Abstract
Small, soluble single-domain fragments derived from the unique variable region of dromedary heavy-chain antibodies (VHHs) against enzymes are known to be potent inhibitors. The immunization of dromedaries with the TEM-1 and BcII β-lactamases has lead to the isolation of such single-domain antibody fragments specifically recognizing and inhibiting those β-lactamases. Two VHHs were isolated that inhibit TEM-1 and one BcII inhibiting VHH was identified. All inhibitory VHHs were tight-binding inhibitors. The 50% inhibitory concentrations were determined for all inhibitors and they were all in the same range as the enzyme concentration used in the assay. Addition of the VHHs to the TEM-1 β-lactamase, expressed on the surface of bacteria, leads to a higher ampicillin sensitivity of the bacteria. This innovative strategy could generate multiple potent inhibitors for all types of β-lactamases.
The production of β-lactamases by nosocomial strains represents the most widespread and often the most efficient mechanism of resistance devised by bacteria to escape the lethal action of β-lactam antibiotics (8, 13, 19). β-Lactamases catalyze the irreversible hydrolysis of these compounds, thus precluding further reaction with the bacterial targets (the DD-transpeptidases, also known as penicillin-binding proteins [7, 10]).
About 300 different enzymes have been described so far, exhibiting a wide range of primary structures and catalytic properties. They are divided into four main groups (18). β-Lactamases which display an essential serine residue can be categorized on the basis of their primary structures into three classes, A, C, and D, while a smaller number of enzymes, referred to as class B β-lactamases, are Zn(II)-dependent enzymes.
The emergence of resistant strains has been a recurrent problem from the very beginning of the clinical utilization of penicillins. This has resulted in the progressive introduction of new molecules which escape the activity of the most common enzymes. Bacteria, however, have responded by developing improved resistance mechanisms. In particular, the appearance of new or modified β-lactamases exhibiting broadened specificity spectra represents a major problem. The situation is complicated by the facts that some strains produce several β-lactamases and that the corresponding genetic materials can easily spread in the bacterial population by horizontal gene transfer (4). Thus, although a rather large number of molecules (1) are now offered by the pharmaceutical industry, none of them can be considered as the “universal drug” which might kill all pathogenic bacteria. Moreover, some bacterial strains have acquired resistance characteristics which make them resistant to all known antibiotics.
Since new β-lactamases appear as an immediate response to the introduction of new β-lactams, the use of non-β-lactam inhibitors or inactivators of the β-lactamases and DD-transpeptidases might be preferable. The latter are choice targets for antibacterial drugs. In this context, peptidomimetics derived from proteinaceous inhibitors that can bind the β-lactamases with high affinities seem to constitute a new and attractive solution. At the present time, only the β-lactamase inhibitor protein BLIP has been isolated and characterized (12, 26, 27).
In this paper we describe an innovative strategy to identify an unlimited number of proteinaceous inhibitors against β-lactamases, based on the isolation of dromedary single-domain antibodies. The Camelidae, besides containing conventional antibodies consisting of heterodimers of a heavy and a light chain, also contain heavy-chain antibodies that are homodimers of heavy chains only (11). Therefore, single-domain antigen-binding fragments can be generated from the variable domain of these heavy-chain antibodies (VHHs). The VHHs are minimally sized, highly soluble entities that bind the antigen with nanomolar affinity (9). In contrast to the antigen-binding fragments of conventional antibodies, it was established that the VHHs are often potent inhibitors of enzymes (16). Hence, we immunized dromedaries with TEM-1 and BcII β-lactamases, representatives of class A and class B β-lactamases, respectively. In both cases, highly inhibitory single-domain VHH antibody fragments were obtained.
MATERIALS AND METHODS
Enzymes.
The TEM-1 and the Bacillus cereus 569/H (BcII) enzymes were purified as described by Raquet et al. (24) and Carfi et al. (2), respectively. All enzyme preparations were at least 95% pure and were stored at −20°C until further use.
Immunization of dromedaries.
Two adult male dromedaries (Camelus dromedarius) kept at the Central Veterinary Research Laboratories facilities (Dubai, United Arab Emirates) received six subcutaneous injections at weekly intervals of 1 mg of TEM-1 β-lactamase or 700 μg of BcII β-lactamase. For the first injection the antigen was mixed with an equal volume of complete Freund's adjuvant, and all subsequent boosts were with incomplete Freund's adjuvant. Forty-five days after the first injection, 50 ml of anticoagulated blood was collected (and transported refrigerated to the Brussels laboratory by courier service) to evaluate the immune response raised against the injected antigens and for the isolation of lymphocytes. Peripheral blood lymphocytes were prepared with Uni-Sep tubes (WAK-Chemie). Lymphocytes were counted under the microscope and aliquots of 5 × 106 cells were pelleted and stored at −80°C until further use.
Fractionation of the IgG subclasses.
