Abstract
We demonstrate that the transfer of fully charged aminoacyl-tRNAs into peptides directed by the MS2 RNA template requires both ATP and GTP, initiation factors (IF1, IF2, and IF3), elongation factors (EF-Tu, EF-Ts, and EF-G), and the ribosomal ATPase (RbbA). The nonhydrolyzable analogue AMPPCP inhibits the reactions, suggesting that hydrolysis of ATP is required for synthesis. The RbbA protein occurs bound to ribosomes and stimulates the ATPase activity of Escherichia coli 70S and 30S particles. The gene encoding RbbA harbors four ATP binding domains; the C-terminal half of the protein bears extensive sequence similarity to EF-3, a ribosome-dependent ATPase. Here, we show that the antibiotic hygromycin B selectively inhibits the ATPase activity of RbbA. Other antibiotics with similar effects on miscoding, streptomycin and neomycin, as well as antibiotics that impair peptide bond synthesis and translocation, had little effect on the ATPase activity of RbbA on 70S ribosomes. Immunoblot analysis indicates that at physiological concentrations, hygromycin B selectively releases RbbA from 70S ribosomes. Hygromycin B protects G1494 and A1408 in the decoding region, and RbbA enhances the reactivity of A889 and G890 of the 16S rRNA switch helix region. Cross-linking and X-ray diffraction data have revealed that this helix switch and the decoding region are in close proximity. Mutations in the switch helix (889-890) region affect translational fidelity and translocation. The binding site of hygromycin B and its known dual effect on the fidelity of decoding and translocation suggest a model for the action of this drug on ribosomes.
Many antibiotics exert their action by inhibiting protein biosynthesis through direct interactions with the ribosomes. A large number of studies indicate that certain antibiotics perturb specific ribosomal events and protect bases on the small- and large-subunit rRNAs. In few cases, there has been a correlation between the inhibitory activity of an antibiotic and specific translation reactions, e.g., the disruption of the elongation factors EF-Tu by kirromycin and EF-G by fusidic acid (9). It is quite possible that most antibiotics exert their effects on functional sites of the ribosome; specifically, they may directly or indirectly disrupt the structure of rRNA. Thus, many mutations in rRNA that confer resistance to antibiotics reside in specific bases of either the 16S or 23S rRNA (9). Of special interest in this regard is a set of antibiotics that bind to the 16S rRNA. This group includes streptomycin, neomycin, paromomycin, tetracycline, spectinomycin, and hygromycin B. All these antibiotics protect bases that are phylogenetically conserved on the 16S rRNA (2, 9). The aminoglycoside antibiotic hygromycin B is thought to have a dual effect on translation by inducing misreading of aminoacyl-tRNAs as well as impairing translocation (5). Of particular interest is the fact that hygromycin B has been shown to protect N7 of G1494 from dimethyl sulfate modification and to significantly enhance the modification of A1408 at N1 (24). These sites are proximal to the sites that confer resistance to hygromycin B (3, 26, 27). These sites occur in the decoding center of the ribosome, as expected from their effects on miscoding. Thus, hygromycin B has been proposed to distort the ribosomal A site, thereby inhibiting translation (3, 5,9, 24, 26, 27).
Recently, the crystal structure of the ribosome enabled the visualization of the tRNA binding sites. The views reveal the RNA backbones of regions that encompass the site of hygromycin B resistance. The region of the ribosome that is of eminent importance in this case is the decoding region encompassing bases 1400 to 1497 (7). Since the A site regulates the entrance of aminoacyl-tRNAs into the ribosome, it also affects the fidelity of the reaction. The process of A-site recognition requires the participation of the EF-Tu–GTP–aminoacyl-tRNA complex. This complex enters the ribosome at a site that is adjacent to the A site—the T or A/T site (17). Hydrolysis of GTP is required for an accommodation of the aminoacyl-tRNA that insures both the proper codon-anticodon recognition and the ejection of incorrect or noncognate tRNAs from the particle (17, 26). Thus, distortion of the A site through binding of hygromycin B to the 16S rRNA can, in principle, explain the action of the drug on the ribosomes. Less clear is the effect of the antibiotic on translocation (5, 17).
Studies on the effect of aminoglycoside antibiotics have revealed that many of these affect the dissociation from ribosomes of the translocase EF-G in its GDP-bound form (6). Hygromycin B, however, does not affect this reaction. It has, in contrast, been suggested that hygromycin B affects the actual translocation subsequent to initiation of poly(rU)-programmed ribosomes (18). The drug does not appear to interact directly with EF-G.
Reconstitution of translation in Escherichia coli has revealed that several new proteins are required for synthesis (12–15, 19–23). One of these, EFP, stimulates the action of the peptidyl transferase on 70S particles (15). Another factor, W, ejects tRNAs from 70S particles during synthesis (13); the W2 protein unwinds the mRNA during initiation (23), and a ribosome-dependent ATPase (RbbA) promotes hydrolysis of ATP by 70S ribosomes (19). These proteins harbor extensive sequence similarity to the eukaryotic factors IF5A, IF4A, and EF-3.
