Learning objectives.
By reading this article you should be able to:
-
•
Describe the main challenges of caring for a patient with major burns in the operating theatre or ICU.
-
•
Explain the ways for optimising intravenous fluid therapy to avoid under- and over-resuscitation.
-
•
Outline how to recognise and manage infectious complications in patients with major burns.
-
•
Illustrate the pharmacological and non-pharmacological techniques available to mitigate the hypermetabolic response to burn injury.
Key points.
-
•
Involvement of the multidisciplinary burns team is vital for the care of patients with major burns.
-
•
Thermoregulation, blood loss and coagulopathy are key considerations for the anaesthetist during surgery for major burns.
-
•
The Parkland formula should be used to guide resuscitation and fluids titrated to urine output, haemodynamic and laboratory variables.
-
•
Human albumin solution, given in addition to crystalloids, may reduce fluid requirements.
-
•
The hypermetabolic response to major burns can be attenuated by early excision, appropriate nutrition and specific pharmacotherapy.
As a result of improvements in the care of patients with major burns, increasing numbers of patients are surviving more severe injuries.1 Many require a protracted stay in intensive care, numerous operative interventions and comprehensive rehabilitation. Engagement of a multidisciplinary burns team is necessary to help maximise quality of life for survivors who may have profound physiological, psychological, functional and social problems.
The initial assessment and treatment of patients with major burns has been covered in a recent accompanying article.2 This article focuses on the ongoing care of an adult patient with a major burn of more than 15% total body surface area (TBSA) in both the operating theatre and the ICU. Previous articles in this journal describe the management of major burns in paediatric patients and smoke inhalation.3,4
Anaesthesia and burn surgery
Since the 1940s it has been recognised that early excision of burn eschar improves mortality.5 This is likely to result from removal of necrotic tissue that can both fuel the intense inflammatory response and act as a culture medium for pathogens. Patients often undergo surgical excision of burnt tissue in the first day or two after the injury. Some patients with full thickness burns will require more immediate decompressive surgery such as escharotomy or fasciotomy. However, many will undergo multiple subsequent operations including temporising cadaveric autografts, xenografts (such as pig skin) or synthetic dermal substitutes until skin coverage with autografts is possible (using unburned patient donor skin). Later elective surgical procedures are frequently required to help restore function and improve cosmesis.
Location of surgery
Burns surgery should be carried out in a dedicated burns operating theatre, ideally in close proximity to intensive care facilities. Theatre personnel should have appropriate training, experience and access to specialist equipment. It is vital that the theatre can be appropriately warmed in order to reduce heat loss. Procedures such as dressing changes may be carried out under sedation led by an anaesthetist, but all the necessary resources to convert to general anaesthesia should be immediately available.
Preoperative assessment
Assessment of a patient for burn surgery should include knowledge of the extent of the burn, associated injuries and details of the planned surgery, including positioning needed and estimated blood loss. Coagulopathy may be present, driven by endothelial injury and the inflammatory cascade, often exacerbated by secondary infection. Its extent can vary from subclinical changes to fulminant disseminated intravascular coagulation (DIC). Therefore, the need for blood products should be anticipated early.
Given the large volumes of i.v. fluids often required and the potential for associated renal injury, a thorough assessment of circulating volume status and electrolyte abnormalities should also be made. Nutritional support should generally be continued throughout the operative period in patients who have been mechanically ventilated before surgery, and fasting times kept to a minimum in those requiring airway intervention.
Airway assessment should include clinical examination and a careful review of previous anaesthetics. Although mechanical ventilation may have already been established before transfer to the operating theatre, particular care is required to prevent accidental extubation, especially in patients with airway burns or inhalation injuries. Formation of a tracheostomy may be indicated for various reasons and is commonly required for patients with complex facial burns in order to maintain skin health around the mouth and nose. In addition, although burns involving the face or neck may not present any airway difficulties initially, subsequent contractures may severely limit neck extension and mouth opening. Advanced techniques such as awake fibreoptic intubation may be indicated. A difficult airway trolley with equipment for emergency front of neck access should always be immediately available.
Intraoperative management
Monitoring can prove challenging. Electrocardiogram electrodes sometimes require sutures or skin clips for reliable contact. Pulse oximetry and blood pressure cuff positioning may also be difficult, and suitable sites for i.v. access and direct arterial monitoring may be limited.
It is mandatory to monitor the patient's core temperature. Methods to minimise heat loss include minimising exposure, using forced air warmers and heat lamps, humidifying anaesthetic gases, infusing warmed i.v. fluids and maintaining an ambient temperature in theatre of 28–33°C.
