Key Points
Question
What is the recommended dose of difelikefalin, a κ-opioid receptor agonist, in Japanese patients with moderate to severe pruritus receiving hemodialysis?
Findings
In this phase 2 randomized clinical trial of 247 patients, 8-week treatment with difelikefalin (0.5 or 1.0 μg/kg) significantly reduced pruritus according to the Numerical Rating Scale score for itch without any safety concerns.
Meaning
Difelikefalin at a dose of 0.5 μg/kg is expected to be a new option with high adherence, safety, and tolerability in the treatment of moderate to severe pruritus for patients receiving hemodialysis.
This randomized clinical trial evaluates the efficacy, dose response, safety, and pharmacokinetics of 3 different doses of difelikefalin compared with placebo for the treatment of moderate to severe pruritus among Japanese patients receiving hemodialysis.
Abstract
Importance
Patients with pruritus receiving hemodialysis frequently experience oppressive physical and psychiatric symptoms that directly affect their quality of life and increase mortality. However, treatment options are limited.
Objective
To determine the clinically recommended dose of difelikefalin, a κ-opioid receptor agonist, based on the efficacy, dose response, safety, and pharmacokinetics.
Design, Setting, and Participants
This randomized, double-blind, placebo-controlled, 4-arm phase 2 trial was conducted from February 1, 2019, to October 22, 2019, at 94 sites in Japan. Patients with moderate to severe pruritus receiving hemodialysis were enrolled.
Interventions
Difelikefalin (0.25, 0.5, and 1.0 μg/kg) and placebo were intravenously administered 3 times a week at the end of each hemodialysis session for 8 weeks.
Main Outcome and Measures
The primary end point was the change from baseline in the weekly mean Worst Itching Intensity Numerical Rating Scale (NRS) score at week 8. Secondary outcomes measured changes in itch-related quality-of-life score using the Skindex-16 and 5-D itch scale. Safety was assessed according to adverse events, laboratory tests, vital signs, body weight, and 12-lead electrocardiogram.
Results
A total of 247 Japanese patients (186 male [75%]; mean [SD] age, 64.5 [11.7] years) were randomized to placebo (n = 63), 0.25 μg/kg of difelikefalin (n = 61), 0.5 μg/kg of difelikefalin (n = 61), or 1.0 μg/kg of difelikefalin (n = 62). The changes from baseline in the adjusted mean (SE) of the 24-hour Worst Itching Intensity NRS score at week 8 were −2.86 (0.29) in the placebo group, −2.97 (0.29) in the 0.25 μg/kg of difelikefalin group, −3.65 (0.30) in the 0.5 μg/kg of difelikefalin group, and −3.64 (0.30) in the 1.0 μg/kg of difelikefalin group. Significant differences were found in the 0.5 μg/kg of difelikefalin group (adjusted mean difference, −0.80; 95% CI, −1.55 to −0.04; P = .04) and the 1.0 μg/kg of difelikefalin group (adjusted mean difference, −0.78; 95% CI, −1.54 to −0.03; P = .04) compared with placebo. The Skindex-16 overall score and 5-D itch scale total score indicated an improvement with treatment with 0.5 and 1.0 μg/kg of difelikefalin (adjusted weekly mean [SE] Skindex-16 overall score at week 8, −27.79 [2.05]; 95% CI, −31.83 to −23.74 for 0.5 μg/kg of difelikefalin and −22.69 [2.04]; 95% CI, −26.71 to −18.68 for 1.0 μg/kg of difelikefalin; adjusted weekly mean [SE] 5-D itch scale total score at week 8, −6.5 [0.4]; 95% CI, −7.2 to −5.8 for 0.5 μg/kg of difelikefalin and −6.8 [0.3]; 95% CI, −7.5 to −6.2 for 1.0 μg/kg of difelikefalin). The incidence of adverse events was 67% (42 of 63 patients) in the placebo group, 72% (44 of 61 patients) in the 0.25 μg/kg of difelikefalin group, 77% (47 of 61 patients) in the 0.5 μg/kg of difelikefalin group, and 85% (53 of 62 patients) in the 1.0 μg/kg of difelikefalin group. No dependency was reported.
Conclusions and Relevance
The findings of this phase 2 randomized clinical trial of difelikefalin suggest that 0.5 μg/kg of difelikefalin should be the clinically recommended dose as a new option for treating moderate to severe pruritus in patients undergoing hemodialysis because of its efficacy, acceptable tolerability, and manageable safety profile.
Trial Registration
ClinicalTrials.gov Identifier: NCT03802617
Introduction
Uremic pruritus is an intractable symptom in patients with end-stage kidney disease (ESKD) undergoing maintenance dialysis.1,2 It is directly associated with reduced quality of life (QoL), depression, poor sleep quality, and increased mortality.3,4,5,6 For the treatment of pruritus in patients receiving dialysis, antihistamines, antiallergic agents, topical moisturizers, and corticosteroids are commonly used.7 Despite the use of these multiple antipruritic medications, approximately 40% of patients receiving hemodialysis still have moderate to severe pruritus.7 In Japan, nalfurafine, an oral κ-opioid receptor (KOR) agonist, has been approved for the treatment of moderate to severe pruritus.8,9,10 However, the oral formulation is problematic for patients receiving dialysis who are already taking many other oral medications for the management of various complications associated with ESKD. Decreased salivary secretion and/or restricted fluid intake in patients receiving hemodialysis may make it more difficult to take oral medications, such as nalfurafine.11 Frequent adverse effects of nalfurafine include insomnia and constipation caused by its activation of KOR in the central nervous system (CNS) and digestive tract.12 Therefore, an alternative treatment option is warranted.