The separation of the different serum immunoglobulin G (IgG) subclasses was performed by differential adsorption on Hitrap-protA and Hitrap-protG columns (Pharmacia). IgG3 and IgG1 subclasses were eluted from a protG column with an acetate buffer (pH 3.5) and a glycine-HCl buffer (pH 2.7), respectively. The flowthrough was subsequently loaded on the Hitrap-protA column to recover the IgG2 subclass during elution with the acetate buffer (pH 3.5) (11). The protein concentration was determined spectrophotometrically, assuming an A278 (1%, 1 cm) of 13.5.
Solid-phase binding enzyme-linked immunosorbent assays (ELISAs).
Maxisorb 96-well plates (Nunc) were coated with both TEM-1 and BcII β-lactamases overnight in phosphate-buffered saline (PBS) (4°C) at a concentration of 1 μg/ml. Residual sites were blocked for 2 h at room temperature with 1% (wt/vol) casein in PBS. After incubation with serial dilutions of purified IgG subclasses, bound dromedary IgG was detected with a rabbit anti-dromedary IgG antiserum. As secondary reagent, a goat anti-rabbit–alkaline phosphatase conjugate (Sigma) was used. After addition of the substrate p-nitrophenyl phosphate (Sigma), the reaction was measured after 10 min at 410 nm. Virion binding was revealed using a horseradish peroxidase–anti-M13 conjugate (Pharmacia). For the detection of Escherichia coli-produced VHH proteins, a mouse antihemagglutinin decapeptide tag (clone 16B12; BAbCO) or an anti-His tag (Serotec) were used as primary reagents in combination with an anti-mouse–alkaline phosphatase conjugate (Sigma).
Vector construction.
The phage display vector pHEN4 has been described previously by Ghahroudi et al. (9). The pHEN4C vector is a derivative of pHEN4 in which the ampicillin resistance gene has been replaced by the chloramphenicol acetyltransferase gene. The pHEN6 vector is equivalent to the pHEN4 vector, except that the hemagglutinin tag and geneIII were replaced by a His6 detection and purification tag. The pHEN6C vector is pHEN6 with a chloramphenicol resistance gene instead of the ampicillin resistance gene. All of these constructs were made by using standard cloning techniques.
VHH library construction and selection of binders.
The mRNA was extracted from the lymphocytes and in a subsequent step cDNA was prepared (Ready-to-Go beads, Pharmacia). With the specific primers CALL001 (5′GTCCTGGCTGCTCTTCTACAAGG3′) and CALL002 (5′GGTACGTGCTGTTGAACTGTTCC3′) annealing at the leader sequence and at the CH2 exon of the heavy chains of all dromedary immunoglobulins, respectively, we amplified the gene fragments coding for the variable domain until the CH2 domain. After reamplification of the VHH gene fragments with nested primers annealing at the framework1 and framework4 regions (9), the final PCR products were cloned into the phagemid vector pHEN4 (for BcII VHHs) or pHEN4C (for TEM-1 VHHs) according to the methods of Ghahroudi et al. (9). The VHH repertoire was expressed on phage after infection with M13K07 helper phages. Specific VHHs against the TEM-1 or BcII β-lactamases were enriched by three consecutive rounds of in vitro selection on microtiter plates coated with antigen (10 μg/well). Bound phage particles were eluted with 100 mM triethylamine (pH 10.0). The eluate was immediately neutralized with Tris-HCl (pH 7.4) and used to infect exponentially growing E. coli TG1 cells. The enrichment of phage particles carrying antigen-specific VHHs was assessed by comparing the number of eluted phages from antigen-coated versus noncoated wells. After the third panning, individual colonies were picked and expression of their cloned VHH as soluble periplasmic protein was induced with 1 mM isopropyl β-d-thiogalactopyranoside (IPTG). The recombinant VHH extracted from the periplasm (25) was tested for antigen recognition in an ELISA.
Expression and purification of the single-domain antibody fragments.
The VHH genes of clones that scored positive in ELISAs were recloned into the expression vectors pHEN6 (for VHHs against BcII) or pHEN6C (for VHHs against TEM-1) by using the restriction enzymes NcoI and BstEII. The plasmid constructs were transformed into E. coli WK6 cells. Large-scale production of the recombinant VHHs was performed in shake flasks by growing the bacteria in Terrific broth supplemented with 0.1% glucose and ampicillin (for VHHs present in pHEN6) or chloramphenicol (for VHHs present in pHEN6C) till an optical density at 600 nm (OD600) of 0.6 to 0.9 was reached and then inducing expression with 1 mM IPTG for 16 h at 28°C. After pelleting the cells, the periplasmic proteins were extracted by osmotic shock (25). This periplasmic extract was loaded on a Ni-nitrilotriacetic acid superflow Sepharose column (Qiagen) and after washing, the bound proteins were eluted with a pH 4.7 acetate buffer. The eluted fraction was concentrated on Vivaspin concentrators with a molecular mass cutoff of 5 kDa (Vivascience) and loaded on a Superdex-75 16/60 gel filtration column (Pharmacia). The purity of the fragments was evaluated in a Coomassie-stained sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The absorption at 280 nm and the extinction coefficient (A280 [0.1%, 1cm] values of 1.455, 1.469, and 2.378 for the cAbBCII10, cAbTem02, and cAbTem13, respectively) as calculated from the amino acid sequence were used to determine the VHH protein concentration.
