Abstract
Aerobic glycolysis plays a key role in cancer cell metabolism and contributes to tumorigenesis, including that of non-small cell lung cancer (NSCLC). Tanshinone IIA (Tan IIA), an active compound of Salvia miltiorrhiza, exhibits antitumor properties. Multiple mechanisms are involved in the antitumor action of Tan IIA in lung cancer, such as inhibiting cell growth, promoting cell apoptosis and influencing cellular metabolism. However, the effects of Tan IIA on NSCLC cells and its mechanisms of action remain unclear. The present study shows Tan IIA dose-dependently attenuated the growth of NSCLC cells and in vitro in a dose-dependent manner. Moreover, Tan IIA markedly decreased the ATP level, glucose uptake and lactate production in the NSCLC cells in vitro. Tan IIA also inhibited tumor growth in a xenograft model in vivo. Mechanically, Tan IIA treatment decreased sine oculis homeobox homolog 1 (SIX1) mRNA and protein levels, thus leading to the downregulation of pyruvate kinase isozyme M2, hexokinase 2 and lactate dehydrogenase A (LDHA) expression in A549 cells. SIX1 knockdown with small interfering-RNA inhibited glycolysis in NSCLC cells, suggesting that SIX1 plays a role in the antitumor effect of Tan IIA on NSCLC cells. More importantly, it was demonstrated that SIX1 expression was stimulated in patients with NSCLC and was positively correlated with the LDH serum level. Finally, SIX1 low expression levels predicted the poor prognosis of patients with NSCLC. In conclusion, the present study showed that Tan IIA functioned as an anti-glycolysis agent in NSCLC cells by downregulating SIX1 expression and inhibiting cell proliferation.
Keywords: Tan IIA, non-small cell lung cancer, aerobic glycolysis, proliferation, SIX1
Introduction
Lung cancer is the leading cause of cancer-associated death and the second most diagnosed cancer worldwide. Approximately 228,820 people are diagnosed with lung cancer and at least 135,700 people succumb to the disease each year (1). Non-small cell lung cancer (NSCLC) is the most common histological type of lung cancer, accounting for ~85% of lung cancer cases (2,3). Although the prognosis of patients with NSCLC has improved over the last decade with the development of target drugs and immune checkpoint inhibitors, NSCLC is still a highly malignant, rapidly progressing and incurable malignant tumor (4,5). The 5-year survival rate of patients with NSCLC is <20% (6). Therefore, elucidating the underlying molecular mechanisms and identifying the therapeutic targets are critical for developing efficient treatments of NSCLC.
Sine oculis homeobox homolog 1 (SIX1) is an important transcription factor for the development of various organs and is not expressed in most normal adult tissues. However, SIX1 expression is frequently found in a number of malignant cells and plays a significant role in tumorigenesis, including cell proliferation, apoptosis, invasion, epithelial to mesenchymal transition and stem cell maintenance (7–11). More importantly, previous studies have confirmed that SIX1 is mainly involved in aerobic glycolysis, a metabolic feature of malignancy, by positively regulating the expression of key glycolytic enzymes at the transcriptional level (12). SIX1 knockdown results in a decrease in glucose uptake, ATP level and lactate production (13). Additionally, SIX1 levels are increased in lung cancer and are correlated with a poor prognosis (14). Hence, finding new compounds to block SIX1 expression in NSLSC may contribute to the development of successful cancer therapy.
Tanshinone IIA (Tan IIA) is an active constituent extracted from the plant Salvia miltiorrhiza, the dried root of which forms the traditional Chinese herb Danshen. Tan IIA is found to exert antitumor effects in multiple human cancer types, including ovarian cancer (15), oral cancer (16), prostate cancer (17), acute leukemia (18) and lung cancer (19). Multiple mechanisms are involved in the antitumor action of Tan IIA in lung cancer. For example, Liao et al (20) reported that Tan IIA induced cell apoptosis through the phosphatidylinositol 3-kinase/protein kinase B pathway. Tan IIA also inhibits NSCLC cell growth by regulating epidermal growth factor receptor signaling (19) and prevents A549 cell proliferation by targeting the kinase domain of vascular endothelial growth factor receptor 2 (21). Additionally, the antitumor effects of Tan IIA have been shown to deplete pyruvate kinase isozyme M2 (PKM2) levels, leading to the inhibition of glycolysis (22). Therefore, we hypothesize that the antitumor activity of Tan IIA in NSCLC cells may involve its antimetabolic role.
The present study investigated the antitumor effects of Tan IIA on NSCLC via the repression of SIX1 expression and the subsequent inhibition of aerobic glycolysis.
Material and methods
Clinical specimens
A total of 30 samples of NSCLC primary tumor tissues and their adjacent noncancerous tissues were obtained from newly diagnosed patients admitted to the Hebei Provincial Chest Hospital (Shijiazhuang, Hebei, China) between September 2017 and August 2019. The tumor tissues were obtained via surgical resection, and the distance between normal lung tissue and corresponding non-cancerous lung tissues was >3 cm. The patient clinical characteristics are shown in Table I. The mean age of the patients was 63.9 years (range, 49-74 years). None of patients received chemotherapy or radiotherapy before surgery. The inclusion criterion was histologically proven non-small cell lung cancer. The exclusion criterion was any patient undergoing chemotherapy or radiotherapy. TNM stage was defined according to TNM Stage Groupings in the Forthcoming (Eighth) Edition of the NM Classification for Lung Cancer (23). All the tissues were stored in the liquid nitrogen immediately for the next experiments. All the protocols of this study were approved by the Ethics Committee of the Hebei Provincial Chest Hospital. Written informed consent was obtained from all patients.
Table I.
Clinicopathological characteristics of the non-small cell lung caner patients.
| Characteristic | Number of cases |
|---|---|
| Age, years | |
| ≤60 | 13 |
| >60 | 17 |
| Sex | |
| Male | 16 |
| Female | 14 |
| Tumor size, cm | |
| ≤3 | 22 |
| >3 | 8 |
| TNM stage | |
| I+II | 24 |
| III+IV | 6 |
| Smoking | |
| Yes | 20 |
| No | 10 |
TNM, Tumor-Node-Metastasis.
Cell culture and transfection
Human NSCLC A549 and H292 cell lines were obtained from the Shanghai Cell Bank of the Chinese Academy of Science. A549 and H292 cells were cultured in Dulbecco's modified Eagle's medium (Gibco; Thermo Fisher Scientific, Inc.) with 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.) and 1% penicillin-streptomycin. Cells were cultured in an incubator with 5% CO2 at saturated humidity at 37°C.