Care should be taken to assess a patient's ventilatory requirements preoperatively and lung protective ventilation should be continued throughout the intraoperative period. Patients who have also suffered an inhalation injury may have increased requirements for oxygen and PEEP. For these patients, it is particularly important to avoid alveolar derecruitment with the loss of PEEP during transfer to a transport or theatre ventilator. As the hypermetabolic response suffered by patients with burns leads to increased oxygen consumption and carbon dioxide production, higher than expected oxygen concentrations and minute volumes may be required.
Blood loss can be as much as 3.4% of total blood volume for each per cent TBSA excised.6 Bleeding risks are increased in patients with infected burn tissue, deeper thickness burns and by prolonged operative time. Bleeding risks may be reduced by using limb tourniquets on extremity burns, topical adrenaline or compression bandages. The use of near-patient coagulation studies such as thromboelastography may be superior to standard laboratory-based tests in detecting coagulation abnormalities and may be of value to direct blood product use intraoperatively.7
General vs regional anaesthesia
Most patients with major burn injuries will require general anaesthesia for surgical interventions. However, regional anaesthesia, either alone or in combination with general anaesthesia, may be suitable. Careful evaluation is required before performing neuraxial anaesthesia because of the increased incidence of coagulopathy and infection in this patient group.
Intensive care management
Fluid management
Appropriate resuscitation with fluids is critical in the first 24–48 h after a burn injury. Under-resuscitation may lead to impaired tissue perfusion, end organ damage and extension of burn depth. However, giving excessive fluids is also harmful; risks include electrolyte disturbances such as hyponatraemia, exacerbation of tissue oedema, pulmonary and cerebral oedema, and abdominal and limb compartment syndromes.
The Parkland formula remains the most commonly used tool to calculate fluid requirements. Concerns have been raised that it may overestimate the volume needed, prompting bodies such as the American Burn Association to recommend less than 4 ml kg−1 TBSA−1.8 However, we advocate using 4 ml kg−1 TBSA−1 for initial calculations and then performing regular clinical reviews to permit escalation or de-escalation of fluid input based on individual patient physiology, as opposed to rigidly adhering to any one formula.
Goal-directed fluid therapy
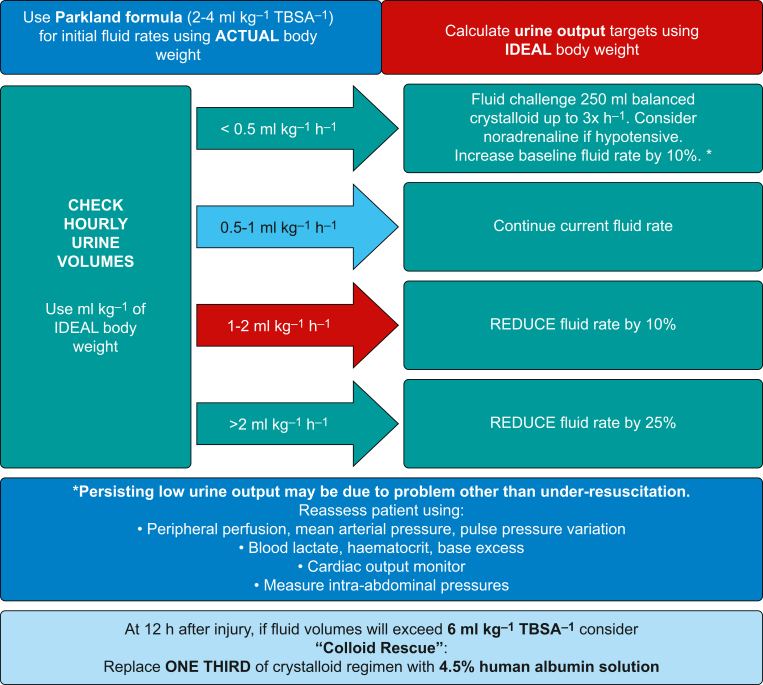
The most common and easy method of ensuring appropriate fluid resuscitation is by targeting an hourly urine output of 0.5–1 ml kg−1 ideal body weight. Failure to meet this target should prompt reassessment and adjustment of fluid delivery as detailed in Figure 1.
Fig 1.
Resuscitation protocol for resuscitation with i.v. fluids in the adult patient with burns (adapted with permission from Care of burns in Scotland [COBIS]).
However, an inadequate urine output is not always caused by volume depletion. Renal failure from acute tubular necrosis or rhabdomyolysis can result in oliguria, as can increased anti-diuretic hormone release in response to injury. Other causes such as vasoplegia, low cardiac output and abdominal compartment syndrome should also be considered. Conversely, urine volumes in excess of targeted values should prompt a reduction in the volumes of fluids given, mitigating against the phenomenon of ‘fluid creep’, whereby more fluid than required is given.
