Key Points
Question
Do uterine cancer mortality trends vary by tumor characteristics according to race and ethnicity?
Findings
In this cohort study of 208 587 women with uterine cancer, linked mortality and cancer registry data showed uterine cancer mortality rate annual increases of 3.4% among Asian women, 3.5% among Black women, 6.7% among Hispanic women, and 1.5% among White women, irrespective of histologic subtype or stage at diagnosis. Mortality rates were found to have increased by 1.8% for uterine cancer overall and 2.7% for nonendometrioid subtypes, whereas mortality rates of less-aggressive endometrioid cancers remained stable.
Meaning
These findings suggest that increasing uterine cancer mortality is associated with increasing rates of aggressive nonendometrioid carcinomas, but racial and ethnic disparities cannot solely be explained by histologic subtype and stage at diagnosis.
Abstract
Importance
Uterine cancer incidence has been increasing, particularly rates of aggressive, nonendometrioid subtypes, which are disproportionately higher among non-Hispanic Black women. The association of subtype-specific trends with uterine cancer mortality and with the role of tumor subtype and stage at diagnosis with racial disparities in uterine cancer deaths at the population-based level are not known.
Objective
To estimate histologic subtype- and stage-specific uterine cancer mortality rates by race and ethnicity, corrected for hysterectomy.
Design, Setting, and Participants
This cohort study used the US Surveillance, Epidemiology, and End Results–18 Incidence-Based Mortality database, representing approximately 26% of the US population and including deaths that occurred from 2000 to 2017. Hysterectomy correction was based on hysterectomy prevalence data from the Behavioral Risk Factor Surveillance System. Uncorrected and corrected rates associated with uterine corpus cancer cases diagnosed between 2000 and 2017 and uterine corpus cancer deaths occurring between 2010 and 2017 were age-adjusted to the 2000 US standard population and are expressed per 100 000 person-years, and annual percent changes in rates were calculated using log-linear regression. Data analysis was performed from March 10 to May 20, 2021.
Exposures
Tumor histologic subtype, cancer stage at diagnosis, and race and ethnicity.
Results
Among 208 587 women diagnosed with uterine cancer during 2000-2017 (15 983 [7.7%] were Asian; 20 302 [9.7%] Black; 23 096 [11.1%] Hispanic; and 149 206 [71.5%] White individuals), there were 16 797 uterine cancer deaths between 2010 and 2017, corresponding to a hysterectomy-corrected mortality rate of 15.7 per 100 000 person-years. Hysterectomy-corrected rates were highest among Black women, overall, by histologic subtype and stage at diagnosis. Among all women, uterine corpus cancer mortality rates increased significantly by 1.8% (95% CI, 1.5%-2.9%) per year from 2010 to 2017, as did rates of nonendometrioid carcinomas (2.7%; 95% CI, 1.8%-3.6%), with increases occurring in Asian (3.4%; 95% CI, 0.3%-6.6%), Black (3.5%; 95% CI, 2.2%-4.9%), Hispanic (6.7%; 95% CI, 1.9%-11.8%), and White women (1.5%; 95% CI, 0.6%-2.4%). In contrast, endometrioid carcinoma mortality rates remained stable.
Conclusions and Relevance
The findings of this cohort study suggest a significant increase of nonendometrioid uterine carcinoma mortality rates, aligning with recent incidence trends. The factors associated with these trends are not well understood and require more investigation of possible mechanisms. Despite stable incidence rates, endometrioid cancer mortality rates have not decreased over the past decade at the population level, suggesting limited progress in treatment for these cancers. The substantial disparities in uterine corpus cancer mortality rates among non-Hispanic Black women cannot be fully explained by subtype distribution and stage at diagnosis.
This cohort study analyzes histologic subtype- and stage-specific uterine cancer mortality rates by race and ethnicity.
Introduction
Uterine cancer is the most common and second deadliest gynecologic cancer in the US, with 65 950 new cases and 12 550 deaths expected in 2022.1 Endometrioid carcinoma is the predominant histologic subtype, accounting for approximately 75% of all cases that are usually diagnosed at an early stage with good prognosis.2 These tumors are associated with obesity as well as hormonal and reproductive factors related to cumulative lifetime estrogen exposure.3,4 Nonendometrioid carcinomas account for approximately 15% to 20% of cases, have been described as estrogen independent,3,4 and are typically diagnosed at later stages with poorer prognosis.2,5 Uterine sarcomas are rare tumors that usually arise in the myometrium and are less well understood.2
Uterine cancer incidence has been increasing2 and is projected to surpass colorectal cancer as the third leading cancer and fourth leading cause of cancer death among women by 2040.6 In a 2019 analysis of hysterectomy-corrected uterine cancer incidence in the US, rates increased by approximately 1% per year (2003-2015), with the sharpest increases observed among Asian, Hispanic, and non-Hispanic Black women. Rates of aggressive nonendometrioid subtypes significantly increased among all women and were twice as high among non-Hispanic Black women compared with other groups.2 Factors explaining these trends, as well as the disproportionately higher rates of nonendometrioid subtypes among non-Hispanic Black women, remain unclear.
Previous analyses based on death certificate data from the National Center for Health Statistics have suggested that uterine cancer mortality has also been increasing, and that rates are twice as high among Black women compared with other groups, often attributed to the higher incidence of nonendometrioid subtypes and more advanced-stage cancers diagnosed among Black women.7 However, an important limitation of these studies is that mortality rates cannot be stratified by tumor characteristics, such as histologic subtype or stage at diagnosis, because death certificates do not record this information.8 These details are necessary for evaluating the extent to which recent increases in the incidence of nonendometrioid carcinomas account for the increase in uterine cancer mortality, as well as understanding the contribution of subtype- and stage-specific differences to racial disparities. To our knowledge, a nationally representative analysis of subtype- and stage-specific uterine cancer mortality rates by race and ethnicity has not been conducted.
To address this gap, we conducted an incidence-based mortality analysis that links mortality records to incident cancer cases in the US Surveillance, Epidemiology, and End Results (SEER) Program. We estimated histologic subtype- and stage-specific mortality rates by race and ethnicity after correcting for hysterectomy prevalence, which has been reported to vary across racial and ethnic groups.2
Methods
Data Sources
SEER Database
We used the SEER 18 Incidence-Based Mortality database, which represents approximately 26% of the US population and includes deaths that occurred from 2000 to 2017 linked to incident cancer cases registered in SEER 18 allowing the evaluation of mortality rates according to characteristics of cancer cases at diagnosis.9 Causes of death and age at death are ascertained from death certificates obtained by the National Center for Health Statistics.10
We included microscopically confirmed malignant cases of corpus uteri and uterine corpus not otherwise specified (excluding cervix uteri) cancers (hereinafter referred to as uterine cancer) diagnosed between 2000 and 2017 among women using the first matching record from the SEER 18 Incidence-Based Mortality database. We excluded cases identified only from autopsy records or death certificates.
