Abstract
Insulin-like growth factor binding protein 6 (IGFBP6) is a secreted protein with a controversial role in human malignancies, being downregulated in most types of human cancer, but upregulated in selected tumors. Ovarian cancer (OC) is a human malignancy characterized by IGFBP6 downregulation; however, the significance of its low expression during ovarian carcinogenesis is still poorly understood. In the present study, IGFBP6 expression and activation of its associated signaling pathway were evaluated in two matched OC cell lines derived from a high-grade serous OC before and after platinum resistance (PEA1 and PEA2 cells, respectively). A whole genome gene expression analysis was comparatively performed in both cell lines upon IGFBP6 stimulation using Illumina technology. IGFBP6 gene expression data from human OC cases were obtained from public datasets. Gene expression data from public datasets confirmed the downregulation of IGFBP6 in primary and metastatic OC tissues compared with in normal ovarian tissues. The comparative analysis of platinum-sensitive (PEA1) and platinum-resistant (PEA2) cell lines showed quantitative and qualitative differences in the activation of IGFBP6 signaling. Notably, IGFBP6 enhanced ERK1/2 phosphorylation only in PEA1 cells, and induced more evident and significant gene expression reprogramming in PEA1 cells compared with in PEA2 cells. Furthermore, the analysis of selected genes modulated by IGFBP6 (i.e., FOS, JUN, TNF, IL6, IL8 and EGR1) exhibited an inverse regulation in PEA1 versus PEA2 cells. In addition, selected hallmarks (TNFA_signaling_via_NFKB, TGF_beta_signaling, P53_pathway) and IL-6 signaling were positively regulated in PEA1 cells, whereas they were inhibited in PEA2 cells in response to IGFBP6. These data suggested that dysregulation of IGFBP6 signaling may serve a role in the progression of OC, and is likely associated with the development of platinum resistance.
Keywords: ovarian serous carcinoma, IGFBP6, platinum resistance, gene expression, IL6 pathway
Introduction
Ovarian cancer (OC) is the most common and fatal gynecological malignancy worldwide with most of patients diagnosed in locally advanced stage due to lack of symptoms (1). Surgery along with platinum-based chemotherapy is the standard treatment recommended by International Guidelines for patients with advanced stage OC. However, 5-year survival is only about 30-40% in most countries (2). Indeed, while OC is considered a platinum-sensitive tumor with significant and prolonged responses to platinum-based chemotherapy, the occurrence of platinum resistance represents a critical step in OC progression, being the prognosis of platinum-resistant OC extremely unfavorable. Thus, the identification of molecular mechanisms of platinum resistance onset and novel reliable therapeutic targets are needed to improve the OC clinical outcome.
Insulin-like growth factor binding proteins (IGFBPs) are a family of secreted proteins originally characterized as passive high-affinity carriers of two Insulin-like Growth Factors (IGFI and II) in the circulation, composed of 7 members (IGFBP1, 2, 3, 4, 5, 6, 7) (3). Apart from functions within the IGF system, they play various roles in the extracellular and intracellular compartment to modulate cell proliferation and apoptosis and survival (4).
The relationships between IGFBPs and cancer is sometimes contradictory and divergent, according to different tumor types (5). Their role has been investigated in many cancers, which may be especially relevant since IGF-II is frequently an autocrine cancer growth factor (6). In most studies, IGFBP6 expression was lower in malignant than in normal cells, consistent with the idea that it may act as an inhibitor of tumorigenic pathways, including those driven by an excess of IGF-II activity. However, few studies showed IGFBP6 upregulation in cancer cells, which may represent a compensatory response to increased IGF-II activity or may reflect IGF-independent actions. IGFBP6 was detected in 38 of 41 OCs (7), and a microarray study showed that IGFBP6 mRNA levels were lower in OC than in non-cancerous tissue (8). Consistently, IGFBP6 plasma levels have been found downregulated in patients with OC compared to those without cancer. In a biological perspective, recent findings suggest that IGFBP6 may have opposing effects on the migration of two OC cell lines, this further supporting heterogeneous responsiveness of OC cells to IGFBP6 (9). Furthermore, several findings suggest that the IGFs/IGFBPs axis is involved in modulating drug sensitivity in cancer cells (10) and, specifically, IGFPB6 is responsible for the proliferation of chemoresistant tumor cells is human glioblastoma (11).
Based on this evidence, our study evaluated the role of IGFBP6 in two matched cell lines of high-grade serous OC (HGSOC) obtained from the same patient before and after the development of clinical platinum resistance (PEA1 and PEA2 cells, respectively) (12). A comparative gene expression profiling of these cell lines was performed to evaluate, at molecular level, the differential response of OC cell lines to IGFBP6 in two different contexts of OC progression, before and after the onset of platinum resistance.
Materials and methods
Cell lines, chemicals
The human ovarian adenocarcinoma cell lines PEA1 and PEA2 were purchased from European Collection of Authenticated Cell Cultures (ECACC). PEA1 and PEA2 cells were derived from a malignant effusion from the peritoneal ascites of the same patient with a HGSOC. PEA1 cell line was collected prior to treatment with cisplatin and prednimustine; PEA2 were collected on relapse after the treatment (12). Both cell lines were cultured in RPMI 1640 medium (Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum (FBS) (Gibco; Thermo Fisher Scientific, Inc.), 1% glutamine (Gibco; Thermo Fisher Scientific, Inc.) and 1% Pen-Strep (Gibco; Thermo Fisher Scientific, Inc.) and grown at 37°C in a 5% CO2 atmosphere (12). In order to study IGFBP6 signaling, PEA1 and PEA2 cells were cultured overnight in serum-free RPMI 1640 medium and subsequently incubated with IGFBP6 recombinant protein (Recombinant Human IGFBP6, Peprotech, Cat. No: 350-07B) at a final concentration of 1 µg/ml.