Enzymatic assays.
The enzymatic activity of the TEM-1 and BcII β-lactamases by the hydrolysis of the nitrocefin substrate, measured spectrophotometrically at 482 nm (ΔɛM = 15,000 M−1 cm−1). In the case of TEM-1, the measurements were performed in PBS (pH 7.0); for BcII the measurements were performed in 10 mM cacodylate buffer (pH 6.5) containing 50 μM ZnCl2. All dilutions were made in buffer containing 0.2 mg of bovine serum albumin/ml.
The inhibitory capacities of the single-domain antibodies were determined by preincubating the enzyme (0.05 μM TEM-1 or 0.2 μM BcII) with various concentrations of antibody solution in a volume of 10 μl for at least 30 min at room temperature. After the addition of 1 ml of 100 μM nitrocefin, the initial rate of hydrolysis was measured as an increase in the OD482.
The 50% inhibitory concentrations (IC50s) for the different antibody fragments were obtained by plotting the initial rate of nitrocefin hydrolysis as a function of increasing antibody fragment concentration.
Expression of TEM-1 on the surface of E. coli
The gene coding for the mature TEM-1 protein was cloned as a fusion protein with OprI (3) in a vector (pBAD; Invitrogen) containing an arabinose-inducible promoter and selectable markers coding for streptomycin and spectinomycin resistance. At an OD600 of 0.5, TEM-1 expression was induced with 0.02% arabinose after 30 min.
RESULTS
Humoral response of the dromedaries after immunization with BcII and TEM-1 β-lactamases.
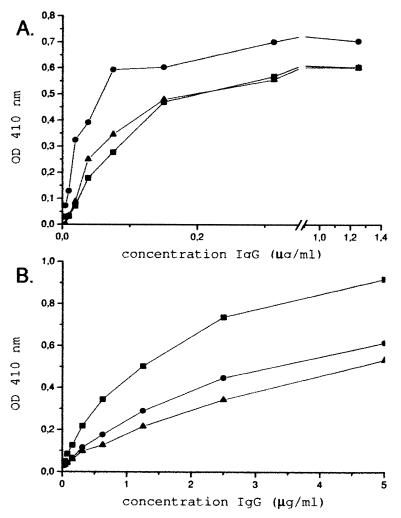
Two dromedaries were injected six times with either BcII or TEM-1 β-lactamase. Blood was collected 45 days after the first injection and shown to contain specific antibodies directed against the BcII or TEM-1 antigens. From the sera, the IgG-subclasses were fractionated into the conventional immunoglobulins IgG1 and the heavy-chain immunoglobulins IgG2 and IgG3 to evaluate the humoral response within each subclass. Serial dilutions of the IgG subclasses were used in a solid-phase ELISA with coated antigen and detected with a rabbit anti-dromedary serum against IgGs (Fig. 1). For both antigens, an antigen-specific response was elicited, in conventional antibodies as well as in the heavy-chain antibodies. A higher signal was noticed for BcII for all subclasses tested, probably due to a better immune response. Moreover, the heavy-chain antibody subclasses against TEM-1 had a weak titer compared to the conventional IgG1 subclass.
FIG. 1.
Analysis of antigen-specific antibodies. (A) Microtiter plates were coated with BcII at a concentration of 1 μg/ml. (B) TEM-1 was immobilized at a concentration of 2 μg/ml. The plates were incubated with serial dilutions of the immunoglobulin subclasses, isolated from serum after immunization. Bound IgG1 (▪), IgG2 (●), and IgG3 (▴) were subsequently detected with a rabbit anti-dromedary IgG antiserum and anti-rabbit IgG-alkaline phosphatase conjugate. OD410 was measured after 10 min.
VHH library construction and selection of specific binders.
From the lymphocytes prepared from anticoagulated blood of the immunized dromedaries, cDNA was prepared and used as template to amplify genes coding for the variable domains of the heavy-chain antibodies. The PCR fragments were ligated into a pHEN4 or pHEN4C phagemid vector and transformed in E. coli TG1 cells. In this way, two VHH libraries of 107 transformants were obtained. More than 85% of the clones within the libraries contained a vector with a VHH gene insert of the proper size as determined by PCR. The VHH repertoires of both VHH libraries were expressed on phages after infection with helper phages and selection of phage particles expressing a specific antigen-binding VHH were performed. After three consecutive rounds of selection on solid-phase coated antigen, a clear enrichment for TEM-1- or BcII-specific phage particles was observed. After the third round of selection on TEM-1 or BcII, 40 and 30 colonies, respectively, were randomly chosen for expression of their VHH as soluble protein. When these crude periplasmic extracts were tested in an ELISA, 19 VHHs out of the 40 extracts were shown to be specific towards TEM-1 and 27 VHHs out of the 30 recognized BcII. The TEM-1 binders did not cross-react with the BcII binders, and vice versa (data not shown). The gene fragments from the clones, positive by ELISA, were digested with the frequent cutter HinfI to identify the differing VHH gene fragments by restriction length polymorphism analysis. Of all clones analyzed, five distinct fingerprints of VHHs were identified for the BcII enzyme, and seven were identified for the TEM-1 enzyme. This was confirmed by DNA sequencing.
