Abstract
Background:
Right heart catheterization for invasive hemodynamics has shown only modest correlation with clinical outcomes. We designed a novel hemodynamic variable that incorporates ventricular output and filling pressure. We anticipated that the aortic pulsatility index (API) would correlate with clinical outcomes in patients with heart failure.
Methods and Results:
We retrospectively analyzed consecutive patients undergoing right heart catheterization with milrinone drug study at our institution (February 2013 to November 2019). The API was calculated as (systolic blood pressure – diastolic blood pressure)/pulmonary capillary wedge pressure. The primary outcome was freedom from advanced therapies, defined as the need for inotropes, temporary mechanical circulatory support, a left ventricular assist device, or orthotopic heart transplantation, or death at 30 days. A total of 224 patient encounters, age 57 years (48–66 years; 34% women; 31% ischemic cardiomyopathy) were included. In univariable analysis, lower baseline API was significantly associated with progression to advanced therapies or death at 30-days (odds ratio 0.43, 95% confidence interval 0.30–0.61; P < .001) compared with those on continued medical management. Receiver operator characteristic analysis specified an optimal cutpoint of 1.45 for API. A Kaplan–Meier analysis indicated an association of API with the primary outcome (79% for API ≥ 1.45 vs 48% for API < 1.45). In multivariable analysis, higher API was strongly associated with freedom from advanced therapies or death (odds ratio 0.38, 95% confidence interval 0.22–0.65, P ≤ .001), even when adjusted for baseline characteristics and routine right heart catheterization measurements.
Conclusions:
The API is a novel invasive hemodynamic measurement that is associated independently with freedom from advanced therapies or death at 30-day follow-up.
Keywords: Heart failure, hemodynamics, cardiogenic shock, outcomes
Right heart catheterization (RHC) for invasive hemodynamics, as measured by a pulmonary arterial catheter, has been an integral aspect of the diagnosis and management of heart failure and cardiogenic shock for decades.1,2 Routine invasive hemodynamics include measurements of filling pressures representing right- and left-sided chambers of the heart, along with the calculation of cardiac output and systemic vascular resistance. However, calculation of cardiac output, whether by the Fick principle or thermodilution, involves assumptions that may not be accurate, and they have shown only modest, and often discrepant, correlation with clinical outcomes.3,4
Prior research on pulmonary arterial catheter use in hemodynamically stable patients admitted with acute decompensated heart failure demonstrated that elevated right atrial (RA) pressure and pulmonary capillary wedge pressure (PCWP) after optimization were associated with long-term adverse events, whereas a decreased cardiac index (CI) was not, indicating the importance of filling pressures on patient outcomes over standard measurements of cardiac function.5 Advanced hemodynamic calculations have since been developed to better represent intrinsic cardiac function under variable loading conditions. Most notably, cardiac power output (CPO) was found to have the strongest independent association of in-hospital mortality in patients with acute myocardial infarction and cardiogenic shock, when compared with routine RHC measurements, for patients with cardiogenic shock.6
We aimed to design and derive a novel hemodynamic variable that would simultaneously incorporate ventricular output and filling pressure, while remaining simple to calculate. The aortic pulsatility index (API) is calculated as systolic blood pressure minus diastolic blood pressure and divided by PCWP. The difference between the systolic blood pressure and diastolic blood pressure (ie, the arterial pulse pressure) in the numerator has been shown to correlate with stroke volume in the setting of a fixed systemic compliance.7 In contrast, the PCWP is reflective of pulmonary pressures, and therefore, represents pulmonary congestion.8–10 The API is analogous to the pulmonary artery pulsatility index (PAPI), which is the best invasive hemodynamic predictor of right ventricular failure and clinical outcomes in patients with advanced heart failure.11 Furthermore, both pulse pressure, defined as the difference between systolic blood pressure and diastolic blood pressure, and left ventricular end-diastolic pressure, for which PCWP serves as a surrogate, have been associated with short-term mortality in patients with acute coronary syndromes.12–14
We hypothesized that the API would correlate with clinical outcomes of a cohort of patients with acute, chronic, and worsening heart failure with invasive hemodynamics consistent with decompensated heart failure.
Methods
This study was approved by the University of Chicago Institutional Review Board. Retrospective data were collected on consecutive patients undergoing RHC at the University of Chicago between January 2013 and November 2019. Patients were retrieved with a coding query via the electronic medical record (Epic 2018, Epic Headquarters, Verona, WI). Included patients had to be 18 years or older and undergoing a milrinone drug study completed by a member of the advanced heart failure team to assess inotropic response in patients with concern for cardiogenic shock. There are no specific criteria for patients to be referred to the advanced heart failure cardiac catheterization laboratory, but in an effort to be inclusive, we included all patients 18 years of age or older undergoing milrinone drug study by the advanced heart failure team. At our institution, patients are referred for RHC by the advanced heart failure team for either acute decompensation of heart failure with concern for cardiogenic shock or chronic heart failure with progressively worsening functional status (ie, Interagency Registry for Mechanically Assisted Circulatory Support [INTER-MACS] profile 4–7) concerning for advancement to American Heart Association stage D heart failure. Milrinone drug studies are done in patients with elevated filling pressures (PCWP of ≥ 15 mm Hg and/or a mean pulmonary arterial pressure of >25 mm Hg) and a CI of less than 2.2 L/min/m2. Milrinone drug studies consist of a bolus of 50μg/kg/min over 10 minutes with repeat invasive hemodynamic measurements after this 10-minute period. Continuation and dosage of milrinone after the study are at the discretion of the treating physician and relate to the individual patient’s response to the medication.