Immunoblot analysis
Total cell lysates were obtained by homogenization of cell pellets in cold lysis buffer [20 mmol/l Tris (pH 7.5) containing 300 mmol/l sucrose, 60 mmol/l KC1, 15 mmol/l NaC1, 5% (v/v) glycerol, 2 mmol/l EDTA, 1% (v/v) Triton X100, 1 mmol/l phenylmethylsulfonylfluoride, 2 mg/ml aprotinin, 2 mg/ml leupeptin, and 0.2% (w/v) deoxycholate] for 30 min on ice and centrifuged at 12,000 rpm for 30 min. Protein concentration was quantified using the Bio-Rad protein assay kit (Bio-Rad Laboratories, Cat. No. 5000006), according to the manufacturer's procedures. 30 µg of protein samples were resolved on SDS-PAGE using polyacrylamide 4–20% precast gels (Mini-PROTEAN TGX Stain-Free Gels, Bio-Rad, Cat. No. 456-8094) and then transferred on nitrocellulose membrane (Trans-Blot Turbo Transfer Pack, Bio-Rad, Cat. No. 1704158). The membrane was incubated for 60 min at room temperature (RT) with Western Blocker Solution (Sigma Aldrich, Cat. No. W0138) and immunoblotted with the following antibodies: Mouse monoclonal anti-Phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204) (E10) (Cell Signaling, Cat. No. #9106), rabbit polyclonal anti-MAPkinase ERK1/2 from (Calbiochem, Cat. No. #442704), mouse monoclonal anti-IGFBP6 (Human IGFBP6 antibody, R&D, Cat. No: MAB8761), rabbit monoclonal anti-IL-6 (D3K2N) (Cell Signaling, Cat. No. #12153); mouse monoclonal anti-Phospho-Stat3 (Tyr705) (3E2) (Cell Signaling, Cat. No. #913); rabbit polyclonal anti-Stat3 (C-20) (Santa Cruz Biotechnology, Inc. Cat. No. sc-482); mouse monoclonal anti-GAPDH (Santa Cruz Biotechnology, Inc. Cat. No: Sc-47724); mouse monoclonal anti-β-Actin (Santa Cruz Biotechnology, Inc. Cat. No: Sc-47778). During the study, anti-Stat3 antibody (C-20) has been discontinued and replaced by mouse monoclonal anti-Stat3 antibody (F-2, Santa Cruz Biotechnology, Inc. Cat. No. sc-8019). The expression of specific proteins was detected by a secondary antibody labeled with peroxidase (Bio Rad, Goat Anti Mouse, (H + L)-HRP Conjugate, Cat. No. #170-6516, Goat Anti Rabbit (H + L)-HRP Conjungate, Cat. No. #170-6515) and the Clarity Western ECL Substrate (Bio-Rad Laboratories, Cat. No. 1705061). The differences in protein expression were quantified through densitometric analysis, using the ImageJ software and normalized according to the expression of housekeeping genes.
RNA extraction and reverse transcription-quantitative PCR (RT-qPCR)
Total RNA extraction was performed by Pure Link RNA Mini Kit (Invitrogen, Cat. No. 12183018A). RNA was first quantified through optical density measurements at 260 and 280 nm, and subsequently converted in complementary DNA (cDNA) using a SuperScript Vilo IV (Life Technologies Cat. No 11756050) Reverse Transcriptase, according to the supplier's instructions. One µl of cDNA was amplified by SsoAdvanced Universal SYBR green Supermix (Bio-Rad, Cat. No. 1725271) and Real-Time PCR CFX96 Touch (Bio-Rad). The reaction was conducted according to the following amplification protocol: 95°C for 3 min, 39 cycles at 95°C for 10 sec, 60°C for 30 sec and 65-95°C for 5 sec. β-Actin and α-Tubulin were chosen as internal controls. The results were calculated using the ∆∆CT (where CT is threshold cycle) method (13). Gene expression was analyzed using CFX manager software (Bio-Rad, Hercules, CA). Primers are reported in Table I.
Table I.
Forward and reverse primers used for all reverse transcription-quantitative PCR analyses.
| Gene | Primer | Brand |
|---|---|---|
| c-FOS | F 5′-CAGTTATCTCCAGAAGAAGAAG-3′ | KiCqStart® |
| R 5′-CTTCTAGTTGGTCTGTCTCC-3′ | Sigma-Aldrich | |
| c-JUN | F 5′-AAAGGATAGTGCGATGTTTC-3′ | KiCqStart® |
| R 5′-TAAAATCTGCCACCAATTCC-3′ | Sigma-Aldrich | |
| IL-8 | F 5′-CCAACACAGAAATTATTGTAAAGC-3′ | Invitrogen |
| R 5′-TGAATTCTCAGCCCTCTTCAA-3′ | ||
| TNF-α | F 5′-CTCCAGGCGGTGCTTGTTC-3′ | Invitrogen |
| R 5′-CAGGCAGAAGAGCGTGGTG-3′ | ||
| IL-6 | F 5′-CAGTTGCCTTCTCCCTGGG-3′ | Eurofin MWG |
| R 5′-TGAGTGGCTGTCTGTGTGGG-3′ | Operon | |
| IGFBP-6 | F 5′-GGAAGCTGAGGGCTGTCTC-3′ | Eurofin MWG |
| R 5′-GTCTCTGCGGTTCACATCCT-3′ | Operon | |
| EGR1 | F 5′-GCAGAGTCTTTTCCTGAC-3′ | KiCqStart® |
| R 5′-TTGGTCATGCTCACTAGG-3′ | Sigma-Aldrich | |
| β-ACT | F 5′-GACGACATGGAGAAAATCTG-3′ | KiCqStart® |
| R 5′-ATGATCTGGGTCATCTTCTC-3′ | Sigma-Aldrich | |
| TUBB | F 5′-CTTTGTATTTGGTCAGTCTGG-3′ | KiCqStart® |
| R 5′-ATCTTGCTGATAAGGAGAGTG-3′ | Sigma-Aldrich |
c-FOS, Fos proto-oncogene, AP-1 transcription factor subunit; c-JUN, Jun proto-oncogene, AP-1 transcription factor subunit; IL-8, interleukin-8; TNF-α, tumor necrosis factor-α; IL-6, interleukin-6; IGFBP6, insulin-like growth factor binding protein 6; EGR1, early growth response 1; β-ACT, β-actin; TUBB, tubulin β class I.
Microarray experiments and data analysis
Total RNA from PEA1 and PEA2 cells treated or not with IGFBP6 (1 µg/ml) for 2 h was extracted using TRIzol reagent (Invitrogen) and evaluated for quality and integrity number by the 2100 Bioanalyzer (Agilent Technologies). For each sample, 300 ng of total RNA were reverse transcribed and used for the synthesis of cDNA and biotinylated cRNA according to the protocol of the Illumina TotalPrep RNA amplification kit (Ambion; Life Technologies Cat. No AMIL1791).
A total of 750 ng of each cRNA were hybridized on the Illumina HumanHT12 V4_0_R2 Expression BeadChip array (Illumina inc.). Staining was performed according to standard protocols (14). The BeadChip was dried and then scanned with the Illumina HiScanSQ System (Illumina Inc.). All analyses were performed in triplicate.
Raw data from Illumina HumanHT-12_V4_0_R2 microarray were normalized using neqc function in limma package and low-quality annotation probes were excluded. Differentially expressed genes (DEGs) were obtained on the linear model fit of the microarray data. The statistical significance was established on P<0.05.
The differences of gene expression levels were shown in terms of LogFC and the cut-off of +/-0.58 was based on its correspondence to +1.5/-1.5 fold-change (2^|0.58|=|1.5|FC), showing the number of upregulated or downregulated genes, respectively (15).
The enrichment analysis was carried out by the Gene Set Enrichment Analysis (GSEA) method in clusterProfiler package (16) and on the base of the gene sets collections (Hallmark, Pathway) in MSigDB (Molecular Signatures Database) (17,18). The statistical significance was established on adjusted P<0.05 for Hallmark collections and on P<0.05 for Pathway collections. All the steps were performed according to the ‘microarray analysis’ best practice using R well known packages (19,20).
