Abstract
Introduction
We conducted a systematic review and meta‐analysis of the cognitive effects of coronavirus disease 2019 (COVID‐19) in adults with no prior history of cognitive impairment.
Methods
Searches in Medline/Web of Science/Embase from January 1, 2020, to December 13, 2021, were performed following Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines. A meta‐analysis of the Montreal Cognitive Assessment (MoCA) total score comparing recovered COVID‐19 and healthy controls was performed.
Results
Oof 6202 articles, 27 studies with 2049 individuals were included (mean age = 56.05 years, evaluation time ranged from the acute phase to 7 months post‐infection). Impairment in executive functions, attention, and memory were found in post‐COVID‐19 patients. The meta‐analysis was performed with a subgroup of 290 individuals and showed a difference in MoCA score between post‐COVID‐19 patients versus controls (mean difference = −0.94, 95% confidence interval [CI] −1.59, −0.29; P = .0049).
Discussion
Patients recovered from COVID‐19 have lower general cognition compared to healthy controls up to 7 months post‐infection.
Keywords: attention, cognition, cognitive dysfunction, COVID‐19, executive functions, neuropsychological test, SARS‐CoV‐2 coronavirus
1. INTRODUCTION
With an increasing number of individuals recovering from severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection, there is an urgent need to study the medium‐ and long‐term consequences of the disease. Growing evidence suggests that some patients exhibit symptoms such as fatigue, “brain fog,” or cognitive complaints after the acute infection stage, commonly referred to as “Long COVID.” 1 A 6‐month study using multidimensional data from the medical records of 73,435 coronavirus disease 2019 (COVID‐19) patients showed that, after the first 30 days of illness, individuals have an increased risk of death, higher health resource utilization, and an increased burden from neurocognitive disorders. 1 Indeed, evidence from previous epidemics shows that subsequent neurological and, particularly, cognitive complications can occur, such as in the severe influenza epidemic from 1918 to 1921 (also known as the Spanish flu). 2 More recently, cases of encephalitis, sensory impairment, coma, and severe neurological damage were reported during the Middle East respiratory syndrome coronavirus (MERS‐CoV) outbreak in 2012 3 and vascular or inflammatory damage of the brain and central nervous system in people affected by the severe acute respiratory syndrome coronavirus (SARS‐CoV) outbreak in 2003. 4
Cognitive dysfunction has a significant impact on functionality and quality of life. 5 Given the high incidence of COVID‐19 and the associated economic, health, and social burden of the epidemic, studying its occurrence and underlying mechanisms is crucial. In the current systematic review, we assess whether there is an increased occurrence of cognitive deficits in adult patients with COVID‐19 who previously had no cognitive impairment.
2. MATERIALS AND METHODS
The protocol of the present study was registered in the International Prospective Register of Systematic Reviews (PROSPERO) (registration number CRD42021243026). This systematic review was reported following the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) recommendations. 6
2.1. Eligibility criteria
The PICOS (Population, Intervention/issue of interest, Comparison, Outcome, and Study design) method was used. 7 The population included COVID‐19 patients with no previous cognitive impairment. The intervention/exposure included being ill with COVID‐19 disease (confirmed by real time‐polymerase chain reaction (RT‐PCR)/nasopharyngeal swabs). The comparison group was specified as healthy controls with no history of COVID‐19 infection or patients enrolled pre‐pandemic. Outcomes included neuropsychological test performance (either during the acute phase of COVID‐19 or after recovery).
The eligibility criteria were as follows: (1) studies including adults with no prior history of cognitive impairment who had been diagnosed with COVID‐19; (2) studies reporting neuropsychological outcomes after COVID‐19 disease; and (3) studies published in English, Spanish, or Portuguese. The search was conducted to identify articles published since the start of the COVID‐19 pandemic; therefore, the search dates ranged from January 1, 2020 to December 13, 2021. We excluded: (1) studies including patients with a history of dementia, mild cognitive impairment, or subjective cognitive impairment; (2) editorials/commentaries, duplicate publications, non–peer‐reviewed publications/gray literature, and review articles; (3) pediatric studies (age <18), and (4) animal or pre‐clinical studies.
2.2. Search strategy
A systematic search of the literature was performed on March 19, 2021 in three electronic databases: Medline, Web of Science, and Embase. The search terms used were devised by an expert group of neurologists, epidemiologists, and neuropsychologists, and included the following keywords: COVID‐19, Coronavirus, COVID19, SARS‐CoV‐2, 2019‐n‐CoV, pandemic, cognition, Cognitive, Memory, Major cognitive disorder, mild cognitive impairment, mild neurocognitive disorder, MCI, cognitive decline, cognitive deficit, major neurocognitive disorder, cognitive impairment, memory impairment, MMSE, MoCA, neuropsychology, neuropsychological impact, executive function, attention deficit, language, visuospatial, dysexecutive syndrome, orientation, concentration, verbal fluency, and processing speed.
Reference lists of publications were also screened to identify additional articles.
2.3. Study inclusion and exclusion criteria
Two reviewers independently screened the titles and abstracts according to the eligibility criteria. Disagreements were discussed with a third reviewer and subsequently resolved via consensus (Figure 1).
FIGURE 1.
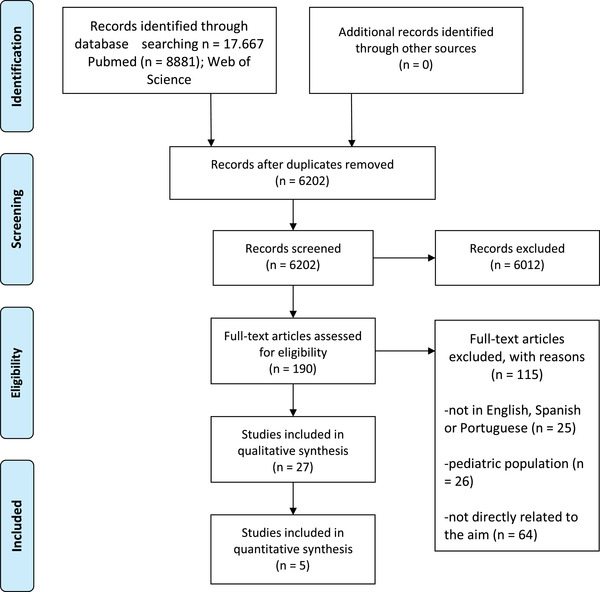
PRISMA flow chart describing the screening and selection of articles
2.4. Data extraction and synthesis and risk of bias
The following data categories were collected when available: study design, sample size, country, patient demographics, population setting, time of assessment related to COVID‐19 infection, cognitive testing instruments, neuropsychological findings, and COVID‐19 disease severity calculated using the World Health Organization (WHO) scale: WHO/2019‐nCoV/clinical/2020.5. 8 One of the reviewers performed the data extraction and the other reviewer assessed the accuracy of the extracted data. We performed a meta‐analysis on articles (minimum n = 3) that included the same outcome (eg, the same neuropsychological test).
2.5. Statistical analysis and assessment of bias
A meta‐analysis was conducted based on the neuropsychological test total scores between individuals who had versus those who did not have COVID‐19. All data were analyzed using R v4.0.5 (March 31, 2021) and the meta and dmetar packages. Heterogeneity was measured through Higgin & Thompson I 2 and DerSimonian‐Laird estimator for tau2 statistics and tested with Cochran Q test. For pooling effect sizes and the estimation of the overall effect size of the studies, we applied a random‐effects model approach. As a summary measure, we calculated the mean difference (MD) between groups. We defined a statistical significance level of P < .05 (two‐sided), and effects and predictions are presented with a 95% confidence interval (CI). We assessed publication bias with a funnel plot and Egger test for asymmetry. Two reviewers independently rated the quality of included studies using the Newcastle‐Ottawa Scale (NOS). 9
RESEARCH IN CONTEXT
Systematic Review: Studies were identified from searches using Medline/Web of Science/Embase sources and following Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines. Although cognitive consequences of coronavirus disease 2019 (COVID‐19) have not been studied widely, several recent publications describe the impact of COVID‐19 on cognition. In addition, a meta‐analysis of the Montreal Cognitive Assessment (MoCA) total score comparing COVID and non‐COVID patients was performed.
