Abstract
Objective
To determine the effects of repeated placement of quantified lyophilised platelet-rich plasma (LPRP) on the soft and hard tissue components.
Methods
Lyophilised platelet-rich plasma was topically placed, and later injected, into fresh sockets using the third molar surgical model, randomised according to the split-mouth approach. The control site received placebo. The application of LPRP was done intraoperatively, one month and two months postoperatively. The measured endpoints included post-operative pain, swelling, trismus, pocket depth at mid-distal adjacent second molar, soft tissue healing, and bone formation (which was assessed radiographically). Fifteen healthy young adults, aged between 21 and 35 years, visiting the Oral and Maxillofacial Surgery Clinic at the University of Malaya were recruited for this study.
Results
There was no significant difference in post-operative pain, swelling size, trismus, and bone healing within their specific timelines during this study. However, the LPRP group showed significant reduction in pocket depth at the two-month post-operative period, suggesting that LPRP improves soft tissue healing.
Conclusion
Soft tissue healing, measured as the change of periodontal pocket depth, showed significant reduction, suggesting the benefit of LPRP for soft tissue healing. However, bone regeneration and reduction of post-operative sequelae showed no improvement even after quantification and repeated LPRP application.
Keywords: Bone healing, Growth factors, Lyophilised platelet-rich plasma, Regeneration, Soft tissue healing
الملخص
أهداف البحث
تحديد تأثير التنسيب المتكرر للبلازما الغنية بالصفائح الدموية المجففة بالتجميد الكمي على مكونات الأنسجة الرخوة والصلبة.
طرق البحث
تم وضع البلازما الغنية بالصفائح موضعيا وحقنها لاحقا في مآخذ جديدة باستخدام نموذج ضرس الرحى الثالثة الجراحي، وتم اختياره عشوائيا وفقا لطريقة تقسيم الفم. تلقى موقع التحكم الدواء الوهمي. تم تطبيق البلازما الغنية بالصفائح أثناء الجراحة، وبعد شهر واحد، وبعد شهرين بعد الجراحة. كانت مقاييس النتائج هي الألم بعد الجراحة، والتورم، والتشقق، وعمق الجيب في منتصف الضرس الثاني المجاور البعيد، وشفاء الأنسجة الرخوة، وتكوين العظام (الذي تم تقييمه شعاعيا). تم اشتراك خمسة عشر من الشباب الأصحاء المراجعين في عيادة الأسنان بجامعة مالايا في هذه الدراسة.
النتائج
لم يكن هناك فرق كبير في ألم ما بعد الجراحة، وحجم التورم، والتشقق وشفاء العظام ضمن الجدول الزمني المحدد لهذه الدراسة. ومع ذلك، أظهرت مجموعة البلازما الغنية بالصفائح انخفاضا كبيرا في عمق الجيب في فترة ما بعد الجراحة لمدة شهرين مما يشير إلى أن البلازما الغنية بالصفائح تحسن التئام الأنسجة الرخوة.
الاستنتاجات
أظهر قياس شفاء الأنسجة الرخوة من خلال تغير عمق الجيب اللثوي انخفاضا كبيرا، مما يشير إلى فائدة البلازما الغنية بالصفائح في التئام الأنسجة الرخوة. ومع ذلك، فإن تجديد العظام وتقليل مضاعفات ما بعد الجراحة إلى البلازما الغنية بالصفائح لم يظهر أي تحسن حتى بعد القياس الكمي والتطبيق المتكرر للبلازما الغنية بالصفائح.
الكلمات المفتاحية: البلازما الغنية بالصفائح الدموية المجففة بالتجميد, التئام العظام, التئام الأنسجة الرخوة, عوامل النمو, التجديد.
Introduction
The loss of hard and soft tissues in the oral cavity following extraction, trauma, or chronic periodontitis is the leading cause of deformity on the alveolar ridge. Therefore, the preservation and reconstruction of the alveolar ridge is the holy grail of research. The use of chairside platelet-rich plasma (PRP) to aid tissue healing is popular among surgeons. Platelets-derived growth factors contained in PRP play a key mediator role by acting as a chemoattractant and mitogen, aiding angiogenesis and tissue repair1; however its clinical application has been inconclusive. Since the introduction of PRP for maxillofacial use in 1997 by Withman,2 there has been no single protocol showing significant reproducible results. A recent systematic review stated that there is limited evidence regarding the effects of PRP in the intraoral bone grafting procedure,3 and scientific evidence for PRP promoting third molar socket healing was poor,4 thus suggesting further research to fully identify its indication and effectiveness in patients. However, Anitua,5 who studied PRP extensively across many disciplines of medicine, concluded that it does promote significant bone healing. These conflicting conclusions prompted us to review 18 PRP6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23 studies on extraction sockets, which showed that 10 studies assessed both soft and hard tissues healing, two assessed soft tissue healing alone, and six assessed hard tissue healing alone. This review showed a spectrum of PRP production protocols as well as healing outcomes with no definitive conclusion.