Sequence alignment of the fragments.
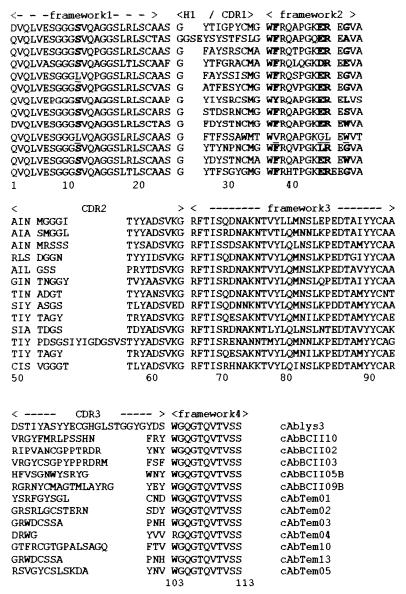
The deduced amino acid sequences of the five different BcII binders and the seven TEM-1 binders aligned with the dromedary VHH cAbLys3 (Fig. 2). cAbLys3 is a VHH that inhibits hen egg-white lysozyme and its structure in complex with the antigen was determined by crystallography (6, 28). With the exception of cAbTem04, all of the β-lactamase-specific fragments are derived from the heavy-chain antibody-specific VHH germ line gene (22), since they contain the hallmark amino acid substitutions in framework1 and -2 (Fig. 2). These amino acids are responsible for VH-VL interactions within a conventional antibody, whereas a heavy-chain antibody is devoid of light chains, and the substitutions occurred to overcome the insolubility of the heavy-chain immunoglobulins (21). Although cAbTem04 does not harbor the VHH hallmark amino acid substitutions, it is probably also derived from a heavy-chain immunoglobulin because the W103R mutation will undoubtedly disrupt VH-VL interaction.
FIG. 2.
Amino acid sequence of the isolated binders against the TEM-1 β-lactamase, named cAbTemxx, and against the BcII β-lactamase, named cAbBCIIxx. Numbering and deoxycytidine (CDR) designations are according to the methods of Kabat et al. (14).
The disulfide bond often occurring between CDR1 and CDR3 in VHHs was present in 8 (cAbTem01, cAbTem02, cAbTem03, cAbTem10, cAbTem13 cAbBCII02, cAbBCII03, and cAbBCII09B) of the 12 clones. A disulfide bridge is probably formed between the CDR3 and a cysteine at position 50 in the CDR2 for cAbTem05. A cysteine at position 50 has never been reported for dromedary VHHs but occurs frequently in llama VHHs (30). The length of the CDR3 varies from 7 to 19 amino acids among the different VHHs. In addition, a number of aberrant CDR lengths are observed; cAbBCII10 has a first antigen binding loop with an insertion of 3 amino acids compared to the usual length, whereas cAbTem10 contains a CDR2 with a length of 26 amino acids compared to the usual 17-amino-acid length. In the cAbTem05 we noticed a remarkable insertion between positions 45 and 46. One clone (cAbBCII05B) has a shorter CDR2 length of only 15 amino acids. These aberrant sizes are not encountered in the dromedary VHH germ line genes and were therefore introduced by somatic mutation or gene conversion (22). It was previously noticed that 30% of the VHH dromedary cDNAs are off-sized but nevertheless functional, whereas in humans most insertions or deletions result in nonfunctional immunoglobulin genes. The insertions and deletions in VHH genes increase the potential antigen binding repertoire (22).
Production and purification of the different binders.
Production of the single-domain antibody fragments as soluble protein was accomplished after recloning into expression vector pHEN6 or pHEN6C and transformation into E. coli WK6 cells. The single-domain antibodies, carrying a His6 tag to facilitate purification, are transported into the periplasm of E. coli. Purification of periplasmic extract by immobilized-metal affinity chromatography followed by an additional gel permeation chromatography yielded >95% pure VHH as determined by Coomassie-stained sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The yield of purified product varied from 0.5 to 5 mg/liter of culture, depending on the actual fragment. The purified proteins were present as monomers, since gel filtration profiles showed only a single peak eluting at a molecular mass of ≈15,000 Da.
Identification of inhibitory VHHs.
The hydrolysis of nitrocefin (Δɛ=15,000 M−1 cm−1) is a measure of the activity of both β-lactamases, since nitrocefin is a substrate for these enzymes. The inhibitory capacity of the isolated VHHs was determined by measuring the initial hydrolysis rate of nitrocefin by an enzyme-VHH mixture. A molar excess of VHH was preincubated with 0.05 μM TEM-1 or 0.2 μM BcII enzyme. One fragment, cAbBCII10, of the five isolated fragments was able to inhibit the BcII β-lactamase, and two fragments, cAbTem02 and cAbTem13, of the seven isolated fragments were able to inhibit the TEM-1 β-lactamase.
