Abstract
Methamphetamine (MA) is a highly addictive psychostimulant that, used in excess, may be neurotoxic. Although the mechanisms that underlie its addictive potential are not completely understood, in animal models matrix metalloproteinase (MMP) inhibitors can reduce behavioral correlates of addiction. In addition, evidence from genome-wide association studies suggests that polymorphisms in synaptic cell-adhesion molecules (CAMs), known MMP substrates, are linked to addictive potential in humans. In the present study, we examined the ability of MA to stimulate cleavage of intercellular adhesion molecule-5 (ICAM-5), a synaptic CAM expressed on dendritic spines in the telencephalon. Previous studies have shown that shedding of ICAM-5 is associated with maturation of dendritic spines, and that MMP-dependent shedding occurs with long term potentiation. Herein, we show that MA stimulates ectodomain cleavage of ICAM-5 in vitro, and that this is abrogated by a broad spectrum MMP inhibitor. We also show that an acute dose of MA, administered in vivo, is associated with cleavage of ICAM-5 in murine hippocampus and striatum. This occurs within 6 h and is accompanied by an increase in MMP-9 protein. In related experiments, we examined the potential consequences of ICAM-5 shedding. We demonstrate that the ICAM-5 ectodomain can interact with β1 integrins, and that it can stimulate β1 integrin-dependent phosphorylation of cofilin, an event that has previously been linked to MMP-dependent spine maturation. Together these data support an emerging appreciation of MMPs as effectors of synaptic plasticity and suggest a mechanism by which MA may influence the same.
Keywords: dendrite, integrin, matrix metalloproteinase, plasticity, telencephalin
Methamphetamine (MA) is the most widely abused illicit drug after cannabis, with 1.3 million users in a National Survey focused on 2007. It is highly addictive, although a complete understanding of the involved mechanisms is lacking.
Addiction to MA likely involves reorganization and strengthening of synaptic connections, or structural plasticity, because structural plasticity is associated with varied forms of long-term behavioral changes that occur with learning (Robinson and Kolb 2004). Consistent with this possibility, MA influences structural changes in brain areas to which MA-sensitive dopaminergic neurons project. For example, in a study using adult gerbils, a one time 25 mg/kg intraperitoneal injection led to dendritic spine alterations in the prefrontal cortex (Dawirs et al. 1991). In a separate study, repeated MA injections led to long-lasting structural changes in the dorsolateral striatum, including an increase in mushroom spines (Jedynak et al. 2007). Although structural changes in synapses have been observed, however, relatively few studies have directly examined the molecular basis of drug-induced structural plasticity (Robinson and Kolb 2004).
One possibility is that matrix metalloproteinases (MMPs) contribute to MA-associated synaptic plasticity. Levels of these enzymes may be increased with MA, and published studies suggest that they may influence morphology of dendritic spines (Tian et al. 2007; Wang et al. 2008). MMP activity has also been linked to varied forms of learning and memory including that associated with addiction. For example, a broad spectrum MMP inhibitor has been shown to reduce multiple forms of hippocampal CA1 plasticity (Meighan et al. 2007), and MMP-9 activity has been implicated in the maintenance of late long-term potentiation (LTP) (Nagy et al. 2006). Moreover, inhibition of MMPs with antisense oligonucleotides has been shown to prevent acquisition in the Morris water maze test (Meighan et al. 2006). In addition, MA-induced behavioral sensitization is reduced in mice lacking MMP-2 or MMP-9 (Mizoguchi et al. 2007a). Proteases have also been shown to contribute to cocaine-associated conditioned place preference (Brown et al. 2007, 2008; Maiya et al. 2009), and in a more recent study, it was shown that a broad spectrum MMP inhibitor could prevent reconsolidation of a fear association memory that was not dependent on contextual cues (Brown et al. 2009).
Although evidence is accumulating to suggest that MMPs play a role in synaptic plasticity as well as learning and memory, the mechanisms by which they do so are not well understood. Many studies of MMP substrates in the CNS have focused on extracellular matrix proteins of the blood–brain barrier (Yong et al. 2001; Rosenberg 2002). In the field of cancer biology, however, several studies suggest that MMPs are important effectors of cell-adhesion molecule (CAM) integrity (Sternlicht and Werb 2001; Gutierrez-Fernandez et al. 2008). As a variety of transmembrane CAMs are expressed at the post-synaptic process, and because their intracellular domains are often linked to effectors of actin polymerization, it is tempting to speculate that MMP-mediated CAM cleavage may play a role in the potential for MA to increase dendritic spine volume.
In the present study, we have focused on the question of whether MA can stimulate the cleavage of one synaptic CAM in particular, intercellular adhesion molecule-5 (ICAM-5). This adhesion molecule is expressed by spiny neurons localized to the telencephalon (neocortex, hippocampus, amygdala, striatum) (Matsuno et al. 2006; Mitsui et al. 2007). The C-terminal intracytoplasmic domain of ICAM-5 is linked to ezrin–radixin–moiesin proteins (Furutani et al. 2007), and a link between shedding of ICAM-5 and spine enlargement has been demonstrated (Tian et al. 2007). Of interest to forms of plasticity including addiction, ICAM-5 expression is higher on thin ‘plasticity’ spines thought to enlarge with LTP (Tada and Sheng 2006; Tian et al. 2007) than it is on more stable mushroom spines that change less with LTP inducing stimuli. This raises the question of whether plasticity spines may be particularly sensitive to the effects of ICAM-5 cleavage.
In terms of the mechanisms by which ICAM-5 can affect spine maturation, several possibilities must be considered. As suggested by experiments with chimeric proteins and deletion mutants, both extracellular and intracellular domains of ICAM-5 may be important to filopodial formation and maintenance (Furutani et al. 2007). MMP-mediated ICAM-5 cleavage might disrupt N- and C-terminal binding interactions and allow filopodia to expand. Another non-mutually exclusive possibility is that the shed N-terminal domain can also influence spine dynamics. This possibility is of particular interest because two groups have shown that MMP dependent effects on synaptic function and/or spine volume may be dependent on β1 integrins (Nagy et al. 2006; Meighan et al. 2007; Wang et al. 2008).
In the present study, we have focused on the potential for MA to stimulate ICAM-5 shedding and on the possibility that the shed N-terminal domain can stimulate intracellular changes linked to spine maturation.
Experimental procedures
Cell culture
MN9D cells were derived from mouse embryonic mesencephalon and were a kind gift from Dr A. Heller (Choi et al. 1991). MN9D cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM; Sigma, St Louis, MO, USA D5648) containing 10% fetal bovine serum.
The B35 rat neuroblastoma cell line (Schubert et al. 1974) was obtained from the American Type Culture Collection (Manassas Virginia), and cultures were maintained in DMEM supplemented with glucose, penicillin/streptomycin, and 10% fetal bovine serum at 5% CO2.
For dissociated neuronal cultures, animals from which cultures were prepared were killed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals prior to the harvesting of tissue. Every effort was made to reduce the number of animals used. Rat hippocampal neurons were plated on polylysine-coated (1 mg/mL) 12 well tissue culture plates at density of 2 × 105 cells/well in the absence of an astrocyte feeder layer. Hippocampal neurons were maintained in neurobasal medium (Gibco, Grand Island, NY, USA) containing 2% B27 supplement (Gibco), and 500 μM l-glutamine (Sigma), and the medium was changed every 3–4 days. These neurons were also treated with 5 μM cytosine arabinoside to inhibit the proliferation of non-neuronal cells. Immunostaining indicates that less than 5% of cells were non-neuronal cells at 7 days in vitro in this culture system. Cultures were used between 10 and 14 days in vitro.
Reagents
The MMP inhibitor GM-6001 was obtained from Calbiochem. Recombinant ICAM-5 was obtained from R and D systems. This recombinant construct contains amino acids 31-828 of the extracellular domain of mouse ICAM-5 fused to the Fc region of human IgG. In terms of antibodies, the rabbit polyclonal ICAM-5 cytoplasmic specific antibody was created using two synthetic peptide antigens that correspond to amino acids 889–902 and 905–917 of mouse ICAM-5. These synthetic peptides were conjugated to keyhole limpet hemocyanin and co-injected into rabbits for production of antisera. The antisera specific for ICAM-5 were then affinity purified. Specificity of this antibody has been demonstrated in a previous publication (Conant et al. 2010). Anti-N terminal ICAM-5 (AF 1173) was obtained from R & D Systems (Minneapolis, MN, USA). Anti- cofilin and anti-phospho-cofilin antibodies were obtained from Cell Signaling Technologies (Beverly, MA, USA). Anti-β1 integrin antibodies were obtained from R & D Systems (AF2405) and Millipore Corporation (Bedford, MA, USAAB1952). Texas-Red conjugated phalloidin was purchased from Molecular Probes.
