Abstract
Introduction
Lung transplantation is the only effective treatment option for many patients with irreversible pulmonary injury, and the demand for lung transplantation is increasing worldwide and expected to continue to outstrip the number of available donors. Regenerative therapy with alveolar epithelial cells (AECs) holds promise as an alternative option to organ transplantation. AECs are usually co-cultured with mouse-derived 3T3 feeder cells, but the use of xenogeneic tissues for regenerative therapy raises safety concerns. Fabrication of AEC sheets under feeder-free conditions would avoid these safety issues. We describe a novel feeder-free method of fabricating AEC sheets that may be suitable for pulmonary regenerative therapy.
Methods
Lung tissues excised from male outbred rats or transgenic rats expressing green fluorescent protein (GFP) were finely minced and dissociated with elastase. The isolated AECs were cultured under four different feeder-free conditions according to whether a rho kinase (ROCK) inhibitor was included in the low-calcium medium (LCM) and whether the tissue culture dish was coated with recombinant laminin-511 E8 fragment (rLN511E8). The expanded cells were cultured on temperature-responsive dishes and subsequently harvested as AEC sheets. Engraftment of GFP-AEC sheets after their transplantation onto a partially resected region of the left lung was assessed in athymic rats.
Results
AECs proliferated and reached confluence when cultured in LCM containing a ROCK inhibitor on tissue culture dishes coated with rLN511E8. When both the ROCK inhibitor and rLN511E8-coated culture dish were used, the number of AECs obtained after 7 days of culture was significantly higher than that in the other three groups. Immunohistochemical analyses revealed that aquaporin-5, surfactant protein (SP)-A, SP-C, SP-D and Axin-2 were expressed by the cultured AECs. AEC sheets were harvested successfully from temperature-responsive culture dishes (by lowering the temperature) when the expanded AECs were cultured for 7 days in LCM + ROCK inhibitor and then for 3 days in LCM + ROCK inhibitor supplemented with 200 mg/L calcium chloride. The AEC sheets were firmly engrafted 7 days after transplantation onto the lung defect and expressed AEC marker proteins.
Conclusions
AEC sheets fabricated under feeder-free conditions retained the features of AECs after transplantation onto the lung in vivo. Further improvement of this technique may allow the bioengineering of alveolar-like tissue for use in pulmonary regenerative therapy.
Keywords: Alveolar epithelial cell, Feeder-free, Cell sheet, Regenerative therapy
Abbreviations: AEC, alveolar epithelial cell; AECI, type I alveolar epithelial cell; AECII, type II alveolar epithelial cell; AEpiCM, alveolar epithelial cell medium; AQP-5, aquaporin-5; Ca2+, ionized calcium; FBS, fetal bovine serum; GFP, green fluorescent protein; HBSS, Hanks' balanced salt solution; HE, hematoxylin and eosin; LCM, medium with a low ionized calcium concentration; PBS, phosphate-buffered saline; rLN511E8, recombinantly expressed laminin-511 E8 fragment; ROCK, rho kinase; SP, surfactant protein
Highlights
-
•
Alveolar epithelial cells were cultured and expanded under feeder-free conditions.
-
•
Alveolar epithelial cell sheets were generated using temperature-responsive dishes.
-
•
Alveolar epithelial cell sheets engrafted after transplantation onto rat lung.
-
•
The sheets retained alveolar epithelial cell characteristics after transplantation.
-
•
These cell sheets potentially could be used for pulmonary regenerative therapy.
1. Introduction
Lung transplantation is the only effective treatment option for many patients with irreversible pulmonary injury, and the demand for lung transplantation is increasing worldwide and expected to continue to outstrip the number of available donors [1]. Regenerative therapy holds great promise as a future alternative option to organ transplantation and would circumvent the limitations of conventional transplantation techniques such as the lack of donor organs, adverse effects due to immunosuppression and organ rejection [2].
Regenerative therapy for lung injury requires the transplantation of bioengineered tissue constructed from appropriate cell types. Gas exchange occurs in pulmonary alveoli, which comprise 90% of the total volume of the lungs [3]. The alveolar epithelium is lined with type I alveolar epithelial cells (AECIs), which are specialized for gas exchange, and type II alveolar epithelial cells (AECIIs), which produce surfactant proteins (SPs) involved in innate immune responses [4]. Although alveolar epithelial cell (AEC) cultures have been established previously, one of their limitations was the unwanted trans-differentiation of AECIIs to AECIs in vitro [5]. The differentiation of AECIIs to AECIs in vivo normally only occurs following injury to the lung and aims to replenish the damaged alveolar epithelium. Initially, AECIIs proliferate to replace the lost cells, although the underlying mechanisms remain only partially understood [6]. Once normal cell numbers are restored, some AECIIs trans-differentiate into AECIs to re-establish the normal alveolar architecture [7]. Recent reports have suggested that Wnt signaling regulates the stemness of AECIIs [[8], [9], [10]].
Approaches to improve the culture of cells in vitro include the use of feeder cells, which secrete various proliferation-promoting factors, and pharmacological agents such as rho kinase (ROCK) inhibitors [11,12]. Although human AECIIs proliferate rapidly when cultured in the presence of mouse-derived 3T3 feeder cells and a ROCK inhibitor, markers of AECIIs become downregulated after the first passage [4]. Furthermore, AECs are difficult to maintain in long-term culture [4]. Importantly, human epithelial cell grafts co-cultured with mouse-derived 3T3 feeder cells are classified by the US Food and Drug Administration as xenogeneic products, which complicates their potential use as a cell therapy or regenerative therapy in the clinical setting. Hence, the establishment of a feeder-free culture system for AECs would facilitate the development of new regenerative therapies for patients with respiratory failure due to irreversible lung injury.
Research is ongoing to optimize the conditions for feeder-free culture of AECs and other cell types. Laminin is a high-molecular-weight protein found in the extracellular matrix, and recombinant laminin-511 isoform, which is a heterotrimer consisting of α5, β1, and γ1 chains, has been shown to improve the long-term culture of human pluripotent stem cells in the absence of feeder cells [13]. Furthermore, recombinant laminin-511 E8 fragment (rLN511E8) also has been reported to support the stable, feeder-free culture of human embryonic stem cells, human induced pluripotent stem cells and ocular epithelial cells [14]. In addition, ionized calcium (Ca2+) is recognized as a differentiation-inducing factor for epithelial cells. For example, the use of culture medium containing a low concentration of Ca2+ (low-Ca2+ medium, LCM) has been demonstrated to enhance the proliferation of human epidermal keratinocytes when cultured in the absence of 3T3 feeder cells [15].