Peripheral perfusion, serum lactate, acid–base balance and haematocrit should also be used to help guide fluid therapy. The additional use of cardiac output monitoring to guide fluid delivery in patients with major burns has demonstrated improvements in cardiac output, oxygen delivery and organ dysfunction, but a mortality benefit has not been identified.9
Choice of fluid
Balanced crystalloids such as Hartmann's solution are the mainstay of fluid resuscitation in major burn injuries. Their use, when compared with 0.9% saline, has been demonstrated to reduce the incidence of significant electrolyte disturbances such as hyperchloraemic metabolic acidosis.10 The use of colloids such as human albumin solution in combination with crystalloids may reduce overall fluid volume requirements, mitigate against ‘fluid creep’ and lessen increases in intra-abdominal pressures compared with crystalloids alone.9 We recommend the addition of 4.5% human albumin solution if fluid resuscitation volumes in the first 24 h are projected to be greater than 6 ml kg−1 %TBSA−1 (Fig. 1). This technique of ‘colloid rescue’ should be continued until 48 h after the burn injury.
Thermoregulation
Major burn injuries are associated with thermodysregulation, with an initial propensity to hypothermia driven by heat and fluid loss from the burn wounds themselves. Steps to reduce heat loss during the initial stages of resuscitation are detailed in Part 1.2 The principles of maintaining an adequate core temperature in the operating theatre also apply to the intensive care environment.
Most patients with major burns will subsequently develop a raised core temperature. This reflects altering of the hypothalamic setpoint for thermoregulation by pyrogens such as interleukin-1 (IL-1) and tumour necrosis factor.11,12 More profound hyperthermia, with temperatures exceeding 40°C, can occasionally occur and may lead to multiorgan failure. Steps to address potentially harmful hyperthermia should be taken in patients with a core temperature above 39.5°C. Techniques include debulking dressings, giving antipyretics such as paracetamol, applying ice to non-burned areas, infusion of cooled i.v. fluids, and irrigating the bladder and stomach with cold fluids. More invasive approaches such as intravascular heat exchange catheters or extracorporeal circuits may also need to be considered.
Nutrition
Basal metabolic rate can increase significantly after a burn injury, more than doubling in patients with burns >40% TBSA.13 If not addressed, this can result in loss of lean body mass, immune compromise and impaired healing. There is a direct correlation between loss of lean body mass and adverse events, including infectious complications and death.14
Timing of nutritional support
Starting enteral nutrition in the hours immediately after injury has been shown to have beneficial effects on stress hormones, improve gut integrity, reduce intensive care length of stay, improve wound healing and reduce wound infections.14 The British Burn Association (BBA) national standards state15:
-
(i)
Enteral nutrition should be started as soon as possible in major burns, ideally within 6–12 h after the injury.
-
(ii)
Total body weight loss should not exceed 10% of the patient's weight at admission.
Route of nutrition
As with most critically ill patients, the enteral route is preferred. Postpyloric feeding may be required if gastric stasis is present and impairing calorie delivery. Postpyloric feeding may also help reduce the risk of aspiration, especially in patients requiring multiple interventions under general anaesthesia. Parenteral nutrition is rarely required, but when used, caution should be exercised because of the associated risks including infection, overfeeding and erratic blood glucose control.
Caloric requirements
The aim of nutritional support in patients with major burns is to meet the substantially increased caloric requirements in these patients while avoiding harmful overfeeding. The complications of overfeeding include hyperglycaemia, hypertriglyceridaemia, hepatic steatosis, hypercapnoea and prolonged duration of mechanical ventilation.14 Various methods have been used to assess caloric needs. The most accurate method is indirect calorimetry, but this remains mainly a research tool. In lieu of this, various formulae have been devised to guide clinicians (Table 1).14,16 Such formulae may under- or overestimate requirements at different times during a patient's admission. Given the complexities of providing optimum nutritional support, specialist input from a dietician within the burns team is essential.
Table 1.
Formulae to calculate daily caloric requirements (kcal day−1) in patients with burn injuries. TBSA, total body surface area.