One of these proteins, RbbA, has been shown to bind near the switch helix (889-890) of the 16S rRNA which neighbors the decoding center of the ribosome at bases 1400 to 1497 (21). This switch helix has, in turn, been shown by mutagenesis to affect the fidelity of the decoding process (22). Mutations in this helix result in either hyperaccurate or error-prone ribosomes (22). Here, we show that hygromycin B directly inhibits the action of RbbA on 70S ribosomes and that the antibiotic releases this protein from the particles. A model for the action of hygromycin B is proposed.
MATERIALS AND METHODS
Poly(U) was purchased from Sigma. MS2 RNA was from Boehringer Mannheim. MRE 600 E. coli cells (RNase deficient) were purchased from the University of Alabama Fermentation Facility. Cells were harvested at mid-log phase and were frozen at −80°C. [35S]methionine (1,100 μCi/μM), [14C]phenylalanine (600 μCi/mmol), and [γ-33P]ATP (400 Ci/mmol) were purchased from ICN.
Poly(U)-programmed polyphenylalanine and MS2 RNA-directed synthesis.
In vitro polyphenylalanine synthesis was conducted in 50-μl incubation mixtures containing 1.5 μg of EF-Tu, 0.11 μg of EF-G, 20 mM Tris-HCl (pH 7.4), 6.0 mM magnesium acetate [Mg(OAc)2], 100 mM NH4Cl, 0.2 mM GTP, 0.6 mM ATP, 22 μg of poly(U), 50 μg of 70S ribosomes (twice washed) and 60,000 dpm of [3H]Phe-tRNA. Reaction times were 15 min at 37°C. Trichloroacetic acid-precipitable counts were measured after hydrolysis of the peptidyl-tRNA in 5% trichloroacetic acid for 15 min at 90°C and washing the filtrates on nitrocellulose filters. Radioactivity was determined on the filters after addition of scintillation fluid. MS2 RNA-directed synthesis was conducted as described previously (12, 13, 16) using [35S]Met-tRNA and 19 cold aminoacyl-tRNAs. The reaction mixtures contained pure IF1, IF2, IF3, EF-Tu, EF-Ts, and EF-G which had been isolated as previously described (14).
Isolation of 70S ribosomes.
E. coli MRE 600 cells were lysed by grinding with twice their weight of alumina. One volume of buffer A [50 mM Tris-HCl (pH 7.5), 10 mM Mg(OAc)2, 30 mM NH4Cl, 1 mM dithiothreitol (DTT)] containing DNase I (RNase free) was added for extraction. After a 30-min incubation on ice, the extract was centrifuged twice at 30,000 × g at 4°C to obtain the S30 supernatant. S30 was layered over a 0.4 to 1.2 M sucrose gradient in buffer A and centrifuged at 100,000 × g for 4 h at 4°C. The resultant supernatant, S100, was removed, and the pellets containing the 70S ribosomes were resuspended in buffer A containing 1 M NH4Cl. The resuspended ribosomes were centrifuged again at 100,000 × g to obtain the 1× ribosomal wash (supernatant) and the 1× 70S ribosomes (pellet). To obtain the 2×, 3×, and 4× ribosomal washes and the 70S ribosomes, the 1 M NH4Cl wash was repeated an additional three times.
Purification of translation factors.
Translation factors were purified from E. coli MRE 600 cells lysed on a French press at 10,000 lb/in2. The ribosomes were used as an affinity matrix for the purification of both initiation and elongation factors as previously described (14).
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis, immunoblot analysis, and gel staining.
Samples were electrophoresed on either 10, 8, or 6% Tris-glycine or 10% Tris-Tricine gels under reducing conditions.
Electroblotting to nitrocellulose was performed in a Novex cell following the manufacturer's protocol. Immunoblot analysis was performed with anti-EF-3 polyclonal antibody, horseradish peroxidase-conjugated anti-immunoglobulin and enhanced chemiluminescence (Amersham) for detection.
Silver stains were performed either with the Bio-Rad Silver Stain Plus kit or by first soaking the gels for 30 min in 50% (vol/vol) methanol–10% (vol/vol) acetic acid. The gels were then rinsed for 10 min in 20% ethanol and 10 min in H2O. Gels were sensitized in 0.02% (wt/vol) sodium thiosulfate. After two 20-s rinses in H2O, gels were stained in 0.2% (wt/vol) silver nitrate. Gels were rinsed once in H2O for 20 s and developed in 2% sodium carbonate–0.04% (vol/vol) formaldehyde. Staining was stopped by rinsing the gels in 5% (vol/vol) acetic acid.
Coomassie stains were performed by shaking the gels in 50% (vol/vol) methanol–10% (vol/vol) acetic acid–0.25% (wt/vol) Coomassie blue R-250 for 4 h. Gels were then destained overnight in 7.5% (vol/vol) acetic acid–5% (vol/vol) methanol.
ATPase assays.