Difelikefalin is a selective KOR agonist, and because of its low membrane permeability and limited transfer to the CNS, it can be expected to have a more favorable safety profile and better tolerability than other KOR agonists.13 Because it is an intravenous formulation, it can be administered directly into the dialysis circuit at the end of the dialysis session under the supervision of a nephrologist; therefore, adherence will be high. The efficacy of difelikefalin has recently been reported in a clinical trial from the US14 of patients with moderate to severe pruritus receiving hemodialysis. However, no clear dose response in relation to efficacy was observed with difelikefalin (0.5, 1.0, and 1.5 μg/kg) in the previous phase 2 trial in the US.15 Factors such as race, age, or physique may not affect the efficacy and safety of difelikefalin. However, in Japan, the dialysis environment is different from that in Western countries; for example, hemodiafiltration is rather more common than hemodialysis, and the clearance of urea multiplied by dialysis duration and normalized for urea distribution volume is lower than that in Western countries.5 Furthermore, nalfurafine is used clinically only in Japan. To determine the clinically recommended dose in Japanese patients with pruritus receiving hemodialysis, we conducted a phase 2 trial to evaluate the efficacy, dose response, safety, and pharmacokinetics of difelikefalin (0.25, 0.5, and 1.0 μg/kg).
Methods
Study Design
This was a multicenter, randomized, double-blind, placebo-controlled, 4-arm, parallel-group phase 2 trial of intravenous difelikefalin in patients with pruritus receiving hemodialysis. The trial was conducted at 94 sites in Japan from February 1, 2019, to October 22, 2019. The trial consisted of a 2-week screening, 8-week double-blind treatment, and 2-week follow-up periods. The trial protocol and the informed consent form were approved by the institutional review board at each participating trial site. The trial protocol and statistical analysis plan can be found in Supplement 1. All patients gave written informed consent before initiation of any study-specific procedures. The trial was conducted in accordance with the ethical principles originating in or derived from the Declaration of Helsinki16 and Good Clinical Practice guidelines. The study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Patients
Japanese male or female patients with ESKD and moderate to severe pruritus who were 20 years or older and undergoing maintenance hemodialysis 3 times a week for at least 12 weeks were enrolled in this trial. Moderate to severe pruritus was defined as a weekly mean score of more than 4 points on the 24-hour Worst Itching Intensity Numerical Rating Scale (NRS).14 The other inclusion criteria were nonresponse to systemic treatment (antihistamines and/or antiallergic drugs) and/or topical antipruritic moisturizers and a moderate or severe baseline Shiratori severity score for 2 days or more in the 7 days preceding the start of treatment. Patients with or without a history of treatment with nalfurafine could be enrolled. Key exclusion criteria were patients having pruritus not associated with chronic kidney disease; those whose condition was complicated by liver cirrhosis; history of phototherapy; history of adverse events (AEs) attributable to nalfurafine; and history of drug hypersensitivity to opioids. Inclusion and exclusion criteria are described in detail in the trial protocol.
Randomization and Intervention
Eligible individuals were randomized in a 1:1:1:1 ratio by a dynamic allocation method to 1 of the following 4 treatment groups: placebo, 0.25 μg/kg of difelikefalin, 0.5 μg/kg of difelikefalin, or 1.0 μg/kg of difelikefalin. The randomization used 2 stratification factors: prior treatment with nalfurafine (use vs no use) and CNS-specific signs or symptoms during the screening period (yes vs no). No information was available on whether previous use of nalfurafine affected the efficacy and safety of difelikefalin; therefore, prior treatment with nalfurafine was set as a stratification factor.
The randomization sequence was generated independently by the trial sponsors. The investigators, patients, and sponsor were masked to treatment assignment throughout the trial. The study drug was administered intravenously at the end of each hemodialysis session 3 times a week for 8 weeks (total of 24 administrations). The administration volume was determined according to the dry weight (eTable 1 in Supplement 2).17
Patients taking nalfurafine underwent a washout period of at least 2 weeks before the screening period. Throughout the trial, nalfurafine, opioids, and phototherapy were prohibited. The use of antipruritus agents, including oral antihistamines and antiallergic agents, topical moisturizers, and corticosteroids, was allowed; however, any change to the regimen of these drugs was prohibited for the duration of the study.
Primary and Secondary Outcomes
The primary end point was the change from baseline in the weekly mean NRS score at week 8. Secondary end points were the proportions of 3-point and 4-point improvement in the weekly mean NRS score at week 8; the change from baseline in the weekly mean Shiratori severity score; the change from baseline in the Skindex-16 overall score at weeks 4 and 8; the change from baseline in the 5-D itch scale total score at weeks 4 and 8; and Patient Global Impression of Change (PGIC) at the end of the treatment period.
Participants were instructed on how to complete an electronic symptom diary, including NRS score and Shiratori severity score, with entries being made from the day after the start of the screening period to the end of the follow-up period. Their NRS score for the worst itching during the preceding 24 hours was recorded in the diary. The NRS is a validated 11-point scale that ranges from 0 to 10, where 0 represents no itching and 10 represents worst itch intensity.6 For the Shiratori severity score, participants recorded the worst itching score during the day and at night during the preceding 24 hours according to a pruritus severity scale from 0 to 4, where 0 represents no symptom and 4 represents severe itching.18 Participants completed itch-related QoL questionnaires at weeks 0, 4, and 8: the Skindex-16 scale, which assesses itch in the preceding week on a scale ranging from 0 to 6, where 0 represents never bothered and 6 represents always bothered19; and the 5-D itch scale, which assesses 5 domains of itch and its impact (duration, degree, direction, body distribution of itch, and disability caused by itch) in the preceding 2 weeks.20 The PGIC assesses the patient’s overall impression of changes in itch at the end of treatment, classified into very much improved, much improved, minimally improved, no change, minimally worse, much worse, and very much worse.21
Safety
Safety was assessed according to AEs, clinical laboratory tests, vital signs, body weight, and 12-lead electrocardiogram. The investigators scored dependency using a dependency questionnaire22 on a 4-level scale (remarkable, moderate, slight, or none) at the end of treatment, and the Dependency Assessment Committee assessed the level of dependency.
Pharmacokinetics
Plasma concentrations of difelikefalin were measured before the first dialysis in weeks 1, 2, 4, 7, and 8; 5 minutes after dosing at weeks 1, 4, and 7; before the second dialysis at weeks 1, 4, and 7; and 1 hour after dosing in week 7. Plasma concentrations of difelikefalin were determined at the bioanalytical laboratory using a liquid chromatography–tandem mass spectrometry method.