Microarray data are available at the Gene Expression Omnibus (GEO) repository under accession number GSE189717.
Analysis of public datasets
IGFBP6 expression analysis in OC samples, compared with normal tissue, was performed using the TNMplot database (https://www.tnmplot.com/). The platform directly compares tumor and/or metastasis and normal samples and performs a Mann-Whitney U or Kruskal-Wallis tests or a paired Wilcoxon test (in case of availability of paired normal and adjacent tumor) for statistical significance (21). The expression correlation between IGFBP6 and selected FOS and EGR1 genes was revealed by R2: Genomics Analysis and Visualization Platform (http://r2.amc.nl) using the Tumor Ovarian Bowtell-285-MAS5.0-u133p2 dataset. The R2 software evaluated the statistical significance with the Pearson's correlation analysis. The expression of FOS and EGR1 genes was analyzed in ovarian serous adenocarcinomas compared to normal tissues using TNMplot (RNA seq data) and GEPIA (TCGA normal vs GTEX data) databases. GEPIA (http://gepia.cancer-pku.cn) is a network-based tool for processing the RNA expression information, collected from carcinomas and healthy specimens from GTEx project and TCGA (22).
Statistical analysis
The unpaired Student's t-test was used to establish the statistical significance in IGFBP6-stimulated versus unstimulated cells. The densitometric analysis data were analyzed by two-way ANOVA and Bonferroni post hoc test. Statistically significant values (P<0.05) are reported in Figure Legends. All experiments were independently performed at least three times.
Results
IGFBP6 is downregulated along ovarian carcinogenesis
Since the role of IGFBP6 in human OC carcinogenesis is controversial, IGFBP6 expression levels were preliminary explored using gene expression data from public datasets. Thus, TNMplot gene chip data were used to analyze the differential expression of IGFBP6 in normal human ovarian samples (n=46), non-metastatic OCs (n=744) and metastatic OCs (n=44). Interestingly, while normal ovarian tissues were characterized by high IGFBP6 expression, a significant downregulation of IGFBP6 mRNA was observed from normal ovarian tissue to non-metastatic OC tissues (Fig. 1) (P=1.77e-07, Kruskal-Wallis test and post hoc Dunn's test). No further down-regulation was observed between primary tumor tissue and metastatic OCs. These data suggest that IGFBP6 expression is downregulated at early stages during ovarian carcinogenesis.
Figure 1.

IGFBP6 mRNA expression in human OCs from public databases. Boxplot graph of IGFBP6 gene expression in OCs comparing normal human ovarian tissues (n=46), primary ovarian tumors (n=744), and metastastic ovarian tumors (n=44) based on gene chip data of TNMplot. The statistical significance of differential expression (P=1.77e-07) was evaluated by the Kruskal-Wallis test and the post hoc Dunn's test. IGFBP6, insulin-like growth factor binding protein 6; OC, ovarian cancer.
Platinum-sensitive PEA1 HGSOC cells are more sensitive to IGFBP6 stimulation compared to platinum-resistant PEA2 HGSOC cells
To study the activity of IGFBP6 during OC progression, IGFBP6 mRNA and protein expression levels were examined in both platinum-sensitive (PEA1) and platinum-resistant (PEA2) OC cell lines by quantitative RT-PCR and immunoblot blot analysis, respectively. IGFPB6 mRNA levels were significantly higher in OC PEA2 cells compared to PEA1 cells (Fig. 2A), while IGFBP6 protein expression was similar in both cell lines (Fig. 2B).
Figure 2.
IGFBP6 expression levels and ERK pathway analysis in HGSOC PEA1 and PEA2 cell lines. IGFBP6 (A) quantitative PCR and (B) immunoblot analysis in PEA1 and PEA2 OC cells lines. β-actin was used as loading marker. *P<0.05. (C) p-ERK1/2 immunoblot analysis in PEA1 and PEA2 cells cultured in serum-free medium overnight and subsequently stimulated with IGFBP6 recombinant protein (1 µg/ml) for 10 min. GAPDH was used as loading marker. (B and C) Histograms represent band intensity ± SD and the ratio of phosphorylated/total proteins from the densitometric analysis of three independent experiments. *P≤0.05. IGFBP6, insulin-like growth factor binding protein 6; ERK, extracellular signal-regulated kinase; HGSOC, high-grade serous ovarian carcinoma; PEA1, pleural effusion adenocarcinoma-1; PEA2, pleural effusion adenocarcinoma-2; PCR, polymerase chain reaction; OC, ovarian cancer; p-ERK1/2, phosphorylated-extracellular signal-regulated kinase; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
In the effort to explore the effects of IGFBP6 on ERK pathway, which is known to play a major role in OC pathogenesis (23), ERK1/2 phosphorylation was evaluated in PEA1 and PEA2 cell lines in response to exogenous IGFBP6. Interestingly, a 10 min stimulation with IGFBP6 recombinant protein increased ERK1/2 phosphorylation in PEA1 cells and induced no effects on ERK pathway activation in PEA2 cell line (Fig. 2C). These preliminary data suggest that IGFBP6 signaling is more active in PEA1 OC cells.
IGFBP6 elicits a wider gene expression reprogramming in platinum-sensitive PEA1 compared to platinum-resistant PEA2 cell lines
To further characterize IGFBP6 activity and identify differentially expressed genes in PEA1 and PEA2 OC cell lines following IGFBP6 perturbation, a full genome gene expression profiling was performed by Illumina technology after cell stimulation with IGFBP6 recombinant protein for 2 h. Data allowed the identification of significantly modulated genes and were also analyzed to understand the role of IGFBP6 in the regulation of pathways of interest.
The analysis of PEA1 cell line treated with IGFBP6 compared to its unstimulated control identified 2,440 significantly modulated genes, 1,348 up- and 1,092 down-regulated (P<0.05). Therefore, performing a filter based on absolute logFC>0.58 (15), this list was restricted to 85 up- and 28 down-regulated genes (Table SI). Likewise, the analysis of gene expression data from PEA2 cell line stimulated with IGFBP6 identified 1,471 genes (P<0.05), 729 up- and 742 down-regulated. Therefore, performing a filter based on absolute logFC>0.58, this list was restricted to 37 up- and 36 down-regulated genes (Table SII).
The enrichment analysis was focuses on the Hallmarks and Pathways categories (Table SIII). We identified 16 hallmarks statistically significant (adjusted P<0.05) and with ES>0.3 upon IGFBP6 stimulation in PEA1 cells and, among them, TNF-α signaling via NF-κB was the most significant (Table II). Conversely, only two hallmarks were statistically significant (adjusted P<0.05) and with ES>0.3 in PEA2 cells stimulated with IGFBP6 (Table III).
Table II.
