Abstract
Swallowing impairment (dysphagia) is a common sequela in patients with motor neuron disease (MND). The purpose of this retrospective, observational pilot investigation was to characterize how pharyngeal swallowing mechanics are impacted in patients with MND using a comparison with healthy, non-dysphagic control group. Computational analysis of swallowing mechanics (CASM) was used to determine covariate biomechanics of pharyngeal swallowing from videofluoroscopic assessment in 15 patients with MND and 15 age- and sex-matched healthy controls. Canonical variant analysis with post hoc discriminate function analysis (DFA) was performed on coordinate data mapping functional muscle groups underlying pharyngeal swallowing. Differences in swallowing mechanics associated with group (MND; control), motor neuron predominance (upper; lower), onset (bulbar; spinal), and swallow task (thin, pudding) were evaluated and visualized. Pharyngeal swallowing mechanics differed significantly in patients with MND compared with healthy controls (D = 2.01, p < 0.0001). Post hoc DFA pairwise comparisons suggest differences in pharyngeal swallow mechanics by motor neuron predominance (D = 5.03, p < 0.0001), onset (D = 2.03, p < 0.0001), and swallow task (D = 1.04, p < 0.0001). Pharyngeal swallowing mechanics of patients with MND differ from and are more heterogeneous than healthy controls. These findings suggest patients with MND may compensate reductions in pharyngeal shortening and tongue base retraction by extending the head and neck and increasing hyolaryngeal excursion. This work and further CASM investigations will lead to further insights into development and evaluation of targeted clinical treatments designed to prolong safe and efficient swallowing function in patients with MND.
Keywords: Motor neuron disease, Deglutition, Deglutition disorders, Morphometric, Modified barium swallow study, Healthy
Introduction
Swallowing impairment (dysphagia) is a well-known sequela in patients with motor neuron disease (MND), including amyotrophic lateral sclerosis, and it has profound effects on quality-of-life [1, 2]. Difficulty swallowing may present as the initial symptom in MND (e.g., bulbar-onset MND) or may appear later as the disease progresses. Ultimately, most patients with MND will demonstrate swallowing impairment as bulbar function declines [3-6], which may result in life-threatening complications. The modified barium swallow study (MBSS) is a common videofluoroscopic diagnostic procedure using real-time X-ray imaging to assess oropharyngeal swallowing function in patients with MND.
Previous literature reports various oral and pharyngeal swallowing impairment in patients with MND (Table 1). These latter pharyngeal functional changes in swallowing mechanics have been evaluated using timing, kinematic and pressure measurements examining movements of the hyoid, larynx, tongue base, pharyngeal shortening, and pharyngoesophageal segment opening. Since the pharyngeal swallow is highly complex and interdependent upon multiple structures and innervations, conventional univariate displacement measures from MBSS imaging are limited to what structure is being investigated.
Table 1.
MND-related oropharyngeal swallowing impairments reported in the literature
Computational analysis of swallowing mechanics (CASM) involves mapping and collecting coordinates of upper aerodigestive tract anatomical landmarks to track and analyze the covariant biomechanics of swallowing observed during MBSS. Anatomical landmarks can be reliably obtained from videofluoroscopic imaging data to map muscles underlying hyoid movement, laryngeal elevation, tongue base retraction, pharyngeal shortening, and head and neck posture [12, 13]. Configurations of coordinates portray the complex interaction of multiple muscle groupings as anatomical landmarks are displaced during swallowing [14]. Shape analysis of landmarks can be compared mathematically to determine shape differences with variables of interest [15].
Once these overall differences are determined, eigenvectors are then utilized to visualize the impact of variables of interest on covariant swallowing mechanics. Traditional distance measurements are thus replaced by eigenvectors that provide information characterizing both direction and magnitude of shape variations. Multivariate morphometric analysis of coordinates provides statistical evaluation and visualization of the shape change representing the mechanics of swallowing within a patient or patient group [16]. The impact of the clinical variable of interest, such as disease state, swallowing task, sex, etc., on the action of multiple muscle groupings is then inferred and allows for quantification and visualization of swallowing mechanics.
The purpose of this retrospective, observational pilot investigation was to characterize how pharyngeal swallowing mechanics are affected in patients with MND using CASM, compared to an age- and sex-matched healthy, non-dysphagic group.
