Abstract
Background
The incidence of breast cancer is rising worldwide. Recent advances in systemic and local treatments have significantly improved survival rates of patients having early breast cancer. In the last decade, great attention has been paid to the prevention and early detection of cardiotoxicity induced by breast cancer treatments. Systemic therapy-related cardiac toxicities have been extensively studied. Radiotherapy, an essential component of breast cancer treatment, can also increase the risk of heart diseases. Consequently, it is important to balance the expected benefits of cancer treatment with cardiovascular risk and to identify strategies to prevent cardiotoxicity and improve long-term outcomes and quality of life for these patients.
Objective
This CardioTox Breast study aims to investigate the use of cardiac imaging, based on cardiac magnetic resonance and echocardiography, and to identify associated circulating biomarkers to assess early tissue changes in chemo-induced and radiation-induced cardiotoxicity in the time window of 12 months after the end of radiotherapy in patients with breast cancer.
Methods
The CardioTox Breast trial is a multicenter observational prospective longitudinal study. We aim to enroll 150 women with stage I-III unilateral breast cancer, treated with breast conserving surgery, who planned to receive radiotherapy with or without systemic therapy. Baseline and follow-up data include cardiac measurements based on cardiac magnetic resonance imaging, echocardiography, and circulating biomarkers of cardiac toxicity.
Results
This study details the protocol of the CardioTox Breast trial. Recruitment started in September 2020. The results of this study will not be published until data are mature for the final analysis of the primary study end point.
Conclusions
The CardioTox Breast study is designed to investigate the effects of systemic and radiation therapy on myocardial function and structure, thus providing additional evidence on whether cardiac magnetic resonance is the optimal screening imaging for cardiotoxicity.
Trial Registration
ClinicalTrials.gov NCT04790266; https://clinicaltrials.gov/ct2/show/NCT04790266
International Registered Report Identifier (IRRID)
DERR1-10.2196/31887
Keywords: breast cancer, cardiotoxicity, cardiac diagnostic imaging, radiotherapy, chemotherapy
Introduction
Incidence of breast cancer is rising worldwide. Due to developments in systemic and local therapies, median survival of early breast cancer patients has increased [1], resulting in decreased risk of local recurrence and breast cancer death, as well as longer life expectancy [2-4]. Consequently, the reduction in treatments’ side effects and the improvement of patients’ quality of life are becoming increasingly important in treatment prescription and planning. The most frequent toxicities described in literature are neurological, hematological, gastrointestinal, cutaneous, and cardiological ones. In the last decade, great attention has been paid to prevention and early detection of cardiotoxicity because heart diseases are the main nononcological cause of death in these patients [5].
Our understanding of the pathophysiology and natural history of iatrogenic cardiotoxicity remains limited, and the diagnosis is carried out frequently only when cardiovascular disease presents clinically [6]. Cardiovascular complications from cancer therapies are widely heterogeneous (eg, myocardial dysfunction, heart failure [HF], coronary artery disease, valvular disease, arrhythmias, and arterial hypertension). Myocardial dysfunction and HF are the most frequent complications after breast cancer treatments. It is believed that cardiotoxicity is a continuous phenomenon beginning with myocardial injury and changes in myocardial strain; subsequently, a progressive left ventricular ejection fraction (LVEF) decline may gradually lead to symptomatic HF [7]. If HF treatment is delayed, it is possible that the cardiac function may never restore to what it was at baseline [8]. It is therefore fundamental to distinguish asymptomatic patients who are at risk for cardiotoxicity and, if possible, give them protective treatment for avoiding side effects.
Therefore, cardio-oncology is a rapidly developing subspecialty within cardiology, which aims to optimize the diagnosis and management of cancer treatment cardiac complications [9].
The American Society of Echocardiography and the European Association of Cardiovascular Imaging define cardiotoxicity as a decline of LVEF ≥10% with a final LVEF <53%.
Anthracyclines-based systemic therapy is considered as the prototype of type I cardiotoxic agents [10]. It is believed that agents belonging to this group cause irreversible continuous progressive decline in LVEF, which is dose dependent and can lead to dilated cardiomyopathy [11]. Anthracycline-induced cardiotoxicity may present during or immediately after the infusion (acute), within the first year of treatment (early) and years after treatment (late) [12,13].
Trastuzumab, an anti-human epidermal growth factor receptor 2 (HER2) agent is considered a standard treatment in breast cancer overexpressing HER2. As a type II cardiotoxic agent, it induces cardiotoxicity that is usually reversible with its interruption or with HF treatment. Toxicity from type II agents is not related to cumulative dose and usually develops during treatment [11].
The incidence of anthracycline-related cardiotoxicity is 3%-48% while it varies from 1.7% to 20.1% with trastuzumab.
Due to their potential cardiotoxicity, anthracycline and trastuzumab are usually not administered concurrently [11].