We hypothesized that it would be possible to culture AECs under feeder-free conditions if a suitable combination of pharmacological agents was used. Therefore, the main objectives of this study were to develop a new method of culturing AECs under feeder-free conditions, optimize the fabrication of AEC sheets, and evaluate whether AEC sheets would engraft onto host tissue after transplantation in vivo. We show that AECs can be cultured under feeder-free conditions using LCM containing a ROCK inhibitor and rLN511E8-coated culture dishes. Furthermore, in vivo experiments in athymic rats confirmed that AEC sheets engrafted successfully after transplantation onto a lung defect.
2. Methods
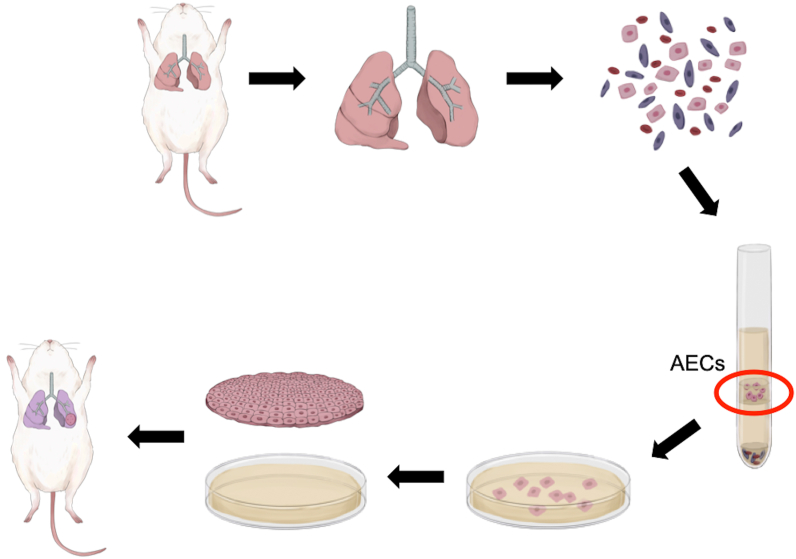
All animal experiments were performed in accordance with the Guidelines of Tokyo Women's Medical University on Animal Use and consistent with the Guide for the Care and Use of Laboratory Animals prepared by the Institute of Laboratory Animal Resources (ILAR). The experimental procedures (isolation and culture of rat AECs followed by fabrication and transplantation of rat AEC sheets) are summarized in Fig. 1.
Fig. 1.
Schematic diagram of the methods used to fabricate and transplant alveolar epithelial cell (AEC) sheets. Lung tissues were carefully excised from male rats and finely minced, and cells were dissociated with elastase. A Percoll gradient was used to separate AECs from other cell types in the cell suspension. The isolated AECs were cultured under feeder-free conditions, and the expanded cells were seeded and cultured on temperature-responsive culture dishes. After subculture, the cells were harvested from the temperature-responsive dish as an AEC sheet by reducing the temperature from 37 °C to 20 °C for 30 min. Each AEC sheet was transplanted onto a partially resected region of the left lung or onto the left gluteal musculature of an athymic rat.
2.1. Isolation of rat AECs
AECs were isolated from 4 to 8 week-old male outbred rats (Slc:SD; Japan SLC Inc., Shizuoka, Japan) or transgenic rats (SD-Tg[CAG-EGFP]; Japan SLC Inc.) expressing green fluorescent protein (GFP) and weighing 100–150 g. The chest wall was incised along the entire length of the sternum and anterior portion of the diaphragm under inhalation anesthesia with isoflurane. The right ventricle and left atrium were cannulated with 20-gauge plastic catheters, and the lungs were perfused with phosphate-buffered saline (PBS; Fujifilm Wako Pure Chemical Corporation, Osaka, Japan) until white in color to ensure that they had been cleared of blood. Lung tissues were carefully excised from the chest cavity and finely minced. Dissociated cells were obtained by gently shaking the lung tissue in Hanks’ balanced salt solution (HBSS; Fujifilm Wako Pure Chemical Corporation) containing 1 mg/mL elastase (Worthington Biochemical Corporation, Lakewood, NJ, USA) at 37 °C for 60 min. After quenching the enzymatic activity with fetal bovine serum (FBS; Thermo Fisher Scientific, Waltham, MA, USA), the digested tissue was filtered through 100-μm and 40-μm cell strainers (Corning Inc., Corning, NY, USA) to collect a cell suspension. After centrifugation at 400×g for 10 min at room temperature, the cell pellet was resuspended in HBSS and layered on a Percoll gradient (1.083 g/mL; Sigma–Aldrich, St. Louis, MO, USA). After centrifugation at 400×g for 30 min at room temperature, the layer of AECs generated on the Percoll gradient was collected.
2.2. Culture of rat AECs
The isolated AECs were suspended in LCM comprising Alveolar Epithelial Cell Medium (AEpiCM; ScienCell, Carlsbad, CA, USA) supplemented with 2% FBS. Primary AECs were cultured under four different feeder-free conditions (see Fig. 2A) according to whether 10 μM Y-27632 (a ROCK inhibitor; Fujifilm Wako Pure Chemical Corporation) was included in the LCM and whether the tissue culture dish was coated with rLN511E8 (iMatrix-511; Matrixome, Osaka, Japan). AECs were seeded at a density of 2.0 × 104 cells/cm2 onto tissue culture dishes (35 mm in diameter) and cultured for 7–10 days at 37 °C in a humidified atmosphere containing 5% CO2. Primary cultured cells were subsequently harvested by treatment with 0.05% trypsin-ethylenediamine tetraacetic acid for 5 min at 37 °C. The expanded cells were seeded at a density of 5.0 × 104 cells/cm2 onto temperature-responsive culture dishes (35 mm in diameter; UpCell, CellSeed Inc., Tokyo, Japan) and cultured for 7–10 days at 37 °C.
Fig. 2.