There are two apparent reasons for this variation in results: first, the quantity of platelet used is not quantifiable prior to placement; second, this method only allows for a single application of PRP at the start of the healing process. This limitation can be addressed by lyophilised platelet-rich plasma (LPRP).
Lyophilised platelet-rich plasma essentially refers to platelet cells in plasma that have been freeze-dried into powder form, thereby allowing platelet quantification, and are suitable for prolonged storage. Studies show that platelet-rich plasma lyophilisation24 enables growth factor preservation and functionality when compared with fresh PRP. In a nutshell, growth factors that are the essence of PRP can be maintained during the storage process.
This study aimed to determine the effects of providing repeated doses of quantified LPRP, available in lyophilised form, on soft and hard tissues healing following surgical removal of third molars. Additional endpoints included assessment of its ability to reduce post-operative sequelae, namely swelling, pain, and trismus. Our null hypothesis is as follows: placement of repeated doses of quantified LPRP will aid in soft and hard tissues healing.
Materials and Methods
A prospective randomised controlled trial using the impacted third molar surgery model was conducted on outpatients attending the Oral and Maxillofacial Surgery Clinic at the Faculty of Dentistry, University of Malaya to investigate the soft and hard tissues healing, and sequelae associated with third molar extraction in LPRP-treated and non-LPRP-treated socket sites.
Patient selection and blood donation
A sample of 15 patients with clinical indications for extraction of bilateral impacted mandibular third molars with similar orientation, depth, and root morphology was identified from a pool of patients presenting at the Oral and Maxillofacial Surgery Clinic at the Faculty of Dentistry, University of Malaya. These patients consisted of healthy individuals aged 21–35 years from both genders, and who complied to the American Society of Anaesthesiologist classification of Class 1 (ASA1).
During the first visit, interested patients were informed of their diagnosis and the need for surgical intervention, with an outline of the treatment plan together with their rights and responsibilities. Patients were sent for Full Blood Count (FBC) to ensure that their haemoglobin and platelet levels were within normal range. During the second visit, they were provided with a written informed consent form specifying the title of the study and the possible risks and complications that may arise because of surgery. Consented patients were then enlisted for blood donation, pre-blood donation vital signs (blood pressure, oxygen saturation, pulse rate, and temperature) were recorded, and blood was collected using the blood donations kits provided by Stemtech International. A blood collection kit contains one JMS® single blood-transfer bag with anticoagulant (Citrate-Phosphate-Dextrose-Adenine), two BD Vacutainer® SSTs (containing silica and polymer gel), and a consent booklet for blood-taking procedure and screening of infectious diseases. Around 300–350 ml of their periphery blood was collected for LPRP processing. An additional 10 ml of blood was collected into two BD Vacutainer® SSTs for screening of infectious diseases prior to LPRP processing. All blood products were labelled with the patient's name and national identification numbers and sealed accordingly. Patients' vital signs were monitored for around one hour after blood transfusion before being discharged.
Lyophilised platelet-rich plasma preparation
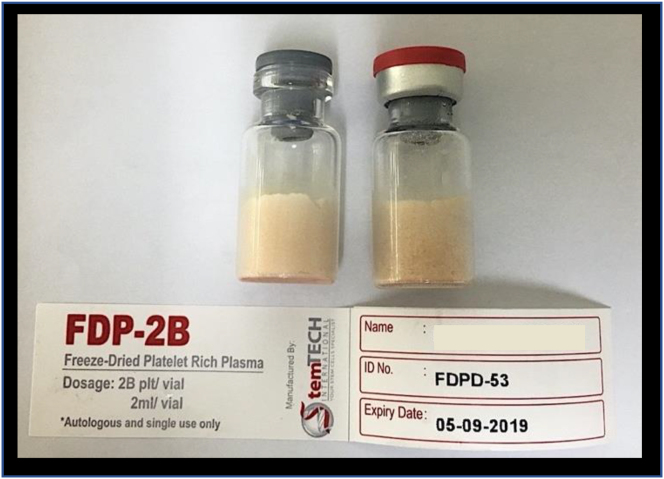
All blood products were screened via serology and nucleic acid test for potential blood-borne infections, such as syphilis, Hepatitis B, Hepatitis C, and HIV. Once cleared, they were centrifuged according to protocol to prepare PRP, after which platelet count was validated. Once the PRP was aliquoted into vials, they were then freeze-dried to produce LPRP. Sterility testing was performed on all vials before being sent out. All preparations were done by Stemtech International, a certified good manufacturing practice (GMP) facility. We received five vials per patient, with each vial containing two billion platelets (Figure 1).
Figure 1.
An example of the LPRP vials used in this experiment.