Assays were performed in ATP buffer [50 mM Tris-HCl (pH 7.5), 5 mM Mg(OAc)2, 50 mM NH4Cl, 1 mM DTT, 500 μg of bovine serum albumin/ml] containing 20 pmol of 70S ribosomes and 20 pmol of RbbA. Reaction volumes were 50 μl, and reactions were started by adding 0.5 mM [γ-32P]ATP. After a 10-min incubation at 37°C, reactions were stopped by adding 100 μl of 0.02 M tungstosilic acid in 0.02 N H2SO4. Free phosphate was extracted as a molybdate-phosphate complex by the method of Conway and Lipmann (8) by the sequential addition of 20 μl of 5 mM phosphate buffer (pH 6.9)–40 μl of 5% ammonium molybdate in 4 N H2SO4–200 μl of water-saturated butanol-benzene (1:1). Thin-layer chromatography of the products was carried out on polyethyleneimine plates using 1 M NH4 formate and 1 N HCl (62:38). The plates were developed for 1.5 to 3.5 h, the nucleotides were visualized with UV light, and the spots were excised from the plates. Radioactivity was measured in a scintillation counter.
Charging of tRNAs.
Total tRNA from E. coli MRE 600 was used as the source for both tRNAPhe (phenylalanine acceptor) and tRNAMet (methionine acceptor). Both tRNAs were charged with their respective labeled amino acids, either [3H]phenylalanine (0.6 Ci/mmol) or [35S]methionine (1,100 Ci/mmol), as previously described (11, 25). Essentially, reaction mixtures contained total tRNA and 150 μCi of the labeled amino acid in a buffer containing 100 mM Tris-HCl (pH 7.5), 10 mM Mg(OAc)2, 100 mM KCl, 5 mM 2-mercaptoethanol, and 3.2 mM ATP, and 1 μM concentrations each of 19 amino acids lacking methionine or phenylalanine. Optimized amounts of S100 were added as the source of aminoacyl-tRNA synthetases. Reaction mixtures were incubated 15 min at 37°C and then made acidic by adding sodium acetate (pH 5.0). The tRNAs were purified as previously described (11, 25).
Antibiotic wash of 70S ribosomes.
70S ribosomes were incubated with either KCl, streptomycin, or hygromycin B at final concentrations of 50 μM in 50 mM Tris-HCl (pH 7.4)–6 mM Mg(OAc)2–30 mM NH4Cl–1 mM DTT. Reaction mixtures were incubated at 37°C for 10 min and then centrifuged 100,000 × g for 2.5 h at 4°C to sediment the ribosomes. Supernatants contained the antibiotic “wash.”
Purification of RbbA.
RbbA was purified from the ribosomal wash of E. coli MRE 600 cells as previously described (19).
RESULTS AND DISCUSSION
Reconstitution of translation directed by a native mRNA template using E. coli ribosomes and each pure initiation and elongation factor has revealed that several proteins are required for synthesis (12–15, 19, 23). Among these proteins is a ribosome-bound ATPase (RbbA) that stimulates 70S ribosomes and 30S subunits to hydrolyze ATP (19). The sequence of the gene encoding RbbA harbors four ATP binding domains (19). Two of these domains are present in a number of ABC transporters and in the yeast elongation factor EF-3. The other two domains are typical of ATP and GTP binding proteins (1). The RbbA protein also harbors an RNA binding motif that is also present in prolyl- and valyl-tRNA synthetases (19). RbbA has been demonstrated to stimulate the synthesis of polyphenylalanine from poly(rU) templates in the presence of elongation factors EF-Tu and EF-G when the reactions contain physiological concentrations of both ATP and GTP (19). Here, we ask whether ATP is required in the reconstituted system and whether the hydrolysis of ATP is promoted by initiation or elongation factors as well.
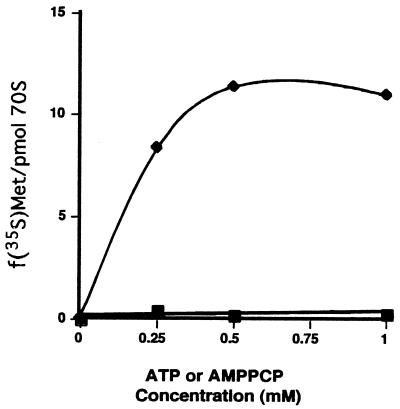
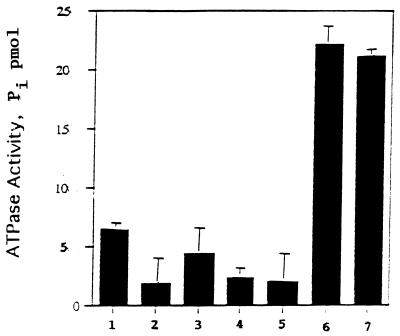
As shown in Fig. 1, the synthesis of the N-terminal peptides of the coat protein directed by MS2 RNA requires the presence of ATP. The nonhydrolyzable form of ATP, AMPPCP, does not promote the reactions, suggesting that ATP hydrolysis is needed. In these experiments the synthesis was conducted in the presence of f[35S]Met-tRNA and each of the cold aminoacyl-tRNAs that occur in the five amino acids of the N terminus of the coat protein, namely, AlaSerAspPheThr. The ATP requirement could be involved in initiation or elongation. However, no requirement for ATP was observed for the binding of the initiator, fMet-tRNA, to the MS2 RNA-programmed ribosomes (data not shown). Thus, ATP may be required in elongation. The system requires each initiation and elongation factor as well as the ribosome-bound RbbA. Controls were performed to ascertain whether any of the initiation or elongation factors promoted hydrolysis of ATP. As shown in Fig. 2, none of the initiation (IF1, IF2, and IF3) or elongation (EF-Tu and EF-G) factors was able to foster ATP hydrolysis. In contrast, RbbA was able to hydrolyze ATP by itself to a limited extent, and ribosomes were able to stimulate this hydrolysis two- or threefold.
