Abstract
With the advent of novel systemic therapies, such as molecular targeted therapy and immune checkpoint inhibitors, the treatment of advanced-stage cancer is markedly transitioning. The treatment for brain metastasis is influenced by these new therapies. Moreover, the frequency of brain metastasis is associated with cancer genetics. Since conventional cytotoxic chemotherapeutic drugs cannot easily cross the brain-blood barrier, radiotherapy plays a major role in the management of brain metastasis. Whole-brain radiotherapy (WBRT) has been frequently used, especially for multiple metastatic brain tumors; however, late adverse effects on cognitive function are a significant clinical problem of WBRT in patients with an otherwise good prognosis and overall survival rate. Some novel systemic agents are effective against brain metastasis. Moreover, advances in radiotherapy technology have made it possible to deliver optimal radiation doses to patients with brain metastasis, with fewer adverse events. Brain metastasis has a significant impact on the quality of life of patients with advanced-stage cancer; therefore, its appropriate management is an important factor in the comprehensive treatment of cancer.
Keywords: brain metastasis, radiotherapy, systemic therapy, immunotherapy, cancer
1. Introduction
Between 10 and 15% of patients with cancer develop metastatic brain tumors (1–3). The most frequent primary site of metastasis is the lung, followed by the breast, gastrointestinal tract, kidneys and skin (4–6). The frequency of brain metastasis is linked to the genetic background of the cancer. Patients with advanced non-small cell lung cancer (NSCLC) with epidermal growth factor receptor (EGFR) mutation have a higher rate of brain metastasis, even after adjusting for differences in survival, compared with wild-type patients (7). Brain metastasis is more prevalent in patients with human epidermal growth factor type 2 (HER2)-positive or triple-negative metastatic breast cancer (8).
With the recent development of molecular targeted therapies and immune checkpoint inhibitors (ICIs), the management of metastatic brain tumors is undergoing major changes with respect to the systemic treatment of cancer. Cytotoxic chemotherapy is ineffective in treating intracranial lesions due to the blood-brain barrier (BBB) (9). Therefore, whole-brain radiotherapy (WBRT) has been the primary treatment for metastatic brain tumors, especially in cases with multiple lesions (10).
Since the development of irradiation techniques, an emphasis has been placed on treatment strategies focusing on long-term outcomes without severe clinical adverse effects (11,12). A linear accelerator, which is widely used in cancer treatment, is a device that speeds up electrons with a small linear accelerator and collides them with a metal target to produce X-rays of 4–10 MV. Recent progress in image-guided radiation therapy and intensity-modulated radiation therapy (IMRT) has made it possible to treat several brain metastases at once in a non-invasive manner (13). The combination of high-dose-delivery mode and volumetric modulated arc therapy, which is an advanced type of IMRT, has made it possible to deliver a high dose of radiation suitable for stereotactic radiosurgery in a short period (14,15).
In recent years, the advent of immunotherapy has revolutionized the systemic treatment of cancer, which has led to the reconsideration of treatment strategies for brain metastasis. We consider that it is necessary to review the management of brain metastasis while factoring in the use of the novel therapeutic agents. The present study reviews the general management of brain metastasis and how it has been impacted by the recent advances in systemic therapy and radiation therapy.
2. Radiotherapy for brain metastasis
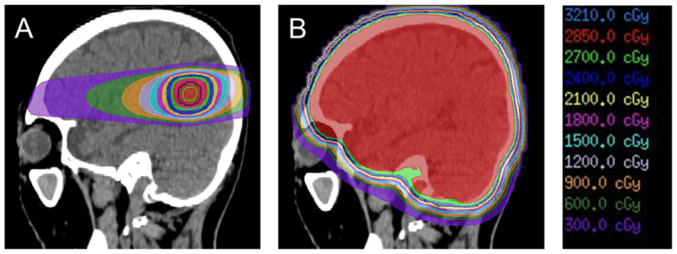
Although WBRT has been the standard treatment for multiple brain metastases, cognitive dysfunction after WBRT is a major clinical problem (16). Recently, the stereotactic irradiation (STI) technique, involving a linear accelerator, Gamma Knife or CyberKnife, was developed. Fig. 1 represents the difference in dose distribution for WBRT and STI in a metastatic brain tumor. The role of STI has become more important, as it makes it possible to focus the radiation on the lesion from multiple directions, while minimizing the dose to the surrounding normal tissue. Radiation necrosis (RN) after STI is one of the most frequent adverse events. The incidence rate of RN after stereotactic radiotherapy is reported to be 6–25% (17–20). Minniti et al (21) analyzed 310 metastatic brain tumors treated by STI and reported 75 cases of RN (24%), consisting of symptomatic 31 cases and asymptomatic 44 cases. The incidence rate of RN is associated with the maximum size of the treatment target tumor (22). According to Radiation Therapy Oncology Group protocol 90–05, the maximum tolerated dose is considered to be 24 Gy when the maximum diameter of the tumor is ≤2 cm (23). Recently, more detailed radiation parameters, such as the normal brain volume receiving a specific dose of 10 Gy or 12 Gy, have been widely used as sophisticated predictors of RN (24). Putz et al (25) revealed that fractionated stereotactic radiotherapy leads to a reduction in both RN risk [hazard ratio (HR), 0.18; P=0.045] and local progression rate (HR, 0.47; P=0.015) compared with single-session radiosurgery.