Surgery and lyophilised platelet-rich plasma placement
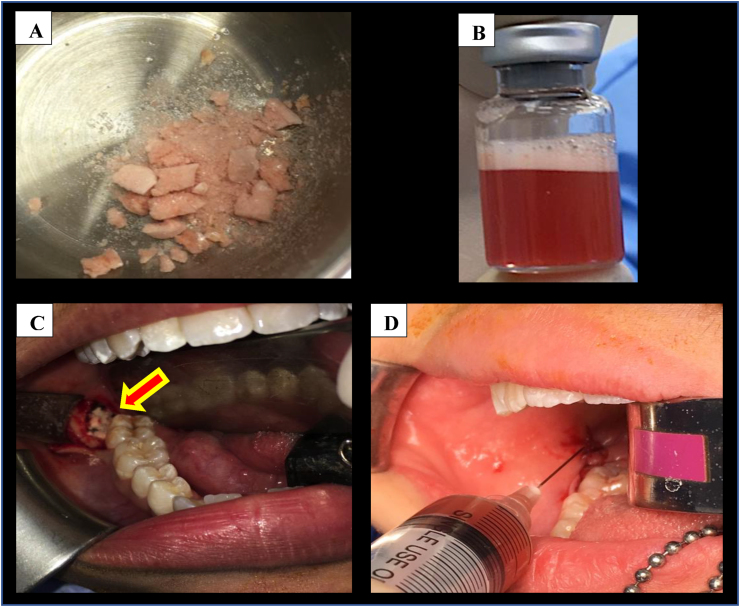
Lyophilised platelet-rich plasma returned from Stemtech International at least three days prior to the day of surgery and was stored at −80 °C. Pre-operative baseline facial measurements and the width of mouth opening were taken immediately before surgery. This protocol follows one that is currently practiced at our centre.25 Standard third molar surgery was performed by a single operator with the sockets randomised to be controlled or as LPRP study site. The height of the distal exposed root to the cementoenamal junction (CEJ) of the adjacent second molar was measured using a periodontal probe immediately post-operatively. Using the split-mouth approach, sockets on one side received LPRP, whereas the ones on the contralateral sockets (control) did not undergo intervention. Lyophilised platelet-rich plasma was placed topically into the extraction sockets of impacted third molars with the aid of Mitchell's osseous trimmer (Figures 2A and 2C). All bony surfaces were completely lined with LPRP and the flap was held with 4/0 polyglactin 910 (Vicryl ®; Johnson & Johnson, USA) simple interrupted sutures, after a clot had formed over it. Another LPRP vial was reconstituted using 2 mm of normal saline (Figure 2B) and was injected into the submucosa as per protocol used to inject steroids into the submucosal region, which was done at our centre25 (Figure 2D).
Figure 2.
(A) Powder form of LPRP prior to topical placement into extraction socket; (B) Reconstituted LPRP in 2 ml normal saline; (C) Topical placement of LPRP into the extraction socket; (D) Submucosal injection of reconstituted LPRP.
Parameters measured
All patients underwent three months of clinical and radiographic follow-up. Reviews were performed on post-operative days one, two, and seven for assessment of healing at structures adjacent to the surgical site. Facial swelling measurements were taken as the sum of the length of two lines along the pre-determined facial reference points from the outer corner of the eye to the angle of mandible and tragus of the ear to the corner of the mouth.25 Facial measurement was taken using a measuring tape. The percentage of facial swelling was then calculated based on the differences between baseline measurements with measurements taken on the three days of the study period. Trismus was measured as the changes in the width of mouth opening (maximum interincisal distance) between pre-operative and post-operative days one, two, and seven. Pain was evaluated and recorded on post-operative days one, two, and seven using a 10 cm visual analogue scale (VAS). The state of soft tissue healing was assessed on post-operative day seven, with periodontal pocketing distal to the second molar measured using a periodontal probe to the CEJ at post-operative day seven and post-operative one and two months. The healing index of Landry and Gonshor26,27 was used to complement these measurements.
During the first and second months of review, another two doses of constituted LPRP were injected into the submucosa adjacent to the socket. Prior to the injections, periodontal pocketing at the mid-distal to the second molar was measured using a periodontal probe. The CEJ of the second molar was used as the upper limit of pocket depth.