Radiotherapy (RT) can also increase the risk of several heart diseases [14]. The relative risk varies from 3.5 (for left-sided) to 1.2 (for right-sided) breast cancer [15]. Radiation-induced cardiotoxicity usually develops even more than 10 years after RT with an interstitial myocardial fibrosis [8,15]. RT may induce ischemic heart disease through the development of severe atherosclerotic and nonatherosclerotic disease, complicated by plaque rupture and thrombosis, and potentially with coronary spasm [11,16]. Cardiac damage has a strict correlation with the mean heart dose, with a 7.4% increase in relative risk of major cardiac event for each additional 1 Gy of mean heart dose [15]. The mean heart dose has to be as low as possible, almost 0 Gy, albeit at the critical portions of the heart, as left ventricle or left anterior descending artery can receive more than 40 Gy in a very limited volume [17]. Technological developments in RT techniques such as intensity-modulated RT or volumetric modulated arc therapy and deep inspiration breath hold have allowed a reduction in cardiac doses, particularly for patients with left-sided breast cancer, lowering the risk of cardiotoxicity.
A synergistic effect on cardiac risk between left breast RT and cardiotoxic chemotherapy has been described even if its actual incidence is difficult to quantify and evaluate.
Cardiotoxicity can be detected by different diagnostic methods. Circulating biomarkers could detect and predict cardiotoxicity. The 2014 American Society of Echocardiography guidelines the recommend measurement of troponin at baseline, before systemic therapy and 24 hours after, to aid in the detection of subclinical cardiotoxicity.
Troponin and brain natriuretic peptide (BNP) have been investigated in many trials, but unfortunately with different results. Several studies have shown that the elevation of Troponin I may predict the development of future LVEF depression, and N-terminal pro b-type natriuretic peptide can predict the risk for radiation-induced cardiotoxicity [7,8]. Of note, 1 study has showed that a reduction in longitudinal strain and an increase in high-sensitivity troponin, after the end of anthracycline therapy, predicted future left ventricular dysfunction [7].
Several epidemiologic studies have shown a significant association between elevated plasma concentrations of high-sensitivity C-reactive protein (hs-CRP) and the prevalence of underlying atherosclerotic vascular disease, as well as the risk of recurrent cardiovascular events among patients with established disease and apparently healthy individuals [18-20].
In 2012, Onitilo and his colleagues [21] published a pilot study about hs-CRP as a biomarker for trastuzumab-induced cardiotoxicity. They showed that abnormal hs-CRP (≥3 mg/L) predicted decreased LVEF with a sensitivity of 92.9%.
Cardiac biomarkers could also be potential candidates to monitor cardiac damage after RT. Echocardiography (ECHO) is currently the standard method for detecting cardiotoxicity, usually by monitoring serial LVEF. A 3-dimensional modality is preferred to a 2-dimensional one because of better reproducibility [22]. LVEF is not directly correlated to early toxicity and usually decreases months after myocardial cell injury has happened. A recent advanced echocardiographic technique, automated 2-dimensional speckle tracking echocardiography (cardiac strain), has been used for detecting and quantifying subclinical changes in left ventricular strain and function. The use of global longitudinal strain (GLS) by speckle tracking echocardiography is strongly recommended because of its feasibility and biological reproducibility [22]. GLS is changing earlier than LVEF, corresponding to myocardial deformation; consequently, this technique could diagnose cardiotoxicity more quickly [8,23].
Current guidelines suggest ECHO at baseline, after the end of anthracycline therapy, before the start of trastuzumab treatment, and every 3 months during trastuzumab treatment.
Cardiac magnetic resonance (CMR) is the most accurate methodology for the evaluation of volumes and function of heart chambers. Additionally, it is exceptionally capable of providing myocardial tissue characterization, including the presence and extension of myocardial oedema (reflected in increased T2-weighted magnetic resonance imaging), hyperemia (assessed by an increment in early enhancement), and fibrosis (visualized with late enhancement techniques) [24].
Serial CMR imaging showed reduction in LVEF, 12 to 24 months after therapy, in women treated for breast cancer with anthracycline-based chemotherapy. Few recent studies have suggested that LVEF could start to decline earlier; however, the prognostic implications of these changes are not yet known. Recent preliminary experimental data suggest that the decline in contractile function is preceded by CMR evidence of myocardial oedema with T2 (transverse relaxation time) sequences and T2 mapping [25-27]. Furthermore, T1 (longitudinal relaxation time) mapping is a promising technique to quantify morphologic tissue injuries, such as interstitial or diffuse myocardial fibrosis. If treated with radiation therapy, patients diagnosed with breast cancer have an increased risk of acute asymptomatic pericardial effusion, which can also be detected by CMR. CMR is the most sensitive and reproducible measure of LVEF.
When biomarkers and cardiac imaging methods (eg, GLS on ECHO and oedema on CMR) are integrated, we could detect preclinical cardiotoxicity and prevent left ventricular dysfunction.
Unfortunately, thus far, these studies have had a small sample size, and therefore we cannot draw any practice changing conclusion.
In our opinion, it is important to better define high-risk patients who need intensive cardiovascular screening during and after cardiotoxic treatment. Our purpose is to detect toxicity when it is still subclinical and reversible and to prevent its deterioration with protective drugs administered to “the right patient at the right time.”