Figure 1.
Dose distribution for a metastatic brain tumor calculated by the treatment planning system of (A) stereotactic radiotherapy and (B) whole brain radiotherapy. The red-colored area is receiving 95% of the prescribed dose.
Brown et al (26) randomized 213 patients with 1–3 brain metastases to undergo treatment using STI alone or STI + WBRT. The combined therapy did not improve the overall survival (OS) rate (HR, 1.02; P=0.92) and increased cognitive dysfunction events (91.7 vs. 63.5%; P=0.04). Yamamoto et al (27) reported the clinical results of STI without WBRT for multiple brain metastases in the JLGK0901 study. Patients with 1 brain metastasis had a better prognosis than others, and no significant difference was observed in the OS rate in patients with 2–4 and 5–10 brain metastases (HR, 0.97; P=0.78). A consensus was not reached on adjuvant therapy for limited isolated brain metastases. In the 1990s, Patchell et al (28,29) revealed that adjuvant WBRT after surgical resection of brain metastases prolonged OS. Recently, adjuvant STI has provided the possibility of a comparable treatment outcome to WBRT for patients with resected brain metastases. In the randomized phase III JCOG0504 trial by Kayama et al (30), after brain metastasis surgery, patients were randomly assigned to WBRT or salvage STI treatment arms, and both arms reported a median OS time of 15.6 months. Brown et al (31) also reported that there was no significant difference in OS time when comparing postoperative STI and WBRT (12.2 vs. 11.6 months, respectively; P=0.70). By contrast, Kepka et al (32) insisted that non-inferiority of postoperative STI compared with postoperative WBRT was not demonstrated. Randall et al (33) reported that the clinical benefit of adjuvant therapy occurred through the use of ICIs after local therapy for isolated brain metastasis.
In patients with SCLC, STI is considered inappropriate for managing brain metastasis. According to the American Society of Clinical Oncology-Society for Neuro-Oncology-American Society for Radiation Oncology collaborative guidelines for the treatment of brain metastases, STI alone, without WBRT, should be offered to patients with 1–4 small brain metastases, excluding cases of SCLC (34). A prospective randomized phase III study is currently comparing STI with hippocampal-avoidance whole brain radiotherapy in patients with 5–20 brain metastases (ClinicalTrials.gov Identifier: NCT03075072). WBRT is the primary method of managing brain metastases in patients with SCLC. However, in a recent multicenter retrospective cohort study, the median OS time after STI did not significantly differ from that after WBRT in propensity score-matched analyses (6.5 vs. 5.2 months, respectively; P=0.003) (35).
Prophylactic cranial irradiation (PCI) has been used for intracranial progression control and OS benefits in patients with limited-stage SCLC (36,37). According to a recent meta-analysis, patients treated with PCI exhibited decreased brain metastasis (HR, 0.45; P<0.001) and prolonged OS times (HR, 0.81; P<0.001) compared with those without PCI treatment (38). However, with the widespread use of diagnostic imaging techniques, such as magnetic resonance imaging (MRI) and high-precision radiotherapy techniques, the use of PCI has become controversial. Pezzi et al (39) reported that PCI did not prolong OS time and increase intracranial control compared with MRI, as assessed using a propensity score-matching analysis. Identification of appropriate patient groups for the use of PCI will be necessary in the future.