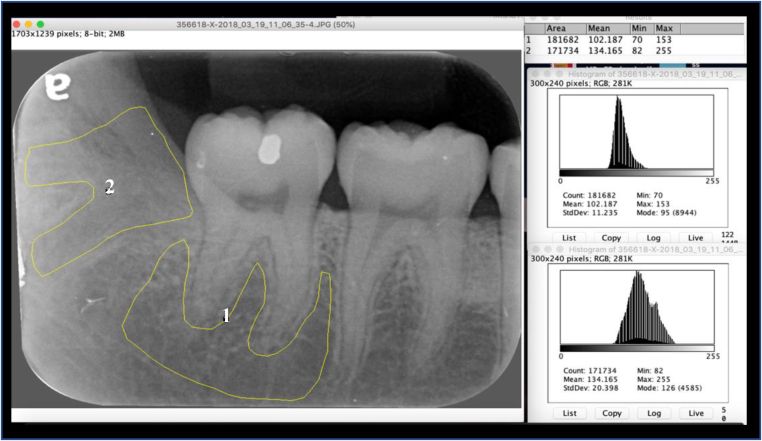
Bone repair was assessed using an intraoral periapical (IOPA) radiograph obtained at the three-month post-operative period. All radiographs were obtained by the same radiographer using the same technique. Changes in bone repair were assessed through image histogram analysis using Image J 1.52a (National Institutes of Health & the Laboratory for Optical and Computational Instrumentation [LOCI], University of Wisconsin, USA). Image J can calculate pixel value statistics of user-defined selections and intensity-thresholded objects, which it then shows as density histograms. The histogram indicates how many pixels of a selected area share the same grey spectrum (0 = pure black and 255 = pure white). Image J 1.52 produced a graft with the x-axis showing grey levels (0–255), and the y-axis, showing their frequency in the selected area. The radiographic densities of the extraction sockets were compared with the densities of the basal bone and interradicular space of the adjacent teeth, with the difference between both serving to distinguish changes in the LPRP and control sides (Figure 3). The difference between the two histograms taken at three months post-surgery (histogram difference [HD]) was calculated. Histogram difference is inversely proportional to new bone formation; that is, the lower the difference, the greater is the degree of bone repair. Therefore, the extraction socket histogram was always compared with the same landmark on the radiograph, in order to compensate for any differences in tone from one film to another. In keeping with the split-mouth design of the study to minimise variability, HDs of both sides of the same patient were compared as well. The median HD was calculated for each radiographic assessment (at three-month follow-up) to show the progress of bone healing in the PRP side as compared with the control side.
Figure 3.
Histogram analysis using Image J 1.52a. (1) An area of basal bone and interradicular space of the adjacent teeth is used as control; (2) An area of previously impacted third molar that has been removed surgically, followed by LPRP placement.
Data analysis
Data obtained were analysed using IBM SPSS Statistics version 25.0 (IBM Corporation, New York, USA). Findings from the two study sites was analysed using Levene's test to show equal homogenous assumption and normality testing prior to statistical analysis. The independent sample t-test was used for the histogram finding and soft tissue healing, followed by paired sample t-test to evaluate changes in histogram and soft tissue healing. The repeated measure ANOVA was used to evaluate pain score and facial swelling. As for the mouth opening, the mean was calculated for comparison with the baseline. The significant value was set at p < 0.05.
Results
The samples recruited consisted of 12 healthy females and three healthy males, with a mean age of 26 (4.90) years (ranging from 21 to 35 years). The processed LPRP vials had a mean platelet concentration of 1126 × 103/uL, with a platelet concentration range of 928–1186 × 103/uL. All serology tests and nucleic acid tests for syphilis, Hepatitis B & C, and Human Immunodeficiency Virus (HIV) were negative. Bacterial and fungal tests on the processed LPRP were also negative.
Sequelae of third molar surgery
Pain, swelling, and trismus are three common sequelae of third molar surgery. Post-operative pain scores on day one (POD1), day two (POD2), and day seven (POD7) were collected and grouped into control (C) and LPRP groups. A summary of pain scores obtained is shown in Table 1(A). There is no difference in pain scores reported between these two groups. However, the group treated with LPRP recorded slightly higher pain score throughout the first seven days.
Table 1.
Comparison of LPRP-treated and control groups parameters.
