Abstract
Endogenous peptide antibiotics are under investigation as inhaled therapeutic agents for cystic fibrosis (CF) lung disease. The bactericidal activities of five cathelicidin peptides (LL37 [human], CAP18 [rabbit], mCRAMP [mouse], rCRAMP [rat], and SMAP29 [sheep]), three novel alpha-helical peptides derived from SMAP29 and termed ovispirins (OV-1, OV-2, and OV-3), and two derivatives of CAP18 were tested by broth microdilution assays. Their MICs were determined for multiply antibiotic-resistant Pseudomonas aeruginosa (n = 24), Burkholderia cepacia (n = 5), Achromobacter xylosoxidans (n = 5), and Stenotrophomonas maltophilia (n = 5) strains isolated from CF patients. SMAP29 was most active and inhibited mucoid and nonmucoid P. aeruginosa strains (MIC, 0.06 to 8 μg/ml). OV-1, OV-2, and OV-3 were nearly as active (MIC, 0.03 to 16 μg/ml), but CAP18 (MIC, 1.0 to 32 μg/ml), CAP18-18 (MIC, 1.0 to >32 μg/ml), and CAP18-22 (MIC, 0.5 to 32 μg/ml) had variable activities. LL37, mCRAMP, and rCRAMP were least active against the clinical isolates studied (MIC, 1.0 to >32 μg/ml). Peptides had modest activities against S. maltophilia and A. xylosoxidans (MIC range, 1.0 to > 32 μg/ml), but none inhibited B. cepacia. However, CF sputum inhibited the activity of SMAP29 substantially. The effects of peptides on bacterial cell membranes and eukaryotic cells were examined by scanning electron microscopy and by measuring transepithelial cell resistance, respectively. SMAP29 caused the appearance of bacterial membrane blebs within 1 min, killed P. aeruginosa within 1 h, and caused a dose-dependent, reversible decrease in transepithelial resistance within 5 h. The tested cathelicidin-derived peptides represent a novel class of antimicrobial agents and warrant further development as prophylactic or therapeutic agents for CF lung disease.
Cystic fibrosis (CF) is the most common autosomal recessive, life-shortening genetic disorder among Caucasians (29). There are approximately 30,000 CF patients in the United States and 60,000 CF patients worldwide, and their average life expectancy is approximately 30 years (11, 12, 15). The major morbidity and mortality in CF are caused by the progressive loss of pulmonary function that results from a cycle of inflammation and infection. Over 80% of CF patients become chronically infected with Pseudomonas aeruginosa (7, 21). Initially, strains are nonmucoid, but over time a biofilm consisting of mucoid strains develops (5, 6). Strains also become increasingly antibiotic resistant due to many prolonged courses of antimicrobial agents used to slow the rate of decline in pulmonary function (3, 30). Furthermore, the microbiology of CF has changed during the past two decades; intrinsically antibiotic-resistant, gram-negative organisms such as Burkholderia cepacia, Stenotrophomonas maltophilia, and Achromobacter (Alcaligenes) xylosoxidans are emerging as CF pathogens (3, 8, 11, 34). Thus, new therapeutic approaches are needed to improve the management of CF lung disease, including both prophylactic strategies to delay chronic infection with P. aeruginosa and the development of new agents to treat antibiotic-resistant pathogens.
In the search for new classes of antibiotics, there has been recent interest in naturally occurring antimicrobial peptides, including the cathelicidins. These endogenous peptides contribute to innate immunity and are expressed in myeloid and epithelial cells (13, 16, 17). The cathelicidins of mammalian origin are synthesized in precursor form, requiring proteolytic processing to release the mature C-terminal antimicrobial peptide (24, 37). They are typically linear, highly cationic, and salt insensitive and display broad-spectrum antimicrobial activities (17, 24, 33, 37). The peptides kill bacteria by thinning and disrupting the bacterial membrane. Cathelicidin peptides are currently under investigation as inhaled therapeutic agents for CF lung disease (16). To identify new bactericidal agents with activity against CF pathogens, we evaluated the in vitro activity of multiple cathelicidin peptides, including designed truncated peptides, against clinical strains of P. aeruginosa, B. cepacia, S. maltophilia, and A. xylosoxidans isolated from CF patients. Possible synergy with conventional antibiotics, the effects of these peptides on bacterial and respiratory epithelial cell membranes, and the impact of CF sputum on the activity of the cathelicidin peptides were evaluated as well.