3. Molecular targeted therapy for brain metastasis
A new generation of molecular targeted therapies is expected to have notable therapeutic efficacy in intracranial lesions (40). Osimertinib, a third-generation EGFR-tyrosine kinase inhibitor (TKI), is highly effective in controlling intracranial lesions, as shown by a subset analysis of the FLAURA trial (41). In this study, 20 out of 22 patients with EGFR mutation-positive advanced NSCLC, who had evaluable intracranial lesions, achieved an intracranial response. Lorlatinib is a third-generation anaplastic lymphoma kinase (ALK)-TKI that was developed to improve the central nervous system (CNS) distribution of alectinib and is expected to have a high suppressive effect on CNS lesions (42). In the recently reported CROWN trial, lorlatinib had an intracranial response efficiency of 82% in patients with advanced ALK-positive NSCLC without prior systemic therapy and with evaluable brain metastases (43). A clinical randomized phase III trial is currently evaluating the clinical advantage of upfront radiotherapy before systemic therapy in patients with driver-mutated NSCLC and asymptomatic brain metastases (ClinicalTrials.gov Identifier: NCT05236946).
Tiramurtinib, a second-generation Bruton's tyrosine kinase inhibitor, has shown a good intracranial response in patients with relapsed and refractory CNS lymphoma (44). Tucatinib, a HER2 inhibitor, improves intracranial response efficiency when included in a regimen consisting of trastuzumab and capecitabine for patients with HER2-positive breast cancer and brain metastasis (45).
4. Novel immunotherapy for brain metastasis
In recent years, cancer treatment has advanced significantly, and prognostic techniques and systemic therapy, especially in advanced-stage cancer, have changed markedly. For example, the development of ICIs was given the Nobel Prize in Physiology or Medicine in 2018 (46). Table I represents frequently used ICIs in cancer therapy. Initially, it was hypothesized that monoclonal antibodies, including ICIs, could not pass through the BBB due to their large molecular size. ICIs were considered to be ineffective against intracranial lesions as they are antibody therapies and do not directly pass through the BBB. However, ICIs have exhibited good results when used for the treatment of brain metastasis, as they not only directly penetrate the brain, but also stimulate a systemic immune response against malignant cells (47). Goldberg et al (48) evaluated the efficacy of pembrolizumab, an anti-programmed cell death-1 antibody, for brain metastases in a phase II trial and reported that 29.7% of patients with programmed cell death-ligand 1 (PD-L1)-positive NSCLC brain metastases responded to the treatment. The CheckMate 204 study reported a response rate of 57% for intracranial lesions in patients with brain metastases from malignant melanoma treated with the anti-PD-L1 antibody nivolumab in combination with the anti-cytotoxic T-lymphocyte-associated protein 4 antibody ipilimumab (49). This result is similar to the response rate of extracranial lesions in the same study. Further studies are needed to identify whether ICIs or STIs should be administered first. An ongoing clinical trial is currently evaluating the timing of STI with respect to ICI (ClinicalTrials.gov Identifier: NCT04650490).
Table I.
Frequently used immune checkpoint inhibitors and their molecular targets.
| Molecular target | Generic drug name | Brand name |
|---|---|---|
| PD-1 | Pembrolizumab | Keytruda |
| Nivolumab | Opdivo | |
| PD-L1 | Atezolizumab | Tecentriq |
| Durvalumab | Imfinzi | |
| Avelumab | Bavencio | |
| CTLA-4 | Ipilimumab | Yervoy |
PD-1, programmed cell death-1; PD-L1, programmed cell death-ligand 1; CTLA-4, cytotoxic T-lymphocyte-associated protein-4.
5. Assessment of patient prognosis
When deciding a treatment strategy for a single patient, one of the principal components to be considered is patient prognosis. In the 1990s, the Radiation Therapy Oncology Group designated patient groups based on clinical trial data and reported on the recursive partitioning analysis (RPA) score (50), which was calculated from Karnofsky Performance Status (51), age and extracranial disease status. The median survival time was 7.1 months for RPA class I, 4.2 months for class II and 2.3 months for class III. Due to its simplicity, the RPA classification has been widely used for the assessment of patient prognosis in a clinical setting. However, its disadvantage is that it does not include the differences in prognosis due to the number of brain lesions and primary cancer sites. Moreover, in the era of its first use, brain metastasis was mainly treated with WBRT.