Mouse ICAM-5 cDNA (Entrez nucleotide database number NM0083192) was purchased from Genscript (Piscataway, NJ, USA). The sequence fidelity of ICAM-5 was earlier verified by DNA sequencing. This was cloned into pcDNA 3.1 (+) (Invitrogen, Carlsbad, CA, USA), with flanking HindIII and XbaI sites.
Transfection
Cells were transfected using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s protocol. After a 1-h incubation at 37°C, the media was changed with fresh DMEM.
Lysates and western blot
Lysates from cultured cells were prepared via the addition of lysis buffer [50 mM Tris–HCl, pH 7.5, 150 mM NaCl, 0.1% sodium dodecyl sulfate, 1% NP-40, 0.5% sodium deoxycholate, 0.2 mM phenylmethylsulfonyl fluoride, 0.5 mM dithiothreitol, 1× protease inhibitor cocktail (Sigma P8340)]. The mixture was placed into a microfuge tube, sonicated for 10 s, kept on ice for 20 min, and then spun at 14 000 rpm for 15 min at 4°C in a microcentrifuge. For lysates from microdissected hippocampus or striatum, one-half of the hippocampus or striatum was placed into 400 μL of lysis buffer and similarly processed. Supernatants were then saved and used in western blot experiments. Western blot was performed using 40 μg of protein per lane, as determined by the Qubit assay (Invitrogen). Prior to analysis, samples were mixed with sample buffer containing 5% β-mercaptoethanol and boiled for 5 min at 95°C. Electrophoresis was performed on Tris-glycine polyacrylamide gradient gels (Bio-Rad, Hercules, CA, USA). Following electrophoretic transfer of the protein to a nitrocellulose membrane (iblot; Invitrogen), membranes were stained with Ponceau S to ensure appropriate loading and transfer. A recent publication compared actin and Ponceau staining by densitometric analysis and concluded that Ponceau could be used as a valid alternative to actin blotting (Romero-Calvo et al. 2010). Membranes were then blocked in 5% non-fat dry milk in phosphate-buffered saline (PBS) with 0.1% Tween (PBST) for 1 h. The blot was then probed with the indicated primary antibody, at a dilution recommended by the manufacturer, overnight at 4°C. After washing the membrane three times (15 min each) in PBST, it was incubated with an appropriate secondary antibody for 1–2 h at 20°C. The membrane was then washed again in PBST and immunoreactive bands were visualized using electrochemiluminescence (Amersham Pharmacia Biotech, Piscataway, NJ, USA).
Concentration of cell culture supernatants
VWR centrifugal filters (cat no. 82031-344) were used to concentrate supernatants. Just prior to cell culture treatment, medium was changed to OPTIMEM-reduced serum media (Gibco). Following treatment, 500 μL of supernatant from culture wells having 5 × 105 cells/mL medium was spun at 14 000 rpm in an eppendorf centrifuge for 20 min at 4°C. Twenty microliters lysis buffer [50 mM Tris–HCl, pH 7.5, 150 mM NaCl, 0.1% sodium dodecyl sulfate, 1% NP-40, 0.5% sodium deoxycholate, 0.2 mM phenylmethylsulfonyl fluoride, 0.5 mM dithiothreitol, 1× protease inhibitor cocktail (Sigma P8340)] was subsequently added to the top of the filter to suspend retained proteins, and this suspension was used for western blot analysis.
Immunoprecipitation
Immunoprecipitation experiments involving murine brain tissue was performed through incubation of lysates (100 μg protein in 100 μL lysis buffer) with the indicated antibody (10 μg) overnite. This mixture was then incubated with pre-washed Protein G-Sepharose 4B conjugate (Invitrogen) for 2 h. Beads were washed 5× in cold PBS, and following the final wash, western blot sample buffer was added to the beads and the mixture heated at 95°C for 5 min. The supernatant was then analyzed by western blot. Antibodies used for IP experiments included anti-ICAM-5 ectodomain (R & D systems AF1173), anti-C terminal ICAM-5 (described above), and anti-β1 (R & D systems AF2405). Immunoprecipitation studies with ICAM-5-treated B35 cells were performed in a similar manner as previously described for recombinant MMP-1 treated cells (Conant et al. 2004).
ELISA
ELISA for total MMP-9 was performed on brain lysates using a commercially available kit (R & D Systems, MMPT90) according to the manufacturer’s instructions.
Immunocytochemistry
Neurons were fixed in 4% paraformaldehyde, 4% sucrose in PBS for 30 min at 20°C, permeabilized in 0.05% triton X-100 for 30 min, and rinsed in PBS, containing 0.05% triton X-100. Non-specific binding sites were blocked by incubation for 30 min at 20°C in Blotto-T (4% non-fat dry milk powder in 20 mM Tris, pH 7.5, 150 mM NaCl, 0.05% Triton X-100). Immunostaining was performed with anti-phospho cofilin (Cell Signaling) overnight at 4°C. Cells were washed three times in Blotto-T to remove excess primary antibodies. Immunostaining of phospho-cofilin was visualized through subsequent incubation with the Alexa Fluor goat anti-rabbit. F-actin visualization with phalloidin was performed as previously described (Lim et al. 2008).
In vivo experiments
C57/Bl6 mice were purchased from Jackson Laboratories. At approximately 6 weeks of age, mice were injected intraperitoneally with 100 μL saline (n = 4) or 40 mg/kg MA in 100 μL saline (n = 4). We used a dose that had been shown to increase MMP expression in previous studies, and one that is often used in studies focused on an acute dose of MA (Sharma and Ali 2006; Kobeissy et al. 2008; Liu et al. 2008). In addition, we focused on young adult mice (6–8 weeks) because substantial plasticity in brain regions important to addiction may persist for up to 3 and ½ months in rodents (Kolb et al. 2003). Moreover, although effects of MA on measures including microglial activation and dopamine depletion occur to a significant extent in both male and female mice, the latter may be relatively increased in males (Wagner et al. 1993; Thomas et al. 2008). An equal number of males and females were therefore used in each group. Following injection, mice were observed regularly over the next 6 h and then killed. Brains were quickly isolated and microdissected, before hippocampal and striatal sections were put into ice cold lysis buffer.
In a separate set of experiments, male mice were injected with 100 μL saline once daily for 5 days, or with 100 μL saline containing 2 mg/kg MA once daily for 5 days. These mice were killed 6 h following the final injection and striatal samples isolated and processed.
Densitometry
Where indicated, densitometric analysis of data was performed using Vision Works Acquisition and Analytical Software, version 6.7.1 (Upland, CA, USA) in accordance with the manufacturer’s instructions.
Statistics
Although Student’s t-test was used for pairwise comparisons, anova with a Bonferroni post hoc test was used to compare the multiple groups examined by densitometric analysis. Graphical data are shown as the mean ± standard error.
Results
MA stimulates MMP-dependent cleavage of ICAM-5 in a midbrain-derived cell line
We have previously observed that MMP-dependent cleavage of ICAM-5 generates a 16 kDa C-terminal fragment (CTF). This fragment can be detected with an antibody that we generated to an intracellular domain epitope. Specificity of the antibody for ICAM-5 has also been demonstrated (Conant et al. 2010).
To determine whether MA might stimulate MMP-dependent cleavage of ICAM-5, we used the MN9D mesencephalic cell line (Choi et al. 1991). These cells have previously been shown to express dopamine receptors and transporters (Su et al. 2008). And although ICAM-5 expression may be limited to glutamatergic neurons in the telencephalon (neocortex, hippocampus, amygdala and striatum), the potential for MA responsive cells to release MMPs in quantities sufficient to cleave ICAM-5 is likely relevant. In vivo, MA-responsive cells project to ICAM-5 expressing cells. Moreover, MMPs may be released from pre-synaptic stores (Miyata et al. 2005).