Methods
Study Design
This CardioTox Breast study (registered with ClinicalTrials; registration number NCT04790266) is a multicenter, observational, prospective, longitudinal study that includes female patients with left-breast and right-breast cancer treated with postoperative RT, with or without chemotherapy or hormonal therapy after primary breast conservative surgery. The patients will be followed for at least 1 year after RT, with cardiac imaging and circulating biomarkers (further detailed below). Three investigating centers are involved in the study: the Oncology Institute of Southern Switzerland (Bellinzona, Switzeralnd), the North Estonia Medical Center (Tallinn, Estonia), and Fondazione IRCCS Policlinico San Matteo (Pavia, Italy). Furthermore, Cardiocentro Ticino is involved in the study as the center of analysis of CMR and echocardiography in Switzerland.
Study Population
We aim to enroll 150 female patients aged ≥18 years with stage I-III unilateral breast cancer treated with breast conserving surgery and planned to receive radiation therapy with or without systemic therapy.
Eligibility Criteria
Inclusion criteria are as follows: (1) written informed consent must be obtained before any assessment is performed; (2) female, aged ≥18 years at visit 1; (3) performance status Eastern Cooperative Oncology Group (ECOG) 0-1; (4) stage I-III histology proven breast cancer (Swiss center allows inclusion of ductal carcinoma in situ); (5) adjuvant RT and neoadjuvant anthracycline and/or trastuzumab-based therapy with or without hormonal therapy; and (6) negative pregnancy test (plasma human chorionic gonadotropin [hCG]) for all female participants of childbearing potential (ie, not permanently sterilized—posthysterectomy or tubal ligation status).
Exclusion criteria are as follows: (1) known metastatic spread of any cancer; (2) known active or recurrent hepatic disorder (cirrhosis, hepatitis), aspartate aminotransferase to alanine aminotransferase ratio of 2 x upper limits of normal; (3) renal function decrease (estimated glomerular filtration rate <30 ml/min); (4) known coronary artery disease; (5) angina pectoris; (6) positive or missing pregnancy test (pre- and perimenopausal women) at enrollment visit; (7) patients with baseline LVEF <53% and GLS <15%; and (8) patients with pacemaker.
Pretreatment Evaluation
The patients will be recruited in participating centers. The investigators ensure that women will meet the inclusion criteria in the study and sign the written informed consent. On inclusion, patient demographics and proper medical history, including current medications and family history of cardiovascular disease will be recorded. A physical examination will be performed, including the measurement of blood pressure and pulse rate.
A baseline assessment will be conducted before the start of adjuvant treatment by checking CMR, ECHO, an electrocardiogram to detect any arrhythmia, and blood sampling to analyze circulating biomarkers.
CMR Protocol
CMR will be performed with Siemens Skyra 3T or a 1.5T scanner. All patients will undergo a standard protocol, including late gadolinium enhancement for estimated glomerular filtration rate >30 ml/min. For native T1 and postcontrast mapping, basal, midventricular, apical short axis, and 4-4-3 and 2 chamber apical images will be acquired by “ECG-triggered Modified Look-Locker Inversion recovery” sequence. Additionally, T2 mapping (T2-prepared True-FISP [fast imaging with steady-state free precession]) will be acquired on the same plane. The CMR images and maps will be analyzed offline. Late gadolinium enhancement will be quantified on short-axis stacks using a semiautomatic approach.
Echocardiography
Transthoracic echocardiography will be performed by using a Philips Epiq or General Electric Vivid E95. Images will be digitally stored for offline analysis on custom software. We will collect the parameters to detect myocardial dysfunction and deformation.
Blood Sampling Procedures and Biochemical Assays
Blood sampling will be carried out to analyze circulating biomarkers of cardiac injury. High-sensitivity cardiac troponin T, high-sensitivity cardiac troponin I, N-terminal pro-BNP, and inflammatory and anti-inflammatory mediators such as hs-CRP will be measured.
Treatment
Chemotherapy
patients will receive adjuvant chemotherapy in accordance with the international guidelines. Patients receiving cardiotoxic chemotherapy will be drawn blood before and, if possible, 24 hours after chemotherapy administration.
Patients who receive anthracycline have an ECHO and electrocardiography after the end of this schedule.
During trastuzumab, blood sample will be taken before every administration (every 3 weeks), and ECHO will be conducted after every 4 cycles (every 3 months).
Radiation Therapy
Patients will undergo a simulation chest computed tomography (CT) in free breathing and breath hold modality. Clinical target volume, the planning target volume, and the organs at risk will be drawn on the breath hold modality imaging if this modality improves the distance between breast and heart or left coronary artery (left anterior descending artery). An appropriate physical dosimetric study will be processed using treatment planning system and will be generated to achieve maximum coverage for the planning target volume region by minimizing the dose to the surrounding healthy tissues.
For each contoured volume, we will analyze the minimum, maximum, and mean dose, and dose volume histogram will be evaluated for obtaining the best treatment.
