Abstract
The effects of the phytohormone strigolactone (SL) and smoke-derived karrikins (KARs) on plants are generally distinct, despite the fact that they are perceived through very similar mechanisms. The homologous receptors DWARF14 (D14) and KARRIKIN-INSENSITIVE2 (KAI2), together with the F-box protein MORE AXILLARY GROWTH2 (MAX2), mediate SL and KAR responses, respectively, by targeting different SMAX1-LIKE (SMXL) family proteins for degradation. These mechanisms are putatively well-insulated, with D14-MAX2 targeting SMXL6, SMXL7, and SMXL8 and KAI2-MAX2 targeting SMAX1 and SMXL2 in Arabidopsis thaliana. Recent evidence challenges this model. We investigated whether D14 can target SMAX1 and whether this occurs naturally. Genetic analysis indicates that the SL analog GR24 promotes D14-SMAX1 crosstalk. Although D14 shows weaker interactions with SMAX1 than with SMXL2 or SMXL7, D14 mediates GR24-induced degradation of SMAX1 in plants. Osmotic stress triggers SMAX1 degradation, which is protective, through SL biosynthesis and signaling genes. Thus, D14-SMAX1 crosstalk may be beneficial and not simply a vestige of the evolution of the SL pathway.
Key words: phytohormone, signaling, proteolysis, crosstalk
The strigolactone analog GR24 elicits a physical interaction between D14 and SMAX1 that leads to the degradation of both proteins and the inhibition of hypocotyl elongation in light-grown seedlings. D14-SMAX1 crosstalk enhances osmotic stress tolerance and suppresses the induction of osmotic-stress-related gene expression in Arabidopsis seedlings in a strigolactone-dependent manner.
Introduction
Strigolactones (SLs) and karrikins (KARs) are two classes of butenolide molecules that regulate diverse aspects of plant development. SLs were discovered in root exudates as germination stimulants of root-parasitic plants (Cook et al., 1966; Bouwmeester et al., 2021). SLs exuded into soil promote symbiotic interactions between roots and arbuscular mycorrhizal fungi, partly by stimulating hyphal branching (Akiyama et al., 2005; Gomez-Roldan et al., 2008; Kobae et al., 2018). SLs are also plant hormones with many roles, including the regulation of shoot branching, root growth, cambial growth, senescence, defense, and anthocyanin biosynthesis (Gomez-Roldan et al., 2008; Umehara et al., 2008; Agusti et al., 2011; Rasmussen et al., 2012; Van Ha et al., 2014; Yamada et al., 2014; Soundappan et al., 2015; Ueda and Kusaba, 2015; Lahari et al., 2019; Nasir et al., 2019; Villaécija-Aguilar et al., 2019; Wang et al., 2020a; Li et al., 2020b; Kalliola et al., 2020). KARs are abiotic signals found in smoke and biochar (Flematti et al., 2004; Kochanek et al., 2016). They promote germination of many plant species after fire but can also stimulate species from non-fire-prone environments such as Arabidopsis thaliana (Flematti et al., 2004; Nelson et al., 2012). In addition, KAR signaling influences seedling photomorphogenesis, mesocotyl elongation, root and root hair growth, and abiotic stress responses (Jain et al., 2006; Nelson et al., 2010; Li et al., 2017, 2020b; Wang et al., 2018; Swarbreck et al., 2019; Villaécija-Aguilar et al., 2019; Zheng et al., 2020).
Despite their different sources and effects, SLs and KARs are perceived similarly (Blázquez et al., 2020). The core SL signaling pathway in angiosperms consists of the receptor DWARF14 (D14)/DECREASED APICAL DOMINANCE2 (DAD2)/RAMOSUS3 (RMS3), the F-box protein DWARF3 (D3)/MORE AXILLARY GROWTH2 (MAX2), and transcriptional co-repressors in the SUPPRESSOR OF MAX2 1 (SMAX1)-LIKE (SMXL) family that are known as DWARF53 (D53) in rice (Oryza sativa) or SMXL6, SMXL7, and SMXL8 in Arabidopsis thaliana (Gomez-Roldan et al., 2008; Umehara et al., 2008; Hamiaux et al., 2012; Waters et al., 2012; Jiang et al., 2013; Stanga et al., 2013; Zhou et al., 2013; de Saint Germain et al., 2016). D14 is an α/β-hydrolase that cleaves an enol-ether-linked methylbutenolide “D-ring” from SLs (Hamiaux et al., 2012; Seto et al., 2019). The D-ring becomes covalently attached to a His residue in the catalytic triad (de Saint Germain et al., 2016; Yao et al., 2016). D14 changes conformation during SL binding or hydrolysis, promoting interactions with D3/MAX2 and D53/SMXL6/7/8 (Jiang et al., 2013; Zhou et al., 2013; Wang et al., 2015; Yao et al., 2016). D14 is central to the formation of the tripartite complex, but D3 and D53 help stabilize the complex (Liang et al., 2016; Shabek et al., 2018). D3/MAX2 functions within an Skp1, Cullin, F-box (SCF)-type E3 ubiquitin ligase complex. SCFMAX2 polyubiquitinates D53/SMXL6/7/8 proteins, which are then rapidly degraded by the 26S proteasome (Jiang et al., 2013; Zhou et al., 2013; Soundappan et al., 2015; Wang et al., 2015; Yao et al., 2016; Shabek et al., 2018). D14 is also degraded after SL activation in a MAX2-dependent manner, but this occurs over hours rather than minutes (Chevalier et al., 2014; Hu et al., 2017).
KAR signaling shares a requirement for MAX2, but the ancient D14 paralog KARRIKIN INSENSITIVE 2 (KAI2)/HYPOSENSITIVE TO LIGHT (HTL) acts as a receptor, and SMAX1 and SMXL2 are downstream targets (Nelson et al., 2011; Sun and Ni, 2011; Waters et al., 2012; Stanga et al., 2013, 2016; Khosla et al., 2020b; Zheng et al., 2020). Similar to SL signaling, the activation of KAI2 triggers its association with MAX2 and SMAX1/SMXL2, leading to SMAX1 and SMXL2 degradation (Yao et al., 2017; Xu et al., 2018; Carbonnel et al., 2020a; Khosla et al., 2020b; Wang et al., 2020b, 2021; Zheng et al., 2020). Polyubiquitination has been demonstrated for SMXL2 and is presumed for SMAX1 (Wang et al., 2020b). KAI2 is also degraded after activation, although unlike D14, this is SMAX1/SMXL2- dependent rather than MAX2-dependent (Waters et al., 2015b; Khosla et al., 2020b). In addition to mediating KAR responses, KAI2 is thought to recognize an endogenous signal, KAI2 ligand (KL), that remains undiscovered (Waters et al., 2015a; Conn and Nelson, 2015). KAI2 is more sensitive to desmethyl butenolide compounds than methylbutenolide compounds, which may give hints about the chemical structure of KL (Yao et al., 2021). KARs themselves are likely to require metabolism in plants for recognition by KAI2 (Waters et al., 2015a; Xu et al., 2018; Khosla et al., 2020b; Wang et al., 2020b; Nelson, 2021).
There is substantial evidence that SL and KAR/KL pathways function independently despite their homology. First, SL and KAR treatments usually affect different aspects of plant growth (Waters et al., 2017). For example, SLs inhibit shoot branching, whereas KARs promote Arabidopsis germination (Nelson et al., 2011; Scaffidi et al., 2014). Second, genetic analysis often shows different roles for SL and KAR/KL pathway genes. SL-insensitive and SL-deficient mutants often have different phenotypes than the KAR/KL-insensitive mutant kai2 (Nelson et al., 2011; Waters et al., 2012; Villaécija-Aguilar et al., 2019). Likewise, smax1 (or smax1 smxl2) and smxl6,7,8 mutants suppress different max2 phenotypes that are associated with KAR/KL and SL insensitivity, respectively (Stanga et al., 2013, 2016; Soundappan et al., 2015; Wang et al., 2015; Swarbreck et al., 2019; Villaécija-Aguilar et al., 2019). In some cases, however, such as drought resistance or mesocotyl elongation, both pathways may influence a trait (Li et al., 2020b; Zheng et al., 2020). Third, promoter-swapping experiments show that KAI2 and D14 are not interchangeable genes whose unique roles arise from different expression patterns (Waters et al., 2015a; Carbonnel et al., 2020b). Fourth, D14 and KAI2 prefer to interact with different SMXL targets (Yao et al., 2017; Khosla et al., 2020b; Wang et al., 2020b; Zheng et al., 2020). Receptor-SMXL interaction specificity is linked to the central D1M domains of SMXL proteins (Khosla et al., 2020b). Fifth, KAR treatment triggers degradation of SMAX1-type, but not D53-type, SMXL proteins (Jiang et al., 2013; Wang et al., 2015; Khosla et al., 2020b; Zheng et al., 2020). Transient co-expression of SL and KAR/KL signaling components from Lotus japonicus in Nicotiana benthamiana also suggests the specific degradation of SMAX1 by KAI2 and a D53-type SMXL by D14 (Carbonnel et al., 2020a). Finally, evolutionary analysis indicates that D14 was derived from KAI2 and D53-type SMXL proteins were derived from SMAX1-type SMXLs (Bythell-Douglas et al., 2017; Walker et al., 2019). Co-evolution of D14 and D53-type SMXLs may have produced an orthogonal SL signaling pathway.
Recent work has challenged the model of insulated SL and KAR pathways. Genetic studies of lateral root development and root skewing initially implied that KAI2 may target SMXL6, SMXL7, and SMXL8 (Swarbreck et al., 2019). However, lateral root development was later shown to be regulated additively by SL and KAR/KL pathways, putatively with shifting contributions from each at different developmental stages (Villaécija-Aguilar et al., 2019). The effect of smxl6,7,8 on root skewing, which is KAI2-regulated, has been inconsistent between different labs (Swarbreck et al., 2019; Villaécija-Aguilar et al., 2019). Thus, there is no strong support for KAI2-SMXL6,7,8 crosstalk. By contrast, there is compelling biochemical evidence that D14 can target SMXL2 (Wang et al., 2020b). SMXL2 co-immunoprecipitates D14 in the presence of GR245DS or GR244DO, synthetic SL analogs of the natural SLs 5-deoxystrigol (5DS) and 4-deoxyorobanchol (4DO). Furthermore, GR244DO promotes the polyubiquitination and degradation of SMXL2 through D14 in the kai2 background (Wang et al., 2020b). This indicates that one-way crosstalk between the SL and KAR pathways is possible, while also raising the question of whether it occurs naturally.
Co-immunoprecipitation of D14 by SMAX1 was not observed, and it is unknown whether D14 can stimulate SMAX1 degradation (Wang et al., 2020b). However, the potential for D14-SMAX1 crosstalk has been suggested by D14-mediated effects of GR24 on hypocotyl elongation, root-hair density, and root-hair elongation, which are controlled by SMAX1 and SMXL2 (Waters et al., 2012; Toh et al., 2014; Stanga et al., 2016; Villaécija-Aguilar et al., 2019). We investigated whether D14 can interact with SMAX1 and target it for degradation. Here, we report that KAI2-independent hypocotyl inhibition in the presence of an SL analog is genetically dependent on D14 and MAX2 and is primarily due to the destabilization of SMAX1. Although the ability of D14 to interact with SMAX1 and SMXL2 may be a little-used vestige of its evolution from KAI2, this crosstalk has physiological relevance for osmotic stress responses in seedlings.
Results
Genetic evidence for D14 crosstalk with SMAX1 and SMXL2 in seedlings
KAR1, KAR2, and rac-GR24 (a racemic mixture of GR245DS and GR24ent−5DS) inhibit hypocotyl elongation of Arabidopsis seedlings grown under continuous red light (Nelson et al., 2010). GR245DS has a D-ring in the stereochemical configuration of natural SLs and signals through D14. Its enantiomer, GR24ent−5DS, has a D-ring configuration that is not found in SLs. GR24ent−5DS signals mostly through KAI2 but can also activate D14 in vitro and in vivo (Scaffidi et al., 2014; Waters et al., 2015a; Flematti et al., 2016). Although kai2 seedlings are insensitive to KAR2 and mostly insensitive to GR24ent−5DS, responses to rac-GR24 and GR245DS remain (Waters et al., 2012; Scaffidi et al., 2014). We first tested whether KAI2-independent responses to GR24 require MAX2. rac-GR24 and GR245DS had no effect on the kai2 max2 hypocotyl, confirming that responses to these compounds are MAX2-dependent (Supplemental Figure 1).