Interpretation: Our systematic review and meta‐analysis findings lead to an integrated hypothesis describing the cognitive consequences of COVID‐19. This hypothesis applies both to the acute phase and to assessment 6 months after recovery from the infection.
Future Directions: Prospective studies should be designed systematically to include pre‐morbid clinical data from COVID‐19 patients and a wide range of severity levels, including asymptomatic cases. Larger numbers of patients should be investigated during more extended follow‐up periods.
3. RESULTS
3.1. Overview of the included studies
After removing duplicates, a total of 6202 records were identified from the databases (Figure 1); 6012 were excluded after screening the titles and abstracts. After reading the full text, 115 were excluded because they were not related to the aims (n = 64); had a pediatric population (n = 26); or the language was not English, Spanish, or Portuguese (n = 25). Thus, 27 studies were included in the final review with 2103 patients and 506 healthy controls. The mean age of COVID cases was 56.05 years range (50.03 to 62.07), and for the controls it was 50.30 years (range 43.56 to 57.05], with no statistically significant age difference (P = .083). There was a female proportion of 0.44 (0.39; 0.48) in patients and 0.50 (0.43; 0.57) in controls. Characteristics of the included studies are presented in Table 1. Thirteen were cohort studies, seven were case‐control studies, five were case series, and two were case reports. Studies included patients with a range of disease severity, from asymptomatic COVID‐19 to severe infection that required admittance to an intensive care unit (ICU). The time from infection to neuropsychological assessment ranged from the acute phase of COVID‐19 10 , 11 , 12 , 13 up to 7 months after infection. 14 A range of general cognitive screening tools as well as more extensive neuropsychological batteries were used to assess cognitive functioning (see Table 1), with some outcomes assessed as continuous variables, whereas other studies 15 , 16 categorized the outcome as cognitive impairment (mild, moderate, or severe) versus no cognitive deficits according to specific criteria. Most studies were from Europe (n = 16), with two from Asia, five from North America (the United States), three from South America, and one from Central America (Table 1).
TABLE 1.
Characteristics of the included studies: methodological summary and main results
| First author, Year | Country | Population settings | Age | Sex (F %) | COVID‐19 cases (n Severity) | Healthy controls (n Definition) | Time of assessment | Cognitive test | Results | Adjustment or corrected values |
|---|---|---|---|---|---|---|---|---|---|---|
| CASE CONTROL | ||||||||||
| Amalakanti 2021 | India | General Hospital | 36.2 ± 11.7 | 52.3 | 93 Mild | 102 PCR | Acute phase of COVID‐19 | MoCA |
Fluency Cases: 0.9 ± 0.6 vs HC: 1.6 ± 0.7; P <.001 Visuo‐perception Cases: 2.4 ± 0.7 vs HC: 2.8 ± 0.7; P = .032 Naming Cases: 3.6 ± 0.5 vs HC: 3.9 ± 0.2; P = .016 No significant difference between cases and controls in MoCA, executive function, orientation, calculation, abstraction, delayed recall, and attention |
Age and sex |
| Manera 2021 | Italy | Hospital | 67 ± 13.2 | 33.5 | 152 Mild, moderate, and severe | No | 3 months after acute infection | MMSE | Impaired MMSE performance was notably more frequently for mild to moderate (26.3%) | Age and education |
| Ortelli 2021 | Italy | Neurorehabilitation Hospital | 67 ± 9.6 | 16.7 | 12 Moderate | 12 HC | 3 months after infection | MoCA, FAB, C‐FSS, BDI, AES, and computerized: VT, SIT, and NT task |
Montreal Cognitive Assessment (MoCA) Cases; 17.8 ± 5.3 vs HC: 26.8 ± 3.1; P <.001 Frontal Assessment Battery ± FAB Cases: 12.3 ± 2.3 vs HC: 16.7 ± 1.2; P < .001 RT in Vigilance Task ± VT Cases: 341.3 ± 86.3 vs HC: 308.8 ± 44.2; P = .541 Percentage of errors in VT Cases: 4.6 ± 0.8 vs HC: 1.2 ± 0.3; P< .001 RT in Stroop Interference Task ± SIT Cases: 969.4 ± 152.1 vs HC: 802.1 ± 122.0; P = .015 Percentage of errors in SIT Cases: 4.6 ± 0.8 vs HC: 1.2 ±0.3; P = .001 RT in Navon Task ± NT Cases: 1327.1 ± 525.3 vs HC: 850.3; P = .046 Percentage of errors in NT Cases: 3.8 ±1.2 vs HC: 1.2 ± 0.3; P < .001 |
Age and sex |
| Raman 2021 | UK | General Hospital | 55 ± 13 | 41.4 | 58 Moderate to severe | 38 Health Subjects | 2‐3 months after infection | MoCA, FSS, GAD‐7, and PHQ‐9 |
Executive/visuospatial score < 4 Cases: 40% vs HC: 16%; P = .010 No significant difference between cases and controls in MoCA |
Age, sex, BMI, BP, smoking, head size |
| Triana 2020 | Cuba | Neuro Hospital | 54.5 ± 12.5 | 52.3 | 42 Hospitalized | 100 Health Subjects | 45 days from covid‐positive | MoCA |
MoCA Cases: 23.43 ± 3.054 vs HC: 25.12 ± 3.367); PP= .007 Digit Series Cases: 1.41 ± 0.631 vs HC: 1.74 ± 0.543; P = .005 Attention Cases: 4.51 ± 1.381 vs HC: 5.07 ± 1.166; P = .026 Abstraction Cases: 1.41 ± 0.706 vs HC: 1.71 ± 0.574; P = .021 Delayed recall Cases: 1.78 ± 1.388 vs HC: 2.78 ± 1.567; P = .001 No significant difference between cases and controls in executive/visuospatial, orientation, language, word search, repeating phrases, subtraction, sustained attention, and fluency |
Age, sex, and education |
| Woo 2020 | Germany | General Hospital | 42.2 ± 14.3 | 57.9 | 18 Mild‐ moderate | 10 Randomly selected | Median 3 months after recovery | TICS‐M, FSS, PHQ‐9 |
TICS‐M Total Score Cases: 38.83 (31‐46) vs HC: 45.8 (43‐50); P = .000 TICS‐M Subscores Attention; P = .029 Language and Concentration; P = .009 Memory; P = .004 No significant difference between cases and controls in orientation |
Age‐matched healthy controls |
| Zhou 2020 | China | General Hospital | 47 ± 10.5 | 37.9 | 29 Moderate | 29 PCR | 2‐3 weeks after infection | iPad‐based online‐ TMT, SCT, CPT, and DST |
Missing Number Cases: 41.55 ± 2.90 vs HC: 39.59 ± 2.31; P = .006 CPT part 3 Correct Number Cases: 6.34 ± 2.50 vs HC: 8.21 ± 1.90; P = .002 Missing Number Cases: 40.38± 3.10 vs HC: 38.45 ± 2.13; P = .008 No significant difference between cases and controls in the Trail Making Test and Sign Coding Test. |
Age, gender, and education |
| COHORT | ||||||||||
| Alemanno 2021 | Italy | Hospital | 67.2 ± 12.8 | 29.0 |
87 Moderate to severe Four severity groups |
No | Acute phase of COVID‐19 and 1 month after infection | MoCA, MMSE, HRSD, and DTS |
Patients were divided in four groups according to the respiratory support they received in the acute phase of the disease Group 1 had higher scores than Group 3 for visuospatial/executive functions (P = .016), naming (P = .024), short‐ and long‐term memory (P = .010; P = .005), abstraction (P = .024), and orientation (P = .034). |
Severity of the respiratory compromise |