|
A. Mean VAS Pain score (SD) [Range] (0–10) |
p-value |
||
| Post-operative | LPRP | Control | |
| Day 1 At 9:00 am | 2.46 (1.24) [0–7.0] |
2.0(1.41) [0–5.0] |
0.564 |
| At 6:00 pm | 2.33 (1.83) [0–5.0] |
2.0 (1.69) [0–5.0] |
0.788 |
| Day 2 At 9:00 am | 3.13 (2.16) [0–7.0] |
2.33 (2.19) [0–7.0] |
0.601 |
| At 6:00 pm | 2.66 (1.87) [0–9.0] |
2.60 (2.13) [0–7.0] |
1 |
| Day 7 At 9:00 am | 1.60 (1.40) [0–6.0] |
1.53 (1.76) [0–4.0] |
0.312 |
| At 6 pm | 1.26 (1.48) [0–5.0] |
1.20 (1.47) [0–7.0] |
0.415 |
|
B. Mean distance from tragus to the corner of the mouth (in mm) (SD) [Range] |
p-value |
||
| Post-operative | LPRP | Control | |
| Baseline | 115.4 (8.5) mm [104–130] |
114.2 (8.0) mm [103–125 mm] |
0.705 |
| Day 1 | 114.1 (29) mm [105–135] |
117.9 (6.9) mm [109–130 mm] |
0.196 |
| Day 2 | 122.6 (10.1) mm [108–140] |
118.1 (5.5) mm [112–127 mm] |
0.263 |
| Day 7 | 116.6 (7.1) mm [104–129] |
115.6 (7.9) mm [104–130 mm] |
0.690 |
|
C. The distance from the outer canthus to the mandible angle (in mm) (SD) [Range] |
p-value |
||
| Post-operative | LPRP | Control | |
| Baseline | 102.8 (6.1) mm [94–115] |
105.0 (6.5) mm [95–118 mm] |
0.871 |
| Day 1 | 120 (12) mm [104–140 mm] |
116.0 (9.3) mm [103–135 mm] |
0.378 |
| Day 2 | 116 (11.0) mm [100–135 mm] |
114.7 (8.3) mm [100–135 mm] |
0.216 |
| Day 7 | 112 (12.1) mm [98–135 mm] |
113.4 (9.5) mm [98–135 mm] |
0.225 |
|
D. Pocket depth (in mm) (SD) [Range] |
p-value |
||
| Post-operative | LPRP | Control | |
| POD7 | 5.67 (0.82) [5.0–7.0] |
5.73 (0.88) [4.0–7.0] |
0.832 |
| PO1M | 4.53 (0.64) [4.0–6.0] |
4.87 (0.83) [4.0–6.0] |
0.230 |
| PO2M | 3.53 (0.52) [3.0–4.0] |
4.40 (0.63) [3.0–5.0] |
a<0.001 |
|
E. IMAGE J DATA (SD) |
p-value |
||
| Post-operative | LPRP (PO3M) | Control | |
| Bone | 87.24 (19.7) | 94.76 (18.2) | 0.287 |
| Socket | 86.71 (26.2) | 90.20 (22.1) | 0.696 |
Significant with alpha = 0.05.
The size of facial swelling measured at POD1, POD2, and POD7 were also grouped into control and LPRP groups. A summary of the size of facial swelling obtained is shown in Table 1(B&C). No difference in facial swelling size is reported between the group treated with LPRP versus the control group. However, the group treated with LPRP recorded slightly bigger swelling throughout the study period.
Interincisal mouth opening was assessed during the first week post-surgery. Mean interincisal baseline was 43.75. There was a significant reduction of interincisal mouth opening at POD1 (17.13 mm) and at POD2 (19.8 mm). The size of mouth opening significantly improved to 27.2 mm at POD7, as compared to the size at POD1 (p < 0.00).
Soft tissue healing and pocket depth at extraction sockets
Soft tissue healing in both groups at POD7 was rated using the healing index score, with a score of 3 being observed for all patients. There was no difference between control and LPRP-treated sockets. There was no significant difference in pocket depth between the LPRP-treated group and the control group throughout the first month of study (Table 1D). However, by the second month, there was a statistically significant improvement noted, that is, reduction in pocket depth in the LPRP-treated group (3.53 mm versus 4.40 mm), as summarised in Table 1D.
Hard tissue healing
The independent sample t-test showed no significant difference between the control socket and its surrounding normal bone (p = 0.542). Similarly, no significant difference was identified between the extraction socket treated with LPRP and its surrounding normal bone (p = 0.950). The derived histogram of control bone and socket are 94.76 (18.2) and 90.20 (22.1), respectively. In contrast, the derived histogram of LPRP treated bone and socket are slightly lower at 87.24 (19.7) and 86.71 (26.2), respectively (Table 1D).
A comparison between the control extraction socket and the LPRP-treated socket also yielded no significant difference (Independent t-test; p = 0.287 at bone, p = 0.696 at socket), suggesting no clinical benefit of repeat application of LPRP.
Discussion
This study investigated whether standardised LPRP preparation in a certified laboratory, which had quantified platelet count combined with repetition submucosal application, will accelerate the healing process and help reduce post-operative sequelae. This effort aimed to standardise our protocol in order to allow clinicians replicate the results in future.
Based on current observations, there is no statistically significant effect of LPRP towards the general healing process, except for the reduction of periodontal pocket depth by the second month. The reduction in periodontal pocket depth observed is in agreement with several authors,8,9,16,20,21 which suggests promising effects of LPRP use for periodontal health regeneration. This is attributed to the presence of platelet-derived growth factors (PDGF) and endothelial growth factors (EGF), which are the main growth factors involved in the migration, attachment, proliferation, and differentiation of periodontal progenitor cells28 and the effect of capillary regeneration.29 Two repetitions of submucosal injection of LPRP over a period of two months might have helped to increase/maintain the level of PDGF over the healing extraction socket. Increased concentrations of these growth factors is the likely reason for the accelerated soft tissue wound healing, which is suggested to be at least 2–3 times faster than normal.5
Currently, no possible explanation exists for the increased experience of pain (although not statistically significant) in LPRP-treated patients. Thanasas30 experienced the same event in patients treated with PRP for chronic lateral elbow epicondylitis. He hypothesised that the increase of white blood cells may have caused the intense inflammation response, thus leading to increased pain score. In contrast to the current study, Haraji31 had reported that post-operative pain was significantly less in sockets treated with PRP, as compared to the control group. The difference in this finding may be the result of using different platelet concentrates and anticoagulants in LPRP, which stimulates local inflammatory responses at different levels, leading to increased levels of pain.