FIG. 1.
Stimulation of protein synthesis by ATP. In vitro synthesis was carried out with ribosomes programmed with the native mRNA from MS2 bacteriophage as described in Materials and Methods. Synthesis reactions were identical except that either ATP (♦) or the nonhydrolyzable form of ATP, AMPPCP (▪), was added at the indicated concentrations. Protein synthesis without added ATP (3.05 pmol of [35S]methionine incorporated) was subtracted.
FIG. 2.
Ribosome stimulation of RbbA ATPase activity. RbbA was assayed for the ATPase activity of RbbA alone (column 1); elongation factors (column 2); initiation factors (column 3); a combination of all factors (column 4); 70S ribosomes (column 5); 70S ribosomes and RbbA (column 6); and 70S ribosomes, initiation factors, elongation factors, and RbbA (column 7).
Hygromycin B inhibits RbbA.
Several antibiotics were tested to learn whether they inhibit the action of RbbA. Figure 3 shows that streptomycin, neomycin, fusidic acid, and chloramphenicol at a concentration of 50 μM cause very little inhibition (from 10 to 20%) of the ribosome-dependent ATPase activity due to RbbA. In marked contrast, the ATPase activity of RbbA was inhibited about 80% by 50 μM hygromycin B. Hygromycin B does not inhibit the residual ATPase activity of RbbA in the absence of ribosomes (Fig. 3). Chloramphenicol was used at 500 μM, which was the concentration observed to give quantitative inhibition of protein synthesis. However, even at these higher concentrations, chloramphenicol had little effect on the ATPase activity of the RbbA bound to 70S ribosomes.
FIG. 3.
Antibiotic effects on the ribosomal ATPase. Antibiotics were added to the ATPase assays containing 20 pmol of 1× washed 70S ribosomes. O, control (no antibiotic); S, streptomycin; N, neomycin;. F, fusidic acid; C, chloramphenicol; H, hygromycin B; CH, hygromycin B control without 70S ribosomes but with RbbA. All antibiotics were added at a final concentration of 50 μM except for chloramphenicol, which was at 500 μM. Data are the averages of three experiments. The control was normalized to 100%.
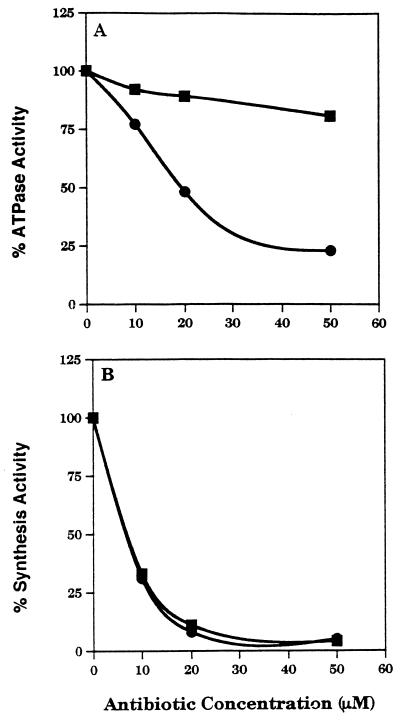
To compare the effect of hygromycin B on synthesis and ATPase activities, hygromycin B was examined for its ability to inhibit both poly(rU)-directed synthesis and the ATPase activity of 70S ribosomes due to RbbA addition. Streptomycin was used as a control. Both antibiotics exhibit similar synthesis inhibition curves, but hygromycin B more effectively inhibits the ribosomal ATPase activity of RbbA. The 50% inhibitory concentration for hygromycin B on the ATPase activity is 20 μM, whereas that for polyphenylalanine synthesis inhibition is about 10 μM (Fig. 4). Hygromycin B must alter the ribosomal particle in a way that specifically affects the ribosomal ATPase activity of RbbA. It may also have other effects that exert a stronger inhibition on polyphenylalanine synthesis (5).
FIG. 4.
Hygromycin B and streptomycin inhibition curves. Hygromycin B (●) and streptomycin (▪) were added at the indicated concentrations to an ATPase assay (A) containing 2× washed 70S ribosomes or to a poly(rU)-programmed polyphenylalanine synthesis assay (B).
Hygromycin B affects the association of RbbA with the ribosomes.
Previous experiments have shown that RbbA accounts for most of the ATPase activity of 70S ribosomes and 30S subunits (19). In this work we demonstrate that the antibiotic hygromycin B can inhibit the 70S ribosome-associated ATPase activity. The ability of hygromycin B and streptomycin to release the RbbA protein from the ribosomes was examined. Ribosomes were incubated under conditions similar to those used for synthesis with and without the antibiotics, and the particles were removed by ultracentrifugation. The resulting supernatants were then analyzed by immunoblots for the presence of RbbA. Figure 5 shows results of an immunoblot of the supernatants that were obtained by these procedures. No RbbA was released from the ribosomes in the absence of antibiotics. Hygromycin B, on the other hand, consistently released RbbA from the ribosomes. Streptomycin released only about 25% of the amount of RbbA that hygromycin B released. This is consistent with the fact that streptomycin also exhibits about 20% inhibition of the ATPase activity of ribosomes compared to that of hygromycin B (Fig. 3). Thus, the effect of hygromycin B on the ribosomal ATPase activity may be due to its ability to effectively release RbbA from the particles.