Statistical Analysis
A sample size of 60 participants per group (total of 240 participants) was established based on the results of the phase 2 trial in non-Japanese patients with pruritus undergoing hemodialysis.15 We assumed that the mean difference in NRS score between the placebo and each of the treatment groups were −0.6 in the 0.25 μg/kg of difelikefalin group, −1.3 in the 0.5 μg/kg of difelikefalin group, and −1.3 in the 1.0 μg/kg of difelikefalin group with a common SD of 2.5. A sample size of 60 participants per group provided at least 80% power to demonstrate the superiority of 0.5 or 1.0 μg/kg of difelikefalin compared with placebo, with a 2-sided significance level of P < .05.
Efficacy was analyzed according to the intent-to-treat principle in the full analysis set and secondarily in the per protocol set. Safety analysis was performed for the safety set. All missing data were handled as missing values, and no imputation by statistical methods was performed. The primary analysis was performed using a mixed-effects model for repeated measures (MMRM) with change from baseline in the mean efficacy variable at each time point as an objective variable; treatment group, time point, and treatment group by time point interaction as fixed effects; and baseline mean NRS score and dynamic allocation factors as covariates were included in the analysis. Estimation was calculated using a restricted maximum likelihood method. An unstructured covariance structure was used to estimate error variance. The adjusted mean change at each time point for each group and its 2-sided 95% CI were presented. The adjusted mean difference between the placebo and difelikefalin (0.25, 0.5, and 1.0 μg/kg) groups in the mean change at each time point, its 2-sided 95% CI, and the P value are presented. For the primary end point and the change from baseline in the weekly mean NRS score at week 2, 6 contrast tests were used to determine the dose-response relationship. Sensitivity analyses were performed using multiple imputation MMRM and placebo multiple imputation MMRM methods. Summary statistics of the change from baseline in the mean efficacy variables at each time point were calculated in each group. The Fisher exact test was used to compare the proportions of patients who made 3-point and 4-point improvements in the weekly mean NRS score between the placebo and each of the difelikefalin (0.25, 0.5, and 1.0 μg/kg) groups. For other efficacy end points, an MMRM was used for comparisons between the placebo and each of the difelikefalin groups according to the method used for the primary end point. Subgroup analysis of prior treatment with or without nalfurafine was performed for primary analysis. For PGIC analysis, the number (percentage) of participants was presented, and a 2-sample Wilcoxon test was used for comparisons between the groups. For plasma concentrations of difelikefalin, summary statistics, geometric means, and geometric coefficient variation were presented. The AEs were coded using the Medical Dictionary for Regulatory Activities Japanese Edition, version 21.1 (International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use). All analyses were performed using SAS software, version 9.4 for Windows (SAS Institute Inc).
Results
Patients
Among a total of 311 Japanese patients who provided informed consent, 247 (186 male [75%] and 61 female [25%]; mean [SD] age, 64.5 [11.7] years) were randomized and received at least 1 dose of the study drug: placebo (n = 63), 0.25 μg/kg of difelikefalin (n = 61), 0.5 μg/kg of difelikefalin (n = 61), and 1.0 μg/kg of difelikefalin (n = 62) (Figure 1). A total of 225 patients completed the study treatment: 59 in the placebo group, 59 in the 0.25 μg/kg of difelikefalin group, 53 in the 0.5 μg/kg of difelikefalin group, and 54 in the 1.0 μg/kg of difelikefalin group. Baseline characteristics in the full analysis set were similar across treatment groups (Table 1). The treatment adherence in all groups was more than 98%.
Figure 1. Enrollment, Randomization, and Treatment Assignment.
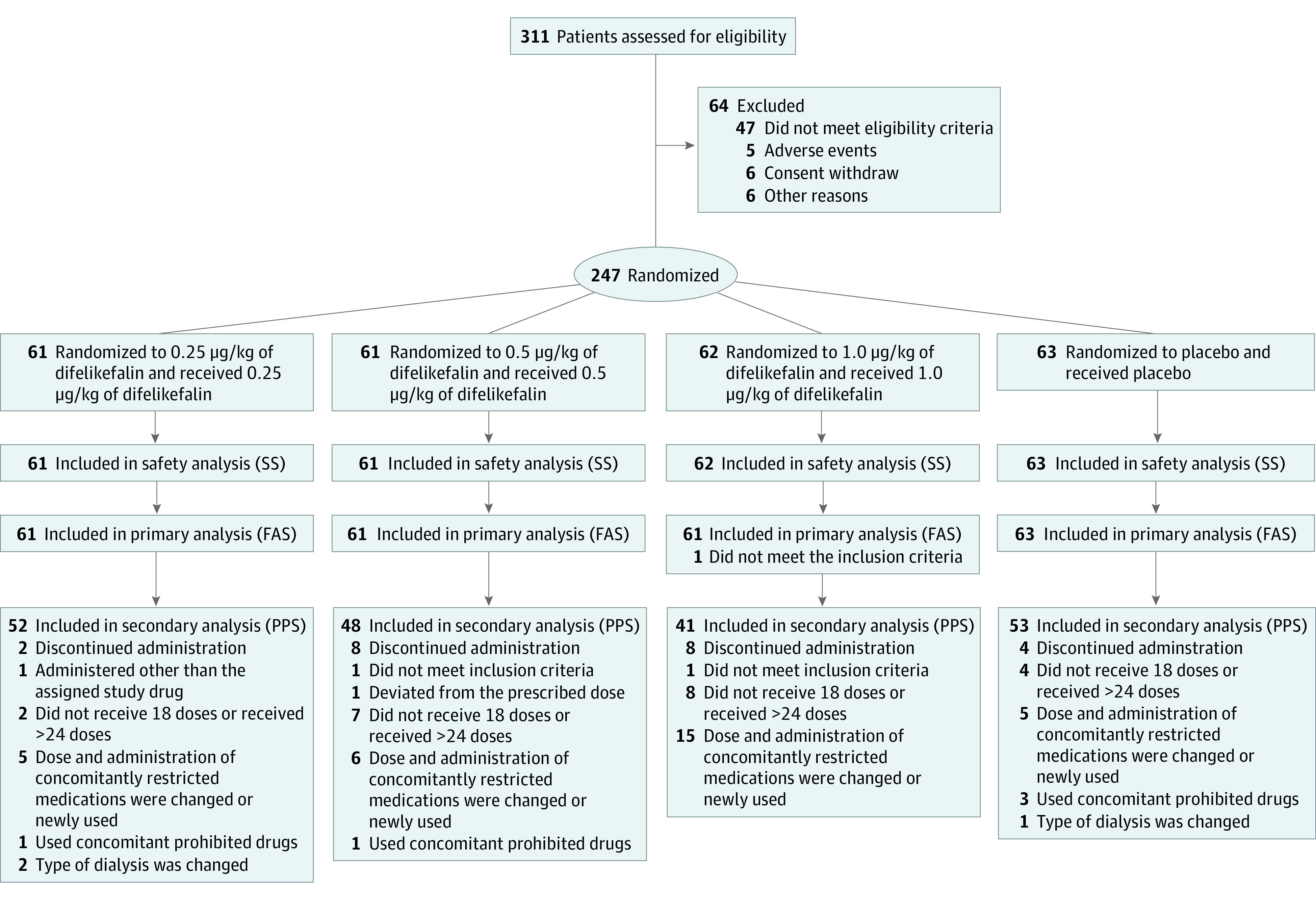
FAS indicates full analysis set; PPS, per protocol set; SS, safety set.