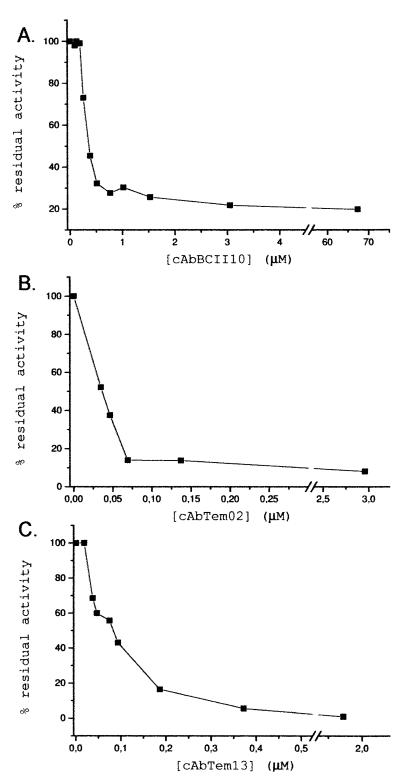
The efficiency of the inhibition of the three inhibitory fragments was estimated from the rate of substrate hydrolysis in the presence of different concentrations of purified antibody fragment. The IC50 was determined. In the experiments, 0.05 μM TEM-1 or 0.2 μM BcII was used and in both cases the IC50s obtained for the different binders corresponded to the concentration of enzyme used in the assay (Fig. 3). The IC50s obtained from the measurements were 0.035, 0.08, and 0.30 μM for cAbTem02, cAbTem13, and cAbBCII10, respectively. Therefore, the three inhibiting antibody fragments were revealed to be tight-binding inhibitors, because the concentration of antibody fragment needed to observe inhibition is in the same range of the enzyme concentration used in the assay.
FIG. 3.
IC50 determinations for the different inhibitors. The residual enzymatic activity was measured as a function of antibody fragment concentration. For all measurements, 100 μM nitrocefin was used as substrate. This was performed for cAbBCII10 (A), cAbTem02 (B), and cAbTem13 (C). For the experiment shown in panel A, 0.2 μM BcII was used, whereas 0.05 μM TEM-1 was used for the experiments shown in panels B and C.
In vivo inhibition of TEM-1.
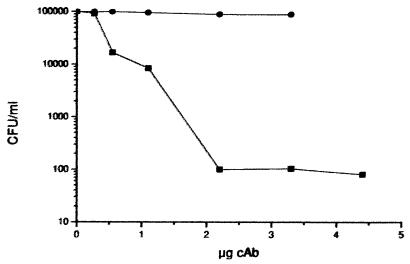
E. coli resistant to ampicillin due to the presence of plasmid-encoded TEM-1 expresses the β-lactamase in the periplasmic space. In view of the size of the single-domain VHH molecules (15,000 Da) and due to the low permeability of the outer membrane for such polypeptides, we inferred that inhibition of TEM-1 with the inhibitory VHHs would not be measurable. To indicate the in vivo enzymatic inhibition of the VHHs, a system was used where the target, in this case TEM-1, was expressed on the outer membrane of bacteria. It has been demonstrated that proteins, such as the Leishmania major gp63 protein and a hepatitis B virus epitope, could be expressed as fusion proteins with OprI, the major lipoprotein from the outer membrane of Pseudomonas aeruginosa (3). We cloned the gene coding for the mature TEM-1 β-lactamase within this expression vector and transformed E. coli cells. Functional expression of TEM-1 on the surface of E. coli cells was demonstrated by the ability of the bacteria to grow in the presence of more than 150 μg of ampicillin/ml and by the hydrolysis of nitrocefin added to the cells. E. coli cells harboring the parental vector failed to grow on agar plates containing ampicillin. The ability of cAbTem13 to inhibit surface-exposed TEM-1 and prevent bacterial growth was assessed by preincubating cells expressing TEM-1 with increasing amounts of the antibody fragment. Survival was determined by counting the number of colonies after plating the bacteria on agar containing ampicillin. The results summarized in Fig. 4 reveal that cells expressing TEM-1 β-lactamase readily grew on agar plates containing the antibiotic but lost their ability to neutralize the antibiotic when preincubated with cAbTem13 before plating. In contrast, cAbBCII10 was unable to inhibit colony formation by E. coli cells on agar plates containing ampicillin. This confirms the ability of cAbTem13 to inhibit the TEM-1 enzyme when present on bacterial cells and to render bacterial cells susceptible to ampicillin.
FIG. 4.