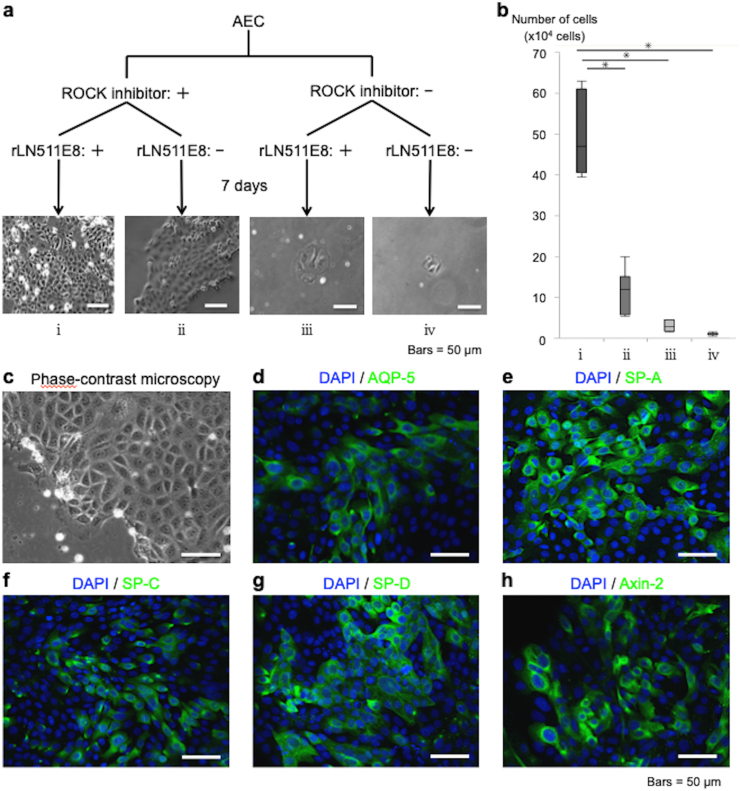
Alveolar epithelial cells (AECs) cultured under feeder-free conditions. (a) Primary AECs were cultured under feeder-free conditions in the absence or presence of a rho kinase (ROCK) inhibitor (10 mM Y-27632) and in the absence or presence of recombinantly expressed laminin-511 E8 fragment (rLN511E8) as a coating on the tissue culture dish (i.e., four experimental groups). The cultured AECs were harvested 7 days later, and the number of cells was counted. (b) Comparison of the number of AECs expanded under each feeder-free condition. The data are displayed as box plots showing the median, interquartile range and range (n = 7). ∗p < 0.05. (c) Phase-contrast microscopy of AECs cultured on an rLN511E8-coated dish in alveolar epithelial cell medium (AEpiCM) containing a ROCK inhibitor. The cultured AECs exhibited a polygonal, cobblestone-like morphology characteristic of epithelial cells. (d–h) Immunohistochemical analyses of AECs cultured on rLN511E8-coated dishes in AEpiCM containing a ROCK inhibitor. The cultured AECs were immunostained using primary antibodies against aquaporin-5 (AQP-5), surfactant protein (SP)-A, SP-C, SP-D and Axin-2.
2.3. Fabrication and transplantation of rat AEC sheets
AECs were cultured on temperature-responsive culture dishes until confluency. In some experiments, the cells were further cultured in medium supplemented with 200 mg/L calcium chloride (to increase the Ca2+ concentration) for 3 days. A support membrane (Cell Shifter; Cell Seed Inc.) was placed into the culture dish, and the culture medium was aspirated. Following incubation at 20 °C for 30 min, the membrane was slowly peeled from the periphery of the dish to harvest the AECs as an intact sheet.
The rat GFP-AEC sheets were transplanted onto the lungs of male athymic rats (F344/NJcl-rnu/rnu; CLEA Japan Inc., Tokyo, Japan) under isoflurane inhalation anesthesia and mechanical ventilation. The rat was placed in the right lateral decubitus position, and a left lateral thoracotomy was performed. A 5 mm lung incision with 3 mm depth was made in the left lung using scissors, and three GFP-AEC sheets were transplanted onto the incised region of the left lung. After the GFP-AEC sheets were transplanted, no air leakage from lung was confirmed and the wound closed without chest tube drainage. Seven days later, the rat was euthanized by exsanguination under isoflurane anesthesia, and the left lung including the transplanted cell sheets was resected for histological analyses.
2.4. Histology and immunohistochemistry
For immunohistochemistry, cultured AECs were fixed in 4% paraformaldehyde and blocked with 5% FBS in PBS for 60 min. Then, the cells were incubated with anti-SP-A rabbit polyclonal antibody (1:300 dilution; bs-10265R; Bioss, Woburn, MA, USA), anti-SP-C rabbit polyclonal antibody (1:300 dilution; bs-10067R; Bioss), anti-SP-D rabbit polyclonal antibody (1:300 dilution; bs-1583R; Bioss), anti-aquaporin-5 (AQP-5) rabbit polyclonal antibody (1:300 dilution; bs-1554R; Bioss), anti-Axin-2 rabbit polyclonal antibody (1:300 dilution; GTX31822; Genetex, Irvine, CA, USA) or anti-E-cadherin rabbit polyclonal antibody (1:300 dilution; GTX477; Genetex) at 4 °C overnight. On the following day, the AECs were incubated with Alexa Fluor 488 goat anti-rabbit IgG antibody (1:500; A11008; Invitrogen, Carlsbad, CA, USA) as the secondary antibody at 20–30 °C for 60 min.
The harvested AEC sheets were fixed in 4% paraformaldehyde and either processed into 5-μm-thick paraffin wax-embedded sections (for cross-sectional observations) or not further processed (planar observations). The sections were treated with anti-SP-A rabbit polyclonal antibody (1:200 dilution; bs-10265R; Bioss), anti-SP-C rabbit polyclonal antibody (1:100 dilution; bs-10067R; Bioss), anti-SP-D rabbit polyclonal antibody (1:200 dilution; bs-1583R; Bioss), anti-AQP-5 rabbit polyclonal antibody (1:200 dilution; bs-1554R; Bioss), anti-Axin-2 rabbit polyclonal antibody (1:100 dilution; GTX31822; Genetex) or anti-E-cadherin rabbit polyclonal antibody (1:100 dilution; GTX477; Genetex) at 4 °C overnight. Subsequently, the sections were incubated with Alexa Fluor 488 goat anti-rabbit IgG antibody (1:500 dilution; A11008; Invitrogen) as the secondary antibody at 20–30 °C for 30 min. In addition, cross-sectional observations of the cell sheets were made after staining with hematoxylin and eosin (HE) using conventional methods.