| Becker 2021 | USA | Health System Institutional | 49.0 ±14.2 | 63 | 740 Mild to severe | No | 7 months after diagnosis | Digit Span Forwards and Backward, TMT, phonological and category fluency, and HVLT‐R. | Hospitalized patients were more likely to have impairments in attention (odds ratio [OR]: 2.8; 95% CI: 1.3‐5.9), executive functioning (OR: 1.8; 95% CI: 1.0‐3.4), category fluency (OR: 3.0; 95% CI: 1.7‐5.2), memory encoding (OR: 2.3; 95% CI: 1.3‐4.1), and memory recall (OR: 2.2; 95% CI: 1.3‐3.8) than those in the outpatient group. Patients treated in the ED were more likely to have impaired category fluency (OR: 1.8; 95% CI: 1.1‐3.1) and memory encoding (OR: 1.7; 95% CI: 1.0‐3.0) than those treated in the outpatient setting. | Age, no history of dementia and spoke English or Spanish |
| Crivelli 2021 | Argentina | Neurological Clinic | 50 ± 43.63 | 49 | 45 Mild to severe | 45 Health subjects | 5 months after illness | MoCA, TMT, Digit Span Forwards and Digit‐Symbol Coding, Craft Story, RAVLT, Benson Figure, WISC, Stroop, MINT, phonological fluency, and HADS | ***Significant differences between groups were found in cognitive composites of memory (p = 0.016, Cohen's d = 0.73), attention (P < 0.001, Cohen's d = 1.2), executive functions (p < 0.001, Cohen's d = 1.4), and language (p = 0.002, Cohen d = 0.87). | Age, sex, and education |
| Dressing 2021 | Germany |
General Hospital |
53.6 ± 12.0 | 64.5 | 31 Mild | No | 3 months after acute infection | MoCA, HVLT, DST, BVMT‐R, TMT, FWIT, SDMT, fluency | The most frequently impaired domain was visual memory (7/31 [23%] patients; other domains ≤ 2/31 [≤ 7%]). Impaired individual tests on single‐subjects level were most frequently observed for verbal and visual memory tests. | None |
| Vannorsdall 2021 | USA | Clinic | 54.5 ± 14.6 | 59.5 | 82 (48 severe, 34 moderate) | No | 4 months after acute infection | RAVLT, TMT, DST, fluency |
67% of patients showed ≥1 abnormal cognitive score. Patients requiring intensive care unit (ICU) stays displayed more severe and heterogenous impairment than those requiring less intensive treatment. Mild/moderate impairment was particularly common on Oral Trail Making Test part A, category‐cued verbal fluency, RAVLT acquisition, and RAVLT delayed recall. |
Age |
| Hellgren 2021 | USA | Hospital | 59 ± 6.4 | 25 | 35 Mild, moderate, and severe | No | 5 months after acute infection | RBANS | Sixteen of 35 patients (46%) showed cognitive impairments; 6 of these (17%) showed mildly/moderately impaired cognition, and 10 patients (29%) had severely impaired cognition. | Age |
| Del Brutto 2021 | Ecuador | Atahualpa Residents | 62.6 ± 11 | 63.0 | 52 Mild | 41 PCR | 6 months after infection | MoCA, PSQI and HADS |
Post‐pandemic MoCA was worse in seropositive mild symptomatic individuals. Cognitive decline was defined as worsening in the post‐pandemic MoCA ≥4 points compared to the reduction experienced between pre‐pandemic baseline and follow‐up MoCA scores. Cognitive decline in 21% seropositive vs 2% seronegative cases. In multivariate analyses, the odds for developing cognitive decline were 18.1 times higher. |
Cardiovascular risk factors, sleep quality, depression and education |
| Ermis 2021 | Germany | Hospital | 63 | 39.6 | 53 Moderate to severe | No | Acute phase of COVID‐19 | MoCA | Cognitive impairment with deficits primarily in executive function, attention, language. and delayed recall. | None |
| Mattioli 2021 | Italy | University Hospital | 53.4 ± 9.2 | 62.7 | 163 Mild and moderate, 52 severe | No | 4 months after acute infection | COWA‐S, ROCFT, CVLT, RAVLT, TOL | Mild and moderate COVID patients show impairment in TOL (in 24 cases, 15%), Rey figure recall (in 13 cases, 8%), and Rey figure copy (in 8 cases, 5%). In severe COVID patients, the impairment also included the verbal memory test (delayed RAVLT in 4 cases (7.7%); immediate RAVLT in 3 patients (5.7%). | Age and education |
| Méndez 2021 | Spain | General Hospital | 57 IQR [49,67] | 41.3 | 179 Moderate to severe | No | 2 months after discharge | Telephone: SCIP, ANT, COWAT, WAIS IV, Digit span forward and backward, GAD‐7, PHQ‐2, and 17 DTS |
Neurocognitive domain impairment was predefined as moderate/severe impairment of any of the four neuropsychological tests. 105 patients (58.7%) met criteria for moderate neurocognitive impairment and 33 (18.4%) for severe neurocognitive impairment Immediate verbal memory impairment 38% moderate, 11.2% severe Delayed memory: 11.8% moderate impairment and 2.8% severe impairment. Semantic verbal fluency 34.6% moderate deficits and 8.4% severe deficit Working memory: 6.1% and severely impaired in 1.1% |
Age and education |
| Miskowiak 2021 | Denmark | Hospital | 56.2 ± 10.6 | 59.0 | 29 Moderate to severe | 100 | 3‐4 months after discharge | SCID‐D, TMT B, WMT, VLT, VFT, PMT, and CFQ |
SCIP total score Cases: 67.4 ± 13.9 vs HC: 75.0 ± 9.1; P = .010 VLT‐L Cases: 19.9 ± 4.2 vs HC: 22.1 ± 3.0; P = .003 WMT Cases: 18.2 ± 4.2 vs HC: 1.9 ± 2.5; P = .040 VFT Cases: 14.3 ± 4.7 vs HC: 16.0 ± 4.5; P = .170 VLT‐D Cases: 6.3 ± 2.8 vs HC: 7.0 ± 1.9; P = .080 PMT Cases: 9.0 ± 3.2 vs HC: 10.1 ± 2.3; P = .090 TMT‐B Cases: 116.2 ± 65.0 vs Normative Score: 80.6 ±18.7: p = .002 No associations between the severity of COVID‐19 and cognitive functioning in terms of cognitive impairments, length of hospitalization, total oxygen requirements and acute illness severity markers. More global cognitive impairment and executive dysfunction both correlated with severity of respiratory symptoms according to the ACQ. (Spearman's rho: SCIP total score deviation: r = −.56; P = .009; TMT‐B deviation: r = 0.44; P = .020) and CAT (Spearman's rho: SCIP total deviation r = −.39; P = .050; TMT B: r = .64; P < .001) More global cognitive impairment also correlated with poorer pulmonary function, as reflected by lower forced expiratory volume in one second (FEV1; Spearman's rho, r = 0.37; P = .049) |
Age, sex and education |
| Hosp 2021 | UK | Hospital | 65.2 ± 14.40 | 38.0 | 29 Moderate | 29 PCR | 1 month after symptom | MoCA, HVLT, TMT, Stoop, Digit span forward and backward, Symbol digit modalities test, and Fluency (animals, s‐words) |
MoCA global score 26 (max 30). MoCA global score 69% MoCA global score 54% MoCA global score 15% MoCA global score 31% HVLT‐R total 50% Stroop test: Word reading 28.6% Stroop test: Color naming 14.3% Stroop test: Interference 14.3% Digit span forward 20% Digit span reverse 40% Symbol digit modalities test 14.3% Categorical (animals) 46.1% Phonemic (s‐words) 23.1% |
Age and presentation of at least one newly acquired neurological symptom |
| CASE REPORT | ||||||||||
| Tolentino 2021 | Brazil | General Hospital | 47 | 0.0 | 1 Moderate | No | Acute phase of COVID‐19 | MMSE, Go/No‐Go task, CVAT, GAD‐7, and PHQ‐9 |