Follow-up
During follow-up, imaging (CMR and ECHO) and biomarkers will be checked 2 weeks after the end of RT and 1 year later. If a patient has symptomatic heart failure during the treatment, or if LVEF declines greater than 10% with a final LVEF <53% on ECHO, the patient will be referred to the cardiologist for a specific treatment. Adverse events will be classified according to the Common Terminology Criteria for Adverse Events. Figure 1 describes the protocol flow chart of the CardioTox Breast study.
Figure 1.
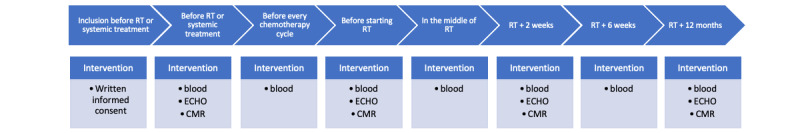
Protocol flow chart description. Blood: blood sample for circulating biomarkers; CMR: cardiac magnetic resonance; ECHO: echocardiography; RT: radiotherapy.
Data Collection
Data will be collected in a dedicated database. An accurate patient history, the type of cancer, and information on the main risk factors for a cardiac event are collected at the inclusion time. Cardiac imaging data (ECHO and CMR) and circulating biomarkers measurements are registered in the database.
Ethical Considerations
This study will be conducted in accordance with the Declaration of Helsinki (amended at the 64th World Medical Association General Assembly, Fortaleza, Brazil, October 2013) and in accordance with the principles of “Good Clinical Practice” and the Medical Research Involving Human Subjects Act. This study was approved by the ethics committee of the Oncology Institute of Southern Switzerland (ID 2019-01395) and by the local ethical committees of the other 2 investigating centers.
Study End Points
Primary End Point
The primary end point is the incidence of cardiotoxicity, as defined by consensus guidelines (decline of LVEF ≥10% points with a final LVEF of <53%), measured on CMR and ECHO over the time window of 12 months from the end of radiation therapy.
Primary Objective
The primary objective is to assess the role of myocardial oedema on CMR (T2 mapping) after radiation and cardiotoxic systemic therapy in predicting the incidence of cardiotoxicity, as defined by consensus guidelines (decline of LVEF ≥10% points with a final LVEF <53%), measured on CMR and ECHO over the time window of 12 months from the end of radiation therapy.
Secondary Objectives
The secondary objectives are the following: (1) detect GLS decrease >15% from baseline, measured on ECHO over the time window of 12 months; (2) see if the changes in biomarkers will correlate with LVEF measurements, assessed by ECHO and CMR; (3) see if the changes in biomarkers will correlate with GLS measurements, assessed by ECHO; (4) compare the time to the biomarkers’ positivity to the time to decrease in GLS >15% or decline of LVEF ≥10% with a final LVEF of <53% measured on ECHO; (5) find out if patients with increased baseline biomarkers will develop cardiotoxicity, and identify predictors of cardiotoxicity by multivariable analysis; (6) detect major cardiovascular events (defined as acute myocardial infarction, hospitalization due to heart failure, atrial flutter or fibrillation, and ventricular tachycardia) or death due cardiac problems during follow-up; (7) assess the role of fibrosis on CMR (T1 mapping with evaluation of extracellular volume) after cardiotoxic radiation therapy or systemic therapy in predicting the incidence of cardiotoxicity; (8) detect the incidence of acute asymptomatic pericarditis after radiation therapy, measured on CMR; (9) investigate if the area of the oedema on CMR correlates with RT dose distribution; and (10) assess the incidence of myocardial oedema on CMR (T2 mapping) after radiation therapy and cardiotoxic systemic therapy, measured on CMR and ECHO over the time window of 12 months from the end of radiation therapy.
Statistical Analysis
Sample Size Calculation
Sample size of this multicenter study is evaluated based on feasibility. We hypothesize to be able to enroll 150 patients satisfying the enrollment criteria and giving consent to participate into the study. We base calculations on the primary end point.
Table 1 summarizes the effect size for the primary end point that can be detected for the expected sample size, given a power of 80% and different rates of prevalence of myocardial oedema after cardiotoxic systemic therapy or radiation therapy.
Table 1.
Effect size computation assuming a rate of cardiac toxicity at 12 months of p1=17% in the cohort without oedema at magnetic resonance. Power 80%, alpha (2-sided) 20%.
| Hypothesized points with MRa oedema at the end of RTb, % | Detectable effect size in the oedema population, % |
| 5 | 52 (25) |
| 10 | 43 (26) |
| 15 | 39 (22) |
| 20 | 37 (20) |
| 25 | 35 (18) |
aMR: magnetic resonance.
bRT: radiotherapy.
We use an alpha level of 10% one-sided test, given the lack of strong evidence in the literature (“early evidence” study). We used Stata 15 (Stata Corp) for computation.
Planned Analysis
Data at enrollment will be described as mean and standard deviation or median and 25th-75th percentiles if continuous and as counts and percent if categorical. They will be compared between groups of patients with and without oedema at the end of RT with the Student t test (or the Mann Whitney U test, based on the distribution) and the Fisher exact test, respectively.