Resected lung tissues were fixed in 4% paraformaldehyde and routinely processed into 5-μm-thick paraffin-embedded sections. For histology, HE staining was performed by conventional methods. For immunohistochemistry, the tissues were blocked with 5% FBS in PBS for 60 min and then incubated with anti-GFP rabbit polyclonal antibody (1:100 dilution; ab290; Abcam, Cambridge, UK), anti-SP-A rabbit polyclonal antibody (1:100 dilution; bs-10265R; Bioss), anti-SP-C rabbit polyclonal antibody (1:100 dilution; bs-10067R; Bioss), anti-SP-D rabbit polyclonal antibody (1:100 dilution; bs-1583R; Bioss), anti-AQP-5 rabbit polyclonal antibody (1:100 dilution; bs-1554R; Bioss) or anti-Axin-2 rabbit polyclonal antibody (1:100 dilution; GTX31822; Genetex) at 4 °C overnight. Then, the tissues were incubated with Opal Polymer Anti-Rabbit HRP (1:5 dilution) and either the Opal 520 (1:100 dilution) or Opal 570 (1:100 dilution) fluorophore (NEL840001KT; Akoya Biosciences Inc., Marlborough, MA, USA) at 20–30 °C for 10 min. Subsequently, microwave treatment was used to remove the primary and secondary antibodies and non-specific staining and to reduce tissue autofluorescence. Another round of staining for additional targets was performed after microwave treatment without the risk of antibody cross-reactivity. The primary antibody was omitted in negative control experiments. Tissue sections stained using immunohistochemical methods were observed using a confocal laser scanning microscope (FluoView FV1200; Olympus Corporation, Tokyo, Japan).
2.5. Statistical analysis
JMP Pro 14.0.0 (SAS Institute, Cary, NC, USA) was used for data analysis. Cell counts are presented as boxplots (showing the median, interquartile range and range) and were compared between groups using the Steel–Dwass test. A p-value less than 0.05 (p < 0.05) was considered statistically significant.
3. Results
3.1. Optimization of the conditions for feeder-free culture of rat AECs
Primary AECs were cultured in AEpiCM under feeder-free conditions in the absence or presence of a ROCK inhibitor and in the absence or presence of rLN511E8 as a coating on the tissue culture dish (i.e., four experimental groups; see Fig. 2a). When both the ROCK inhibitor and rLN511E8-coated culture dish were used, the number of AECs obtained after 7 days of culture was significantly higher than that in the other three groups (Fig. 2b). Phase-contrast microscopy demonstrated that the cultured AECs exhibited a polygonal, cobblestone-like morphology characteristic of epithelial cells (Fig.2c). Furthermore, immunohistochemical analyses (Fig. 2d–h) revealed that the cultured AECs expressed AQP-5 (a marker of AECIs), SP-A, SP-C, SP-D (markers of AECIIs) and Axin-2 (a marker of a subpopulation of AECIIs capable of trans-differentiation into AECIs) [8,16,17].
3.2. Optimization of the conditions for fabrication of rat AEC sheets
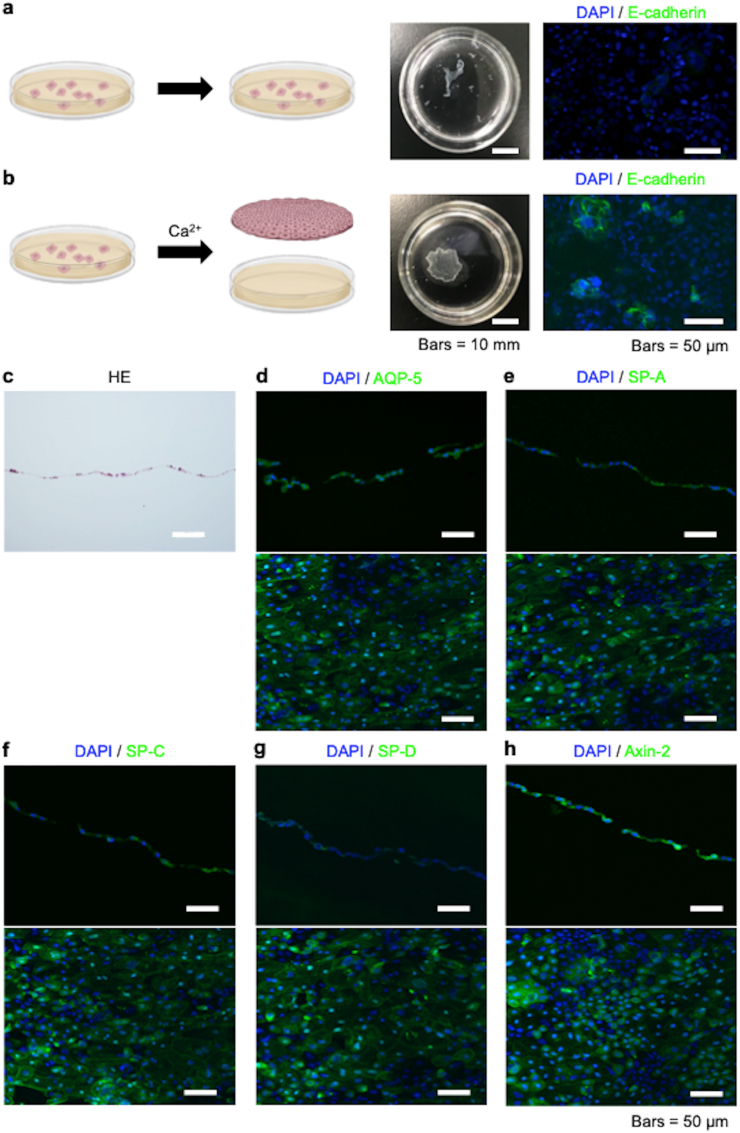
In our initial attempts to fabricate rat AEC sheets, AECs were subcultured on temperature-responsive culture dishes in AEpiCM containing a ROCK inhibitor. However, after 7 days of subculture, confluent AECs could not be harvested as a cell sheet from the temperature-responsive culture dish when the temperature was reduced from 37 °C to 20 °C for 30 min (Fig. 3a). Therefore, we altered the protocol by adding an additional stage. First, the AECs were subcultured for 7 days as described above and confirmed to be confluent. Then, the AECs were cultured for a further 3 days in AEpiCM containing a ROCK inhibitor and 200 mg/L calcium chloride. Using this approach, it was possible to harvest the AECs from the temperature-responsive culture dish as an intact cell sheet by reducing the temperature from 37 °C to 20 °C for 30 min (Fig. 3b). Staining with HE showed that the AEC sheet was composed of a single layer of cells (Fig. 3c). Immunohistochemical analyses revealed that the cells expressed E-cadherin when cultured in the presence of calcium chloride but not when cultured in the absence of calcium chloride (Fig. 3a and b). Additionally, the AEC sheets expressed AQP-5, SP-A, SP-C, SP-D and Axin-2 (Fig. 3d–h).