Cause of death was selected as corpus uteri and uterine corpus not otherwise specified (excluding cervix uteri) cancer. We excluded women younger than 40 years given the low number of deaths occurring in younger age groups. Because many uterine cancer deaths included in the National Center for Health Statistics database from 2000 to 2017 occurred among cases diagnosed before 2000 (when incidence data and tumor characteristics were not available from all 18 registries), we restricted the analysis to uterine cancer deaths occurring between 2010 and 2017 among cases diagnosed between 2000 and 2017 to allow for adequate follow-up time and avoid underestimating uterine cancer mortality rates (eMethods and eFigure 1 in the Supplement).
We used data from the SEER 18 incidence file (unlinked to mortality records) to estimate the distribution of all cases diagnosed between 2000 and 2017 using the same criteria described above. This study was exempt from institutional review board approval and the need for informed consent because data are deidentified and publicly available. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Race and Ethnicity
Race and ethnicity data are originally abstracted from the medical records and submitted to regional or state cancer registries; these data are grouped by SEER into race and origin categories using standardized algorithms.11 We included women of Hispanic and non-Hispanic origin or ethnicity; the non-Hispanic groups comprised Asian or Pacific Islander (hereafter referred to as Asian), Black, and White women; non-Hispanic American Indian or Alaska Native and unknown race categories were excluded because of small numbers.
Histologic Subtype and Stage at Diagnosis
We used histologic diagnoses defined by the International Classification of Diseases for Oncology, 3rd Edition, histologic codes according to SEER and classified histologic subtypes as endometrioid and nonendometrioid carcinomas, sarcomas, and all other types as other (eTable 1 in the Supplement). We excluded cases classified as other that were not likely to be of uterine corpus cancer origin (n = 10). Deaths caused by adenocarcinoma not otherwise specified (n = 1128) were reclassified according to the observed distribution of endometrioid and nonendometrioid deaths by year, age, race and ethnicity, and stage as previously described (eMethods in the Supplement).2 We classified stage at diagnosis using SEER Summary Stage 2000 as localized, regional, distant, or unstaged/unknown.
Behavioral Risk Factor Surveillance System
We used data from the nationally representative Behavioral Risk Factor Surveillance System to estimate hysterectomy prevalence, as previously described.12 In brief, we calculated survey-weighted estimates of hysterectomy prevalence for women aged 40 to 80 or more years. Because the Behavioral Risk Factor Surveillance System obtains information on hysterectomy only in even-numbered years, we calculated a population-weighted average of the neighboring years to estimate prevalence in odd-numbered years. To ensure stable estimates of hysterectomy prevalence, we used data from women residing in all 50 states. We estimated smoothed survey-weighted hysterectomy prevalence as previously described.2
Statistical Analysis
Data analysis was conducted from March 10 to May 20, 2021. Age-adjusted incidence-based mortality rates, uncorrected and corrected for hysterectomy prevalence, were calculated using SEER*Stat software, version 8.3.9, overall and by histologic subtype and stage at diagnosis, stratified by 5-year age groups (ie, 40-44, 45-49, and continuing to ≥85), year of death, and race and ethnicity. The numerator in the calculation of incidence-based mortality includes uterine cancer–specific deaths among uterine cancer cases diagnosed during 2000-2017 in the SEER 18 registries and the denominator is the general population of women at risk at the time of death in SEER 18 areas. For the hysterectomy-corrected rates, we removed the proportion of women with a hysterectomy from the denominator. Rates were age adjusted to the 2000 US standard population and are expressed per 100 000 person-years.
We used the National Cancer Institute Joinpoint Regression Analysis program, version 4.8.0.1, to calculate annual percentage changes (APCs) and 95% CIs to quantify trends in mortality over time using t tests to determine whether APCs were significantly different from 0. The program also selects the best-fitting log-linear regression model to identify years when APCs significantly changed, providing a minimum number of joinpoints necessary to fit the data. In most cases, a single segment best fit our data. If more than 1 APC was estimated, trends were summarized by the average APC. Trends were plotted using a semilogarithmic scale.13 For plots stratified by race and ethnicity and for sarcomas, we plotted the 2-year averages to stabilize the rates and make comparisons easier to visualize; the corresponding APC estimates were based on annual rates. All statistical tests were 2 sided, and statistical significance was assessed at an α level of P < .05. Unless otherwise stated, we report hysterectomy-corrected rates in the results.
Results
Hysterectomy-Corrected Age-Adjusted Uterine Cancer Incidence-Based Mortality Rates
Among 208 587 women diagnosed with uterine cancer during 2000-2017 (15 983 [7.7%] were Asian; 20 302 [9.7%] Black; 23 096 [11.1%] Hispanic; and 149 206 [71.5%] White individuals), a total of 16 797 uterine cancer deaths occurred between 2010 and 2017, corresponding to a hysterectomy-corrected incidence-based mortality rate of 15.7 per 100 000 person-years (Table 1). Although approximately 9.7% of the cases diagnosed between 2000 and 2017 occurred in Black women, the proportion of uterine cancer deaths among Black women was 17.7%. Both the uncorrected- and hysterectomy-corrected mortality rates among Black women were twice those among White women. Both uncorrected and corrected mortality rates were lowest among Asian women. With respect to histologic subtype, endometrioid carcinomas accounted for approximately 73.8% of incident cases and 40.5% of uterine cancer deaths, with a corresponding corrected incidence-based mortality rate of 6.3 per 100 000 person-years, whereas nonendometrioid carcinomas accounted for approximately 19.8% of incident cases and 45.1% of the total deaths with a rate of 7.3 per 100 000 person-years. Sarcomas accounted for 4.4% of the cases and 9.6% of the deaths (rate, 1.4 per 100 000 person-years) and other uterine cancers accounted for 2.0% of the cases and 4.8% of the deaths (rate, 0.7 per 100 000 person-years). Most uterine cancer deaths occurred among women diagnosed with regional (35.0%) or distant (33.4%) stage disease, with corresponding mortality rates of 5.6 and 5.1 per 100 000 person-years. Most cases (67.8%) were localized stage at diagnosis, and 26% of the deaths occurred among those cases, with a corresponding mortality rate of 4.2 per 100 000 person-years.