On day 1 of illness, the patient reported a subjective attention impairment. On day 3, the patient performed worse than the 75th percentile in two subdomains (variability of reactions time [VRT] and recreation time {RT]), indicating a moderate attention impairment. On day 6, the patient performed worse than the 75th percentile in all variables of the CVAT except commission errors (CE), indicating a severe impairment. VRT is the most affected variable, followed by omission errors (OE). Thus, the sustained‐focused subdomain is the most affected subdomain. On day 10, there was a mild deficit on only one variable (OE). On day 16, his performance was within the normal range. |
Sex and age |
| Yesilkaya 2021 | Turkey | General Hospital | 29 | 0.00 | 1 Mild | No | 3 months after the initial diagnosis | FAB, GDS, TMT, and CVLT |
A number of errors were detected in both the A and B parts of TMT and the scores were 2 and 4, respectively. The patient repeated 7 words in his first trial of CVLT. Overall, the results suggested impairment in varying spheres of cognition including memory, executive functioning, motor programming, attention, and concentration. After 3 months: The FAB score was 16. No error was detected in part A while the patient made 2 errors in part B of TMT. He repeated 11 words in CVLT. No neurological or cognitive deficits were detected at the patient's follow‐ups. |
None |
| CASE SERIES | ||||||||||
| Beaud 2020 | Switzerland | General Hospital | 64.8 ± 7.6 | 23.0 | 13 Severe | No | Mean 5.5 (SD 2.4) days from ICU discharge | MoCA and FAB |
MoCA Cases: 19.7 ± 7.5 FAB scores Cases: 10.9 ± 5.5 |
None |
| Groiss 2020 | Germany | General Hospital | 59.5 ±17.6 | 0.0 | 4 Severe | No | 3 weeks after dismissal | MoCA, MMSE, SDMT, and 4AT |
MoCA total score Patient 1 Impaired (21) Patient 2 impaired (16) MMSE total score Patient 1 Impaired (−18.81) Patient 2 Impaired (−4.29) Patient 3 Impaired (14) |
None |
| Hellmuth 2021 | USA | Memory Clinical and Telemedicine | 44.5 ± 11.5 | 100.0 | 2 Mild | No | 149 days after infection | MoCA, CVLT, MMSE, WAIS IV Digit span forward and backwards, D‐KEFS fluency, TMT, ROCFT, Color word interference, and NAB |
Rey Osterrieth Complex Copy Cases: 33/36 low average Figure 2 min delay Cases: 16/36 below average Backward Span Cases. 4 low average Inhibition/switching Cases: 77 low average |
None |
| Negrini 2021 | Italy | Rehabilitation Hospital | 48.5 | 33.3 | 9 Moderate | No | 1 month after hospitalization | MMSE, FAB, BDI, and STAI | General cognitive decline was observed in three patients (33.3%), who had pathological MMSE scores. All these patients had low scores in the domain of attention and calculation, short‐term memory, and written language. Only one patient (11.1%) showed decay of executive frontal functioning, as measured by the pathological scores at the FAB test, with deficits in conceptualization, lexical fluency, and motor programming. | Age, sex and education |
| Whiteside 2021 | USA | Rehabilitation Hospital | 70 ± 7.0 | 33.3 | 3 Severe | No | 2 months after acute infection | Telephone: WAIS‐IV (Vocabulary Subtest), RDS, HVLT‐R, RBANS, BDAE, O‐TMT, TSAT, ILS, BAI, and GDS | Neurocognitive deficits after severe COVID‐19 infection, particularly in encoding and verbal fluency. | Age and education |
References Instruments: MoCA (Montreal Cognitive Assessment); FAB (Frontal Assessment Battery); FSS (Fatigue Severity Scale); BDI (Beck Depression Inventory); AES (Apathy Evaluation Scale); GAD‐7 (General Anxiety Disorder Scale); PHQ (Patient Health Questionnaire) ; TICS‐M (The modified telephone interview for cognitive status); TMT (Trail Making Test); SCT (Sign Coding Test); CPT (Continuous Performance Test); DST (Digit Span Test); CVAT (Continuous Visual Attention Test); MMSE (Mini‐Mental State Examination); SDMT (Symbol Digit Modalities Test); CVLT (California Verbal Learning Test); NAB (Neuropsychological Assessment Battery); STAI (The State Trait Anxiety Inventory); HVLT‐R (Hopkins Verbal Learning Test Revised); RBANS (Repeatable Battery for the Assessment of Neuropsychological Status); O‐TMT (Oral Trail Making Test); BDAE (Boston Diagnostic Aphasia Examination); HRSD (Hamilton Depression Rating Scale); BNT (Boston Naming Test); ROCFT (Rey Osterrieth Complex Figure Test); HADS (Hospital Anxiety and Depression Scale); PSQI (Pittsburgh Sleep Quality Index); SCIP (Screen for Cognitive Impairment in Psychiatry); ANT (Animal Naming Test); COWAT (Controlled Oral Word Association Test); DTS (Davidson Trauma Scale); VT (Vigilance Task); SIT (Stoop Interference Task); NT (Navan Task); 4AT (Rapid clinical test for delirium); SCID‐D (Cognitive impairment in psychiatry Danish version); CFQ (Cognitive Failures Questionnaire); VLT (Verbal learning Test); WMT (Working Memory Test); VFT (Verbal Fluency Test); PMT (Psychomotor Speed Test); BMT (Babcock Memory Test); PCL (Post‐traumatic stress disorder checklist); BMET (Brief Memory and Executive Test); HVLT (Hopkins Verbal Learning Test); BVMT‐R (Brief Visuospatial Memory Test‐Revised); FWIT (Color‐Word Interference Test); COWA‐S (Controlled Oral Word Association Test); TOL (Tower of London Test); GDS (Geriatric Depression Scale); MINT (Multilingual Naming Test); WISC (Wisconsin Card Sorting Test); WAIS IV (Weschler Adult Intelligence Scale); RAVLT (Rey Auditory Verbal Learning Test); TSAT (Test of Sustained Attention and Tracking); ILS (Independent Living Scale); BAI (Beck Anxiety Inventory).
All COVID‐19 cases were defined with RT‐PCR except Beaud et al. 2020, who used the definition of PCR and ARD
3.2. Cognitive functioning during the acute phase of COVID‐19
Five studies examined patients in the acute stage of COVID‐19. 10 , 11 , 12 , 13 A case report by Tolentino et al. 13 followed up a patient with moderate COVID‐19 throughout the disease course and found that cognitive deficits increased until day 10, after which the cognitive functioning began to improve until a normal performance was achieved on day 16. Another case‐control study including young asymptomatic patients (mean age 36.2 ± 11.7) found differences between cases and healthy controls in fluency (P ≤.001), visual‐perception (P = .032), and naming (P = .016). 11 The occurrence of cognitive impairment in the acute COVID‐19 phase ranged from 61.5% in a cohort of mild to moderate patients in a general hospital 12 to 80% in a cohort of moderate to severe patients in a rehabilitation clinic, 40% of them with mild to moderate depression. 10
Case reports and case series (n = 7) 13 , 17 , 18 , 19 , 20 , 21 , 22 explored cognition in‐depth and reported low scores on executive functions, attention, memory, and verbal fluency during the acute phase of COVID‐19. A case report 22 described a young patient with new‐onset transient attention and memory deficits following a SARS‐CoV‐2 infection that had normalized completely at 3‐months follow‐up.