Patients were excluded if they were on any vasoactive medication at baseline (because this would directly affect the hemodynamic measurements), were on temporary or permanent mechanical circulatory support, or had undergone an orthotopic heart transplant. An ejection fraction cutoff was not used. Finally, we excluded patients if the RHC was completed by a physician who was not a member of the advanced heart failure team. This strategy allowed for decreased variability in regard to patient phenotypes, invasive hemodynamic measurement interpretation, and the use of milrinone drug studies for resuscitation of low-output states because this practice was a standard of care for diagnostic and therapeutic assessment for RHC competed by the advanced heart failure team.
Routine RHC and advanced hemodynamic measurements were measured at the start of the procedure, before the milrinone drug infusion. RHC measurements included RA pressure, right ventricular pressure, pulmonary arterial pressure, PCWP, cardiac output, and CI, as measured by both the Fick principle and thermodilution, systemic vascular resistance, pulmonary vascular resistance, pulmonary artery saturation, systemic saturation, and hemoglobin. The PCWP was recorded at end-expiration after advancing the catheter into the wedge position via the left or right pulmonary artery. All hemodynamic measurements were recorded as the average of three beats, or 5 beats if the patient was in atrial fibrillation. Fick cardiac output and CI were measured using the difference between systemic oxygenation (via pulse oximeter) and the mixed venous oxygen saturation (drawn from the pulmonary artery), and used in the standard Fick equation in which oxygen consumption (ie, VO2) was assumed to be 125 multiplied by the body surface area. Cardiac output by thermodilution was recorded as the average of 3 measurements. Blood pressure was measured noninvasively and was recorded during the measurement of the pulmonary artery oxygen saturation, because this is when the Fick cardiac output and systemic vascular resistance are measured. Moderate sedation, with fentanyl and/or midazolam, was used as needed for patients for anxiety.
In addition to the API, indices derived from hemodynamic measurements included left ventricular CPO, left ventricular stroke work index, right ventricular stroke work index, left ventricular pressure ratio (systolic blood pressure/PCWP), and PAPI were calculated (Supplementary Table 1). Baseline medical diagnoses, heart failure specific guideline-directed medical therapy, and renal function data were collected. Finally, outcomes were stratified by noninotrope medical management or the need for advanced heart failure therapies, defined as a need for continuous inotrope medication, temporary mechanical circulatory support, left ventricular assist device implantation, or orthotopic heart transplantation. Individual patient outcomes then were recorded at 30 days after the date of procedure. The primary outcome was freedom from advanced therapies, defined as the need for inotropes, temporary mechanical circulatory support, a left ventricular assist device, or orthotopic heart transplantation, or death at 30 days compared with continued medical management.
Statistical Analysis
Differences in baseline medical diagnoses, heart failure specific guideline-directed medical therapy, and renal function data were expressed as means ± standard deviations or medians with interquartile ranges and compared with either Student t tests or Mann Whitney U (Wilcoxon) tests depending on normality as determined by Shapiro–Wilk tests. Categorical variables were expressed as relative counts and percentages and compared with χ2 tests of association or Fisher exact tests. Receiver operator characteristic (ROC) curves were used to determine the appropriate cutoff value for API, CPO, Fick CI, RA pressure, and PCWP for association of continued medical management, versus advanced therapies or death. The API ROC curve was then compared with the remaining ROC curves using a χ2 test adjusted with the Sidak adjustment for multiple testing. In another set of comparisons, the CPO ROC curve was compared with the other ROC curves using a χ2 test with the Sidak adjustment for multiple testing. Separate ROC analyses were done to determine the optimal cutoff for API including only patients with the outcome of medical management, left ventricular assist device, orthotopic heart transplant, or death (ie, excluding patients on inotropes), because these are hard, time-to-event outcomes suitable for the generation of Kaplan–Meier time-to-event analyses. Log-rank testing was not done because there was not an independent dataset with which to validate the cutoff from the ROC.
Univariable logistic regressions were used to determine which routine or advanced hemodynamic measurements explained the events of continued medical management compared with the events of advanced therapies or death at 30 days, whereby results were presented as odds ratios (OR) and 95% confidence intervals (CI). A 1-point increase in value was used to calculate the OR of the continuous variables. Independent parameters were checked for multicollinearity using Spearman rank correlations before multivariable logistic regressions were conducted to determine which routine or advanced hemodynamic measurements best explained the events of continued medical management compared with the events of advanced therapies or death at 30 days, when adjusting for age and sex. Not all hemodynamic measurements could be included in the same multivariable analysis (specifically CPO and Fick CI) because both parameters are calculated with cardiac output. Therefore, 2 separate multivariable analyses were conducted, one including the API and CPO, but not the Fick CI, and the other including the API and the Fick CI, but not CPO. Tests were 2 tailed and considered statistically significant with a Pvalue of less than .05. All statistical analyses were conducted using STATA MP version 15 (College Station, TX).