Hallmarks significantly enriched in PEA1 cells stimulated with IGFBP6.
| Hallmarks | ES | P-value | NES |
|---|---|---|---|
| TNFA_SIGNALING_VIA_NFKB | 0.609 | 1E-10 | 2.53 |
| UV_RESPONSE_UP | 0.503 | 7.28E-08 | 2.029 |
| HYPOXIA | 0.456 | 4.57E-07 | 1.899 |
| TGF_BETA_SIGNALING | 0.546 | 0.0006 | 1.829 |
| P53_PATHWAY | 0.431 | 1.09E-05 | 1.792 |
| MYC_TARGETS_V2 | 0.518 | 0.0018 | 1.773 |
| MITOTIC_SPINDLE | 0.399 | 0.0001 | 1.664 |
| INFLAMMATORY_RESPONSE | 0.384 | 0.0008 | 1.596 |
| IL6_JAK_STAT3_SIGNALING | 0.423 | 0.0083 | 1.557 |
| APOPTOSIS | 0.373 | 0.0065 | 1.505 |
| ESTROGEN_RESPONSE_EARLY | 0.362 | 0.0029 | 1.504 |
| EPITHELIAL_MESENCHYMAL_TRANSITION | 0.361 | 0.0031 | 1.503 |
| CHOLESTEROL_HOMEOSTASIS | 0.418 | 0.0015 | 1.496 |
| UV_RESPONSE_DN | 0.372 | 0.0088 | 1.482 |
| IL2_STAT5_SIGNALING | 0.353 | 0.0059 | 1.468 |
| MYOGENESIS | 0.348 | 0.0079 | 1.450 |
ES, enrichment score; NES, normalized enrichment score.
Table III.
Hallmarks significantly enriched in PEA2 cells stimulated with IGFBP6.
| Hallmarks | ES | P-value | NES |
|---|---|---|---|
| MYC_TARGET_V1 | 0.329 | 0.0018 | 1.475 |
| G2M_CHECKPOINT | 0.329 | 0.0018 | 1.483 |
ES, enrichment score; NES, normalized enrichment score.
In order to further study the differential activation of IGFBP6 pathway in OC cell lines, six genes (i.e., FOS, JUN, EGR1, TNF, IL-6 and IL-8) were selected among the top 20 significantly upregulated genes in PEA1 data set (Tables IV and SI). Considerably, all the selected genes enriched the 16 hallmarks reported in Table II, while three of them (i.e., FOS, JUN and EGR1) were also in the list of IGFBP6-modulated genes in PEA2 cells (Tables V and SII). Noteworthy, while all these genes were significantly upregulated in PEA1 cells upon IGFBP6 stimulation (Fig. 3A; Table IV), FOS, JUN and EGR1 were downregulated in PEA2 cells after exogenous IGFBP6 stimulation (Fig. 3B; Table V). The expression of these genes was further validated by quantitative RT-PCR in PEA1 and PEA2 cells stimulated with IGFBP6, confirming the significantly upregulation of all genes in PEA1 cells (Fig. 3A) and the downregulation of FOS and EGR1 in PEA2 cells (Fig. 3B).
Table V.
Expression levels of selected genes in PEA2 data set.
| Symbol | Name | Entrez ID | logFC | P-value | Fold change |
|---|---|---|---|---|---|
| EGR1 | Early growth response 1 | 1958 | −0.661 | 0.003 | 0.632 |
| FOS | Fos proto-oncogene, AP1 trancription factor subunit | 2353 | −1.299 | 0.0002 | 0.406 |
| JUN | Jun proto-oncogene, AP1 trancription factor subunit | 3725 | −0.388 | 0.026 | 0.764 |
Figure 3.
Gene expression profiles of OC platinum-sensitive PEA1 and platinum-resistant PEA2 cells stimulated with IGFBP6. Comparative gene expression (microarray vs. quantitative PCR) of genes reported in Tables IV and V in (A) PEA1 and (B) PEA2 cells. P-values respect to unstimulated control: *P<0.05; **P<0.001; ***P<0.0001. (C) Statistical correlation between the expression of IGFBP6 and FOS (R=0.222, P=1.61e-04, T=3.826, degrees of freedom=283) or EGR1 (R=0.232, P=7.69e-05, T=4.013 degrees of freedom=283) based on the Tumor Ovarian-Bowtell dataset and analyzed via R2: Genomics Analysis and Visualization Platform. OC, ovarian cancer; PEA1, pleural effusion adenocarcinoma-1; PEA2, pleural effusion adenocarcinoma-2; IGFBP6, insulin-like growth factor binding protein 6; FOS, proto-oncogene, AP-1 transcription factor subunit; EGR1, early growth response 1.
Table IV.
Expression levels of selected genes in PEA1 data set.
| Symbol | Name | Entrez ID | logFC | P-value | Fold change |
|---|---|---|---|---|---|
| EGR1 | Early growth response 1 | 1958 | 2.829 | 2.2E-10 | 7.106 |
| FOS | Fos proto-oncogene, AP1 trancription factor subunit | 2353 | 3.874 | 2.9E-10 | 14.662 |
| JUN | Jun proto-oncogene, AP1 trancription factor subunit | 3725 | 1.451 | 8.7E-08 | 2.734 |
| TNF | Tumor Necrosis Factor | 7124 | 1.133 | 3.9E-08 | 2.193 |
| IL-6 | Interelukin 6 | 3569 | 0.679 | 4.7E-06 | 1.601 |
| CXCL8 | C-X-C motif chemokine ligand 8 | 3576 | 1.198 | 1.4E-07 | 2.294 |
Since these data suggest that IGFBP6 induces a wider gene expression reprogramming in platinum-sensitive PEA1 compared to platinum-resistant PEA2 cells, we comparatively evaluated gene expression data at basal level between PEA2 and PEA1 cell lines. This comparative analysis of PEA2 versus PEA1 cell lines in basal conditions identified 9,372 significantly modulated genes, 4,477 up- and 4,895 down-regulated (P<0.05). Moreover, performing a filter based on absolute logFC>0.58, 2,871 genes up-regulated and 3,238 down-regulated were identified. Hallmarks enrichment analysis in PEA2 versus PEA1 cells identified 16 positive and 9 negative statistically significant hallmarks (adjusted P<0.05, ES>0.3, ES<-0.3) and, among other, the TNF-α signaling via NF-κB pathway, which is known to favor platinum resistance (24) (Tables SIV and SV). Consistently, the comparative expression analysis of FOS, JUN, EGR1, TNF, IL-6 and IL-8 showed that these genes are upregulated in platinum-resistant PEA2 compared to platinum-sensitive PEA1 cells (Tables SVI and SVII). Altogether, these data suggest that IGFBP6 stimulation of platinum-sensitive PEA1 cells results in the activation of gene/pathways that are already constitutive active in platinum-resistance PEA2 cells and that this is a potential explanation of the poorer response of PEA2 cells to IGFBP6 stimulation.