Table 1. Uterine Cancer Incidence (2000-2017) and Incidence-Based Mortality (2010-2017) in US Women Aged 40 Years or Older From SEER 18.
| Variable | No. (%) | Mortality rate (95% CI)a | ||
|---|---|---|---|---|
| Cases (2000-2017) | Deaths (2010-2017) | Uncorrected | Corrected | |
| Total | 208 587 (100.0) | 16 797 (100.0) | 9.1 (9.0-9.3) | 15.7 (15.5-16.0) |
| Race and ethnicity | ||||
| Asian | 15 983 (7.7) | 1228 (7.3) | 6.7 (6.3-7.1) | 9.0 (8.4-9.5) |
| Black | 20 302 (9.7) | 2973 (17.7) | 16.0 (15.5-16.6) | 31.4 (30.3-32.6) |
| Hispanic | 23 096 (11.1) | 1842 (11.0) | 7.9 (7.5-8.3) | 12.3 (11.8-12.9) |
| White | 149 206 (71.5) | 10 754 (64.0) | 8.6 (8.5-8.8) | 15.2 (15.0-15.5) |
| Histologic subtype | ||||
| Endometrioid | 153 979 (73.8) | 6811 (40.5) | 3.7 (3.6-3.8) | 6.3 (6.1-6.5) |
| Nonendometrioid | 41 259 (19.8) | 7571 (45.1) | 4.1 (4.0-4.2) | 7.3 (7.1-7.5) |
| Sarcomas | 9219 (4.4) | 1614 (9.6) | 0.9 (0.9-0.9) | 1.4 (1.4-1.4) |
| Other | 4130 (2.0) | 801 (4.8) | 0.4 (0.4-0.5) | 0.7 (0.7-0.8) |
| Stage at diagnosis | ||||
| Localized | 141 439 (67.8) | 4394 (26.2) | 2.4 (2.3-2.5) | 4.2 (4.0-4.4) |
| Regional | 42 288 (20.3) | 5886 (35.0) | 3.2 (3.1-3.3) | 5.6 (5.4-5.7) |
| Distant | 17 857 (8.6) | 5607 (33.4) | 3.1 (3.0-3.1) | 5.1 (5.0-5.2) |
| Unknown | 7003 (3.3) | 910 (5.4) | 0.5 (0.4-0.5) | 0.9 (0.7-0.9) |
Abbreviation: SEER, Surveillance, Epidemiology, and End Results 18 Database.
Incidence-based mortality rates were age adjusted to the 2000 US standard population and are expressed per 100 000 person-years. Includes microscopically confirmed cases of corpus and uterus, not otherwise specified cancer. Rates shown as uncorrected and corrected for hysterectomy prevalence.
Table 2 reports uterine cancer incidence-based mortality rates by race and ethnicity according to histologic subtype and stage at diagnosis. Among all histologic subtypes combined, rates among Black women were notably higher for each stage of disease compared with other groups. Rates for distant stage disease were higher than other stages among Asian and Black women, whereas rates for regional stage disease were highest among Hispanic and White women. Among women with endometrioid carcinomas, mortality rates were highest among Black women overall and for each stage of disease, followed by White, Hispanic, and Asian women. The rates for women with localized disease were similar to or exceeded those among regional or distant-stage disease in most racial and ethnic groups. Among women with nonendometrioid carcinomas, mortality rates among Black women were approximately 3 times those among White women overall and for each stage of disease. Rates among Asian and Hispanic and women were somewhat lower than those among White women. Among all racial and ethnic groups, both regional- and distant-stage disease rates were each higher than the localized disease rates. Although much rarer, uterine sarcoma mortality rates were also notably higher among Black women compared with those of other racial and ethnic groups.
Table 2. Uterine Cancer Incidence-Based Mortality Rates (2010-2017) by Race and Ethnicity According to Histologic Subtype and Stage at Diagnosis in US Women Aged 40 Years or Older From SEER 18.
| Variable | Deaths, No. (%) | Mortality rate (95% CI)a | |
|---|---|---|---|
| Uncorrected | Corrected | ||
| All histologic subtypes combined | |||
| Asian | |||
| Localized | 291 (23.7) | 1.6 (1.4-1.8) | 2.1 (1.9-2.5) |
| Regional | 414 (33.7) | 2.3 (2.0-2.5) | 3.0 (2.7-3.4) |
| Distant | 460 (37.5) | 2.5 (2.3-2.7) | 3.3 (3.0-3.6) |
| Unknown | 63 (5.1) | 0.3 (0.3-0.4) | 0.5 (0.4-0.5) |
| Black | |||
| Localized | 656 (22.1) | 3.7 (3.4-4.0) | 7.3 (6.8-8.0) |
| Regional | 994 (33.4) | 5.4 (5.1-5.8) | 10.7 (10.1-11.5) |
| Distant | 1153 (38.8) | 6.0 (5.7-6.4) | 11.4 (10.8-12.2) |
| Unknown | 170 (5.7) | 1.0 (0.8-1.1) | 2.0 (1.2-2.2) |
| Hispanic | |||
| Localized | 449 (24.4) | 2.0 (1.8-2.2) | 3.1 (2.8-3.5) |
| Regional | 635 (34.5) | 2.8 (2.5-3.0) | 4.3 (3.9-4.7) |
| Distant | 660 (35.8) | 2.7 (2.5-3.0) | 4.2 (3.8-4.6) |
| Unknown | 98 (5.3) | 0.4 (0.4-0.5) | 0.7 (0.6-0.8) |
| White | |||
| Localized | 2998 (27.9) | 2.4 (2.3-2.5) | 4.2 (4.1-4.4) |
| Regional | 3843 (35.7) | 3.1 (3.0-3.2) | 5.4 (5.2-5.6) |
| Distant | 3334 (31.0) | 2.7 (2.6-2.8) | 4.6 (4.4-4.7) |