In vivo inhibition of TEM-1 when expressed as a fusion protein with OprI on the surface of E. coli. A total of 4 × 107 cells were preincubated for 1 h with 0 to 3.5 μg of cAb in a final volume of 100 μl. The cells were plated on ampicillin-containing agar plates. Shown are the CFU (per milliliter) results obtained in the presence of increasing amounts of cAbTem13, ranging from 0 to 3.5 μg (▪), and the results obtained in the presence of increasing amounts of cAbBCII10, ranging from 0 to 3.5 μg (●).
DISCUSSION
At the present time, the mechanism-based inhibitors tazobactam, sulbactam, and clavulanic acid are used to fight β-lactamases, and several β-lactam–β-lactamase inhibitor mixtures are commercially available for the treatment of common infections. However, the strong selection pressure exerted has resulted in the appearance of inhibitor-resistant β-lactamases (18). Thus, the development of novel, drastically different inhibitors would provide new opportunities for the treatment of bacterial infections with existing antibiotics. Here, an innovative approach is presented to find potent inhibitors of β-lactamases.
Antibodies are known to be a source of specific binders towards any antigen. However, the antigen-binding fragments of camel heavy-chain antibodies were proven to be a much better choice to obtain small-sized enzyme inhibitors (16). The absence of the light chain in dromedary heavy-chain antibodies is compensated by the presence of a longer CDR3 in the VHH relative to that of a VH, with novel architectures that extend the strategies to interact with the antigen. As shown in the case of cAbLys3, a VHH against lysozyme where part of the 24-amino-acid-long CDR3 protrudes from the antigen-binding site and inserts into the active site of lysozyme (6). A large concave protruding antigen-binding loop has only been observed in VHHs; the antigen-binding loops of VH-VL pairs form either a cavity or a flat surface. In addition, the protruding loop of the cAb-Lys3 mimics the natural substrate of lysozyme and is therefore a competitive inhibitor (28).
Dromedaries immunized with two different β-lactamases, TEM-1 and BcII, raised a good immune response in the heavy-chain antibody subclasses. The β-lactamases are immunogenic and it can be assumed that each type of β-lactamase will elicit a humoral response in the heavy-chain antibody isotypes. For the two types of β-lactamases described in this paper, each a good representative of its class, we demonstrated that specific inhibitors against the different enzymes could be obtained by immunization of dromedaries. The inhibitors were isolated from phage libraries containing the genes coding for the variable domains of the heavy-chain antibodies. In this case, one out of five and two out of seven identified VHHs specific towards BcII and TEM-1, respectively, were able to inhibit the β-lactamase enzyme. The isolated inhibitory proteins, cAbBCII10, cAbTem02, and cAbTem13, behave as tight-binding inhibitors with high affinities. According to the measurements, both inhibitory single-domain fragments have an IC50 around the enzyme concentration used in the assay, resulting in effective inhibitors at low concentrations. It was further demonstrated that in the presence of ampicillin, growth of E. coli cells expressing a fusion protein of TEM-1 on their outer membrane was prohibited by addition of the inhibitor cAbTem13. This clearly illustrates the in vivo potential of isolated VHHs to revert the antibiotic resistance based on β-lactamases. The isolation of cAbBCII10-inhibiting BcII lactamase is of particular interest since no clinically useful inhibitors are currently available for the class B metalloenzymes (23).
No cross-reactivity was observed between the various VHHs, reflecting the specificity of each antibody fragment towards its corresponding enzyme, as demonstrated in vitro and in vivo. This high specificity of the VHHs is an advantage in cases where they would be employed as a diagnostic tool in biosensors or in ELISAs for the fast identification of lactamases that confer antibiotic resistance to infective pathogens. The high expression levels of the recombinant VHHs, their good stability and robustness even in denaturing solutions, and their easy immobilization on solid supports (20) are additional benefits for their application in biosensors.
Due to the diversity of β-lactamases, immunization of dromedaries with individual enzymes would be needed in order to obtain inhibitors that would be effective against all of the β-lactamase classes. Because the techniques needed for isolation of the antibody fragments are already optimized, the time needed to identify an inhibitor would not be a limiting factor. Moreover, the large repertoire of antibodies with a wide diversity of antigen-binding fragments gives access to an almost endless list of possible inhibitors. This is illustrated with the high diversity in loop length and amino acid sequence of the identified VHHs, all binding the same antigen with nanomolar affinity (9, 16).
Obviously, the direct use of these single-domain antibodies is not the optimal strategy for combating β-lactamases, because their size would still prevent easy access to the target. However, the design of small peptidic or peptidomimetic inhibitors (15, 29) derived from VHHs might be a far better option. Recently, a loop-mimetic inhibitor of the NS3 hepatitis C virus protease was designed from a synthetic minibody, a single-domain fragment with two antigen-binding loops. This cyclic hexapeptide mimicking the bioactive loop of the parent macromolecule was used as a lead compound to form a second generation of inhibitors readily obtained through solid-phase combinatorial chemistry (17). Similarly, the VHHs also offer good opportunities for such an approach to generate peptidomimetics. The antigen-binding site of the VHH comprises only three antigen-binding loops, and a VHH that uses only a single CDR loop to interact with the antigen with nanomolar affinity was solved recently by crystallography (5).