Sperduto et al (52) reported a new prognostic index, the graded prognostic assessment (GPA) score, which revealed a higher effectiveness than the previous indexes, including the RPA score. In 2010, the GPA score was refined to the diagnosis-specific GPA (DS-GPA) score, which was classified into five types of primary cancer sites: NSCLC, breast cancer, renal cell cancer, gastrointestinal cancer and malignant melanoma (53). However, since the DS-GPA is limited to five types of cancer, brain metastasis from another cancer was diagnosed as per the original GPA. The DS-GPA of each primary site was updated by incorporating recent data for cancer treatment. The lung-molGPA was a revision created by incorporating EGFR or ALK alteration data into the lung DS-GPA (54). In 2020, breast GPA was updated, reflecting the results of the retrospective multi-institutional analysis of 2,473 patients with newly diagnosed brain metastasis (55).
6. Future direction
For the treatment of brain metastases, it is necessary to carefully consider the merits and drawbacks of various treatments based on patient prognosis, primary pathology, size and number of tumors, general patient condition, systemic therapy options and performance status. According to the results of the QUARTZ trial, additional WBRT for steroid administration provides little benefit to patients, with a poor prognosis (56).
FLASH radiotherapy, applying irradiation at an extremely high dose rate that is >1,000 times higher than the conventional dose rate, is a potentially powerful therapeutic modality for cancer treatment; it reduces associated side effects and delivers safer radiation therapy (57). Montay-Gruel et al (58) reported that FLASH radiotherapy has neuroprotective effects and suppresses neurocognitive deficits after radiation therapy.
Treatment strategy for brain metastasis should be discussed by a multidisciplinary cancer board, where physicians, neurosurgeons, pathologists, radiologists and radiation oncologists work together. Combination treatment, such as a combination of ICIs and STI, augments the potential clinical efficacy in patients with advanced cancer and brain metastasis (59). An ongoing prospective cohort trial is evaluating the synergetic efficacy of the treatment response to STI and ICI in patients with NSCLC and malignant melanoma (ClinicalTrials.gov Identifier: NCT03458455). In conclusion, since brain metastasis has a significant impact on the quality of life of patients with advanced-stage cancer, appropriate management is critical.
Acknowledgements
Not applicable.
Funding Statement
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
AK wrote the manuscript and performed the literature search. HY conceived this study and participated in its design and coordination. HY and AK revised this report critically for important intellectual content. Both authors have read and approved the final manuscript. Data authentication is not applicable.
Patient consent for publication
Written informed consent was obtained from the patient for publication of the data in Fig. 1.
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Nayak L, Lee EQ, Wen PY. Epidemiology of brain metastases. Curr Oncol Rep. 2012;14:48–54. doi: 10.1007/s11912-011-0203-y. [DOI] [PubMed] [Google Scholar]
- 2.Lin X, DeAngelis LM. Treatment of brain metastases. J Clin Oncol. 2015;33:3475–3484. doi: 10.1200/JCO.2015.60.9503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sacks P, Rahman M. Epidemiology of brain metastases. Neurosurg Clin N Am. 2020;31:481–488. doi: 10.1016/j.nec.2020.06.001. [DOI] [PubMed] [Google Scholar]
- 4.Bhambhvani HP, Granucci M, Rodrigues A, Kakusa BW, Hayden Gephart M. The primary sites leading to brain metastases: Shifting trends at a tertiary care center. J Clin Neurosci. 2020;80:121–124. doi: 10.1016/j.jocn.2020.08.006. [DOI] [PubMed] [Google Scholar]
- 5.Ostrom QT, Wright CH, Barnholtz-Sloan JS. Brain metastases: Epidemiology. Handb Clin Neurol. 2018;149:27–42. doi: 10.1016/B978-0-12-811161-1.00002-5. [DOI] [PubMed] [Google Scholar]