| Unknown | 579 (5.4) | 0.4 (0.4-0.5) | 0.8 (0.7-0.9) |
| Endometrioidb | |||
| Asian | |||
| All stages | 500 (43.4) | 2.7 (2.5-2.9) | 3.5 (3.2-3.8) |
| Localized | 144 (29.8) | 0.8 (0.7-0.9) | 1.0 (0.9-1.2) |
| Regional | 203 (42.0) | 1.1 (1.0-1.3) | 1.5 (1.3-1.8) |
| Distant | 136 (28.2) | 0.7 (0.6-1.0) | 0.9 (0.8-1.2) |
| Black | |||
| All stages | 784 (27.5) | 4.3 (4.1-4.7) | 8.4 (8.0-9.3) |
| Localized | 268 (37.1) | 1.5 (1.3-1.7) | 3.0 (2.6-3.5) |
| Regional | 249 (34.4) | 1.3 (1.1-1.5) | 2.6 (2.2-3.0) |
| Distant | 206 (28.5) | 1.1 (0.9-1.2) | 2.0 (1.6-2.3) |
| Hispanic | |||
| All stages | 688 (39.4) | 2.9 (2.7-3.2) | 4.6 (4.2-4.9) |
| Localized | 211 (32.9) | 1.0 (0.8-1.1) | 1.5 (1.4-1.7) |
| Regional | 251 (39.2) | 1.1 (1.0-1.2) | 1.6 (1.5-1.8) |
| Distant | 179 (27.9) | 0.7 (0.6-0.8) | 1.1 (0.9-1.2) |
| White | |||
| All stages | 4839 (47.2) | 3.9 (3.8-4.0) | 6.8 (6.6-7.0) |
| Localized | 1729 (37.8) | 1.4 (1.3-1.4) | 2.5 (2.3-2.5) |
| Regional | 1783 (39.0) | 1.4 (1.3-1.4) | 2.5 (2.3-2.5) |
| Distant | 1058 (23.2) | 0.9 (0.8-0.9) | 1.5 (1.3-1.5) |
| Nonendometrioidb | |||
| Asian | |||
| All stages | 528 (43.4) | 2.9 (2.6-3.1) | 4.0 (3.6-4.3) |
| Localized | 94 (18.6) | 0.5 (0.4-0.6) | 0.8 (0.6-0.9) |
| Regional | 179 (35.4) | 1.0 (0.9-1.2) | 1.4 (1.2-1.7) |
| Distant | 232 (46.0) | 1.3 (1.2-1.5) | 1.7 (1.6-2.1) |
| Black | |||
| All stages | 1705 (59.8) | 9.3 (8.8-9.8) | 18.5 (17.6-19.6) |
| Localized | 300 (18.4) | 1.7 (1.5-1.9) | 3.4 (3.0-3.8) |
| Regional | 660 (40.6) | 3.6 (3.4-3.9) | 7.2 (6.8-7.9) |
| Distant | 667 (41.0) | 3.5 (3.2-3.8) | 7.0 (6.3-7.4) |
| Hispanic | |||
| All stages | 810 (46.4) | 3.7 (3.4-4.0) | 5.9 (5.5-6.4) |
| Localized | 153 (19.6) | 0.7 (0.6-0.8) | 1.1 (1.0-1.3) |
| Regional | 323 (41.4) | 1.5 (1.3-1.6) | 2.3 (2.0-2.5) |
| Distant | 304 (39.0) | 1.3 (1.1-1.5) | 2.1 (1.8-2.3) |
| White | |||
| All stages | 4528 (44.2) | 3.6 (3.5-3.7) | 6.5 (6.3-6.7) |
| Localized | 934 (21.4) | 0.7 (0.6-0.7) | 1.4 (1.2-1.4) |
| Regional | 1776 (40.8) | 1.4 (1.4-1.5) | 2.6 (2.6-2.8) |
| Distant | 1645 (37.8) | 1.3 (1.2-1.4) | 2.3 (2.2-2.5) |
| Sarcomas (all stages) | |||
| Asian | 123 (11.0) | 0.7 (0.5-0.8) | 0.8 (0.6-1.0) |
| Black | 362 (12.8) | 1.8 (1.7-2.0) | 3.2 (3.0-3.5) |
| Hispanic | 246 (14.1) | 0.9 (0.8-1.0) | 1.3 (1.1-1.4) |
| White | 883 (8.6) | 0.8 (0.7-0.8) | 1.2 (1.1-1.3) |
Abbreviation: SEER, Surveillance, Epidemiology, and End Results 18 Database.
Incidence-based mortality rates were age adjusted to the 2000 US standard population and expressed per 100 000 person-years, uncorrected and corrected for hysterectomy prevalence.
Percentages for the all-stages category correspond to the overall proportion of deaths by histologic subtype (endometrioid, nonendometrioid, and sarcomas) among each racial and ethnic group; the denominator includes unknown or unstaged disease. Percentages by stage for endometrioid and nonendometrioid subtypes represent the proportion of deaths by stage, excluding unknown or unstaged disease.
Trends in Hysterectomy-Corrected Age-Adjusted Uterine Cancer Incidence-Based Mortality Rates
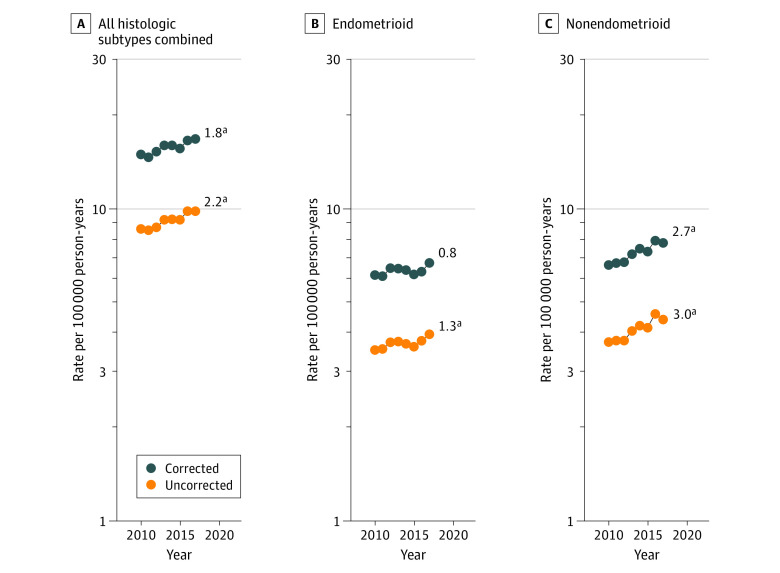
Among all women, hysterectomy-corrected uterine cancer incidence-based mortality rates significantly increased by approximately 1.8% (95% CI, 1.5%-2.9%) per year during 2010-2017 (Figure 1A). With respect to histologic subtype, although uncorrected rates of endometrioid carcinomas were significantly increasing (1.3%; 95% CI, 0.3%-2.3%), hysterectomy-corrected rates were stable from 2010 to 2017 (Figure 1B). In contrast, both uncorrected and corrected mortality rates of nonendometrioid carcinomas significantly increased among all women by approximately 2.7% per year (95% CI, 1.8%-3.6%) for corrected rates (Figure 1C; eTable 2 in the Supplement).
Figure 1. Uterine Cancer Incidence-Based Mortality Trends From Surveillance, Epidemiology, and End Results 18 Database (2010-2017), Uncorrected and Corrected for Hysterectomy and Corrected for Hysterectomy Prevalence.
Trends in age-adjusted incidence-based mortality rates of microscopically confirmed uterine cancer among US women aged 40 years or older. All trends are summarized by a single annual percentage change estimate (numbers reported at the end of the lines).
aSignificantly different from 0 at P < .05.