Table 1. Baseline Demographics and Clinical Characteristics (Full Analysis Set).
| Characteristic | Difelikefalin | Placebo (n = 63) | ||
|---|---|---|---|---|
| 0.25 μg/kg (n = 61) | 0.5 μg/kg (n = 61) | 1.0 μg/kg (n = 61) | ||
| Sex, No. (%) | ||||
| Male | 50 (82) | 45 (74) | 47 (77) | 43 (68) |
| Female | 11 (18) | 16 (26) | 14 (23) | 20 (32) |
| Age, mean (SD), y | 64.2 (11.2) | 65.6 (11.4) | 64.4 (11.7) | 64.1 (12.7) |
| Dry weight, mean (SD), kg | 61.25 (13.86) | 59.98 (11.22) | 62.85 (13.39) | 60.63 (12.71) |
| Primary disease-caused ESKD (overlapping), No. | ||||
| Diabetic nephropathy | 28 | 32 | 32 | 27 |
| Glomerulonephritis chronic | 12 | 11 | 13 | 10 |
| Nephrosclerosis | 8 | 14 | 7 | 10 |
| Polycystic kidney | 2 | 0 | 4 | 3 |
| Other | 5 | 4 | 3 | 8 |
| Unspecified | 8 | 1 | 3 | 8 |
| Type of dialysis, No. (%) | ||||
| Hemodialysis | 23 (38) | 27 (44) | 30 (49) | 24 (38) |
| Off-line hemodiafiltration | 1 (2) | 2 (3) | 1 (2) | 1 (2) |
| Online hemodiafiltration | 29 (48) | 25 (41) | 22 (36) | 30 (48) |
| Intermittent infusion hemodiafiltration | 8 (13) | 7 (11) | 8 (13) | 8 (13) |
| Duration of dialysis, mean (SD), y | 7.0 (6.5) | 6.7 (7.2) | 7.7 (6.5) | 6.8 (6.1) |
| Single-pool Kt/V, mean (SD) | 1.435 (0.267) | 1.511 (0.309) | 1.516 (0.415) | 1.498 (0.343) |
| Urea reduction ratio, mean (SD), % | 68.8 (7.2) | 70.2 (6.6) | 70.6 (8.4) | 70.2 (7.6) |
| Disease duration of itch, mean (SD), y | 3.7 (3.5) | 4.5 (4.4) | 4.8 (4.9) | 4.3 (4.4) |
| Prior treatment with nalfurafine, No. (%) | 30 (49) | 30 (49) | 33 (54) | 34 (54) |
| Concomitant antipruritus agents, No. (%) | ||||
| Corticosteroids | 24 (39) | 27 (44) | 23 (38) | 21 (33) |
| Antihistamines | 47 (77) | 46 (75) | 46 (75) | 51 (81) |
| Moisturizers | 40 (66) | 38 (62) | 38 (62) | 33 (52) |
| Others | 19 (31) | 13 (21) | 19 (31) | 19 (30) |
| Specific signs or symptoms at screening, No. (%) | 5 (8) | 5 (8) | 6 (10) | 7 (11) |
| Weekly NRS score, mean (SD) | 6.35 (1.24) | 6.83 (1.40) | 6.47 (1.29) | 6.53 (1.31) |
Abbreviations: ESKD, end-stage kidney disease; Kt/V, clearance of urea multiplied by dialysis duration and normalized for urea distribution volume; NRS, Numerical Rating Scale.