To further validate the relationship between IGFBP6 and the expression of selected genes observed in PEA1 and PEA2 cells, gene expression correlation analysis of IGFBP6 with FOS and EGR1 genes, was performed using R2 software. In particular, the correlation analysis was carried out interrogating the microarray dataset Tumor Ovarian Bowtell, which provides the gene expression profiling of 285 OC samples (25). Interestingly, the analysis revealed positive association between mRNA levels of IGFBP6 and expression of both FOS (R=0.222, P=1.61e-04) and EGR1 (R=0.232, P=7.69e-05) in OC specimens (Fig. 3C). In addition, since IGFBP6 is downregulated from normal ovarian tissue to OC, we evaluated the hypothesis that FOS and EGR1 may have a similar behavior being IGFBP6-modulated. The expression of FOS and EGR1 genes was analyzed in ovarian serous cystadenocarcinomas in TNMplot database (RNA seq data), comparing normal tissue (133 samples) to tumor tissue (374 samples) (FOS P=1.52e-11, EGR1 P=3.07e-07). The down-regulation of FOS and EGR1 expression was observed in tumors compared to normal samples (Fig. S1A). These results were further confirmed analyzing 426 tumor tissue samples (OC) and 88 normal tissue samples in the GEPIA (TCGA normal vs GTEX data) dataset(|logFc| 0.58; P-value cutoff:0.05) (Fig. S1B).
IGFBP6 activity is qualitatively different in platinum-sensitive PEA1 compared to platinum-resistant PEA2 cell lines
Since our data suggest a different response of platinum-sensitive and platinum-resistant OC cell lines to IGFBP6 stimulation, signaling pathways significantly deregulated (ES>0.4, P<0.05) by IGFBP6 in PEA1 versus PEA2 cells were subsequently analyzed. The analysis identified 1240 and 502 pathways in PEA1 and PEA2 cells, respectively (Fig. 4A) and among them, 156 in common between the two cell lines (Fig. 4B). Of note, the comparison of the top 10 common pathways in Table SIII showed a positive regulation in PEA1 cells and a negative regulation in PEA2 cells exposed to IGFBP6 (Fig. 4C).
Figure 4.
Qualitative comparison of signaling pathways between PEA1 and PEA2 datasets. (A) Number of pathways significantly regulated in PEA1 and PEA2 datasets. (B) Venn diagram reporting the overlap between pathways significantly modulated by IGFBP6 in PEA1 and PEA2 datasets. (C) Bar chart reporting the top ten common pathways between PEA1 and PEA2 datasets referred to normalized enrichment scores. PEA1, pleural effusion adenocarcinoma-1; PEA2, pleural effusion adenocarcinoma-2; IGFBP6, insulin-like growth factor binding protein 6.
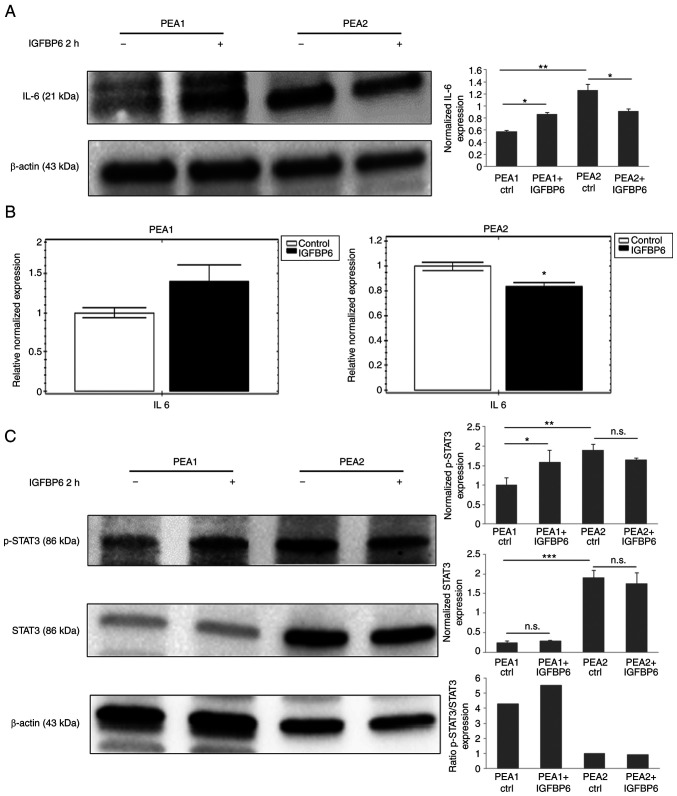
Since the statistical analysis identified IL6-JAK-STAT3 signaling as an enriched hallmark in IGFBP6 stimulated PEA1 cells (Table II), and IL6 among genes differently regulated by IGFBP6 in PEA1 cells (Fig. 3A), this pathway was further investigated to characterize the divergent response of platinum-sensitive and platinum-resistant OC cells to IGFBP6. Thus, IL6 protein and mRNA levels were analyzed in both cell lines exposed to IGFBP6 and STAT3 phosphorylation was used as a signaling event downstream to IL6 stimulation. IL6 protein (Fig. 5A) and mRNA levels (Fig. 5B) resulted both up-regulated in IGFBP6-stimulated PEA1, while significantly downregulated in PEA2 cells (Fig. 5A and B). In line with an increased activity of IL6 pathway in PEA1 after IGFBP6 stimulation, recombinant IGFBP6 increased STAT3 phosphorylation in PEA1 cells, while STAT3 phosphorylation was moderately reduced in PEA2 cells exposed to IGFBP6 (Fig. 5C). Consistently with data showing a constitutive activation of selected pathways in PEA2 versus PEA1 cells, higher levels of IL6, STAT3 and phosphoSTAT3 were observed in PEA2 compared to PEA1 cells.
Figure 5.
Analysis of IL6 pathway in PEA1 and PEA2 cells exposed to IGFBP6. IL6 (A) immunoblot and (B) quantitative PCR expression analysis in PEA1 and PEA2 cells stimulated with IGFBP6 recombinant protein (1 µg/ml) for 2 h. (C) Total and phosphorylated STAT3 immunoblot analysis in PEA1 and PEA2 cells stimulated with IGFBP6 recombinant protein (1 µg/ml) for 2 h. β-actin was used as loading marker. (A and C) Histograms represent band intensity ± SD and the ratio of phosphorylated/total proteins from the densitometric analysis of three independent experiments. *P≤0.05; **P≤0.001; ***P≤0.0001. IL6, interleukin 6; PEA1, pleural effusion adenocarcinoma-1; PEA2, pleural effusion adenocarcinoma-2; IGFBP6, insulin-like growth factor binding protein 6; PCR, polymerase chain reaction; STAT3, signal transducer and activator of transcription 3.
Concluding, these data suggest that OC cell response to IGFBP6 stimulation is divergent in platinum-sensitive PEA1 cells compared to platinum-resistant PEA2 cells.
Discussion
The role of IGFBP6 in human malignancies and specifically in OC is still controversial (9). The majority of studies suggests that IGFBP6 is downregulated in human tumors compared to normal tissues, this supporting the hypothesis that IGFBP6 is characterized by oncosuppressive functions, stimulating apoptosis and inhibiting cell proliferation (6). Again, IGFBP6 expression is increased in selected tumors (26–28), along with evidence that IGFBP6 positively regulates cell proliferation and migration.