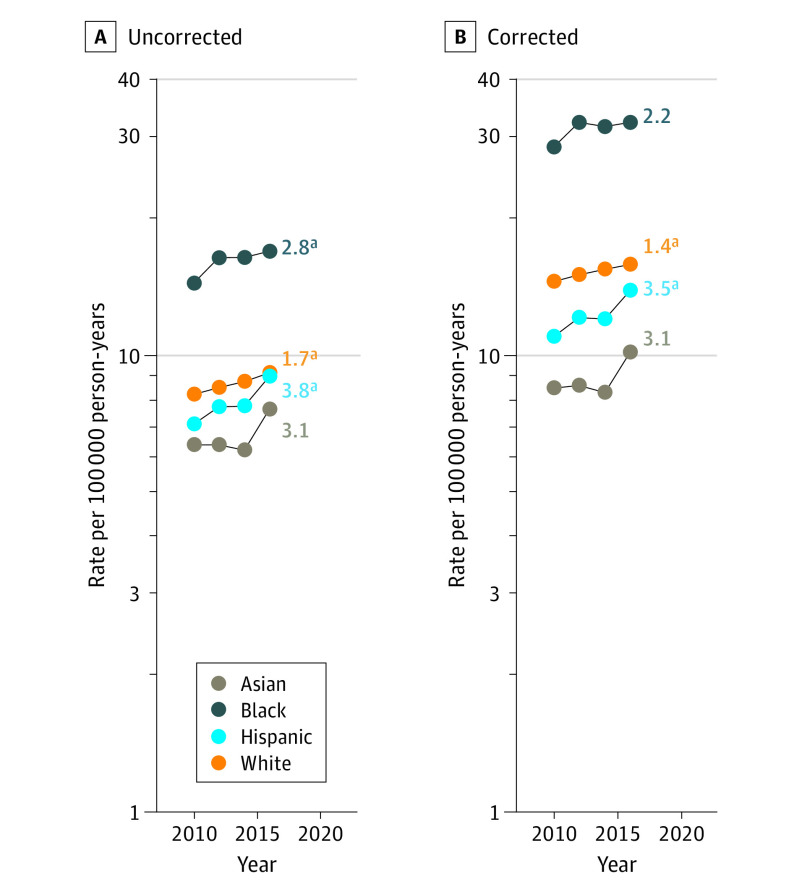
With respect to trends by race and ethnicity, hysterectomy-corrected rates for uterine cancers overall increased significantly among Hispanic (APC, 3.5%; 95% CI, 1.2%-5.8%) and White (APC, 1.4%; 95% CI, 0.3%-2.4%) women, whereas trends were stable among Asian (APC, 3.1%; 95% CI, −3.1% to 9.5%) and Black (APC, 2.2%; 95% CI, −0.3% to 4.8%) women (Figure 2; eTable 2 in the Supplement). The average APC for Black women based on uncorrected rates was significant (APC, 2.8%, 95% CI, 0% to 5.6%), but not after hysterectomy correction. Mortality rates among Black women consistently were more than twice those among the other racial and ethnic groups. Both uncorrected and corrected rates of endometrioid carcinomas were stable among all racial and ethnic groups (Figure 3). In contrast, both uncorrected and corrected rates of nonendometrioid carcinomas were significantly increasing among all racial and ethnic groups, with the highest increase observed among Hispanic women (APC, 6.7%; 95% CI, 1.9% to 11.8%), followed by Black women (average APC, 3.5%; 95% CI, 2.2% to 4.9%), Asian women (APC, 3.4%; 95% CI, 0.3% to 6.6%), and White women (APC, 1.5%; 95% CI, 0.6% to 2.4%) (Figure 3; eTable 2 in the Supplement). For both endometrioid and nonendometrioid subtypes, mortality rates remained higher among Black women during the study period compared with women of other racial and ethnic groups. Among all women, uncorrected and corrected mortality rates of sarcomas were stable (eFigure 2 in the Supplement).
Figure 2. Uterine Cancer Incidence-Based Mortality Trends From Surveillance, Epidemiology, and End Results Database (2010-2017), Uncorrected and Corrected for Hysterectomy Prevalence, by Race and Ethnicity.
Trends in age-adjusted incidence-based mortality rates of microscopically confirmed uterine cancer among US women aged 40 years or older. Two-year averages of uncorrected and corrected rates are plotted. All trends are summarized by a single annual percentage change estimate based on annual rates, except for uncorrected and corrected rates among Asian and Black women, which are summarized by the average annual percentage change (numbers reported at the end of the lines).
aSignificantly different from 0 at P < .05.
Figure 3. Endometrioid and Nonendometrioid Cancer Incidence-Based Mortality Trends, From Surveillance, Epidemiology, and End Results 18 Database (2010-2017), Uncorrected and Corrected for Hysterectomy Prevalence, by Race and Ethnicity.
