiological outcomes with those classified as having unsuccessful microbiological outcomes, it is interesting that the patients classified as having failed therapy were, on average, younger (44 versus 53 years) and had higher estimated creatinine clearances (117 versus 100 ml/min). These factors result in greater drug clearance rates and, therefore, a lower level of drug exposure. Further, the MICs for pneumococcal isolates from patients classified as having failed therapy tended to be higher (i.e., each being 1.0 μg/ml) than those for isolates from patients classified as having been cured. Obviously, these factors combined result in low free-drug AUC24/MIC ratios.
In the present study, the correlation between clinical response and the free-drug AUC24/MIC ratio was not significant. This lack of significance should not be interpreted to mean that these two variables are unrelated; it is often difficult to determine if a lack of clinical improvement is due to failure of the antibiotic to kill the pathogen or due to some other factor. Reasons known to complicate clinical response determinations in clinical trials include comorbid conditions, underlying viral infection, and/or a clinician's lack of familiarity with the patient's baseline health status. In the present study, the mean free-drug AUC24/MIC ratio of the patients who failed to show a clinical response above a ratio of 33.7 was 472. These levels of exposure would be clearly expected to eradicate S. pneumoniae in animal and in vitro models of infection; therefore, considering these high drug exposures and that these patients had positive microbiological responses, it is possible that other factors may be responsible for the lack of clinical improvement.
In summary, a relationship between free-drug AUC24/MIC ratio and microbiological response in patients with community-acquired respiratory tract infections involving S. pneumoniae was observed in this study. The free-drug AUC24/MIC ratio associated with a high probability of bacterial eradication in this patient population was >33.7, which is significantly lower than AUC/MIC ratios associated with positive outcomes in severely ill patients with infections involving gram-negative microorganisms. These findings may provide a minimum target free-drug AUC24/MIC ratio for the treatment of infection involving S. pneumoniae with fluoroquinolone antibiotics and provide a paradigm for the selection of fluoroquinolones to be brought forward from drug discovery into clinical development and dose selection for clinical trials. Further, when target free-drug AUC24/MIC ratios are used in conjunction with stochastic modeling techniques, these findings may be used to support susceptibility breakpoints for fluoroquinolone antibiotics and S. pneumoniae.
ACKNOWLEDGMENTS
This work was supported by a grant from the Bristol-Myers Squibb Company.
The analysis presented herein benefited from communication with George L. Drusano.
REFERENCES
- 1.Ambrose P G, Grasela D M. The use of Monte Carlo simulation to examine pharmacodynamic variance of drugs: fluoroquinolone pharmacodynamics against Streptococcus pneumoniae. Diagn Microbiol Infect Dis. 2000;38:151–157. doi: 10.1016/s0732-8893(00)00185-1. [DOI] [PubMed] [Google Scholar]
- 2.Chow A W, Hall C B, Klein J O. General guidelines for the evaluation of new anti-infective drugs for the treatment of respiratory infections. Clin Infect Dis. 1992;15(Suppl. 1):S62–S88. doi: 10.1093/clind/15.Supplement_1.S62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Craig W A. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing in mice and men. Clin Infect Dis. 1998;26:1–12. doi: 10.1086/516284. [DOI] [PubMed] [Google Scholar]
- 4.Drusano G L. Role of pharmacokinetics in the outcome of infection. Antimicrob Agents Chemother. 1988;32:289–297. doi: 10.1128/aac.32.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eagle H, Fleischman R, Levy M. Continuous vs. discontinuous therapy with penicillin. N Engl J Med. 1953;248:481–488. doi: 10.1056/NEJM195303192481201. [DOI] [PubMed] [Google Scholar]
- 6.Fantin B, Leggett J, Ebert S, Craig W A. Correlation between in vitro and in vivo activity of antimicrobial agents against gram-negative bacilli in a murine infection model. Antimicrob Agents Chemother. 1991;35:1413–1422. doi: 10.1128/aac.35.7.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forrest A, Chodash S, Amantea M A, Collins D A, Schentag J J. Pharmacokinetics and pharmacodynamics of oral grepafloxacin in patients with acute bacterial exacerbations of chronic bronchitis. J Antimicrob Chermother. 1997;40(Suppl. A):45–57. doi: 10.1093/jac/40.suppl_1.45. [DOI] [PubMed] [Google Scholar]
- 8.Forrest A, Nix D E, Ballow C H, Goss T F, Birmingham M C, Schentag J J. Pharmacodynamics of intravenous ciprofloxacin in seriously ill patients. Antimicrob Agents Chemother. 1993;37:1073–1081. doi: 10.1128/aac.37.5.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.LaCreta F P, Kollia G D, Duncan G, Behr D, Grasela D M. Age and gender effects on the pharmacokinetics of gatifloxacin. Pharmacotherapy. 2000;20:675–755. doi: 10.1592/phco.20.8.67s.35185. [DOI] [PubMed] [Google Scholar]
- 10.Lacy M A, Lu W, Xu X, Tessier P R, Nicolau D P, Quintiliani R, Nightingale C H. Pharmacodynamic comparisons of levofloxacin, ciprofloxacin, and ampicillin against Streptococcus pneumoniae in an in vitro model of infection. Antimicrob Agents Chemother. 1999;43:672–677. doi: 10.1128/aac.43.3.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lister P D, Sanders C C. Pharmacodynamics of levofloxacin and ciprofloxacin against Streptococcus pneumoniae. J Antimicrob Chemother. 1999;43:79–86. doi: 10.1093/jac/43.1.79. [DOI] [PubMed] [Google Scholar]
- 12.NCCLS. Performance standards for antimicrobial susceptibility testing. Standard M100–S8. Wayne, Pa: NCCLS; 1998. [Google Scholar]
- 13.Preston S L, Drusano D L, Berman A L, Fowler C L, Chow A T, Dornseif B, Reichi V, Natarajan J, Wong W A, Corrado M. Levofloxacin population pharmacokinetics and creation of a demographic model for prediction of individual drug clearance in patients with serious community-acquired infection. Antimicrob Agents Chemother. 1998;42:1098–1104. doi: 10.1128/aac.42.5.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Preston, S. L., D. L. Drusano, A. L. Berman, C. L. Fowler, A. T. Chow, B. Dornseif, V. Reichi, J. Natarajan, and M. Corrado. Pharmacodynamics of levofloxacin: a new paradigm for early clinical trials. JAMA 279:125–129. [DOI] [PubMed]
- 15.Ramirez A, Molina J, Dolman A, Fogarty C, DeAbate C A, Breen J, Skuba K. Gatifloxacin treatment in patients with acute exacerbations of chronic bronchitis: clinical trial results. J Respir Dis. 1999;20(Suppl. 11A):30–39. [Google Scholar]
- 16.Stokes M E, Davis C S, Koch G G. Logistic regression I: dichotomous response. In: Stokes M E, Davis C S, Koch G G, editors. Categorical data analysis using the SAS system. Cary, N.C: SAS Institute, Inc.; 1995. pp. 165–214. [Google Scholar]
- 17.Sullivan J G, McElroy A D, Honsinger R W, McAdoo M, Harrison B J, Plouffe J F, Gotfried M, Mayer H. Treating community-acquired pneumonia with once-daily gatifloxacin vs. once-daily levofloxacin. J Respir Dis. 1999;20(Suppl. 11A):49–59. [Google Scholar]