Abstract
Targeting type I interferon immune responses is a potential strategy for the treatment of systemic lupus erythematosus. Although a phase 2 clinical trial of anifrolumab did not meet its primary end point, further studies are needed to assess the effects of interferon blockade on flare rates of lupus nephritis. However, the observed higher risk of herpes zoster associated with anifrolumab use suggests that caution is warranted with this strategy.
Subject terms: Kidney, Lupus nephritis
Refers to Jayne, D. et al. Phase II randomised trial of type I interferon inhibitor anifrolumab in patients with active lupus nephritis. Ann. Rheum. Dis. 81, 496–506 (2022).
Lupus nephritis (LN) is a frequent complication of systemic lupus erythematosus (SLE), and suppression of SLE activity therefore represents an important therapeutic strategy to prevent LN development and relapse, irreversible kidney damage and progression of chronic kidney disease (CKD). The pathogenesis of SLE involves all components of the adaptive immune system, and hence traditional treatment regimens involved the use of nonselective immunosuppressants. The past decade, however, has witnessed the introduction of highly specific biological drugs, such as the inhibitor of B cell activating factor belimumab (which is approved for SLE and LN) and anifrolumab, an inhibitor of the type I interferon-α receptor 1 (IFNαR1). Despite robust evidence of the benefit of anifrolumab for patients with SLE1,2, a new trial of anifrolumab in patients with LN did not reach its primary end point3. Although longer-term data may still reveal benefits of anifrolumab on renal outcomes, available data from these trials suggest that anifrolumab increases the risk of herpes zoster and influenza, suggesting that blocking antiviral immunity may more broadly increase the risk of viral infections in these patients.
Blocking antiviral immunity may more broadly increase the risk of viral infections in these patients
The US FDA approved anifrolumab for the treatment of adults with moderate-to-severe SLE receiving standard therapy on the basis of findings from two phase 3 trials: TULIP-1 and TULIP-2. Both of these trials demonstrated therapeutic efficacy using clinical scores of non-renal SLE disease activity1,2. The phase 2 TULIP-LN trial has now assessed the efficacy and safety of anifrolumab therapy in adult patients with SLE and biopsy-proven proliferative LN (biopsy class III or IV + /− class V) and a proteinuria-to-creatinine ratio of >1 mg/mg (ref.3). The trial investigators randomly assigned 147 patients with LN in a 1:1:1 ratio to receive placebo, a basic regime of monthly intravenous 300 mg anifrolumab or an intensified regime with 900 mg anifrolumab for three months and 300 mg thereafter for 12 months. Standard-of-care therapy included initial intravenous methylprednisolone pulses, oral glucocorticoids with a mandatory taper regime over 6 months and mycophenolate mofetil at a target dose of 2 g per day. The trial did not reach its prespecified primary end point of a relative reduction in proteinuria from baseline to week 52. However, a significantly higher number of patients on the intensified regime reached a complete response rate as defined by a 24-h urine protein-to-creatinine ratio of ≤0.7 mg/mg, estimated glomerular filtration rate ≥ 60 ml/min/1.73 m2 or no decrease of ≥20% from baseline, an inactive urinary sediment (<10 red blood cells per high power field), no drug discontinuation and no use of restricted medications (18 of 44 (41.0%) of patients on the intensified anifrolumab regimen versus 6 of 46 (13.3%) patients in the placebo group; P = 0.003). The significance of this association was lost, however, when the urinary sediment was omitted as a criterion of complete response.
Numbers of adverse events or serious adverse events were slightly higher among patients receiving anifrolumab than in those on placebo. In particular, rates of herpes zoster (16.7% versus 8.2%) and influenza (8.3% versus 2.0%) episodes were notably higher among patients on anifrolumab. Episodes of urinary tract infections (16.7 versus 10.2%), nasopharyngitis (15.6 versus 18.4%), upper respiratory tract infection (15.6 versus 16.3%), diarrhoea (7.3 versus 20.4%), oral herpes (6.3 versus 4.1%) and herpes simplex (5.2 versus 4.1%) were also noted.
The type I IFN system has a central pathogenic role in SLE4, and inborn errors of immunity that cause enhanced IFN signalling lead to SLE-like systemic autoimmune disease. In SLE, nucleic acid autoantigens or nucleic acids that are attached to nucleoproteins activate innate viral nucleic acid recognition receptors and initiate a type I IFN-driven antiviral-like immune response (Fig. 1). Hence, the symptoms of SLE resemble those of viral infections; the majority of patients show an antiviral gene expression signature in white blood cells and tissues, including the kidney5,6. Intrarenal IFN signalling can lead to the presence of tubuloreticular inclusions as visualized on transmission electron microscopy; in podocytes this process is thought to drive podocyte loss7. Thus, a scientific rationale suggests that blocking type I IFN signalling might reduce proteinuria and kidney damage in patients with LN.