| Formula | Calculation (kcal day−1) | Comments |
|---|---|---|
| Harris–Benedict | Men: 66.5 + 13.75 (weight in kg) + 5 (height in cm) – 6.76 (age in yrs) Women: 66 + 9.56 (weight in kg) + 1.85 (height in cm) – 4.68 (age in yrs) |
Multiplied by factor determined by burn size: <20% TBSA ×1.5 20–40% TBSA ×1.6 >40% TBSA ×1.7 |
| Toronto | –4343 + 10.5 (%TBSA) + 0.23 (caloric intake in last 24 h) + 0.84 (Harris–Benedict unadjusted) + 114 (temp in °C) – 4.5 (days after injury) | Can be adjusted by activity factor: Confined to bed: ×1.2 Minimal ambulation: ×1.3 Moderate activity: ×1.4 |
| Curreri | 25 (weight in kg) + 40 (%TBSA) | Can overestimate caloric needs |
| Hangang | 867.542–5.546 (age in yrs) + 13.297 (weight in kg) + 4.879 (% TBSA) – 9.844 (PBD) + 500.612 (V) | Adjusted for each post-burn day (PBD) and if mechanically ventilated (V=1 if ventilated, V=0 if not) |
Macronutrients
The three main macronutrients – carbohydrates, proteins and lipids – provide substrates for adenosine triphosphate (ATP) biosynthesis, wound repair, immune function and maintenance of lean body mass. Carbohydrates are generally the preferred energy source, preventing a reliance on muscle proteolysis. However, relying solely on carbohydrates to meet caloric needs would result in hyperglycaemia caused by glucose delivery exceeding the rate at which it can be used. A relative insulin resistance, commonly observed in critically ill patients, may also exacerbate hyperglycaemia. Glucose should be limited to 55% of total energy requirements and hyperglycaemia managed with supplemental insulin as required.16
Excessive lipid delivery can result in accumulation in the liver and impaired immune function.14 Therefore, lipids should account for no more than 30% of energy delivered, although some centres recommend a maximum of 15%.14,16 Given the potentially high sedation requirements of these patients, the lipid content of propofol should also be accounted for.
Protein plays a crucial role in wound repair and maintenance of lean body mass. Increasing protein intake to supra-physiological values does not prevent catabolism of existing protein stores but does prevent a negative nitrogen balance and improve protein synthesis. Protein should be delivered at 1.5–2 g kg−1 day−1 in adults. This often requires protein-enriched feeds prescribed under the guidance of a dietetic team.16
Micronutrients
Reserves of trace elements including copper, selenium, zinc and vitamins B, C, D and E may become depleted because of the intense inflammatory response, exudative losses and haemodilution resulting from resuscitation with i.v. fluids.17 Micronutrients are essential for antioxidant defences, wound healing and immune function. Adequate supplementation often requires supraphysiological doses to be given, often parenterally because enteral absorption may be limited.16 Early replacement of these elements may reduce infectious complications, improve wound healing and reduce intensive care length of stay.17
Infection
The loss of skin, the primary barrier to infection, coupled with relative immunosuppression in patients with major burns lead to an increased risk of infectious complications. Infections are an important contributor to the high morbidity and mortality rates after major burns, accounting for an estimated 42–65% of deaths after burn injury.18
Burn wound colonisation and infection
Patients can become colonised with multiple, often resistant, organisms.18 Such colonisation occurs with low bacterial concentrations on the surface of wounds, without surrounding erythema or cellulitis. In contrast, invasive wound infection is characterised by cellulitis of surrounding healthy tissue, extension of existing burn depth, eschar separation or necrosis. Patients may develop worsening pyrexia or raised inflammatory markers, but this can be difficult to distinguish from the inflammatory response after burns. Consequently, specific criteria for the diagnosis of sepsis in patients with burns have been proposed19:
-
(i)
Temperature >39°C or <36.5°C
-
(ii)
Heart rate >110 beats min−1
-
(iii)
Ventilatory frequency >25 bpm (or minute ventilation >12 L min−1 if invasively ventilated)
-
(iv)
Thrombocytopenia <100×109 L−1 (>3 days after initial resuscitation)
-
(v)
Hyperglycaemia >11.1 mmol L−1 or insulin infusion dose requirement >7 units h−1
-
(vi)
Intolerance of enteral feed
With such difficulty in relying on clinical signs and traditional biomarkers such as C-reactive protein (CRP), other markers such as procalcitonin (PCT) may have better discriminatory capacity in diagnosing sepsis in patients with major burns.20 There is also increasing interest in the use of genomic variants, cytokine profiles and epigenetic markers.21
Patients with infections should be treated with appropriate antibiotics, ideally as advised by the medical microbiology team and based on colonising organisms. Surgical debridement may be required. Patients with a delayed presentation or delayed excision of burn wounds are at highest risk of infective complications.
Common pathogens
The most common pathogens, particularly early in the admission, are Gram-positive bacteria such as Staphylococcus aureus, Streptococcus and Enterococcus species. Gram-negative bacteria such as Pseudomonas aeruginosa can translocate from the gastrointestinal tract or the environment and thrive in the moist environment of a burn wound. Infections from Pseudomonas will have a typically green/yellow colour and foul smell and can lead to invasive infection with necrosis. Other Gram-negative pathogens including Acinetobacter, Escherichia coli and Klebsiella can also cause invasive infections. Multiresistant strains of pathogens including P. aeruginosa, Acinetobacter species and vancomycin-resistant Enterococcus (VRE) are an increasing concern. Recognised risk factors include the use of broad-spectrum antibiotics, colonisation at hospital admission, need for escharotomy, prolonged hospital or intensive care stay and multiple surgical procedures.22 Fungal colonisation and invasive fungal infections are also significant problems. Fungal wound infection is independently associated with increased mortality.23 Candida albicans is the most common pathogen, although Aspergillus and non-albicans Candida such as Candida tropicalis and Candida krusei are becoming more common.22 Regular clinical review and sampling of wounds and other potential sources of colonisation and infection should guide antimicrobial therapy.