The small interfering (si)RNAs specifically targeting SIX1 were designed by Suzhou GenePharma Co., Ltd., and had the following the sequences: siSIX1-1 forward, 5′-GGUGGACUUUCACAAAUAUUU-3′ and reverse, 5′-AUAUUUGUGAAAGUCCACCUU-3′; and siSIX1-2 forward, 5′-CAGGUCAGCAACUGGUUUAUU-3′ and reverse, 5′-UAAACCAGUUGCUGACCUGUU-3′. A scrambled sequence was used as the negative control, which had the following sequences: Forward, 5′-GCUGCUUCUACUCGUAAGUTT-3′ and reverse, 5′-AUUCAGGAGUAGAGAGACCTT-3′. Cells were transfected with siRNA using Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's instructions. Briefly, 20 pmol siRNA oligomer and 1 µl Lipofectamine 2000 were diluted in 50 µl Opti-MEM medium (Gibco; Thermo Fisher Scientific, Inc.) without serum, respectively. After a 5-min incubation at room temperature, the diluted oligomer with the diluted Lipofectamine 2000 were combined and incubated for 20 min at room temperature. The oligomer-Lipofectamine 2000 complexes were then added to each well containing cells and cultured at 37°C in a 5% CO2 incubator. After 6 h, cells were introduced to normal medium. Transfected cells were incubated at 37°C in a 5% CO2 incubator for 24 h and then collected to be used in the subsequent experiments.
Cell viability assay
Cell viability was assessed with the CCK-8 Kit (Bestbio) following the manufacturer's instructions. Briefly, 1×106 cells were seeded in 100 µl culture medium on 96-well plates and then treated with different concentrations of Tan IIA (0.25, 0.5, 1, 2, 4, 8, 16 and 32 µM). After 48 h, 10 µl CCK-8 assay solution was added to each well and the cells were further incubated at 37°C with 5% CO2 for 2 h. The optical density values were measured using an ELx808 absorbance reader (BioTek Instruments, Inc.) at 450 nm.
Protein extraction and western blotting
Cultured cells or tissues were lysed with RIPA buffer (Abcam). The BCA method was used for protein determination. Then 10–30 µg total protein was separated by 10% SDS-PAGE and electrotransferred to polyvinylidene fluoride membranes (MilliporeSigma). Membranes were blocked with 5% non-fat milk for 2 h at 37°C and then incubated with the primary antibodies overnight at 4°C with primary antibody recognizing SIX1 (catalog no. 10709-1-AP; 1:1,000), hexokinases 2 (HK2; catalog no. 66974-1-Ig; 1:1,000), PKM2 (catalog no. 15822-1-AP; 1:1,000), lactate dehydrogenase A (LDHA; catalog no. 19987-1-AP; 1:1,000), hypoxia inducible factor 1α (HIF1α; catalog no. 20960-1-AP 1:1,000) and β-actin (catalog no. 66009-1-Ig; 1:2,000) (all Wuhan Sanying Biotechnology). The next day, after washing with TBST (containing 0.05% Tween-20), the membranes were incubated with the horse radish peroxidase-conjugated secondary antibodies (1:5,000; anti-mouse, catalog no. ab205719; anti-rabbit, catalog no. ab205718; Abcam). Finally, the blots were treated with the Immobilo™ Western (MilliporeSigma) and imaged with ECL Fuazon Fx (Vilber Lourmat Deutschland GmbH) on FusionCapt Advance Fx5 software (Vilber Lourmat Deutschland GmbH).
RNA extraction and reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from tissues and cells using QIAzol Lysis Reagent (Qiagen GmbH) following the manufacturer's protocol. The M-MLV First-Strand Kit (Thermo Fisher Scientific, Inc.) was used for the reverse transcription of RNA (2 µg) to cDNA according to the manufacturer's instructions. The Platinum SYBR Green qPCR Super Mix UDG Kit (Invitrogen; Thermo Fisher Scientific, Inc.) was used for the PCR on a CFX96TM Real-Time System (Bio-Rad Laboratories, Inc.) with the following primers: SIX1 forward, 5′-CCAGGTCAGCAACTGGTTTAAG-3′ and reverse, 5′-ATAGTTTGAGCTCCTGGCGT-3′; PKM2 forward, 5′-GTGGCTCGTGGTGATCTAGG-3′ and reverse, 5′-GTGGAGTGACTTGAGGCTCG-3′; HK2 forward, 5′-GCCATCCTGCAACACTTAGGGCTTGAG-3′ and reverse, 5′-GTGAGGATGTAGCTTGTAGAGGGTCCC-3′; LDHA forward, 5′-ATGGCAACTCTAAAGGATCA-3′ and reverse, 5′-GCAACTTGCAGTTCGGGC-3′; HIF1α forward, 5′-TACTCAGCACTTTTAGATGCTGTT-3′ and reverse, 5′-ACGTTCAGAACTTATCCTACCAT-3′; and β-actin forward, 5′-GAGCTACGAGCTGCCTGAC-3′ and reverse, 5′-GGTAGTTTCGTGGATGCCACAG-3′. The qPCR conditions were as follows: Initial denaturation at 95°C for 10 min, followed by 40 cycles of 95°C for 5 sec, 60°C for 30 sec and 72°C for 20 sec, with a final extension at 72°C for 5 min. All the relative gene expression levels were calculated using the 2−ΔΔCq formula (24).
Glucose uptake, lactate production and ATP assays
Glucose uptake and lactate production were assessed using a Glucose Uptake Colorimetric Assay Kit (catalog no. ab136955; Abcam) and a Lactate Assay Kit II (catalog no. K267-100; BioVision, Inc.), respectively, following the manufacturers' instructions. Briefly, 5×105 cells were seeded into 96-well plates and treated with Tan IIA (5 µM) for 48 h. After washing with PBS, the cells were preincubated with 100 ml Krebs-Ringer-Phosphate-HEPES buffer containing 2% BSA for 40 min at room temperature. Next, 2-deoxy-D-glucose was added, and the cells were incubated for 20 min at 37°C. Afterward, cells were lysed with 90 ml extraction buffer and then 10 ml neutralization buffer was added. After centrifugation (1,500 × g, for 10 min at room temperature), the supernatant was used to measure glucose uptake at 412 nm and lactate production at 450 nm in a microplate reader (BioTek Instruments, Inc.).
For ATP level analysis, cells were lysed in the same manner as for the glucose uptake and lactate production assays. The cells were collected and extracted in 100 ml of the ATP Assay Buffer from the ATP calorimetric assay kit (BioVision, Inc.). After centrifugation (1,500 × g, for 10 min at room temperature), the supernatants were incubated for 30 min at room temperature, and then absorbance at 570 nm was measured using a microplate reader (BioTek Instruments, Inc.).
Xenograft model
A total of 12 male BALB/c-nu/nu nude male mice (4–6 weeks old; weight, 22–25 g) were purchased from Vital River Laboratory Animal Technology Co., Ltd. Mice were maintained in specific-pathogen-free conditions under a constant humidity of 60–70% and room temperature of 18–20°C in the Laboratory Animal Center of Hebei Medical University (Shijiazhuang, Hebei, China). A total of 5×106 A549 cells were mixed with 50% Matrigel matrix (BD Biosciences) and subcutaneously injected into the nude mice. On the 8th day after injection, the mice were then randomly divided into two groups (6 mice in each group), namely, the Tan IIA-treated and control groups. In the Tan IIA-treated group, Tan IIA (20 mg/kg) was administered to the mice intraperitoneally every day for 2 weeks, as described previously (25). The control group was injected with the same amount of saline as the negative control. After 28 days, the mice were euthanized using carbon dioxide asphyxiation as follows: The mice were placed into a euthanasia box (20×12×10 cm) and then carbon dioxide (concentration of 99.9%) was injected into the box at a flow rate of 1 l/min (~33% of the box volume/min). The mice were exposed to the carbon dioxide until a complete cessation of breathing was determined (usually within 4-8 min) and observed for at least 2 min afterwards. The mice were then taken out of the box and cervical dislocation was performed to ensure death. The tumors were excised from the mice for further study. Tumor volume was calculated as follows: Volume = (length × width2)/2. All animal experiments were approved by the Institutional Animal Care and Use Committee of Hebei Medical University (approval no. HebMU 20,080,026).