MATERIALS AND METHODS
Peptides.
The activities of the following five parent compounds derived from mammalian species were evaluated: LL37 (human [1]), CAP18 (rabbit [22, 23]), mCRAMP (mouse [13, 27]), rCRAMP (rat), and SMAP29 (sheep [2, 20, 26]). In addition, the activities of five shorter peptides were investigated, including three novel alpha-helical peptides derived from SMAP29 and termed ovispirins (OV-1, OV-2, and OV-3) and two truncated forms of CAP18 (CAP18-18 and CAP 18-22). The ovispirins are 14- to 18-amino-acid linear peptides designed to mimic the antimicrobial activity of SMAP29, which has 29 amino acids. The rat sequence (rCRAMP) was identified by a homology search with the mouse protein (mCRAMP) sequence and corresponded to GenBank accession number AA998531. The amino acid sequences of the peptides used in this study are shown in Table 1.
TABLE 1.
Sequences of cathelicidin peptides tested against clinical isolates from CF patients
| Peptide | Species | Peptide sequencea |
|---|---|---|
| LL37 | Human | LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES |
| CAP-18 | Rabbit | GLRKRLRKFRNKIKEKLKKIGQKIQGLLPKLAPRTDY |
| CAP-18-18 | Rabbit | KRLRKFRNKIKEKLKKIG |
| CAP18-22 | Rabbit | RKRLRKFRNKIKEKLKKIGQKI |
| mCRAMP | Mouse | GLLRKGGEKIGEKLKKIGQKIKNFFQKLVPQPEQ |
| rCRAMP | Rat | GLVRKGGEKFGEKLRKIGQKIKEFFQKLALEIEQ |
| SMAP29 | Sheep | RGLRRLGRKIAHGVKKYGPTVLRIIRIAG |
| OV-1 | Sheep | KNLRRIIRKIIHIIKKYG |
| OV-2 | Sheep | LRRIIRKIIHIIKK-NH2b |
| OV-3 | Sheep | IRRIIRKIIHIIKK-NH2b |
Underlined amino acids are charged residues.
NH2 indicates C-terminal amidation.
The peptides were synthesized on an Applied Biosystems model 433A synthesizer at the 0.1 mM scale, using solid-phase Fastmoc chemistry as described previously (33). Peptides were purified by reverse-phase high-pressure liquid chromatography on a Vydac 218TP1022 column. Selected fractions were pooled and lyophilized for further characterization by mass spectrometry and capillary electrophoresis. Peptide concentrations were determined by quantitative amino acid analysis on a Beckman 6300 amino acid analyzer.
Clinical isolates.
The activities of the peptides were tested in vitro using antibiotic-resistant CF clinical isolates sent to the CF Referral Center at Columbia University (30). Strains were stored at −70°C and subcultured once on blood agar prior to use in this study. Twenty-four P. aeruginosa strains (including 13 mucoid and 11 nonmucoid isolates), 5 B. cepacia, 5 S. maltophilia, and 5 A. xylosoxidans strains were studied. Each isolate was from a single patient and resistant to several conventional antimicrobial agents (Table 2). Species identification was confirmed by selective media, the bacterial identification system API 20 NE (bioMérieux, Hazelwood, Mo.), and the P. aeruginosa-specific exoA probe (31). Identification of the B. cepacia strains was further confirmed by the CF Referral Laboratory using genus-specific primers (25).
TABLE 2.
Resistance of clinical isolates from CF patients to conventional antimicrobial agentsa
| Pathogen (no. of strains) | % Resistance to:
|
||||||
|---|---|---|---|---|---|---|---|
| TIM | CAZ | MEM | CIP | TOB | AMK | SXT | |
| P. aeruginosa(n = 24) | 67 | 71 | ND | 33 | 63 | 67 | ND |
| B. cepacia(n = 5) | 67 | 100 | 100 | 100 | ND | ND | 100 |
| S. maltophilia(n = 5) | 100 | 100 | 100 | 80 | 100 | ND | 100 |
| A. xylosoxidans(n = 5) | 80 | 40 | ND | 80 | 100 | 100 | ND |
Abbreviations: TIM, Timentin; CAZ, ceftazidime; MEM, meropenem; CIP, ciprofloxacin; TOB, tobramycin; AMK, amikacin; SXT, trimethoprim-sulfamethosoxazole; ND, not determined.