The increase in swelling was noticeable for the first two days post-operatively, and at POD7, swellings for the control and LPRP groups were similar. On the contrary, Rutkowski13 showed that there was significantly less facial oedema over the site that received PRP. We hypothesised that the increase in swelling is caused by the same inflammatory mechanism that contributed to the increase in pain in case of LPRP use.
Bone production was fundamental to this study; however, there was no significant improvement. Human and animal study on PRP echoed the same findings for bone production.23,32,33 Butterfield et al.34 reported an animal model study that showed no increase in histologic total bone, bone formation rate, or bone density, despite the addition of bone grafting material. They suggested that the short, five-days lifespan of platelets was unable to render enough direct influence on bone formation. However, Rutkowski13 showed an increase in bone density in PRP-treated socket, suggesting a greater volume of new bone formation. They reported that the control side required 16 weeks to reach the same radiographic density as the PRP-treated socket, which achieved this improvement in eight weeks. There is no clear evidence for the lack of bone formation in our study, but this may be attributed to the optimal amount and concentration of PRP needed for significant bone healing. The risk of over saturating the socket with platelets cannot be ruled out either.
An important limitation of this study is the fact that the growth factor concentration in each vial was not quantified via ELISA testing, and the biological activity of available growth factors was not assessed thoroughly, even though the platelets were quantified. This shortcoming may clarify the current inconclusive finding. The small sample size—caused by high LPRP processing cost—and an unbalanced gender ratio are also limitations of this study. Additionally, there might be a potential recall bias as the clinician performing the clinical measurement post-surgery is aware of the LPRP placement site. For future studies, we suggest that the concentration of platelet-derived growth factors and the biological activity of platelets be assessed prior to usage, and different clinicians be associated with surgery and clinical measurements.
Conclusion
In conclusion, the soft tissue healing, measured as the change of periodontal pocket depth, showed significant reduction, suggesting the benefit of LPRP for soft tissue healing. However, the regeneration of bone and reduction of post-operative sequelae showed no improvement even after quantification and repeated application of LPRP.
Source of funding
University of Malaya Dental Postgraduate Research Fund (DPRG/24/17). Stemtech International Sdn Bhd supported this study by preparing the LPRP for all patients.
Conflict of interest
The authors have no conflicts of interest to declare.
Ethical approval
Ethical approval for the study was obtained from the Medical Ethics Committee, Faculty of Dentistry, University of Malaya on 30 January, 2018 (No: DF OS1801/0002(P).
Authors contributions
JR designed the study, conducted research, collected and organised data, and wrote the initial and final drafts. WCN conceived the idea, analysed and interpreted data, and proofread the article. NI conceived the radiological assessment idea, interpreted the radiological data, and proofread the article. All authors have critically reviewed and approved the final draft and are responsible for the content and similarity index of the manuscript.
Acknowledgment
We thank Stemtech International Sdn Bhd for preparing the LPRP for all patients.
Footnotes
Peer review under responsibility of Taibah University.