TCGA analysis
Kaplan-Meier plot analysis of TCGA data was performed by using OncoLnc (http://www.oncolnc.org/kaplan). A total of 489 patients with LUAD were contained in the database. The cut-off value was 25%.
Statistical analysis
Data are presented as the mean ± standard deviation of three independent experiments. Student's t-test was used to determine the significance of differences between two groups. One-way ANOVA followed by Bonferroni's post hoc test was used for multigroup comparisons. Correlations between SIX1 expression and LDH level were analyzed using Pearson's correlation test. P<0.05 was considered to indicate a statistically significant difference. Statistical analyses were performed using GraphPad Prism (version 8; GraphPad Software, Inc.).
Results
Tan IIA inhibits cell proliferation and glycolysis in NSCLC cells
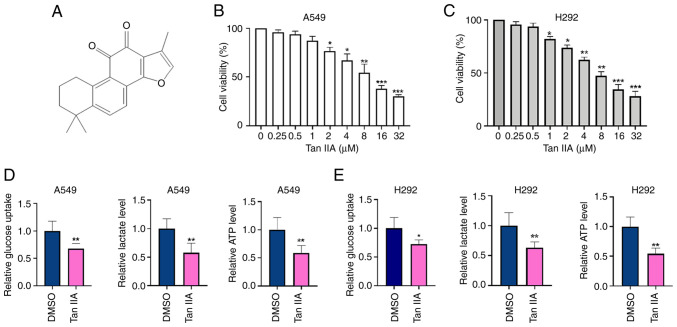
The structure of Tan IIA is shown in Fig. 1A. To investigate the antitumor effect of Tan IIA in NSCLC cells, A549 and H292 cells were treated with different concentrations of Tan IIA for 48 h and then cell viability was detected by CCK-8 assay. As shown in Fig. 1B and 1C, Tan IIA significantly inhibited the proliferation of NSCLC cells in a dose-dependent manner. The half maximal inhibitory concentration of Tan IIA was 5.45 µM in A549 cells and 5.78 µM in H292 cells. To clarify the effects of Tan IIA on the glycolysis of NSCLC cells, the levels of glucose consumption, lactate production and ATP were detected. As shown in Fig. 1D and E, glucose uptake, lactate production and ATP levels were significantly decreased by 30, 40 and 52% in A549 cells, and 25, 38 and 50% in H292 cells, respectively, in the Tan IIA-treated group compared with those of the DMSO group. These results indicate that Tan IIA inhibits cell viability and suppresses cell glycolysis activity in NSCLC cells in vitro.
Figure 1.
Tan IIA inhibits cell proliferation and glycolysis in non-small cell lung cancer cells. (A) The structure of Tan IIA. (B and C) A549 and H292 cells were treated with different concentrations of Tan IIA for 48 h. CCK-8 assay was used to detect cell viability. *P<0.05, **P<0.01 and ***P<0.001 vs. DMSO. (D and E) A549 cells and H292 cells were treated with 5 µM Tan IIA for 48 h. Glucoses uptake, lactate production and ATP level were measured by corresponding assays. *P<0.05 and **P<0.01 vs. DMSO. Tan IIA, Tanshinone IIA.
Tan IIA downregulates SIX1 expression in NSCLC cells
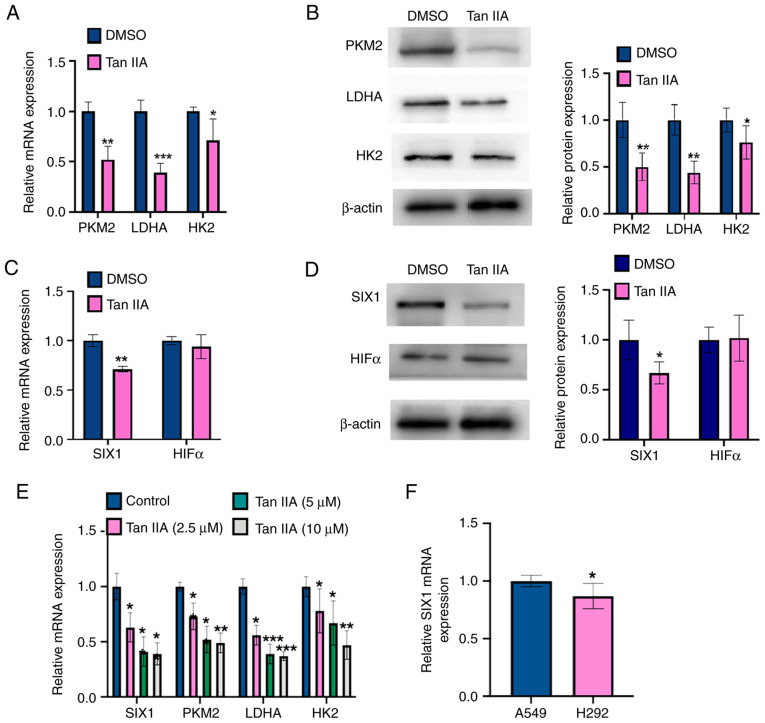
To detect whether Tan IIA inhibited the glycolysis in NSCLC cells, the mRNA and protein levels of glycolysis rate-limiting enzymes (PKM2, HK2 and LDHA) were measured in Tan IIA-treated A549 cells using RT-qPCR and western blot analysis, respectively. As shown in Fig. 2A, Tan IIA treatment markedly decreased the mRNA levels of PKM2, HK2 and LDHA compared with those measured in DMSO-treated controls. Consistent with the RT-qPCR results, the western blotting results showed that Tan IIA-treated A549 cells contained lower protein levels of PKM2, HK2 and LDHA compared with DMSO-treated controls (Fig. 2B), suggesting that Tan IIA inhibited glycolysis by repressing the activity of enzymes involved in aerobic glycolysis. A previous study reported that HIF1α and SIX1 contribute to regulate the levels of enzymes involved in aerobic glycolysis level in multiple cancer types (13). Therefore, the present study analyzed the mRNA and protein levels of SIX1 and HIF1α by RT-qPCR and western blot analysis, respectively. As shown in Fig. 2C and D, SIX1 mRNA and protein levels were markedly decreased by Tan IIA, whereas HIF1α expression was not affected. Additionally, A549 cells were treated with Tan IIA (2.5, 5 and 10 µM) and then RT-qPCR was used to detect the expression levels of PKM2, LDHA, HK2 and SIX1. As shown in Fig. 2E, the treatment with Tan IIA decreased the mRNA expression levels of PKM2, LDHA, HK2, and SIX1. However, there was no significant different between Tan IIA used at 5 and 10 µM concentrations. Furthermore, it was found that SIX1 mRNA expression in the A549 cell line was higher than that in the H292 cell line (Fig. 2F). Therefore, the A549 cell line was chosen for the following experiments. Together, these data suggest that Tan IIA may influence glycolysis activity by regulating SIX1 expression.