- 6.Tabouret E, Chinot O, Metellus P, Tallet A, Viens P, Gonçalves A. Recent trends in epidemiology of brain metastases: An overview. Anticancer Res. 2012;32:4655–4662. [PubMed] [Google Scholar]
- 7.Hsu F, De Caluwe A, Anderson D, Nichol A, Toriumi T, Ho C. Patterns of spread and prognostic implications of lung cancer metastasis in an era of driver mutations. Curr Oncol. 2017;24:228–233. doi: 10.3747/co.24.3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pestalozzi BC, Zahrieh D, Price KN, Holmberg SB, Lindtner J, Collins J, Crivellari D, Fey MF, Murray E, Pagani O, et al. Identifying breast cancer patients at risk for Central Nervous System (CNS) metastases in trials of the International Breast Cancer Study Group (IBCSG) Ann Oncol. 2006;17:935–944. doi: 10.1093/annonc/mdl064. [DOI] [PubMed] [Google Scholar]
- 9.Arvanitis CD, Ferraro GB, Jain RK. The blood-brain barrier and blood-tumour barrier in brain tumours and metastases. Nat Rev Cancer. 2020;20:26–41. doi: 10.1038/s41568-019-0205-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McTyre E, Scott J, Chinnaiyan P. Whole brain radiotherapy for brain metastasis. Surg Neurol Int. 2013;4((Suppl 4)):S236–S244. doi: 10.4103/2152-7806.111301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehta SR, Suhag V, Semwal M, Sharma N. Radiotherapy: Basic concepts and recent advances. Med J Armed Forces India. 2010;66:158–162. doi: 10.1016/S0377-1237(10)80132-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gelband H, Jha P, Sankaranarayanan R, Horton S, editors. Third Edition. The International Bank for Reconstruction and Development/The World Bank; Washington, D.C.: 2015. Cancer: Disease Control Priorities. (Volume 3) [PubMed] [Google Scholar]
- 13.Xing L, Thorndyke B, Schreibmann E, Yang Y, Li TF, Kim GY, Luxton G, Koong A. Overview of image-guided radiation therapy. Med Dosim. 2006;31:91–112. doi: 10.1016/j.meddos.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 14.Otto K. Volumetric modulated arc therapy: IMRT in a single gantry arc. Med Phys. 2008;35:310–317. doi: 10.1118/1.2818738. [DOI] [PubMed] [Google Scholar]
- 15.Sapkaroski D, Osborne C, Knight KA. A review of stereotactic body radiotherapy-is volumetric modulated arc therapy the answer? J Med Radiat Sci. 2015;62:142–151. doi: 10.1002/jmrs.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeAngelis LM, Delattre JY, Posner JB. Radiation-induced dementia in patients cured of brain metastases. Neurology. 1989;39:789–796. doi: 10.1212/WNL.39.6.789. [DOI] [PubMed] [Google Scholar]
- 17.Ali FS, Arevalo O, Zorofchian S, Patrizz A, Riascos R, Tandon N, Blanco A, Ballester LY, Esquenazi Y. Cerebral radiation necrosis: Incidence, pathogenesis, diagnostic challenges, and future opportunities. Curr Oncol Rep. 2019;21:66. doi: 10.1007/s11912-019-0818-y. [DOI] [PubMed] [Google Scholar]
- 18.Kim JM, Miller JA, Kotecha R, Xiao R, Juloori A, Ward MC, Ahluwalia MS, Mohammadi AM, Peereboom DM, Murphy ES, et al. The risk of radiation necrosis following stereotactic radiosurgery with concurrent systemic therapies. J Neurooncol. 2017;133:357–368. doi: 10.1007/s11060-017-2442-8. [DOI] [PubMed] [Google Scholar]
- 19.Donovan EK, Parpia S, Greenspoon JN. Incidence of radionecrosis in single-fraction radiosurgery compared with fractionated radiotherapy in the treatment of brain metastasis. Curr Oncol. 2019;26:e328–e333. doi: 10.3747/co.26.4749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vellayappan BA, McGranahan T, Graber J, Taylor L, Venur V, Ellenbogen R, Sloan AE, Redmond KJ, Foote M, Chao ST, et al. Radiation necrosis from stereotactic radiosurgery-how do we mitigate? Curr Treat Options Oncol. 2021;22:57. doi: 10.1007/s11864-021-00854-z. [DOI] [PubMed] [Google Scholar]