References
- 1.Hollinger J.O., Hart C.E., Hirsch S.N., Lynch S., Friedlaender G.E. Recombinant human platelet-derived growth factor: biology and clinical applications. J Bone Jt Surg Am. 2008;90(Suppl 1):48–54. doi: 10.2106/JBJS.G.01231. [DOI] [PubMed] [Google Scholar]
- 2.Whitman D.H., Berry R.L., Green D.M. Platelet gel: an autologous alternative to fibrin glue with applications in oral and maxillofacial surgery. J Oral Maxillofac Surg. 1997;55(11):1294–1299. doi: 10.1016/s0278-2391(97)90187-7. [DOI] [PubMed] [Google Scholar]
- 3.Dragonas P., Schiavo J.H., Avila-Ortiz G., Palaiologou A., Katsaros T. Plasma rich in growth factors (PRGF) in intraoral bone grafting procedures: a systematic review. J Cran-Maxillo-Fac Surg. 2019;47(3):443–453. doi: 10.1016/j.jcms.2019.01.012. [DOI] [PubMed] [Google Scholar]
- 4.Barona-Dorado C., Gonzalez-Regueiro I., Martin-Ares M., Arias-Irimia O., Martinez-Gonzalez J.M. Efficacy of platelet-rich plasma applied to post-extraction retained lower third molar alveoli. A systematic review. Med Oral, Patol Oral Cirugía Bucal. 2014;19(2):e142–e148. doi: 10.4317/medoral.19444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anitua E., Andia I., Ardanza B., Nurden P., Nurden A.T. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemostasis. 2004;91(1):4–15. doi: 10.1160/TH03-07-0440. [DOI] [PubMed] [Google Scholar]
- 6.Simon D., Manuel S., Geetha V., Naik B.R. Potential for osseous regeneration of platelet-rich plasma--a comparative study in mandibular third molar sockets. Indian J Dent Res. 2004;15(4):133–136. [PubMed] [Google Scholar]
- 7.Sammartino G., Tia M., Marenzi G., di Lauro A.E., D'Agostino E., Claudio P.P. Use of autologous platelet-rich plasma (PRP) in periodontal defect treatment after extraction of impacted mandibular third molars. J Oral Maxillofac Surg. 2005;63(6):766–770. doi: 10.1016/j.joms.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 8.Mozzati M., Scoletta M., Gallarato I. Clinical application of autologous platelet rich plasma (P.R.P.) in the extraction of third impacted mandibular molar. Revista Romana De Stomatologie. 2007;LIII(NR. 2):81–88. [Google Scholar]
- 9.Sammartino G., Tia M., Gentile E., Marenzi G., Claudio P.P. Platelet-rich plasma and resorbable membrane for prevention of periodontal defects after deeply impacted lower third molar extraction. J Oral Maxillofac Surg. 2009;67(11):2369–2373. doi: 10.1016/j.joms.2009.04.093. [DOI] [PubMed] [Google Scholar]
- 10.Gawande P.D., Halli R. Efficacy of platelet rich plasma in bone regeneration after surgical removal of impacted bilateral mandibular third molars: pilot study. J Maxillofac Oral Surg. 2009;8(4):301–307. doi: 10.1007/s12663-009-0074-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vivek G.K., Sripathi Rao B.H. Potential for osseous regeneration of platelet rich plasma: a comparitive study in mandibular third molar sockets. J Maxillofac Oral Surg. 2009;8(4):308–311. doi: 10.1007/s12663-009-0075-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arenaz-Bua J., Luaces-Rey R., Sironvalle-Soliva S., et al. A comparative study of platelet-rich plasma, hydroxyapatite, demineralized bone matrix and autologous bone to promote bone regeneration after mandibular impacted third molar extraction. Med Oral, Patol Oral Cirugía Bucal. 2010;15(3):e483–e489. doi: 10.4317/medoral.15.e483. [DOI] [PubMed] [Google Scholar]
- 13.Rutkowski J.L., Johnson D.A., Radio N.M., Fennell J.W. Platelet rich plasma to facilitate wound healing following tooth extraction. J Oral Implantol. 2010;36(1):11–23. doi: 10.1563/AAID-JOI-09-00063. [DOI] [PubMed] [Google Scholar]
- 14.Ogundipe O.K., Ugboko V.I., Owotade F.J. Can autologous platelet-rich plasma gel enhance healing after surgical extraction of mandibular third molars? J Oral Maxillofac Surg. 2011;69(9):2305–2310. doi: 10.1016/j.joms.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 15.Celio-Mariano R., de Melo W.M., Carneiro-Avelino C. Comparative radiographic evaluation of alveolar bone healing associated with autologous platelet-rich plasma after impacted mandibular third molar surgery. J Oral Maxillofac Surg. 2012;70(1):19–24. doi: 10.1016/j.joms.2011.03.028. [DOI] [PubMed] [Google Scholar]