Figure 2.
Tan IIA downregulates SIX1 expression in NSCLC cells. (A) A549 cells were treated with 5 µM Tan IIA for 48 h. The mRNA levels of PKM2, LDHA and HK2 were detected by RT-qPCR. *P<0.05, **P<0.01 and ***P<0.001 vs. DMSO. (B) A549 cells were treated as in (A), and the protein levels of PKM2, LDHA and HK2 were detected by western blot analysis. The right panel shows the densitometric analysis of three independent experiments. *P<0.05 and **P<0.01 vs. DMSO. (C) A549 cells were treated as in (A), and the mRNA levels of SIX1 and HIF1α were detected by RT-qPCR. **P<0.01 vs. DMSO. (D) A549 cells were treated as in (A), and the protein levels of SIX1 and HIF1α were detected by western blot analysis. The right panel shows the densitometric analysis of three independent experiments. *P<0.05 vs. DMSO. (E) A549 cells were treated with Tan IIA (2.5, 5 and 10 µM) and mRNA levels of PKM2, LDHA, HK2 and SIX1 were detected by RT-qPCR. *P<0.05, **P<0.01 and ***P<0.001 vs. DMSO. (F) RT-qPCR was used to detect the mRNA level of SIX1 in A549 and H292 cell lines. *P<0.05 vs. A549 cell line. Tan IIA, Tanshinone IIA; SIX1, sine oculis homeobox homolog 1; PKM2, pyruvate kinase subtype M2; HK2, hexokinases 2; LDHA, lactate dehydrogenase A; HIF1α, hypoxia inducible factor 1α; RT-qPCR, reverse transcription-quantitative PCR.
Knockdown of SIX1 represses glycolysis in NSCLC cells
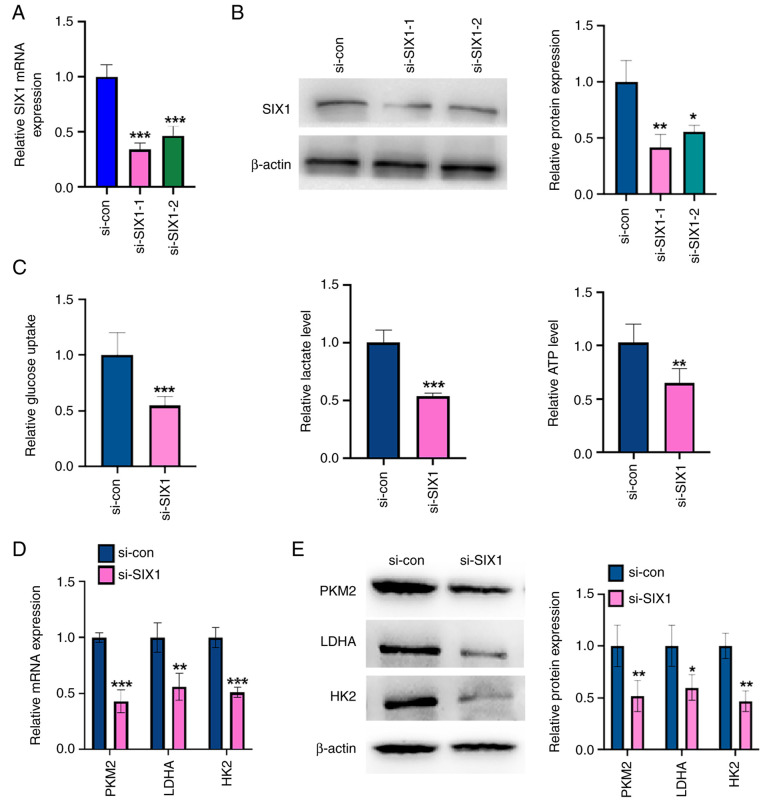
To investigate the role of SIX1 in glycolysis in NSCLC cells, loss-of-function experiments were performed. First, SIX1 expression was knocked down in A549 cells using SIX1-specific siRNA. The transfection of siRNAs against SIX1 (si-SIX1-1 and si-SIX1-2) efficiently decreased SIX1 mRNA and protein levels of SIX1 compared with those in the negative control (Fig. 3A and B), with si-SIX1-1 knocking down 70% of SIX1 mRNA expression. Therefore, si-SIX1-1 was chosen for all subsequent experiments. The knockdown of SIX1 decreased glucose uptake level, lactate production level and ATP level in A549 cells compared with those found in the negative control (Fig. 3C). Western blotting and RT-qPCR results showed that SIX1 depletion led to a significant decrease in PKM2, HK2 and LDHA expression at the mRNA and protein levels compared with those of the negative control (Fig. 3D and E). These results demonstrate that depletion of SIX1 decreases glycolysis in an NSCLC cell line.
Figure 3.
Knockdown of SIX1 represses glycolysis in non-small cell lung cancer cells. (A) A549 cells were transfected with si-SIX1 (si-SIX1-1 and si-SIX1-2) or si-con, respectively. The mRNA level of SIX1 was detected by RT-qPCR. ***P<0.001 vs. si-con. (B) A549 cells were treated as in (A), and the protein level of SIX1 was measured by western blot analysis. The right panel shows the densitometric analysis of three independent experiments. *P<0.05 and **P<0.01 vs. si-con. (C) A549 cells were transfected with si-SIX1 or si-con respectively. Glucose uptake, lactate production and ATP level were measured by corresponding assays. **P<0.01 and ***P<0.001 vs. si-con. (D) A549 cells were treated as in (C), and the mRNA levels of PKM2, LDHA and HK2 were detected by RT-qPCR. **P<0.01 and ***P<0.001 vs. si-con. (E) A549 cells were treated as in (C), and the protein levels of PKM2, LDHA and HK2 were detected by western blot analysis. The right panel shows the densitometric analysis of three independent experiments. *P<0.05 and **P<0.01 vs. si-con. Tan IIA, Tanshinone IIA; SIX1, sine oculis homeobox homolog 1; PKM2, pyruvate kinase subtype M2; HK2, hexokinases 2; LDHA, lactate dehydrogenase A; RT-qPCR, reverse transcription-quantitative PCR; si-, small interfering; con, control.