Toxic shock syndromes
Toxin-producing strains of S. aureus can cause toxic shock syndrome (TSS). Streptococcal toxic shock syndrome (STSS), caused by group A Streptococcus, has a similar presentation. In addition to the non-specific clinical signs suggestive of infection described previously, patients with TSS or STSS may also exhibit a diffuse macular rash, vomiting and diarrhoea, thrombocytopenia, lymphopenia and deranged liver function tests. In addition to standard management of infections, antibiotics that directly reduce exotoxin production, such as clindamycin and linezolid, should be considered. The use of i.v. immunoglobulin has also been reported.24
Hypermetabolic and inflammatory response
Although changes in the metabolic and inflammatory response are most pronounced in the acute phase, some changes can persist for several years after a burn injury has healed.25 Changes include:
-
(i)
Increased resting energy expenditure
-
(ii)
Increased serum and urine cortisol and catecholamines
-
(iii)
Increased cytokines including IL-6, IL-8 and granulocyte colony-stimulating factor (G-CSF)
These persisting metabolic, inflammatory and immune changes can result in an increased risk of developing later problems (Table 2).26 Appropriate skin closure, nutritional support and analgesia are fundamental to mitigating this response. Several therapies have been proposed, some of which are discussed further.
Table 2.
Long-term effects of burn injuries.25
| Organ system | Effects |
|---|---|
| Immune | Increased incidence of respiratory infections (influenza, pneumonia) Increased hospital admissions with infective diseases Increased mortality from infections |
| Cardiovascular | Increased risk of ischaemic heart disease, hypertension, heart failure and stroke Reduced exercise tolerance Myocardial fibrosis |
| Gastrointestinal | Increased risk of disease of alimentary tract, gallbladder, biliary tract and pancreas Increased hospital admissions with diabetes mellitus |
| Musculoskeletal | Increased fracture risk Joint pain and stiffness Reduced mobility Increased hospital admissions with musculoskeletal disorders |
| Central Nervous | Increased hospital admissions with epilepsy, migraine and nerve problems |
| Miscellaneous | Increased all-cause mortality Increased cancer risk (perhaps worse in females) |
Beta-blockers
Beta-adrenergic blockade with drugs, such as propranolol, suppress the catabolic effects of a burn by reducing energy expenditure, limiting insulin resistance, preventing muscle wasting and acting as anti-inflammatory agents. However, they must be used with caution in intensive care because of the risk of cardiovascular instability. A recent systematic review of the use of beta-blockers in patients with major burns showed no benefit in terms of mortality, length of hospital stay or incidence of sepsis.27 However, other studies have demonstrated improved wound healing and reduced muscle catabolism.28
Oxandrolone
Oxandrolone is an androgen receptor agonist, which stimulates protein synthesis and muscle growth with much less virilising activity than testosterone, making it more suitable for use in women and children. Oxandrolone has been used from around Day 5 after burns, at a dose of 10 mg enterally twice daily in patients with ≥30% TBSA burns, to minimise weight loss, improve urinary nitrogen balance, increase muscle strength and reduce healing time. Some studies have shown benefits such as reduced ICU and hospital duration of stay, maintained lean body mass and improved whole body mass.29 Adverse events include hepatic injury with increased liver transaminases, renal injury and skin complications such as cellulitis.
Pain management
The pain experienced from a burn injury can be excruciating and is often difficult to manage. Some studies suggest higher pain scores during hospital admission are associated with poorer long-term outcomes such as increased mental health problems.30 Given the complexities and challenges of effective pain management, a specialist pain service should be an integral part of the burns team.15
Types of pain
Although the burn injury is usually the most significant source of pain, there are many other causes. These include pain from associated injuries, tracheal tubes, invasive lines and catheters, skin autograft donor sites, pressure areas and interventions including position changes. Clinicians should adopt a structured approach to management of acute burns pain, addressing the three main types of pain: background, breakthrough and procedural pain.
Background pain
Patients will experience a degree of persistent pain after a burn injury and multimodal analgesia should be prescribed to maintain adequate control. Infusions should be titrated to a targeted effect, maximising clinical benefit while avoiding unwanted adverse effects. Multiple methods for pain assessment exist including the VAS, designed for cooperative and communicative patients, and the Critical Care Pain Observation Tool (CPOT) for use in the ICU.31 Non-pharmacological measures such as appropriate dressings, comfortable positioning, cutaneous stimulation, acupuncture and techniques such as cognitive behavioural therapy and music therapy may also help alleviate this form of pain.