Analysis of the Primary End Point
The number of patients with cardiac toxicity at 12 months will be compared between groups with the Fisher exact test. The mean difference in proportions of cardiac toxicity at 12 months and its 80% confidence interval will be reported. Logistic regression will be used to adjust for potential confounders. We do not expect losses to follow-up. A sensitivity analysis will classify them as no cardiac toxicity patients. If the mortality or losses to follow-up are above 10%, we will also compare groups using survival analysis methods to account for the different follow-ups between patients, using the same strategies.
Analysis of the Secondary Objectives
The data will be compared as described above. The time-to-event end points will be compared using survival analysis methods. The association of changes in biomarkers and changes in cardiac function will be assessed with linear regression models. Data transformation will be applied as needed. The details are provided in Table 2.
Table 2.
Analysis of the secondary end points.
| Secondary end point | Analysis |
| Detect GLSa decrease of >15% from baseline, measured on ECHOb over the time window of 12 months. | The number of patients with a >15% decrease will be compared between groups with the Fisher exact test. The mean difference in proportions at 12 months and its 80% CI will be reported. |
| See if the changes in biomarkers will correlate with LVEFc measurements, assessed by ECHO and CMRd. | The association of changes in biomarkers and LVEF will be assessed with a linear regression model, while adjusting for oedema. |
| See if the changes in biomarkers will correlate with GLS measurements, assessed by ECHO. | The association of changes in biomarkers and GLS will be assessed with a linear regression model, while adjusting for oedema. |
| Compare the time to biomarkers’ positivity to the time to decrease in GLS >15% or decline in LVEF ≥10% in points with a final LVEF of <53% measured on ECHO. | The times will be compared with the Mann Whitney U test. |
| Find out if patients with increased baseline biomarkers will develop cardiotoxicity; identify predictors of cardiotoxicity by multivariable analysis. | A univariable and multivariable logistic model will be used. |
| Detect MACEe (defined as acute myocardial infarction, hospitalization due to heart failure, atrial flutter or fibrillation, and ventricular tachycardia) or death due cardiac problems during follow-up. | The rate of each overall MACE and that of each event will be computed per 100 person-year with 80% CI. Kaplan Meier curves will be plotted. |
| Assess the role of fibrosis on CMR (T1f mapping with evaluation of extracellular volume) after cardiotoxic radiation therapy or systemic therapy in predicting the incidence of cardiotoxicity. | A univariable and multivariable logistic model will be used. |
| Detect incidence of acute asymptomatic pericarditis after RTg, measured on CMR. | The proportion of patients with acute asymptomatic pericarditis and 80% CI will be computed. |
| Investigate if the area of the oedema on CMR correlates with RT dose distribution. | The Spearman correlation coefficient and 80% CI will be computed. |
| To assess the incidence of myocardial oedema on CMR (T2h mapping) after radiation therapy and cardiotoxic systemic therapy measured on CMR and ECHO over the time window of 12 months from the end of radiation therapy. | The proportion of patients with oedema and 80% CI will be computed. |
aGLS: global longitudinal strain.
bECHO: echocardiography.
cLVEF: left ventricular ejection fraction.
dCMR: cardiac magnetic resonance.
eMACE: major cardiovascular events.
fT1: longitudinal relaxation time.
gRT: radiotherapy.
hT2: transverse relaxation time.
Multivariable analysis to identify potential predictors of cardiac toxicity will be performed, while considering a predictor-to-events ratio of 1:10 to avoid overfitting.
Results
Recruitment started in September 2020. The results of this study will not be published until data are mature for the final analysis of the primary study end point.
Discussion
Summary
CardioTox Breast is a multicenter prospective longitudinal study testing the role of CMR in predicting cardiotoxicity in patients with breast cancer. The study is designed to combine both cardiac imaging information regarding potential early myocardial dysfunction and anatomical coronary changes, as well as variations in circulating cardiac damage biomarkers.
We will investigate the effects of systemic therapy and radiation therapy on myocardial function and structure, thus providing additional evidence on whether CMR is the optimal screening tool for cardiotoxicity. CMR is very promising for assessing the function and structure of the cardiovascular system and is starting to be investigated further in prospective studies [28].
Conclusions
Cardiotoxicity can affect the quality of life of breast cancer survivors, whose numbers are increasing. It is important to distinguish high-risk patients who need intensive cardiovascular screening during and after cardiotoxic treatment.
Cardiotox Breast results should improve the prediction and prevention of potential lesions to normal cardiac tissue and ultimately enhance patients’ care and quality of life.
To our knowledge, this study is one of the first longitudinal studies with the primary aim of identifying any change in cardiac imaging (based on CMR and ECHO) and circulating biomarkers to predict the incidence of cardiotoxicity.