Patients and Methods
Participants
This study received institutional approval for access of previously collected data. All data were de-identified prior to analysis. Inclusion criteria included patients with a diagnosis of MND who underwent a MBSS between January 1, 2013 and March 31, 2016 as part of their medical management. Confirmation of MND was performed by author AC, neuromuscular specialist and director of a multidisciplinary MND clinic. Swallow studies were extracted from the digital swallowing station by author KG. For patients with repeat MBSSs, only the initial MBSS was extracted. This resulted in 23 patients meeting inclusion criteria. Eight patients were removed from analysis secondary to one of the following reasons: missing at least one swallowing task; anatomical landmarks were out of view to map coordinates; or lack of an exact age-matched control from the normative database. The final sample included 15 patients with MND for analysis. Each patient with MND was age- and sex-matched with a healthy, non-dysphagic adult control derived from a large normative MBSS adult database for comparison. Two swallow tasks from each subject (5-ml thin liquid and 5-ml pudding) were included in the sample, yielding a total of 60 swallowing tests for analysis.
Patient demographic and clinical information were extracted from medical chart reviews by two authors (KG and AC). Demographic and clinical information was extracted from the most recent MND clinic visit temporally associated to the MBSS. Most of the patients had MBSS on the day of MND clinic visit, with the exception of three patients (#s 12, 17, and 29). Their clinical information was extracted from a clinic visit within 2 weeks of the MBSS.
Videofluoroscopic Examination of Swallowing
MBSSs were performed under continuous fluoroscopy and recorded at 30 frames per second using a standardized protocol by an experienced speech-language pathologist in collaboration with a radiologist per institutional policy [17]. Video clips of 5-ml thin liquid and 5-ml pudding swallowing tasks were extracted by RS for the purposes of this study. Each MBSS video was converted from .avi to .mov into 60 brief video clips [15 subjects × 2 groups (MND vs. control) × 2 swallowing tasks (thin vs. pudding)] capturing oral bolus transport and completion of pharyngeal swallow.
Coordinate Mapping of Anatomical Landmarks
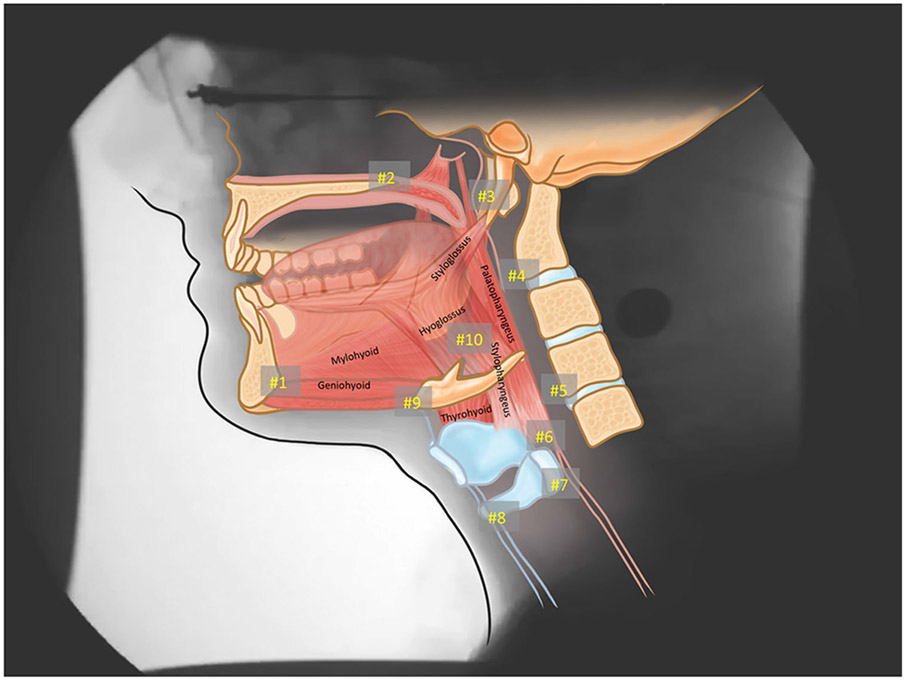
A medical student (RS) trained to annotate coordinates of anatomical landmarks using a semi-automated MATLAB tracker tool achieved mastery on a training set of MBSS videos (inter-rater r > 0.95 for all coordinates). The rater used this tool to annotate ten anatomical landmarks (Fig. 1) in each frame to capture the swallowing event for all patients and healthy controls [18]. The pharyngeal swallow was defined at the start of brisk anterior hyoid movement and ending at the first closure of the pharyngoesophageal segment. The semi-automated MATLAB tracking tool produced an .mp4 video depicting the annotation for review and a corresponding .txt file with coordinates of each point and respective frame. All annotated files were reviewed by a head and neck anatomist (WP) experienced in CASM for quality assurance. MorphoJ software was used to evaluate the sample for outliers as an indication of measurement error and none were observed.