SIX1 expression is upregulated in NSCLC tissues and correlates with LDH level in patients with NSCLC
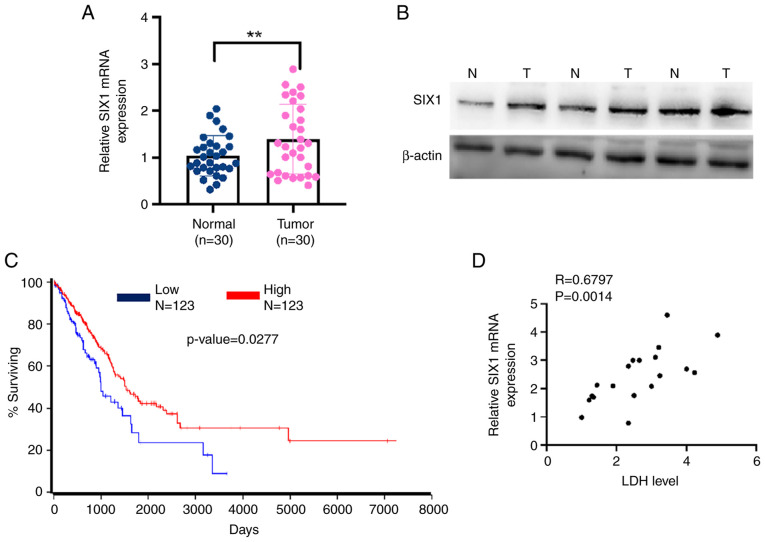
Previous studies have shown that SIX1 expression is correlated with the prognosis of multiple types of cancer (26,27). Therefore, the present study investigated SIX1 expression in NSCLC tissues. As shown in Fig. 4A and B, SIX1 expression was markedly upregulated in NSCLC tissues compared with the levels found in adjacent noncancerous lung tissues. More importantly, Kaplan-Meier survival analysis of TCGA data using OncoLnc (http://www.oncolnc.org/) revealed that a low level of SIX1 was associated with a lower survival rate in patients with NSCLC (Fig. 4C). A positive correlation was also found between SIX1 expression and LDH level (R=0.6797). These data support the fact that lower expression of SIX1 correlates with the increased LDH level and the poor prognosis of patients with NSCLC.
Figure 4.
SIX1 is upregulated in NSCLC tissues and correlates with LDH level in patients with NSCLC. (A) mRNA levels of SIX1 in NSCLC tissues (n=30) and normal lung tissues (n=30) were detected by reverse transcription-quantitative PCR. **P<0.01 vs. normal lung tissue. (B) The protein levels of SIX1 in NSCLC tissues and normal lung tissues were detected by western blot analysis. (C) The overall survival of NSCLC patients with low or high levels of SIX1 was analyzed by Kaplan-Meier analysis of data from The Cancer Genome Atlas database (P=0.00319). (D) The correlation between mRNA expression level of SIX1 and serum LDH level in patients with NSCLC was analyzed by Pearson's correlation analysis (R=0.6796, P=0.0014). NSCLC, non-small cell lung cancer; SIX1, sine oculis homeobox homolog 1; T, tumor; N, normal; LDH, lactate dehydrogenase.
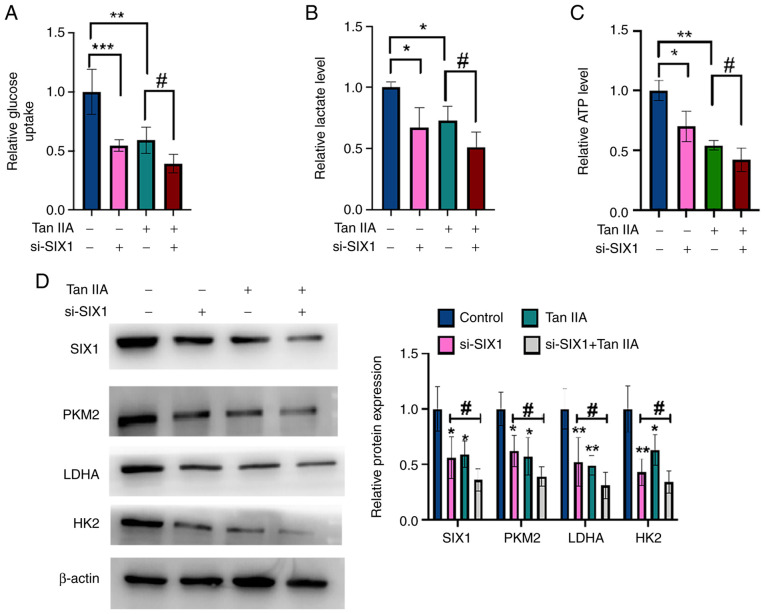
Tan IIA decreases glycolysis in NSCLC cells through SIX1
To investigate whether SIX1 plays a role in the Tan IIA-induced decrease in glycolysis, rescue experiments were performed. A549 cells were transfected with si-SIX1 or negative control and then treated with Tan IIA or DMSO. As shown in Fig. 5A-C, glycolysis in NSCLC cells was inhibited by Tan IIA treatment and this inhibitory effect was enhanced by the simultaneous knockdown of SIX1. Western blotting and RT-qPCR data revealed that Tan IIA treatment decreased the protein and mRNA levels of SIX1, PKM2, HK2 and LDHA, and that the knockdown of SIX1 combined with Tan IIA treatment significantly enhanced this effect compared with Tan IIA treatment alone (Fig. 5D). Collectively, these findings demonstrate that SIX1 plays a crucial role in the decrease of glycolysis induced by Tan IIA in NSCLC cells.
Figure 5.
Tan IIA decreases glycolysis in non-small cell lung cancer cells through SIX1. (A) A549 cells were transfected with si-SIX1 or si-con and then treated with Tan IIA or DMSO. Glucose uptake was detected by glucose assay kit. (B) A549 cells were treated as in (A), and lactate level was detected by lactate assay kit. (C) A549 cells were treated as in (A), and ATP level was detected by ATP assay kit. (D) A549 cells were treated as in (A), and the protein levels of SIX1, PKM2, LDHA and HK2 were detected by western blot analysis. The right panel shows the densitometric analysis of three independent experiments. *P<0.05, ***P<0.001, **P<0.01 and ##P<0.05 vs. corresponding control. SIX1, sine oculis homeobox homolog 1; Tan IIA, Tanshinone IIA; si-, small interfering; con, control; PKM2, pyruvate kinase subtype M2; HK2, hexokinases 2; LDHA, lactate dehydrogenase A.
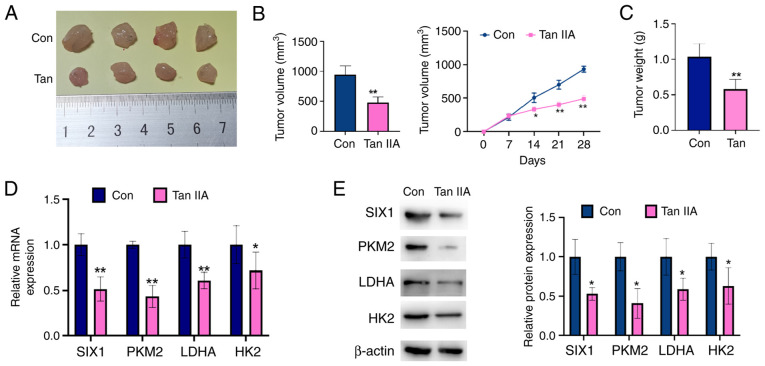
Tan IIA suppresses NSCLC cell growth in vivo
To further confirm the antitumor role of Tan IIA in NSCLC, a nude mouse xenograft model of NSCLC was established. First, A549 cells were implanted into nude mice. After 8 days, the mice were administered Tan IIA. As shown in Fig. 6A-C, the Tan IIA-treated group presented with smaller tumor volumes and lower tumor weights compared with those measured in the control group. In addition, the expression levels of SIX1, PKM2, HK2 and LDHA were determined in the tumor tissues by RT-qPCR and western blot analysis. As shown in Fig. 6D and E, SIX1, PKM2, HK2 and LDHA levels were significantly decreased in the tumors of the Tan IIA-treated group compared with those of the controls. These data reveal that Tan IIA treatment decreases tumor growth and suppresses SIX1 expression in vivo.