3.3. Cognitive functioning following COVID‐19 recovery
The case‐control studies reported mainly consistent results; all found significantly lower scores in cognition in the post‐COVID‐19 patient group compared to controls. Although some studies found deficits in global scores of screening measures 23 , 24 and sub‐scores of attention, memory, and executive functions, others 25 found deficits in only specific cognitive domains, principally attention. A study focusing on a young population (mean age 42.2 ± 14.3 years) reported cognitive impairment on Montreal Cognitive Assessment (MoCA) in COVID‐19 patients, which did not correlate with neuropsychiatric symptoms or disease severity. 26
Cohort studies showed a high occurrence of moderate cognitive impairment in post‐COVID‐19 patients, exceeding 50% in all studies that reported prevalence, 12 , 15 , 16 , 27 ranging from 54% in a cohort of consecutive patients admitted to hospital with moderate COVID‐19 15 to 65% in a cohort of moderate to severe patients. 27 A study 16 on patients with moderate to severe COVID‐19 (mean age 57, interquartile range [IQR] 49‐67) reported that 58.7% met criteria for moderate neurocognitive impairment and 18.4% for severe neurocognitive impairment 2 months after discharge. Specifically, the cognitive domains mostly impaired were immediate verbal memory impairment (38% moderate, 11.2% severe) and semantic verbal fluency (34.6% moderate deficits and 8.4% severe). In addition, studies found cognitive deficits in verbal fluency, attention, executive functions, and delayed memory. 12 , 15 , 27 , 28 , 29 In a study by Hosp et al., 15 neurological symptoms and executive deficits correlated with frontoparietal hypometabolism in fluorodeoxyglucose (FDG)–positron emission tomography (PET). Conversely, a recent cohort study 30 in patients (n = 31) in the long‐term phase after COVID‐19 (202 ± 58 days after positive PCR) with self‐reported symptoms of Long COVID showed minor cognitive impairments only on the single‐patient level. In contrast, cerebral F‐FDG‐PET failed to reveal a distinct pathological signature; however, a high prevalence of fatigue was found in the sample. Ortelli et al. 2021 23 also found evidence for abnormal neuromuscular fatigue, cognitive fatigue, apathy, and executive dysfunction in post‐COVID‐19 patients compared to healthy controls.
The study that assessed patients in the longest term since infection (7 months) 14 included 749 young patients (mean age 49, IQR 38‐59) with moderate to severe infection and found a high frequency of cognitive impairment. The most prominent deficits were in processing speed (18%, n = 133), executive function (16%, n = 118), phonemic fluency (15%, n = 111) and category fluency (20%, n = 148), memory encoding (24%, n = 178), and memory recall (23%, n = 170).
FIGURE 2.
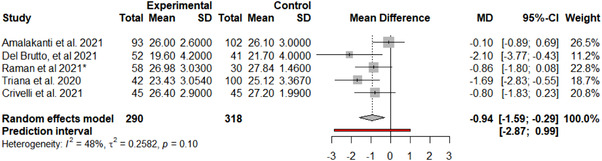
MoCA meta‐analysis forest plot
3.3.1. Longitudinal studies on global cognition
Two cohort studies had longitudinal measures over two time points. 10 , 31 One study from Ecuador included pre‐pandemic measures of cognitive functioning 31 ; seropositive mildly symptomatic COVID‐19 patients had a significantly larger cognitive decline over 6 months in MoCA scores than seronegative individuals (21% vs 2%). The risk of cognitive decline was 18.1 times higher among SARS‐CoV‐2‐seropositive individuals (95% CI 1.7, 188; P = .015) after adjustment for cardiovascular risk factors, sleep quality, depression, and education. The study that reported the higher prevalence of cognitive impairment during the acute phase of COVID‐19 10 followed up the cohort 1 month after discharge and found that MoCA and Mini‐Mental Status Examination (MMSE) total scores were significantly higher than at admission.
3.4. Effect of disease severity and symptoms
There is an insufficient number of published papers to make conclusions about how the severity of COVID‐19 or types of disease symptoms differentially affect cognition. A study that tested moderate to severe hospitalized patients with functional dependence 1 month after discharge found that patients who received treatment with mechanical ventilation had better cognitive performance than those who only received oxygen therapy. 10 The former group of subjects had significantly higher performance in visuospatial/executive functions, naming, short‐ and long‐term memory, abstraction, and orientation but were also significantly younger than the latter group. Similarly, a cohort study by Manera et al. 32 found that patients presenting with adult respiratory distress syndrome (ARDS) who underwent intensive care suffered less from cerebral hypoxia and thus had less cognitive sequels than those treated with non‐invasive ventilation.
Woo et al. 33 studied 18 young patients with mild to moderate post‐COVID‐19 (mean age 42.11 years) and assessed disease severity by the length of sickness, length of inpatient stay, and the number of sustained somatic symptoms, and they found that none of the variables correlated with cognitive performance. Likewise, Miskowaik et al. 27 reported no association between the severity of COVID‐19 and cognitive functioning (3‐4 months after recovery) in terms of length of hospitalization, total requirement of oxygen during hospitalization, or other acute severity markers. However, global cognitive impairment and executive dysfunction both correlated with the severity of respiratory symptoms and poorer pulmonary function. Furthermore, higher maximum d‐dimer levels correlated with poorer verbal recall and psychomotor speed. Conversely, a cohort study on moderate to severe hospitalized patients 10 to 35 days after hospital discharge found that patients who required oxygen had lower punctuations in verbal memory (P = .030), visual memory (P = .050), attention (P = .002), working memory (P = .036), complex working memory (P = .027), processing speed (P = .035), and the global cognitive index (P = .010), compared to ICU patients who only had worse executive functions (P = .037). 28 Accordingly, two studies 34 , 35 compared ICU versus non‐ICU post‐COVID patients and found significantly more severe and broad impairment in ICU patients.
3.5. Meta‐analysis on Montreal Cognitive Assessment (MoCA)
A total of five studies reporting MoCA results, including COVID‐19 patients versus a control group, 11 , 24 , 29 , 31 , 36 were included in the meta‐analysis. There was evidence of an effect of COVID‐19 infection on the total MoCA score (MD = −0.94, 95% CI −1.59, −0.29; P = .0049). In addition, although influential assessment reports no outliers, it is interesting to note that the study by Amalakanti 11 was large and may influence the overall findings (see Figure 2). This is the only study that included asymptomatic young patients (mean age 36.2 ± 11.7) and did not find cognitive differences in MoCA but found deficits in a more specific cognitive assessment. The heterogeneity was: I 2 = 48.3%, 95% CI 0.0%, ‐81.0%; Q = 7.73, P = .10. Results from this meta‐analysis can be seen in Figure 3. The test of publication bias showed significant asymmetry: Egger test = −4.959, 95% CI −7.67, −2.25; P = .037. The asymmetry in the figure (see Figure 4) indicates publication bias, the possibility that negative studies have not been submitted due to being of less interest to the journals to be published. However, it must be considered that Egger test may lack the statistical power to detect bias when the number of studies is small (ie, k < 10). In addition, a meta‐regression analysis based on age was performed to study the effects of age on MoCA of post‐COVID‐19 patients (see Figure 5). We found an estimated change in MoCA total score of −0.064, 95% CI: −0.012, −0.116 for an increase of 1 year in age (z = −2.4148; P = .0157).
FIGURE 3.
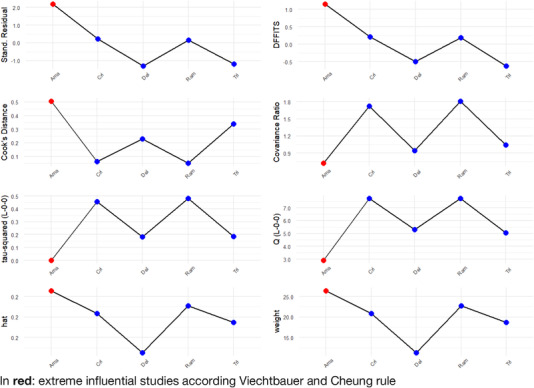
Influential Assessment
FIGURE 4.
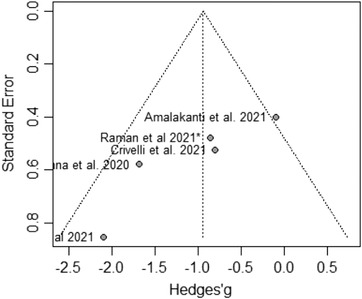
MoCA meta‐analysis publication bias
FIGURE 5.