Breakthrough pain
Breakthrough pain occurs on top of well-controlled background pain, either as an exacerbation of background pain or originating from another source. This can be evoked, spontaneous, predictable or unpredictable. It can be managed with boluses of rapid-acting agents, increases in the rate of opioid infusions or, if predictable, anticipatory doses of longer-acting agents.
Procedural pain
Procedural interventions include dressing changes and mobilisation. Analgesic interventions should be timed appropriately to gain the maximum benefit from the agent being used. Appropriate agents include opioid boluses, inhaled agents including nitrous oxide and methoxyflurane or analgosedative agents such as ketamine. Other non-pharmacological methods may be beneficial, including hypnosis, virtual reality systems and other distraction techniques.
Pharmacological management
Opioids
Opioids remain the mainstay of pain management in burn injuries. The choice of opioid and method of delivery will depend on local preferences and patient factors including renal function and conscious level. Patient-controlled analgesia (PCA) is commonly used but relies on a conscious and alert patient. Patients with significant burn injuries often require large doses of opioids. Adverse effects include ileus, respiratory depression, delirium, hypotension and potentially dependence. Because of this, a multimodal analgesic approach incorporating advice from a pain specialist is advised.
Non-steroidal anti-inflammatory drugs
Non-steroidal anti-inflammatory drugs such as ibuprofen and diclofenac are infrequently used in critically ill patients because of the potential risks of gastrointestinal haemorrhage and renal impairment. Although they may be of benefit in selected patients, they should be used with caution.
Ketamine
Ketamine is an N-methyl-d-aspartate (NMDA) receptor antagonist with potent analgesic effects. Ketamine can be given as an i.v. infusion, often to reduce opioid requirements, or in sedative or anaesthetic doses for painful interventions. Ketamine may also have a role in preventing the development of neuropathic pain and has been shown to reduce secondary hyperalgesia and ‘wind-up’ phenomenon in healthy volunteers.32
Gabapentinoids
Gabapentin and pregabalin have been used in the management of burn pain and pruritus, both acutely and in those who develop chronic symptoms. The evidence suggesting benefit from these drugs is mainly from observational studies, which demonstrated improved pain scores and reduced opioid consumption.33 However, given the increasing evidence of harm from dependence, abuse and overdose, they should be used with caution, especially in patients with a history of alcohol or drug misuse.34
Dexmedetomidine
Dexmedetomidine is a highly selective α2 receptor agonist with both analgesic and sedative effects. It can be used in the ICU as part of an analgosedative regimen, in combination general anaesthesia, as a sedative for painful procedures and also as an adjunct in PCA. Common adverse effects of dexmedetomidine are bradycardia and hypotension. As recent studies have highlighted the risk of pyrexia associated with dexmedetomidine use, caution is advised in the context of hypermetabolism after burns.35
Psychological sequelae of burn injuries
Survivors of burn injuries can be left with long-lasting mental health problems such as depression, anxiety, suicidal ideation and post-traumatic stress disorder (PTSD). Other consequences include cognitive impairment, physical limitations, chronic pain and pruritus. Many of these sequelae are recognised in intensive care survivors as post-intensive care syndrome (PICS), although such effects are likely to be amplified in survivors of major burn injuries, regardless of whether or not they were managed in the ICU. Patients with major burns often have a prolonged hospital admission, numerous invasive procedures, multiple risk factors for developing delirium and significant pain issues. Furthermore, the event causing the burn injury is frequently distressing.
Psychological support staff should form part of the multidisciplinary team. Routine psychological assessment is advised and robust care pathways should be in place in order to provide help and support to burn victims and their families. This should include access to support groups, charities, websites and opportunities for peer support.15 Early psychosocial screening may help identify patients at the highest risk of developing problems after burn injury, allowing prompt intervention to address the psychological, emotional and social challenges these patients may face.
Conclusions
As a result of vast improvements in burn care in recent decades, clinicians are now responsible for managing patients who have suffered burn injuries of increasing severity and complexity. It is challenging to provide high-quality anaesthetic and intensive care. These patients often require numerous and complex surgical interventions. Areas that require special focus include airway management, management of blood and fluid loss, thermoregulation and overcoming monitoring difficulties. Patients who have suffered major burns are also at high risk of infection, excessive catabolism, significant pain and psychological distress, all of which are associated with adverse long-term consequences. Managing this myriad of complex issues requires expert input from a wide multidisciplinary team.
Acknowledgements
The authors thank Drs Martin Hughes and Richard Cowan of Glasgow Royal Infirmary and Jamie Nimmo at Care of Burns in Scotland (COBIS) for permission to reproduce the i.v. fluids protocol, and Katrina Dalgarno, specialist dietician in intensive care and surgery, for guidance in writing the section on nutrition.