- 21.Minniti G, Clarke E, Lanzetta G, Osti MF, Trasimeni G, Bozzao A, Romano A, Enrici RM. Stereotactic radiosurgery for brain metastases: Analysis of outcome and risk of brain radionecrosis. Radiat Oncol. 2011;6:48. doi: 10.1186/1748-717X-6-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kohutek ZA, Yamada Y, Chan TA, Brennan CW, Tabar V, Gutin PH, Yang TJ, Rosenblum MK, Ballangrud Å, Young RJ, et al. Long-term risk of radionecrosis and imaging changes after stereotactic radiosurgery for brain metastases. J Neurooncol. 2015;125:149–156. doi: 10.1007/s11060-015-1881-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shaw E, Scott C, Souhami L, Dinapoli R, Kline R, Loeffler J, Farnan N. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: Final report of RTOG protocol 90-05. Int J Radiat Oncol Biol Phys. 2000;47:291–298. doi: 10.1016/S0360-3016(99)00507-6. [DOI] [PubMed] [Google Scholar]
- 24.Loo M, Clavier JB, Attal Khalifa J, Moyal E, Khalifa J. Dose-Response effect and dose-toxicity in stereotactic radiotherapy for brain metastases: A review. Cancers (Basel) 2021;13:6086. doi: 10.3390/cancers13236086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Putz F, Weissmann T, Oft D, Schmidt MA, Roesch J, Siavooshhaghighi H, Filimonova I, Schmitter C, Mengling V, Bert C, et al. FSRT vs. SRS in brain metastases-differences in local control and radiation Necrosis-A volumetric study. Front Oncol. 2020;10:559193. doi: 10.3389/fonc.2020.559193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown PD, Jaeckle K, Ballman KV, Farace E, Cerhan JH, Anderson SK, Carrero XW, Barker FG, II, Deming R, Burri SH, et al. Effect of radiosurgery alone vs. radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: A randomized clinical trial. JAMA. 2016;316:401–409. doi: 10.1001/jama.2016.9839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamamoto M, Serizawa T, Shuto T, Akabane A, Higuchi Y, Kawagishi J, Yamanaka K, Sato Y, Jokura H, Yomo S, et al. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): A multi-institutional prospective observational study. Lancet Oncol. 2014;15:387–395. doi: 10.1016/S1470-2045(14)70061-0. [DOI] [PubMed] [Google Scholar]
- 28.Patchell RA, Tibbs PA, Regine WF, Dempsey RJ, Mohiuddin M, Kryscio RJ, Markesbery WR, Foon KA, Young B. Postoperative radiotherapy in the treatment of single metastases to the brain: A randomized trial. JAMA. 1998;280:1485–1489. doi: 10.1001/jama.280.17.1485. [DOI] [PubMed] [Google Scholar]
- 29.Patchell RA, Tibbs PA, Walsh JW, Dempsey RJ, Maruyama Y, Kryscio RJ, Markesbery WR, Macdonald JS, Young B. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990;322:494–500. doi: 10.1056/NEJM199002223220802. [DOI] [PubMed] [Google Scholar]
- 30.Kayama T, Sato S, Sakurada K, Mizusawa J, Nishikawa R, Narita Y, Sumi M, Miyakita Y, Kumabe T, Sonoda Y, et al. Effects of surgery with salvage stereotactic radiosurgery versus surgery with whole-brain radiation therapy in patients with one to four brain metastases (JCOG0504): A phase III, noninferiority, randomized controlled trial. J Clin Oncol. 2018:JCO2018786186. doi: 10.1200/JCO.2018.78.6186. [DOI] [PubMed] [Google Scholar]
- 31.Brown PD, Ballman KV, Cerhan JH, Anderson SK, Carrero XW, Whitton AC, Greenspoon J, Parney IF, Laack NNI, Ashman JB, et al. Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC·3): A multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017;18:1049–1060. doi: 10.1016/S1470-2045(17)30441-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kępka L, Tyc-Szczepaniak D, Bujko K, Olszyna-Serementa M, Michalski W, Sprawka A, Trąbska-Kluch B, Komosińska K, Wasilewska-Teśluk E, Czeremszyńska B. Stereotactic radiotherapy of the tumor bed compared to whole brain radiotherapy after surgery of single brain metastasis: Results from a randomized trial. Radiother Oncol. 2016;121:217–224. doi: 10.1016/j.radonc.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 33.Randall Patrinely J, Jr, Funck-Brentano E, Nguyen K, Rapisuwon S, Salem JE, Gibney GT, Carlino M, Johnson DB. A multicenter analysis of immune checkpoint inhibitors as adjuvant therapy following treatment of isolated brain metastasis. Oncologist. 2021;26:e505–e507. doi: 10.1002/onco.13608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vogelbaum MA, Brown PD, Messersmith H, Brastianos PK, Burri S, Cahill D, Dunn IF, Gaspar LE, Gatson NTN, Gondi V, et al. Treatment for brain metastases: ASCO-SNO-ASTRO guideline. J Clin Oncol. 2022;40:492–516. doi: 10.1200/JCO.21.02314. [DOI] [PubMed] [Google Scholar]