Figure 6.
Tan IIA suppresses non-small cell lung cancer cell growth in vivo. A549 cells were injected into the right posterior ankle of the nude mice to establish xenograft tumors. From the eighth day, mice were intraperitoneally injected with Tan IIA (20 mg/kg) for 2 weeks. (A) Representative tumor sizes in each group of mice. (B) Tumor volumes were monitored by direct measurement. *P<0.05 and **P<0.01 vs. control group. (C) Xenograft tumor wet weight in each group of mice. **P<0.01 vs. control group. (D) The mRNA levels of SIX1, PKM2, LDHA and HK2 in xenograft tumor tissues were detected by reverse transcription-quantitative PCR. *P<0.05 and **P<0.01 vs. control group. (E) The protein levels of SIX1, PKM2, LDHA and HK2 in xenograft tumor tissues were detected by western blot analysis. The right panel shows the densitometric analysis of three independent experiments. *P<0.05 vs. control group. SIX1, sine oculis homeobox homolog 1; Tan IIA, Tanshinone IIAcon, control; PKM2, pyruvate kinase subtype M2; HK2, hexokinases 2; LDHA, lactate dehydrogenase A.
Discussion
It is well known that the uncontrolled proliferation of tumor cells is mainly due to their monoclonality. However, increasing evidence indicates that malignancy is not only a clonal disease, but also a metabolic disease (28). Indeed, the rapid growth of tumor cells depends on their unique metabolic pathways, especially in glucose metabolism (29). The Warburg effect allows tumor cells to undergo aerobic glycolysis to provide energy, even in the presence of sufficient oxygen, leading to increased glucose uptake and lactate production (30,31). This characteristic of glucose metabolism renders tumor cells more adaptable to hypoxia and contributes to cancer progression (32,33). Therefore, inhibiting aerobic glycolysis and then blocking the energy metabolism of neoplastic cells may constitute an effective antitumor treatment.
In the present study, it was demonstrated that Tan IIA, a constituent of Salvia miltiorrhiza, significantly inhibited NSCLC cell growth in vivo and in vitro, which was consistent with earlier studies (19,34). Moreover, Tan IIA decreased the glucose uptake, ATP level and lactate production of the NSCLC cells suggesting that Tan IIA exerts an anti-Warburg effect in NSCLC. Previous studies showed that Tan IIA inhibited the Warburg effect in various neoplasms. For example, Liu et al (35) reported that Tan IIA treatment led to decreased glycolysis in cervical cancer and subsequently to cell apoptosis. Li et al (36) revealed that Tan IIA exerted its antitumor effect by suppressing HK2-mediated glycolysis in oral squamous cell carcinoma. In the present study, it was demonstrated that Tan IIA treatment effectively suppressed glycolysis by interfering with glucose uptake, lactate production and ATP level. Moreover, Zhang et al (37) reported that Tan IIA suppressed PKM2 expression in esophageal cancer cells by upregulating the microRNA (miR)-122 level. Liu et al (35) also showed that treatment of Tan IIA downregulated PKM2 and HK2 expression in cervical cancer cells, thus leading to cell apoptosis. The present study confirmed that Tan IIA treatment decreased PKM2, LDHA and LDHA mRNA and protein levels. These are key enzymes in the glycolytic pathway in A549 cells. Although the anticancer effect of Tan IIA in NSCLC cells was shown in vivo and in vitro, clinical trials to test the safety and efficacy of Tan IIA in patients with cancer are needed.
Since Li et al (13) first demonstrated that SIX1 was a critical transcription factor involved in the Warburg effect and contributing to tumorigenesis, research has focused on suppressing SIX1 gene expression. For example, Nie et al (38) reported that miR-140-5p targeted SIX1 directly in leukemia cells. Translation of miR-140-5p mimics markedly downregulated SIX1 level and inhibited leukemia cell growth. In addition, miR-23a-3p negatively regulated the post-transcription level of SIX1 in head and neck squamous cell carcinomas (HNSCC). SIX1 expression was decreased and HNSCC cell growth was suppressed in vitro by transfection of miR-23a-3p mimics (39). Furthermore, Zhou et al revealed that SIX1 was highly modified by O-GlcNAcylation in HCC cells, which could inhibit the ubiquitination degradation of SIX1 (40). In addition, Chu et al (41) reported that the compound 8430 decreased SIX1 transcriptional activity and MCF7 cell proliferation in vitro, indicating that 8430 might serve as a promising adjuvant in the future. In the present study, it was demonstrated that Tan IIA treatment downregulated the mRNA and protein levels of SIX1 in A549 cells, indicating that Tan IIA might function as a SIX1 inhibitor. Knockdown of SIX1 expression downregulated the mRNA and protein levels of PKM2, HK2 and LDHA. Importantly, depletion of SIX1 expression led to a decrease in ATP level, lactate production and glucose uptake. These results further explained the mechanisms by which Tan IIA regulated PKM2, HK2 and LDHA expression. Previous studies found that Tan IIA decreased the HIF1α level in colorectal cancer and breast cancer (42,43). However, in the present study, Tan IIA treatment (5 µM) did not affect the HIF1α expression in A549 cells, possibly as a different cell line was used, in which Tan IIA might trigger different mechanisms. Therefore, the underlying mechanism involved in the regulation of SIX1 expression by Tan IIA in NSCLC cells needs to be further investigated.
The high expression of SIX1 is linked to the prognosis of patients with cancer. For example, in a previous study, SIX1 expression was increased in prostate cancer tissues compared with that in normal tissues, The high level of SIX1 was an independent prognostic indicator and correlated with a high histological grade and clinical stage in prostate cancer (44). In glioma, the higher expression of SIX1 is more frequent in high-grade glioma and is correlated with a poor clinical outcome (22). Consistent with these previous studies, the present study found increased SIX1 expression levels in NSCLC tissues and cell lines. TCGA database analysis revealed that lower expression of SIX1 was closely associated with the poor prognosis of patients with NSCLC. Notably, increased SIX1 expression was positively correlated with the LDH serum levels of the patients with NSCLC. These results also confirmed the important role of SIX1 in metabolism. The association between SIX1 and LDH in NSCLC tissues provides a basis for the future selection of treatment for clinical patients. Indeed, patients with higher serum LDH levels might benefit from Tan IIA treatment.