Fig. 3.
Fabrication of alveolar epithelial cell (AEC) sheets. (a) AECs were subcultured on 35-mm-diameter temperature-responsive culture dishes in alveolar epithelial cell medium (AEpiCM) containing a rho kinase (ROCK) inhibitor (10 mM Y-27632). However, after 7 days of subculture, confluent AECs could not be harvested as a cell sheet when the temperature was reduced from 37 °C to 20 °C for 30 min. Immunohistochemical analysis revealed that cells cultured under these conditions did not express E-cadherin. (b) AECs were subcultured on 35-mm-diameter temperature-responsive culture dishes in AEpiCM containing a ROCK inhibitor, and the cells were confirmed to be confluent after 7 days. Then, the AECs were cultured for a further 3 days in AEpiCM containing a ROCK inhibitor and supplemented with 200 mg/L calcium chloride. Under these conditions, the AECs could be harvested as a cell sheet by reducing the temperature from 37 °C to 20 °C for 30 min. Immunohistochemical analysis revealed that cells cultured using this protocol expressed E-cadherin. (c–h) Hematoxylin and eosin (HE) staining and immunofluorescence staining of AEC sheets fabricated under the feeder-free conditions described in (b). Positive staining for aquaporin-5 (AQP-5), surfactant protein (SP)-A, SP-C, SP-D and Axin-2 showed that the cultured cells were AECs. Cross-sectional (upper panel) and planar (lower panel) views were obtained for the immunohistochemical analyses.
3.3. Transplantation of rat AEC sheets
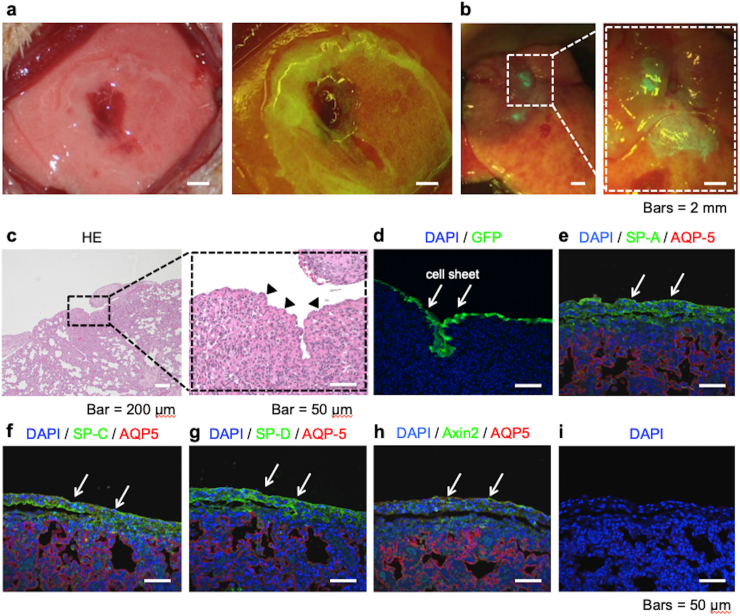
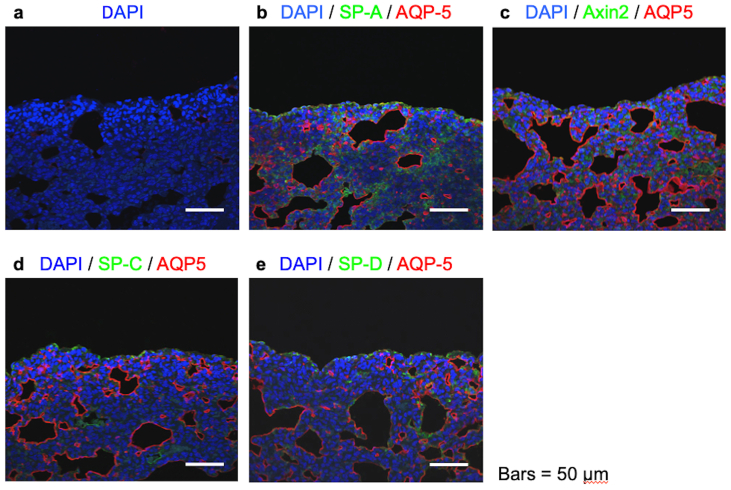
Seven days after the transplantation of three GFP-AEC sheets onto a partially resected region of the left lung of an athymic rat, illumination of the transplantation region with fluorescent light (488 nm) demonstrated the presence of GFP-positive cells that were derived from the transplanted cell sheets (Fig. 4a and b). Staining with HE revealed that the transplanted AEC sheets were tightly adhered to the lung tissue with no air spaces found between the cell sheets and pulmonary parenchyma (Fig. 4c). Immunohistochemical analyses revealed that cells on the surface of the lung expressed GFP and that the same region also expressed AQP-5, SP-A, SP-C, SP-D and Axin-2 (Fig. 4d–h). Positive staining was not observed when the primary antibodies were omitted in negative control experiments (Fig. 4i). Immunohistochemical analyses of lung tissues without transplanted GFP-AEC sheets are shown in Supplemental Fig. 1.
Fig. 4.
Analyses of green fluorescent protein (GFP)-expressing alveolar epithelial cell (AEC) sheets (GFP-AEC sheets) after transplantation onto the lung. (a) Three GFP-AEC sheets were transplanted onto a partially resected area of the left lung of an athymic rat. (b) Seven days later, illumination of the transplantation region with fluorescent light revealed the presence of GFP-positive cells, which were derived from the transplanted cell sheets. (c–i) Histological and immunohistochemical analyses of lung tissues and transplanted GFP-AEC sheets. The lung tissues and transplanted GFP-AEC sheets were subjected to HE staining or immunofluorescence staining for GFP, aquaporin-5 (AQP-5), surfactant protein (SP)-A, SP-C, SP-D or Axin-2. Staining with HE demonstrated that the transplanted AEC sheets were tightly adhered to the lung tissue, and no air spaces were found between the cell sheets and the pulmonary parenchyma (arrowheads). The immunohistochemical analyses revealed that cells on the lung surface expressed GFP (white arrows), and the same region also expressed AQP-5, SP-A, SP-C, SP-D and Axin-2. No staining was observed when the primary antibodies were omitted in negative control experiments.