Fig. 1.
Ten coordinates mapping anatomical landmarks to characterize the actions of muscles underlying pharyngeal swallowing mechanics. Coordinates #s 1–3 represent the proximal muscle attachments (#1—mandible; #2—hard palate; #3—styloid process). Coordinates #s 4, 5 map C2 and C4, respectively, to evaluate head and neck posture. Coordinates #6–10 map distal muscle attachments and actions including #6—palatopharyngeus (pharyngeal shortening); #7—stylopharyngeus (posterior elevation of larynx); #s 8, 9—mylohyoid, geniohyoid, and thyrohyoid (anterosuperior movement of hyolaryngeal complex); and #10—styloglossus and hyoglossus (tongue base retraction)
Computational Analysis of Swallowing Mechanics
The coordinate .txt files were concatenated and each set of ten coordinates per frame (n = 1595 frames) assigned a unique identifier. Each unique identifier was assigned associated classifier variables, including: group (MND; control); predominant motor neuron presentation (upper motor neuron, UMN; lower motor neuron, LMN; both); onset (bulbar; spinal; unknown) and swallow task (thin; pudding) (Table 3). Predominant motor neuron presentation was extracted from physician record during medical chart review by author AC.
Table 3.
Patient demographic and clinical information
| ID | MND | Age | Sex | MBSS date (month/year) |
Symptom onset (month/year) |
Onset and initial symptom |
Diagnosis date (month/year) |
UMN or LMN predominance |
FVC | ALSFS-R score (bulbar subscore) |
FOIS level [23] |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 01 | ALS | 65 | M | 03/16 | 01/14 | Spinal—LUE | 05/15 | LMN | 48% | 15 (9) | 7 |
| 02 | PLS | 53 | F | 04/15 | 03/11 | Bulbar—Speech | Unknown | UMN | Unknown | Unknown | 3 |
| 03 | ALS | 71 | F | 06/15 | 07/13 | Spinal—LLE | 05/15 | Unknown | 69% | 37 (9) | 7 |
| 04 | PLS | 67 | F | 02/15 | Unknown | Unknown | Unknown | UMN | Unknown | Unknown | 7 |
| 11 | PLS | 48 | M | 04/15 | Unknown | Unknown | Unknown | UMN | Unknown | N/A | 7 |
| 12 | PBP | 66 | F | 06/15 | 01/13 | Bulbar—Speech | 11/13 | UMN | 57% | 41 (7) | 7 |
| 13 | PLS | 65 | F | 09/15 | Unknown | Unknown | Unknown | UMN | Unknown | N/A | 5 |
| 16 | ALS | 68 | F | 11/13 | 01/13 | Spinal—LLE | 06/13 | Unknown | Unknown | 40 (8) | 5 |
| 17 | ALS | 76 | F | 05/15 | 03/15 | Bulbar—Voice | 02/16 | Unknown | Unknown | Unknown | 7 |
| 21 | ALS | 52 | M | 10/14 | 03/08 | Spinal—UE | 01/10 | Unknown | 95%a | 36 (unknown) | 7 |
| 22 | ALS | 71 | M | 06/15 | 11/13 | Spinal—UE | 11/14 | LMN | 82% | Unknown | 7 |
| 23 | ALS | 66 | M | 09/14 | 01/08 | Spinal—LLE | 11/11 | Unknown | 76% | 36 (unknown) | 7 |
| 26 | ALS | 68 | F | 06/15 | 04/14 | Spinal—LUE | 09/14 | Unknown | 43% | 28 (unknown) | 1 |
| 27 | ALS | 62 | M | 09/15 | 04/15 | Bulbar—Speech | 09/15 | Unknown | 29% | Unknown | 6 |
| 29 | ALS | 51 | F | 07/15 | –/09 | Spinal—LLE | –/11 | Unknown | 29% | 11 (4) | 3 |
MND motor neuron disease, ALS amyotrophic lateral sclerosis, PLS primary lateral sclerosis, PBP progressive pseudobulbar palsy, MBSS modified barium swallow study, LUE left upper extremity, RUE right upper extremity, LLE left lower extremity, RLE right lower extremity, UMN upper motor neuron, LMN lower motor neuron, FVC forced vital capacity, ALSFRS-R amyotrophic lateral sclerosis functional rating scale-revised, FOIS functional oral intake scale, FOIS 7 total oral intake with no restrictions, FOIS 6 total oral intake with no special preparation, but must avoid specific food items, FOIS 5 total oral intake of multiple consistencies requiring special preparation, FOIS 3 tube supplements with consistent oral intake
Measurement taken 6 months prior to MBSS
Data Analysis
MorphoJ software was used to analyze and visualize changes in coordinate position indicating swallowing mechanics [19]. Following a Procrustes fit, canonical variant analysis (CVA) with post hoc discriminate function analysis (DFA) was performed to determine multivariate mechanics of swallowing associated classifier variables related to group (MND; control), predominant motor neuron presentation (UMN; LMN; both), onset (bulbar; spinal; unknown), and swallow task (thin; pudding). To account for multiple comparisons of ten coordinates, a Bonferroni correction was applied and statistical significance was set at a p value of ≤0.005 or less for all analysis. In a CVA of k coordinates and G independent variables, the total sample size must be greater than ([2k – 4] + [G – 1]) [20]. Thus, our present study that includes 60 swallows exceeds the requisite 20 samples needed.