Primary and Secondary Outcomes
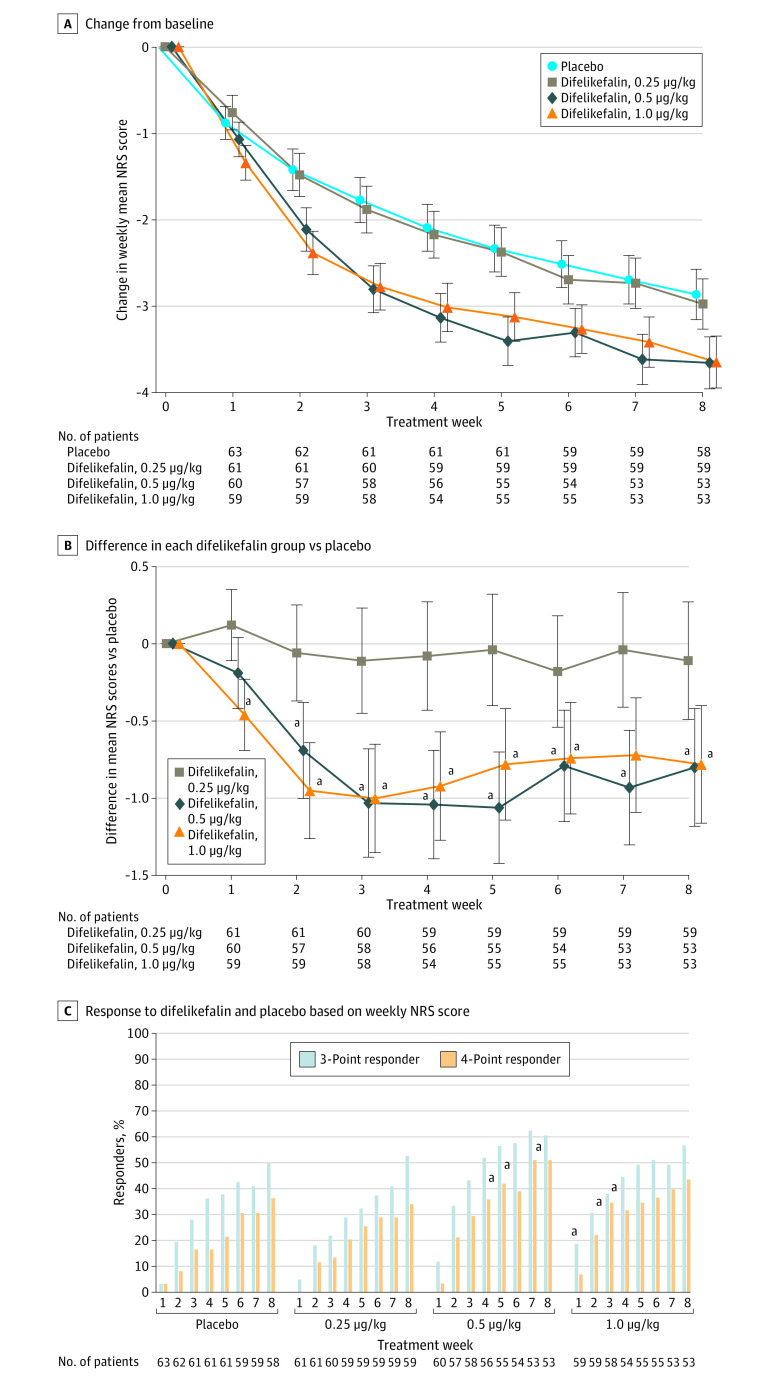
For the primary end point, the change from baseline in the weekly mean NRS score (adjusted mean [SE] change) at week 8 was −2.86 (0.29) in the placebo group, −2.97 (0.29) in the 0.25 μg/kg of difelikefalin group, −3.65 (0.30) in the 0.5 μg/kg of difelikefalin group, and −3.64 (0.30) in the 1.0 μg/kg of difelikefalin group. Compared with placebo, significant differences were found for 0.5 μg/kg of difelikefalin (adjusted mean difference, −0.80; 95% CI, −1.55 to −0.04; P = .04) and 1.0 μg/kg of difelikefalin (adjusted mean difference, −0.78; 95% CI, −1.54 to −0.03; P = .04). Similar results were obtained in the sensitivity analyses (eTable 2 in Supplement 2). Significant reductions in weekly mean NRS scores were observed throughout the study in the 0.5- and 1.0-μg/kg of difelikefalin groups compared with placebo (Figure 2A). The differences in the change from baseline in the weekly mean NRS score at week 2 were −0.06 (0.31) for the 0.25 μg/kg of difelikefalin group, −0.69 (0.31) for the 0.5 μg/kg of difelikefalin group, and −0.95 (0.31) for the 1.0 μg/kg of difelikefalin group, and those at week 8 were −0.11 (0.38) for the 0.25 μg/kg of difelikefalin group, −0.80 (0.38) for the 0.5 μg/kg of difelikefalin group, and −0.78 (0.38) for the 1.0 μg/kg of difelikefalin group (Figure 2B). The most applicable contrast pattern of the dose response was 1 for the placebo group, 1 for the 0.25 μg/kg of difelikefalin group, −1 for the 0.5 μg/kg of difelikefalin group, and −1 for the 1.0 μg/kg of difelikefalin group (P < .001 at week 2 and P = .007 at week 8), indicating that the effect of difelikefalin was consistent at 0.5 μg/kg or more of difelikefalin. In a subgroup analysis by prior treatment with and without nalfurafine, results were consistent with the primary end point (eTable 3 in Supplement 2). The proportions of 3-point improvement in the NRS score at week 8 were 50% (n = 29 of 58) in the placebo group, 53% (n = 31 of 59) in the 0.25 μg/kg of difelikefalin group, 60% (n = 32 of 53) in the 0.5 μg/kg of difelikefalin group, and 57% (n = 30 of 53) in the 1.0 μg/kg of difelikefalin group, and the 4-point improvements were 36% (n = 21 of 58) in the placebo group, 34% (n = 20 of 59) in the 0.25 μg/kg of difelikefalin group, 51% (n = 27 of 53) in the 0.5 μg/kg of difelikefalin group, and 43% (n = 23 of 53) in the 1.0 μg/kg of difelikefalin group (Figure 2C). Both the 0.5- and 1.0-μg/kg doses of difelikefalin significantly reduced Shiratori severity score values from baseline to week 8 (adjusted mean difference, −0.31; 95% CI, −0.60 to −0.02; P = .04 for 0.5 μg/kg of difelikefalin and −0.31; 95% CI, −0.60 to −0.02; P = .03 for 1.0 μg/kg of difelikefalin) (eFigure in Supplement 2). The Skindex-16 overall score and 5-D itch scale total score indicated an improvement with treatment with 0.5 and 1.0 μg/kg of difelikefalin (adjusted weekly mean [SE] Skindex-16 overall score at week 8, −27.79 [2.05]; 95% CI, −31.83 to −23.74 for 0.5 μg/kg of difelikefalin and −22.69 [2.04]; 95% CI, −26.71 to −18.68 for 1.0 μg/kg of difelikefalin; adjusted weekly mean [SE] 5-D itch scale total score at week 8, −6.5 [0.4]; 95% CI, −7.2 to −5.8 for 0.5 μg/kg of difelikefalin and −6.8 [0.3]; 95% CI, −7.5 to −6.2 for 1.0 μg/kg of difelikefalin) (eTable 4 in Supplement 2). The PGIC ratings in the 0.5 and 1.0 μg/kg groups showed an improvement over the placebo group (22 patients [37.3%] in the 0.5 μg/kg of difelikefalin group and 19 patients [31.7%] in the 1.0 μg/kg of difelikefalin group were very much improved) (eTable 5 in Supplement 2).