In conclusion, the present study revealed the antitumor effect of Tan IIA in lung cancer in vitro and in vivo. Tan IIA decreased SIX1 expression and inhibited glycolysis in NSCLC cells. The results indicate that Tan IIA is an anti-Warburg effect agent and may constitute a novel treatment for patients with NSCLC.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- TCGA
The Cancer Genome Atlas
- NSCLC
non-small cell lung cancer
- Tan IIA
Tanshinone IIA
- SIX1
sine oculis homeobox homolog 1
- PKM2
pyruvate kinase subtype M2
- HK2
hexokinases 2
- LDHA
lactate dehydrogenase A
- RT-qPCR
reverse transcription-quantitative PCR
Funding Statement
This study was supported by the Scientific Research Foundation of Hebei Administration of Traditional Chinese Medicine (grant no. 2022383).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request. The data showing the association between SIX1 and the prognosis of patients with lung cancer are available from OncoLnc (http://www.oncolnc.org/kaplan).
Authors' contributions
YL was responsible for the conception and design of the study. HQ, ZC and XW were responsible for study data acquisition. HQ, ZZ and YQ was involved in the development of the study methodology, analysis and interpretation of the data. HQ, ZC, YQ and YL were involved in the writing, reviewing and revision of the article, and analyzed the relevant literature. HQ and YL confirm the authenticity of the raw data. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
All the protocols of this study were approved by the Ethics Committee of Hebei Chest Hospital. All patients provided written informed consent. All animal experiments were approved by the Institutional Animal Care and Use Committee of Hebei Medical University (approval no. HebMU 20,080,026). Hebei Provincial Chest Hospital is an affiliated hospital of Hebei Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21601. [DOI] [PubMed] [Google Scholar]
- 2.Ettinger DS, Wood DE, Aggarwal C, Aisner DL, Akerley W, Bauman JR, Bharat A, Bruno DS, Chang JY, Chirieac LR, et al. NCCN guidelines insights: Non-small cell lung cancer, version 1.2020. J Natl Compr Canc Netw. 2019;17:1464–1472. doi: 10.6004/jnccn.2019.0059. [DOI] [PubMed] [Google Scholar]
- 3.Chen Z, Fillmore CM, Hammerman PS, Kim CF, Wong KK. Non-small-cell lung cancers: A heterogeneous set of diseases. Nat Rev Cancer. 2014;14:535–546. doi: 10.1038/nrc3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature. 2018;553:446–454. doi: 10.1038/nature25183. [DOI] [PubMed] [Google Scholar]
- 5.Rosner S, Reuss JE, Forde PM. PD-1 blockade in early-stage lung cancer. Annu Rev Med. 2019;70:425–435. doi: 10.1146/annurev-med-050217-025205. [DOI] [PubMed] [Google Scholar]
- 6.Pastorino U, Buyse M, Friedel G, Ginsberg RJ, Girard P, Goldstraw P, Johnston M, McCormack P, Pass H, Putnam JB, Jr, International Registry of Lung Metastases Long-term results of lung metastasectomy: Prognostic analyses based on 5206 cases. J Thorac Cardiovasc Surg. 1997;113:37–49. doi: 10.1016/S0022-5223(97)70397-0. [DOI] [PubMed] [Google Scholar]
- 7.Wu W, Ren Z, Li P, Yu D, Chen J, Huang R, Liu H. Six1: A critical transcription factor in tumorigenesis. Int J Cancer. 2015;136:1245–1253. doi: 10.1002/ijc.28755. [DOI] [PubMed] [Google Scholar]
- 8.Chu Y, Chen Y, Li M, Shi D, Wang B, Lian Y, Cheng X, Wang X, Xu M, Cheng T, et al. Six1 regulates leukemia stem cell maintenance in acute myeloid leukemia. Cancer Sci. 2019;110:2200–2210. doi: 10.1111/cas.14033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coletta RD, Christensen KL, Micalizzi DS, Jedlicka P, Varella-Garcia M, Ford HL. Six1 overexpression in mammary cells induces genomic instability and is sufficient for malignant transformation. Cancer Res. 2008;68:2204–2213. doi: 10.1158/0008-5472.CAN-07-3141. [DOI] [PubMed] [Google Scholar]
- 10.Coletta RD, McCoy EL, Burns V, Kawakami K, McManaman JL, Wysolmerski JJ, Ford HL. Characterization of the Six1 homeobox gene in normal mammary gland morphogenesis. BMC Dev Biol. 2010;10:4. doi: 10.1186/1471-213X-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y, Wang S, Liu Z, Yang L, Liu J, Xiu M. Increased Six1 expression in macrophages promotes hepatocellular carcinoma growth and invasion by regulating MMP-9. J Cell Mol Med. 2019;23:4523–4533. doi: 10.1111/jcmm.14342. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Yang C, Xu W, Gong J, Chai F, Cui D, Liu Z. Six1 overexpression promotes glucose metabolism and invasion through regulation of GLUT3, MMP2 and snail in thyroid cancer cells. Onco Targets Ther. 2020;13:4855–4863. doi: 10.2147/OTT.S227291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li L, Liang Y, Kang L, Liu Y, Gao S, Chen S, Li Y, You W, Dong Q, Hong T, et al. Transcriptional regulation of the Warburg effect in cancer by SIX1. Cancer Cell. 2018;33:368–385.e7. doi: 10.1016/j.ccell.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 14.Liu Q, Li A, Tian Y, Liu Y, Li T, Zhang C, Wu JD, Han X, Wu K. The expression profile and clinic significance of the SIX family in non-small cell lung cancer. J Hematol Oncol. 2016;9:119. doi: 10.1186/s13045-016-0339-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li N, Yang L, Zhang B, Chen S. Tanshinone IIA effects on ovarian cancer cell line. J Pharm Pharmacol. 2018;70:1369–1377. doi: 10.1111/jphp.12961. [DOI] [PubMed] [Google Scholar]
- 16.International BR. Retracted: Tanshinone IIA induces apoptosis in human oral cancer KB cells through a mitochondria-dependent pathway. Biomed Res Int. 2017;2017:9496485. doi: 10.1155/2017/9496485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiu SC, Huang SY, Chen SP, Su CC, Chiu TL, Pang CY. Tanshinone IIA inhibits human prostate cancer cells growth by induction of endoplasmic reticulum stress in vitro and in vivo. Prostate Cancer Prostatic Dis. 2013;16:315–322. doi: 10.1038/pcan.2013.38. [DOI] [PubMed] [Google Scholar]