- 16.Kaul R.P., Godhi S.S., Singh A. Autologous platelet rich plasma after third molar surgery: a comparative study. J Maxillofac Oral Surg. 2012;11(2):200–205. doi: 10.1007/s12663-011-0311-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Antonello Gde M., Torres do Couto R., Giongo C.C., Correa M.B., Chagas Junior O.L., Lemes C.H. Evaluation of the effects of the use of platelet-rich plasma (PRP) on alveolar bone repair following extraction of impacted third molars: prospective study. J Cranio-Maxillo-Fac Surg. 2013;41(4):e70–e75. doi: 10.1016/j.jcms.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 18.Dutta S.R., Singh P., Passi D., Patter P. Mandibular third molar extraction wound healing with and without platelet rich plasma: a comparative prospective study. J Maxillofac Oral Surg. 2015;14(3):808–815. doi: 10.1007/s12663-014-0738-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nathani D.B., Sequeira J., Rao B.H. Comparison of platelet rich plasma and synthetic graft material for bone regeneration after third molar extraction. Ann Maxillofac Surg. 2015;5(2):213–218. doi: 10.4103/2231-0746.175762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doiphode A.M., Hegde P., Mahindra U., Santhosh Kumar S.M., Tenglikar P.D., Tripathi V. Evaluation of the efficacy of platelet-rich plasma and platelet-rich fibrin in alveolar defects after removal of impacted bilateral mandibular third molars. J Int Soc Prev Community Dent. 2016;6(Suppl 1):S47–S52. doi: 10.4103/2231-0762.181167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gandevivala A., Sangle A., Shah D., et al. Autologous platelet-rich plasma after third molar surgery. Ann Maxillofac Surg. 2017;7(2):245–249. doi: 10.4103/ams.ams_108_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhujbal R, Malik N.A, Kumar N., Kv S., Parkar M.I., Mb J. Comparative evaluation of platelet rich plasma in socket healing and bone regeneration after surgical removal of impacted mandibular third molars. J Dent Res Dent Clin Dent Prospects. 2018;12(3):153–158. doi: 10.15171/joddd.2018.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gurbuzer B., Pikdoken L., Tunali M., Urhan M., Kucukodaci Z., Ercan F. Scintigraphic evaluation of osteoblastic activity in extraction sockets treated with platelet-rich fibrin. J Oral Maxillofac Surg. 2010;68(5):980–989. doi: 10.1016/j.joms.2009.09.092. [DOI] [PubMed] [Google Scholar]
- 24.da Silva L.Q., Montalvao S.A.L., Justo-Junior A.D.S., et al. Platelet-rich plasma lyophilization enables growth factor preservation and functionality when compared with fresh platelet-rich plasma. Regen Med. 2018;13(7):775–784. doi: 10.2217/rme-2018-0035. [DOI] [PubMed] [Google Scholar]
- 25.Lim D., Ngeow W.C. A comparative study on the efficacy of submucosal injection of dexamethasone versus methylprednisolone in reducing postoperative sequelae after third molar surgery. J Oral Maxillofac Surg. 2017;75(11):2278–2286. doi: 10.1016/j.joms.2017.05.033. [DOI] [PubMed] [Google Scholar]
- 26.G Landry RST R., Howley T. Effectiveness of benzydamine HCl in the treatment of periodontal post-surgical patients. Res Clin Forums. 1988;10:105–118. [Google Scholar]
- 27.Gonshor A. Technique for producing platelet-rich plasma and platelet concentrate: background and process. Int J Periodontics Restor Dent. 2002;22(6):547–557. [PubMed] [Google Scholar]
- 28.Giannobile W.V. Periodontal tissue engineering by growth factors. Bone. 1996;19(1 Suppl):23s–37s. doi: 10.1016/s8756-3282(96)00127-5. [DOI] [PubMed] [Google Scholar]
- 29.Lindeboom J.A., Mathura K.R., Aartman I.H., Kroon F.H., Milstein D.M., Ince C. Influence of the application of platelet-enriched plasma in oral mucosal wound healing. Clin Oral Implants Res. 2007;18(1):133–139. doi: 10.1111/j.1600-0501.2006.01288.x. [DOI] [PubMed] [Google Scholar]
- 30.Thanasas C., Papadimitriou G., Charalambidis C., Paraskevopoulos I., Papanikolaou A. Platelet-rich plasma versus autologous whole blood for the treatment of chronic lateral elbow epicondylitis: a randomized controlled clinical trial. Am J Sports Med. 2011;39(10):2130–2134. doi: 10.1177/0363546511417113. [DOI] [PubMed] [Google Scholar]
- 31.Haraji A., Lassemi E., Motamedi M.H., Alavi M., Adibnejad S. Effect of plasma rich in growth factors on alveolar osteitis. Natl J Maxillofac Surg. 2012;3(1):38–41. doi: 10.4103/0975-5950.102150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aghaloo T.L., Moy P.K., Freymiller E.G. Investigation of platelet-rich plasma in rabbit cranial defects: a pilot study. J Oral Maxillofac Surg. 2002;60(10):1176–1181. doi: 10.1053/joms.2002.34994. [DOI] [PubMed] [Google Scholar]
- 33.Raghoebar G.M., Schortinghuis J., Liem R.S., Ruben J.L., van der Wal J.E., Vissink A. Does platelet-rich plasma promote remodeling of autologous bone grafts used for augmentation of the maxillary sinus floor? Clin Oral Implants Res. 2005;16(3):349–356. doi: 10.1111/j.1600-0501.2005.01115.x. [DOI] [PubMed] [Google Scholar]
- 34.Butterfield K.J., Bennett J., Gronowicz G., Adams D. Effect of platelet-rich plasma with autogenous bone graft for maxillary sinus augmentation in a rabbit model. J Oral Maxillofac Surg. 2005;63(3):370–376. doi: 10.1016/j.joms.2004.07.017. [DOI] [PubMed] [Google Scholar]