Figure 2. Course of the Primary Outcomes During 8-Week Treatment Period.
A and B, A mixed-effects model for repeated measures was used to compare the difelikefalin (0.25, 0.5, and 1.0 μg/kg) and placebo groups. C, A comparison between the difelikefalin (0.25, 0.5, and 1.0 μg/kg) and placebo groups was performed using the Fisher exact test. Error bars indicate SEs. NRS indicates Numerical Rating Scale.
aP < .05.
Safety
The incidences of AEs during the treatment period were 67% (42 of 63 patients) in the placebo group, 72% (44 of 61 patients) in the 0.25 μg/kg of difelikefalin group, 77% (47 of 61 patients) in the 0.5 μg/kg of difelikefalin group, and 85% (53 of 62 patients) in the 1.0 μg/kg of difelikefalin group. The incidence of AEs increased in a dose-dependent manner, and the incidence was significantly higher in the 1.0 μg/kg of difelikefalin group than in the placebo group (53 [85%] vs 42 [67%]; P = .02) (Table 2).
Table 2. Summary of Adverse Events (Safety Set).
| Event | No. (%) | |||
|---|---|---|---|---|
| Difelikefalin | Placebo (n = 63) | |||
| 0.25 μg/kg (n = 61) | 0.5 μg/kg (n = 61) | 1.0 μg/kg (n = 62) | ||
| Adverse events | 44 (72) | 47 (77) | 53 (85) | 42 (67) |
| Adverse drug reactions | 9 (15) | 9 (15) | 17 (27) | 7 (11) |
| Death | 0 | 0 | 0 | 0 |
| Other serious adverse events | 3 (5) | 8 (13) | 5 (8) | 2 (3) |
| Other serious adverse drug reactions | 0 | 1 (2) | 1 (2) | 0 |
| Adverse events leading to | ||||
| Discontinuation | 0 | 4 (7) | 5 (8) | 1 (2) |
| Interruption | 0 | 5 (8) | 5 (8) | 2 (3) |
| Most frequently reported adverse events (≥5% in any group) | ||||
| Nasopharyngitis | 8 (13) | 6 (10) | 3 (5) | 7 (11) |
| Somnolence | 2 (3) | 3 (5) | 6 (10) | 3 (5) |
| Dizziness | 3 (5) | 3 (5) | 5 (8) | 3 (5) |
| Constipation | 5 (8) | 3 (5) | 7 (11) | 0 |
| Vomiting | 1 (2) | 1 (2) | 4 (6) | 3 (5) |
| Nausea | 0 | 1 (2) | 4 (6) | 1 (2) |
| Arthralgia | 3 (5) | 5 (8) | 2 (3) | 2 (3) |
| Malaise | 2 (3) | 2 (3) | 4 (6) | 0 |
| Pyrexia | 2 (3) | 4 (7) | 0 | 0 |
| Blood pressure decreased | 0 | 4 (7) | 3 (5) | 2 (3) |
| Procedural hypotension | 4 (7) | 3 (5) | 2 (3) | 3 (5) |
Central nervous system AEs, such as somnolence and dizziness, were more frequent in the 1.0 μg/kg of difelikefalin group; however, the incidence of these AEs in the 0.25- and 0.5-μg/kg groups was comparable to placebo. Most AEs were mild and occurred relatively early in treatment, and patients improved or recovered without discontinued or suspended use of the study drug.
No deaths were reported. Other serious AEs occurred in 3 patients (5%) in the 0.25 μg/kg of difelikefalin group, 8 patients (13%) in the 0.5 μg/kg of difelikefalin group, and 5 patients (8%) in the 1.0 μg/kg of difelikefalin group. Altered state of consciousness in the 0.5 μg/kg of difelikefalin group and obstruction in the small intestine in the 1.0 μg/kg of difelikefalin group were reported as drug-related serious AEs, but all of those patients recovered after discontinuing or suspending use of the study drug. No notable changes in clinical laboratory tests, vital signs, and 12-lead electrocardiogram were observed. The Dependency Assessment Committee reviewed all study participants and concluded that dependency on difelikefalin did not develop.
Pharmacokinetics
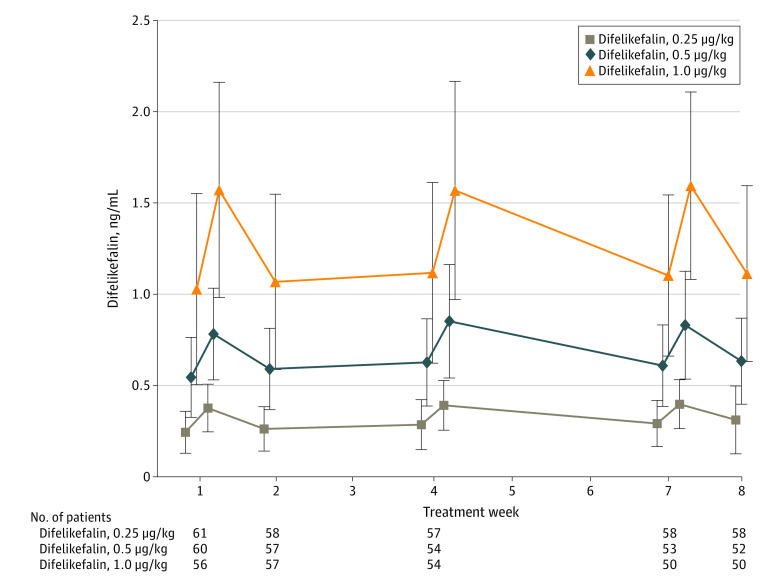
A dose-dependent increase in plasma difelikefalin concentration was observed 5 minutes after administration, before the second or first dialysis sessions of the week commenced (Figure 3). No significant differences in plasma difelikefalin concentrations were observed in relation to difference in body weight classification at weeks 1, 4, and 7.