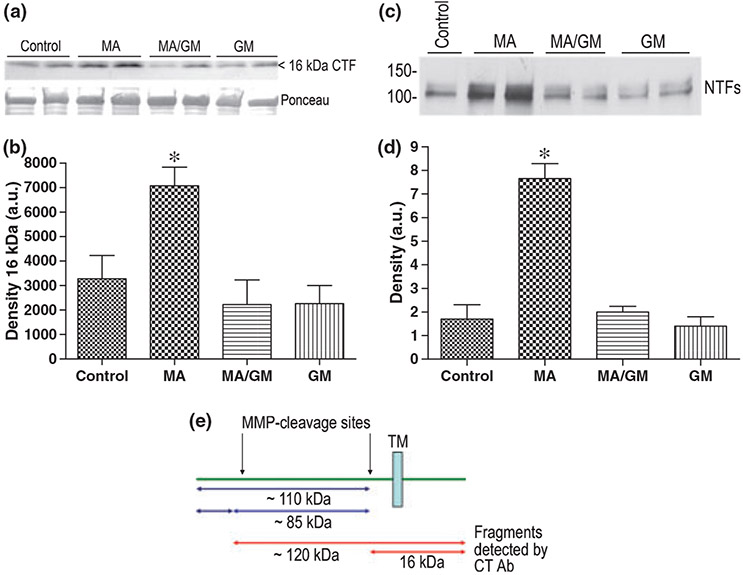
MN9D cells were transfected with ICAM-5. At 48 h following transfection, cell cultures were treated with 100 μM MA, in the presence or absence of 5 μM of the broad spectrum MMP inhibitor GM-6001, for 90 min. The concentration of MA was based on published plasma and oral fluid levels of MA treated volunteers which can be in the low micromolar range (Schepers et al. 2003), and on concentrations used in previous publications (Higuchi et al. 2008). Results are shown in Fig. 1 and demonstrate an increase in the intensity of the 16 kDa CTF in MA-treated cells. This increase was abrogated in extracts from cells that received GM-6001. Data were analyzed by anova with a Bonferroni post hoc test and the difference between control and MA was significant (p < 0.05). In separate experiments, ICAM-5-transfected cells were similarly treated for 6 h and supernatants then tested for the presence of shed ICAM-5 using an antibody to the ectodomain. Although the CTF is not stable over long periods, this timing allowed N terminal fragments (NTFs) to accumulate in MA treated cell culture supernatants. A representative image is shown in panel c. Two bands can be appreciated which may represent two products, or a differentially glycosylated version of the same. Densitometric analysis (1D) showed that the difference between control and MA, as well as that between MA and MA/GM, was significant (p < 0.001). A diagrammatic representation of ICAM-5 cleavage sites including that which generates the 16 kDa CTF is shown in 1E.
Fig. 1.
MA stimulates MMP-dependent effects on ICAM-5 expressing mesencephalic cells. To examine effects of MA on mesencephalic cells in vitro, MN9D mesencephalic cells, which are known to express dopamine receptors and transporters (Luo et al. 2008), were transiently transfected with an ICAM-5 construct. MA (100 μM, 90 min) stimulated an increase in the 16 kDa ICAM-5 CTF and this effect was inhibited by pre-administration (5 μM) of the broad spectrum MMP inhibitor GM-6001 (a). Ponceau S staining is shown in the lower section of panel a. Densitometry results are shown in panel b (mean + SEM). Data were analyzed by anova with a Bonferroni post hoc test and the difference between control and MA was significant (*p < 0.05). In separate experiments, ICAM-5 transfected cells were similarly treated for 6 h and supernatants then tested for the presence of shed ICAM-5. A representative blot from an experiment performed twice is shown (c). Two bands can be appreciated which may represent two products, or a differentially glycosylated version of the same. Densitometric analysis was performed on both blots, values normalized, and data analyzed by anova with a Bonferroni post hoc test. Results (mean + SEM) are shown in panel d, and the difference between control and MA was again significant (*p < 0.001), as was that between MA and MA/GM. A schematic diagram showing MMP cleavage sites is shown in panel e.
II. MA stimulates increased MMP protein levels in vivo
In vitro data suggested that midbrain neurons may respond to MA by releasing GM-6001 sensitive MMPs capable of cleaving ICAM-5. While the use of cultured cells provides data that is relatively easy to interpret as compared to data from a more complex in vivo system, in vitro experiments do not allow us to see effects of MA on MMP expression and CAM integrity that may be mediated by the interplay between several CNS systems. For example, hippocampal glutamate levels may be increased in association with MA through changes that involve more than one transmitter system (Mark et al. 2007). Moreover, MA-sensitive cells may or may not release MMPs in the proximity of ICAM-5 bearing neurons.
To determine whether an acute dose of MA could increase MMP levels in vivo and in a temporal manner relevant to this study, we administered MA to mice by intraperitoneal injection. We focused on MMP-9 because this MMP has been implicated in multiple forms of plasticity including that associated with MA (Mizoguchi et al. 2007b), and it is an MMP that has been well linked to the cleavage of ICAM-5 (Tian et al. 2007; Conant et al. 2010). In terms of CNS regions, we focused on the striatum because midbrain dopaminergic neurons project to this area, and MA-associated spine and LTP changes have been noted in the same (Jedynak et al. 2007). We also focused on the hippocampus because previous work has shown that glutamate receptor agonists can stimulate increased MMP levels (Zhang et al. 1998; Michaluk et al. 2007), and MA affects an increase in hippocampal glutamate (Mark et al. 2007). Moreover, this brain region is increasingly recognized as being important to addiction (Ricoy and Martinez 2009).
Shown in Fig. 2 are results comparing MMP levels in saline control (n = 4) and MA (n = 4) injected animals. Mice were killed 6 h following injection, at which time brain regions were isolated by micro-dissection and lysates prepared. As shown, MA affected an increase in MMP-9 levels as determined by ELISA. The difference between control and MA striatal samples was significant (p < 0.05), and although the difference between hippocampal samples was not significant (p = 0.13), there was an appreciable trend towards increased MMP-9 in the hippocampal lysates from MA-treated animals. Taken together, these results are consistent with an earlier report that the same dose of MA could increase MMP-9 at the mRNA level in brain specimens (Liu et al. 2008). Published work has also shown an increase in MMP-9 protein, as determined by western blot, in the frontal cortex and nucleus accumbens of chronically MA treated (2 mg/kg × 5 days) rats (Mizoguchi et al. 2007b).
Fig. 2.
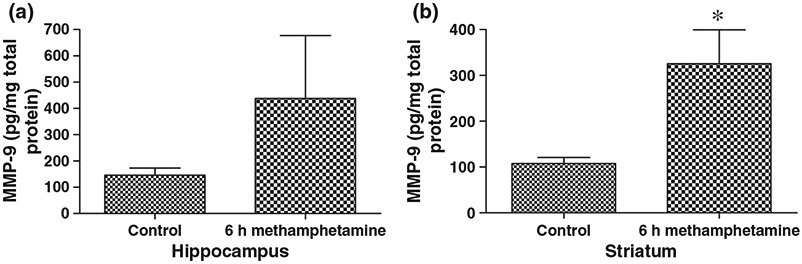
MMP-9 levels are increased in brain lysates from MA-treated mice. Mice were killed at 6 h following a single intraperitoneal dose of 100 μL saline (control, n = 4), or MA dissolved in 100 μL saline (40 mg/kg, n = 4). Hippocampal and striatal lysates were then prepared as described (St Hillaire et al. 2005) and analyzed for total MMP-9 levels by ELISA (R & D Systems). Values were corrected for total protein concentration in the lysates (Qubit; Invitrogen) and ng MMP-9/mg total protein was graphed (mean + SEM). The difference between control and MA striatal samples was significant (*p < 0.5, Student’s t-test), and while the difference between hippocampal samples was not significant (p = 0.13, Student’s t-test), there was there was an appreciable trend towards increased MMP-9 in the hippocampal lysates from MA-treated animals.