Abbreviations
- BNP
brain natriuretic peptide
- CMR
cardiac magnetic resonance
- CT
computed tomography
- ECHO
echocardiography
- ECOG
Eastern Cooperative Oncology Group
- FISP
fast imaging with steady-state free precession
- GLS
global longitudinal strain
- hCG
human chorionic gonadotropin
- HER2
human epidermal growth factor receptor 2
- HF
heart failure
- hs-CRP
high-sensitivity C-reactive protein
- LVEF
left ventricular ejection fraction
- RT
radiotherapy
Footnotes
Conflicts of Interest: None declared.
References
- 1.Waks AG, Winer EP. Breast cancer treatment: a review. JAMA. 2019 Jan 22;321(3):288–300. doi: 10.1001/jama.2018.19323.2721183 [DOI] [PubMed] [Google Scholar]
- 2.Allemani C, Weir HK, Carreira H, Harewood R, Spika D, Wang X, Bannon F, Ahn JV, Johnson CJ, Bonaventure A, Marcos-Gragera R, Stiller C, Azevedo e Silva Gulnar, Chen W, Ogunbiyi OJ, Rachet B, Soeberg MJ, You H, Matsuda T, Bielska-Lasota M, Storm H, Tucker TC, Coleman MP, CONCORD Working Group Global surveillance of cancer survival 1995-2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2) Lancet. 2015 Mar 14;385(9972):977–1010. doi: 10.1016/S0140-6736(14)62038-9.S0140-6736(14)62038-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asselain B, Barlow W, Bartlett J, Bergh J, Bergsten-Nordström E, Bliss J, Boccardo F, Boddington C, Bogaerts J, Bonadonna G, Bradley R, Brain E, Braybrooke J, Broet P, Bryant J, Burrett J, Cameron D, Clarke M, Coates A, Coleman R, Coombes RC, Correa C, Costantino J, Cuzick J, Danforth D, Davidson N, Davies C, Davies L, Di Leo A, Dodwell D, Dowsett M, Duane F, Evans V, Ewertz M, Fisher B, Forbes J, Ford L, Gazet J, Gelber R, Gettins L, Gianni L, Gnant M, Godwin J, Goldhirsch A, Goodwin P, Gray R, Hayes D, Hill C, Ingle J, Jagsi R, Jakesz R, James S, Janni W, Liu H, Liu Z, Lohrisch C, Loibl S, MacKinnon L, Makris A, Mamounas E, Mannu G, Martín M, Mathoulin S, Mauriac L, McGale P, McHugh T, Morris P, Mukai H, Norton L, Ohashi Y, Olivotto I, Paik S, Pan H, Peto R, Piccart M, Pierce L, Poortmans P, Powles T, Pritchard K, Ragaz J, Raina V, Ravdin P, Read S, Regan M, Robertson J, Rutgers E, Scholl S, Slamon D, Sölkner L, Sparano J, Steinberg S, Sutcliffe R, Swain S, Taylor C, Tutt A, Valagussa P, van de Velde C, van der Hage J, Viale G, von Minckwitz G, Wang Y, Wang Z, Wang X, Whelan T, Wilcken N, Winer E, Wolmark N, Wood W, Zambetti M, Zujewski JA. Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: meta-analysis of individual patient data from ten randomised trials. The Lancet Oncology. 2018 Jan;19(1):27–39. doi: 10.1016/S1470-2045(17)30777-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Early Breast Cancer Trialists' Collaborative Group (EBCTCG) Darby S, McGale P, Correa C, Taylor C, Arriagada R, Clarke M, Cutter D, Davies C, Ewertz M, Godwin J, Gray R, Pierce L, Whelan T, Wang Y, Peto R. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011 Nov 12;378(9804):1707–16. doi: 10.1016/S0140-6736(11)61629-2. https://linkinghub.elsevier.com/retrieve/pii/S0140-6736(11)61629-2 .S0140-6736(11)61629-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barish R, Lynce F, Unger K, Barac A. Management of cardiovascular disease in women with breast cancer. Circulation. 2019 Feb 19;139(8):1110–1120. doi: 10.1161/CIRCULATIONAHA.118.039371. [DOI] [PubMed] [Google Scholar]
- 6.Albini A, Pennesi G, Donatelli F, Cammarota R, De Flora Silvio, Noonan D. Cardiotoxicity of anticancer drugs: the need for cardio-oncology and cardio-oncological prevention. J Natl Cancer Inst. 2010 Jan 06;102(1):14–25. doi: 10.1093/jnci/djp440. http://europepmc.org/abstract/MED/20007921 .djp440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cardinale D, Ciceri F, Latini R, Franzosi MG, Sandri MT, Civelli M, Cucchi G, Menatti E, Mangiavacchi M, Cavina R, Barbieri E, Gori S, Colombo A, Curigliano G, Salvatici M, Rizzo A, Ghisoni F, Bianchi A, Falci C, Aquilina M, Rocca A, Monopoli A, Milandri C, Rossetti G, Bregni M, Sicuro M, Malossi A, Nassiacos D, Verusio C, Giordano M, Staszewsky L, Barlera S, Nicolis EB, Magnoli M, Masson S, Cipolla CM, ICOS-ONE Study Investigators Anthracycline-induced cardiotoxicity: A multicenter randomised trial comparing two strategies for guiding prevention with enalapril: The International CardioOncology Society-one trial. Eur J Cancer. 2018 May;94:126–137. doi: 10.1016/j.ejca.2018.02.005.S0959-8049(18)30174-6 [DOI] [PubMed] [Google Scholar]