Results
The mean age (± SD) of this cohort was 63 (± 8.3) years. Only one patient (#29) had a PEG tube at the time of the MBSS with use for supplementation to her oral diet. Three patients (#s 03, 27, and 29) were using nocturnal non-invasive ventilation. Median time between MND diagnosis and MBSS was 23 months (range 2–79 months; n = 11) (Table 2).
Table 2.
Each patient grouping with corresponding variables and number of observations included in each group
| Groups number | Classifier variables | Number of observations |
|---|---|---|
| 1 | MND, both, bulbar, pudding | 60 |
| 2 | MND, both, spinal, pudding | 122 |
| 3 | MND, both, unknown, pudding | 19 |
| 4 | MND, spinal, LMN, pudding | 50 |
| 5 | MND, UMN, bulbar, pudding | 43 |
| 6 | MND, UMN, unknown, pudding | 89 |
| 7 | MND, both, bulbar, thin | 45 |
| 8 | MND, both, spinal, thin | 106 |
| 9 | MND, both, unknown, thin | 22 |
| 10 | MND, LMN, spinal, thin | 44 |
| 11 | MND, UMN, bulbar, thin | 68 |
| 12 | MND, UMN, unknown, thin | 68 |
| 13 | Control, pudding | 450 |
| 14 | Control, thin | 409 |
| Total | 1595 |
MND motor neuron disease, LMN lower motor neuron, UMN upper motor neuron
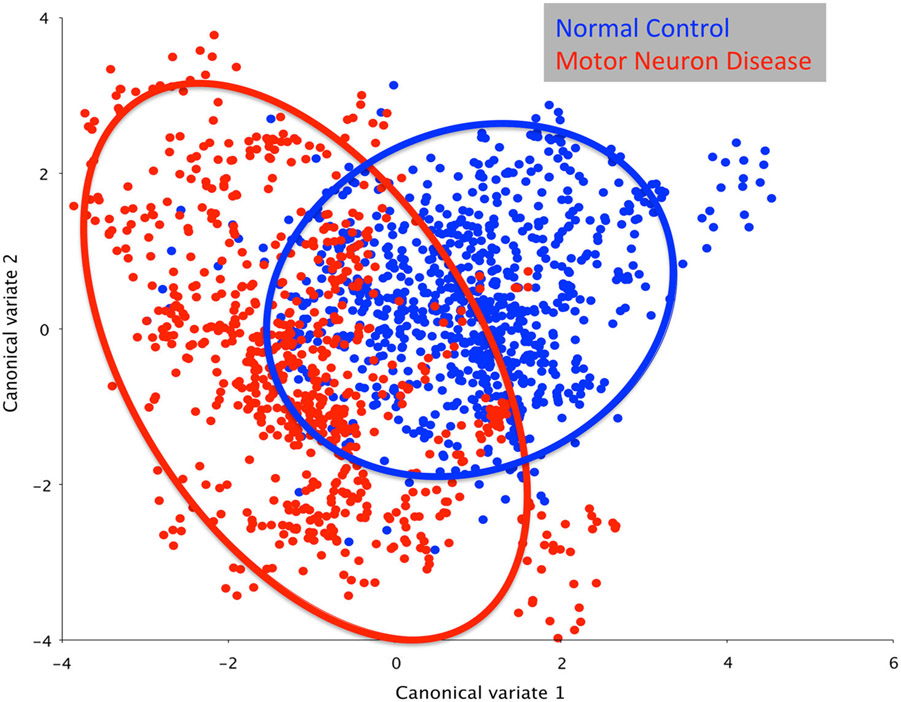
CVA of pharyngeal swallowing mechanics naming group (MND; control), motor neuron predominance (UMN; LMN; both), onset (bulbar; spinal; unknown), and swallow task (thin; pudding) yielded seven canonical variates that explained 95% of the variance. The first canonical variate (CV1) was associated with group (MND; control), defining 33% of the variance (Fig. 2). Onset and motor neuron predominance clustered in CV2 and CV3, defining 23 and 16% of the variance, respectively. Swallow task was associated with CV6 defining 5% of the variance, leaving CV4 and CV5, or 23% of the variance, undetermined (Table 3).