MA stimulates increased ICAM-5 cleavage in vivo
As MMP-9 was elevated in brain regions known to express ICAM-5 (Mitsui et al. 2007), we next examined the integrity of this molecule in lysates we had used to examine MMP-9. We hoped to detect ICAM-5 fragments that would accumulate over several hours but that might not be stable over a longer period. Four control and four MA hippocampal samples were analyzed, along with two control and two striatal samples. We again used a C-terminal antibody to ICAM-5 because, as compared with commercially available N-terminal antibodies, this antibody is particularly sensitive. We did not detect the 16 kDa fragment in lysates, consistent with tissue culture experiments in which the short fragment is not stable for this long of a period (K. Conant and S. T. Lim, unpublished observations). We did, however, observe an increase in a 120 kDa CTF, with representative blots shown in Fig. 3(a) and (c). A CTF of this size would be expected to result from cleavage at one of two previously identified MMP-9 cleavage sites that are targeted in the ectodomain to generate 85 and 110 kDa NTFs, as previously shown in Fig. 1e. Densitometric analysis of the 120 kDa fragment was subsequently performed for each western blot. Values were then normalized so that the average control value for each blot was set at 1. Statistical analysis of the resulting control and MA values showed that the difference between the value in control and MA hippocampal samples was statistically significant (p < 0.05). Statistical analysis was not performed on the striatal samples because of the smaller sample size.
Fig. 3.
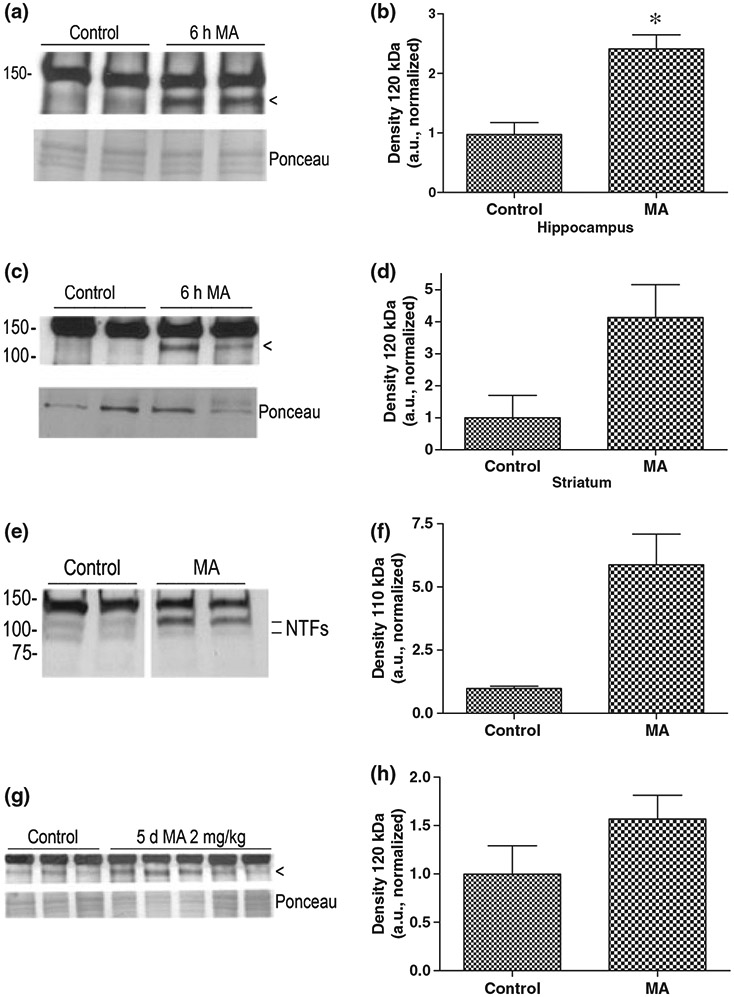
ICAM-5 in MA-treated mice. Mice were injected with an acute dose of MA (40 mg/kg) in 100 μL saline (n = 4), or an equivalent volume of saline alone (n = 4), and then killed 6 h later. A separate group of mice was treated with MA (2 mg/kg) in 100 μL saline once daily for 5 days (n = 3) or an equivalent volume of saline alone once daily for 5 days (n = 5) and killed 6 h following the final dose (chronic regimen). Lysates were made from hippocampus and/or striatum and analyzed for ICAM-5 by western blot. Representative images for the acute experiment are shown in panels a and c. As can be appreciated, immunoreactivity for a potential 120 kDa C-terminal cleavage fragment (arrowheads) appears increased in lysates in association with MA. Based on previously published data, this sized fragment would be consistent with MMP-mediated cleavage (Tian et al. 2007). A loading control, showing the major band(s) seen with Ponceau S staining, is shown in the lower panels for a and c. Densitometric analysis of the 120 kDa CTF was performed for each western blot and results were subsequently normalized so that the average control value for each was set at 1 (b and d). Statistical analysis of the resulting control and MA values showed that the difference between the value in control and MA hippocampal samples was statistically significant (*p < 0.05). Two control and two MA hippocampal samples were also analyzed using a commercially available antibody to the ectodomain. Results are shown in panel e and demonstrate an increase in intensity for a 110 kDa NTF. Densitometric analysis is shown in panel f. In (g), a western blot is shown that compares striatal lysates from control and chronically MA-treated mice for the presence of the 120 kDa CTF. Densitometric analysis shows an increase in the intensity of this band in association with MA (h). The difference between control and chronic MA values was significant only for a cutoff of p < 0.1 (Student’s t-test, p = 0.097).
Although sample quantity was limiting, we also analyzed two control and two MA hippocampal samples for presence of NTFs using a commercially available antibody to the ectodomain (Fig. 3e). Samples were run on the same gel but not in adjacent lanes. Bands at approximately 110 and 85 kDa were observed, with the former particularly increased in association with MA. That we did not observe a similar increase in the 85 kDa band could be related to differential stability of the two NTFs. Comparison of the 110 kDa band intensity by densitometry is shown in Fig. 3f.
As it has been demonstrated that chronic MA (2 mg/kg × 5 days) can increase MMP-9 protein levels in the striatum of rodents (Mizoguchi et al. 2007b), we also treated a separate set of animals with 100 μL saline by intraperitoneal injection, or 100 μL saline with 2 mg/kg MA by intraperitoneal injection, once per day for 5 days. Mice were killed 6 h following the final injection and striatal lysates examined by western blot for the presence of the 120 kDa ICAM-5 C-terminal cleavage fragment. Results are shown in Fig. 3g. Of note is that the fragment is relatively more appreciable in samples from the control mice used for this chronic experiment, as compared with samples from control mice used for acute studies. This could relate to the stress of repeated intraperitoneal injections in that stress hormones are known to induce MMP expression (Cury et al. 2007; Lutgendorf et al. 2008). Densitometric results are shown in Fig. 3h. The difference between control and MA was significant using a cutoff of p < 0.1 (p = 0.097).
The ICAM-5 NTF interacts with β1 integrins
We next focused on the potential of the ICAM-5 N terminal domain to interact with β1 integrins because β1 has been implicated as being an important effector of plasticity in a varied studies including those focused on plasticity mediated by MMP-9 (Chavis and Westbrook 2001; Huang et al. 2006; Kramar et al. 2006; Shi and Ethell 2006; Wang et al. 2008).
All ICAMs including ICAM-5 bind to αLβ2 integrin (Gahmberg et al. 2008), which is expressed on leukocytes, via their first Ig domain at the extreme N terminus. Whether ICAM-5 can interact with β1 integrins and which region may be involved in this interaction is unknown. As MMPs generate several NTFs (~25, 85 and 110 kDa), we tested a recombinant construct that includes the entire ectodomain.
To determine whether this construct could interact with β1, we treated a neuronal cell line that expresses β1 (B35 cells) with exogenous ICAM-5. Cells were subsequently washed and lysates prepared for immunoprecipitation as described. As shown in Fig. 3, when antibodies to β1 were used to pull down β1 with interacting proteins, we detected ICAM-5 in the immunoprecipitates (Fig. 4a). Two antibodies to β1 were tested and showed similar results. Anti-β1 also pulled down β1 as expected (4B). Control IgG did not pull down β1 or ICAM-5 (Fig. 4a and b).
Fig. 4.