Several data suggest that IGFBP6 is downregulated along OC progression (8,29), while other authors report that IGFBP6 promotes migration in SKOV3 OC cells by activation of MAP kinase signaling, while represses migration in HEY OC cells through both IGF-dependent and IGF-independent mechanisms (9). The IGFBPs family revealed distinct prognosis in patients with OCs (30). Indeed, it was recently reported that increased IGFBP6 mRNA levels negatively affect overall survival and progression-free survival in OC patients. Interestingly, aberrant IGFBP6 mRNA expression was correlated with significantly worse prognosis in patients receiving different chemotherapeutic regimens, suggesting IGFBP6 as a useful prognostic predictor for chemotherapeutic effect in OCs (30).
To shed light on this conflicting evidence, the role of IGFBP6 was evaluated by using gene expression data from public datasets of human OCs and by an established OC cell model of two matched cell lines derived from the same patient with HGSOC, before and after rising platinum resistance. Our data suggest that i) IGFBP6 is downregulated from normal ovarian tissues to primary OCs, and that ii) IGFBP6 signaling is quantitatively and qualitatively different in platinum-sensitive PEA1 compared to platinum-resistant PEA2 cells. Indeed, data showed a more abundant and significant gene expression reprogramming in PEA1 compared to PEA2 cells and the analysis of specific genes and signaling pathways suggest a divergent response to IGFBP6 in platinum-sensitive PEA1 versus platinum-resistant PEA2 cells. Among them, IGFBP6 activated ERK1/2 signaling only in PEA1 cells, while IL6 signaling pathway was positively regulated in PEA1 and conversely inhibited in PEA2 cells, upon IGFBP6 stimulation.
This evidence supports the hypothesis that IGFBP6 signaling is deregulated during ovarian carcinoma progression, likely playing a role in the transition from platinum-sensitive to platinum-resistant conditions. Actually, platinum-based chemotherapy is the mainstay of OC treatment in locally advanced and metastatic stages (31,32). OC is considered a chemo-responsive neoplasm, but, despite this, most patients ultimately develop recurrent disease and resistance to platinum-based chemotherapy with parallel worsening of clinical outcome (33). In such context, our data, although limited to a single model of platinum resistant/sensitive OC cell lines, suggest that the deregulation of IGFBP6 signaling may represent a mechanism associated to the development of platinum resistance and that its modulation may be ultimately a strategy to prevent/delay its onset.
Intriguingly, we observed both quantitative and qualitative differences in gene expression reprogramming between platinum-sensitive PEA1 and platinum-resistant PEA2 cell lines upon IGFBP6 stimulation. Since the differential expression of IGFBP6 between the two cell lines is minimal, it does not explain the differences observed in signal transduction and gene expression. Indeed, the mechanism used by IGFPB6 to activate its downstream signaling is complex and still largely unknown (34). The extracellular activity of IGFBP6 presents both IGF-dependent and IGF-independent mechanisms. As observed for others IGFBPs, IGFBP6 binds and inhibit IGFs preventing their interaction with the receptors, with a relatively higher propensity towards IGF-II than IGF-I. Furthermore, IGFBP6 is capable of actions independent of IGF-II, including regulation of proliferation, apoptosis, angiogenesis and cell migration upon binding with other receptors, mostly unknown (35,36). In such a context, IGFBP6 binding to prohibitin-2 (PBH2) has been proposed as a mechanism responsible for IGF-independent inhibition of cancer cell migration (36). Thus, it is reasonable to hypothesize that modifications in IGFBP6 signaling transduction machinery are responsible for the qualitative different response of PEA2 compared to PEA1 cells and are likely involved in the onset of platinum resistance.
A relevant observation is the evidence that IGFBP6 stimulation of platinum sensitive PEA1 cells induces several genes/pathways/hallmarks that are already constitutively active in platinum-resistant PEA2 cells. While this result may account for the lower response of PEA2 cells to IGFBP6 stimulation, it may also suggest that IGFBP6 is responsible for promoting platinum resistance by activating specific pathways responsible for escape from cytotoxic agents. Interestingly, ERK signaling is among signaling pathways differentially active between PEA1 and PEA2 and this, in our preliminary experiments, was used to establish that PEA2 cells are poorly sensitive to IGFBP6 stimulation. Since ERK signaling is one of the most relevant signaling pathway whose activation is modulated upon IGFBP6 stimulation (37), the differential activation of ERK signaling between the two cell lines may be responsible for the difference in gene expression after IGFBP6 stimulation and likely drug resistance. In line with this hypothesis, the expression of JUN and FOS, two well established downstream targets of ERK signaling (38), is upregulated in PEA1 and downregulated/unchanged in PEA2 cells upon IGFPB6 stimulation. However, further studies are needed to address the specific role of ERK signaling in IGFBP6-dependent reprogramming of gene expression and its relationship with drug resistance.
Intriguingly, our data suggest a qualitative difference between drug-resistant PEA2 and drug-sensitive PEA1 cells lines in terms of response to IGFBP6. Indeed, IGFBP6 regulates specific genes and pathways involved in inflammation and their expression/activation was inhibited in PEA2 cells where IGFBP6 signaling is contextually downregulated. More specifically, several hallmarks/pathways involved in inflammatory processes, as IL6-JAK-STAT3 and TGF-B signaling pathways (39) are active in PEA1 and conversely inhibited in PEA2 cells, after IGFBP6 stimulation. This evidence is consistent with several studies, which suggest that inflammatory pathways along with metabolic remodeling are involved in the development of platinum resistance (40–42). In addition, specific inflammatory indexes are predictive of platinum sensitivity in OC (43,44) and are differentially expressed in platinum-sensitive versus resistant OC cell models (45). However, although the literature demonstrates that IL6-JAK-STAT3 pathway is known to favor platinum resistance in OC (46), our data failed to demonstrate that IL6-JAK-STAT3 axis activation/inhibition is the main mechanism responsible for modulation of platinum sensitivity in response to IGFBP6 in OC cells (data not shown). Thus, based on our results, we suggest that it is unlikely that a single pathway/hallmark is responsible for modulation of platinum sensitivity in response to IGFBP6, but rather that the vast remodeling induced by IGFBP6 with activation/inhibition of several pathways may modulate platinum sensitivity in PEA1/PEA2 ovarian carcinoma cells. However, it is important to underline that this study does was performed using a single model of platinum sensitive/resistant ovarian carcinoma cell lines and does not provide a final answer about the specific molecular mechanism responsible for this process. Thus, further studies are needed to further validate these results and clarify the role of IGFBP6 in platinum resistance/sensitivity.
In conclusion, this study suggests a role of IGFBP6 in OC progression, highlighting that the deregulation of its actions is likely involved in the remodeling of the gene expression response and the occurrence of platinum resistance. Further studies are needed to evaluate whether IGFBP6 may represent a target to slowdown OC progression toward drug resistance and characterize this signaling pathway as a novel source of biomarkers.