The successful generation of small, potent proteinaceous inhibitors derived from the heavy-chain antibodies of dromedaries immunized with β-lactamases was demonstrated. With this approach it should be possible to obtain an almost unlimited number of yet-unexplored β-lactamase inhibitors that can be used as leads in the design of peptidomimetics combating antibiotic resistance where conventional antibiotics fail.
ACKNOWLEDGMENTS
This work was supported by Fonds voor Wetenschappelijk Onderzoek Vlaanderen and by Vlaams Interuniversitair Instituut voor Biotechnology. The work in Liège was supported by the Belgian Government in the frame of the Pôles d'Attraction Interuniversitaires (PAI P4/03). A.M. is a Research Associate of the National Fund for Scientific Research, Belgium.
REFERENCES
- 1.Burton G, Osborne N F, Pearson M J, Southgate R. The beta-lactam antibiotics. In: Wolff M E, editor. Burger's medicinal chemistry and drug discovery. 5th ed. New York, N.Y: John Wiley & Sons, Inc.; 1998. pp. 257–263. [Google Scholar]
- 2.Carfi A, Pares S, Duée E, Galleni M, Duez C, Frère J M, Dideberg O. The 3-D structure of a zinc metallo-β-lactamase from Bacillus cereus reveals a new type of protein fold. EMBO J. 1995;14:4914–4921. doi: 10.1002/j.1460-2075.1995.tb00174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cornelis P, Cote Sierra J, Lim A, Malur A, Tungpradabkul S, Tazka H, Leitao A, Martins C V, di Perna C, Brys L, De Baetselier P, Hamers R. Development of new cloning vectors for the production of immunogenic outer membrane fusion proteins in Escherichia coli. Bio/Technology. 1996;14:203–208. doi: 10.1038/nbt0296-203. [DOI] [PubMed] [Google Scholar]
- 4.Davies J. Inactivation of antibiotics and the dissemination of resistance genes. Science. 1994;264:375–381. doi: 10.1126/science.8153624. [DOI] [PubMed] [Google Scholar]
- 5.Desmyter, A., K. Decanniere, S. Muyldermans, and L. Wyns. One hypervariable loop of camel single-domain antibody sufficient for specific antigen recognition. J. Biol. Chem., in press. [DOI] [PubMed]
- 6.Desmyter A, Transue T R, Ghahroudi M A, Dhao-Thi M H, Poortmans F, Hamers R, Muyldermans S, Wyns L. Crystal structure of a camel single-domain VH antibody fragment in complex with lysozyme. Nat Struct Biol. 1996;3:803–811. doi: 10.1038/nsb0996-803. [DOI] [PubMed] [Google Scholar]
- 7.Frère J M, Joris B. Penicillin-sensitive enzymes in peptidoglycan biosynthesis. CRC Crit Rev Microbiol. 1985;11:299–396. doi: 10.3109/10408418409105906. [DOI] [PubMed] [Google Scholar]
- 8.Frère J M. Beta-lactamases and bacterial resistance to antibiotics. Mol Microbiol. 1995;16:385–395. doi: 10.1111/j.1365-2958.1995.tb02404.x. [DOI] [PubMed] [Google Scholar]
- 9.Ghahroudi M A, Desmyter A, Wyns L, Hamers R, Muyldermans S. Selection and identification of single-domain antibody fragments from camel heavy-chain antibodies. FEBS Lett. 1997;414:521–526. doi: 10.1016/s0014-5793(97)01062-4. [DOI] [PubMed] [Google Scholar]
- 10.Ghuysen J M. Serine beta-lactamases and penicillin-binding proteins. Annu Rev Microbiol. 1991;45:37–67. doi: 10.1146/annurev.mi.45.100191.000345. [DOI] [PubMed] [Google Scholar]
- 11.Hamers-Casterman C, Atarhouch T, Muyldermans S, Robinson G, Hamers C, Songa E B, Bendahman N, Hamers R. Naturally occurring antibodies devoid of light chains. Nature. 1993;363:446–448. doi: 10.1038/363446a0. [DOI] [PubMed] [Google Scholar]
- 12.Huang W, Zhang Z, Palzkill T. Design of potent β-lactamase inhibitors by phage display of β-lactamase inhibitory protein. J Biol Chem. 2000;275:14964–14968. doi: 10.1074/jbc.M001285200. [DOI] [PubMed] [Google Scholar]
- 13.Jacoby G A. Extrachromosomal resistance in gram-negative organisms: the evolution of beta-lactamase. Trends Microbiol. 1994;2:357–360. doi: 10.1016/0966-842x(94)90611-4. [DOI] [PubMed] [Google Scholar]
- 14.Kabat E A, Wu T T, Perry H M, Gottesman K S, Foeller C. Seqeunce of proteins of immunological interest. Publication 91-3242. U.S. Bethesda, Md: Public Health Service; 1991. [Google Scholar]