- 8.Zagar TM, Cardinale DM, Marks LB. Breast cancer therapy-associated cardiovascular disease. Nat Rev Clin Oncol. 2016 Mar;13(3):172–84. doi: 10.1038/nrclinonc.2015.171.nrclinonc.2015.171 [DOI] [PubMed] [Google Scholar]
- 9.Pudil R. The future role of cardio-oncologists. Card Fail Rev. 2017;3(2):140–142. doi: 10.15420/cfr.2017:16:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Curigliano G, Cardinale D, Suter T, Plataniotis G, de Azambuja E, Sandri MT, Criscitiello C, Goldhirsch A, Cipolla C, Roila F, ESMO Guidelines Working Group Cardiovascular toxicity induced by chemotherapy, targeted agents and radiotherapy: ESMO Clinical Practice Guidelines. Ann Oncol. 2012 Oct;23 Suppl 7:vii155–66. doi: 10.1093/annonc/mds293. https://linkinghub.elsevier.com/retrieve/pii/S0923-7534(19)37674-4 .S0923-7534(19)37674-4 [DOI] [PubMed] [Google Scholar]
- 11.Zamorano JL, Lancellotti P, Rodriguez Muñoz Daniel, Aboyans V, Asteggiano R, Galderisi M, Habib G, Lenihan DJ, Lip GYH, Lyon AR, Lopez Fernandez T, Mohty D, Piepoli MF, Tamargo J, Torbicki A, Suter TM, ESC Scientific Document Group 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC) Eur Heart J. 2016 Sep 21;37(36):2768–2801. doi: 10.1093/eurheartj/ehw211.ehw211 [DOI] [PubMed] [Google Scholar]
- 12.Curigliano G, Cardinale D, Dent S, Criscitiello C, Aseyev O, Lenihan D, Cipolla CM. Cardiotoxicity of anticancer treatments: Epidemiology, detection, and management. CA Cancer J Clin. 2016 Jul 26;66(4):309–25. doi: 10.3322/caac.21341. doi: 10.3322/caac.21341. [DOI] [PubMed] [Google Scholar]
- 13.Cardinale D, Colombo A, Bacchiani G, Tedeschi I, Meroni CA, Veglia F, Civelli M, Lamantia G, Colombo N, Curigliano G, Fiorentini C, Cipolla CM. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation. 2015 Jun 02;131(22):1981–8. doi: 10.1161/CIRCULATIONAHA.114.013777.CIRCULATIONAHA.114.013777 [DOI] [PubMed] [Google Scholar]
- 14.Jacob S, Ferrières Jean. Breast cancer radiotherapy: A case of double jeopardy. Arch Cardiovasc Dis. 2016 Nov;109(11):587–590. doi: 10.1016/j.acvd.2016.09.001. https://linkinghub.elsevier.com/retrieve/pii/S1875-2136(16)30151-6 .S1875-2136(16)30151-6 [DOI] [PubMed] [Google Scholar]
- 15.Darby SC, Ewertz M, McGale P, Bennet AM, Blom-Goldman U, Brønnum D, Correa C, Cutter D, Gagliardi G, Gigante B, Jensen M, Nisbet A, Peto R, Rahimi K, Taylor C, Hall P. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013 Mar 14;368(11):987–998. doi: 10.1056/nejmoa1209825. [DOI] [PubMed] [Google Scholar]
- 16.Little MP, Tawn EJ, Tzoulaki I, Wakeford R, Hildebrandt G, Paris F, Tapio S, Elliott P. Review and meta-analysis of epidemiological associations between low/moderate doses of ionizing radiation and circulatory disease risks, and their possible mechanisms. Radiat Environ Biophys. 2010 May 28;49(2):139–53. doi: 10.1007/s00411-009-0250-z. http://europepmc.org/abstract/MED/19862545 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cahlon O, Khan AJ. Cardiac toxicity: the more we learn, the less we know. Int J Radiat Oncol Biol Phys. 2017 Dec 01;99(5):1162–1165. doi: 10.1016/j.ijrobp.2017.08.048.S0360-3016(17)33845-2 [DOI] [PubMed] [Google Scholar]
- 18.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000 Mar 23;342(12):836–843. doi: 10.1056/nejm200003233421202. [DOI] [PubMed] [Google Scholar]
- 19.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008 Nov 20;359(21):2195–2207. doi: 10.1056/nejmoa0807646. [DOI] [PubMed] [Google Scholar]
- 20.Ridker PM, MacFadyen JG, Everett BM, Libby P, Thuren T, Glynn RJ, CANTOS Trial Group Relationship of C-reactive protein reduction to cardiovascular event reduction following treatment with canakinumab: a secondary analysis from the CANTOS randomised controlled trial. Lancet. 2018 Jan 27;391(10118):319–328. doi: 10.1016/S0140-6736(17)32814-3.S0140-6736(17)32814-3 [DOI] [PubMed] [Google Scholar]
- 21.Onitilo AA, Engel JM, Stankowski RV, Liang Hong, Berg RL, Doi SAR. High-sensitivity C-reactive protein (hs-CRP) as a biomarker for trastuzumab-induced cardiotoxicity in HER2-positive early-stage breast cancer: a pilot study. Breast Cancer Res Treat. 2012 Jul;134(1):291–8. doi: 10.1007/s10549-012-2039-z. [DOI] [PubMed] [Google Scholar]
- 22.Galderisi M, Lancellotti P. What Is The Best Imaging Tool In Cardio-oncology? European Society of Cardiology. 2019. Feb 20, [2022-01-10]. https://www.escardio.org/Journals/E-Journal-of-Cardiology-Practice/Volume-16/What-is-the-best-imaging-tool-in-cardio-oncology .