Results
Baseline
A total of 224 procedures were analyzed from 224 individual patients. At the time of procedure, average age was 57 years (48–66 years), and 33.5% were women, 39.3% Caucasian, and 31.3% had underlying ischemic cardiomyopathy. Patients with continued medical management were more likely to have had a history of stroke at baseline (20.3% vs 8.7%, P = .01). Additional data regarding baseline characteristics and medical regimens can be seen in Table 1. Moderate sedation was used in 103 procedures (45.6%), including 29 of the 74 patients (39.2%) with the medical management outcome at 30 days, compared with 74 of the 150 patients (49.3%) with advanced therapies outcome at 30 days (P = .15). The baseline hemodynamics of patients on medical management versus the need for advanced therapies or death at both 30 days are listed in Table 2. There were significant differences in multiple hemodynamic measurements when stratified by 30-day outcomes for medical management and advanced therapies (Table 2).
Table 1.
Baseline Characteristics
| Characteristics | Advanced Therapies or Death (n = 150) | Medical Management (n = 74) | P Value |
|---|---|---|---|
| Age, years | 57(48–66) | 58 (47–64) | .56 |
| Female | 101 (67.3) | 48 (64.9) | .71 |
| Race | .56 | ||
| Caucasian or White | 63 (42.0) | 25 (33.8) | |
| African American or Black | 76 (50.7) | 43 (58.1) | |
| Asian | 2(1.3) | 2(2.7) | |
| Other | 9(6.0) | 4(5.4) | |
| Nonischemic cardiomyopathy | 108 (72.0) | 46 (62.2) | .14 |
| Coronary artery disease | 57(38.0) | 31 (41.9) | .58 |
| Hypertension | 68 (45.3) | 44 (59.5) | .05 |
| Hyperlipidemia | 38 (25.3) | 21 (28.4) | .63 |
| Atrial fibrillation | 59(39.3) | 30 (40.5) | .86 |
| Diabetes mellitus | 60 (40.0) | 25 (33.8) | .37 |
| Chronic obstructive pulmonary disease | 14(9.3) | 5 (6.8) | .52 |
| Stroke | 13(8.7) | 15 (20.3) | .93 |
| Chronic kidney disease | 66 (44.0) | 33 (44.6) | .89 |
| ACE-I | 50(33.3) | 24(32.4) | .85 |
| ARB | 30 (20.0) | 14(18.9) | .46 |
| ARNI | 26(17.3) | 10(13.5) | .38 |
| ACE-I, ARB, or ARNI | 106 (70.7) | 48(64.9) | .87 |
| Beta-blocker | 123(82.0) | 60(81.1) | .16 |
| Aldosterone antagonist | 95 (63.8) | 40(54.1) | .85 |
| Hydralazine | 15(10.0) | 8(10.8) | .34 |
| Isosorbide dinitrate | 9 (6.0) | 7 (9.5) | .34 |
| Digoxin | 37 (24.7) | 14(18.9) | .93 |
| Left ventricular ejection fraction, % | 20.1 (15.2–26.0) | 21.7(17.1–30.6) | .07 |
| Creatinine, mg/dL | 1.3(1.1–1.6) | 1.4(1.1–1.8) | .18 |
| Estimated glomerular filtration rate, mL/min/1.73 m2 | 55(38–70) | 47(32–68) | .17 |
| Moderate Sedation during Procedure | 74 (49.3) | 29 (39.2) | .15 |
Values are median (IQR) or number (%). ACE-I, angiotensin-converting enzyme inhibitor, ARB, angiotensin receptor blocker, ARNI, angiotensin receptor neprolysin inhibitor, IQR, interquartile range.
Table 2.
Baseline Hemodynamics for 30-Day Outcomes
| Characteristics | Advanced Therapies or Death (n = 150) | Medical Management (n = 74) | P |
|---|---|---|---|
| RA (mmHg) median (IQR) | 13 (10–19) | 13 (8–17) | 0.21 |
| RV Systolic (mmHg), mean ± SD | 56 ± 13 | 57 ± 17; n= 73 | 0.88 |
| RV Diastolic (mmHg) median (IQR) | 15 (10–20) | 13 (10–18); n = 73 | 0.14 |
| PA Systolic (mmHg) median (IQR) | 60 (49–66) | 59(45–70) | 0.74 |
| PA Diastolic (mmHg), mean ± SD | 30 ±9 | 29 ±9 | 0.25 |
| Mean PA Pressure (mmHg), mean ± SD | 40 ± 10 | 38 ± 11 | 0.15 |
| PCWP (mmHg), mean ± SD | 27 ± 8 | 24 ± 8 | 0.03 |
| PA saturation (%), mean ± SD | 51 ± 10.0 | 55 ±9 | 0.004 |
| Sp02 (%) median (IQR) | 97 (95–99) | 97 (95–99) | 0.81 |
| Fick CO (L/min) median (IQR) | 3.44 (3.00–4.00) | 3.76(3.10–4.65) | 0.002 |
| Fick CI (L/min/m2) median (IQR) | 1.80 (1.50–1.97) | 1.90(1.73–2.20) | 0.001 |
| Thermodilution CO (L/min) median (IQR) | 3.20 (2.50–3.80); n = 109 | 3.50 (2.90–4.42); n = 60 | 0.02 |
| Thermodilution CI (L/min/m2) median (IQR) | 1.60(1.36–1.90); n = 109 | 1.80(1.50–2.07); n = 60 | 0.01 |
| Systolic BP (mmHg) median (IQR) | 103 (95–111) | 119(104–135) | <0.001 |
| Diastolic BP (mmHg) median (IQR) | 70 (63–76) | 73(65–85) | 0.03 |