Susceptibility testing.
The MICs of each peptide were determined using broth microdilution assays (Microtech Medical Systems, Aurora, Oreg.). This assay has been shown to be comparable to agar dilution for testing the antimicrobial susceptibility of multiply antibiotic-resistant P. aeruginosa isolated from patients with CF (31). Microtiter plates were prepared immediately prior to use with serial twofold dilutions of peptides (0.06 to 32 μg/ml) suspended in Mueller-Hinton (MH) broth ([Na+] = 128 mM; [Cl−] = 108 mM). Peptide concentrations were increased to 128 μg/ml when testing B. cepacia strains. Microtiter wells were inoculated with 105 CFU of bacteria per ml, incubated at 35°C, and read at 20 to 24 h (31). Each assay was performed at least twice. The MIC range and the MIC at which 50% of the strains tested were inhibited (MIC50) were determined for each peptide. Reproducibility studies with SMAP29, CAP18, and mCRAMP using four clinical strains of P. aeruginosa (two mucoid and two nonmucoid strains) and P. aeruginosa strain ATCC 27853 were performed in triplicate on each of three days.
Synergy studies. (i) Time-kill studies.
The P. aeruginosa laboratory strain PAO1 (18) and a nonmucoid, tobramycin-susceptible CF clinical isolate, 50BK, were grown to a 0.5 McFarland standard in Trypticase soy broth. The MICs of tobramycin and SMAP29 for PAO1 were 2 and 4 μg/ml, respectively, and the MICs of tobramycin and SMAP29 for 50BK were 2 and 1 μg/ml, respectively. Various concentrations of tobramycin (0.5 to 1.0 μg/ml) or SMAP29 (0.5 to 2.0 μg/ml) or a combination of tobramycin and SMAP29 were suspended in cation-supplemented MH broth, and 105 CFU of bacteria per ml were added and incubated at 37°C. Samples were taken at 0, 1, 4, 8 and 24 h, and suitable dilutions were made in 0.9% NaCl, plated on sheep blood agar, and incubated for 24 h at 37°C to determine CFU/ml. Synergy was defined as a ≥100-fold increase in killing at 4 h (9).
(ii) Checkerboard dilution studies.
The activities of ceftazidime (1 to 16 μg/ml), piperacillin (4 to 64 μg/ml), ciprofloxacin (0.125 to 2 μg/ml), or tobramycin (0.5 to 8 μg/ml) paired with increasing concentrations of SMAP29 (0.125 to 2 μg/ml) were tested against the 24 isolates of P. aeruginosa. Serial twofold dilutions of the peptide were added to commercially prepared microtiter plates containing the four conventional antibiotics (Microtech Medical Systems). The fractional inhibitory concentrations were calculated as previously described using an inoculum of 105 CFU/ml (30). Synergy was defined as a ≥4-fold decrease in the MIC of both the peptide and the conventional drug when tested together compared with the MIC of each agent tested alone. Synergy studies were performed using the five B. cepacia strains and testing four peptides (SMAP29, OV-1, OV-2, and OV-3) in concentrations between 16 and 128 μg/ml paired with the same four conventional antibiotics.
Efficacy of SMAP29 against bacteria in CF sputum.
The antimicrobial effect of SMAP29 against clinical isolates in CF sputum was evaluated. Sputum was obtained from a CF patient, placed on ice, and processed within 1 h. Two strains of P. aeruginosa were identified: one mucoid strain and one multiply antibiotic-resistant, nonmucoid strain. The MICs of tobramycin for the mucoid and nonmucoid strains were <0.5 and 16 μg/ml, respectively, and the MIC of SMAP29 for both strains was 0.5 μg/ml. The sputum was mixed with sterile, filtered 20% glycerol (1 mg/ml) and then divided into 75-μl aliquots and frozen at −80°C for later use.
Three aliquots of the sputum sample were thawed, and 10 μl of each aliquot was serially diluted 1:10 in PBS to obtain 1:101 to 1:105 dilutions. The 1:103 to 1:105 dilutions were plated on sheep blood agar plates to determine the initial CFU/ml. The remaining 65 μl of each sputum aliquot was mixed with 65 μl of PBS and tobramycin (final concentration, 250 μg/ml) or SMAP29 (final concentration, 250 or 1,000 μg/ml), vortexed for 15 s, and incubated at 37°C. Ten microliters of sample was removed from each experimental condition at 10, 60, and 240 min of incubation, serially diluted, and plated as described above to determine the CFU/ml.