FIG. 5.
Antibiotic wash of 70S ribosomes. Hygromycin B or streptomycin (50 μM each) was added to 20 pmol of 70S ribosomes to induce the release of protein(s) from the ribosomes. The ribosomes were isolated by ultracentrifugation, and the supernatants were analyzed by immunoblotting as described in Materials and Methods. The data were scanned densitometrically, and the total RbbA released into the supernatant was taken as 100%. Column 1, control ribosomes (not washed) in a 5× excess; column 2, no antibiotic added; column 3, streptomycin wash; column 4, hygromycin B wash.
Binding site of RbbA on ribosomes.
The RbbA protein has been shown to occur bound to 70S ribosomes as well as to 30S subunits (19, 20). In this work, we demonstrate that hygromycin B inhibits the 70S-associated ATPase activity. The RbbA protein binds to free labeled 16S rRNA, and the binding of rRNA can be competed with unlabeled 16S rRNA but not appreciably with 23S rRNA, tRNA, or mRNA. Thus, the binding of RbbA to 16S rRNA appears to be specific, suggesting that 16S rRNA bases comprise part of the binding site for RbbA on both 30S and 70S particles. 16S rRNA protection and footprinting experiments indicate that RbbA enhances the reactivity of the bases A889 and G890 of the 16S rRNA to the action of diethyl pyrocarbonate and RNase T1, respectively (21). Thus, RbbA binds near the region of the 16S rRNA that harbors bases A889 and G890. Since the extent of modification of these bases can be enhanced by RbbA, they may neighbor the RbbA binding site. Indeed, RbbA protects G925, which is within 34 Å of the A889-G890 switch (3). RbbA has strong affinity for poly(G) and not for other polymers, suggesting that it binds to G residues (19). The 889-890 base pair region of 16S rRNA also neighbors a poly(G) stretch in the 16S rRNA (G885 to G888). This region of 16S rRNA has been demonstrated by Lodmell and Dahlberg (22) to exist in two different conformations. Conformation A results in hyperaccurate ribosomes; the A site becomes very stringent for the binding of cognate aminoacyl-tRNAs. In conformation B, the ribosomes become prone to errors and the A site becomes more “open” to aminoacyl-tRNAs.
The 16S rRNA of wild-type ribosomes can and does exist in both conformations. Lodmell and Dahlberg (22) suggested that the ribosomal proteins S5 and S12 probably facilitate this conformational switch that affects tRNA binding at both the A and P sites because these proteins have been implicated in the ribosomal hyperaccurate and error-prone (ram) phenotypes.
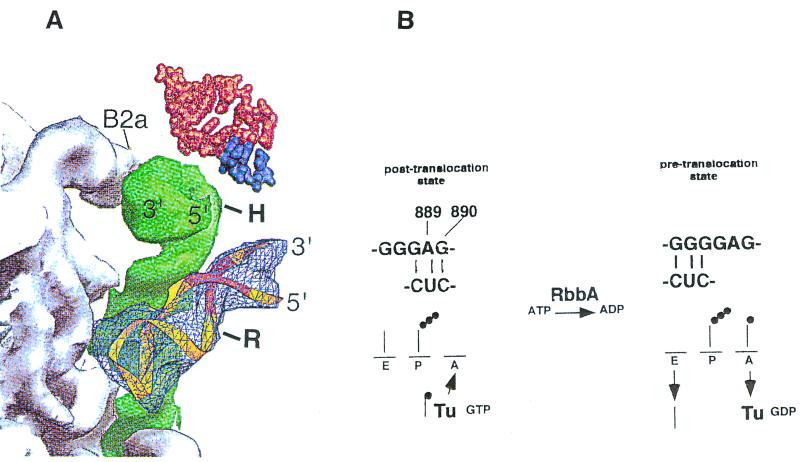
RbbA binds strongly to poly(G) sequences (21). G885-G888 is conserved among all small-subunit rRNAs (22); thus, the poly(G) stretch in 16S rRNA is likely to be essential for ribosome function. Based on binding experiments with 16S rRNA, RbbA may bind to this region of the rRNA, and the poly(G) stretch of the 16S rRNA which is close to G925 that is protected by RbbA, too, is probably a binding site for RbbA. Because RbbA enhances the reactivity of A889 and G890, it is tempting to suggest that RbbA facilitates the conformational switch discussed above. Both RNase T1 and diethyl pyrocarbonate preferentially modify unpaired bases. Conformation B (error prone) leaves these two bases unpaired. The second conformation, A (hyperaccurate), pairs them with U911 and C910. RbbA may drive the conformation of the rRNA towards the unpairing of A889 and G890 (Fig. 6). RbbA also protected G925 from RNase T1 (21). This is also part of the stretch of conserved G residues on the 16S rRNA. In the structural models of 16S rRNA, the G890 and G925 regions are within 34 to 38 Å of each other (3, 7).
FIG. 6.