4. Discussion
The present study has described the successful fabrication of AEC sheets under feeder-free conditions. Furthermore, the fabricated cell sheets continued to express AEC-specific proteins after transplantation onto the lung. We propose that the AEC sheets bioengineered using our technique may have potential for development into a new regenerative therapy for lung injury.
One of the challenges facing researchers striving to develop regenerative therapies for respiratory diseases is the structural complexity of the pulmonary system, which is highly specialized for gas exchange. Previously, we reported that temperature-responsive culture dishes could be used to fabricate transplantable cell sheets without the need for additional synthetic or biological materials such as scaffolds [18,19]. Various cell types can attach to, proliferate on and spread along these unique surfaces when cultured under standard conditions at 37 °C, with cell growth comparable to that seen on ordinary tissue culture dishes. However, a major advantage of temperature-responsive dishes is that cultured cells together with their extracellular matrix can be harvested non-invasively as intact sheets (without the need for proteolytic enzymes) simply by reducing the temperature to 20 °C. This non-destructive method of harvesting maintains the cell-to-cell junctions and extracellular matrix proteins in the cell sheet construct [18,19]. Notably, cell sheet-based regenerative therapies for various organs have been applied in the clinical setting [[20], [21], [22]]. Furthermore, cell sheet-based therapy was reported to be superior to cell infusion-based therapy in terms of cell viability, number of engrafted cells and transplanted cell function [23]. Our previous research in the field of respiratory medicine showed that autologous dermal fibroblast sheets harvested from temperature-responsive culture dishes could be used as a pleural substitute to seal intraoperative air leaks in the lungs in both animal models and clinical studies [[24], [25], [26], [27]]. The AEC sheet described in the present study was fabricated using cell sheet-based tissue engineering. These results indicate that further development of this technique will allow AEC sheets to be used clinically to promote lung regeneration and thereby treat pulmonary disorders that currently require lung transplantation.
Because safety is of paramount importance, major concerns have been raised about regenerative therapies based on cells derived from different species. Therefore, the objectives of this study were to develop a method for feeder-free cultivation of AECs and to find suitable conditions for the fabrication of AEC sheets for transplantation in vivo. Feeder-free culture on an rLN511E8-coated dish in LCM containing a ROCK inhibitor promoted rapid AEC growth. In the culture method of this study, AECII did not completely trans-differentiate into AECI in vitro and remained after transplantation, possibly preserving the characteristics of both AECI and AECII. ROCK-induced Rho signaling leads to the phosphorylation and activation of non-muscle myosin light chain II and hence muscle contraction [28,29]. Non-muscle myosin light chain II is a key modulator of cell behavior that has been implicated in cell migration, proliferation and differentiation [30,31]. ROCK inhibitors have been widely used in the culture of several cell types because of their ability to suppress apoptosis, and pharmacological inhibition of ROCK has been shown to enhance the proliferation of keratinocytes from foreskin, ectocervical and vaginal tissues [32]. Ca2+ induces calmodulin-mediated signaling that regulates the activity of the myosin light chain [33]. Ca2+ is also recognized as a trans-differentiation-inducing factor for epithelial cells and is essential for cell–cell adhesion via E-cadherin [34,35]. LCM prevents the differentiation of epithelial cells and promotes cell expansion [36]. Remarkably, the combination of LCM and a ROCK inhibitor has been reported to cause a 1012-fold increase in epithelial cell numbers [37]. rLN511E8 strongly supports the long-term, feeder-free culture of several cell types. For example, a previous study utilized a feeder-free culture system and recombinant laminin-coated dishes to generate human pluripotent stem cells that could be differentiated into retinal pigment epithelial cells and corneal limbal epithelial stem cells, and the authors developed cryopreservation protocols for all three cell types [14]. In the present study, AECs were also able to grow rapidly on rLN511E8-coated dishes when cultured in LCM containing a ROCK inhibitor under feeder-free conditions. This new method of culturing AECs under feeder-free conditions is straightforward and would circumvent safety issues regarding the use of xenogeneic feeder cells if used as the basis of a regenerative therapy.
AECs cultured in LCM containing a ROCK inhibitor could not be harvested as a cell sheet from the temperature-responsive culture dish due to insufficient cell–cell adhesion. Since Ca2+ is essential for cell–cell adhesion via E-cadherin, we altered the protocol so that the confluent AECs were further cultured in LCM supplemented with Ca2+. AECs cultured in LCM containing additional Ca2+ exhibited expression of E-cadherin (unlike cells not cultured in Ca2+-supplemented medium) and could be harvested as a cell sheet, likely due to the strengthening of cell–cell adhesions via E-cadherin. A potential disadvantage of this approach is that Ca2+ is a differentiation-inducing factor for epithelial cells. However, immunohistochemical analyses showed that the cell sheets harvested after culture in Ca2+-supplemented medium for 3 days exhibited localized expression of AEC-specific proteins.
GFP-AEC sheets transplanted onto rat lung continued to express AQP-5 (a marker of AECIs), SP-A, SP-C, SP-D (markers of AECIIs) and Axin-2 (a marker of a subpopulation of AECIIs capable of trans-differentiation into AECIs), which indicates that the cell sheets retained the characteristics of AECs after transplantation. There is a paucity of data regarding the fate of AECs after transplantation onto the lung in vivo. Lung fibroblasts reside beneath the alveolar epithelium and make contact with AECs through gaps in the basement membrane [38]. Pulmonary fibroblasts secrete important soluble factors that have been reported to limit the trans-differentiation and promote the proliferation of AECs [39]. In addition, trans-differentiation is slowed when AECs are grown under air-liquid interface conditions in vitro [4]. Therefore, environmental factors such as the presence of pulmonary fibroblasts and an air-liquid interface likely inhibit the differentiation of AECs in vivo.
This study has some limitations. First, AEC sheet engraftment was only evaluated 7 days after transplantation in vivo, so it remains unclear whether the transplanted cells would survive as AECs for a longer period of time. Whether AEC sheets are maintained long-term after transplantation will require additional evaluation. Second, although engraftment of the AEC sheet onto the lung was demonstrated from a structural perspective, the functionality of the AEC sheet after transplantation was not assessed. Further research will be needed to establish whether transplanted AEC sheets exert functional effects such as improvements in pulmonary function or suppression of lung disease progression. In addition, although feeder-free culture on an rLN511E8-coated dish in LCM containing a ROCK inhibitor promoted rapid AEC growth, the effects of each on AECs and differentiation have not been investigated. Pharmacological aspects of the effects on AECs will be needed to investigate.