- 23.Charbonnel C, Convers-Domart R, Rigaudeau S, Taksin AL, Baron N, Lambert J, Ghez S, Georges J, Farhat H, Lambert J, Rousselot P, Livarek B. Assessment of global longitudinal strain at low-dose anthracycline-based chemotherapy, for the prediction of subsequent cardiotoxicity. Eur Heart J Cardiovasc Imaging. 2017 Apr 01;18(4):392–401. doi: 10.1093/ehjci/jew223.jew223 [DOI] [PubMed] [Google Scholar]
- 24.Friedrich MG, Sechtem U, Schulz-Menger J, Holmvang G, Alakija P, Cooper LT, White JA, Abdel-Aty H, Gutberlet M, Prasad S, Aletras A, Laissy J, Paterson I, Filipchuk NG, Kumar A, Pauschinger M, Liu P, International consensus group on cardiovascular magnetic resonance in myocarditis Cardiovascular magnetic resonance in myocarditis: A JACC White Paper. J Am Coll Cardiol. 2009 Apr 28;53(17):1475–87. doi: 10.1016/j.jacc.2009.02.007. https://linkinghub.elsevier.com/retrieve/pii/S0735-1097(09)00496-3 .S0735-1097(09)00496-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farhad H, Staziaki PV, Addison D, Coelho-Filho OR, Shah RV, Mitchell RN, Szilveszter B, Abbasi SA, Kwong RY, Scherrer-Crosbie M, Hoffmann U, Jerosch-Herold M, Neilan TG. Characterization of the changes in cardiac structure and function in mice treated with anthracyclines using serial cardiac magnetic resonance imaging. Circ Cardiovasc Imaging. 2016 Dec;9(12):1–20. doi: 10.1161/CIRCIMAGING.115.003584. http://europepmc.org/abstract/MED/27923796 .CIRCIMAGING.115.003584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Galán-Arriola Carlos, Lobo M, Vílchez-Tschischke Jean Paul, López Gonzalo J, de Molina-Iracheta A, Pérez-Martínez Claudia, Agüero Jaume, Fernández-Jiménez Rodrigo, Martín-García Ana, Oliver E, Villena-Gutierrez R, Pizarro G, Sánchez Pedro L, Fuster V, Sánchez-González Javier, Ibanez B. Serial magnetic resonance imaging to identify early stages of anthracycline-induced cardiotoxicity. J Am Coll Cardiol. 2019 Feb 26;73(7):779–791. doi: 10.1016/j.jacc.2018.11.046. https://linkinghub.elsevier.com/retrieve/pii/S0735-1097(18)39536-6 .S0735-1097(18)39536-6 [DOI] [PubMed] [Google Scholar]
- 27.Jordan JH, D’Agostino RB, Hamilton CA, Vasu S, Hall ME, Kitzman DW, Thohan V, Lawrence JA, Ellis LR, Lash TL, Hundley WG. Longitudinal assessment of concurrent changes in left ventricular ejection fraction and left ventricular myocardial tissue characteristics after administration of cardiotoxic chemotherapies using T1-weighted and T2-weighted vardiovascular magnetic resonance. Circ Cardiovasc Imaging. 2014 Nov;7(6):872–879. doi: 10.1161/circimaging.114.002217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walker V, Crijns A, Langendijk J, Spoor D, Vliegenthart R, Combs SE, Mayinger M, Eraso A, Guedea F, Fiuza M, Constantino S, Tamarat R, Laurier D, Ferrières Jean, Mousseaux E, Cardis E, Jacob S. Early detection of cardiovascular changes after radiotherapy for breast cancer: protocol for a European multicenter prospective cohort study (MEDIRAD EARLY HEART Study) JMIR Res Protoc. 2018 Oct 01;7(10):e178. doi: 10.2196/resprot.9906. https://www.researchprotocols.org/2018/10/e178/ v7i10e178 [DOI] [PMC free article] [PubMed] [Google Scholar]