Switch helix in the 30S subunit. A view of the packing interactions from the platform side of the A-site tRNA is shown. (A) Model for the action of hygromycin B and of RbbA on 70S ribosomes. The 889-890 base pair switch helix in the 30S subunit is shown. The diagram (from reference 7) shows the penultimate stem (green) between the 50S subunit (gray) and the switch helix (bp 900) (light blue), and the remainder of the 30S subunit with A-site tRNA (red) and mRNA (dark purple) above. B2a, interface contact, shown for orientation; H, approximate location of base 1408, which is protected by hygromycin B from dimethyl sulfate modification; R, site of binding of RbbA to base G925 of the switch helix region. A ribbon representation of the 889-890 stem-loop region of 16S rRNA containing the S turn is in yellow, with the switch helix region in orange. (B) Proposed model for RbbA. The conformational switch in 16S rRNA demonstrated by Lodmell and Dahlberg is at the top (22). RbbA enhances modification by diethyl pyrocarbonate of A889 and RNase T1 cleavage of G890 (21), suggesting a switch to the conformation on the right that increases binding to the A site. The conformation on the left restricts binding to the A site. RbbA-mediated hydrolysis of ATP may be an energy requirement for the transition. The transition may also be mediated by the EF-Tu–GTP-dependent binding of aminoacyl-tRNA to the A site, since RbbA can bind EF-Tu. The binding of hygromycin B to bases 1494 to 1408 could affect the bp 900 switch helix, preventing the binding of RbbA to this region.
RbbA has also been shown to cross-link to ribosomal protein S1 on the 30S subunit (20). S1, in turn, has been cross-linked to A892 of 16S rRNA (4). S5, S8, and S16 closely neighbor S1 on the 30S subunit. S1, in turn, is known to be an RNA-dependent helicase that helps recruit the mRNA to the 30S subunit (4). S1 is positioned in the decoding center of the ribosome. All this evidence argues for the fact that the RbbA binding site is close to or at the decoding center of the 16S rRNA of the ribosomes.
Possible mechanism of hygromycin B action.
Hygromycin B binds to subunit and protects bases A1408 and G1494, which lie in the decoding center of the 16S rRNA (3, 26, 27). Hygromycin B affects two different steps of elongation. First, hygromycin B, like other aminoglycosides, induces misreading (24, 26). This is an effect which is more likely due to a distortion of the A site (decoding center). Hygromycin B also affects the translocation process (5, 18). The mRNA is often mistranslocated (i.e., moved more or less than three bases) in the presence of hygromycin B. This, too, causes misreading at the A site. Hygromycin B inhibits at least 75 to 80% of the ATPase activity of 70S ribosomes (Fig. 3 and 4). Antibiotics of similar function, e.g., streptomycin, which footprints to base 902 of the 16S rRNA (24), and neomycin, exert lesser effects (25 and 10%, respectively) on the ATPase activity. Hygromycin B is also capable of removing RbbA from the ribosome (Fig. 5). This could be due to direct competition for the binding site, or more likely, based on comparative binding site data, hygromycin B may induce conformational changes within the ribosome.
Recent X-ray diffraction analysis of complexes of 70S ribosomes and hygromycin B indicate that the drug binds to the top of helix 44 of the 16S rRNA in a region that contains the A, P, and E sites of the 30S subunit (3) (Fig. 6A). The molecule was observed to be in the major groove of helix 44 and to contact nucleotides from both strands of the RNA in the 1490-to-1500 and the 1400-to-1410 regions. Ring IV of the hygromycin B molecule is within 4 Å of the second base of the P-site-bound mRNA codon. The antibiotic also appears to be in close contact with base 1498 of the 16S rRNA, which is known to bind the P-site-bound mRNA (3).
Part of helix 44, to which hygromycin B binds, has been suggested to be involved in movement of the ribosome relative to the mRNA during translocation (10). This effect could prevent the movement of the tRNA from the A site to the P site, thus restricting the tRNA in the A site. This action of hygromycin B on the A-site-bound tRNA could alter the balance between the ram and the error-restrictive states of the ribosome (22). The X-ray diffraction data obtained by Cate et al. (7) of the penultimate stem of the 16S rRNA shown in Fig. 6A reveals that the helix 44 region and the switch helix encompassing bases 889-890 and G925 are indeed in close proximity to each other, although they occur in quite distal positions in the secondary structure models of the 16S rRNA. Of interest is the recent work by Wilms et al. (28) that demonstrates cross-linking between the bp 900 region (RbbA binding site) and position 1408 (hygromycin B binding site) of the 16S rRNA.