Fig. 2.
Canonical variate analysis showing pharyngeal mechanics differs by group (MND vs. control; D = 2.01, p < 0.0001)
A post hoc DFA showed significant differences between groups (D = 2.01, p < 0.0001). This significant D-value, or Mahalanobis distance, indicated an overall difference in pharyngeal mechanics in patients with MND compared with healthy, non-dysphagic controls. Relative differences in the various elements of swallowing mechanics, visualized by DFA eigenvectors, indicated an increase in hyolaryngeal elevation and head neck extension in the MND group. Further, patients with MND demonstrated slightly reduced tongue base retraction and pharyngeal shortening compared with healthy controls (Fig. 3).
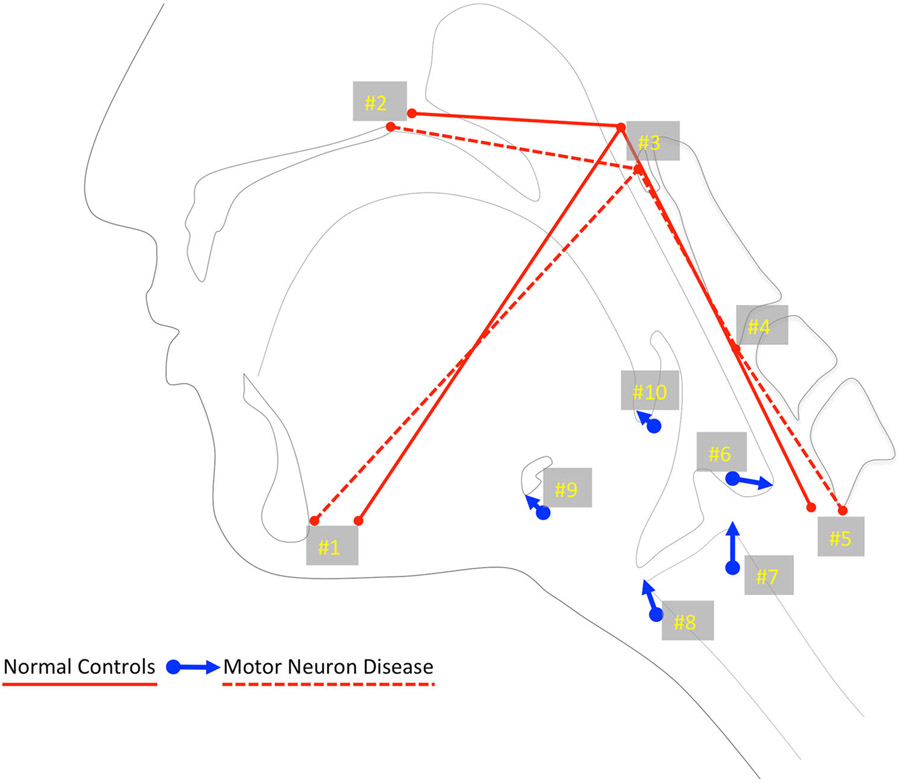
Fig. 3.