Figure 3. Mean (SD) Plasma Concentrations of Difelikefalin .
Plasma concentrations of difelikefalin (0.25, 0.5, and 1.0 μg/kg) were measured before the first dialysis session at weeks 1, 2, 4, 7, and 8 and before the second dialysis session at weeks 1, 4, and 7. Error bars indicate SDs.
Discussion
This randomized, double-blind, placebo-controlled, parallel trial demonstrated that, compared with placebo, 0.5 and 1.0 μg/kg of difelikefalin were effective in treating moderate to severe pruritus in patients receiving hemodialysis when intravenously administered 3 times a week at the end of each dialysis session during a period of 8 weeks. The efficacy of difelikefalin was confirmed by the NRS score, Shiratori severity score, and itch-related QoL. To our knowledge, this was the first report to evaluate the efficacy of 0.25 μg/kg of difelikefalin as the lowest dose. Dose-response analysis showed clearly that the response to difelikefalin was inadequate at 0.25 μg/kg and that a dose of at least 0.5 μg/kg is required.
In terms of safety, the incidence of AEs increased in a dose-dependent manner. Most AEs were mild and occurred relatively early in treatment. Difelikefalin was well tolerated up to a dose of 1.0 μg/kg. Central nervous system AEs, such as somnolence and dizziness, were more frequent in the 1.0-μg/kg group; however, the incidence of these AEs was comparable across the 0.25-μg/kg, 0.5-μg/kg, and placebo groups. The current efficacy and safety data indicate that a dose of 0.5 μg/kg should be the clinically recommended dose of difelikefalin for Japanese patients receiving dialysis. This finding is in accordance with those of previous clinical studies conducted in the US14,15 and indicates that the typical dialysis conditions in Japan did not affect the efficacy of difelikefalin, just as other factors, such as race, age, and physique, also have no effect.
Because the access of difelikefalin to the CNS is limited, it selectively activates KORs distributed in peripheral and immune cells.13 Discomfort and hallucinations, which are frequently reported as AEs associated with centrally acting KOR agonists, were not observed.23,24,25 In addition, the incidence of insomnia and constipation in patients taking difelikefalin was absent or lower than that reported for nalfurafine.12 However, CNS-related AEs potentially associated with difelikefalin should be considered based on a report of altered states of consciousness in the 0.5 μg/kg of difelikefalin group. Low adherence to oral drugs, in part because of polypharmacy, contributes to a reduction in treatment efficacy in patients receiving hemodialysis.26 This reduction in efficacy is not a concern with difelikefalin because it is administered intravenously into the dialysis circuit during routine dialysis. Furthermore, 0.5 μg/kg of difelikefalin was effective in patients regardless of prior treatment with nalfurafine.
Limitations
This trial has some limitations. First, the placebo effect was relatively large because subjective symptoms of itching are evaluated based solely on patient-reported outcomes. In addition, depression and anxiety might be involved in the placebo effect, but we did not evaluate these variables in the current study. However, the efficacy of 0.5 and 1.0 μg/kg of difelikefalin was consistently observed by the Shiratori severity scores and QoL assessments, such as the 5-D itch scale, Skindex-16, and PGIC, suggesting that a change of 0.8 in NRS score is clinically meaningful. Second, objective end points, such as skin findings, were not evaluated. Third, this trial was conducted in a small number of individuals for a short duration. To fully evaluate the efficacy, safety, and drug dependence in long-term use, a phase 3, long-term (52 weeks) study of 0.5 μg/kg of difelikefalin in Japanese patients is currently under way.
Conclusions
This phase 2 trial in Japanese patients with pruritus receiving hemodialysis demonstrated that, compared with placebo, 0.5 μg/kg of difelikefalin significantly improved moderate to severe pruritus and this dose can be considered to be the clinically recommended dose. Difelikefalin at a dose of 0.5 μg/kg is expected to be a new option for the treatment of moderate to severe pruritus in patients receiving hemodialysis.
Clinical Study Protocol and Statistical Analysis Plan
eTable 1. Administration Volume
eTable 2. Sensitivity Analyses in the Primary Endpoint
eTable 3. Subgroup Analyses in the Primary Endpoint
eTable 4. Skindex-16 Overall Score and 5-D Itch Scale Total Score at Week 4 and 8
eTable 5. Patient Global Impression of Change at the End of the Treatment Period
eFigure. Time Course of the Adjusted Weekly Mean Change ± Standard Error in the Shiratori Severity Score
Nonauthor Collaborators
Data Sharing Statement
References
- 1.Mettang T, Kremer AE. Uremic pruritus. Kidney Int. 2015;87(4):685-691. doi: 10.1038/ki.2013.454 [DOI] [PubMed] [Google Scholar]
- 2.Combs SA, Teixeira JP, Germain MJ. Pruritus in kidney disease. Semin Nephrol. 2015;35(4):383-391. doi: 10.1016/j.semnephrol.2015.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Narita I, Alchi B, Omori K, et al. Etiology and prognostic significance of severe uremic pruritus in chronic hemodialysis patients. Kidney Int. 2006;69(9):1626-1632. doi: 10.1038/sj.ki.5000251 [DOI] [PubMed] [Google Scholar]
- 4.Kimata N, Fuller DS, Saito A, et al. Pruritus in hemodialysis patients: results from the Japanese Dialysis Outcomes and Practice Patterns Study (JDOPPS). Hemodial Int. 2014;18(3):657-667. doi: 10.1111/hdi.12158 [DOI] [PubMed] [Google Scholar]