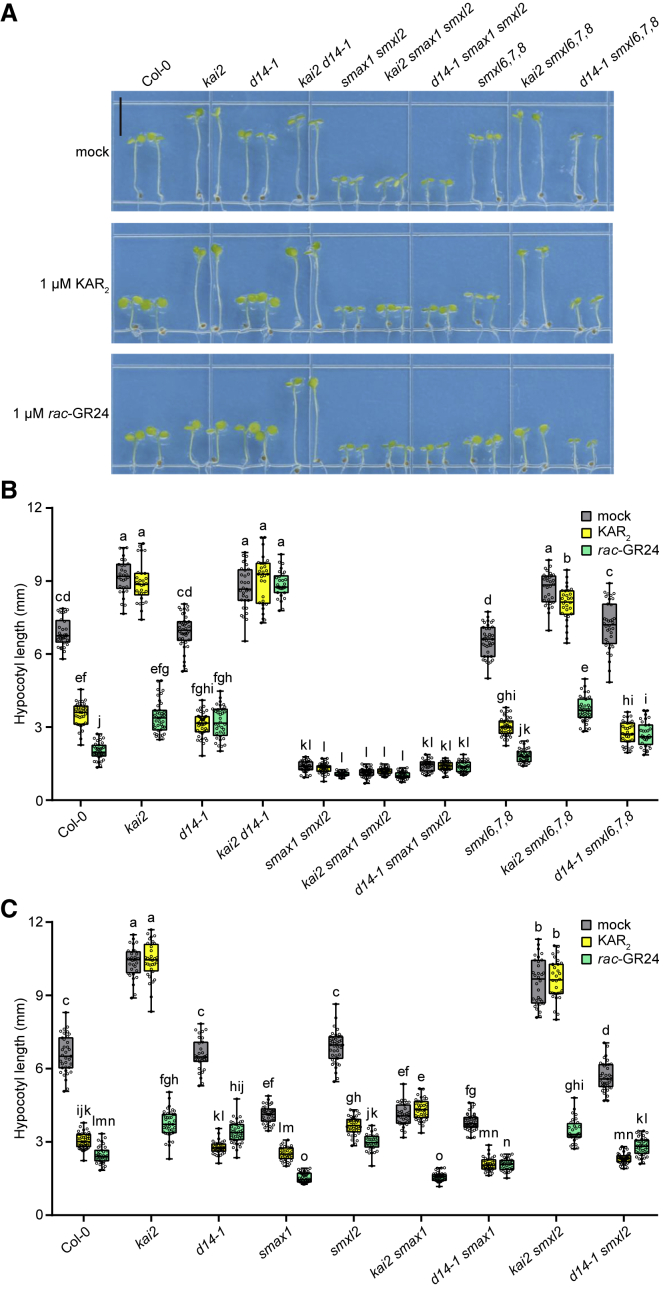
We next examined genetic interactions among kai2, d14-1, smax1, and smxl mutants to determine which SMXL genes are epistatic to KAI2 and D14 (Figures 1A and 1B; Supplemental Figure 2). As shown previously, d14-1 showed wild-type hypocotyl elongation under control conditions, implying that endogenous SLs do not affect hypocotyl growth. By contrast, kai2 had elongated hypocotyls, and smax1 smxl2 hypocotyls were very short (Waters et al., 2012; Stanga et al., 2016). The kai2 d14-1 double mutant was similar to kai2 but was also insensitive to GR24 treatments, indicating that KAI2-independent responses to GR24 occur through D14 (Scaffidi et al., 2014). The kai2 smax1 smxl2 and d14-1 smax1 smxl2 triple mutants showed dramatically decreased hypocotyl lengths that were not further affected by KAR2 or GR24 treatments, similar to smax1 smxl2. This indicated that SMAX1 and SMXL2 are epistatic to KAI2 (Figures 1A and 1B).
Figure 1.
D14 inhibits hypocotyl growth after GR24 treatment via SMAX1 and SMXL2.
(A) Images of representative 5-day-old seedlings of Col-0 (wild type), kai2, d14-1, kai2 d14-1, smax1 smxl2, kai2 smax1 smxl2, d14-1 smax1 smxl2, smxl 6,7,8, kai2 smxl6,7,8, and d14-1 smxl6,7,8 grown under continuous red light for 4 days on 0.5× MS agar medium containing 1 μM KAR2, 1 μM rac-GR24, or acetone. Bar, 5 mm.
(B) Hypocotyl lengths of the seedlings shown in (A).
(C) Hypocotyl lengths of 5-day-old seedlings of Col-0, kai2, d14-1, smax1, smxl2, kai2 smax1, d14-1 smax1, kai2 smxl2, and d14-1 smxl2 grown under continuous red light for 4 days on 0.5× MS medium containing 1 μM KAR2, 1 μM rac-GR24, or acetone. Box-and-whisker plots with the same letter are not significantly different from one another (Tukey’s honest significant difference [HSD], p < 0.05, n ≥ 30).
Because hypocotyl elongation of d14-1 is similar to that of the wild type, however, the d14-1 smax1 smxl2 triple mutant did not clarify whether SMAX1 and SMXL2 also act downstream of D14 or function in a separate pathway. We found evidence for the former idea by excluding a role for SMXL6, SMXL7, and SMXL8 in hypocotyl growth. We did not observe an appreciable difference between smxl6,7,8 and wild-type seedlings under the mock condition or in their responses to KAR2 or GR24 (Figures 1A and 1B). Moreover, smxl6,7,8 mutations did not substantially affect the length of kai2 or d14-1 hypocotyls under the mock condition or their responses to KAR2 and GR24 treatments, in clear contrast to smax1 smxl2. Therefore, D14-mediated responses to rac-GR24 and GR245DS in seedling hypocotyls are not due to SMXL6,7,8 degradation. Instead, D14 is likely to target SMAX1 and/or SMXL2 for degradation in the presence of GR24.
SMAX1 is the primary regulator of hypocotyl growth targeted by KAI2 and D14
Given the biochemical evidence for D14 interactions with SMXL2, but not SMAX1, we hypothesized that D14 may target SMXL2 for degradation more effectively than SMAX1 (Wang et al., 2020b). To assess whether KAI2 and D14 differentially target SMAX1 and SMXL2 during hypocotyl elongation, we compared the growth of d14-1 smax1, d14-1 smxl2, kai2 smax1, and kai2 smxl2 seedlings (Figure 1C). Consistent with the larger role of SMAX1 in hypocotyl elongation, smax1 dramatically suppressed the elongated hypocotyl phenotype of kai2, whereas smxl2 had little effect (Stanga et al., 2016). Responses to rac-GR24 and GR245DS were similarly strong in kai2 smxl2 and kai2, putatively reflecting the ability of D14 to act upon SMAX1 (Figure 1C and Supplemental Figure 3). Interestingly, the average hypocotyl length of seedlings treated with rac-GR24 was slightly shorter for kai2 smax1 (in which D14 and SMXL2 remain) than for d14-1 smax1 (in which KAI2 and SMXL2 remain), suggesting that D14 may target SMXL2 better than KAI2. Conversely, the hypocotyl length of seedlings treated with rac-GR24 was slightly longer for kai2 smxl2 than for d14-1 smxl2, suggesting that KAI2 may target SMAX1 better than D14 (Figure 1C). A similar pattern of results was observed in treatments with purified GR24 stereoisomers (Supplemental Figure 3).
SMAX1 is degraded after GR245DS treatment by D14-SCFMAX2 signaling
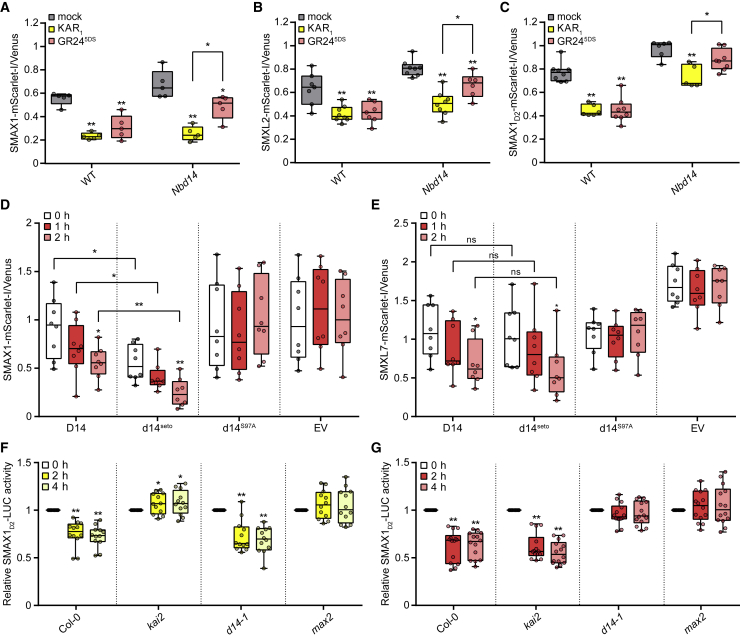
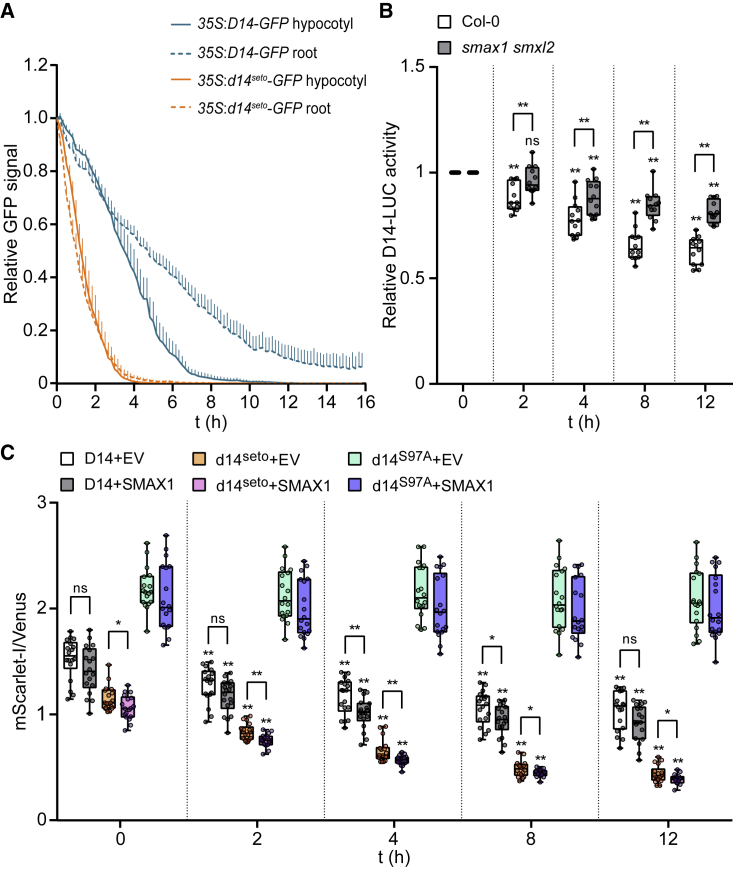
We next used a ratiometric reporter system to investigate whether D14 can induce degradation of Arabidopsis SMAX1 and SMXL2 proteins (Figure 2) (Khosla et al., 2020a, 2020b). We transiently expressed pRATIO1212-SMAX1, -SMXL2, and -SMAX1D2 (a C-terminal domain of SMAX1 sufficient for degradation; see below) dual-fluorescent reporter constructs in wild-type N. benthamiana leaves and tested the effects of 10 μM KAR1 and GR245DS treatments on excised leaf discs. The ratio of mScarlet-I/Venus fluorescence decreased for all constructs in response to both treatments, indicating the degradation of SMAX1-, SMXL2-, and SMAX1D2-mScarlet-I fusion proteins (Figures 2A–2C). The extent of degradation induced by GR245DS was similar to that induced by KAR1. Although GR245DS responses are predominantly mediated by D14 in Arabidopsis, we could not assume that GR245DS-induced degradation of SMAX1 and SMXL2 in N. benthamiana was due to D14 alone. Therefore, we also tested these constructs in an N. benthamiana d14a d14b double mutant (Nbd14) background (White et al., 2021). The SMXL7 reporter was unaffected by rac-GR24 in Nbd14, indicating that its degradation is specifically mediated by N. benthamiana D14 proteins and not by KAI2 (White et al., 2021). In Nbd14 leaves, we observed 55% and 51% less degradation of SMAX1 and SMXL2 reporters, respectively, after 12 h treatment with GR245DS compared with KAR1 (Figures 2A and 2B). At an earlier 4-h time point, GR245DS had very little effect on SMAX1 degradation compared with KAR1 in the Nbd14 mutant, but it was effective in the wild type (Supplemental Figure 4). These results indicated that N. benthamiana D14 proteins mediate much, although not all, of the GR245DS-induced degradation of SMAX1 and SMXL2.