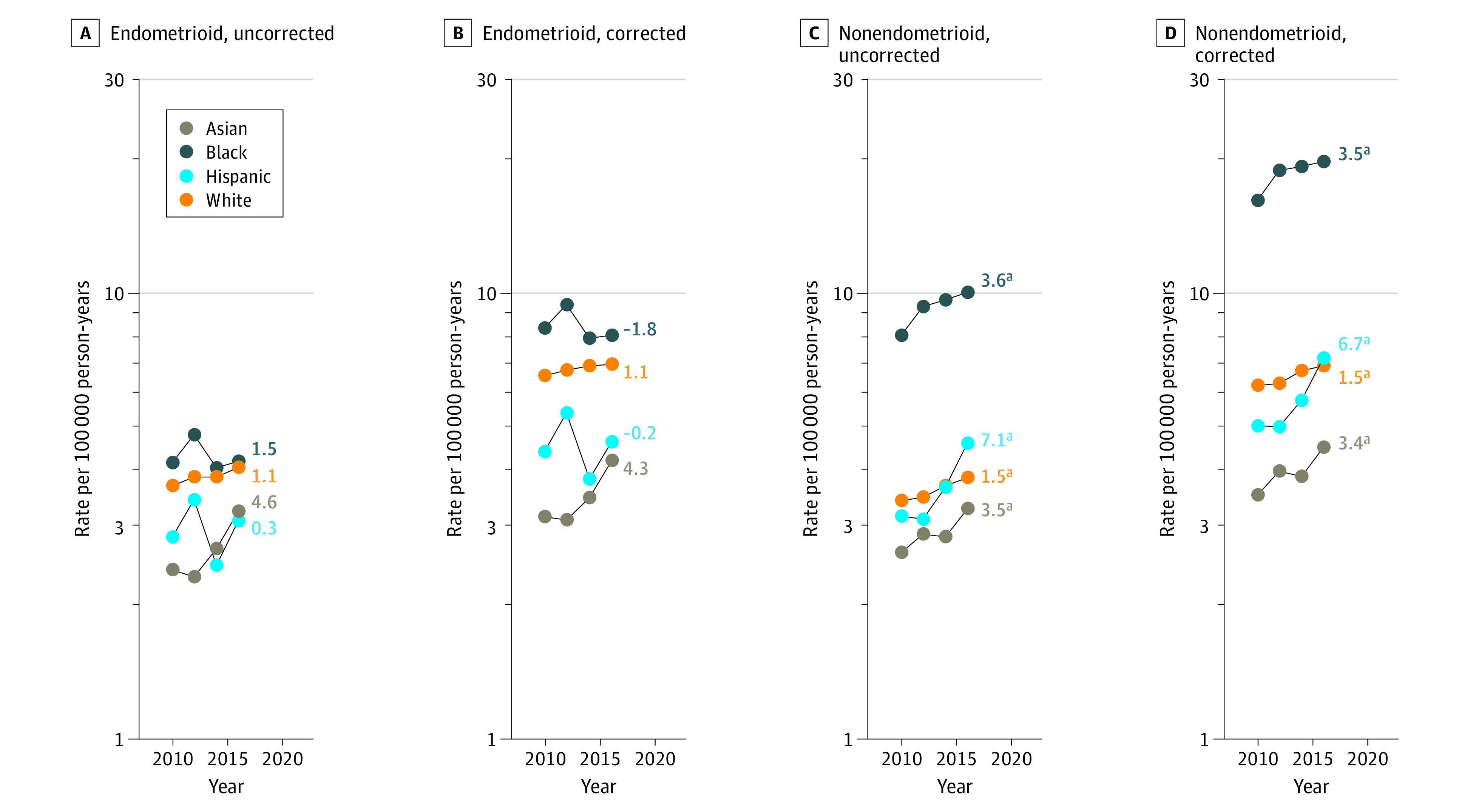
Trends in age-adjusted incidence-based mortality rates of microscopically confirmed endometrioid and nonendometrioid cancers among US women aged 40 years or older. Two-year averages of uncorrected and corrected rates are plotted. All trends are summarized by a single annual percentage change estimate based on annual rates, except for corrected rates of nonendometrioid cancers among Black women, which are summarized by the average annual percentage change (numbers reported at the end of the lines).
aSignificantly different from 0 at P < .05.
Discussion
Our findings suggest that hysterectomy-corrected uterine cancer mortality rates have been increasing significantly by approximately 1.8% per year from 2010 to 2017. These trends are associated with rates of nonendometrioid carcinomas, which have been increasing by about 2.7% per year among all women, with the sharpest increases occurring among Hispanic women (6.7%), followed by Black (3.5%), Asian (3.4%), and White women (1.5%). Increasing nonendometrioid carcinoma mortality rates align with recent incidence trends of these aggressive subtypes, which have been increasing over the past 2 decades.2 In contrast, mortality rates for endometrioid carcinomas have not changed substantially over time among all racial and ethnic groups.
Our analysis revealed notable racial disparities in uterine cancer mortality rates. Despite representing less than 10% of incident cases, nearly 18% of all uterine cancer deaths occurred in Black women. In addition, Black women had rates more than twice those among other racial and ethnic groups for uterine cancer overall and for nonendometrioid subtypes, and higher rates for endometrioid carcinomas, which are relatively less common among Black women.2 Across both subtypes, Black women had higher mortality rates irrespective of stage at diagnosis compared with other racial and ethnic groups, in line with analyses of uterine cancer survival.2,14 Our data emphasize the importance of determining the underlying reasons for the disproportionately higher incidence of aggressive, nonendometrioid subtypes among Black women, as well as the need to better understand nonbiological, modifiable factors underlying disparities in uterine cancer mortality.15 For example, some,16,17 but not all, studies18,19,20 have suggested disparities in receipt of guideline-concordant treatment among Black women with uterine cancer. Some studies have also shown that Black women have a higher risk of recurrence compared with White women,21 even after controlling for adjuvant treatment and socioeconomic status.22 Other factors, including comorbidities, health care facility characteristics, patient and health care professional communication, health care professional bias, discrimination and structural racism, and patient adherence need to be better understood in terms of how they influence racial disparities in uterine cancer mortality.15,17
In contrast to the strong racial disparities that have been reported for uterine cancer among Black women, less is known about Hispanic and Asian populations. Comparisons of uterine cancer survival have suggested lower or similar survival among Hispanic compared with White women,2,23,24,25 and similar or higher survival in Asian women compared with other racial and ethnic groups.2,26 In our analysis, mortality rates among Hispanic and Asian women were lower overall compared with White women, aligning with lower incidence rates. However, our findings suggest that mortality rates have been disproportionately increasing among Asian and Hispanic women, particularly for nonendometrioid subtypes among Hispanic women. More research is needed to understand the reasons underlying these recent trends.
The increasing mortality rates among patients diagnosed with nonendometrioid carcinomas in the US and elsewhere27 warrant clinical attention. Surgery followed by adjuvant treatment with radiotherapy or chemotherapy has been the standard of care for high-risk uterine cancers, including early-stage nonendometrioid subtypes, for decades, and trials evaluating combined adjuvant chemotherapy and radiotherapy treatment for patients at higher risk with early-stage nonendometrioid cancer have not demonstrated an overall survival benefit compared with standard approaches.28,29 More recently, studies evaluating molecular subtyping of endometrial tumors have shown promising results for directing treatment for advanced and high-risk disease according to The Cancer Genome Atlas subtypes, representing an important avenue for future research.30,31,32,33
Notably, endometrioid carcinomas tend to be diagnosed at localized stages with favorable prognosis; they accounted for approximately 41% of the total uterine cancer deaths observed in our study, and more than 25% of deaths occurred among patients diagnosed with localized disease. Stable mortality rates for endometrioid cancers align with incidence trends2 and suggest that clinical management has not improved outcomes on a population level over the past 2 decades. Several studies have attempted to identify women with early-stage endometrioid cancers who are at higher risk of recurrence (eg, those with high-grade tumors and >50% myometrial invasion) and develop adjuvant therapies for these patients to improve overall survival; however, no such strategies have proven effective to date.5,28,29,34
Strengths and Limitations
Our study has several strengths. We used an incidence-based mortality approach that allows estimating uterine cancer mortality rates by tumor characteristics. We corrected for hysterectomy prevalence, which enables unbiased comparisons over time and across different racial and ethnic groups. In addition, we used proportional reallocation of unclassified adenocarcinomas to reduce misclassification of histologic subtype.