| MAP (mmHg) median (IQR) | 82(75–89) | 89(83–101) | <0.001 |
| Pulse Pressure, median (IQR) | 31 (25–41) | 43 (34–52) | <0.001 |
| SVR (dynes-sec-cm5) median (IQR) | 1576(1338–1918) | 1611 (1283–2133) | 0.59 |
| PVR (Woods Units) median (IQR) | 3.9 (2.6–5.1) | 4.0 (2.6–5.21 | 0.98 |
| Heart Rate, median (IQR) | 80 (69–89); n = 144 | 78 (68–87) | 0.26 |
| CPO (Watts) median (IQR) | 0.64 (0.51–0.74) | 0.77 (0.65–0.95) | <0.001 |
| API, median (IQR) | 1.18(0.91–1.68) | 1.85 (1.35–2.50) | <0.001 |
| LVSWI, (g*m/m2) median (IQR) | 24 (19–28); n = 144 | 30 (25–37) | <0.001 |
| RVSWI. (g*m/m2) median (IQR) | 7.9(5.4–10.1); n = 144 | 8.50 (6.6–10.9); n = 71 | 0.17 |
| PAPI, median (IQR) | 2.00(1.33–2.74) | 2.28(1.60–3.42) | 0.10 |
API = aortic pulsatility index, BP = systemic blood pressure. Cl = cardiac index, CO = cardiac output, CPO = cardiac power output, IQR = interquartile range, PA = pulmonary artery, PCWP = pulmonary capillary wedge pressure, PVR = pulmonary vascular resistance, RA = right atrium, RV = right ventricle, SD = standard deviation, SpO2 = systemic oxygen saturation, SVR = systemic vascular resistance
Thirty-Day Outcomes
At 30 days, 74 patients were continued on medical management and 91 patients were on inotropes; 35 underwent left ventricular assist device implantation, 16 received orthotopic heart transplant, and 10 patients died. A univariable analysis indicated multiple standard hemodynamic measurements were associated with medical management at 30 days, in addition to 4 advanced hemodynamic measurements: API (OR 0.43, 95% CI 0.30–0.61, P < .001), CPO (OR 0.02, 95%CI 0.01–0.07, P < .001), left ventricular stroke work index (OR 0.93, 95% CI 0.90–0.96, P < .001), and PAPI (OR 0.83, 95% CI 0.71–0.98, P = .02) (Table 3). In separate multivariable analyses API, CPO, and age were found to be associated with medical management at 30 days, even when adjusted for CI (as measured by Fick or thermodilution), right ventricular function (as measured by PAPI), age, and sex (Table 4).
Table 3.
Univariable Analysis of Hemodynamic Variables on 30- Day Outcomes
| Characteristic Hemodynamic Variable |
n | 30 Days |
||
|---|---|---|---|---|
| OR | 95%CI | P | ||
| RA (mmHg) | 224 | 1.03 | (0.99–1.08) | 0.16 |
| RV Systolic (mmHg) | 221 | 1.00 | (0.98–1.02) | 0.88 |
| RV Diastolic (mmHg) | 221 | 1.03 | (0.99–1.08) | 0.15 |
| PA Systolic (mmHg) | 224 | 1.01 | (0.99–1.03) | 0.73 |
| PA Diastolic (mmHg) | 224 | 1.02 | (0.99–1.06) | 0.25 |
| Mean PA Pressure (mmHg) | 224 | 1.02 | (1.00–1.05) | 0.15 |
| PCWP (mmHg) | 224 | 1.04 | (1.01–1.08) | 0.03 |
| PA saturation (%) | 224 | 0.96 | (0.93–0.99) | 0.01 |
| Sp02 (%) | 224 | 1.00 | (0.90–1.12) | 1.00 |
| Fick CO (L/min) | 224 | 0.59 | (0.43–0.81) | 0.001 |
| Fick Cl (L/min/m2) | 224 | 0.33 | (0.17–0.66) | 0.002 |
| Thermodilution CO (L/min) | 169 | 0.69 | (0.53–0.90) | 0.01 |
| Thermodilution Cl (L/min/m2) | 169 | 0.43 | (0.23–0.79) | 0.01 |
| Systolic BP (mmHg) | 224 | 0.95 | (0.93–0.97) | <0.001 |
| Diastolic BP (mmHg) | 224 | 0.98 | (0.96–1.00) | 0.038 |
| MAP (mmHg) | 224 | 0.94 | (0.92–0.96) | <0.001 |
| Pulse Pressure | 224 | 0.95 | (0.93–0.97) | <0.001 |
| SBP/PCWP | 224 | 0.72 | (0.61–0.85) | <0.001 |
| SVR (dynes-sec-cm5) | 222 | 1.00 | (1.00–1.00) | 0.66 |
| PVR (Woods Units) | 220 | 1.12 | (0.89–1.42) | 0.33 |
| Heart Rate | 218 | 1.01 | (0.99–1.03) | 0.26 |
| Left Ventricular EF (%) | 212 | 0.97 | (0.95–1.00) | 0.04 |
| CPO (Watts) | 224 | 0.02 | (0.00–0.07) | <0.001 |
| API | 224 | 0.43 | (0.30–0.61) | <0.001 |
| LVSWI (g*m/m2) | 214 | 0.93 | (0.90–0.96) | <0.001 |
| RVSWI (g*m/m2) | 217 | 0.94 | (0.87–1.01) | 0.10 |
| PAPI | 222 | 0.83 | (0.71–0.98) | 0.02 |
API = aortic pulsatility index, BP = systemic blood pressure. Cl = cardiac index, CO = cardiac output, CPO = cardiac power output, EF = ejection fraction, PA = pulmonary artery, PAPI = pulmonary artery pulsatility index, PCWP = pulmonary capillary wedge pressure, PVR = pulmonary vascular resistance, RA = right atrium, RV = right ventricle, SBP = systolic blood pressure, SpO2 = systemic oxygen saturation, SVR = systemic vascular resistance
Table 4.