5. Conclusions
We have bioengineered AEC sheets under feeder-free conditions that retain the morphological features of AECs after transplantation onto the lung in vivo. The ultimate objective of our research is to develop a regenerative therapy that can reconstruct the alveolar structure in an injured lung, and the research described in this study is part of that process. One of our future aims is to fabricate three-dimensional lung tissue using AEC sheets and enlarge it step-by-step in vitro. Furthermore, we will investigate whether the transplantation of AEC sheets onto decellularized pulmonary tissue would generate lung constructs that could be used as a regenerative therapy.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Tatsuya Shimizu is a shareholder of CellSeed Inc. Tokyo Women's Medical UniversityTokyo Women's Medical University received research funding from CellSeed Inc. The other authors have no competing interests to declare.
Acknowledgements
This work was supported by JSPS KAKENHI grant number JP21K08913. The authors thank Dr. Tamami Isaka (Department of Thoracic Surgery, Tokyo Women's Medical University, Tokyo, Japan) for supporting this study. We also thank OXMEDCOMMS (www.oxmedcomms.com) for help with the writing of this manuscript.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.reth.2022.01.005.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Supplemental Fig. 1.
Immunohistochemical analyses of lung tissues without transplanted green fluorescent protein (GFP)-expressing alveolar epithelial cell (AEC) sheets. (a) No staining was observed when the primary antibodies were omitted in negative control experiments. (b–e) Immunofluorescence staining of lung tissues for aquaporin-5 (AQP-5), surfactant protein (SP)-A, SP-C, SP-D and Axin-2.
References
- 1.Neizer H., Singh G.B., Gupta S., Singh S.K. Addressing donor-organ shortages using extended criteria in lung transplantation. Ann Cardiothorac Surg. 2020;9:49–50. doi: 10.21037/acs.2019.10.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edgar L., Pu T., Porter B., Aziz J.M., La Pointe C., Asthana A., et al. Regenerative medicine, organ bioengineering and transplantation. Br J Surg. 2020;107:793–800. doi: 10.1002/bjs.11686. [DOI] [PubMed] [Google Scholar]
- 3.Knudsen L., Ochs M. The micromechanics of lung alveoli: structure and function of surfactant and tissue components. Histochem Cell Biol. 2018;150:661–676. doi: 10.1007/s00418-018-1747-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qian Z., Travanty E.A., Oko L., Edeen K., Berglund A., Wang J., et al. Innate immune response of human alveolar type II cells infected with severe acute respiratory syndrome-coronavirus. Am J Respir Cell Mol Biol. 2013;48:742–748. doi: 10.1165/rcmb.2012-0339OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bove P.F., Dang H., Cheluvaraju C., Jones L.C., Liu X., O'Neal W.K., et al. Breaking the in vitro alveolar type II cell proliferation barrier while retaining ion transport properties. Am J Respir Cell Mol Biol. 2014;50:767–776. doi: 10.1165/rcmb.2013-0071OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanjore H., Degryse A.L., Crossno P.F., Xu X.C., McConaha M.E., Jones B.R., et al. β-catenin in the alveolar epithelium protects from lung fibrosis after intratracheal bleomycin. Am J Respir Crit Care Med. 2013;187:630–639. doi: 10.1164/rccm.201205-0972OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Desai T.J., Brownfield D.G., Krasnow M.A. Alveolar progenitor and stem cells in lung development, renewal and cancer. Nature. 2014;507:190–194. doi: 10.1038/nature12930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nabhan A.N., Brownfield D.G., Harbury P.B., Krasnow M.A., Desai T.J. Single-cell Wnt signaling niches maintain stemness of alveolar type 2 cells. Science. 2018;359:1118–1123. doi: 10.1126/science.aam6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zacharias W.J., Frank D.B., Zepp J.A., Morley M.P., Alkhaleel F.A., Kong J., et al. Regeneration of the lung alveolus by an evolutionarily conserved epithelial progenitor. Nature. 2018;555:251–255. doi: 10.1038/nature25786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hogan B. Stemming lung disease? N Engl J Med. 2018;378:2439–2440. doi: 10.1056/NEJMcibr1803540. [DOI] [PubMed] [Google Scholar]
- 11.Llames S., García-Pérez E., Meana Á., Larcher F., del Río M. Feeder layer cell actions and applications. Tissue Eng B Rev. 2015;21:345–353. doi: 10.1089/ten.teb.2014.0547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aslanova A., Takagi R., Yamato M., Okano T., Yamamoto M. A chemically defined culture medium containing Rho kinase inhibitor Y-27632 for the fabrication of stratified squamous epithelial cell grafts. Biochem Biophys Res Commun. 2015;460:123–129. doi: 10.1016/j.bbrc.2015.02.120. [DOI] [PubMed] [Google Scholar]
- 13.Rodin S., Domogatskaya A., Ström S., Hansson E.M., Chien K.R., Inzunza J., et al. Long-term self-renewal of human pluripotent stem cells on human recombinant laminin-511. Nat Biotechnol. 2010;28:611–615. doi: 10.1038/nbt.1620. [DOI] [PubMed] [Google Scholar]
- 14.Hongisto H., Ilmarinen T., Vattulainen M., Mikhailova A., Skottman H. Xeno- and feeder-free differentiation of human pluripotent stem cells to two distinct ocular epithelial cell types using simple modifications of one method. Stem Cell Res Ther. 2017;8:291. doi: 10.1186/s13287-017-0738-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boyce S.T., Ham R.G. Calcium-regulated differentiation of normal human epidermal keratinocytes in chemically defined clonal culture and serum-free serial culture. J Invest Dermatol. 1983;81:33s–40s. doi: 10.1111/1523-1747.ep12540422. [DOI] [PubMed] [Google Scholar]