Biographies
Christopher McGovern MRCEM FRCA FFICM is a specialty trainee in anaesthesia and intensive care medicine and a clinical research fellow at the University of Glasgow.
Kathryn Puxty MD MRCP FRCA FFICM is a consultant in anaesthesia and intensive care at Glasgow Royal Infirmary. She is an honorary clinical associate professor at the University of Glasgow and a CSO career research fellow.
Lia Paton BMedSci (Hons) FRCA FFICM is a consultant in anaesthesia and intensive care and lead clinician for burns intensive care at Glasgow Royal Infirmary, a tertiary referral centre for burns in Scotland. She is also chair of the Care of Burns in Scotland (COBIS) data group.
Matrix codes: 1B04, 1D01, 1D02, 2A02, 3A10, 3C00, 3H00
Declaration of interests
The authors declare that they have no conflicts of interest.
MCQs
The associated MCQs (to support CME/CPD activity) will be accessible at www.bjaed.org/cme/home by subscribers to BJA Education.
References
- 1.Capek K.D., Sousse L.E., Hundeshagen G., et al. Contemporary burn survival. J Am Coll Surg. 2018;226:453–463. doi: 10.1016/j.jamcollsurg.2017.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCann C., Barnes D., Watson A. Major burns: part one. Epidemiology, pathophysiology and initial management. BJA Educ. 2022;22:94–103. doi: 10.1016/j.bjae.2021.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suman A., Owen J. Update on the management of burns in paediatrics. BJA Educ. 2020;20:103–110. doi: 10.1016/j.bjae.2019.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gill P., Martin R.V. Smoke inhalation injury. BJA Educ. 2015;15:143–148. [Google Scholar]
- 5.Cope O., Langohr J.L. Expeditious care of full-thickness burn wounds by surgical excision and grafting. Ann Surg. 1947;125:1–22. [PubMed] [Google Scholar]
- 6.Bhananker S.M., Cullen B.F., Burns, Fleisher L.A., editors. Anesthesia and uncommon diseases. 6th Edn. Saunders; Philadelphia: 2012. pp. 526–536. [Google Scholar]
- 7.Marsden N.J., Van M., Dean S., et al. Measuring coagulation in burns: an evidence-based systematic review. Scars Burn Heal. 2017;3 doi: 10.1177/2059513117728201. 205951311772820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pham T.N., Bettencourt A.P., Bozinko G.M., et al. American Burn Association; Chicago: 2017. 2018 ABLS provider manual 1. [Google Scholar]
- 9.Guilabert P., Usúa G., Martín N., Abarca L., Barret J.P., Colomina M.J. Fluid resuscitation management in patients with burns: update. Br J Anaesth. 2016;117:284–296. doi: 10.1093/bja/aew266. [DOI] [PubMed] [Google Scholar]
- 10.Powell-Tuck J., Gosling P., Lobo D.N., et al. British consensus guidelines on intravenous fluid therapy for adult surgical patients (GIFTASUP) BMJ. 2009;338:b2418. doi: 10.1111/j.1365-2044.2009.05886_1.x. [DOI] [PubMed] [Google Scholar]
- 11.Jeschke M.G., Mlcak R.P., Finnerty C.C., et al. Burn size determines the inflammatory and hypermetabolic response. Crit Care. 2007;11:R90. doi: 10.1186/cc6102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herndon D.N., Tompkins R.G. Support of the metabolic response to burn injury. Lancet. 2004;363:1895–1902. doi: 10.1016/S0140-6736(04)16360-5. [DOI] [PubMed] [Google Scholar]
- 13.Williams F.N., Herndon D.N., Jeschke M.G. The hypermetabolic response to burn injury and interventions to modify this response. Clin Plast Surg. 2009;36:583–596. doi: 10.1016/j.cps.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clark A., Imran J., Madni T., Wolf S.E. Nutrition and metabolism in burn patients. Burn Trauma. 2017;5:11. doi: 10.1186/s41038-017-0076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.British Burn Association . British Burn Association; 2018. National standards for provision and outcomes in adult and paediatric burn care; pp. 1–83. (November) [Google Scholar]
- 16.Rousseau A.F., Losser M.R., Ichai C., Berger M.M. ESPEN endorsed recommendations: nutritional therapy in major burns. Clin Nutr. 2013;32:497–502. doi: 10.1016/j.clnu.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 17.Nordlund M.J., Pham T.N., Gibran N.S. Micronutrients after burn injury: a review. J Burn Care Res. 2014;35:121–133. doi: 10.1097/BCR.0b013e318290110b. [DOI] [PubMed] [Google Scholar]