Multivariable Analysis of Variables on 30-Day Outcomes
| Multivariable Analysis A Hemodynamic Variable |
30 Days (n=158) |
||
|---|---|---|---|
| OR | 95% CI | p | |
| API (1-point increase) | 0.38 | (0.22–0.65) < | 0.001 |
| CPO (1-point increase) | 0.02 | (0.001–0.25) | 0.002 |
| PAPI (1-point increase) | 0.79 | (0.60–1.04) | 0.09 |
| Thermodilution CI (1-point increase) | 1.15 | (0.50–2.65) | 0.74 |
| Left Ventricular EF (1-point increase) | 0.99 | (0.95–1.03) | 0.63 |
| Age (1-point increase) | 1.04 | (1.01–1.07) | 0.01 |
| Male Sex | 1.28 | (0.57–2.88) | 0.55 |
| Multivariable Analysis B Hemodynamic Variable |
30 Days (n=169) |
||
| OR | 95% CI | p | |
|
| |||
| API (1-point increase) | 0.38 | (0.23–0.63) | <0.001 |
| PAPI (1-point increase) | 0.81 | (0.63–1.04) | 0.10 |
| Fick CI (1-point increase) | 0.74 | (0.25–2.19) | 0.59 |
| Thermodilution CI (1-point increase) | 0.63 | (0.28–1.41) | 0.26 |
| Age (1-point increase) | 1.03 | (1.00–1.06) | 0.02 |
| Male Sex | 1.10 | (0.50–2.39) | 0.81 |
API = aortic pulsatility index, CI= cardiac index, CPO = cardiac power output, EF = ejection fraction, PAPI = pulmonary artery pulsatility index, RA = right atrial pressure
Clinical Outcomes Associated With API
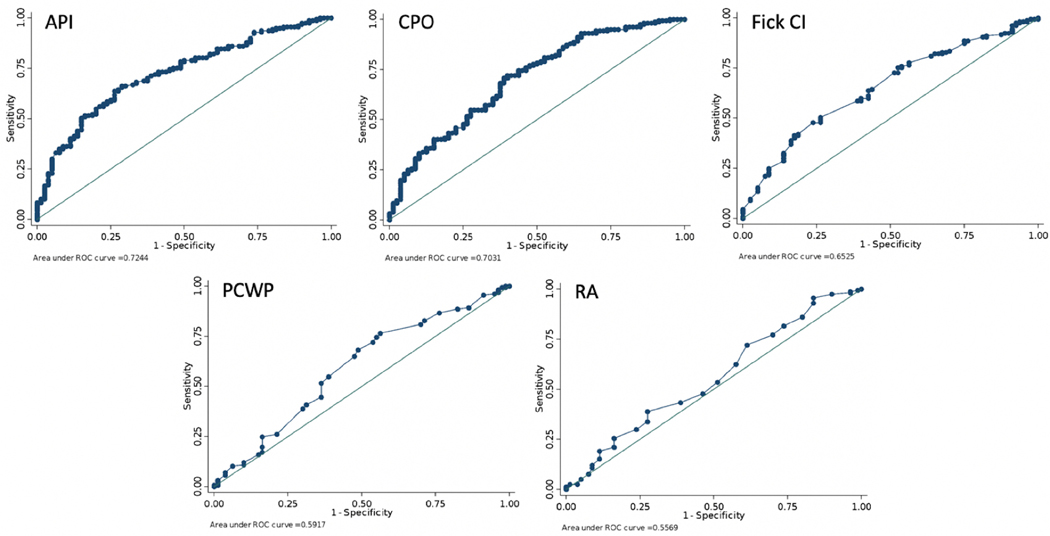
A ROC analysis indicated a cutoff of 1.45 for API was most associated with continued medical management at 30 days, 0.71 Watts for CPO, 1.67 L/min/m2 for Fick CI, 11 mm Hg for RA pressure, and 21 mm Hg for PCWP compared with the combined endpoint of advanced therapies or death (Table 5, Fig. 1). Head-to-head area under the curve (AUC) comparisons of API versus the other variables noted the API to have a significantly greater AUC than that of RA or PCWP, but not in comparison with CPO or Fick CI. Head-to-head AUC comparisons of CPO versus the other variables noted that it was significantly greater than that of RA, but not in comparison with the API, Fick CI, or PCWP (Table 6).