The most recent X-ray crystallography maps of the 70S ribosome also show a close interaction between the two rRNA regions (the decoding center and the 889-890 base helix switch) (7). All of the above considerations suggest a role for hygromycin B in the decoding process of protein synthesis. The codon-anticodon interactions of the mRNA and tRNAs must remain intact during the translocation event. The footprinting data of Green and Noller (17) suggest that the peptidyl-tRNA remains tightly bound to the P site of the 50S subunit before the translocation event. The peptidyl-tRNA anticodon end is in the 30S subunit A site (the hybrid A/P site). Since the tRNA is firmly anchored on the ribosome, it is suggested that during translocation the 30S subunit is moved relative to the 50S subunit. The head and the body of the 30S subunit are held together by a single-stranded region of rRNA that could facilitate this movement (17). This suggestion has been confirmed by cryoelectron microscopy studies of the ribosomes in the pre- and posttranslocational states (10). RbbA binds near this site and is a 30S-subunit ATPase (21). Hygromycin B, binding at 1408 on the adjacent decoding center, is known to pleiotropically affect the 889-890 base pair helix. Thus, the binding of hygromycin B to the decoding center which neighbors or overlaps the RbbA binding site may initiate a cascade of events resulting in the release of RbbA. The mechanism of RbbA action is not yet known. It is possible that RbbA helps accommodate the aminoacyl-tRNA from the T or A/T site to the A site. Thus, in the absence of RbbA and ATP hydrolysis, incorrect aminoacyl-tRNAs could be incorporated into proteins. The improper accommodation could, depending on the strength of the codon-anticodon interaction, be mistranslocated, as occurs in the presence of hygromycin B. Alternatively, RbbA due to its interaction with EF-Tu–GDP, may help eject the EF-Tu-GDP from the ribosomes (20). The resulting tighter association of EF-Tu–GDP on the ribosome may result in errors, since the final proofreading function of EF-Tu would remain incomplete. Finally, this distortion of the decoding region and of the 889-890 base pair helix switch would alter the translocation event as well by impairing the binding of EF-G and EF-Tu, since both proteins bind to the same site of the ribosome (7). Thus, a single contact of hygromycin B with a base, e.g., 1408 of the 30S subunit, could distort the A site and result in misreading, improper translocation, and ejection of RbbA, which along with EF-Tu and EF-G safeguards these processes on the ribosome.
ACKNOWLEDGMENTS
We are grateful to the Natural Science and Engineering Council, to the Medical Research Council of Canada, and to the Pharmacia Corporation for financial support.
We are thankful to H. Aoki for much expert technical advice and to A. J. Becker for discussion of this work. We also thank K. Chakraburtty for a generous gift of yeast anti-EF-3 antibodies.
ADDENDUM IN PROOF
Recent work in our laboratory with intact 30S subunits has shown that RbbA protects base A937 of 16S rRNA from chemical modification by diethyl pyrocarbonate; bases G844 and G869 have enhanced reactivity to chemical modification by Kethoxal due to the binding of RbbA. These bases are adjacent to the 889-890 helix. This is compatible with the model we have presented in the manuscript.
Footnotes
M.C.G. dedicates this work to the memory of Clelia H. Finney.
REFERENCES
- 1.Ames G F, Mimura C S, Holbrook S R, Shyamala V. Traffic ATPases: a superfamily of transport proteins operating from Escherichia coli to humans. Adv Enzymol Relat Areas Mol Biol. 1992;65:1–47. doi: 10.1002/9780470123119.ch1. [DOI] [PubMed] [Google Scholar]
- 2.Brimacombe R, Atmadja J, Stiege W, Schuler D. A detailed model of the three-dimensional structure of Escherichia coli 16S ribosomal RNA in situ in the 30S subunit. J Mol Biol. 1988;119:115–136. doi: 10.1016/0022-2836(88)90383-x. [DOI] [PubMed] [Google Scholar]
- 3.Brodersen D E, Clemons W M, Jr, Carter R J, Morgan-Warren A P, Wimberly B T, Ramakrishnam V. The structural basis for the action of the antibiotics tetracycline, pactamycin and hygromycin B on the 30 S ribosomal subunit. Cell. 2000;103:1143–1154. doi: 10.1016/s0092-8674(00)00216-6. [DOI] [PubMed] [Google Scholar]
- 4.Bycroft M, Hubbard T J, Proctor M, Freund S M, Murzin A G. The solution structure of the S1 RNA binding domain: a member of an ancient nucleic acid-binding fold. Cell. 1997;88:235–242. doi: 10.1016/s0092-8674(00)81844-9. [DOI] [PubMed] [Google Scholar]
- 5.Cabaras M J, Vásquez D, Modolell J. Dual interference of hygromycin with ribosomal translocation and with aminoacyl-tRNA recognition. Eur J Biochem. 1978;8:21–27. doi: 10.1111/j.1432-1033.1978.tb12347.x. [DOI] [PubMed] [Google Scholar]
- 6.Campuzano S, Vásquez N, Modolell J. Dissociation of guanosine nucleotide-elongation factor G ribosome complexes. Biochemistry. 1979;18:1570–1574. doi: 10.1021/bi00575a029. [DOI] [PubMed] [Google Scholar]