Figure 2.
SL triggers SMAX1 and SMXL2 degradation through D14.
(A–C) Relative fluorescence from the SMAX1-mScarlet-I reporter (A), the SMXL2-mScarlet-I reporter (B), or the SMAX1D2-mScarlet-I reporter (C) and the Venus reference after transient expression of the ratiometric system in wild-type (WT) tobacco and Nbd14 is shown. Leaf discs were treated with acetone, 10 μM KAR1, or 10 μM GR245DS for 12 h before measurement. n = 5–8 leaf discs. Asterisks indicate significant differences from each acetone control or between compared pairs using Student's t test (∗p < 0.05 and ∗∗p < 0.01).
(D and E) Relative fluorescence from the SMAX1-mScarlet-I reporter (D) or the SMXL7-mScarlet-I reporter (E) along with D14, d14seto, d14S97A, or an empty vector (EV) expressed in Nbd14 at 0, 1, and 2 h after 10 μM GR245DS treatment. n = 12 leaf discs. ns, no significance. ∗p < 0.05, ∗∗p < 0.01, Student's t test comparisons with the relative fluorescence at 0 h or between compared pairs.
(F and G) SMAX1D2-luciferase (LUC) transgenic seedlings in the Col-0, kai2, d14-1, and max2 backgrounds were treated with 5 μM KAR2(F), 5 μM GR245DS(G), or acetone for 4 h. Bioluminescence is shown as relative LUC activity at 0, 2, and 4 h after treatment. n = 12–14 seedlings. ∗p < 0.05, ∗∗p < 0.01, Student's t test comparisons with each genotype/treatment at 0 h.
To verify that D14 can cause SMAX1 degradation, we rescued the Nbd14 mutant by transient expression of Arabidopsis D14. As a negative control, we tested the d14S97A mutant, which has no SL hydrolysis or signaling activity (Waters et al., 2015a; Seto et al., 2019). We also tested the seto5/d14-2 allele of Arabidopsis D14 (referred to here as d14seto to avoid confusion with the Osd14-2 allele in rice). The d14seto mutant has increased axillary bud outgrowth, similar to the loss-of-function T-DNA insertion allele d14-1 (Chevalier et al., 2014). Co-expression of D14 restored the degradation of SMAX1 and SMXL7 reporters following GR245DS treatment in Nbd14 leaves (Figures 2D and 2E). By contrast, d14S97A failed to restore GR245DS-induced degradation of SMAX1 and SMXL7. Interestingly, d14seto enabled GR245DS-induced degradation of SMAX1 and SMXL7, similar to D14. Moreover, in the absence of GR245DS treatment, d14seto co-expression reduced the accumulation of the SMAX1 reporter relative to D14 co-expression (Figures 2D and 2E). Therefore, the d14seto allele does not cause a complete loss of function and may be more effective at triggering SMAX1 degradation.
We next investigated whether SMAX1 degradation in Arabidopsis also involves D14. We have not yet been successful in detecting full-length SMAX1 in Arabidopsis (Khosla et al., 2020b). However, the C-terminal D2 domain of SMAX1 (SMAX1D2) is more stable than SMAX1 and is necessary and sufficient for MAX2-mediated degradation if full-length SMAX1 and/or SMXL2 proteins are also present. SMAX1D2 lacks the central D1M domains that mediate interactions between SMXL proteins and their receptor partners, KAI2 or D14, and it is therefore likely to be targeted for degradation indirectly through association with SMAX1 or SMXL2 (Khosla et al., 2020b). D14-mediated, GR245DS-induced degradation of the SMAX1D2 ratiometric reporter in N. benthamiana was similar to that of the full-length SMAX1 and SMXL2 reporters (Figure 2C). Therefore, we crossed kai2 and d14-1 mutations into a stable transgenic SMAX1D2-luciferase (LUC) reporter line in Arabidopsis to analyze KAR2- and GR245DS-induced degradation responses (Khosla et al., 2020b). KAR2 caused a significant decline in SMAX1D2-LUC bioluminescence within 4 h in wild-type and d14-1 seedlings but had no effect on kai2 or max2 seedlings (Figure 2F). By contrast, GR245DS caused a decline in the abundance of SMAX1D2-LUC reporters in wild-type and kai2 seedlings but not in d14-1 or max2 seedlings (Figure 2G). This result demonstrated that GR245DS-induced degradation of SMAX1D2 (and, by proxy, SMAX1 and/or SMXL2) in Arabidopsis is due to D14 and MAX2 activity.
GR245DS promotes interactions of D14 with SMAX1 and SMXL2
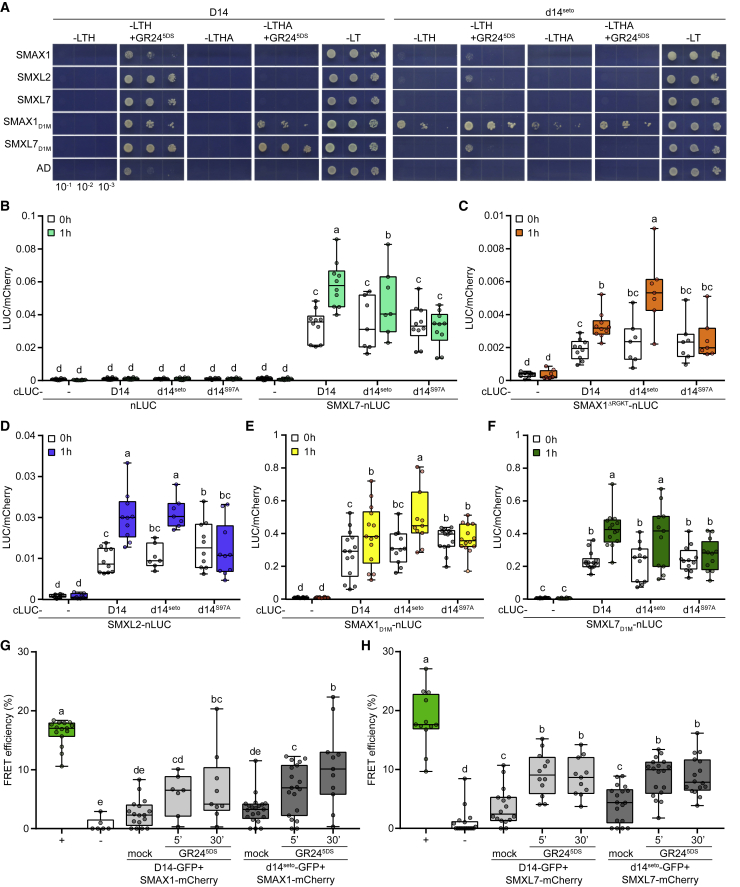
To determine whether D14 targets SMAX1 and SMXL2 directly, we investigated interactions among these proteins. In yeast two-hybrid (Y2H) assays, GR245DS stimulated protein-protein interactions between D14 and SMAX1, SMXL2, and SMXL7. Based upon the relative growth rates of yeast under low-stringency histidine dropout selection, D14-SMAX1 interactions were weaker than D14-SMXL2 and D14-SMXL7 interactions and not very different from a GAL4 activation domain (AD) negative control. In the presence of GR245DS, D14 had stronger interactions with the D1M domains of SMAX1 and SMXL7 (SMAX1D1M and SMXL7D1M) than the full-length proteins, as indicated by yeast growth under higher-stringency histidine and adenine dropout selection. Again, D14 showed a stronger interaction with SMXL7D1M than with SMAX1D1M (Figure 3A). As a negative control, we tested d14S97A and observed no interactions (Supplemental Figure 5).
Figure 3.
D14 and SMAX1 proteins can physically interact.
(A) Yeast two-hybrid assays for D14 and d14seto interactions with SMAX1, SMXL7, and their D1M domains. D14 and d14seto were fused to GAL4-BD. SMAX1, SMXL7, and their domains were fused to GAL4-AD. Serial 10-fold dilutions of yeast cultures were spotted onto selective growth medium (-L, -Leu; -T, -Trp; -H, -His; -A, -Ade) supplemented with 2 μM GR245DS or acetone (control).
(B–F) Split-LUC complementation assay for interactions between SMXL7 (B), SMAX1ΔRGKT(C), SMXL2 (D), and D1M domains of SMAX1 (E) and SMXL7 (F) with D14, d14seto, or d14S97A. N. benthamiana leaves were transiently co-transformed with Agrobacterium tumefaciens strains carrying cLUC, nLUC, or the indicated fusions, as well as a strain carrying an mCherry transgene as a transformation control. Luminescence was measured before and 1 h after treatment with 10 μM GR245DS and was normalized against mCherry fluorescence. Box-and-whisker plots with the same letter are not significantly different from one another (Student's t test, p < 0.05, n = 7–15 leaf discs).
(G) FRET-ABP assay for interactions of SMAX1 with D14. N. benthamiana leaves were transiently co-transformed with Agrobacterium tumefaciens strains carrying SMAX1-GFP-mCherry or the indicated fusions. The FRET efficiency is shown as the percentage increase in donor fluorescence compared with that before receptor bleaching. + (dark green box) and – (white box) indicate SMAX1-GFP-mCherry as a positive control and the SMAX1-mCherry/Myc-GFP pair as a negative control, respectively. Acetone-treated leaf discs were used as mock controls. Box-and-whisker plots with the same letter are not significantly different from one another (Student's t test, p < 0.05, n = 6–21 leaf discs).
(H) FRET-ABP assay for interactions of SMXL7 with D14. N. benthamiana leaves were transiently co-transformed with Agrobacterium tumefaciens strains carrying SMXL7-GFP-mCherry or the indicated fusions. + (dark green box) and – (white box) indicate SMXL7-GFP-mCherry as a positive control and the SMXL7-mCherry/Myc-GFP pair as a negative control, respectively. Acetone-treated leaf discs were used as mock controls. Box-and-whisker plots with the same letter are not significantly different from one another (Student's t test, p < 0.05, n = 6–18 leaf discs).
We also investigated SMAX1 and SMXL7 interactions with d14seto, which has an amino acid substitution at the solvent-exposed surface of a D14 cap helix that may influence protein-protein interactions (Chevalier et al., 2014). We observed that d14seto had highly reduced or abolished Y2H interactions with SMAX1, SMXL2, SMXL7, SMXL7D1M, and the GAL4 AD itself in the presence of GR245DS compared with D14. Unexpectedly, d14seto maintained the interaction with SMAX1D1M and, furthermore, interacted with SMAX1D1M in the absence of GR245DS (Figure 3A).
To validate the Y2H results in a plant system, we examined D14 interactions with SMXL proteins using split-LUC assays in N. benthamiana leaves. N- and C-terminal portions of firefly LUC were fused, respectively, to the C-termini of SMXL proteins and the N-termini of D14, d14seto, or d14S97A. To normalize transformation efficiencies across samples, the fluorescent protein mCherry was co-expressed with the split-LUC constructs. These assays were performed in Nbd14 leaves to avoid possible interference from native NbD14 proteins. The ratio of LUC to mCherry signal produced by cLUC-D14 and SMXL7-nLUC was significantly higher than that produced with unfused cLUC or nLUC negative controls. GR245DS further increased the LUC/mCherry ratio for D14-SMXL7, consistent with enhanced protein-protein interaction. Although d14S97A produced a similar interaction with SMXL7 as D14 before treatment, GR245DS had no effect (Figure 3B). In contrast to the Y2H experiments, d14seto appeared to interact with SMXL7 similarly to D14, albeit with a putatively reduced response to GR245DS. We next tested D14 interactions with SMAX1 and SMXL2. We were unable to detect a LUC/mCherry signal for D14-SMAX1 above that of the negative controls, even in the presence of GR245DS (Supplemental Figure 6). This may reflect the instability of SMAX1 (Khosla et al., 2020b). Deletion of a conserved P-loop motif (RGKT) causes resistance to SCFMAX2-mediated degradation in D53-type SMXL proteins as well as SMAX1 and SMXL2 (Jiang et al., 2013; Zhou et al., 2013; Soundappan et al., 2015; Wang et al., 2015, 2020b; Liang et al., 2016; Khosla et al., 2020b). Therefore, we tested interactions between SMAX1ΔRGKT and D14. This enabled the detection of a GR245DS-responsive interaction with D14, although with a much lower signal than D14-SMXL7 or D14-SMXL2. SMAX1ΔRGKT and SMXL2 interactions with D14, d14seto, and d14S97A were qualitatively similar to those observed for SMXL7, with a positive GR245DS response maintained for d14seto but not for d14S97A (Figures 3C and 3D). SMAX1D1M and SMXL7D1M showed a pattern of interactions with D14 and d14 mutant proteins that was similar to that of full-length SMXL proteins but produced stronger luminescence signals (Figures 3E and 3F, Supplemental Figure 7). In contrast to the Y2H experiments, we did not observe reduced interactions between SMXL7D1M and d14seto compared with D14 in the split-LUC assays.