Table 5.
ROC Analyses of Primary Outcome
| Outcomes | Optimal Cutoff Value | Sensitivity (%) | Specificity (%) | Correctly Classified (%) | AUC |
|---|---|---|---|---|---|
| API | 1.45 | 66.00 | 71.62 | 67.86 | 0.73 |
| CPO | 0.71 | 71.62 | 62.16 | 68.47 | 0.71 |
| Fick Cl | 1.67 | 41.33 | 82.43 | 54.91 | 0.65 |
| RA | 11 | 71.33 | 39.19 | 60.71 | 0.55 |
| PCWP | 21 | 76.67 | 43.24 | 65.62 | 0.60 |
Fig. 1.
ROC analyses of the primary outcome.
Table 6.
Direct Comparison of ROC Analyses
| Hemodynamic Variables |
n | AUC | Standard Error |
95% CI | p |
|---|---|---|---|---|---|
| API | |||||
| API | 224 | 0.73 | 0.03 | 0.66–0.80 | 0.998 |
| CPO | 224 | 0.72 | 0.04 | 0.64–0.79 | |
| API | 224 | 0.73 | 0.03 | 0.66–0.80 | 0.28 |
| Fick Cl | 224 | 0.65 | 0.04 | 0.57–0.72 | |
| API | 224 | 0.73 | 0.03 | 0.66–0.80 | 0.001 |
| RA | 224 | 0.55 | 0.04 | 0.47–0.63 | |
| API | 224 | 0.73 | 0.03 | 0.66–0.80 | 0.001 |
| PCWP | 224 | 0.6 | 0.04 | 0.51–0.68 | |
| CPO | |||||
| CPO | 224 | 0.71 | 0.04 | 0.64–0.79 | 0.26 |
| Fick Cl | 224 | 0.65 | 0.04 | 0.57–0.72 | |
| CPO | 224 | 0.71 | 0.04 | 0.64–0.79 | 0.02 |
| RA | 224 | 0.56 | 0.04 | 0.47–0.63 | |
| CPO | 224 | 0.71 | 0.04 | 0.64–0.79 | 0.19 |
| PCWP | 224 | 0.6 | 0.04 | 0.51–0.68 |
API = aortic pulsatility index, CI= cardiac index, CPO = cardiac power output, PCWP = pulmonary capillary wedge pressure. RA = right atrial pressure
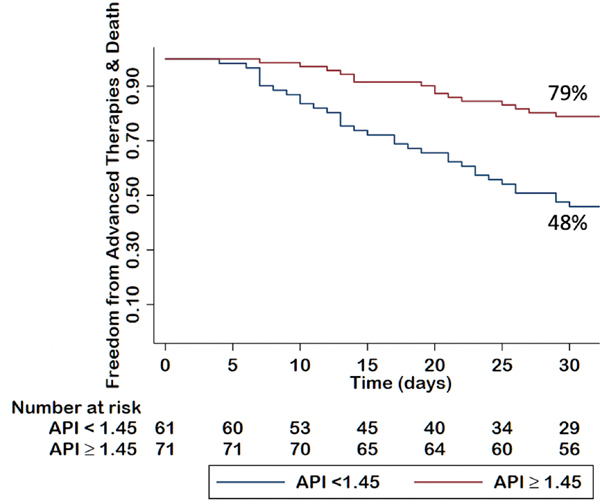
A Kaplan–Meier survival analysis was completed after excluding the outcome of continued inotrope (ie, only including patients on medical management or with a left ventricular assist device, orthotopic heart transplant, or death). Using this API cutoff of 1.45, a Kaplan–Meier survival analysis illustrated an association of the API with the primary outcome with survival 79% for an API of 1.45 or greater and 48% for an API of less than 1.45 (Fig. 2). A Kaplan–Meier survival analysis also illustrated associations with 30-day outcomes for CPO (76% for CPO ≥ 0.71 vs 55% for CPO < 0.71), Fick CI (69% ≥1.67 L/min/m2 vs 53% < 1.67 L/min/m2), PCWP (80% for PCWP ≥ 21 mm Hg vs 57% for PCWP < 21 mm Hg), and RA pressure (65% ≥ 11 mm Hg vs 64% ≤ 11 mm Hg) (Supplemental Figs. 1–4). Log-rank tests were not performed because there was not a separate validation cohort, and the Kaplan–Meier curves are meant to be illustrative and hypothesis generating.
Fig. 2.
Kaplan–Meier analysis of API on the freedom from advanced therapies or death at 30 days
Discussion
In this study, we introduce and derive the API, a novel hemodynamic measurement in patients with acute, chronic, and worsening heart failure, which is significantly associated with adverse clinical outcomes. API is associated with advanced therapies or death at 30 days with a reasonable degree of sensitivity and specificity.
The API was designed to simultaneously represent cardiac function and filling pressures. It accomplishes this goal in 2 ways: (1) by clinical intuition, because medical professionals understand that a low, narrow pulse pressure and signs and symptoms of congestion, which would result in a low API, represent decompensated heart failure or cardiogenic shock, and (2) the API reflects changes in both pressure and stroke volume, and therefore cardiac output. Furthermore, the PCWP correlates with the left ventricular end-diastolic pressure, and informs on pulmonary pressures and congestion.8–10 No other advanced hemodynamic measure so clearly reflects the relationship seen in a pressure–volume loop.