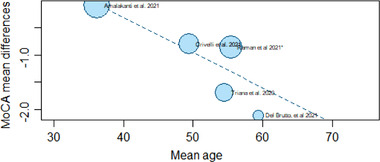
Meta‐regression Model: MoCA by age
3.6. Risk of bias assessment
Two reviewers independently rated the quality of included studies using the Newcastle‐Ottawa Scale (or NOS) 9 (see Table 2). The quality of case‐control and cohort studies was assessed judging three categories: the selection of the study groups, the comparability of the groups; and the ascertainment of either the exposure or outcome of interest for case‐control or cohort studies, respectively. In summary, when using the Agency for Health Research and Quality (AHRQ) threshold standards, 9 of the 20 studies were good, 15 , 23 , 25 , 27 , 29 , 31 , 32 , 33 , 36 1 was fair, 28 and 10 were poor. 10 , 11 , 12 , 14 , 16 , 24 , 30 , 34 , 35 , 37
TABLE 2.
Study designs and quality scoring using Newcastle‐Ottawa Scale for non‐randomized studies in meta‐analyses and classification according to AHRQ standards
| Study | Design | Bias Rating Newcastle Ottawa | AHRQ | ||
|---|---|---|---|---|---|
| Selection | Comparability | Outcome/exposure | |||
| Amalakanti. et al. 2021 | Case control | ** | * | * | Poor |
| Manera et al. 2021 | Case control | *** | * | ** | Good |
| Ortelli et al. 2021 | Case control | *** | ** | ** | Good |
| Raman et al. 2021 | Case control | **** | ** | ** | Good |
| Triana. et al. 2020 | Case control | *** | ** | * | Poor |
| Woo, M.S. et al. 2020 | Case control | *** | * | *** | Good |
| Zhou, H. T., et al. 2020 | Case control | *** | ** | *** | Good |
| Alemanno, F., et al. 2021 | Cohort | * | * | ** | Poor |
| Almeria et al. 2020 | Cohort | ** | * | ** | Fair |
| Becker et al. 2021 | Cohort | ** | ‐ | * | Poor |
| Crivelli et al. 2021 | Cohort | **** | ** | ** | Good |
| Dressing et al. 2021 | Cohort | ** | ‐ | ** | Poor |
| Del Brutto et al. 2021 | Cohort | **** | * | *** | Good |
| Ermis et al. 2021 | Cohort | ** | ‐ | ** | Poor |
| Mattioli et al. 2021 | Cohort | ** | ‐ | ** | Poor |
| Méndez et al. 2021 | Cohort | ** | ‐ | ** | Poor |
| Miskowiak et al. 2021 | Cohort | *** | ** | *** | Good |
| Hellgren et al. 2021 | Cohort | *** | ‐ | ** | Poor |
| Hosp et al. 2021 | Cohort | *** | ** | ** | Good |
| Vannorsdall et al. 2021 | Cohort | **** | ‐ | * | Poor |
| Yesilkaya et al. 2021 | Case report | ||||
| Tolentino, J. C., et al. 2021 | Case report | ||||
| Beaud et al. 2020 | Case series | ||||
| Groiss, S. J., et al. 2020 | Case series | ||||
| Hellmuth et al. 2021 | Case series | ||||
| Negrini et al. 2021 | Case series | ||||
| Whiteside, D. M., et al. 2021 | Case series | ||||
4. DISCUSSION
4.1. Summary of main findings
Our systematic review highlighted that the evidence assessing the consequences of COVID‐19 on cognition is scarce. There are currently few studies in the literature examining differences in cognitive functioning between patients with and without COVID‐19, with only 27 studies published to date in people with no previous cognitive impairment. Study designs—particularly, time of assessment, disease severity, and neurological tests used for assessment—differed considerably, making conclusions difficult. However, the results appear to suggest some form of cognitive deficits associated with COVID‐19 in the acute and short‐term follow‐up phase.
Our meta‐analysis revealed that people with COVID‐19 had poorer general cognitive functioning, as measured with the MoCA, compared to people without COVID‐19 between assessment in the acute phase and 6 months after infection. There was a mean difference of −0.94, corresponding to an ≈1‐point difference on the MoCA. Although a 1‐point score may not seem to have large clinical significance, it is worth noting that the mean age of participants was generally relatively low, between 36.2 years in one study and 62.6 years in another; therefore, a 1‐point difference in adults of this age may be relevant to their functioning and subjective cognition. It is worth highlighting that meta‐regression analysis by age reported that an increase in age correlates with enhanced cognitive disfunction.
Although few studies assess specific cognitive domains, generally the early results suggest that executive function, memory, and attention are the domains that more frequently show differences between COVID‐19 patients and healthy controls up to 3 months after illness. 23 , 27 , 33 Deficits were also seen in some studies for working memory, learning, delayed control, inhibitory control, set‐shifting, phonological verbal fluency, and processing speed.
4.2. Interpretation of main findings
The pathological mechanisms that might underlie the potential cognitive impairment associated with COVID‐19 are still unclear, but may include the direct effects of cellular damage due to viral invasion, secondary inflammatory responses, decreased angiotensin‐converting enzyme 2 (ACE2) activity that regulates neuroprotective and neuro‐immunomodulatory functions; oxidative stress 38 ; and hypoxia, sepsis, and/or multi‐organ damage related to severe COVID‐19. Cognitive impairment in ARDS survivors ranges from 70% to 100% at hospital discharge, 46% to 80% at 1 year, and 20% at 5 years. 39 Studies on hypoxia have demonstrated its negative effect on cognition, 40 which can present with heterogeneous patterns and severities. A metanalysis compared ADRS patients with mixed ICU patients at discharge, 41 and found that cognitive deficits were significantly more frequent in patients with ARDS (82%, 95% CI 78%, 86%] vs 48%, 95% CI 44%, 52%).
In the case of post‐COVID‐19 cognitive impairment, this association is less clear. Two studies included in this review reported a relationship between cognitive impairment and poorer pulmonary function, 27 , 28 suggesting that reduced oxygen delivery to the brain may play a role. However, not all studies report whether or not their subjects have had ARDS, and in those that do report it, the association between severity of ARDS (and therefore hypoxia) is not established, 27 or they present conflicting results, ie, patients with higher severity having better function. 10 The interaction between severity and age is something that should always be considered when interpreting the results, as COVID‐19 severity is age dependent and age is a risk factor for cognitive impairment in the general population.
Another aspect that may underlie the onset of cognitive impairment is vascular involvement. Only one of the studies reported d‐dimer levels, 27 which were elevated in subjects with cognitive impairment. Five of the reviewed studies reported imaging 12 , 15 , 22 , 31 , 36 and no acute vascular lesions were reported. However, this was not the main focus of the studies included in this review, and future studies should focus on the role of d‐dimer and vascular consequences of COVID‐19 and their role in cognitive functioning.
It is possible that delirium may play a role in the association between cognitive impairment and COVID‐19. According to WHO, consciousness and/or confusion can be a core symptom of COVID‐19 at presentation (World Health Organization and International Severe Acute Respiratory and Emerging Infection Consortium, COVID‐19 Core Case Report). Furthermore, a rapid review reported that more than half of COVID‐19 patients admitted to ICUs have delirium, 42 which is consistent with similar coronaviruses such as MERS and SARS, where delirium is frequently observed, especially in older persons, patients with severe respiratory symptoms, and patients with pre‐existing cognitive impairment/dementia. 43
However, in our review, we excluded people with pre‐existing cognitive impairment, a population especially susceptible to delirium. Furthermore, we described separately the studies performed in the acute phase where delirium could have been present. In contrast, the remaining studies were performed 2 weeks to 7 months after SAR‐CoV‐2 infection and, therefore, any reported cognitive impairment is unlikely to be due to COVID‐19‐related delirium. Moreover, in the current review, there was evidence of significant cognitive impairment even in asymptomatic and mild cases of COVID‐19. In the acute phase of COVID‐19, the etiology of delirium is likely to be multifactorial. Clinical complications often seen in severe COVID‐19, such as pneumonia, ADRS, hypoxia, and respiratory failure are also independent risk factors for delirium. 42
Furthermore, interventions to treat ARDS can lead to delirium. Other possible mechanisms are systematic inflammation infecting the central nervous system (CNS) or a storm of intracranial cytokines mediated by blood‐brain barrier permeabilization. 42 These mechanisms could also contribute to cognitive impairment in patients in the acute phase of COVID‐19.