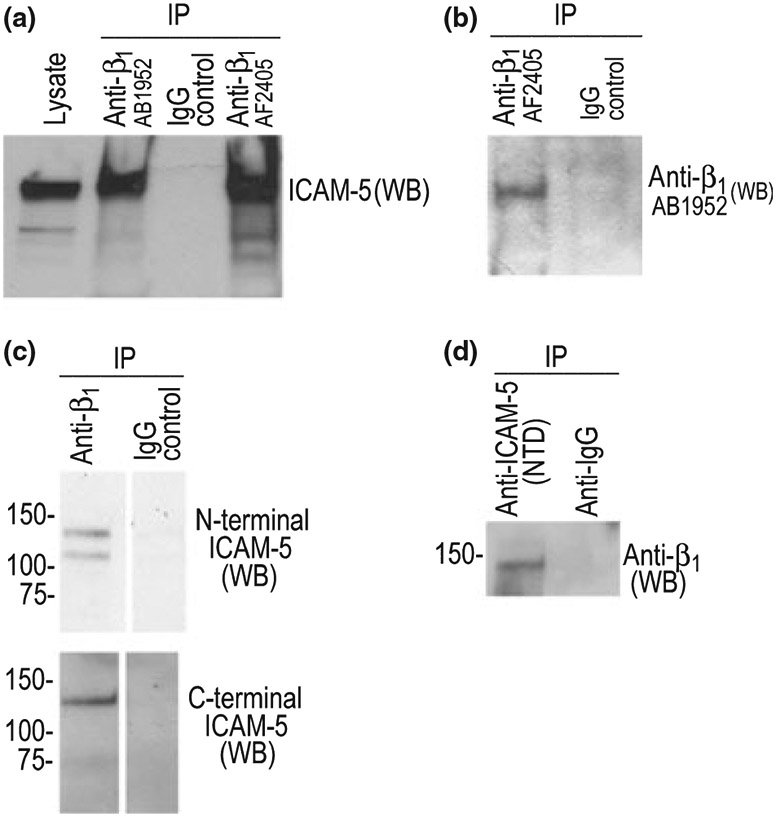
The ectodomain of ICAM-5 and β1 co-immunoprecipitate B35 cells, which had previously been determined to express β1 but not ICAM-5, were treated with recombinant ICAM-5. B35 cells were treated with 1 μg/mL ICAM-5 for 30 min. prior to lysate preparation. Anti-integrin or control (IgG) antibodies (Millipore) were then used to pull out β1 and potential interacting proteins from the ICAM-5 treated B35 cells. Western blot was later performed for ICAM-5 (a) or, as an additional control, β1 (b). ICAM-5 treatment of cells and immunoprecipitation was performed as previously described for MMP-1 (Conant et al. 2004). To determine whether ICAM-5 might interact with β1 in vivo, we also performed studies using lysate from MA treated (6 h) murine hippocampus. As shown in panel c, anti-β1 pulled down both the full length (detected by both N- and C-terminal ICAM-5 antibodies) and ectodomain (detected only by the N-terminal antibody) of ICAM-5. As shown (d), an antibody to the N-terminal domain of ICAM-5 can pull down β1.
To determine whether ICAM-5 might interact with β1 in vivo, we also performed studies using lysate from MA-treated murine hippocampus. This was one of the 6 h samples that had been used in Fig. 3e. As shown in Fig. 4c, Anti-β1 pulled down both the full length (detected by both N- and C-terminal ICAM-5 antibodies) and ectodomain (detected only by the N-terminal antibody) of ICAM-5. Presumably, shed ICAM-5 can interact with unengaged β1. We also did the reverse experiment and show that an antibody to the N terminal domain of ICAM-5 can pull down β1 (Fig. 4d).
ICAM-5 NTF stimulates β1 integrin-dependent phosphorylation of cofilin
We next tested the potential for the soluble ICAM-5 to influence β1 integrin-dependent changes linked to spine maturation. We focused on the possibility that ICAM-5 could stimulate β1 integrin-dependent phosphorylation of cofilin. Cofilin has the ability to depolymerize actin and it is negatively regulated by phosphorylation at a single site. An increase in the phosphorylation of cofilin allows for actin polymerization and it has been observed in association with both LTP and MMP-9 stimulated spine enlargement (Chen et al. 2007; Wang et al. 2008).
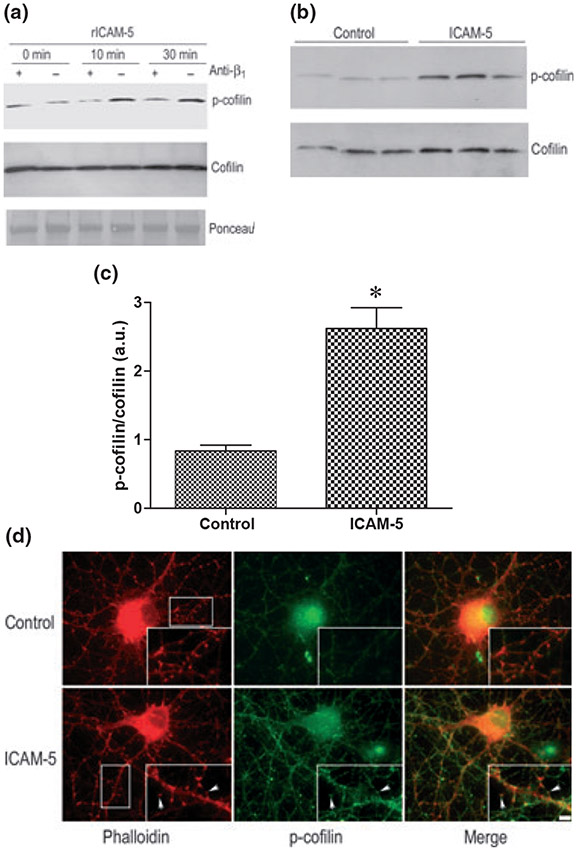
In these experiments, we used an antibody to β1 which has been shown to block β1 integrin-dependent process including semaphorin 7A enhancement of axon outgrowth (Pasterkamp et al. 2003). B35 cells were treated with recombinant ICAM-5, in the presence or absence of previously applied blocking antibody. Lysates were made at varied time points and tested by Western blot for phospho- and total cofilin. As can be appreciated (Fig. 5a), ICAM-5 stimulated an increase in phospho cofilin that was inhibited by the β1-blocking antibody.
Fig. 5.
The ICAM-5 ectodomain stimulates β1 integrin-dependent phosphorylation of cofilin. B35 cells treated with 1 μg/mL recombinant murine ICAM-5 exodomain (rICAM-5, R & D Systems) for 0–30 min. as indicated (a). As also indicated, select cells were pre-treated with a neutralizing Ab to β1 (Pharmingen, San Diego, CA, USA). It can be appreciated that rICAM-5 stimulated an increase in phospho-cofilin that was inhibited by pre-treatment of cells with the neutralizing antibody. In a separate experiment, primary neurons were treated with 1 μg/mL recombinant murine ICAM-5 as indicated. Lysates from three control and three stimulated culture wells were prepared 60 min later and analyzed by western blot for phospho- and total cofilin (b). Ratios of normalized densitometric data were compared (c), and differences between control and ICAM-5 stimulated were significant (*p < 0.02). In panel d, primary neurons (14 days in vitro) grown on cover slips, were treated for 60 min with medium alone or medium containing 1 μg/mL rICAM-5. Neurons were then fixed and stained using phalloidin or anti-phospho-cofilin as indicated. Although the ICAM-5-associated increase in phospho-cofilin is somewhat generalized, as shown in the merged staining inset, an increase can be appreciated in actin-rich structures having the appearance of dendritic spines (representative spines are indicated by arrows). The scale bar (inset) represents 5 μm.
We also examined the potential for ICAM-5 to stimulate the phosphorylation of cofilin in primary neurons. Results from western blot experiments are shown in Fig. 5b, and are consistent with results from B35 cells. The ratio of normalized phospho- to total cofilin is shown in Fig. 5c and the difference is significant (p < 0.02). Results from subsequent immunostaining experiments performed to localize changes, in which phospho cofilin staining was combined with actin staining, showed that ICAM-5 stimulated an overall increase in signal, as well as a localized increase in signal in actin-rich structures having the morphology of dendritic spines (Fig. 5d, arrows).