Supplementary Material
Acknowledgements
Not applicable.
Funding Statement
The present study was supported by the LILT grant 5×1000-2020 (grant no. CUP D79J21003680005) to ML. SV was supported by PON AIM R&I (National Operational Programme-Attraction and International Mobility-Research&Innovation) 2014-2020-1879351-3 (grant no. CUP D74I18000160006) and GB was supported by PON AIM R&I (National Operational Programme-Attraction and International Mobility-Research&Innovation) 2014-2020-1879351-2 (grant no. CUP D74I18000140006). This manuscript has been published with the financial support of the Dept. of Medical and Surgical Sciences of the University of Foggia.
Availability of data and materials
Gene expression data generated in this study are available at the GEO repository under accession number GSE189717 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE189717).
Authors' contributions
ML and AL designed the study. AP, VC, MP, FC and SV performed the experiments. AP, VC, FC, ARDC, GB, GC, GG and DT analyzed the data. AP and VC confirmed the authenticity of the raw data. ML and AP wrote the manuscript. AL, SV, GB, GG, VC, FC, MP and DT revised the paper. All authors approved the final version of the manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Gaona-Luviano P, Medina-Gaona LA, Magaña-Pérez K. Epidemiology of ovarian cancer. Chin Clin Oncol. 2020;9:47. doi: 10.21037/cco-20-34. [DOI] [PubMed] [Google Scholar]
- 2.Torre LA, Islami F, Siegel RL, Ward EM, Jemal A. Global cancer in women: Burden and trends. Cancer Epidemiol Biomarkers Prev. 2017;26:444–457. doi: 10.1158/1055-9965.EPI-16-0858. [DOI] [PubMed] [Google Scholar]
- 3.Bach LA. IGF-binding proteins. J Mol Endocrinol. 2018;61:T11–T28. doi: 10.1530/JME-17-0254. [DOI] [PubMed] [Google Scholar]
- 4.Firth SM, Baxter RC. Cellular actions of the insulin-like growth factor binding proteins. Endocr Rev. 2002;23:824–854. doi: 10.1210/er.2001-0033. [DOI] [PubMed] [Google Scholar]
- 5.Baxter RC. IGF binding proteins in cancer: Mechanistic and clinical insights. Nat Rev Cancer. 2014;14:329–341. doi: 10.1038/nrc3720. [DOI] [PubMed] [Google Scholar]
- 6.Bach LA, Fu P, Yang Z. Insulin-like growth factor-binding protein-6 and cancer. Clin Sci (Lond) 2013;124:215–229. doi: 10.1042/CS20120343. [DOI] [PubMed] [Google Scholar]
- 7.Walker G, MacLeod K, Williams ARW, Cameron DA, Smyth JF, Langdon SP. Insulin-like growth factor binding proteins IGFBP3, IGFBP4, and IGFBP5 predict endocrine responsiveness in patients with ovarian cancer. Clin Cancer Res. 2007;13:1438–1444. doi: 10.1158/1078-0432.CCR-06-2245. [DOI] [PubMed] [Google Scholar]
- 8.Bahrani-Mostafavi Z, Tickle TL, Zhang J, Bennett KE, Vachris JC, Spencer MD, Mostafavi MT, Tait DL. Correlation analysis of HOX, ERBB and IGFBP family gene expression in ovarian cancer. Cancer Invest. 2008;26:990–998. doi: 10.1080/07357900802074349. [DOI] [PubMed] [Google Scholar]
- 9.Yang Z, Bach LA. Differential effects of insulin-like growth factor binding protein-6 (IGFBP-6) on migration of two ovarian cancer cell lines. Front Endocrinol (Lausanne) 2015;5:231. doi: 10.3389/fendo.2014.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amutha P, Rajkumar T. Role of insulin-like growth factor, insulin-like growth factor receptors, and insulin-like growth factor-binding proteins in ovarian cancer. Indian J Med Paediatr Oncol. 2017;38:198–206. doi: 10.4103/ijmpo.ijmpo_3_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oliva CR, Halloran B, Hjelmeland AB, Vazquez A, Bailey SM, Sarkaria JN, Griguer CE. IGFBP6 controls the expansion of chemoresistant glioblastoma through paracrine IGF2/IGF-1R signaling. Cell Commun Signal. 2018;16:61. doi: 10.1186/s12964-018-0273-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Langdon SP, Lawrie SS, Hay FG, Hawkes MM, McDonald A, Hayward IP, Schol DJ, Hilgers J, Leonard RC, Smyth JF. Characterization and properties of nine human ovarian adenocarcinoma cell lines. Cancer Res. 1988;48:6166–6172. [PubMed] [Google Scholar]
- 13.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C (T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 14.Avolio R, Järvelin AI, Mohammed S, Agliarulo I, Condelli V, Zoppoli P, Calice G, Sarnataro D, Bechara E, Tartaglia GG, et al. Protein syndesmos is a novel RNA-binding protein that regulates primary cilia formation. Nucleic Acid Res. 2018;46:12067–12086. doi: 10.1093/nar/gky873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao B. Erwin A, Xue B. How many differentially expressed genes: A perspective from the comparison of genotypic and phenotypic distances. Genomics. 2018;110:67–73. doi: 10.1016/j.ygeno.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 16.Yu G, Wang LG, Han Y, He QY. clusterProfiler: An R package for comparing biological themes among gene clusters. OMICS. 2012;16:284–287. doi: 10.1089/omi.2011.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102:15545–50. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liberzon A, Subramanian A, Pinchback R, Thorvaldsdóttir H, Tamayo P, Mesirov JP. Molecular signatures database (MSigDB) 3.0. Bioinformatics. 2011;27:1739–1740. doi: 10.1093/bioinformatics/btr260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.R Core Team R, corp-author. R Foundation for Statistical Computing; Vienna, Austria: 2020. A language and environment for statistical computing. [Google Scholar]
- 20.Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bartha A, Győrffy B. TNMplot.com: A web tool for the comparison of gene expression in normal, tumor and metastatic tissues. Int J Mol Sci. 2021;22:2622. doi: 10.3390/ijms22052622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang Z, Li C, Kang B, Gao G, Li C, Zhang Z. GEPIA: A web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Research. 2017;45:W98–W102. doi: 10.1093/nar/gkx247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo YJ, Pan WW, Liu SB, Shen ZF, Xu Y, Hu LL. ERK/MAPK signalling pathway and tumorigenesis. Exp Ther Med. 2020;19:1997–2007. doi: 10.3892/etm.2020.8454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fujioka S, Son K, Onda S, Schmidt C, Scrabas GM, Okamoto T, Fujita T, Chiao PJ, Yanaga K. Desensitization of NFκB for overcoming chemoresistance of pancreatic cancer cells to TNF-α or paclitaxel. Anticancer Res. 2012;32:4813–4821. [PubMed] [Google Scholar]