4.3. Strengths and limitations
The strength of our study was that the systematic review process was conducted according to PRISMA guidelines. However, several limitations should be noted. First, the available evidence was obtained from a small number of studies conducted with small sample sizes. Second, some studies used cognitive screening tools and measures of general cognitive functioning rather than a comprehensive battery of domain‐specific tests. Third, we focused on people who had been cognitively intact prior to being infected with SARS‐CoV‐2, but the adverse effects of age and other pre‐existing comorbidities were not considered. Fourth, due to heterogeneity of outcome assessments, we were only able to perform a meta‐analysis on a small subsample of studies and were unable to assess dichotomous categories of clinically significant cognitive impairment. Furthermore, the heterogeneity of studies prevents us from drawing firm conclusions, as there were differences between study populations and designs in terms of disease severity and time of assessment, among others. Finally, due to the fact that the pandemic is a recent event, long‐term follow‐ups to establish how long cognitive impairment persists after COVID‐19 recovery are still not possible.
4.4. Future research
There is an urgent need for more studies on the topic of the cognitive consequences of COVID‐19, as there is currently insufficient evidence in the literature. Prospective studies comparing healthy controls with recovered COVID‐19 patients should be designed systematically and include a wide range of severity levels, including asymptomatic cases, and mild, moderate, and severe COVID‐19 patients. Larger numbers of patients should be investigated at the end of the acute phase and during longer follow‐up periods to verify the duration of symptoms and any improvement or stabilization of cognitive functioning. The patients' pre‐morbid demographic and clinical profile should be described and, along with the COVID‐19 spectrum, correlated with the presence, severity, and duration of cognitive impairment. Several registries and databases are now active worldwide. 44 , 45 Existing studies and cohorts with pre‐pandemic data may also help to establish intra‐individual changes in people who later develop COVID‐19. Because we are still in the early phases of the pandemic, there is currently limited follow‐up time to effectively establish the long‐term effects. Thus, studies with longer follow‐up, up to a year and beyond, are needed. Study protocols should use comprehensive cognitive batteries and periodical long‐term cognitive assessment, preferably standardized to allow for cross‐country comparisons. There was only one study from low‐ and middle‐income countries (LMICs) 11 ; therefore, more extensive research into these regions is needed, especially due to the potential role of education on cognitive reserve. Low formal education has been found to have a deleterious impact on cognition. 46 This is why it would be interesting to include and promote post‐COVID‐19 cognitive studies in LMICs where education levels are low, and to study how cognitive reserve may interact with post‐COVID‐19 cognitive impairment.
4.5. Relevance and implications
The results of the review have several implications from clinical, individual, and public health perspectives. It has been suggested that there may be a “Long‐COVID” syndrome of which cognitive dysfunction might be a symptom. 47 Until now, although rapid guidelines for managing long‐term symptoms of COVID‐19 have been published, 48 there are no internationally established diagnostic criteria for long‐term COVID syndrome. The research described in the current review may provide important insights into which cognitive deficits should be evaluated in any future diagnostic criteria, although more research is needed.
Furthermore, from an individual perspective, cognitive impairments associated with COVID‐19 may affect quality of life and functioning (eg, more than 80% of patients reported experiencing severe cognitive difficulties in daily life 4 months after hospital discharge 27 ). This highlights the importance of systematic cognitive screening in COVID‐19 patients after illness, which may be an important element of post‐COVID‐19 care and management. MoCA was the most commonly used tool in the studies reported in this review, so this may be a relevant screening tool for post‐COVID‐19 cognitive assessment. However, the recent development of other digital cognitive screening tools may provide other alternatives. 49
As our knowledge base grows, it may be relevant to develop and assess potential interventions for post‐COVID‐19 patients with persistent cognitive impairments. As mentioned previously, there is still not sufficient follow‐up time to establish how long the potential cognitive effects of COVID‐19 last in affected individuals, so these implications may differ as time progresses.
5. CONCLUSION
Our systematic review highlighted a lack of studies investigating the effect of COVID‐19 on cognitive functioning, particularly with regard to specific cognitive domains. Although the meta‐analysis suggests that patients with COVID‐19 have lower general cognition compared to healthy controls after they have recovered, evidence is still lacking, and no firm conclusions can be drawn. However, this preliminary evidence suggests that individuals may experience cognitive impairment after recovery from COVID‐19, and future studies will need to further clarify how long these symptoms persist and whether they are associated with specific characteristics of the patient.
CONFLICT OF INTEREST
None.
ACKNOWLEDGEMENTS
The authors acknowledge the contribution of all the members of WHO's Neurology and COVID‐19 Global Forum working group on Follow‐up and Long‐term Impact.
Crivelli L, Palmer K, Calandri I, et al. Changes in cognitive functioning after COVID‐19: A systematic review and meta‐analysis. Alzheimer's Dement. 2022;18:1047–1066. 10.1002/alz.12644
Lucia Crivelli and Katie Palmer are joint first authors.
REFERENCES
- 1. Al‐Aly Z, Xie Y, Bowe B. High‐dimensional characterization of post‐acute sequelae of COVID‐19. Nature. 2021;594(7862):259‐264. [DOI] [PubMed] [Google Scholar]
- 2. Hoffman LA, Vilensky JA. Encephalitis lethargica: 100 years after the epidemic. Brain. 2017;140(8):2246‐2251. [DOI] [PubMed] [Google Scholar]
- 3. Verstrepen K, Baisier L, De Cauwer H. Neurological manifestations of COVID‐19, SARS and MERS. Acta Neurol Belg. 2020;120(5):1051‐1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gu J, Gong E, Zhang B, et al. Multiple organ infection and the pathogenesis of SARS. J Exp Med. 2005;202(3):415‐424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Saracli O, Akca AS, Atasoy N, et al. The Relationship between Quality of Life and Cognitive Functions, Anxiety and Depression among Hospitalized Elderly Patients. Clin Psychopharmacol Neurosci. 2015;13(2):194‐200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int J Surg. 2021;88:105906. [DOI] [PubMed] [Google Scholar]
- 7. Aslam S, Emmanuel P. Formulating a researchable question: A critical step for facilitating good clinical research. Indian J Sex Transm Dis AIDS. 2010;31(1):47‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. WHO . Clinical management of COVID‐19: interim guidance, 27 May 2020. World Health Organization. https://apps.who.int/iris/handle/10665/332196. 2020. [Google Scholar]
- 9. Wells GSB, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. 2013, http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. 2013