Discussion
Although well studied for their role in CNS pathology when levels are particularly elevated, MMPs are increasingly recognized as important contributors to normal brain physiology. They play a role in the processing and/or bioavailability of factors important to glial and neuronal survival including brain-derived neurotrophic factor and insulin-like growth factor-1 (Fowlkes et al. 1995; Lee et al. 2001). Accumulating evidence suggests that they may also play a relatively direct role in varied forms of learning and memory including those associated with addiction (Mizoguchi et al. 2007b).
In the present study, we observed that MA could stimulate increased levels of MMP-9 protein in areas relevant to addiction. MMP-9 levels were increased in the hippocampus, a region increasingly recognized as playing a role in addiction (Ricoy and Martinez 2009), as well as in the striatum, an area that receives projections from MA-sensitive midbrain neurons. MMP-9 was quantified because it is one of the MMPs known to influence ICAM-5 integrity, and earlier reports had indicated that CNS expression or levels may be increased in association with slightly different experimental paradigms (Mizoguchi et al. 2007b; Liu et al. 2008). It should be noted, however, that several MMPs including MMP-2, −3, and −9, have been implicated in neuronal plasticity (Meighan et al. 2006; Cho et al. 2008; Mizoguchi et al. 2008; Pauly et al. 2008) and these too could contribute to MA-dependent effects on synaptic CAM integrity.
In terms of the means by which MA may increase MMP-9 levels, several possibilities exist. MA is thought to increase extracellular dopamine through mechanisms that include a redistribution of the catecholamine from synaptic vesicles to the cytoplasm and reverse flux through cell surface dopamine transporters. One possibility is that MA associated-increases in catecholamines, and dopamine in particular, will lead to catecholamine receptor-dependent increased gene transcription. Catecholamines have been linked to increased DNA-binding activity of AP-1 and to increased MMP expression (Takemoto et al. 1999; Guillaumond et al. 2002; Speidl et al. 2004; Spiegel et al. 2007; Maolood et al. 2008). MA has also been linked to increased hippocampal glutamate, a transmitter well known to stimulate MMP expression. NMDA receptor activation has also been shown to stimulate rapid MMP-dependent CAM cleavage. As MMPs may be present in pre-formed synaptic stores (Miyata et al. 2005; Sbai et al. 2008), and released via a soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) dependent mechanism (Kean et al. 2009), it is possible that MA-associated changes in glutamate might also stimulate rapid release of pre-formed enzymes.
In the present study, we have also investigated the cleavage of ICAM-5 in the hippocampus and striatum. The shedding of ICAM-5 has been well linked to the maturation of dendritic spines. In one study, developmental reductions in ICAM-5 were associated with spine maturation whereas in another, NMDA-dependent shedding was associated with the same (Matsuno et al. 2006; Tian et al. 2007).
Others have shown that both the N- and C-terminal domains of ICAM-5 may be important to the formation and maintenance of filopodia (Furutani et al. 2007). Cleavage of ICAM-5 localized to filopodia might thus better allow for a filopodia to spine transition. Herein, we examined the non-mutually exclusive possibility that the shed N terminus, from filopodia or thin spines, might promote changes that have been linked to spine expansion. We have focused on its ability to interact with β1 integrins in that two groups have suggested that MMP-dependent effects on measures of learning and memory are β1-integrin dependent (Meighan et al. 2007; Wang et al. 2008). We have also focused on the possibility that the shed N terminus stimulates β1 integrin-dependent phosphorylation of cofilin. Cofilin has F-actin depolymerizing activity and is negatively regulated by phosphorylation. In addition, an increase in the phosphorylation of cofilin has been observed in association with both LTP, and MMP-9-stimulated spine enlargement (Chen et al. 2007; Wang et al. 2008).
Here, we showed that the ICAM-5 NTF can stimulate phosphorylation of cofilin via a β1 integrin-dependent mechanism. We focused on the entire N-terminal domain and therefore cannot say with complete certainty whether specific fragments shed in association with MA do the same. MA can, however, stimulate cleavage at sites that would release fragments containing domains that have been implicated in integrin binding.
We also showed ICAM-5-associated phosphorylation of cofilin occurs in actin-rich protrusions with the appearance of dendritic spines. In these studies, we again applied ICAM-5 in an exogenous and somewhat artificial manner which likely allowed it to reach a variety of neuronal regions. It should be noted, however, that in vivo, expression of the molecule is limited to dendrites. Stimulated cleavage of the NTF might therefore have a predominant effect on phospho-cofilin in structures such as dendritic filopodia and spines.
The ability of a CAM fragment to stimulate β1-dependent changes in neuronal morphology is not without precedent. For example, Maness and colleagues showed that metalloproteinase-dependent cleavage of neural CAM-stimulated integrin-dependent cell migration (Hinkle et al. 2006), a process in which actin dynamics and changes in cell shape are critical. In related experiments, it has been shown that shedding of L1 releases a soluble ectodomain that binds to integrins in trans to stimulate cell migration (Mechtersheimer et al. 2001).
Although we studied effects of the NTF in a defined system, in vivo effects may be dependent on variables including developmental stage. There are at least three potential receptors for shed ICAM-5 including full-length ICAM-5, β1 and lymphocyte function-associated antigen 1, although the latter may not be expressed on neurons to the extent that it is expressed on glia (Gahmberg et al. 2008). Therefore, depending on factors including the maturity of spines, the NTF might have diverse effects.
Overall, we hypothesize that while cleavage of ICAM-5 disrupts N- and C-terminal interactions of the full-length molecule that are important to the maintenance of filopodia (Furutani et al. 2007), an ICAM-5 NTF, possibly in combination with other CAM NTFs that are concomitantly generated by MA-stimulated MMP activity, will contribute to spine expansion. The possibility that MA-associated plasticity might be a process in which a specific subset of synaptic CAMs plays a role is intriguing, especially in light of emerging data from genome wide association studies that implicates varied CAM single nucleotide polymorphisms in addiction (Uhl et al. 2008). Of additional interest is a recent study that linked a polymorphism that is associated with higher transcription of MMP-9 to alcohol abuse (Samochowiec et al. 2010).
Although additional studies will be necessary to confirm this possibility, the data herein contribute to an ever-growing literature implicating MMPs in synaptic plasticity, including that associated with addiction.
Acknowledgements
This work was supported by DA024447, AG027233, and funds from the von Matsch Professorship in Neurological Disease. The authors have no conflicts of interest to declare.