- 18.Lachiewicz A.M., Hauck C.G., Weber D.J., Cairns B.A., van Duin D. Bacterial infections after burn injuries: impact of multidrug resistance. Clin Infect Dis. 2017;65:2130–2136. doi: 10.1093/cid/cix682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greenhalgh D.G., Saffle J.R., Holmes J.H., et al. American Burn Association consensus conference to define sepsis and infection in burns. J Burn Care Res. 2007;28:776–790. doi: 10.1097/BCR.0b013e3181599bc9. [DOI] [PubMed] [Google Scholar]
- 20.Egea-Guerrero J.J., Martínez-Fernández C., Rodríguez-Rodríguez A., et al. The utility of C-reactive protein and procalcitonin for sepsis diagnosis in critically burned patients: a preliminary study. Plast Surg (Oakv) 2015;23:239–243. [PMC free article] [PubMed] [Google Scholar]
- 21.Muñoz B., Suárez-Sánchez R., Hernández-Hernández O., Franco-Cendejas R., Cortés H., Magaña J.J. From traditional biochemical signals to molecular markers for detection of sepsis after burn injuries. Burns. 2019;45:16–31. doi: 10.1016/j.burns.2018.04.016. [DOI] [PubMed] [Google Scholar]
- 22.Norbury W., Herndon D.N., Tanksley J., Jeschke M.G., Finnerty C.C. Infection in burns. Surg Infect (Larchmt) 2016;17:250–255. doi: 10.1089/sur.2013.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horvath E.E., Murray C.K., Vaughan G.M., et al. Fungal wound infection (not colonization) is independently associated with mortality in burn patients. Ann Surg. 2007;245:978–985. doi: 10.1097/01.sla.0000256914.16754.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raithatha A.H., Bryden D.C. Use of intravenous immunoglobulin therapy in the treatment of septic shock, in particular severe invasive group A streptococcal disease. Indian J Crit Care Med. 2012;16:37–40. doi: 10.4103/0972-5229.94433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeschke M.G., Gauglitz G.G., Kulp G.A., et al. Long-term persistence of the pathophysiologic response to severe burn injury. PLoS One. 2011;6:e21245. doi: 10.1371/journal.pone.0021245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barrett L.W., Fear V.S., Waithman J.C., Wood F.M., Fear M.W. Understanding acute burn injury as a chronic disease. Burn Trauma. 2019;7:23. doi: 10.1186/s41038-019-0163-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hassoun-Kheir N., Henig O., Avni T., Leibovici L., Paul M. The effect of β-blockers for burn patients on clinical outcomes: systematic review and meta-analysis. J Intensive Care Med. 2021;36:945–953. doi: 10.1177/0885066620940188. [DOI] [PubMed] [Google Scholar]
- 28.Herndon D.N., Hart D.W., Wolf S.E., Chinkes D.L., Wolfe R.R. Reversal of catabolism by beta-blockade after severe burns. N Engl J Med. 2001;345:1223–1229. doi: 10.1056/NEJMoa010342. [DOI] [PubMed] [Google Scholar]
- 29.Wolf S.E., Edelman L.S., Kemalyan N., et al. Effects of oxandrolone on outcome measures in the severely burned: a multicenter prospective randomized double-blind trial. J Burn Care Res. 2006;27:131–139. doi: 10.1097/01.BCR.0000202620.55751.4F. [DOI] [PubMed] [Google Scholar]
- 30.Patterson D.R., Tininenko J., Ptacek J.T. Pain during burn hospitalization predicts long-term outcome. J Burn Care Res. 2006;27:719–726. doi: 10.1097/01.BCR.0000238080.77388.FE. [DOI] [PubMed] [Google Scholar]
- 31.Buttes P., Keal G., Cronin S.N., Stocks L., Stout C. Validation of the critical-care pain observation tool in adult critically ill patients. Dimens Crit Care Nurs. 2014;33:78–81. doi: 10.1097/DCC.0000000000000021. [DOI] [PubMed] [Google Scholar]
- 32.Mcguinness S.K., Wasiak J., Cleland H., et al. A systematic review of ketamine as an analgesic agent in adult burn injuries. Pain Med. 2011;12:1551–1558. doi: 10.1111/j.1526-4637.2011.01220.x. [DOI] [PubMed] [Google Scholar]
- 33.Cuignet O., Pirson J., Soudon O., Zizi M. Effects of gabapentin on morphine consumption and pain in severely burned patients. Burns. 2007;33:81–86. doi: 10.1016/j.burns.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 34.Evoy K.E., Morrison M.D., Saklad S.R. Abuse and misuse of pregabalin and gabapentin. Drugs. 2017;77:403–426. doi: 10.1007/s40265-017-0700-x. [DOI] [PubMed] [Google Scholar]
- 35.Grayson K.E., Bailey M., Balachandran M., et al. The effect of early sedation with dexmedetomidine on body temperature in critically ill patients. Crit Care Med. 2021;49:1118–1128. doi: 10.1097/CCM.0000000000004935. [DOI] [PubMed] [Google Scholar]