- 16.Beers M.F., Moodley Y. When is an alveolar type 2 cell an alveolar type 2 cell? A conundrum for lung stem cell biology and regenerative medicine. Am J Respir Cell Mol Biol. 2017;57:18–27. doi: 10.1165/rcmb.2016-0426PS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flodby P., Li C., Liu Y., Wang H., Rieger M.E., Minoo P., et al. Cell-specific expression of aquaporin-5 (Aqp5) in alveolar epithelium is directed by GATA6/Sp1 via histone acetylation. Sci Rep. 2017;7:3473. doi: 10.1038/s41598-017-03152-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shimizu T., Yamato M., Isoi Y., Akutsu T., Setomaru T., Abe K., et al. Fabrication of pulsatile cardiac tissue grafts using a novel 3-dimensional cell sheet manipulation technique and temperature-responsive cell culture surfaces. Circ Res. 2002;90:e40. doi: 10.1161/hh0302.105722. [DOI] [PubMed] [Google Scholar]
- 19.Yamato M., Okano T. Cell sheet engineering. Mater Today. 2004;7:42–47. [Google Scholar]
- 20.Nishida K., Yamato M., Hayashida Y., Watanabe K., Yamamoto K., Adachi E., et al. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N Engl J Med. 2004;351:1187–1196. doi: 10.1056/NEJMoa040455. [DOI] [PubMed] [Google Scholar]
- 21.Ohki T., Yamato M., Ota M., Takagi R., Murakami D., Kondo M., et al. Prevention of esophageal stricture after endoscopic submucosal dissection using tissue-engineered cell sheets. Gastroenterology. 2012;143:582–588.e2. doi: 10.1053/j.gastro.2012.04.050. [DOI] [PubMed] [Google Scholar]
- 22.Iwata T., Yamato M., Washio K., Yoshida T., Tsumanuma Y., Yamada A., et al. Periodontal regeneration with autologous periodontal ligament-derived cell sheets – a safety and efficacy study in ten patients. Regen Ther. 2018;9:38–44. doi: 10.1016/j.reth.2018.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sekine H., Shimizu T., Dobashi I., Matsuura K., Hagiwara N., Takahashi M., et al. Cardiac cell sheet transplantation improves damaged heart function via superior cell survival in comparison with dissociated cell injection. Tissue Eng. 2011;17:2973–2980. doi: 10.1089/ten.tea.2010.0659. [DOI] [PubMed] [Google Scholar]
- 24.Kanzaki M., Takagi R., Washio K., Kokubo M., Yamato M. Bio-artificial pleura using an autologous dermal fibroblast sheet. NPJ Regen Med. 2017;2:26. doi: 10.1038/s41536-017-0031-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanzaki M., Takagi R., Washio K., Kokubo M., Mitsuboshi S., Isaka T., et al. Bio-artificial pleura using autologous dermal fibroblast sheets to mitigate air leaks during thoracoscopic lung resection. NPJ Regen Med. 2021;6:2. doi: 10.1038/s41536-020-00113-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanzaki M., Yamato M., Yang J., Sekine H., Kohno C., Takagi R., et al. Dynamic sealing of lung air leaks by the transplantation of tissue engineered cell sheets. Biomaterials. 2007;28:4294–4302. doi: 10.1016/j.biomaterials.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 27.Kanzaki M., Yamato M., Yang J., Sekine H., Takagi R., Isaka T., et al. Functional closure of visceral pleural defects by autologous tissue engineered cell sheets. Eur J Cardio Thorac Surg. 2008;34:864–869. doi: 10.1016/j.ejcts.2008.05.048. [DOI] [PubMed] [Google Scholar]
- 28.Wilkinson S., Paterson H.F., Marshall C.J. Cdc42-MRCK and Rho-ROCK signalling cooperate in myosin phosphorylation and cell invasion. Nat Cell Biol. 2005;7:255–261. doi: 10.1038/ncb1230. [DOI] [PubMed] [Google Scholar]
- 29.Schwartz M. Rho signalling at a glance. J Cell Sci. 2004;117:5457–5458. doi: 10.1242/jcs.01582. [DOI] [PubMed] [Google Scholar]
- 30.Nguyen-Ngoc K.V., Silvestri V.L., Georgess D., Fairchild A.N., Ewald A.J. Mosaic loss of non-muscle myosin IIA and IIB is sufficient to induce mammary epithelial proliferation. J Cell Sci. 2017;130:3213–3221. doi: 10.1242/jcs.208546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boraas L.C., Pineda E.T., Ahsan T. Actin and myosin II modulate differentiation of pluripotent stem cells. PLoS One. 2018;13 doi: 10.1371/journal.pone.0195588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chapman S., Liu X., Meyers C., Schlegel R., McBride A.A. Human keratinocytes are efficiently immortallyzed by a Rho kinase inhibitor. J Clin Invest. 2010;120:2619–2626. doi: 10.1172/JCI42297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scholey J.M., Taylor K.A., Kendrick-Jones J. Regulation of non-muscle myosin assembly by calmodulin-dependent light chain kinase. Nature. 1980;287:233–235. doi: 10.1038/287233a0. [DOI] [PubMed] [Google Scholar]
- 34.Ma X.L., Liu H.Q. Effect of calcium on the proliferation and differentiation of murine corneal epithelial cells in vitro. Int J Ophthalmol. 2011;4:247–249. doi: 10.3980/j.issn.2222-3959.2011.03.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.D'Souza S.J., Pajak A., Balazsi K., Dagnino L. Ca2+ and BMP-6 signaling regulate E2F during epidermal keratinocyte differentiation. J Biol Chem. 2011;276:23531–23538. doi: 10.1074/jbc.M100780200. [DOI] [PubMed] [Google Scholar]
- 36.Peehl D.M., Stamey T.A. Serum-free growth of adult human prostatic epithelial cells. Vitro Cell Dev Biol. 1986;22:82–90. doi: 10.1007/BF02623537. [DOI] [PubMed] [Google Scholar]
- 37.Zhang C., Lee H.J., Shrivastava A., Wang R., McQuiston T.J., Challberg S.S., et al. Long-term in vitro expansion of epithelial stem cells enabled by pharmacological inhibition of PAK1-ROCK-myosin II and TGF-beta signaling. Cell Rep. 2018;25:598–610. doi: 10.1016/j.celrep.2018.09.072. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sirianni F.E., Chu F.S., Walker D.C. Human alveolar wall fibroblasts directly link epithelial type 2 cells to capillary endothelium. Am J Respir Crit Care Med. 2003;168:1532–1537. doi: 10.1164/rccm.200303-371OC. [DOI] [PubMed] [Google Scholar]
- 39.Ushakumary M.G., Riccetti M., Perl A.T. Resident interstitial lung fibroblasts and their role in alveolar stem cell niche development, homeostasis, injury, and regeneration. Stem Cells Transl Med. 2021;10:1021–1032. doi: 10.1002/sctm.20-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]