- 7.Cate J H, Yusupov M M, Yusupova G Z, Earnest T N, Noller H F. X-ray crystal structures of 70S ribosome functional complexes. Science. 1999;285:2095–2104. doi: 10.1126/science.285.5436.2095. [DOI] [PubMed] [Google Scholar]
- 8.Conway T W, Lipmann F. Characterization of a ribosome-linked guanosine triphosphatase in Escherichia coli extracts. Proc Natl Acad Sci USA. 1964;52:1402–1409. doi: 10.1073/pnas.52.6.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cundliffe E. Involvement of specific portions of ribosomal RNA in defined ribosomal functions: a study utilizing antibiotics. In: Hardesty B, Kramer G, editors. Structure, function and genetics of ribosomes. New York, N.Y: Springer-Verlag; 1986. pp. 586–604. [Google Scholar]
- 10.Frank J, Agrawal R K. A ratchet-like intersubunit reorganization of the ribosome during translocation. Nature. 2000;408:318–322. doi: 10.1038/35018597. [DOI] [PubMed] [Google Scholar]
- 11.Ganoza M C, Barraclough N, Wong J T. Purification and properties of an N-formylmethionyl-tRNA hydrolase. Eur J Biochem. 1976;65:613–622. doi: 10.1111/j.1432-1033.1976.tb10379.x. [DOI] [PubMed] [Google Scholar]
- 12.Ganoza M C, Cunningham C, Green R M. Isolation and point of action of a factor from Escherichia coli required to reconstruct translation. Proc Natl Acad Sci USA. 1985;82:1648–1652. doi: 10.1073/pnas.82.6.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ganoza M C, Cunningham C, Green R M. A new factor from Escherichia coli affects translocation of mRNA. J Biol Chem. 1995;270:26377–26381. doi: 10.1074/jbc.270.44.26377. [DOI] [PubMed] [Google Scholar]
- 14.Ganoza M C, Aoki H, Burkhardt N, Murphy B J. The ribosome as affinity matrix: efficient purification scheme for translation factors. Biochimie. 1996;78:51–61. doi: 10.1016/0300-9084(96)81329-0. [DOI] [PubMed] [Google Scholar]
- 15.Glick B R, Chladek S, Ganoza M C. Peptide bond formation stimulated by protein synthesis factor EF-P depends on the aminoacyl moiety of the acceptor. Eur J Biochem. 1979;97:23–28. doi: 10.1111/j.1432-1033.1979.tb13081.x. [DOI] [PubMed] [Google Scholar]
- 16.Green R H, Glick B R, Ganoza M C. Requirements for in vitro reconstruction of protein synthesis. Biochem Biophys Res Commun. 1985;126:792–798. doi: 10.1016/0006-291x(85)90254-2. [DOI] [PubMed] [Google Scholar]
- 17.Green R, Noller H F. Ribosomes and translation. Annu Rev Biochem. 1997;66:679–716. doi: 10.1146/annurev.biochem.66.1.679. [DOI] [PubMed] [Google Scholar]
- 18.Hausner T P, Geigenmuller U, Nierhaus K H. The allosteric three-site model for the ribosomal elongation cycle. New insights into the inhibition mechanisms of aminoglycosides, thiostrepton, and viomycin. J Biol Chem. 1988;263:13103–13111. [PubMed] [Google Scholar]
- 19.Kiel M C, Ganoza M C. Identification of a ribosomal ATPase in E. coli cells. Biochimie. 1999;8:1097–1108. doi: 10.1016/s0300-9084(99)00352-1. [DOI] [PubMed] [Google Scholar]
- 20.Kiel M C, Ganoza M C. Functional interactions of an Escherichia coli ribosomal ATPase. Eur J Biochem. 2000;268:1–10. doi: 10.1046/j.1432-1033.2001.01873.x. [DOI] [PubMed] [Google Scholar]
- 21.Kiel M C. Identification and characterization of an Escherichia coli ribosomal ATPase. Thesis. Toronto, Canada: University of Toronto Press; 1999. [Google Scholar]
- 22.Lodmell J S, Dahlberg A E. A conformational switch in Escherichia coli 16S ribosomal RNA during decoding of messenger RNA. Science. 1997;277:1262–1267. doi: 10.1126/science.277.5330.1262. [DOI] [PubMed] [Google Scholar]
- 23.Lu J, Aoki H, Ganoza M C. Molecular characterization of a prokaryotic translation factor homologous to the eukaryotic initiation factor eIF4A. Int J Biochem Cell Biol. 1999;31:215–229. doi: 10.1016/s1357-2725(98)00142-3. [DOI] [PubMed] [Google Scholar]
- 24.Moazed D, Noller H F. Interaction of antibiotics with functional sites in 16S ribosomal RNA. Nature. 1987;327:389–394. doi: 10.1038/327389a0. [DOI] [PubMed] [Google Scholar]
- 25.Rheinberger H J, Geigenmuller U, Wedde M, Nierhaus K H. Parameters for the preparation of Escherichia coli ribosomes and ribosomal subunits active in tRNA binding. Methods Enzymol. 1988;164:658–670. doi: 10.1016/s0076-6879(88)64076-6. [DOI] [PubMed] [Google Scholar]
- 26.Spahn C M, Prescott C D. Throwing a spanner in the works: antibiotics and the translation apparatus. J Mol Med. 1996;74:423–439. doi: 10.1007/BF00217518. [DOI] [PubMed] [Google Scholar]
- 27.Spangler E A, Blackburn E H. The nucleotide sequence of the 17S ribosomal RNA gene of Tetrahymena thermophila and the identification of point mutations resulting in resistance to the antibiotics paromomycin and hygromycin. J Biol Chem. 1985;260:6334–6340. [PubMed] [Google Scholar]
- 28.Wilms C, Noah J W, Zhong D, Wallenzien P. Exact determination of UV-induced crosslinks in 16S ribosomal RNA in 30S ribosomal subunits. RNA. 1997;3:603–612. [PMC free article] [PubMed] [Google Scholar]