The differing results in Y2H and split-LUC assays led us to further examine D14 and d14seto interactions with SMAX1 and SMXL7 by measuring Förster resonance energy transfer after acceptor photobleaching (FRET-APB) (Day et al., 2001). This technique determines FRET efficiency, which is a measure of protein-protein interactions, by comparing the fluorescence of the donor (e.g., GFP) before and after photobleaching of the acceptor (e.g., mCherry). We performed FRET-APB assays with D14-GFP, d14seto-GFP, and SMAX1-mCherry fusion proteins co-expressed in N. benthamiana leaves. Photobleaching of SMAX1-mCherry caused a negligible change in fluorescence of a myc-GFP negative control, indicating an absence of FRET between these two proteins (Figure 3G). By contrast, FRET was detected between D14-GFP and SMAX1-mCherry. After 5 min of treatment with a solvent control, SMAX1-mCherry photobleaching caused a small increase in D14-GFP fluorescence. Treatment with GR245DS for 5 or 30 min increased the FRET efficiency approximately 2- to 3-fold above the solvent control. Similar results were obtained for d14seto-GFP and SMAX1-mCherry. The average FRET efficiency between d14seto-GFP and SMAX1-mCherry was higher than that between D14-GFP and SMAX1-mCherry after 30 min of GR245DS treatment (10.4% versus 6.9%), although this difference was not statistically significant (p = 0.24, Student's t test). We also examined D14-SMXL7 interactions with FRET-APB. The FRET efficiency between D14 and SMXL7 peaked within 5 min of GR245DS treatment. Similar FRET efficiencies in the presence and absence of GR245DS were observed between d14seto and SMXL7 (Figure 3H).
Together, these experiments indicate that D14 and SMAX1 can associate in the presence of GR245DS. Y2H and split-LUC experiments suggest that D14 can interact better with SMXL2 than with SMAX1, although this may be due, at least in part, to the instability of SMAX1 (Figure 3 and Supplemental Figure 7). The effect of d14seto is less clear. Although Y2H experiments suggested that d14seto was less able to interact with SMAX1, SMXL2, and SMXL7, this was not supported by split-LUC and FRET-APB assays in plants. The differences could be a consequence of overexpression or of the effects of other proteins in the plant cell environment (e.g., MAX2) on D14 signaling, interactions, and stability. Regardless, d14seto was not as deficient as d14S97A in its interactions with SMXL proteins or the GR245DS response, suggesting that it is hypomorphic rather than amorphic.
A hypomorphic d14 protein is more active when SMAX1 and SMXL2 are absent
Although D14 can induce degradation of SMAX1 and SMXL2, it is unclear whether this is only an artifact of treatments with an exogenous SL analog. If D14-mediated degradation of SMAX1 and SMXL2 has physiological significance, we can expect d14 to affect growth processes controlled by SMAX1 and SMXL2 and/or smax1 smxl2 to at least partially suppress d14 phenotypes. As noted above, d14-1 seedlings are phenotypically similar to the wild type. We found that d14seto hypocotyls were slightly shorter than those of the wild type, suggesting that SMAX1/SMXL2 may be partially reduced (Supplemental Figure 8). However, d14seto and kai2 d14seto showed little response to GR245DS, implying that any such targeting by d14seto may reflect promiscuous activity rather than an SL response, as suggested by the Y2H results (Figure 3A and Supplemental Figure 8).
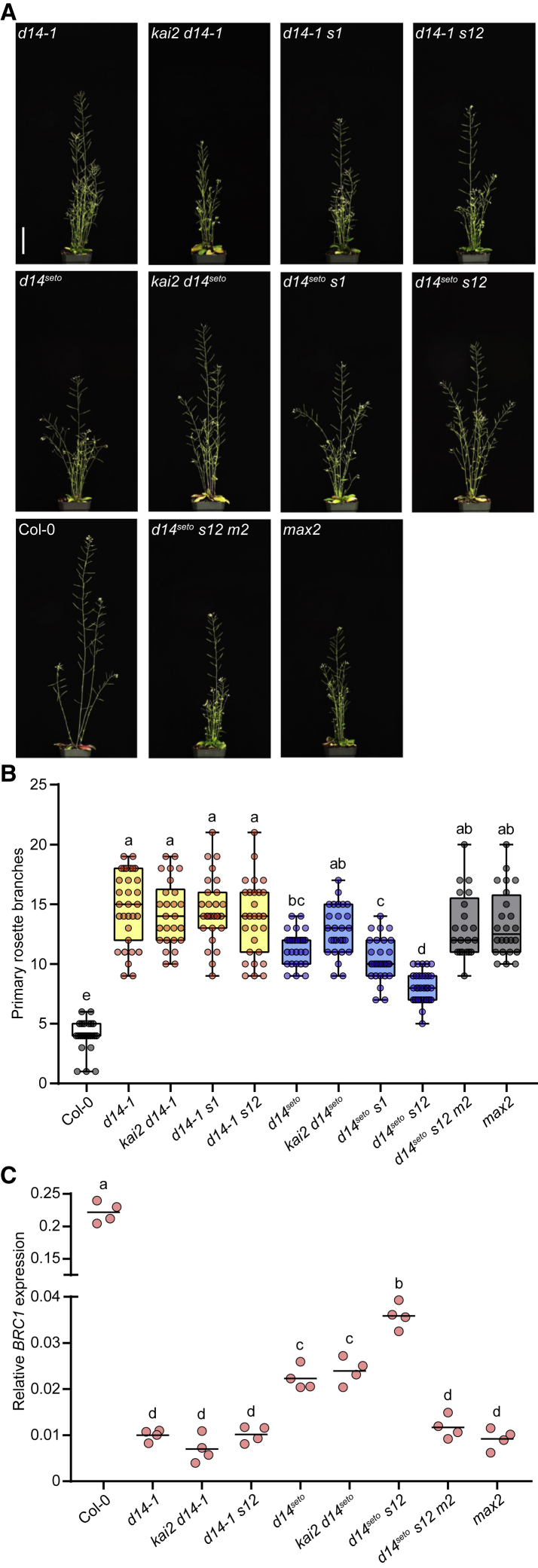
We next examined the effects of KAI2, SMAX1, and SMXL2 on the excess shoot-branching phenotype of d14. A recent study based on the overexpression of SMAX1 proposed that SMAX1 suppresses axillary shoot branching (Zheng et al., 2021). Contrary to this result, we did not observe any effect of kai2, which overaccumulates SMAX1 and SMXL2, or smax1 smxl2 on the excess branching phenotype of d14-1 (Figures 4A and 4B). We also investigated genetic interactions between d14seto and KAR signaling mutants. The excess branching phenotype of d14seto was weaker than that of d14-1, consistent with d14seto causing a partial loss of function. Interestingly, branching number was increased to d14-1 and max2 levels in the kai2 d14seto mutant and reduced in d14seto smax1 smxl2. Because max2 was epistatic in the d14seto smax1 smxl2 max2 mutant, SMAX1 and SMXL2 are unlikely to regulate shoot branching downstream of MAX2. Instead, these data suggest that SMAX1 and SMXL2 negatively affect the ability of d14seto to target SMXL6, SMXL7, and SMXL8 for degradation.
Figure 4.
d14seto is hypomorphic and more active in an smax1 smxl2 background.
(A) Adult shoot morphology of Col-0, d14-1, kai2 d14-1, d14-1 smax1, d14-1 smax1 smxl2, d14seto, kai2 d14seto, d14setosmax1, d14setosmax1 smxl2, d14setosmax1 smxl2 max2, and max2 plants. Bar, 5 cm.
(B) The number of primary rosette branches of plant materials in (A). Box-and-whisker plots with the same letter are not significantly different from one another (Tukey’s HSD, p < 0.05, n = 21–34).
(C) qRT-PCR analysis of BRC1/TCP18 gene expression in non-elongated axillary buds of Col-0, d14-1, kai2 d14-1, d14-1 smax1 smxl2, d14seto, kai2 d14seto, d14setosmax1 smxl2, d14setosmax1 smxl2 max2, and max2 plants collected 10 days after anthesis. Expression of BRC1 is relative to the CACS internal reference gene. Scatter dot plots with the same letter are not significantly different from one another (bar indicates mean; n = 4 pooled tissue samples, three plants per pool; Student's t test, p < 0.05).
We found further support for this idea from analysis of BRANCHED1 (BRC1) expression in non-elongated axillary buds. BRC1 is a transcription factor that represses axillary bud outgrowth and whose expression is negatively regulated by SMXL6, SMXL7, and SMXL8 (Aguilar-Martínez et al., 2007; Soundappan et al., 2015; Wang et al., 2020a). Consistent with the shoot-branching data, smax1 smxl2 did not increase BRC1 expression in the d14-1 background. BRC1 expression was higher in d14seto buds than in d14-1 buds, and the addition of smax1 smxl2 mutations further increased BRC1 expression in a MAX2-dependent manner (Figure 4C).
SMAX1 and SMXL2 may enhance D14 turnover after SL perception
One way that SMAX1 and SMXL2 might affect the activity of d14seto is by reducing its abundance. D14 and KAI2 are both degraded within hours after activation (Chevalier et al., 2014; Waters et al., 2015b; Hu et al., 2017). KAI2 degradation after KAR treatment is MAX2-independent and probably occurs through association with SMAX1 and SMXL2, which are unstable (Waters et al., 2015b; Khosla et al., 2020b). D14 degradation after GR24 treatment is MAX2-dependent in Arabidopsis (Chevalier et al., 2014). If d14seto is more prone to interactions with SMAX1 (Figures 3A and 3C), however, it may undergo increased turnover compared with wild-type D14. This led us to test the degradation of D14-GFP and d14seto-GFP fusions expressed in wild-type seedlings after treatment with rac-GR24. We observed a faster rate of decline for d14seto-GFP than for D14-GFP in both hypocotyl and root tissues of seedlings after rac-GR24 treatment (Figure 5A).
Figure 5.
D14 degradation after GR245DS treatment is enhanced by SMAX1 and SMXL2.
(A) The relative GFP signal from D14-GFP or d14seto-GFP transgenic plants was measured every 10 min in the presence of 5 μM rac-GR24. The curve was generated from the mean value per genotype/treatment at each time point. Bar indicates SE of the mean (n = 6 seedlings).
(B)UBQ:D14-LUC transgenic seedlings in the Col-0 and smax1 smxl2 backgrounds were treated with 5 μM GR245DS or acetone for 12 h. Bioluminescence is shown as relative LUC activity at 0, 2, 4, 8, and 12 h after treatment. n = 10–12 seedlings. Asterisks indicate significant differences relative to each group at 0 h or between compared pairs using Student's t test (∗p < 0.05 and ∗∗p < 0.01; ns, no significance).
(C) Time-course assay of D14, d14seto, and d14S97A stability in N. benthamiana under 10 μM GR245DS treatment. Relative fluorescence from the D14-mScarlet-I reporter, the d14seto-mScarlet-I reporter, or the d14S97A-mScarlet-I reporter and the Venus reference after transient co-expression of the ratiometric system and SMAX1 effector in tobacco is shown. Leaf discs were treated for 12 h to monitor D14, d14seto, and d14S97A stability. n = 14 leaf discs. Asterisks indicate significant differences relative to each group at 0 h or between compared pairs using Student's t test (∗p < 0.05 and ∗∗p < 0.01; ns, no significance).