Abbreviations used:
- CTF
C-terminal fragment
- CAM
cell-adhesion molecule
- DMEM
Dulbecco’s modified Eagle’s medium
- ICAM-5
intercellular adhesion molecule-5
- MA
methamphetamine
- MMP
matrix metalloproteinase
- NTF
N-terminal fragment
- LTP
long-term potentiation
- PBS
phosphate-buffered saline
- PBST
PBS with 0.1% Tween
References
- Brown TE, Forquer MR, Cocking DL, Jansen HT, Harding JW and Sorg BA (2007) Role of matrix metalloproteinases in the acquisition and reconsolidation of cocaine-induced conditioned place preference. Learn. Mem 14, 214–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TE, Forquer MR, Harding JW, Wright JW and Sorg BA (2008) Increase in matrix metalloproteinase-9 levels in the rat medial prefrontal cortex after cocaine reinstatement of conditioned place preference. Synapse 62, 886–889. [DOI] [PubMed] [Google Scholar]
- Brown TE, Wilson AR, Cocking DL and Sorg BA (2009) Inhibition of matrix metalloproteinase activity disrupts reconsolidation but not consolidation of a fear memory. Neurobiol. Learn. Mem 91, 66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavis P and Westbrook G (2001) Integrins mediate functional pre- and postsynaptic maturation at a hippocampal synapse. Nature 411, 317–321. [DOI] [PubMed] [Google Scholar]
- Chen LY, Rex CS, Casale MS, Gall CM and Lynch G (2007) Changes in synaptic morphology accompany actin signaling during LTP. J. Neurosci 27, 5363–5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho RW, Park JM, Wolff SB et al. (2008) mGluR1/5-dependent long-term depression requires the regulated ectodomain cleavage of neuronal pentraxin NPR by TACE. Neuron 57, 858–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HK, Won LA, Kontur PJ, Hammond DN, Fox AP, Wainer BH, Hoffmann PC and Heller A (1991) Immortalization of embryonic mesencephalic dopaminergic neurons by somatic cell fusion. Brain Res. 552, 67–76. [DOI] [PubMed] [Google Scholar]
- Conant K, St Hillaire C, Nagase H, Visse R, Gary D, Haughey N, Anderson C, Turchan J and Nath A (2004) Matrix metalloproteinase 1 interacts with neuronal integrins and stimulates dephosphorylation of Akt. J. Biol. Chem 279, 8056–8062. [DOI] [PubMed] [Google Scholar]
- Conant K, Wang Y, Szklarczyk A, Dudak A, Mattson MP and Lim ST (2010) Matrix metalloproteinase-dependent shedding of intercellular adhesion molecule-5 occurs with long-term potentiation. Neuroscience 166, 508–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cury PR, Araujo VC, Canavez F, Furuse C and Araujo NS (2007) Hydrocortisone affects the expression of matrix metalloproteinases (MMP-1, −2, −3, −7, and −11) and tissue inhibitor of matrix metalloproteinases (TIMP-1) in human gingival fibroblasts. J. Periodontol 78, 1309–1315. [DOI] [PubMed] [Google Scholar]
- Dawirs RR, Teuchert-Noodt G and Busse M (1991) Single doses of methamphetamine cause changes in the density of dendritic spines in the prefrontal cortex of gerbils (Meriones unguiculatus). Neuropharmacology 30, 275–282. [DOI] [PubMed] [Google Scholar]
- Fowlkes JL, Thrailkill KM, Serra DM, Suzuki K and Nagase H (1995) Matrix metalloproteinases as insulin-like growth factor binding protein-degrading proteinases. Prog. Growth Factor Res 6, 255–263. [DOI] [PubMed] [Google Scholar]
- Furutani Y, Matsuno H, Kawasaki M, Sasaki T, Mori K and Yoshihara Y (2007) Interaction between telencephalin and ERM family proteins mediates dendritic filopodia formation. J. Neurosci 27, 8866–8876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahmberg CG, Tian L, Ning L and Nyman-Huttunen H (2008) ICAM-5 - a novel two-facetted adhesion molecule in the mammalian brain. Immunol. Lett 117, 131–135. [DOI] [PubMed] [Google Scholar]
- Guillaumond F, Becquet D, Bosler O and Francois-Bellan AM (2002) Adrenergic inducibility of AP-1 binding in the rat pineal gland depends on prior photoperiod. J. Neurochem 83, 157–166. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Fernandez A, Fueyo A, Folgueras AR et al. (2008) Matrix metalloproteinase-8 functions as a metastasis suppressor through modulation of tumor cell adhesion and invasion. Cancer Res. 68, 2755–2763. [DOI] [PubMed] [Google Scholar]
- Higuchi M, Suzuki Y, Yatani Y, Kitagawa Y, Nagayasu K, Shirakawa H, Nakagawa T and Kaneko S (2008) Augmentation of serotonin release by sustained exposure to MDMA and methamphetamine in rat organotypic mesencephalic slice cultures containing raphe serotonergic neurons. J. Neurochem 106, 2410–2420. [DOI] [PubMed] [Google Scholar]
- Hinkle CL, Diestel S, Lieberman J and Maness PF (2006) Metalloprotease-induced ectodomain shedding of neural cell adhesion molecule (NCAM). J. Neurobiol 66, 1378–1395. [DOI] [PubMed] [Google Scholar]
- Huang Z, Shimazu K, Woo NH, Zang K, Muller U, Lu B and Reichardt LF (2006) Distinct roles of the beta 1-class integrins at the developing and the mature hippocampal excitatory synapse. J. Neurosci 26, 11208–11219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedynak JP, Uslaner JM, Esteban JA and Robinson TE (2007) Methamphetamine-induced structural plasticity in the dorsal striatum. Eur. J. Neurosci 25, 847–853. [DOI] [PubMed] [Google Scholar]
- Kean MJ, Williams KC, Skalski M, Myers D, Burtnik A, Foster D and Coppolino MG (2009) VAMP3, syntaxin-13 and SNAP23 are involved in secretion of matrix metalloproteinases, degradation of the extracellular matrix and cell invasion. J. Cell Sci 122, 4089–4098. [DOI] [PubMed] [Google Scholar]
- Kobeissy FH, Jeung JA, Warren MW, Geier JE and Gold MS (2008) Changes in leptin, ghrelin, growth hormone and neuropeptide-Y after an acute model of MDMA and methamphetamine exposure in rats. Addict. Biol 13, 15–25. [DOI] [PubMed] [Google Scholar]
- Kolb B, Gorny G, Li Y, Samaha AN and Robinson TE (2003) Amphetamine or cocaine limits the ability of later experience to promote structural plasticity in the neocortex and nucleus accumbens. Proc. Natl Acad. Sci. USA 100, 10523–10528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramar EA, Lin B, Rex CS, Gall CM and Lynch G (2006) Integrin-driven actin polymerization consolidates long-term potentiation. Proc. Natl Acad. Sci. USA 103, 5579–5584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee R, Kermani P, Teng KK and Hempstead BL (2001) Regulation of cell survival by secreted proneurotrophins. Science 294, 1945–1948. [DOI] [PubMed] [Google Scholar]
- Lim ST, Lim KC, Giuliano RE and Federoff HJ (2008) Temporal and spatial localization of nectin-1 and l-afadin during synaptogenesis in hippocampal neurons. J. Comp. Neurol 507, 1228–1244. [DOI] [PubMed] [Google Scholar]
- Liu Y, Brown S, Shaikh J, Fishback JA and Matsumoto RR (2008) Relationship between methamphetamine exposure and matrix metalloproteinase 9 expression. Neuroreport 19, 1407–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Xing F, Guiliano R and Federoff HJ (2008) Identification of a novel nurr1-interacting protein. J. Neurosci 28, 9277–9286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutgendorf SK, Lamkin DM, Jennings NB et al. (2008) Biobehavioral influences on matrix metalloproteinase expression in ovarian carcinoma. Clin. Cancer Res 14, 6839–6846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiya R, Zhou Y, Norris EH, Kreek MJ and Strickland S (2009) Tissue plasminogen activator modulates the cellular and behavioral response to cocaine. Proc. Natl Acad. Sci. USA 106, 1983–1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maolood N, Hardin-Pouzet H and Grange-Messent V (2008) Matrix metalloproteinases MMP2 and MMP9 are upregulated by noradrenaline in the mouse neuroendocrine hypothalamus. Eur. J. Neurosci 27, 1143–1152. [DOI] [PubMed] [Google Scholar]
- Mark KA, Quinton MS, Russek SJ and Yamamoto BK (2007) Dynamic changes in vesicular glutamate transporter 1 function and expression related to methamphetamine-induced glutamate release. J. Neurosci 27, 6823–6831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuno H, Okabe S, Mishina M, Yanagida T, Mori K and Yoshihara Y (2006) Telencephalin slows spine maturation. J. Neurosci 26, 1776–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechtersheimer S, Gutwein P, Agmon-Levin N et al. (2001) Ectodomain shedding of L1 adhesion molecule promotes cell migration by autocrine binding to integrins. J. Cell Biol 155, 661–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meighan SE, Meighan PC, Choudhury P, Davis CJ, Olson ML, Zornes PA, Wright JW and Harding JW (2006) Effects of extracellular matrix-degrading proteases matrix metalloproteinases 3 and 9 on spatial learning and synaptic plasticity. J. Neurochem 96, 1227–1241. [DOI] [PubMed] [Google Scholar]