Eigenvectors of MDN compared with healthy controls. These vectors indicate that increased hyolaryngeal excursion (#s 7–9) with an extended head and neck posture (red lines connecting #s 1–5) may be compensating for slight reductions in tongue base retraction (#10) and pharyngeal shortening (#6)
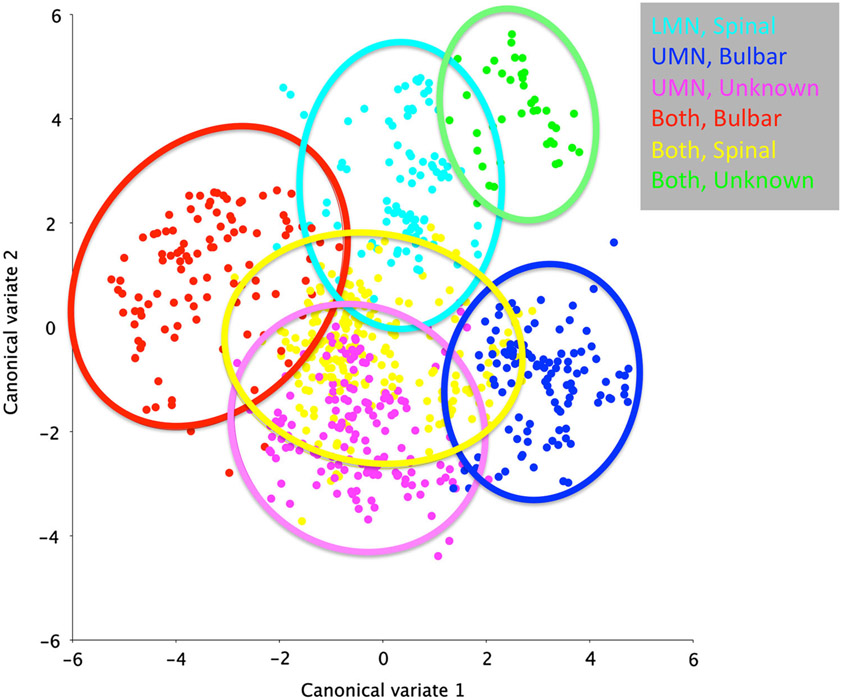
In the ALS subset of MND patients, a CVA of swallowing mechanics showed a clustering of results by motor neuron predominance and onset type (Fig. 4). Post hoc DFA pairwise comparisons suggest differences in pharyngeal swallow mechanics by motor neuron predominance (D = 5.03, p < 0.0001), onset (D = 2.03, p < 0.0001), and swallow task (D = 1.04, p < 0.0001).
Fig. 4.
Canonical variate analysis of MND sample with onset type and motor neuron predominance as named variables of interest show a clustering of swallowing mechanics by disease characteristics
Discussion
MNDs are progressive, degenerative neuromuscular diseases involving motor neurons in the cortex, brainstem, and spinal cord. One of the consequences of impaired corti-cobulbar control resulting from the disease process is disordered volitional swallows. This pilot investigation revealed that pharyngeal swallowing mechanics in patients with MND are heterogeneous within the group, and the mechanics in MND patients differ from those in healthy, non-dysphagic controls.
The heterogeneity of MND swallowing mechanics is appreciated in Fig. 2, with the MND group far less clustered than the control group. By using an age- and sex-matched healthy, non-dysphagic control group, we were able to account for potential variance explained by age and sex. Swallowing tasks (CV6) only accounted for 5% of the variance. Further, group assignment (CV1) only accounted for 33% of the variance, whereas site of onset and motor neuron predominance (CV2 and CV3) was associated with 23 and 16% of the variance, respectively. Undetermined sources, which may include patient-specific adaptations and other attributes of MND impairments, likely comprise the remaining 23% of variance.
Eigenvectors comparing MND cohort and healthy controls indicated that overall alterations in pharyngeal swallowing mechanics in this sample of MND patients allowed compensation for reductions in pharyngeal shortening and tongue base retraction by extending the head and neck, as well as increasing hyolaryngeal excursion. However, while statistically the sample demonstrated a mean difference in gestalt swallowing mechanics, the heterogeneity of swallowing mechanics in the MND cohort offers little guidance for rapid translation in the clinical setting. This finding was not surprising since MND comprises various forms of disease—each with variable clinical features and different progressive patterns. Swallowing physiology is multifactorial, and the mechanism appears to adapt in multiple ways in the MND population in order to protect the airway and provide life-sustaining nourishment. These findings suggest that a one-size-fits-all approach to swallowing impairment in this heterogeneous population is potentially a flawed approach to patient management—what ultimately may be needed in the future is a robust multivariate patient-specific analysis of swallowing mechanics to help establish individualized targets to prolong swallowing function [21].