- 5.Pisoni RL, Wikström B, Elder SJ, et al. Pruritus in haemodialysis patients: international results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol Dial Transplant. 2006;21(12):3495-3505. doi: 10.1093/ndt/gfl461 [DOI] [PubMed] [Google Scholar]
- 6.Mathur VS, Lindberg J, Germain M, et al. ; ITCH National Registry Investigators . A longitudinal study of uremic pruritus in hemodialysis patients. Clin J Am Soc Nephrol. 2010;5(8):1410-1419. doi: 10.2215/CJN.00100110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rayner HC, Larkina M, Wang M, et al. International comparisons of prevalence, awareness, and treatment of pruritus in people on hemodialysis. Clin J Am Soc Nephrol. 2017;12(12):2000-2007. doi: 10.2215/CJN.03280317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inui S. Nalfurafine hydrochloride to treat pruritus: a review. Clin Cosmet Investig Dermatol. 2015;8:249-255. doi: 10.2147/CCID.S55942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumagai H, Ebata T, Takamori K, Muramatsu T, Nakamoto H, Suzuki H. Effect of a novel kappa-receptor agonist, nalfurafine hydrochloride, on severe itch in 337 haemodialysis patients: a Phase III, randomized, double-blind, placebo-controlled study. Nephrol Dial Transplant. 2010;25(4):1251-1257. doi: 10.1093/ndt/gfp588 [DOI] [PubMed] [Google Scholar]
- 10.Miyamoto Y, Oh T, Aihara E, Ando A. Clinical profiles of nalfurafine hydrochloride for the treatment of pruritus patients. Handb Exp Pharmacol. 2022;271:455-472. doi: 10.1007/164_2020_400 [DOI] [PubMed] [Google Scholar]
- 11.Ghimire S, Castelino RL, Lioufas NM, Peterson GM, Zaidi ST. Nonadherence to medication therapy in haemodialysis patients: a systematic review. PLoS One. 2015;10(12):e0144119. doi: 10.1371/journal.pone.0144119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumagai H, Ebata T, Takamori K, et al. Efficacy and safety of a novel ĸ-agonist for managing intractable pruritus in dialysis patients. Am J Nephrol. 2012;36(2):175-183. doi: 10.1159/000341268 [DOI] [PubMed] [Google Scholar]
- 13.Albert-Vartanian A, Boyd MR, Hall AL, et al. Will peripherally restricted kappa-opioid receptor agonists (pKORAs) relieve pain with less opioid adverse effects and abuse potential? J Clin Pharm Ther. 2016;41(4):371-382. doi: 10.1111/jcpt.12404 [DOI] [PubMed] [Google Scholar]
- 14.Fishbane S, Jamal A, Munera C, Wen W, Menzaghi F; KALM-1 Trial Investigators . A phase 3 trial of difelikefalin in hemodialysis patients with pruritus. N Engl J Med. 2020;382(3):222-232. doi: 10.1056/NEJMoa1912770 [DOI] [PubMed] [Google Scholar]
- 15.Fishbane S, Mathur V, Germain MJ, et al. ; Trial Investigators . Randomized controlled trial of difelikefalin for chronic pruritus in hemodialysis patients. Kidney Int Rep. 2020;5(5):600-610. doi: 10.1016/j.ekir.2020.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 17.Kooman JP, van der Sande FM, Leunissen KM. Wet or dry in dialysis—can new technologies help? Semin Dial. 2009;22(1):9-12. doi: 10.1111/j.1525-139X.2008.00533.x [DOI] [PubMed] [Google Scholar]
- 18.Shiratori A. Therapeutic outcomes of the use of mequitazine (LM-209) in severe dermatological diseases. Nishinihon J Dermatol. 1983;45(3):470-473. doi: 10.2336/nishinihonhifu.45.470 [DOI] [Google Scholar]
- 19.Higaki Y, Kawamoto K, Kamo T, Horikawa N, Kawashima M, Chren MM. The Japanese version of Skindex-16: a brief quality-of-life measure for patients with skin diseases. J Dermatol. 2002;29(11):693-698. doi: 10.1111/j.1346-8138.2002.tb00205.x [DOI] [PubMed] [Google Scholar]
- 20.Takahashi N, Yoshizawa T, Okubo A, et al. Usefulness of the Japanese version of the 5-D itch scale for rating pruritus experienced by patients undergoing hemodialysis. Ren Replace Ther. 2018;4:26. doi: 10.1186/s41100-018-0167-6 [DOI] [Google Scholar]
- 21.Dworkin RH, Turk DC, Farrar JT, et al. ; IMMPACT . Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113(1-2):9-19. doi: 10.1016/j.pain.2004.09.012 [DOI] [PubMed] [Google Scholar]
- 22.Kurihara M, Jimbo M, Hirose T, et al. Double-blind comparison of clinical effects of ID-540 (fludiazepam), diazepam, and placebo on psychoneurotic patients and a tentative draft of dependency questionnaire. Clinical Evaluation. 1977;5(2):341-368. [Google Scholar]
- 23.Roth BL, Baner K, Westkaemper R, et al. Salvinorin A: a potent naturally occurring nonnitrogenous kappa opioid selective agonist. Proc Natl Acad Sci U S A. 2002;99(18):11934-11939. doi: 10.1073/pnas.182234399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chavkin C. The therapeutic potential of κ-opioids for treatment of pain and addiction. Neuropsychopharmacology. 2011;36(1):369-370. doi: 10.1038/npp.2010.137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Butelman ER, Kreek MJ. Salvinorin A, a κ-opioid receptor agonist hallucinogen: pharmacology and potential template for novel pharmacotherapeutic agents in neuropsychiatric disorders. Front Pharmacol. 2015;6:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmid H, Hartmann B, Schiffl H. Adherence to prescribed oral medication in adult patients undergoing chronic hemodialysis: a critical review of the literature. Eur J Med Res. 2009;14(5):185-190. doi: 10.1186/2047-783X-14-5-185 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Clinical Study Protocol and Statistical Analysis Plan
eTable 1. Administration Volume
eTable 2. Sensitivity Analyses in the Primary Endpoint
eTable 3. Subgroup Analyses in the Primary Endpoint
eTable 4. Skindex-16 Overall Score and 5-D Itch Scale Total Score at Week 4 and 8
eTable 5. Patient Global Impression of Change at the End of the Treatment Period
eFigure. Time Course of the Adjusted Weekly Mean Change ± Standard Error in the Shiratori Severity Score
Nonauthor Collaborators
Data Sharing Statement