Standard RHC measurements of cardiac output, by both the Fick principle and thermodilution, have inherent flaws and have shown only modest, but frequently discrepant, correlation with clinical outcomes.3,4 CPO was shown to correlate with outcomes, specifically in patients with cardiogenic shock.6 We were able to show that both the API and CPO were associated significantly with continued medical management at 30 days. Importantly, in our clinical experience, CPO is not routinely measured, incorporates another nonintuitive calculation during a cardiac catheterization procedure, and has only been validated in the setting of acute coronary syndromes, which are different from the population being evaluated in this study.
All of the patients in this analysis were being evaluated by the advanced heart failure team at a tertiary care center and were predominantly referred for the evaluation of advanced options for refractory heart failure. CPO has been validated in patients admitted with acute myocardial infarction and cardiogenic shock, whereas the API was recently shown to be prognostic in heart failure patients without cardiogenic shock from the Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness (ESCAPE) Trial, both of which were markedly different from the population evaluated in our study.6,15 Patients with advanced heart failure, American Heart Association Stage C or D, have a poor prognosis, and risk stratification for consideration of advanced therapies use remains elusive.16,17 Furthermore, PCWP, which was shown to be associated with outcomes in acute decompensated heart failure patients in the ESCAPE Trial, remained associated with the primary outcome in our group of patients, but the AUC was significantly lower than that of the API.18
The API continued to be independently associated with the primary outcome, even when accounting for standard RHC hemodynamic measurements (ie, Fick CI, thermodilution CI, RA, and PAPI) in multivariable logistic analysis, and remained significant when compared against the accepted advanced hemodynamic metric of cardiac function, CPO, at the 30-day outcome mark. Of note, because both the API and CPO are continuous variables, the ORs were calculated from 1-point increases in their respective values. It is, therefore, not surprising that the CPO resulted in a significantly lower OR with a 1-point increase compared with the significantly improved OR seen with a 1-point increase in the API, because the CPO values use absolute lower numbers and changes of less than 1 point have significant effect, although both were significant.
The API still requires the use of a pulmonary arterial catheter to measure a PCWP. However, the PCWP is used as a reflection of left ventricular end-diastolic pressure, which can presumably be substituted for the PCWP in the API equation so as to calculate an API during a left heart catheterization. In fact, the systolic blood pressure to left ventricular end-diastolic pressure ratio has recently been shown to correlate with in-hospital mortality or escalation of therapy in patients with heart failure, or those admitted with an ST elevation myocardial infarction.19,20 Future research of API calculated with a left ventricular end-diastolic pressure has promise to help predict clinical outcomes and potentially assist in decisions regarding medical and temporary mechanical circulatory support treatment of patients with cardiogenic shock, including those presenting with an acute coronary syndrome as potentially an additional RHC would not be needed.
Limitations
This study is limited by its retrospective nature. Additionally, all RHC evaluations were done by a single group of physicians, which limits interoperator variability, and may decrease the applicability to patients not evaluated in our center. Likewise, a statistical limitation is that the sample size did not provide the ability to validate the ROC cutoff points by splitting the dataset into training and testing datasets; future research would require the testing these cutoff points with new sets of patients with similar characteristics. Our analysis includes patients dating back to 2013, and with the significant change in management of cardiogenic shock in the interim, the prognostic findings may be limited. It is also important to note that these patients were not presenting with acute coronary syndromes, and thus direct application of this hemodynamic variable to the acute coronary syndrome population may not be accurate. Finally, we acknowledge that the noninvasive brachial blood pressures measured for calculation of the API may vary from central aortic pressures, which were not measured during these venous access procedures. Future research into the use of the API using central aortic pressures would be warranted.
Conclusions
The API is a novel invasive hemodynamic measurement that is independently associated freedom from advanced therapies or death at 30-day follow-up.
LAY SUMMARY
The API is a novel metric to assess the severity of illness in a patient heart failure.
In patients with heart failure, with an API of less than 1.45 was associated with implantation of a left ventricular assist device, heart transplantation, the need for continuous use of vasoactive medications, or temporary mechanical circulatory support devices, or death within 30 days.
The API may help in assessing the need for advanced therapies in patients with advanced heart failure by informing on their 30-day risk.
Heart failure is a progressive disease that can be attenuated by oral, guideline-directed medical therapies. Some patients progress to advanced disease, requiring powerful intravenous medications, temporary or permanent heart pumps, or a heart transplant, despite these medications. Assessing a patient’s risk of progression to advanced disease remains imperfect. The novel hemodynamic metric, API, described in this study may be associated with clinical outcomes 30 days following the procedure. Use of the API may help to assess risk and potentially help inform clinical decisions.
Supplementary Material
Footnotes
Disclosures
The authors disclose no conflicts.
Supplementary materials
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.cardfail.2021.05.010.