- 25.Tothill RW, Tinker AV, George J, Brown R, Fox SB, Lade S, Johnson DS, Trivett MK, Etemadmoghadam D, Locandro B, et al. Novel molecular subtypes of serous and endometrioid ovarian cancer linked to clinical outcome. Clin Cancer Res. 2008;14:5198–5208. doi: 10.1158/1078-0432.CCR-08-0196. [DOI] [PubMed] [Google Scholar]
- 26.Drivdahl RH, Sprenger C, Trimm K, Plymate SR. Inhibition of growth and increased expression of insulin-like growth factor-binding protein-3 (IGFBP-3) and −6 in prostate cancer cells stably transfected with antisense IGFBP-4 complementary deoxyribonucleic acid. Endocrinology. 2001;142:1990–1998. doi: 10.1210/endo.142.5.8158. [DOI] [PubMed] [Google Scholar]
- 27.Nordqvist AC, Mathiesen T. Expression of IGF-II, IGFBP-2, −5, and −6 in meningiomas with different brain invasiveness. J Neurooncol. 2002;57:19–26. doi: 10.1023/A:1015765613544. [DOI] [PubMed] [Google Scholar]
- 28.Cacalano NA, Le D, Paranjpe A, Wang MY, Fernandez A, Evazyan T, Park NH, Jewett A. Regulation of IGFBP6 gene and protein is mediated by the inverse expression and function of c-jun N-terminal kinase (JNK) and NFkappaB in a model of oral tumor cells. Apoptosis. 2008;13:1439–1449. doi: 10.1007/s10495-008-0270-1. [DOI] [PubMed] [Google Scholar]
- 29.Gunawardana CG, Kuk C, Smith CR, Batruch I, Soosaipillai A, Diamandis EP. Comprehensive analysis of conditioned media from ovarian cancer cell lines identifies novel candidate markers of epithelial ovarian cancer. J Proteome Res. 2009;8:4705–4713. doi: 10.1021/pr900411g. [DOI] [PubMed] [Google Scholar]
- 30.Zheng R, Chen W, Xia W, Zheng J, Zhou Q. The prognostic values of the insulin-like growth factor binding protein family in ovarian cancer. Biomed Res Int. 2020;2020:7658782. doi: 10.1155/2020/7658782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pavlakis G, Mountzios G, Terpos E, Leivaditou A, Papadopoulos G, Papasavas P. Recurrent ovarian cancer metastatic to the sternum, costae, and thoracic wall after prolonged treatment with platinum-based chemotherapy: A case report and review of the literature. Int J Gynecol Cancer. 2006;16((Suppl 1)):S299–S303. doi: 10.1111/j.1525-1438.2006.00208.x. [DOI] [PubMed] [Google Scholar]
- 32.Vergote I, Tropé CG, Amant F, Kristensen GB, Ehlen T, Johnson N. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. 2010;363:943–953. doi: 10.1056/NEJMoa0908806. [DOI] [PubMed] [Google Scholar]
- 33.Lisio MA, Fu L, Goyeneche A, Gao ZH, Telleria C. High-grade serous ovarian cancer: Basic Sciences, clinical and therapeutic standpoints. Int J Mol Sci. 2019;20:952. doi: 10.3390/ijms20040952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bach LA. Recent insights into the actions of IGFBP-6. J Cell Commun Signal. 2015;9:189–200. doi: 10.1007/s12079-015-0288-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liso A, Capitanio N, Gerli R, Conese M. From fever to immunity: A new role for IGFBP-6? J Cell Mol Med. 2018;22:4588–4596. doi: 10.1111/jcmm.13738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bach LA. Current ideas on the biology of IGFBP-6: More than an IGF-II inhibitor? Growth Horm IGF Res. 2016;30-31:81–86. doi: 10.1016/j.ghir.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 37.Fu P, Liang GJ, Khot SS, Phan R, Bach LA. Cross-talk between MAP kinase pathways is involved in IGF-independent, IGFBP-6-induced Rh30 rhabdomyosarcoma cell migration. J Cell Physiol. 2010;224:636–643. doi: 10.1002/jcp.22156. [DOI] [PubMed] [Google Scholar]
- 38.Plotnikov A, Zehorai E, Procaccia S, Seger R. The MAPK cascades: Signaling components, nuclear roles and mechanisms of nuclear translocation. Biochim Biophys Acta. 2011;1813:1619–1633. doi: 10.1016/j.bbamcr.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 39.Heinrich PC, Behrmann I, Haan S, Hermanns HM, Müller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J. 2003;374:1–20. doi: 10.1042/bj20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Amoroso MR, Matassa DS, Agliarulo I, Avolio R, Maddalena F, Condelli V, Landriscina M, Esposito F. Stress-adaptive response in ovarian cancer drug resistance: Role of TRAP1 in oxidative metabolism-driven inflammation. Adv Protein Chem Struct Biol. 2017;108:163–198. doi: 10.1016/bs.apcsb.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 41.Wang Y, Niu XL, Qu Y, Wu J, Zhu YQ, Sun WJ. Autocrine production of interleukin-6 confers cisplatin and paclitaxel resistance in ovarian cancer cells. Cancer Lett. 2010;295:110–123. doi: 10.1016/j.canlet.2010.02.019. [DOI] [PubMed] [Google Scholar]
- 42.Matassa DS, Amoroso MR, Lu H, Avolio R, Arzeni D, Procaccini C, Faicchia D, Maddalena F, Simeon V, Agliarulo I, et al. Oxidative metabolism drives inflammation-induced platinum resistance in human ovarian cancer. Cell Death Differ. 2016;23:1542–1554. doi: 10.1038/cdd.2016.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Farolfi A, Scarpi E, Greco F, Bergamini A, Longo L, Pignata S, Casanova C, Cormio G, Bologna A, Orditura M, et al. Inflammatory indexes as predictive factors for platinum sensitivity and as prognostic factors in recurrent epithelial ovarian cancer patients: A MITO24 retrospective study. Sci Rep. 2020;10:18190. doi: 10.1038/s41598-020-75316-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao Q, Zhong J, Lu P, Feng X, Han Y, Ling C, Guo W, Zhou W, Yu F. DOCK4 is a platinum-chemosensitive and prognostic-related biomarker in ovarian cancer. PPAR Res. 2021;2021:6629842. doi: 10.1155/2021/6629842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grabosch S, Bulatovic M, Zeng F, Ma T, Zhang L, Ross M, Brozick J, Fang Y, Tseng G, Kim E, et al. Cisplatin-induced immune modulation in ovarian cancer mouse models with distinct inflammation profiles. Oncogene. 2019;38:2380–2393. doi: 10.1038/s41388-018-0581-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Browning L, Patel MR, Horvath EB, Tawara K, Jorcyk CL. IL-6 and ovarian cancer: Inflammatory cytokines in promotion of metastasis. Cancer Manag Res. 2018;10:6685–6693. doi: 10.2147/CMAR.S179189. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Gene expression data generated in this study are available at the GEO repository under accession number GSE189717 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE189717).