These findings support that patient-specific data can be used in the aggregate to determine patterns of swallowing mechanics in MND population by classification. The CVA of the MND cohort shows a clustering of data defined by motor neuron predominance and site of onset. The post hoc DFA revealed that MND categories can be statistically differentiated in this sample and eigenvectors can then show mechanical differences between categories. Unfortunately, the small sample size in combination with missing clinical information precludes reporting of these post hoc comparisons by further classifications, such as location onset and pulmonary function. Interestingly, the two clusters that overlapped included an unknown onset classification, suggesting that aggregate analysis of a larger data set may allow for an objective source of classification where it is unknown in the clinical setting.
Limitations
A primary limitation of this preliminary study includes its retrospective, cross-sectional design using a small heterogeneous patient sample referred for MBSS for suspected dysphagia. The small sample size and incomplete dataset also limited the MND only comparisons, such as disease onset type and motor neuron predominance. Further, smaller sample sizes potentially confound findings resulting from possible skewed data and influence from other unknown variables, such as patient-specific adaptive behaviors. Previous studies have demonstrated variability among patients with MND with the incidence and progression of dysphagia [10, 22], although swallowing dysfunction often continues to progress over time [10].
Another limitation inherent when using CASM is that it does not provide kinematic information, such as how far the hyoid moves during one swallow compared with another swallow. CASM, however, does provide a gestalt visualization of how the various elements of swallowing mechanics interact with each other associated with any variable of interest, such as changes in the swallowing mechanism in response to a targeted rehabilitative effort.
Conclusions
Pharyngeal swallowing mechanics of patients with MND differ from and are more heterogeneous than healthy controls. These findings suggest that patients with MND may compensate for reductions in pharyngeal shortening and tongue base retraction by extending the head and neck and increasing hyolaryngeal excursion. Future studies using CASM, including patient-specific data as completed in this study, should investigate longitudinal analyses of change in pharyngeal swallowing mechanics as disease progresses and associations with other clinical variables of interest, e.g., results from pulmonary function testing and ALSFRS-R. CASM can also be used to associate changes in pharyngeal mechanics to functional outcomes, such as oral intake status and patient-reported quality-of-life measures. Lastly, CASM may be used in future comparative studies between MND and other neurological populations, including stroke, to detail specifically changes unique to MND. This work will lead to further insights into development and evaluation of targeted clinical treatments designed to prolong safe and efficient swallowing function in patients with MND.
Funding
This study was partially supported by the following grant mechanisms: Veterans Affairs, RR&D 1IK1RX001628-01A1 (PI: Garand); National Institutes of Health, NCATS TL1R000061 (PI: Brady: Project PI: (Focht) Garand; National Institutes of Health, NIDCD 1K24DC12801 (PI: Martin-Harris); and American Speech-Language-Hearing Foundation (Recipient: Garand). Further, this study was partially supported by the J. Harold Harrison M.D. Scholars Program and MCG Medical Scholars Program at Augusta University.
Footnotes
Conflicts of interest The authors declare that they have no conflict of interest.
References
- 1.Paris G, Martinaud O, Petit A, Cuvelier A, Hannequin D, Roppeneck P, et al. Oropharyngeal dysphagia in amyotrophic lateral sclerosis alters quality of life. J Oral Rehabil. 2013;40:199–204. doi: 10.1111/joor.12019. [DOI] [PubMed] [Google Scholar]
- 2.Tabor L, Gaziano J, Watts S, Robison R, Plowman EK. Defining swallowing-related quality of life profiles in individuals with amyotrophic lateral sclerosis. Dysphagia. 2016;31:376–82. doi: 10.1007/s00455-015-9686-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robbins J Swallowing in ALS and motor neuron disorders. Neurol Clin. 1987;5:213–29. [PubMed] [Google Scholar]