To assess whether SMAX1 and SMXL2 influence GR245DS-induced degradation of D14, we next introduced a UBQ:D14-LUC transgene into wild-type and smax1 smxl2 backgrounds. The decline in bioluminescence from D14-LUC following GR245DS treatment was slowed in smax1 smxl2 at all time points compared with the wild type, suggesting that D14-LUC was partially stabilized by the absence of SMAX1 and SMXL2 (Figure 5B). We then transiently expressed D14, d14seto, and d14S97A ratiometric reporters with or without Arabidopsis SMAX1 in Nbd14 leaves (Figure 5C). The d14S97A reporter was the most stable of the three variants; it showed the highest relative abundance and was unaffected by GR245DS treatment. D14 and d14seto reporters both declined in the 12 h after GR245DS treatment. As in Arabidopsis, d14seto showed a faster rate of decline in tobacco. Co-expression of SMAX1 caused a small but significant increase in GR245DS-induced turnover of D14 at two time points and of d14seto at all time points. This suggested that the interaction of D14 with SMAX1 and SMXL2 may reduce its abundance in the presence of GR24; increased availability of a partially active d14seto protein may explain why the d14seto smax1 smxl2 mutant showed partially recovered shoot branching.
D14-SCFMAX2 mediates SMAX1 degradation induced by osmotic stress
Although D14 and KAI2 often affect different developmental traits, this is not always the case. For example, both the SL and KAR/KL pathways promote drought tolerance in Arabidopsis (Li et al., 2017, 2020a, 2020b; Haider et al., 2018). Our data suggest that D14 has no effect on SMAX1 and SMXL2 degradation in response to endogenous SLs during seedling photomorphogenesis or shoot branching. We reasoned that D14-mediated degradation of SMAX1 and SMXL2 might be physiologically relevant for some traits regulated by both pathways or under conditions in which endogenous SL levels are sufficiently high. SL biosynthesis genes are induced by dehydration or mild drought in Arabidopsis and rice, leading to increased SL, at least in rice roots (Van Ha et al., 2014; Haider et al., 2018).
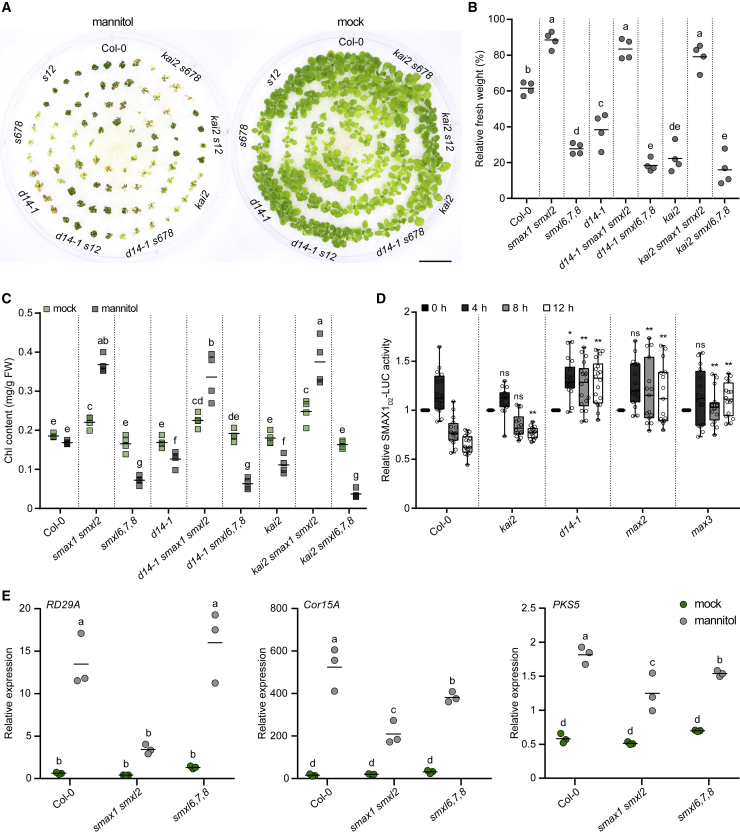
Therefore, as an alternative means of imposing drought/water deficit, we examined the response of KAR and SL signaling pathway mutants to osmotic stress. Wild-type seedlings grown in the presence of 300 mM mannitol showed a 40% reduction in fresh weight compared with seedlings grown on standard medium (Figures 6A and 6B). Growth inhibition by mannitol was enhanced in d14 and kai2 seedlings, and mannitol also caused a reduction in chlorophyll content (Figures 6A and 6C). We found that smxl6,7,8 seedlings were even more strongly affected by mannitol than kai2 and d14. By contrast, smax1 smxl2 seedlings were resistant to mannitol, showing only a 10% reduction in fresh weight and an increase in chlorophyll content under mannitol treatment. Intriguingly, SMAX1 and SMXL2 contributed differently to osmotic stress tolerance. Under mannitol treatment, we observed less reduction in biomass in smax1 seedlings and higher chlorophyll content in smxl2 seedlings compared with the wild type (Supplemental Figure 9).
Figure 6.
D14 targets SMAX1 and SMXL2 under osmotic stress.
(A) Twenty-one-day-old seedlings of Col-0, smax1 smxl2, smxl 6,7,8, d14-1, d14-1 smax1 smxl2, d14-1 smxl6,7,8, kai2, kai2 smax1 smxl2, and kai2 smxl6,7,8 grown under mock or 300 mM mannitol conditions for 14 days. Bar, 2 cm.
(B) Relative fresh weights of plant materials used in (A) after application of 300 mM mannitol. The weights of aerial parts from plants grown on 0.5× MS agar medium with 300 mM mannitol are scaled to those from plants grown on 0.5× MS agar medium. Scatter dot plots with the same letter are not significantly different from one another (bar indicates mean; n = 4; Student's t test, p < 0.05).
(C) Chlorophyll (Chl) contents in the aerial parts of Arabidopsis seedlings used in (A). Others are as in (B).
(D) Bioluminescence of SMAX1D2-LUC in Col-0, kai2, d14-1, max2, and max3 backgrounds. Seedlings were treated with 300 mM mannitol or water (control) for 12 h. Bioluminescence is shown as relative LUC activity at 0, 4, 8, and 12 h after treatment. n = 16–18 seedlings. ns, no significance. ∗p < 0.05, ∗∗p < 0.01, Student's t test comparisons with the Col-0 control at each time point.
(E) Expression of RD29A, Cor15A, and PKS5 relative to the CACS internal reference in Col-0, smax1 smxl2, and smxl6,7,8 grown for 7 days under a long-day photoperiod (16 h light/8 h dark) after 3 h mock or 300 mM mannitol treatment. Scatter dot plots with the same letter are not significantly different from one another (bar indicates mean; n = 3; Student's t test, p < 0.05).
To assess the effect of the smax1 smxl2 or smxl6,7,8 mutant on osmotic-stress-induced gene expression, we performed quantitative RT-PCR of RD29A, Cor15A, and PKS5 (Fujii et al., 2011; Liu et al., 2014). Induction of RD29A, Cor15A, and PKS5 transcripts in response to mannitol treatment was impaired in smax1 smxl2 seedlings. By comparison, RD29A showed normal upregulation in response to mannitol treatment in smxl6,7,8 seedlings. Cor15A and PKS5 were not as highly induced by mannitol in smxl6,7,8 seedlings than in the wild type but were more highly induced than in smax1 smxl2 (Figure 6E).
Because smax1 smxl2 had phenotypes opposite to those of d14 and kai2 and was epistatic to both, we hypothesized that D14 might contribute to SMAX1 and SMXL2 degradation during mannitol treatment. To test this possibility, we compared degradation of the SMAX1D2-LUC reporter after mannitol treatment in Col-0, kai2, d14-1, max2, and the SL biosynthetic mutant max3. We observed degradation of SMAX1D2-LUC within 8 h of mannitol treatment in wild-type and kai2 seedlings but not in d14-1, max2, or SL-deficient max3 seedlings (Figure 6D). SMXL7-LUC was also destabilized in a D14-dependent manner under mannitol treatment, supporting the idea that the level of endogenous SL and/or D14-SCFMAX2 signaling is induced by osmotic stress (Supplemental Figure 10). These results suggest that SL-induced degradation of SMAX1 and SMXL2 via D14-SCFMAX2 is not just an artificial consequence of GR24 application but can also occur under specific environmental conditions.
Discussion
Although there are strong similarities between the KAR/KL and SL signaling pathways, genetic and biochemical studies have suggested that they are well insulated by specific receptor-target interactions, enabling distinct developmental responses to KAR/KL and SL (Soundappan et al., 2015; Villaécija-Aguilar et al., 2019). Contradicting this model, here we have shown that D14 can target SMAX1 for degradation after SL analog treatments. Genetic tests indicated that SMAX1 and, to a lesser degree, SMXL2 regulate hypocotyl elongation, but SMXL6, SMXL7, and SMXL8 do not (Figure 1). This result implied that the D14-mediated effect of GR24 on hypocotyl elongation is due to D14-SMAX1 crosstalk. This idea was supported by the observation that an SMAX1 ratiometric reporter was degraded in N. benthamiana after GR245DS treatment in a partially D14-dependent manner (Figure 2A). GR245DS-induced degradation of an SMAX1D2 reporter in Arabidopsis thaliana was also blocked in the d14 background (Figure 2G). Physical interactions between D14 and SMAX1, however, are weak at best (Figure 3). SMXL proteins, which are distantly related to HSP101 heat-shock proteins that form hexamers, may form multimeric complexes (Khosla et al., 2020b). If heterogeneous complexes form (e.g., composed of SMAX1 and non-SMAX1 subunits), it is possible that SMAX1 could be indirectly targeted for proteolysis by a non-cognate receptor (i.e., D14) that interacts with SMXL2 or SMXL7. However, SMAX1 degradation by D14 does not require the presence of SMXL2, as demonstrated by the GR24 response of kai2 smxl2 seedlings (Figure 2), nor does GR24-induced degradation of SMAX1 and SMXL2 by D14 require SMXL6, SMXL7, or SMXL8, as shown by kai2 smxl6,7,8 seedlings (Figure 1C).
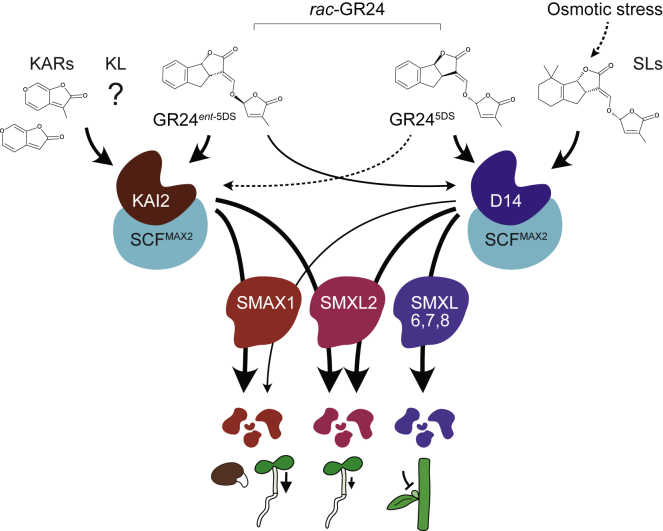
Therefore, our data suggest that a direct interaction between D14-SCFMAX2 and SMAX1 can occur when an SL analog is applied. Similarly, D14 can crosstalk with SMXL2 in the presence of SL analogs (Wang et al., 2020b). By contrast, there is no indication that KAR application can cause KAI2-SCFMAX2 to target D53 or SMXL7 for degradation, and the current genetic evidence for such crosstalk is controversial (Jiang et al., 2013; Wang et al., 2015; Swarbreck et al., 2019; Villaécija-Aguilar et al., 2019; Khosla et al., 2020b). We propose an update of the fully insulated KAR/KL and SL signaling models to include one-way promiscuity, in which D14 crosstalk with SMAX1 and SMXL2 is a putative remnant of its evolution from a KAI2 paralog (see below; Figure 7).
Figure 7.
Model for crosstalk between SL and KAR/KL signaling pathways.