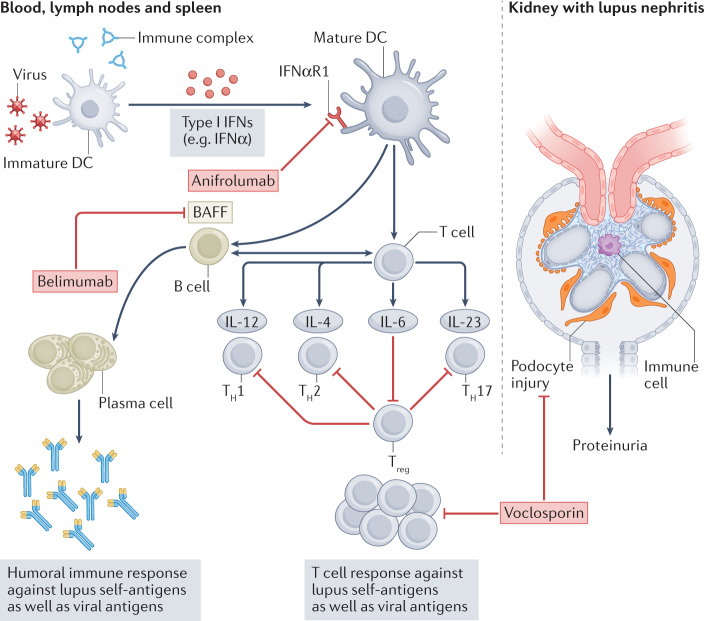
Fig. 1. Pathomechanisms and therapeutic targets of SLE.
Systemic lupus erythematosus (SLE) is an autoimmune disease, the triggers of which include the activation of innate viral nucleic acid recognition receptors by nucleic acid autoantigens or nucleic acids attached to nucleoproteins, leading to activation of immature dendritic cells (DCs) and the release of type I interferons (IFNs). The activation of antigen-presenting DCs by type I IFNs via IFNα receptors (IFNαR1 and IFNαR2) promotes their capacity to effectively present antigens (including self antigens) to T and B cells. The generation of T effector cells (T helper 1 (TH1), TH2 and TH17 cells, as well as regulatory T (Treg) cells) results in the production of cytokines such as interleukin (IL)-12, IL-4, IL-6 and IL-23) and the expression of cell surface molecules that support amplification of a self-directed immune response as well as inflammation via (auto)-antigen-specific T cells. In addition, the production of B cell activating factor (BAFF) by DCs induces the survival, proliferation and differentiation of B cells into plasma cells that ultimately drives autoantibody production. Current therapies include anifrolumab, which blocks IFNαR1 on non-immune and immune cells, including DCs; belimumab, which neutralizes BAFF to limit B cell growth and functions; or the calcineurin inhibitor voclosporin, which targets the cytoskeleton of podocytes at the filtration barrier as well as T cell activation and proliferation.
Findings from TULIP-LN demonstrated that administration of anifrolumab on top of oral steroids and mycophenolate mofetil had little effect on proteinuria — a finding that contrasts with the additive effects of belimumab or voclosporin on top of similar background therapies. Although the primary end point of TULIP-LN — proteinuria at 52 weeks — is certainly a relevant predictor of long-term outcomes in patients with LN, it is important to note that some drugs are more likely to affect proteinuria than others. For example, voclosporin has direct effects on podocytes at the filtration barrier and therefore reduces proteinuria much faster than would be expected on the basis of its immunosuppressive properties8. Although it is attractive to speculate that a rapid and profound decline in proteinuria helps to minimize irreversible kidney damage, the long-term prognosis of LN also depends on other factors, including the extent of systemic SLE activity, number of LN flares, and time to recurrent active LN. Through its inhibition of type I interferon signalling, anifrolumab would be expected to favour superiority over standard-of care for such end points; however, a 52-week treatment period as used in TULIP-LN is likely too short to assess the effect of therapy on flare rate.