References
- 1.Thakkar AB, Desai SP. Swan, Ganz, and their catheter: its evolution over the past half century. Ann Intern Med 2018;169:636–42. [DOI] [PubMed] [Google Scholar]
- 2.Saxena A, Garan AR, Kapur NK, O’Neill WW, Lindenfeld J, Pinney SP, et al. Value of hemodynamic monitoring in patients with cardiogenic shock undergoing mechanical circulatory support. Circulation 2020;141:1184–97. [DOI] [PubMed] [Google Scholar]
- 3.Taylor SH, Silke B. Is the measurement of cardiac output useful in clinical practice? Br J Anaesth 1988;60(Suppl 1):90S–8S. [DOI] [PubMed] [Google Scholar]
- 4.Opotowsky AR, Hess E, Maron BA, Brittain EL, Baron AE, Maddox TM, et al. Thermodilution vs estimated Fick cardiac output measurement in clinical practice: an analysis of mortality from the Veterans Affairs Clinical Assessment, Reporting, and Tracking (VA CART) program and Vanderbilt University. JAMA Cardiol 2017;2:1090–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooper LB, Mentz RJ, Stevens SR, Felker GM, Lombardi C, Metra M, et al. Hemodynamic predictors of heart failure morbidity and mortality: fluid or flow? J Card Fail 2016;22:182–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fincke R, Hochman JS, Lowe AM, Menon V, Slater JN, Webb JG, et al. Cardiac power is the strongest hemodynamic correlate of mortality in cardiogenic shock: a report from the SHOCK trial registry. J Am Coll Cardiol 2004;44:340–8. [DOI] [PubMed] [Google Scholar]
- 7.Chemla D, Hebert JL, Coirault C, Zamani K, Suard I, Colin P, et al. Total arterial compliance estimated by stroke volume-to-aortic pulse pressure ratio in humans. Am J Physiol 1998;274:H500–5. [DOI] [PubMed] [Google Scholar]
- 8.Katz AM. Influence of altered inotropy and lusitropy on ventricular pressure-volume loops. J Am Coll Cardiol 1988;11:438–45. [DOI] [PubMed] [Google Scholar]
- 9.Klotz S, Hay I, Dickstein ML, Yi GH, Wang J, Maurer MS, et al. Single-beat estimation of end-diastolic pressure-volume relationship: a novel method with potential for noninvasive application. Am J Physiol Heart Circ Physiol 2006;291: H403–12. [DOI] [PubMed] [Google Scholar]
- 10.Bakkestrom R, Banke A, Pecini R, Irmukhamedov A, Nielsen SK, Andersen MJ, et al. Cardiac remodelling and haemodynamic characteristics in primary mitral valve regurgitation. Open Heart 2018;5:e000919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kochav SM, Flores RJ, Truby LK, Topkara VK. Prognostic impact of Pulmonary Artery Pulsatility Index (PAPi) in patients with advanced heart failure: insights from the ESCAPE trial. J Card Fail 2018;24:453–9. [DOI] [PubMed] [Google Scholar]
- 12.El-Menyar A, Zubaid M, Almahmeed W, Alanbaei M, Rashed W, Al Qahtani A, et al. Initial hospital pulse pressure and cardiovascular outcomes in acute coronary syndrome. Arch Cardiovasc Dis 2011;104:435–43. [DOI] [PubMed] [Google Scholar]
- 13.Planer D, Mehran R, Witzenbichler B, Guagliumi G, Peruga JZ, Brodie BR, et al. Prognostic utility of left ventricular end-diastolic pressure in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Am J Cardiol 2011;108:1068–74. [DOI] [PubMed] [Google Scholar]
- 14.Bagai A, Armstrong PW, Stebbins A, Mahaffey KW, Hochman JS, Weaver WD, et al. Prognostic implications of left ventricular end-diastolic pressure during primary percutaneous coronary intervention for ST-segment elevation myocardial infarction: findings from the Assessment of Pexelizumab in Acute Myocardial Infarction study. Am Heart J 2013;166:913–9. [DOI] [PubMed] [Google Scholar]
- 15.Belkin MN AF, Besser S, Nguyen A, Chung BB, Smith B, Kalantari S, et al. Aortic pulsatility index predicts clinical outcomes in heart failure: a sub-analysis of the ESCAPE trial. ESC Heart Fail 2021;8:1522–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation 2019;139:e56–e528. [DOI] [PubMed] [Google Scholar]
- 17.Baumwol J. I need help”-a mnemonic to aid timely referral in advanced heart failure. J Heart Lung Transplant 2017;36:593–4. [DOI] [PubMed] [Google Scholar]
- 18.Binanay C, Califf RM, Hasselblad V, O’Connor CM, Shah MR, Sopko G, et al. Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: the ESCAPE trial. JAMA 2005;294:1625–33. [DOI] [PubMed] [Google Scholar]
- 19.Sola M, Venkatesh K, Caughey M, Rayson R, Dai X, Stouffer GA, et al. Ratio of systolic blood pressure to left ventricular end-diastolic pressure at the time of primary percutaneous coronary intervention predicts in-hospital mortality in patients with ST-elevation myocardial infarction. Catheter Cardiovasc Interv 2017;90:389–95. [DOI] [PubMed] [Google Scholar]
- 20.Almousa SW, Belkin MN, Allan T, et al. Left ventricular pressure ratio predicts in-hospital outcomes in hospitalized heart failure with reduced ejection fraction. Journal of Invasive Cardiology 2021. In Press. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.