KAI2 recruits the SCFMAX2 E3 ubiquitin ligase complex upon the perception of KAR/KL or GR24ent−5DS to target SMAX1 and SMXL2 for degradation. SL or GR245DS induces association of D14 with SCFMAX2 and SMXL7, SMXL2, and, to a lesser extent, SMAX1. This subsequently causes MAX2-dependent degradation of the targets. GR24ent−5DS activates D14 more weakly than GR245DS. GR245DS may trigger KAI2 signaling to a limited degree (dotted line), although evidence of ligand-binding and in vitro activation is missing. Degradation of SMXL7 represses shoot branching, whereas degradation of SMAX1 represses seed germination and hypocotyl elongation. SMXL2 plays a minor role in hypocotyl elongation compared with SMAX1. In seedlings, endogenous SL is insufficient to trigger crosstalk between D14 and SMAX1. It occurs, however, in the presence of GR24 and under some conditions, such as osmotic stress, that may raise SL levels.
SMAX1 can be targeted by D14 in Arabidopsis, but less well than SMXL2
It is likely that D14 has lower affinity for SMAX1 than for SMXL2. Although both SMAX1 and SMXL2 are able to co-immunoprecipitate KAI2 from Arabidopsis protoplasts in the presence of an agonist, only SMXL2 is effective at co-immunoprecipitation of D14 (Wang et al., 2020b). SMAX1 also did not interact with D14 in vitro in a pull-down assay (Yao et al., 2017). Likewise, we observed weaker Y2H interactions between D14 and SMAX1 than between D14 and SMXL2 (Figure 3A). In addition, we saw negligible luminescence in split-LUC assays for D14-SMAX1 interactions compared with D14-SMXL2 or D14-SMXL7. This may be due to MAX2-dependent and/or -independent degradation of SMAX1 that causes high turnover (Khosla et al., 2020b). The luminescence signal was increased in split-LUC assays between D14 and a degradation-resistant SMAX1ΔRGKT mutant protein, although it was still weaker than that produced by D14-SMXL2 interactions (Figure 3 and Supplemental Figure 6). Finally, we note that although we observed a strong effect of 500 nM GR245DS on hypocotyl elongation of both smax1 and smxl2, 2020b) observed different D14-mediated responses to 100 nM GR244DO treatments in these mutants (Figure 1 and Supplemental Figure 3). The 100 nM GR244DO treatment had only a small effect on hypocotyl elongation of smxl2 but had a large effect on smax1 seedlings, implying that SMAX1 may be less effectively degraded than SMXL2. Lower concentrations of SL may be required to induce D14 crosstalk with SMXL2 than with SMAX1. For developmental processes such as root-hair elongation, in which SMXL2 has a more prominent role than SMAX1, or root skewing, to which SMXL2 and SMAX1 contribute non-redundantly, endogenous SLs may be more likely to have an effect via D14-mediated crosstalk (Villaécija-Aguilar et al., 2019). It is currently unknown whether D14 can crosstalk with SMAX1 orthologs in other species. At least in rice, OsSMAX1 (LOC_Os08g15230) does not appear to be an interaction partner or target of D14 (Zheng et al., 2020).
Evolution of target preferences in KAR/KL and SL signaling pathways
Regardless of whether non-cognate interactions between D14 and SMAX1/SMXL2 affect development under physiological conditions, it is clear that the cognate interactions between D14 and D53-type SMXL proteins are important for SL-regulated growth in plants. This raises the question of how D14 and D53-type SMXL proteins evolved a specificity in their interactions that largely prevents crosstalk between the homologous SL and KAR/KL pathways in angiosperms. SLs have ancient origins in the land plant lineage (Yoneyama et al., 2018; Walker et al., 2019). However, D14 orthologs are observed only in the seed-bearing lineage (gymnosperms and angiosperms) (Bythell-Douglas et al., 2017). Gymnosperms have putative SMAX1 orthologs, but D53 orthologs are only found in angiosperms (Walker et al., 2019). Thus, the canonical D14-SCFMAX2-D53 SL signaling mechanism is a feature of angiosperms. An attractive hypothesis, however, is that KAI2-like proteins function as SL receptors that target SMAX1 for degradation in other land plants. This is quite plausible given that such a mechanism is used by the seeds of obligate parasitic plants in the Orobanchaceae to sense host-derived SLs and germinate (Nelson, 2021).
One way that selective protein-protein interactions between KAI2-SMAX1 and D14-SMXL7 could have evolved is via mutations in an SL-responsive KAI2 paralog (a proto-D14) that disrupt SMAX1 interactions, combined with compensatory mutations in an SMAX1 paralog (a proto-SMXL7) that establish an orthogonal interaction with the proto-D14. However, this evolutionary path involves an intermediate phase during which the proto-D14 is a pseudogene and/or the proto-SMXL7 is misregulated, with potentially detrimental effects. Bacterial toxin-antitoxin systems have revealed an alternative way in which duplicated protein pairs may evolve selective interactions: via a promiscuous intermediate state (Aakre et al., 2015). According to a promiscuity-based model, proto-D14 might first acquire a mutation that broadens its potential interaction specificity. This would enable proto-SMXL7 to acquire a mutation that blocks interactions with KAI2 but maintains interactions with proto-D14, without negatively affecting fitness. Subsequently, proto-D14 may acquire another mutation that narrows its interaction specificity to proto-SMXL7 alone. Throughout this process, SMXL7 regulation would continue. Substantial work will be needed to evaluate this hypothesis. However, we propose that the ability of D14 to engage in a non-preferred interaction with SMAX1 could be a remnant of such an evolutionary process.
Effects of the d14seto allele
The d14seto allele, which causes a Pro169Leu substitution, appears to reduce the selectivity of D14 against SMAX1 interactions (Figures 3A and 3C). Pro169 is a highly conserved (>90%) surface residue found within a small motif that distinguishes D14 and KAI2 proteins (ADV—P versus GDMDS, respectively) (Chevalier et al., 2014; Bythell-Douglas et al., 2017). As such, it has been hypothesized to be a specificity-determining position (Chevalier et al., 2014). Alternatively, this motif may influence SL perception. The motif containing Pro169 comprises most of a short loop that joins the ɑT2 and ɑT3 helices (also known as ɑE and ɑF) of D14. The composition of this loop affects the rigidity of the ligand-binding pocket, which in turn affects ligand affinities (Bürger et al., 2019).
Our results suggested that d14seto causes a partial loss of function in SL signaling, as it had weaker branching and leaf morphology phenotypes than the null T-DNA insertion allele d14-1 (Figure 4B and Supplemental Figure 11). This result implied that d14seto was less effective at triggering SL-induced degradation of SMXL6, SMXL7, and SMXL8. However, in transient expression experiments in N. benthamiana, d14seto showed an ability similar to that of wild-type D14 to interact with SMXL7 and cause its degradation (Figures 2E, 3B, 3F, and 3H). Therefore, we hypothesized that the d14seto protein may have reduced function because of higher instability. Supporting this notion, we found that d14seto was more rapidly degraded following GR245DS treatment than wild-type D14 in Arabidopsis and N. benthamiana (Figures 5A and 5C).
KAI2 degradation after KAR treatment is MAX2-independent and is probably driven by association with unstable SMAX1 and/or SMXL2 proteins (Waters et al., 2015b; Khosla et al., 2020b). We found that D14 instability was reduced in the smax1 smxl2 background (Figure 5B), suggesting that it may also be degraded by association with SMAX1 and/or SMXL2. This led us to hypothesize that enhanced d14seto turnover after SL perception might be caused by stronger association with SMAX1 and/or SMXL2 compared with wild-type D14. Indeed, co-expression of SMAX1 slightly enhanced d14seto degradation in N. benthamiana (Figure 5C). This hypothesis also predicts that the phenotypes of d14seto will be affected by SMAX1/SMXL2 abundance. Consistent with this prediction, the branching phenotype of d14seto was increased by the addition of kai2. Overaccumulation of SMAX1 and SMXL2 in kai2 might further reduce d14seto abundance (Figure 4B). Conversely, the excess branching of d14seto was partially suppressed by smax1 smxl2, perhaps indicating that the d14seto protein had been stabilized. Similarly, smax1 smxl2 partially suppressed the reduced BRC1 expression in d14seto (Figures 4B and 4C). By comparison, smax1 smxl2 had no effect on branching or BRC1 expression in the null d14-1 background (Figures 4B and 4C).
The physiological relevance of D14-SMAX1 crosstalk
Although D14 can target SMAX1 and SMXL2 for degradation after SL treatment, SL-deficient and SL-insensitive mutants do not show phenotypes associated with SMAX1 and SMXL2 overaccumulation, suggesting that this crosstalk does not normally occur (Nelson et al., 2011; Waters et al., 2012; Soundappan et al., 2015). Alternatively, SL levels that are sufficiently high to stimulate D14 crosstalk may occur only in limited developmental contexts. SL biosynthesis is induced by various stresses such as drought and phosphate starvation (López-Ráez et al., 2008; Van Ha et al., 2014; Haider et al., 2018). This led us to explore whether D14-SMAX1 crosstalk occurs during water stress. Interestingly, although smxl6,7,8 plants have enhanced resistance to water deficit, opposite to d14 and SL-deficient mutants, we found that smxl6,7,8 seedlings are more susceptible to osmotic stress (Van Ha et al., 2014; Li et al., 2020a, 2020b) (Figures 6A–6C). This was particularly surprising because d14 was also more susceptible to osmotic stress than the wild type. By contrast, smax1 smxl2 had enhanced resistance to osmotic stress and was epistatic to d14 and kai2 for this trait (Figures 6A–6C). Defective induction of RD29A and Cor15A expression in smax1 smxl2 may confer osmotic stress tolerance by strengthening photosynthesis and seedling growth (Msanne et al., 2011; Liu et al., 2014). Alternatively, given that smax1 smxl2 seedlings showed better growth under mannitol treatment than the wild type, the reduced upregulation of RD29A, Cor15A, and PKS5 by mannitol may indicate that smax1 smxl2 is less susceptible to osmotic stress. Although we cannot yet explain smxl6,7,8 phenotypes, this result suggested that D14 might target SMAX1 and SMXL2 under osmotic stress. Indeed, we observed enhanced degradation of an SMAX1D2 reporter following osmotic stress—without GR24 treatments—that was dependent on D14 and the SL biosynthesis gene MAX3. It is also notable that KAR-responsive genes are upregulated under osmotic stress (Shah et al., 2020). This implies a reduction in SMAX1 and SMXL2 levels, which could potentially be due to SL signaling activity. In conclusion, we propose that under some environmental conditions or developmental contexts, D14 crosstalk initiated by SLs may broaden the ability of plants to fine-tune SMAX1 and SMXL2 regulation.
Methods
Plant materials
The Arabidopsis thaliana mutants d14-1, d14seto, htl-3 (a kai2 allele), d14-1 htl-3, max2-1, smax1-2, smxl2-1, smax1-2 smxl2-1, smxl6-4 smxl7-3 smxl8-1, and max3-11 have been described previously (Waters et al., 2012; Stanga et al., 2013, 2016; Chevalier et al., 2014; Toh et al., 2014; Soundappan et al., 2015). All lines are in the Col-0 ecotype. Genotyping primers are listed in Supplemental Table 1. Detailed methods are found in the supplemental information.
Funding
We gratefully acknowledge funding support from US National Science Foundation (NSF) grants IOS-1737153, IOS-1740560, and IOS-1856741 to D.C.N. and Spanish Ministry of Science and Innovation grants BIO2017-84363-R and PID2020-112779RB-I00 and FESF Investing in Your Future to P.C.
Author contributions
Experiments were designed, carried out, and analyzed by Q.L., E.S.M.-F., A.K., A.R.F.W., and S.C. Figures were prepared by Q.L. The manuscript was prepared by Q.L. and D.C.N. with contributions and final approval from all authors. The project was designed by Q.L., E.S.M.-F., P.C., and D.C.N. Funding to support the project was secured by P.C. and D.C.N.
Acknowledgments
We thank Dr. Gavin Flematti and Dr. Adrian Scaffidi (University of Western Australia) for supplying KAR1, KAR2, rac-GR24, and purified GR24 enantiomers. No conflict of interest declared.
Published: January 31, 2022
Footnotes
Published by the Plant Communications Shanghai Editorial Office in association with Cell Press, an imprint of Elsevier Inc., on behalf of CSPB and CEMPS, CAS.
Supplemental information is available at Plant Communications Online.
Supporting citations
The following references appear in the Supplemental Information: Gietz and Woods, 2002; Goddard-Borger et al., 2007.