- 18.Nie ZY, Zhao MH, Cheng BQ, Pan RF, Wang TR, Qin Y, Zhang XJ. Tanshinone IIA regulates human AML cell proliferation, cell cycle, and apoptosis through miR-497-5p/AKT3 axis. Cancer Cell Int. 2020;20:379. doi: 10.1186/s12935-020-01468-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao F, Li M, Liu W, Li W. Inhibition of EGFR signaling and activation of mitochondrial apoptosis contribute to tanshinone IIA-mediated tumor suppression in non-small cell lung cancer cells. Onco Targets Ther. 2020;13:2757–2769. doi: 10.2147/OTT.S246606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liao XZ, Gao Y, Huang S, Chen ZZ, Sun LL, Liu JH, Chen HR, Yu L, Zhang JX, Lin LZ. Tanshinone IIA combined with cisplatin synergistically inhibits non-small-cell lung cancer in vitro and in vivo via down-regulating the phosphatidylinositol 3-kinase/Akt signalling pathway. Phytother Res. 2019;33:2298–2309. doi: 10.1002/ptr.6392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xie J, Liu J, Liu H, Liang S, Lin M, Gu Y, Liu T, Wang D, Ge H, Mo SL. The antitumor effect of tanshinone IIA on anti-proliferation and decreasing VEGF/VEGFR2 expression on the human non-small cell lung cancer A549 cell line. Acta Pharm Sin B. 2015;5:554–563. doi: 10.1016/j.apsb.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang X, Xu R. Six1 expression is associated with a poor prognosis in patients with glioma. Oncol Lett. 2017;13:1293–1298. doi: 10.3892/ol.2017.5577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chassagnon G, Bennani S, Revel MP. New TNM classification of non-small cell lung cancer. Rev Pneumol Clin. 2017;73:34–39. doi: 10.1016/j.pneumo.2016.12.006. (In French) [DOI] [PubMed] [Google Scholar]
- 24.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 25.Yen JH, Huang ST, Huang HS, Fong YC, Wu YY, Chiang JH, Su YC. HGK-sestrin 2 signaling-mediated autophagy contributes to antitumor efficacy of Tanshinone IIA in human osteosarcoma cells. Cell Death Dis. 2018;9:1003. doi: 10.1038/s41419-018-1016-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chao L, Liu J, Zhao D. Increased Six1 expression is associated with poor prognosis in patients with osteosarcoma. Oncol Lett. 2017;13:2891–2896. doi: 10.3892/ol.2017.5803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li W, Qin Y, Zhou R, Liu Y, Zhang G. High expression of SIX1 is an independent predictor of poor prognosis in endometrial cancer. Am J Transl Res. 2021;13:2840–2848. [PMC free article] [PubMed] [Google Scholar]
- 28.Bose S, Le A. Glucose metabolism in cancer. Adv Exp Med Biol. 2018;1063:3–12. doi: 10.1007/978-3-319-77736-8_1. [DOI] [PubMed] [Google Scholar]
- 29.Luengo A, Gui DY, Vander Heiden MG. Targeting metabolism for cancer therapy. Cell Chem Biol. 2017;24:1161–1180. doi: 10.1016/j.chembiol.2017.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pascale RM, Calvisi DF, Simile MM, Feo CF, Feo F. The Warburg effect 97 years after its discovery. Cancers (Basel) 2020;12:2819. doi: 10.3390/cancers12102819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vaupel P, Schmidberger H, Mayer A. The Warburg effect: Essential part of metabolic reprogramming and central contributor to cancer progression. Int J Radiat Biol. 2019;95:912–919. doi: 10.1080/09553002.2019.1589653. [DOI] [PubMed] [Google Scholar]
- 32.Ishibashi K, Egami R, Nakai K, Kon S. An anti-tumorigenic role of the Warburg effect at emergence of transformed cells. Cell Struct Funct. 2018;43:171–176. doi: 10.1247/csf.18018. [DOI] [PubMed] [Google Scholar]
- 33.Bar-Or D, Carrick M, Tanner A, II, Lieser MJ, Rael LT, Brody E. Overcoming the Warburg effect: Is it the key to survival in sepsis? J Crit Care. 2018;43:197–201. doi: 10.1016/j.jcrc.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 34.Li Z, Zhang Y, Zhou Y, Wang F, Yin C, Ding L, Zhang S. Tanshinone IIA suppresses the progression of lung adenocarcinoma through regulating CCNA2-CDK2 complex and AURKA/PLK1 pathway. Sci Rep. 2021;11:23681. doi: 10.1038/s41598-021-03166-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Z, Zhu W, Kong X, Chen X, Sun X, Zhang W, Zhang R. Tanshinone IIA inhibits glucose metabolism leading to apoptosis in cervical cancer. Oncol Rep. 2019;42:1893–1903. doi: 10.3892/or.2019.7294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li M, Gao F, Zhao Q, Zuo H, Liu W, Li W. Tanshinone IIA inhibits oral squamous cell carcinoma via reducing Akt-c-Myc signaling-mediated aerobic glycolysis. Cell Death Dis. 2020;11:381. doi: 10.1038/s41419-020-2579-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang HS, Zhang FJ, Li H, Liu Y, Du GY, Huang YH. Tanshinone IIA inhibits human esophageal cancer cell growth through miR-122-mediated PKM2 down-regulation. Arch Biochem Biophys. 2016;598:50–56. doi: 10.1016/j.abb.2016.03.031. [DOI] [PubMed] [Google Scholar]
- 38.Nie ZY, Liu XJ, Zhan Y, Liu MH, Zhang XY, Li ZY, Lu YQ, Luo JM, Yang L. miR-140-5p induces cell apoptosis and decreases Warburg effect in chronic myeloid leukemia by targeting SIX1. Biosci Rep. 2019;39:BSR20190150. doi: 10.1042/BSR20190150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang H, Xue W, Ouyang W, Jiang X, Jiang X. miR-23a-3p/SIX1 regulates glucose uptake and proliferation through GLUT3 in head and neck squamous cell carcinomas. J Cancer. 2020;11:2529–2539. doi: 10.7150/jca.30995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou H, Blevins MA, Hsu JY, Kong D, Galbraith MD, Goodspeed A, Culp-Hill R, Oliphant MUJ, Ramirez D, Zhang L, et al. Identification of a small-molecule inhibitor that disrupts the SIX1/EYA2 complex, EMT, and metastasis. Cancer Res. 2020;80:2689–2702. doi: 10.1158/0008-5472.CAN-20-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chu Y, Jiang M, Wu N, Xu B, Li W, Liu H, Su S, Shi Y, Liu H, Gao X, et al. O-GlcNAcylation of SIX1 enhances its stability and promotes hepatocellular carcinoma proliferation. Theranostics. 2020;10:9830–9842. doi: 10.7150/thno.45161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sui H, Zhao J, Zhou L, Wen H, Deng W, Li C, Ji Q, Liu X, Feng Y, Chai N, et al. Tanshinone IIA inhibits β-catenin/VEGF-mediated angiogenesis by targeting TGF-β1 in normoxic and HIF-1α in hypoxic microenvironments in human colorectal cancer. Cancer Lett. 2017;403:86–97. doi: 10.1016/j.canlet.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 43.Li G, Shan C, Liu L, Zhou T, Zhou J, Hu X, Chen Y, Cui H, Gao N. Tanshinone IIA inhibits HIF-1α and VEGF expression in breast cancer cells via mTOR/p70S6K/RPS6/4E-BP1 signaling pathway. PLoS One. 2015;10:e0117440. doi: 10.1371/journal.pone.0117440. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 44.Zeng J, Shi R, Cai CX, Liu XR, Song YB, Wei M, Ma WL. Increased expression of Six1 correlates with progression and prognosis of prostate cancer. Cancer Cell Int. 2015;15:63. doi: 10.1186/s12935-015-0215-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request. The data showing the association between SIX1 and the prognosis of patients with lung cancer are available from OncoLnc (http://www.oncolnc.org/kaplan).