- 35.Rusthoven CG, Yamamoto M, Bernhardt D, Smith DE, Gao D, Serizawa T, Yomo S, Aiyama H, Higuchi Y, Shuto T, et al. Evaluation of first-line radiosurgery vs. whole-brain radiotherapy for small cell lung cancer brain metastases: The FIRE-SCLC Cohort Study. JAMA Oncol. 2020;6:1028–1037. doi: 10.1001/jamaoncol.2020.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aupérin A, Arriagada R, Pignon JP, Le Péchoux C, Gregor A, Stephens RJ, Kristjansen PE, Johnson BE, Ueoka H, Wagner H, Aisner J. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. Prophylactic Cranial Irradiation Overview Collaborative Group. N Engl J Med. 1999;341:476–484. doi: 10.1056/NEJM199908123410703. [DOI] [PubMed] [Google Scholar]
- 37.Arriagada R, Le Chevalier T, Borie F, Rivière A, Chomy P, Monnet I, Tardivon A, Viader F, Tarayre M, Benhamou S. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. J Natl Cancer Inst. 1995;87:183–190. doi: 10.1093/jnci/87.10.766. [DOI] [PubMed] [Google Scholar]
- 38.Yin X, Yan D, Qiu M, Huang L, Yan SX. Prophylactic cranial irradiation in small cell lung cancer: A systematic review and meta-analysis. BMC Cancer. 2019;19:95. doi: 10.1186/s12885-018-5251-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pezzi TA, Fang P, Gjyshi O, Feng L, Liu S, Komaki R, Lin SH. Rates of overall survival and intracranial control in the magnetic resonance imaging Era for patients with limited-stage small cell lung cancer with and without prophylactic cranial irradiation. JAMA Netw Open. 2020;3:e201929. doi: 10.1001/jamanetworkopen.2020.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhong L, Li Y, Xiong L, Wang W, Wu M, Yuan T, Yang W, Tian C, Miao Z, Wang T, Yang S. Small molecules in targeted cancer therapy: Advances, challenges, and future perspectives. Signal Transduct Target Ther. 2021;6:201. doi: 10.1038/s41392-021-00572-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramalingam SS, Vansteenkiste J, Planchard D, Cho BC, Gray JE, Ohe Y, Zhou C, Reungwetwattana T, Cheng Y, Chewaskulyong B, et al. Overall Survival with Osimertinib in Untreated, EGFR-Mutated Advanced NSCLC. N Engl J Med. 2020;382:41–50. doi: 10.1056/NEJMoa1913662. [DOI] [PubMed] [Google Scholar]
- 42.Ando K, Manabe R, Kishino Y, Kusumoto S, Yamaoka T, Tanaka A, Ohmori T, Sagara H. Comparative efficacy and safety of lorlatinib and alectinib for ALK-Rearrangement positive advanced non-small cell lung cancer in asian and non-asian patients: A systematic review and network meta-analysis. Cancers (Basel) 2021;13:3704. doi: 10.3390/cancers13153704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shaw AT, Bauer TM, de Marinis F, Felip E, Goto Y, Liu G, Mazieres J, Kim DW, Mok T, Polli A, et al. First-Line lorlatinib or crizotinib in advanced ALK-positive lung cancer. N Engl J Med. 2020;383:2018–2029. doi: 10.1056/NEJMoa2027187. [DOI] [PubMed] [Google Scholar]
- 44.Narita Y, Nagane M, Mishima K, Terui Y, Arakawa Y, Yonezawa H, Asai K, Fukuhara N, Sugiyama K, Shinojima N, et al. Phase I/II study of tirabrutinib, a second-generation Bruton's tyrosine kinase inhibitor, in relapsed/refractory primary central nervous system lymphoma. Neuro Oncol. 2021;23:122–133. doi: 10.1093/neuonc/noaa145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murthy RK, Loi S, Okines A, Paplomata E, Hamilton E, Hurvitz SA, Lin NU, Borges V, Abramson V, Anders C, et al. Tucatinib, trastuzumab, and capecitabine for HER2-Positive metastatic breast cancer. N Engl J Med. 2020;382:597–609. doi: 10.1056/NEJMoa1914609. [DOI] [PubMed] [Google Scholar]