The study has limitations. First, data on socioeconomic factors and health care access cannot be linked on an individual level in SEER; thus, additional studies are needed to better understand social determinants of health and other health care professional–, patient-, and system-level factors that might explain the mortality differences observed in our study. Second, although SEER registries use standardized codes and procedures for classifying race and ethnicity data, initial collection of this information is carried out by health care facilities, and misclassification is possible. Third, race and ethnicity are documented only at an aggregate level in both SEER and the Behavioral Risk Factor Surveillance System, precluding more refined identification of racial and ethnic subgroups.35 Fourth, we were not able to evaluate other tumor characteristics, such as grade and extent of both invasion and treatment, because these data are not reliably collected by SEER.
Conclusions
To our knowledge, this cohort study represents the first analysis of hysterectomy-corrected incidence-based uterine cancer mortality rates in a large, representative US population. We noted that hysterectomy-corrected uterine cancer mortality rates have increased significantly by approximately 1.8% per year from 2010 to 2017, and that this increase is associated with nonendometrioid carcinoma mortality rates. Our study suggests racial disparities in uterine cancer mortality that appear to be multifactorial, including a higher incidence of aggressive subtypes and worse outcomes independent of subtype and stage among Black women. Increasing uterine cancer mortality rates and persistent racial disparities underscore the need for new research in diverse populations to identify risk factors and improve early detection approaches for nonendometrioid subtypes, develop new and effective strategies for risk assessment and treatment for women diagnosed with uterine cancer, and identify modifiable patient-, health care professional–, and system-level factors associated with uterine cancer disparities among Black women.
eMethods. Detailed Methods
eFigure 1. Corpus and Uterus, NOS Cancer Observed NCHS Mortality and Incidence-Based Mortality, SEER 18 (2000-2017)
eTable 1. Histologic Subtype Groupings Among Corpus and Uterus, NOS Cancer Deaths in SEER 18 Incidence-Based Mortality File, 2010-2017, (N = 16 797)
eFigure 2. Uterine Sarcoma Incidence-Based Mortality, SEER 18 (2010-2017), Uncorrected and Corrected for Hysterectomy
eTable 2. Uterine Cancer Incidence-Based Mortality Rates by Year (2010 and 2017), Subtype, and Race and Ethnicity, Uncorrected and Corrected for Hysterectomy, SEER 18
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7-33. doi: 10.3322/caac.21708 [DOI] [PubMed] [Google Scholar]
- 2.Clarke MA, Devesa SS, Harvey SV, Wentzensen N. Hysterectomy-corrected uterine corpus cancer incidence trends and differences in relative survival reveal racial disparities and rising rates of nonendometrioid cancers. J Clin Oncol. 2019;37(22):1895-1908. doi: 10.1200/JCO.19.00151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Setiawan VW, Yang HP, Pike MC, et al. ; Australian National Endometrial Cancer Study Group . Type I and II endometrial cancers: have they different risk factors? J Clin Oncol. 2013;31(20):2607-2618. doi: 10.1200/JCO.2012.48.2596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suarez AA, Felix AS, Cohn DE. Bokhman Redux: Endometrial cancer “types” in the 21st century. Gynecol Oncol. 2017;144(2):243-249. doi: 10.1016/j.ygyno.2016.12.010 [DOI] [PubMed] [Google Scholar]
- 5.Lu KH, Broaddus RR. Endometrial cancer. N Engl J Med. 2020;383(21):2053-2064. doi: 10.1056/NEJMra1514010 [DOI] [PubMed] [Google Scholar]
- 6.Rahib L, Wehner MR, Matrisian LM, Nead KT. Estimated projection of US cancer incidence and death to 2040. JAMA Netw Open. 2021;4(4):e214708. doi: 10.1001/jamanetworkopen.2021.4708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henley SJ, Miller JW, Dowling NF, Benard VB, Richardson LC. Uterine cancer incidence and mortality—United States, 1999-2016. MMWR Morb Mortal Wkly Rep. 2018;67(48):1333-1338. doi: 10.15585/mmwr.mm6748a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chu KC, Miller BA, Feuer EJ, Hankey BF. A method for partitioning cancer mortality trends by factors associated with diagnosis: an application to female breast cancer. J Clin Epidemiol. 1994;47(12):1451-1461. doi: 10.1016/0895-4356(94)90089-2 [DOI] [PubMed] [Google Scholar]
- 9.Surveillance, Epidemiology, and End Results (SEER) Program. SEER*Stat database: incidence-based mortality-SEER Research Plus Data, 18 registries, November 2019. (2000-2019). Accessed April 21, 2021. http://www.seer.cancer.gov
- 10.Surveillance, Epidemiology, and End Results (SEER) Program. SEER cause of death recode. Accessed April 21, 2021. https://seer.cancer.gov/codrecode/
- 11.Surveillance, Epidemiology, and End Results (SEER) Program. Race recode changes for the 1973-2005 SEER Research Data (November 2007) Submission and later releases. Accessed March 15, 2019. https://seer.cancer.gov/seerstat/variables/seer/race_ethnicity/
- 12.Centers for Disease Control and Prevention . Behavioral Risk Factor Surveillance System. Survey data & documentation. Accessed January 28, 2022. https://www.cdc.gov/brfss/data_documentation/index.htm
- 13.Devesa SS, Donaldson J, Fears T. Graphical presentation of trends in rates. Am J Epidemiol. 1995;141(4):300-304. doi: 10.1093/aje/141.4.300 [DOI] [PubMed] [Google Scholar]
- 14.Cote ML, Ruterbusch JJ, Olson SH, Lu K, Ali-Fehmi R. The growing burden of endometrial cancer: a major racial disparity affecting Black women. Cancer Epidemiol Biomarkers Prev. 2015;24(9):1407-1415. doi: 10.1158/1055-9965.EPI-15-0316 [DOI] [PubMed] [Google Scholar]
- 15.Doll KM, Snyder CR, Ford CL. Endometrial cancer disparities: a race-conscious critique of the literature. Am J Obstet Gynecol. 2018;218(5):474-482.e2. doi: 10.1016/j.ajog.2017.09.016 [DOI] [PubMed] [Google Scholar]
- 16.Elshaikh MA, Munkarah AR, Robbins JR, et al. The impact of race on outcomes of patients with early stage uterine endometrioid carcinoma. Gynecol Oncol. 2013;128(2):171-174. doi: 10.1016/j.ygyno.2012.10.016 [DOI] [PubMed] [Google Scholar]