Type I interferon signalling is not only a key pathway of SLE activity but is also a central mechanism of antiviral immunity4. It therefore comes as no surprise that all three TULIP trials consistently indicated a drug-related risk of herpes zoster — a condition that can cause postherpetic neuralgia. Notably, herpes zoster affected 8.2% of patients with LN treated with placebo in the TULIP-LN trial as compared to 1.1% in patients with non-renal SLE in TULIP-2 and 2% in TULIP-1 (refs1–3). This surprisingly high background incidence of herpes zoster may be a consequence of several factors, including CKD-related secondary immunodeficiency, the use of different background therapies or the longer duration of SLE in participants of TULIP-LN, which was on average 10 years longer than that of patients in TULIP-1 and TULIP-2 (ref.9). Indeed, herpes zoster is frequent in patients with SLE and the rates of this complication increase with time10. A possible explanation for this association may be an enhanced state of acquired immunodeficiency as indicated by the high prevalence of IgM deficiency in patients with long-term SLE. In all three trials, anifrolumab therapy further increased herpes zoster rates: from 2% to 6% in TULIP-1 (ref.1), 1.1% to 7.2% in TULIP-2 (ref.2) and 8.2% to 16.7% in TULIP-LN3, mirroring increased rate of influenza infections from 2% in patients on placebo to 8.3% in patients on anifrolumab in TULIP-LN3. Data on COVID-19 were not reported, but blocking antiviral immunity may confer a particular risk for more severe disease.
Thus, despite not reaching its primary end point in TULIP-LN, anifrolumab may still have beneficial effects on long-term outcomes of LN that may only be evident with longer-term studies. Not unexpectedly, anifrolumab therapy implies a significant risk of viral infections such as herpes zoster and influenza, raising concerns about a higher risk of more severe COVID-19 in patients on this therapy; however, further studies are needed to assess this possibility.
Acknowledgements
H.-J.A. is supported by the Deutsche Forschungsgemeinschaft (AN372/30-1).
Competing interests
H.-J.A. has received consultancy fees from Bayer, Janssen, GSK, Novartis, Boehringer, AstraZeneca and PreviPharma. S.S. has received research funding from Eleva Ltd.
References
- 1.Furie RA, et al. Type I interferon inhibitor anifrolumab in active systemic lupus erythematosus (TULIP-1): a randomised, controlled, phase 3 trial. Lancet Rheumatol. 2019;1:E208–E219. doi: 10.1016/S2665-9913(19)30076-1. [DOI] [PubMed] [Google Scholar]
- 2.Morand EF, et al. Trial of anifrolumab in active systemic lupus erythematosus. N. Engl. J. Med. 2020;382:211–221. doi: 10.1056/NEJMoa1912196. [DOI] [PubMed] [Google Scholar]
- 3.Jayne D, et al. Phase II randomised trial of type I interferon inhibitor anifrolumab in patients with active lupus nephritis. Ann. Rheum. Dis. 2022;81:496–506. doi: 10.1136/annrheumdis-2021-221478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crow MK, Olferiev M, Kirou KA. Type I interferons in autoimmune disease. Annu. Rev. Pathol. 2019;14:369–393. doi: 10.1146/annurev-pathol-020117-043952. [DOI] [PubMed] [Google Scholar]
- 5.Der E, et al. Tubular cell and keratinocyte single-cell transcriptomics applied to lupus nephritis reveal type I IFN and fibrosis relevant pathways. Nat. Immunol. 2019;20:915–927. doi: 10.1038/s41590-019-0386-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anders HJ, Lichtnekert J, Allam R. Interferon-alpha and -beta in kidney inflammation. Kidney Int. 2010;77:848–854. doi: 10.1038/ki.2010.71. [DOI] [PubMed] [Google Scholar]
- 7.Migliorini A, et al. The antiviral cytokines IFN-α and IFN-β modulate parietal epithelial cells and promote podocyte loss: implications for IFN toxicity, viral glomerulonephritis, and glomerular regeneration. Am. J. Pathol. 2013;183:431–440. doi: 10.1016/j.ajpath.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 8.Rovin BH, et al. Efficacy and safety of voclosporin versus placebo for lupus nephritis (AURORA 1): a double-blind, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2021;397:2070–2080. doi: 10.1016/S0140-6736(21)00578-X. [DOI] [PubMed] [Google Scholar]
- 9.Steiger S, Rossaint J, Zarbock A, Anders HJ. Secondary immunodeficiency related to kidney disease (SIDKD) — definition, unmet need, and mechanisms. J. Am. Soc. Nephrol. 2022;33:259–278. doi: 10.1681/ASN.2021091257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwan A, et al. Herpes zoster in SLE: prevalence, incidence and risk factors. Lupus Sci. Med. 2022;9:e000574. doi: 10.1136/lupus-2021-000574. [DOI] [PMC free article] [PubMed] [Google Scholar]