Supplemental information
References
- Aakre C.D., Herrou J., Phung T.N., Perchuk B.S., Crosson S., Laub M.T. Evolving new protein-protein interaction specificity through promiscuous intermediates. Cell. 2015;163:594–606. doi: 10.1016/j.cell.2015.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar-Martínez J.A., Poza-Carrión C., Cubas P. Arabidopsis BRANCHED1 acts as an integrator of branching signals within axillary buds. Plant Cell. 2007;19:458–472. doi: 10.1105/tpc.106.048934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agusti J., Herold S., Schwarz M., Sanchez P., Ljung K., Dun E.A., Brewer P.B., Beveridge C.A., Sieberer T., Sehr E.M., et al. Strigolactone signaling is required for auxin-dependent stimulation of secondary growth in plants. Proc. Natl. Acad. Sci. U S A. 2011;108:20242–20247. doi: 10.1073/pnas.1111902108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama K., Matsuzaki K.-I., Hayashi H. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature. 2005;435:824–827. doi: 10.1038/nature03608. [DOI] [PubMed] [Google Scholar]
- Blázquez M.A., Nelson D.C., Weijers D. Evolution of plant hormone response pathways. Annu. Rev. Plant Biol. 2020;71:327–353. doi: 10.1146/annurev-arplant-050718-100309. [DOI] [PubMed] [Google Scholar]
- Bouwmeester H., Li C., Thiombiano B., Rahimi M., Dong L. Adaptation of the parasitic plant lifecycle: germination is controlled by essential host signaling molecules. Plant Physiol. 2021;185:1292–1308. doi: 10.1093/plphys/kiaa066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bürger M., Mashiguchi K., Lee H.J., Nakano M., Takemoto K., Seto Y., Yamaguchi S., Chory J. Structural basis of karrikin and non-natural strigolactone perception in physcomitrella patens. Cell Rep. 2019;26:855–865.e5. doi: 10.1016/j.celrep.2019.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bythell-Douglas R., Rothfels C.J., Stevenson D.W.D., Graham S.W., Wong G.K.-S., Nelson D.C., Bennett T. Evolution of strigolactone receptors by gradual neo-functionalization of KAI2 paralogues. BMC Biol. 2017;15:52. doi: 10.1186/s12915-017-0397-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonnel S., Das D., Varshney K., Kolodziej M.C., Villaécija-Aguilar J.A., Gutjahr C. The karrikin signaling regulator SMAX1 controls Lotus japonicus root and root hair development by suppressing ethylene biosynthesis. Proc. Natl. Acad. Sci. U S A. 2020;117:21757–21765. doi: 10.1073/pnas.2006111117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonnel S., Torabi S., Griesmann M., Bleek E., Tang Y., Buchka S., Basso V., Shindo M., Boyer F.-D., Wang T.L., et al. Lotus japonicus karrikin receptors display divergent ligand-binding specificities and organ-dependent redundancy. PLoS Genet. 2020;16:e1009249. doi: 10.1371/journal.pgen.1009249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier F., Nieminen K., Sánchez-Ferrero J.C., Rodríguez M.L., Chagoyen M., Hardtke C.S., Cubas P. Strigolactone promotes degradation of DWARF14, an α/β hydrolase essential for strigolactone signaling in Arabidopsis. Plant Cell. 2014;26:1134–1150. doi: 10.1105/tpc.114.122903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn C.E., Nelson D.C. Evidence that KARRIKIN-INSENSITIVE2 (KAI2) receptors may perceive an unknown signal that is not karrikin or strigolactone. Front. Plant Sci. 2015;6:1219. doi: 10.3389/fpls.2015.01219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook C.E., Whichard L.P., Turner B., Wall M.E., Egley G.H. Germination of witchweed (Striga lutea Lour.): isolation and properties of a potent stimulant. Science. 1966;154:1189–1190. doi: 10.1126/science.154.3753.1189. [DOI] [PubMed] [Google Scholar]
- Day R.N., Periasamy A., Schaufele F. Fluorescence resonance energy transfer microscopy of localized protein interactions in the living cell nucleus. Methods. 2001;25:4–18. doi: 10.1006/meth.2001.1211. [DOI] [PubMed] [Google Scholar]
- de Saint Germain A., Clavé G., Badet-Denisot M.-A., Pillot J.-P., Cornu D., Le Caer J.-P., Burger M., Pelissier F., Retailleau P., Turnbull C., et al. An histidine covalent receptor and butenolide complex mediates strigolactone perception. Nat. Chem. Biol. 2016;12:787–794. doi: 10.1038/nchembio.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flematti G.R., Ghisalberti E.L., Dixon K.W., Trengove R.D. A compound from smoke that promotes seed germination. Science. 2004;305:977. doi: 10.1126/science.1099944. [DOI] [PubMed] [Google Scholar]
- Flematti G.R., Scaffidi A., Waters M.T., Smith S.M. Stereospecificity in strigolactone biosynthesis and perception. Planta. 2016;243:1361–1373. doi: 10.1007/s00425-016-2523-5. [DOI] [PubMed] [Google Scholar]
- Fujii H., Verslues P.E., Zhu J.-K. Arabidopsis decuple mutant reveals the importance of SnRK2 kinases in osmotic stress responses in vivo. Proc. Natl. Acad. Sci. U S A. 2011;108:1717–1722. doi: 10.1073/pnas.1018367108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gietz R.D., Woods R.A. Transformation of yeast by lithium acetate/single-stranded carrier DNA/polyethylene glycol method. Methods Enzymol. 2002;350:87–96. doi: 10.1016/s0076-6879(02)50957-5. [DOI] [PubMed] [Google Scholar]
- Goddard-Borger E.D., Ghisalberti E.L., Stick R.V. Synthesis of the germination stimulant 3-methyl-2H-furo[2,3-c]pyran-2-one and analogous compounds from carbohydrates. Eur. J. Org. Chem. 2007;38:3925–3934. [Google Scholar]
- Gomez-Roldan V., Fermas S., Brewer P.B., Puech-Pagès V., Dun E.A., Pillot J.-P., Letisse F., Matusova R., Danoun S., Portais J.-C., et al. Strigolactone inhibition of shoot branching. Nature. 2008;455:189–194. doi: 10.1038/nature07271. [DOI] [PubMed] [Google Scholar]
- Haider I., Andreo-Jimenez B., Bruno M., Bimbo A., Floková K., Abuauf H., Ntui V.O., Guo X., Charnikhova T., Al-Babili S., et al. The interaction of strigolactones with abscisic acid during the drought response in rice. J. Exp. Bot. 2018;69:2403–2414. doi: 10.1093/jxb/ery089. [DOI] [PubMed] [Google Scholar]
- Hamiaux C., Drummond R.S.M., Janssen B.J., Ledger S.E., Cooney J.M., Newcomb R.D., Snowden K.C. DAD2 is an α/β hydrolase likely to be involved in the perception of the plant branching hormone, strigolactone. Curr. Biol. 2012;22:2032–2036. doi: 10.1016/j.cub.2012.08.007. [DOI] [PubMed] [Google Scholar]
- Hu Q., He Y., Wang L., Liu S., Meng X., Liu G., Jing Y., Chen M., Song X., Jiang L., et al. DWARF14, A receptor covalently linked with the active form of strigolactones, undergoes strigolactone-dependent degradation in rice. Front. Plant Sci. 2017;8:1935. doi: 10.3389/fpls.2017.01935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain N., Kulkarni M.G., van Staden J. A butenolide, isolated from smoke, can overcome the detrimental effects of extreme temperatures during tomato seed germination. Plant Growth Regul. 2006;49:263–267. [Google Scholar]
- Jiang L., Liu X., Xiong G., Liu H., Chen F., Wang L., Meng X., Liu G., Yu H., Yuan Y., et al. DWARF 53 acts as a repressor of strigolactone signalling in rice. Nature. 2013;504:401–405. doi: 10.1038/nature12870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalliola M., Jakobson L., Davidsson P., Pennanen V., Waszczak C., Yarmolinsky D., Zamora O., Palva E.T., Kariola T., Kollist H., et al. Differential role of MAX2 and strigolactones in pathogen, ozone, and stomatal responses. Plant Direct. 2020;4:e00206. doi: 10.1002/pld3.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosla A., Rodriguez-Furlan C., Kapoor S., Van Norman J.M., Nelson D.C. A series of dual-reporter vectors for ratiometric analysis of protein abundance in plants. Plant Direct. 2020;4:e00231. doi: 10.1002/pld3.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosla A., Morffy N., Li Q., Faure L., Chang S.H., Yao J., Zheng J., Cai M.L., Stanga J., Flematti G.R., et al. Structure–function analysis of SMAX1 reveals domains that mediate its karrikin-induced proteolysis and interaction with the receptor KAI2. Plant Cell. 2020;32:2639–2659. doi: 10.1105/tpc.19.00752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobae Y., Kameoka H., Sugimura Y., Saito K., Ohtomo R., Fujiwara T., Kyozuka J. Strigolactone biosynthesis genes of rice are required for the punctual entry of arbuscular mycorrhizal fungi into the roots. Plant Cell Physiol. 2018;59:544–553. doi: 10.1093/pcp/pcy001. [DOI] [PubMed] [Google Scholar]
- Kochanek J., Long R.L., Lisle A.T., Flematti G.R. Karrikins identified in biochars indicate post-fire chemical cues can influence community diversity and plant development. PLoS One. 2016;11:e0161234. doi: 10.1371/journal.pone.0161234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahari Z., Ullah C., Kyndt T., Gershenzon J., Gheysen G. Strigolactones enhance root-knot nematode (Meloidogyne graminicola) infection in rice by antagonizing the jasmonate pathway. New Phytol. 2019;224:454–465. doi: 10.1111/nph.15953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Nguyen K.H., Chu H.D., Van Ha C., Watanabe Y., Osakabe Y., Leyva-González M.A., Sato M., Toyooka K., Voges L., et al. The karrikin receptor KAI2 promotes drought resistance in Arabidopsis thaliana. PLoS Genet. 2017;13:e1007076. doi: 10.1371/journal.pgen.1007076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Nguyen K.H., Tran C.D., Watanabe Y., Tian C., Yin X., Li K., Yang Y., Guo J., Miao Y., et al. Negative roles of strigolactone-related SMXL6, 7 and 8 proteins in drought resistance in Arabidopsis. Biomolecules. 2020;10:607. doi: 10.3390/biom10040607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Nguyen K.H., Chu H.D., Watanabe Y., Osakabe Y., Sato M., Toyooka K., Seo M., Tian L., Tian C., et al. Comparative functional analyses of DWARF14 and KARRIKIN INSENSITIVE 2 in drought adaptation of Arabidopsis thaliana. Plant J. 2020;103:111–127. doi: 10.1111/tpj.14712. [DOI] [PubMed] [Google Scholar]
- Liang Y., Ward S., Li P., Bennett T., Leyser O. SMAX1-LIKE7 signals from the nucleus to regulate shoot development in Arabidopsis via partially EAR motif-independent mechanisms. Plant Cell. 2016;28:1581–1601. doi: 10.1105/tpc.16.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D., Li W., Cheng J., Hou L. Expression analysis and functional characterization of a cold-responsive gene COR15A from Arabidopsis thaliana. Acta Physiol. Plant. 2014;36:2421–2432. [Google Scholar]
- López-Ráez J.A., Charnikhova T., Gómez-Roldán V., Matusova R., Kohlen W., De Vos R., Verstappen F., Puech-Pages V., Bécard G., Mulder P., et al. Tomato strigolactones are derived from carotenoids and their biosynthesis is promoted by phosphate starvation. New Phytol. 2008;178:863–874. doi: 10.1111/j.1469-8137.2008.02406.x. [DOI] [PubMed] [Google Scholar]
- Msanne J., Lin J., Stone J.M., Awada T. Characterization of abiotic stress-responsive Arabidopsis thaliana RD29A and RD29B genes and evaluation of transgenes. Planta. 2011;234:97–107. doi: 10.1007/s00425-011-1387-y. [DOI] [PubMed] [Google Scholar]
- Nasir F., Tian L., Shi S., Chang C., Ma L., Gao Y., Tian C. Strigolactones positively regulate defense against Magnaporthe oryzae in rice (Oryza sativa) Plant Physiol. Biochem. 2019;142:106–116. doi: 10.1016/j.plaphy.2019.06.028. [DOI] [PubMed] [Google Scholar]