- 10. Alemanno F, Houdayer E, Parma A, et al. COVID‐19 cognitive deficits after respiratory assistance in the subacute phase: A COVID rehabilitation unit experience. PLoS ONE. 2021;16(2 February). 10.1371/journal.pone.0246590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Amalakanti S, Arepalli KVR, Jillella JP. Cognitive assessment in asymptomatic COVID‐19 subjects. Virusdisease. 2021;32:1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ermis U, Rust MI, Bungenberg J, et al. Neurological symptoms in COVID‐19: a cross‐sectional monocentric study of hospitalized patients. Neurol Res Pract. 2021;3(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tolentino JC, Gjorup ALT, Schmidt GJ, Schmidt SL. Early attention impairment in a patient with COVID‐19. Psychiatry and Clinical Neurosciences. 2021;75(2):66‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Becker JH, Lin JJ, Doernberg M, et al. Assessment of Cognitive Function in Patients After COVID‐19 Infection. JAMA Netw Open. 2021;4(10):e2130645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hosp JA, Dressing A, Blazhenets G, et al. Cognitive impairment and altered cerebral glucose metabolism in the subacute stage of COVID‐19. Brain. 2021;144(4):1263‐1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Méndez R, Balanzá‐Martínez V, Luperdi SC, et al. Short‐term Neuropsychiatric Outcomes and Quality of Life in COVID‐19 Survivors. J Intern Med. 2021;290:621‐631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Beaud V, Crottaz‐Herbette S, Dunet V, et al. Pattern of cognitive deficits in severe COVID‐19. J Neurol Neurosurg Psychiatry. 2020;92. 10.1136/jnnp-2020-325173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Groiss SJ, Balloff C, Elben S, et al. Prolonged Neuropsychological Deficits, Central Nervous System Involvement, and Brain Stem Affection After COVID‐19‐A Case Series. Frontiers in Neurology. 2020;11. 10.3389/fneur.2020.574004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hellmuth J, Barnett TA, Asken BM, et al. Persistent COVID‐19‐associated neurocognitive symptoms in non‐hospitalized patients. J Neurovirol. 2021;27(1):191‐195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Negrini F, Ferrario I, Mazziotti D, et al. Neuropsychological Features of Severe Hospitalized Coronavirus Disease 2019 Patients at Clinical Stability and Clues for Postacute Rehabilitation. Arch Phys Med Rehabil. 2021;102(1):155‐158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Whiteside DM, Oleynick V, Holker E, Waldron EJ, Porter J, Kasprzak M. Neurocognitive deficits in severe COVID‐19 infection: Case series and proposed model. Clin Neuropsychol. 2021;35:1‐20. [DOI] [PubMed] [Google Scholar]
- 22. Yesilkaya UH, Sen M, Balcioglu YH. COVID‐19‐related cognitive dysfunction may be associated with transient disruption in the DLPFC glutamatergic pathway. J Clin Neurosci. 2021;87:153‐155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ortelli P, Ferrazzoli D, Sebastianelli L, et al. Neuropsychological and neurophysiological correlates of fatigue in post‐acute patients with neurological manifestations of COVID‐19: Insights into a challenging symptom. J Neurol Sci. 2021;420:117271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Triana RM, Martínez CC, Almeida TM, González MÁÁ, Vaillant TZ, Barreto YR. Cognitive performance in convalescent covid‐19 patients. Revista Cubana de Hematologia, Inmunologia y Hemoterapia. 2020;36(special issue):1‐17 [Google Scholar]
- 25. Zhou J, Liu C, Sun Y, Huang W, Ye K. Cognitive disorders associated with hospitalization of COVID‐19: Results from an observational cohort study. Brain, Behavior, and Immunity. 2020. 10.1016/j.bbi.2020.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Woo MS, Malsy J, Pottgen J, et al. Frequent neurocognitive deficits after recovery from mild COVID‐19. Brain Communications. 2020;2(2):fcaa205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Miskowiak KW, Johnsen S, Sattler SM, et al. Cognitive impairments four months after COVID‐19 hospital discharge: Pattern, severity and association with illness variables. Eur Neuropsychopharmacol. 2021;46:39‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Almeria M, Cejudo JC, Sotoca J, Deus J, Krupinski J. Cognitive profile following COVID‐19 infection: Clinical predictors leading to neuropsychological impairment. Brain Behav Immun Health. 2020;9:100163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Crivelli L, Calandri I, Corvalan N, et al. Cognitive consequences of COVID‐19: results of a cohort study from South America. Arq Neuropsiquiatr. 2021. 10.1590/0004-282X-ANP-2021-0320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dressing A, Bormann T, Blazhenets G, et al. Neuropsychological profiles and cerebral glucose metabolism in neurocognitive Long COVID‐syndrome. J Nucl Med. 2021. 10.2967/jnumed.121.262677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Del Brutto OH, Wu S, Mera RM, Costa AF, Recalde BY, Issa NP. Cognitive decline among individuals with history of mild symptomatic SARS‐CoV‐2 infection: A longitudinal prospective study nested to a population cohort. Eur J Neurol. 2021;28(10):3245‐3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Manera MR, Fiabane E, Pain D, et al. Clinical features and cognitive sequelae in COVID‐19: a retrospective study on N = 152 patients. Neurol Sci. 2021. 10.1007/s10072-021-05744-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Woo MS, Malsy J, Pöttgen J, et al. Frequent neurocognitive deficits after recovery from mild COVID‐19. Brain Commun. 2020;2(2):fcaa205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mattioli F, Piva S, Stampatori C, et al. Neurologic and cognitive sequelae after SARS‐CoV2 infection: Different impairment for ICU patients. J Neurol Sci. 2021;432:120061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vannorsdall TD, Brigham E, Fawzy A, et al. Rates of Cognitive Dysfunction, Psychiatric Distress, and Functional Decline After COVID‐19. J Acad Consult Liaison Psychiatry. 2021. 10.1016/j.jaclp.2021.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Raman B, Cassar MP, Tunnicliffe EM, et al. Medium‐term effects of SARS‐CoV‐2 infection on multiple vital organs, exercise capacity, cognition, quality of life and mental health, post‐hospital discharge. EClinicalMedicine. 2021;31:100683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hellgren L, Birberg Thornberg U, Samuelsson K, Levi R, Divanoglou A, Blystad I. Brain MRI and neuropsychological findings at long‐term follow‐up after COVID‐19 hospitalisation: an observational cohort study. BMJ Open. 2021;11(10):e055164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bandala C, Cortes‐Altamirano JL, Reyes‐Long S, Lara‐Padilla E, Ilizaliturri‐Flores I, Alfaro‐Rodriguez A. Putative mechanism of neurological damage in COVID‐19 infection. Acta Neurobiol Exp (Wars). 2021;81(1):69‐79. [DOI] [PubMed] [Google Scholar]
- 39. Herridge MS, Moss M, Hough CL, et al. Recovery and outcomes after the acute respiratory distress syndrome (ARDS) in patients and their family caregivers. Intensive Care Med. 2016;42(5):725‐738. [DOI] [PubMed] [Google Scholar]
- 40. McMorris T, Hale BJ, Barwood M, Costello J, Corbett J. Effect of acute hypoxia on cognition: A systematic review and meta‐regression analysis. Neurosci Biobehav Rev. 2017;74(Pt A):225‐232. [DOI] [PubMed] [Google Scholar]
- 41. Honarmand K, Lalli RS, Priestap F, et al. Natural History of Cognitive Impairment in Critical Illness Survivors. A Systematic Review. Am J Respir Crit Care Med. 2020;202(2):193‐201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hawkins M, Sockalingam S, Bonato S, et al. A rapid review of the pathoetiology, presentation, and management of delirium in adults with COVID‐19. J Psychosom Res. 2021;141:110350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rogers JP, Chesney E, Oliver D, et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta‐analysis with comparison to the COVID‐19 pandemic. Lancet Psychiatry. 2020;7(7):611‐627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dong E, Du H, Gardner L. An interactive web‐based dashboard to track COVID‐19 in real time. Lancet Infect Dis. 2020;20(5):533‐534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hasell J, Mathieu E, Beltekian D, et al. A cross‐country database of COVID‐19 testing. Sci Data. 2020;7(1):345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Farfel JM, Nitrini R, Suemoto CK, et al. Very low levels of education and cognitive reserve: a clinicopathologic study. Neurology. 2013;81(7):650‐657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mendelson M, Nel J, Blumberg L, et al. Long‐COVID: An evolving problem with an extensive impact. S Afr Med J. 2020;111(1):10‐12. [DOI] [PubMed] [Google Scholar]
- 48. Shah W, Hillman T, Playford, ED , Hishmeh L. Managing the long term effects of covid‐19: summary of NICE, SIGN, and RCGP rapid guideline. BMJ. 2021;372:n136. [DOI] [PubMed] [Google Scholar]
- 49. Liu X, Chen X, Zhou X, et al. Validity of the MemTrax Memory Test Compared to the Montreal Cognitive Assessment in the Detection of Mild Cognitive Impairment and Dementia due to Alzheimer's Disease in a Chinese Cohort. J Alzheimers Dis. 2021;80(3):1257‐1267. [DOI] [PubMed] [Google Scholar]