- 4.Hillel AD, Miller R. Bulbar amyotrophic lateral sclerosis: patterns of progression and clinical management. Head Neck. 1989;11:51–9. [DOI] [PubMed] [Google Scholar]
- 5.Hadjikoutis S, Wiles CM. Respiratory complications related to bulbar dysfunction in motor neuron disease. Acta Neurol Scand. 2001;103:207–13. [PubMed] [Google Scholar]
- 6.Corcia P, Pradat P-F, Salachas F, Bruneteau G, le Forestier N, Seilhean D, et al. Causes of death in a post-mortem series of ALS patients. Amyotroph Lateral Scler. 2008;9:59–62. [DOI] [PubMed] [Google Scholar]
- 7.Kawai S, Tsukuda M, Mochimatsu I, et al. A study of the early stage of dysphagia in amytrophic lateral sclerosis. Dysphagia. 2003;18(1):8. doi: 10.1007/s00455-002-0074-3. [DOI] [PubMed] [Google Scholar]
- 8.Palovcak M, Mancinelli JM, Elman LB, McCluskey L. Diagnostic and therapeutic methods in the management of dysphagia in the MND population: issues in efficacy for the out-patient setting. NeuroRehabilitation. 2007;22:417–23. [PubMed] [Google Scholar]
- 9.Ruoppolo G, Schettino I, Frasca V, et al. Dysphagia in amyotrophic lateral sclerosis: prevalence and clinical findings. Acta Neurol Scand. 2013;128:397–401. doi: 10.1111/ane.12136. [DOI] [PubMed] [Google Scholar]
- 10.Higo R, Tayama N, Nito T. Longitudinal analysis of progression of dysphagia in amyotrophic lateral sclerosis. Auris Nasus Larynx. 2004;31:247–54. [DOI] [PubMed] [Google Scholar]
- 11.Higo R, Tayama N, Watanabe T, Nitou T. Videomanofluorometric study in amyotrophic lateral sclerosis. Laryngoscope. 2005;5:911–7. doi: 10.1016/j.anl.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 12.Thompson ZT, Obeidin F, Davidoff AA, Hightower CL, Johnson CZ, Rice SL, et al. Coordinate mapping of hyolaryngeal mechanics in swallowing. J Vis Exp. 2014;87. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4132872/. Accessed 22 June 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pearson WG, Taylor BK, Blair J, et al. Computational analysis of swallowing mechanics underlying impaired epiglottic inversion. Laryngoscope. 2016;126:1854–8. doi: 10.1002/lary.25788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pearson WG, Zumwalt AC. Visualising hyolaryngeal mechanics in swallowing using dynamic MRI. Comput Methods Biomech Biomed Eng Imaging Vis. 2014;2:208–16. doi: 10.1080/21681163.2013.846231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Webster M, Sheets HD, Alroy J, et al. A practical introduction to landmark-based geometric morphometrics. Qant Meth Paleobiol Palentol Soc Papers. 2010;16:163–88. [Google Scholar]
- 16.May NH, Pisegna JM, Marchina S, Langmore SE, Kumar S, Pearson WG. Pharyngeal swallowing mechanics secondary to hemispheric stroke. J Stroke Cardiovasc Dis. 2017;26:952–61. doi: 10.1016/j.jstrokecerebrovasdis.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin-Harris B, Brodsky MB, Michel Y, Castell DO, Schleicher M, Sandidge J, et al. MBS measurement tool for swallow impairment-MBSImp: establishing a standard. Dysphagia. 2008;23:392–405. doi: 10.1007/s00455-008-9185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Natarajan R, Stavness I, Pearson W Jr. Semi-automatic tracking of hyolaryngeal coordinates in videofluoroscopic swallowing studies. Comput Methods Biomech Biomed Eng Imaging Vis. 2015;2015:1–11. [Google Scholar]
- 19.Klingenberg CP. MorphoJ: an integrated software package for geometric morphometrics. Mol Ecol Resour. 2011;11:353–7. doi: 10.1111/j.1755-0998.2010.02924.x. [DOI] [PubMed] [Google Scholar]
- 20.Webster M, Sheets DH. A practical introduction to landmark-based geometric morphometrics. In: Alroy J, Hunt G, editors. Quantitative methods in paleobiology. Boulder: Paleontological Society; 2010. p. 163–88. [Google Scholar]
- 21.Tran TTA, Martin-Harris B, Pearson WG. Improvements resulting from respiratory-swallow phase training visualized in patient-specific computational analysis of swallowing mechanics. Comput Methods Biomech Biomed Eng Imaging Vis. 2016;2016:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strand EA, Miller RM, Yorkston KM, Hillel AD. Management of oral-pharyngeal dysphagia symptoms in amyotrophic lateral sclerosis. Dysphagia. 1996;11:129–39. [DOI] [PubMed] [Google Scholar]
- 23.Crary MA, Mann GD, Groher ME. Initial psychometric assessment of functional oral intake scale for dysphagia in stroke patients. Arch Phys Med Rehabil. 2005;86:1516–20. [DOI] [PubMed] [Google Scholar]