- 17.Kaspers M, Llamocca E, Quick A, Dholakia J, Salani R, Felix AS. Black and Hispanic women are less likely than White women to receive guideline-concordant endometrial cancer treatment. Am J Obstet Gynecol. 2020;223(3):398.e1-398.e18. doi: 10.1016/j.ajog.2020.02.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dholakia J, Llamocca E, Quick A, Salani R, Felix AS. Guideline-concordant treatment is associated with improved survival among women with non-endometrioid endometrial cancer. Gynecol Oncol. 2020;157(3):716-722. doi: 10.1016/j.ygyno.2020.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Felix AS, Cohn DE, Brasky TM, et al. Receipt of adjuvant endometrial cancer treatment according to race: an NRG Oncology/Gynecologic Oncology Group 210 study. Am J Obstet Gynecol. 2018;219(5):459.e1-459.e11. doi: 10.1016/j.ajog.2018.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bregar AJ, Alejandro Rauh-Hain J, Spencer R, et al. Disparities in receipt of care for high-grade endometrial cancer: a National Cancer Data Base analysis. Gynecol Oncol. 2017;145(1):114-121. doi: 10.1016/j.ygyno.2017.01.024 [DOI] [PubMed] [Google Scholar]
- 21.Collins Y, Holcomb K, Chapman-Davis E, Khabele D, Farley JH. Gynecologic cancer disparities: a report from the Health Disparities Taskforce of the Society of Gynecologic Oncology. Gynecol Oncol. 2014;133(2):353-361. doi: 10.1016/j.ygyno.2013.12.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Felix AS, Brasky TM, Cohn DE, et al. Endometrial carcinoma recurrence according to race and ethnicity: an NRG Oncology/Gynecologic Oncology Group 210 Study. Int J Cancer. 2018;142(6):1102-1115. doi: 10.1002/ijc.31127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cook LS, Nelson HE, Cockburn M, Olson SH, Muller CY, Wiggins CL. Comorbidities and endometrial cancer survival in Hispanics and non-Hispanic Whites. Cancer Causes Control. 2013;24(1):61-69. doi: 10.1007/s10552-012-0090-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mahdi H, Hou H, Kowk LL, Moslemi-Kebria M, Michener C. Type II endometrial cancer in Hispanic women: tumor characteristics, treatment and survival compared to non-Hispanic White women. Gynecol Oncol. 2014;133(3):512-517. doi: 10.1016/j.ygyno.2014.03.562 [DOI] [PubMed] [Google Scholar]
- 25.Malagon-Blackwell EM, Seagle BL, Nieves-Neira W, Shahabi S. The Hispanic paradox in endometrial cancer: a National Cancer Database study. Gynecol Oncol. 2017;146(2):351-358. doi: 10.1016/j.ygyno.2017.05.026 [DOI] [PubMed] [Google Scholar]
- 26.Zhang MM, Cheung MK, Osann K, et al. Improved survival of Asians with corpus cancer compared with whites: an analysis of underlying factors. Obstet Gynecol. 2006;107(2, pt 1):329-335. doi: 10.1097/01.AOG.0000195062.75199.7d [DOI] [PubMed] [Google Scholar]
- 27.Gustafson LW, Booth BB, Kahlert J, et al. Trends in hysterectomy-corrected uterine cancer mortality rates during 2002 to 2015: mortality of nonendometrioid cancer on the rise? Int J Cancer. 2021;148(3):584-592. doi: 10.1002/ijc.33219 [DOI] [PubMed] [Google Scholar]
- 28.Randall ME, Filiaci V, McMeekin DS, et al. Phase III trial: adjuvant pelvic radiation therapy versus vaginal brachytherapy plus paclitaxel/carboplatin in high-intermediate and high-risk early stage endometrial cancer. J Clin Oncol. 2019;37(21):1810-1818. doi: 10.1200/JCO.18.01575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Boer SM, Powell ME, Mileshkin L, et al. ; PORTEC study group . Adjuvant chemoradiotherapy versus radiotherapy alone for women with high-risk endometrial cancer (PORTEC-3): final results of an international, open-label, multicentre, randomised, phase 3 trial. Lancet Oncol. 2018;19(3):295-309. doi: 10.1016/S1470-2045(18)30079-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loukovaara M, Pasanen A, Bützow R. Mismatch repair protein and MLH1 methylation status as predictors of response to adjuvant therapy in endometrial cancer. Cancer Med. 2021;10(3):1034-1042. doi: 10.1002/cam4.3691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.León-Castillo A, de Boer SM, Powell ME, et al. ; TransPORTEC Consortium . Molecular classification of the PORTEC-3 trial for high-risk endometrial cancer: impact on prognosis and benefit from adjuvant therapy. J Clin Oncol. 2020;38(29):3388-3397. doi: 10.1200/JCO.20.00549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van den Heerik ASVM, Horeweg N, de Boer SM, Bosse T, Creutzberg CL. Adjuvant therapy for endometrial cancer in the era of molecular classification: radiotherapy, chemoradiation and novel targets for therapy. Int J Gynecol Cancer. 2021;31(4):594-604. doi: 10.1136/ijgc-2020-001822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leon-Castillo A, Horeweg N, Peters EEM, et al. Prognostic relevance of the molecular classification in high-grade endometrial cancer for patients staged by lymphadenectomy and without adjuvant treatment. Gynecol Oncol. Published online January 22, 2022. doi: 10.1016/j.ygyno.2022.01.007 [DOI] [PubMed] [Google Scholar]
- 34.Findley R, Kooy J, Lester B, et al. Adjuvant chemotherapy and radiation for patients with high-risk stage I endometrial cancer treated with curative intent surgery: impact on recurrence and survival. Int J Gynecol Cancer. Published online January 25, 2022. doi: 10.1136/ijgc-2021-003087 [DOI] [PubMed] [Google Scholar]
- 35.Clarke MA, Devesa SS, Wentzensen N. Reply to M. Schlumbrecht et al. J Clin Oncol. 2019;37(35):3466-3467. doi: 10.1200/JCO.19.02109 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Detailed Methods
eFigure 1. Corpus and Uterus, NOS Cancer Observed NCHS Mortality and Incidence-Based Mortality, SEER 18 (2000-2017)
eTable 1. Histologic Subtype Groupings Among Corpus and Uterus, NOS Cancer Deaths in SEER 18 Incidence-Based Mortality File, 2010-2017, (N = 16 797)
eFigure 2. Uterine Sarcoma Incidence-Based Mortality, SEER 18 (2010-2017), Uncorrected and Corrected for Hysterectomy
eTable 2. Uterine Cancer Incidence-Based Mortality Rates by Year (2010 and 2017), Subtype, and Race and Ethnicity, Uncorrected and Corrected for Hysterectomy, SEER 18