- 46.Huang PW, Chang JW. Immune checkpoint inhibitors win the 2018 Nobel Prize. Biomed J. 2019;42:299–306. doi: 10.1016/j.bj.2019.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Corroyer-Dulmont A, Jaudet C, Frelin AM, Fantin J, Weyts K, Vallis KA, Falzone N, Sibson NR, Chérel M, Kraeber-Bodéré F, et al. Radioimmunotherapy for brain metastases: The potential for inflammation as a target of choice. Front Oncol. 2021;11:714514. doi: 10.3389/fonc.2021.714514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goldberg SB, Gettinger SN, Mahajan A, Chiang AC, Herbst RS, Sznol M, Tsiouris AJ, Cohen J, Vortmeyer A, Jilaveanu L, et al. Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: Early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol. 2016;17:976–983. doi: 10.1016/S1470-2045(16)30053-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tawbi HA, Forsyth PA, Hodi FS, Algazi AP, Hamid O, Lao CD, Moschos SJ, Atkins MB, Lewis K, Postow MA, et al. Long-term outcomes of patients with active melanoma brain metastases treated with combination nivolumab plus ipilimumab (CheckMate 204): Final results of an open-label, multicentre, phase 2 study. Lancet Oncol. 2021;22:1692–1704. doi: 10.1016/S1470-2045(21)00545-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gaspar L, Scott C, Rotman M, Asbell S, Phillips T, Wasserman T, McKenna WG, Byhardt R. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37:745–751. doi: 10.1016/S0360-3016(96)00619-0. [DOI] [PubMed] [Google Scholar]
- 51.Schag CC, Heinrich RL, Ganz PA. Karnofsky performance status revisited: Reliability, validity, and guidelines. J Clin Oncol. 1984;2:187–193. doi: 10.1200/JCO.1984.2.3.187. [DOI] [PubMed] [Google Scholar]
- 52.Sperduto PW, Berkey B, Gaspar LE, Mehta M, Curran W. A new prognostic index and comparison to three other indices for patients with brain metastases: An analysis of 1,960 patients in the RTOG database. Int J Radiat Oncol Biol Phys. 2008;70:510–514. doi: 10.1016/j.ijrobp.2007.06.074. [DOI] [PubMed] [Google Scholar]
- 53.Sperduto PW, Kased N, Roberge D, Xu Z, Shanley R, Luo X, Sneed PK, Chao ST, Weil RJ, Suh J, et al. Summary report on the graded prognostic assessment: An accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol. 2012;30:419–425. doi: 10.1200/JCO.2011.38.0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sperduto PW, Yang TJ, Beal K, Pan H, Brown PD, Bangdiwala A, Shanley R, Yeh N, Gaspar LE, Braunstein S, et al. Estimating survival in patients with lung cancer and brain metastases: An update of the graded prognostic assessment for lung cancer using molecular markers (Lung-molGPA) JAMA Oncol. 2017;3:827–831. doi: 10.1001/jamaoncol.2016.3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sperduto PW, Mesko S, Li J, Cagney D, Aizer A, Lin NU, Nesbit E, Kruser TJ, Chan J, Braunstein S, et al. Beyond an updated graded prognostic assessment (Breast GPA): A prognostic index and trends in treatment and survival in breast cancer brain metastases from 1985 to today. Int J Radiat Oncol Biol Phys. 2020;107:334–343. doi: 10.1016/j.ijrobp.2020.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mulvenna P, Nankivell M, Barton R, Faivre-Finn C, Wilson P, McColl E, Moore B, Brisbane I, Ardron D, Holt T, et al. Dexamethasone and supportive care with or without whole brain radiotherapy in treating patients with non-small cell lung cancer with brain metastases unsuitable for resection or stereotactic radiotherapy (QUARTZ): Results from a phase 3, non-inferiority, randomised trial. Lancet. 2016;388:2004–2014. doi: 10.1016/S0140-6736(16)30825-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lin B, Gao F, Yang Y, Wu D, Zhang Y, Feng G, Dai T, Du X. FLASH radiotherapy: History and future. Front Oncol. 2021;11:644400. doi: 10.3389/fonc.2021.644400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Montay-Gruel P, Acharya MM, Petersson K, Alikhani L, Yakkala C, Allen BD, Ollivier J, Petit B, Jorge PG, Syage AR, et al. Long-term neurocognitive benefits of FLASH radiotherapy driven by reduced reactive oxygen species. Proc Natl Acad Sci USA. 2019;116:10943–10951. doi: 10.1073/pnas.1901777116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shepard MJ, Xu Z, Donahue J, Eluvathingal Muttikkal TJ, Cordeiro D, Hansen L, Mohammed N, Gentzler RD, Larner J, Fadul CE, Sheehan JP. Stereotactic radiosurgery with and without checkpoint inhibition for patients with metastatic non-small cell lung cancer to the brain: A matched cohort study. J Neurosurg. 2019;133:685–692. doi: 10.3171/2019.4.JNS19822. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.