- 15.Laune D, Molina F, Ferrieres G, Mani J C, Cohen P, Simon D, Bernardi T, Piechaczyk M, Pau B, Granier C. Systematic exploration of the antigen binding activity of synthetic peptides isolated from the variable regions of immunoglobulins. J Biol Chem. 1997;272:30937–30944. doi: 10.1074/jbc.272.49.30937. [DOI] [PubMed] [Google Scholar]
- 16.Lauwereys M, Ghahroudi M A, Desmyter A, Kinne J, Hölzer W, De Genst E, Wyns L, Muyldermans S. Potent enzyme inhibitors derived from dromedary heavy-chain antibodies. EMBO J. 1998;117:3512–3520. doi: 10.1093/emboj/17.13.3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin F, Steinkühler C, Brunetti M, Pessi A, Cortese R, De Fransesco R, Sollazzo M. A loop mimetic inhibitor of the HCV-NS3 protease derived from a minibody. Protein Eng. 1999;12:1005–1011. doi: 10.1093/protein/12.11.1005. [DOI] [PubMed] [Google Scholar]
- 18.Matagne A, Dubus A, Galleni M, Frère J M. The beta-lactamase cycle: a tale of selective pressure and bacterial ingenuity. Nat Prod Rep. 1999;16:1–19. doi: 10.1039/a705983c. [DOI] [PubMed] [Google Scholar]
- 19.McManus M C. Mechanisms of bacterial resistance to antimicrobial agents. Am J Health-Syst Pharm. 1997;54:1420–1433. doi: 10.1093/ajhp/54.12.1420. [DOI] [PubMed] [Google Scholar]
- 20.Muyldermans S. Single domain camel antibodies: current status. Rev Mol Bio/Technol. 2001;74:277–302. doi: 10.1016/s1389-0352(01)00021-6. [DOI] [PubMed] [Google Scholar]
- 21.Muyldermans S, Atarhouch T, Saldanha J, Barbosa J A, Hamers R. Sequence and structure of VH domain from naturally occurring camel heavy chain immunoglobulins lacking light chains. Protein Eng. 1994;7:1129–1135. doi: 10.1093/protein/7.9.1129. [DOI] [PubMed] [Google Scholar]
- 22.Nguyen V K, Hamers R, Wyns L, Muyldermans S. Camel heavy-chain antibodies: diverse germline VHH and specific mechanisms enlarge the antigen-binding repertoire. EMBO J. 2000;19:921–930. doi: 10.1093/emboj/19.5.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prosperi-Meys C, Llabres G, de Seny D, Soto R P, Valladares M H, Laraki N, Frère J M, Galleni M. Interaction between class B β-lactamases and suicide substrates of active-site serine β-lactamases. FEBS Lett. 1999;443:109–111. doi: 10.1016/s0014-5793(98)01689-5. [DOI] [PubMed] [Google Scholar]
- 24.Raquet X, Lamotte-Brasseur J, Fonzé E, Goussard S, Courvalin P, Frère J M. TEM β-lactamase mutants hydrolysing third-generation cephalosporins. A kinetic and molecular modelling analysis. J Mol Biol. 1994;244:625–639. doi: 10.1006/jmbi.1994.1756. [DOI] [PubMed] [Google Scholar]
- 25.Skerra A, Plückthun A. Assembly of functional immunoglobulin Fv fragments in Escherichia coli. Science. 1988;240:1038–1041. doi: 10.1126/science.3285470. [DOI] [PubMed] [Google Scholar]
- 26.Strynadka N C, Jensen S E, Johns K, Blanchard H, Page M, Matagne A, Frère J M, James M N G. Structural and kinetic characterization of a β-lactamase-inhibitor protein. Nature. 1994;368:657–660. doi: 10.1038/368657a0. [DOI] [PubMed] [Google Scholar]
- 27.Strynadka N C, Jensen S E, Alzari P M, James M N. A potent new mode of beta-lactamase inhibition revealed by the 1.7 Å X-ray crystallographic structure of the TEM-1-BLIP complex. Nat Struct Biol. 1996;3:290–297. doi: 10.1038/nsb0396-290. [DOI] [PubMed] [Google Scholar]
- 28.Transue T R, De Genst E, Ghahroudi M A, Wyns L, Muyldermans S. Camel single-domain antibody inhibits enzyme by mimicking carbohydrate substrate. Proteins Struct Funct Genet. 1998;32:515–522. doi: 10.1002/(sici)1097-0134(19980901)32:4<515::aid-prot9>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 29.Van Regenmortel M H, Muller S. D-peptides as immunogens and diagnostic reagents. Curr Opin Biotechnol. 1998;9:377–382. doi: 10.1016/s0958-1669(98)80011-6. [DOI] [PubMed] [Google Scholar]
- 30.Vu K B, Ghahroudi M A, Wyns L, Muyldermans S. Comparison of llama VH sequences from conventional and heavy chain antibodies. Mol Immunol. 1997;34:1121–1131. doi: 10.1016/s0161-5890(97)00146-6. [DOI] [PubMed] [Google Scholar]