- Nelson D.C. The mechanism of host-induced germination in root parasitic plants. Plant Physiol. 2021;185:1353–1373. doi: 10.1093/plphys/kiab043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D.C., Flematti G.R., Riseborough J.-A., Ghisalberti E.L., Dixon K.W., Smith S.M. Karrikins enhance light responses during germination and seedling development in Arabidopsis thaliana. Proc. Natl. Acad. Sci. U S A. 2010;107:7095–7100. doi: 10.1073/pnas.0911635107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D.C., Scaffidi A., Dun E.A., Waters M.T., Flematti G.R., Dixon K.W., Beveridge C.A., Ghisalberti E.L., Smith S.M. F-box protein MAX2 has dual roles in karrikin and strigolactone signaling in Arabidopsis thaliana. Proc. Natl. Acad. Sci. U S A. 2011;108:8897–8902. doi: 10.1073/pnas.1100987108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D.C., Flematti G.R., Ghisalberti E.L., Dixon K.W., Smith S.M. Regulation of seed germination and seedling growth by chemical signals from burning vegetation. Annu. Rev. Plant Biol. 2012;63:107–130. doi: 10.1146/annurev-arplant-042811-105545. [DOI] [PubMed] [Google Scholar]
- Rasmussen A., Mason M.G., De Cuyper C., Brewer P.B., Herold S., Agusti J., Geelen D., Greb T., Goormachtig S., Beeckman T., et al. Strigolactones suppress adventitious rooting in Arabidopsis and pea. Plant Physiol. 2012;158:1976–1987. doi: 10.1104/pp.111.187104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaffidi A., Waters M.T., Sun Y.K., Skelton B.W., Dixon K.W., Ghisalberti E.L., Flematti G.R., Smith S.M. Strigolactone hormones and their stereoisomers signal through two related receptor proteins to induce different physiological responses in Arabidopsis. Plant Physiol. 2014;165:1221–1232. doi: 10.1104/pp.114.240036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto Y., Yasui R., Kameoka H., Tamiru M., Cao M., Terauchi R., Sakurada A., Hirano R., Kisugi T., Hanada A., et al. Strigolactone perception and deactivation by a hydrolase receptor DWARF14. Nat. Commun. 2019;10:191. doi: 10.1038/s41467-018-08124-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabek N., Ticchiarelli F., Mao H., Hinds T.R., Leyser O., Zheng N. Structural plasticity of D3-D14 ubiquitin ligase in strigolactone signalling. Nature. 2018;563:652–656. doi: 10.1038/s41586-018-0743-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah F.A., Wei X., Wang Q., Liu W., Wang D., Yao Y., Hu H., Chen X., Huang S., Hou J., et al. Karrikin improves osmotic and salt stress tolerance via the regulation of the redox homeostasis in the oil plant sapium sebiferum. Front. Plant Sci. 2020;11:216. doi: 10.3389/fpls.2020.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soundappan I., Bennett T., Morffy N., Liang Y., Stanga J.P., Abbas A., Leyser O., Nelson D.C. SMAX1-LIKE/D53 family members enable distinct MAX2-dependent responses to strigolactones and karrikins in Arabidopsis. Plant Cell. 2015;27:3143–3159. doi: 10.1105/tpc.15.00562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanga J.P., Smith S.M., Briggs W.R., Nelson D.C. SUPPRESSOR OF MORE AXILLARY GROWTH2 1 controls seed germination and seedling development in Arabidopsis. Plant Physiol. 2013;163:318–330. doi: 10.1104/pp.113.221259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanga J.P., Morffy N., Nelson D.C. Functional redundancy in the control of seedling growth by the karrikin signaling pathway. Planta. 2016;243:1397–1406. doi: 10.1007/s00425-015-2458-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X.-D., Ni M. HYPOSENSITIVE TO LIGHT, an alpha/beta fold protein, acts downstream of ELONGATED HYPOCOTYL 5 to regulate seedling de-etiolation. Mol. Plant. 2011;4:116–126. doi: 10.1093/mp/ssq055. [DOI] [PubMed] [Google Scholar]
- Swarbreck S.M., Guerringue Y., Matthus E., Jamieson F.J.C., Davies J.M. Impairment in karrikin but not strigolactone sensing enhances root skewing in Arabidopsis thaliana. Plant J. 2019;98:607–621. doi: 10.1111/tpj.14233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh S., Holbrook-Smith D., Stokes M.E., Tsuchiya Y., McCourt P. Detection of parasitic plant suicide germination compounds using a high-throughput Arabidopsis HTL/KAI2 strigolactone perception system. Chem. Biol. 2014;21:988–998. doi: 10.1016/j.chembiol.2014.07.005. [DOI] [PubMed] [Google Scholar]
- Ueda H., Kusaba M. Strigolactone regulates leaf senescence in concert with ethylene in Arabidopsis. Plant Physiol. 2015;169:138–147. doi: 10.1104/pp.15.00325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umehara M., Hanada A., Yoshida S., Akiyama K., Arite T., Takeda-Kamiya N., Magome H., Kamiya Y., Shirasu K., Yoneyama K., et al. Inhibition of shoot branching by new terpenoid plant hormones. Nature. 2008;455:195–200. doi: 10.1038/nature07272. [DOI] [PubMed] [Google Scholar]
- Van Ha C., Leyva-González M.A., Osakabe Y., Tran U.T., Nishiyama R., Watanabe Y., Tanaka M., Seki M., Yamaguchi S., Van Dong N., et al. Positive regulatory role of strigolactone in plant responses to drought and salt stress. Proc. Natl. Acad. Sci. U S A. 2014;111:851–856. doi: 10.1073/pnas.1322135111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villaécija-Aguilar J.A., Hamon-Josse M., Carbonnel S., Kretschmar A., Schmidt C., Dawid C., Bennett T., Gutjahr C. SMAX1/SMXL2 regulate root and root hair development downstream of KAI2-mediated signalling in Arabidopsis. PLoS Genet. 2019;15:e1008327. doi: 10.1371/journal.pgen.1008327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker C.H., Siu-Ting K., Taylor A., O’Connell M.J., Bennett T. Strigolactone synthesis is ancestral in land plants, but canonical strigolactone signalling is a flowering plant innovation. BMC Biol. 2019;17:70. doi: 10.1186/s12915-019-0689-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Wang B., Jiang L., Liu X., Li X., Lu Z., Meng X., Wang Y., Smith S.M., Li J. Strigolactone signaling in Arabidopsis regulates shoot development by targeting D53-like SMXL repressor proteins for ubiquitination and degradation. Plant Cell. 2015;27:3128–3142. doi: 10.1105/tpc.15.00605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Waters M.T., Smith S.M. Karrikin-KAI2 signalling provides Arabidopsis seeds with tolerance to abiotic stress and inhibits germination under conditions unfavourable to seedling establishment. New Phytol. 2018;219:605–618. doi: 10.1111/nph.15192. [DOI] [PubMed] [Google Scholar]
- Wang L., Wang B., Yu H., Guo H., Lin T., Kou L., Wang A., Shao N., Ma H., Xiong G., et al. Transcriptional regulation of strigolactone signalling in Arabidopsis. Nature. 2020;583:277–281. doi: 10.1038/s41586-020-2382-x. [DOI] [PubMed] [Google Scholar]
- Wang L., Xu Q., Yu H., Ma H., Li X., Yang J., Chu J., Xie Q., Wang Y., Smith S.M., et al. Strigolactone and karrikin signaling pathways elicit ubiquitination and proteolysis of SMXL2 to regulate hypocotyl elongation in Arabidopsis. Plant Cell. 2020;32:2251–2270. doi: 10.1105/tpc.20.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Yao R., Du X., Guo L., Chen L., Xie D., Smith S.M. Molecular basis for high ligand sensitivity and selectivity of strigolactone receptors in Striga. Plant Physiol. 2021;185:1411–1428. doi: 10.1093/plphys/kiaa048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters M.T., Nelson D.C., Scaffidi A., Flematti G.R., Sun Y.K., Dixon K.W., Smith S.M. Specialisation within the DWARF14 protein family confers distinct responses to karrikins and strigolactones in Arabidopsis. Development. 2012;139:1285–1295. doi: 10.1242/dev.074567. [DOI] [PubMed] [Google Scholar]
- Waters M.T., Scaffidi A., Moulin S.L.Y., Sun Y.K., Flematti G.R., Smith S.M. A Selaginella moellendorffii ortholog of KARRIKIN INSENSITIVE2 functions in Arabidopsis development but cannot mediate responses to karrikins or strigolactones. Plant Cell. 2015;27:1925–1944. doi: 10.1105/tpc.15.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters M.T., Scaffidi A., Flematti G., Smith S.M. Substrate-induced degradation of the α/β-Fold hydrolase KARRIKIN INSENSITIVE2 requires a functional catalytic triad but is independent of MAX2. Mol. Plant. 2015;8:814–817. doi: 10.1016/j.molp.2014.12.020. [DOI] [PubMed] [Google Scholar]
- Waters M.T., Gutjahr C., Bennett T., Nelson D.C. Strigolactone signaling and evolution. Annu. Rev. Plant Biol. 2017;68:291–322. doi: 10.1146/annurev-arplant-042916-040925. [DOI] [PubMed] [Google Scholar]
- White A.R.F., Mendez J.A., Khosla A., Nelson D.C. Rapid analysis of strigolactone receptor activity in a Nicotiana benthamiana dwarf14 mutant. Preprint at Biorxiv. 2021 doi: 10.1101/2021.05.11.443507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Miyakawa T., Nosaki S., Nakamura A., Lyu Y., Nakamura H., Ohto U., Ishida H., Shimizu T., Asami T., et al. Structural analysis of HTL and D14 proteins reveals the basis for ligand selectivity in Striga. Nat. Commun. 2018;9:3947. doi: 10.1038/s41467-018-06452-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y., Furusawa S., Nagasaka S., Shimomura K., Yamaguchi S., Umehara M. Strigolactone signaling regulates rice leaf senescence in response to a phosphate deficiency. Planta. 2014;240:399–408. doi: 10.1007/s00425-014-2096-0. [DOI] [PubMed] [Google Scholar]
- Yao R., Ming Z., Yan L., Li S., Wang F., Ma S., Yu C., Yang M., Chen L., Chen L., et al. DWARF14 is a non-canonical hormone receptor for strigolactone. Nature. 2016;536:469–473. doi: 10.1038/nature19073. [DOI] [PubMed] [Google Scholar]
- Yao R., Wang F., Ming Z., Du X., Chen L., Wang Y., Zhang W., Deng H., Xie D. ShHTL7 is a non-canonical receptor for strigolactones in root parasitic weeds. Cell Res. 2017;27:838–841. doi: 10.1038/cr.2017.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J.R., Scaffidi A., Meng Y.J., Melville K.T., Komatsu A., Khosla A., Nelson D.C., Kyozuka J., Flematti G.R., Waters M.T. Desmethyl butenolides are optimal ligands for karrikin receptor proteins. New Phytol. 2021;230:1003–1016. doi: 10.1111/nph.17224. [DOI] [PubMed] [Google Scholar]
- Yoneyama K., Mori N., Sato T., Yoda A., Xie X., Okamoto M., Iwanaga M., Ohnishi T., Nishiwaki H., Asami T., et al. Conversion of carlactone to carlactonoic acid is a conserved function ofMAX1 homologs in strigolactone biosynthesis. New Phytol. 2018;218:1522–1533. doi: 10.1111/nph.15055. [DOI] [PubMed] [Google Scholar]
- Zheng J., Hong K., Zeng L., Wang L., Kang S., Qu M., Dai J., Zou L., Zhu L., Tang Z., et al. Karrikin signaling acts parallel to and additively with strigolactone signaling to regulate rice mesocotyl elongation in darkness. Plant Cell. 2020;32:2780–2805. doi: 10.1105/tpc.20.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X., Yang X., Chen Z., Xie W., Yue X., Zhu H., Chen S., Sun X. Arabidopsis SMAX1 overaccumulation suppresses rosette shoot branching and promotes leaf and petiole elongation. Biochem. Biophys. Res. Commun. 2021;553:44–50. doi: 10.1016/j.bbrc.2021.03.006. [DOI] [PubMed] [Google Scholar]
- Zhou F., Lin Q., Zhu L., Ren Y., Zhou K., Shabek N., Wu F., Mao H., Dong W., Gan L., et al. D14-SCF(D3)-dependent degradation of D53 regulates strigolactone signalling. Nature. 2013;504:406–410. doi: 10.1038/nature12878. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.