- Meighan PC, Meighan SE, Davis CJ, Wright JW and Harding JW (2007) Effects of matrix metalloproteinase inhibition on short- and long-term plasticity of schaffer collateral/CA1 synapses. J. Neurochem 102, 2085–2096. [DOI] [PubMed] [Google Scholar]
- Michaluk P, Kolodziej L, Mioduszewska B, Wilczynski GM, Dzwonek J, Jaworski J, Gorecki DC, Ottersen OP and Kaczmarek L (2007) beta -dystroglycan as a target for MMP-9 in response to enhanced neuronal activity. J. Biol. Chem 282, 16036–16041. [DOI] [PubMed] [Google Scholar]
- Mitsui S, Saito M, Mori K and Yoshihara Y (2007) A transcriptional enhancer that directs telencephalon-specific transgene expression in mouse brain. Cereb. Cortex 17, 522–530. [DOI] [PubMed] [Google Scholar]
- Miyata S, Nakatani Y, Hayashi N and Nakashima T (2005) Matrix-degrading enzymes tissue plasminogen activator and matrix metalloprotease-3 in the hypothalamo-neurohypophysial system. Brain Res. 1058, 1–9. [DOI] [PubMed] [Google Scholar]
- Mizoguchi H, Yamada K, Mouri A et al. (2007a) Role of matrix metalloproteinase and tissue inhibitor of MMP in methamphetamine-induced behavioral sensitization and reward: implications for dopamine receptor down-regulation and dopamine release. J. Neurochem 102, 1548–1560. [DOI] [PubMed] [Google Scholar]
- Mizoguchi H, Yamada K, Niwa M et al. (2007b) Reduction of methamphetamine-induced sensitization and reward in matrix metalloproteinase-2 and −9-deficient mice. J. Neurochem 100, 1579–1588. [DOI] [PubMed] [Google Scholar]
- Mizoguchi H, Yamada K and Nabeshima T (2008) Neuropsychotoxicity of abused drugs: involvement of matrix metalloproteinase-2 and −9 and tissue inhibitor of matrix metalloproteinase-2 in methamphetamine-induced behavioral sensitization and reward in rodents. J. Pharmacol. Sci 106, 9–14. [DOI] [PubMed] [Google Scholar]
- Nagy V, Bozdagi O, Matynia A et al. (2006) Matrix metalloproteinase-9 is required for hippocampal late-phase long-term potentiation and memory. J. Neurosci 26, 1923–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasterkamp RJ, Peschon JJ, Spriggs MK and Kolodkin AL (2003) Semaphorin 7A promotes axon outgrowth through integrins and MAPKs. Nature 424, 398–405. [DOI] [PubMed] [Google Scholar]
- Pauly T, Ratliff M, Pietrowski E, Neugebauer R, Schlicksupp A, Kirsch J and Kuhse J (2008) Activity-dependent shedding of the NMDA receptor glycine binding site by matrix metalloproteinase 3: a PUTATIVE mechanism of postsynaptic plasticity. PLoS ONE 3, e2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricoy UM, Martinez JL Jr (2009) Local hippocampal methamphetamine-induced reinforcement. Front. Behav. Neurosci 3, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE and Kolb B (2004) Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology 47(Suppl 1), 33–46. [DOI] [PubMed] [Google Scholar]
- Romero-Calvo I, Ocon B, Martinez-Moya P, Suarez MD, Zarzuelo A, Martinez-Augustin O and de Medina FS (2010) Reversible Ponceau staining as a loading control alternative to actin in Western blots. Anal. Biochem 401, 318–320. [DOI] [PubMed] [Google Scholar]
- Rosenberg GA (2002) Matrix metalloproteinases in neuroinflammation. Glia 39, 279–291. [DOI] [PubMed] [Google Scholar]
- Samochowiec A, Grzywacz A, Kaczmarek L, Bienkowski P, Samochowiec J, Mierzejewski P, Preuss UW, Grochans E and Ciechanowicz A (2010) Functional polymorphism of matrix metalloproteinase-9 (MMP-9) gene in alcohol dependence: family and case control study. Brain Res. 1327, 103–106. [DOI] [PubMed] [Google Scholar]
- Sbai O, Ferhat L, Bernard A et al. (2008) Vesicular trafficking and secretion of matrix metalloproteinases-2, −9 and tissue inhibitor of metalloproteinases-1 in neuronal cells. Mol. Cell. Neurosci 39, 549–568. [DOI] [PubMed] [Google Scholar]
- Schepers RJ, Oyler JM, Joseph RE Jr, Cone EJ, Moolchan ET and Huestis MA (2003) Methamphetamine and amphetamine pharmacokinetics in oral fluid and plasma after controlled oral methamphetamine administration to human volunteers. Clin. Chem 49, 121–132. [DOI] [PubMed] [Google Scholar]
- Schubert D, Heinemann S, Carlisle W, Tarikas H, Kimes B, Patrick J, Steinbach JH, Culp W and Brandt BL (1974) Clonal cell lines from the rat central nervous system. Nature 249, 224–227. [DOI] [PubMed] [Google Scholar]
- Sharma HS and Ali SF (2006) Alterations in blood-brain barrier function by morphine and methamphetamine. Ann. N Y Acad. Sci 1074, 198–224. [DOI] [PubMed] [Google Scholar]
- Shi Y and Ethell IM (2006) Integrins control dendritic spine plasticity in hippocampal neurons through NMDA receptor and Ca2+/calmodulin-dependent protein kinase II-mediated actin reorganization. J. Neurosci 26, 1813–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speidl WS, Toller WG, Kaun C et al. (2004) Catecholamines potentiate LPS-induced expression of MMP-1 and MMP-9 in human monocytes and in the human monocytic cell line U937: possible implications for peri-operative plaque instability. FASEB J. 18, 603–605. [DOI] [PubMed] [Google Scholar]
- Spiegel A, Shivtiel S, Kalinkovich A et al. (2007) Catecholaminergic neurotransmitters regulate migration and repopulation of immature human CD34+ cells through Wnt signaling. Nat. Immunol 8, 1123–1131. [DOI] [PubMed] [Google Scholar]
- St Hillaire C, Vargas D, Pardo CA, Gincel D, Mann J, Rothstein JD, McArthur JC and Conant K (2005) Aquaporin 4 is increased in association with human immunodeficiency virus dementia: implications for disease pathogenesis. J. Neurovirol 11, 535–543. [DOI] [PubMed] [Google Scholar]
- Sternlicht MD and Werb Z (2001) How matrix metalloproteinases regulate cell behavior. Annu. Rev. Cell Dev. Biol 17, 463–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su X, Maguire-Zeiss KA, Giuliano R, Prifti L, Venkatesh K and Federoff HJ (2008) Synuclein activates microglia in a model of Parkinson’s disease. Neurobiol. Aging 29, 1690–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada T and Sheng M (2006) Molecular mechanisms of dendritic spine morphogenesis. Curr. Opin. Neurobiol 16, 95–101. [DOI] [PubMed] [Google Scholar]
- Takemoto Y, Yoshiyama M, Takeuchi K, Omura T, Komatsu R, Izumi Y, Kim S and Yoshikawa J (1999) Increased JNK, AP-1 and NF-kappa B DNA binding activities in isoproterenol-induced cardiac remodeling. J. Mol. Cell. Cardiol 31, 2017–2030. [DOI] [PubMed] [Google Scholar]
- Thomas DM, Francescutti-Verbeem DM and Kuhn DM (2008) Methamphetamine-induced neurotoxicity and microglial activation are not mediated by fractalkine receptor signaling. J. Neurochem 106, 696–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L, Stefanidakis M, Ning L et al. (2007) Activation of NMDA receptors promotes dendritic spine development through MMP-mediated ICAM-5 cleavage. J. Cell Biol 178, 687–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhl GR, Drgon T, Liu QR et al. (2008) Genome-wide association for methamphetamine dependence: convergent results from 2 samples. Arch. Gen. Psychiatry 65, 345–355. [DOI] [PubMed] [Google Scholar]
- Wagner GC, Tekirian TL and Cheo CT (1993) Sexual differences in sensitivity to methamphetamine toxicity. J. Neural Transm 93, 67–70. [DOI] [PubMed] [Google Scholar]
- Wang XB, Bozdagi O, Nikitczuk JS, Zhai ZW, Zhou Q and Huntley GW (2008) Extracellular proteolysis by matrix metalloproteinase-9 drives dendritic spine enlargement and long-term potentiation coordinately. Proc. Natl Acad. Sci. USA 105, 19520–19525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong VW, Power C, Forsyth P and Edwards DR (2001) Metalloproteinases in biology and pathology of the nervous system. Nat. Rev. Neurosci 2, 502–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JW, Deb S and Gottschall PE (1998) Regional and differential expression of gelatinases in rat brain after systemic kainic acid or bicuculline administration. Eur. J. Neurosci 10, 3358–3368. [DOI] [PubMed] [Google Scholar]