After 240 min of incubation, the remaining 100 μl of each aliquot was divided into two 50-μl aliquots. Aliquots containing tobramycin (concentration, 250 μg/ml), SMAP29 (250 μg/ml), or SMAP29 (1,000 μg/ml) each received an additional dose of tobramycin or SMAP29 to obtain final concentrations of 500, 500, and 2,000 μg/ml, respectively. All samples were then incubated at 37°C, and 10 μl of sample was removed at 250, 300, and 480 min from the start of the experiment, serially diluted, and plated as described above. All plates were incubated for 2 days at 37°C. Resulting colonies were manually counted and multiplied by the appropriate dilution factor(s) to determine the initial CFU/ml. Studies were performed three times.
Scanning electron microscopy of P. aeruginosa treated with cathelicidin peptides or tobramycin.
A single colony of P. aeruginosa PAO1 grown on Luria-Bertani (LB) agar was incubated overnight at 37°C in LB media. Bacteria were then diluted (1:10) into fresh LB media and agitated at 37°C for 4 h, and cell density was determined by measuring the optical density at 600 nm. Following centrifugation at 3,000 × g for 10 min, the bacteria were resuspended in 10 mM KPi and 1% LB medium to a concentration of 108 CFU per 200 μl. Bacteria were then exposed to SMAP29 (0.5 μg/ml), tobramycin (5 μg/ml), or media alone. Bacteria (200 μl) from each exposure were placed on a Millicell polycarbonate filter (0.4-μm pore size; Millipore Corporation, Bedford, Mass.) and incubated at 37°C. After incubation periods of 1 min, 2 h, and 16 h, the liquid was removed from the filter by gravitational force and the bacteria were fixed on the filter with 2% paraformaldehyde–2% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.2). Samples were postfixed in 1% osmium tetroxide, dehydrated through a graded ethanol series, dried with hexamethyldisilizane, mounted on Cambridge-style stubs, sputter coated with a 60/40 mixture of gold/palladium, and imaged using a Hitachi S-4000 FESEM (Tokyo, Japan). Images were captured using a Kevex Sigma digital-image capture system.
Assessment of epithelial toxicity.
Primary cultures of fully differentiated human airway epithelia were exposed to LL37, SMAP29, OV-1, and OV-2 to determine if the peptides affected the transepithelial resistance. Human tracheal epithelia were grown at the air-liquid interface on permeable supports as previously described (35, 36) for ≥14 days to ensure that the cells were fully differentiated. Peptides in concentrations of 0, 1, 10, and 100 μg/ml were suspended in PBS and applied to the apical surfaces of the airway epithelia for an exposure period of 2.5 h (for SMAP29, OV-1, and OV-2) or 4 h (for LL37) in a 50-μl volume. The transepithelial resistance was measured using an EVOM (World Precision Inst., Sarasota, Fla.) Volt-Ohm meter.
RESULTS
Activity of cathelicidin peptides against P. aeruginosa
The sheep peptide SMAP29 (MIC50, 1 μg/ml) and the rabbit peptide CAP18 (MIC50, 4 μg/ml) demonstrated the greatest activity against the 24 clinical strains of P. aeruginosa (Table 3). In contrast, LL37, mCRAMP, and rCRAMP were less active (MIC50, 32 μg/ml). Reproducibility studies with three peptides (SMAP29, CAP18, and mCRAMP) testing five strains in triplicate on each of three days demonstrated that these results were highly consistent (data not shown).
TABLE 3.
Activity of cathelicidin peptides against P. aeruginosa, S. maltophilia, and A. xylosoxidans isolated from patients with CF
| Peptides |
P. aeruginosa
|
S. maltophilia
|
A. xylosoxidans
|
||
|---|---|---|---|---|---|
| MIC range (μg/ml) | MIC50 (μg/ml) | MIC90 (μg/ml) | MIC range (μg/ml) | MIC range (μg/ml) | |
| LL37 | 4–64 | 32 | 64 | 2–32 | >32 |
| CAP18 | 1–32 | 4 | 16 | 2–16 | 2–32 |
| CAP18-18 | 1–64 | 16 | 64 | NTa | NT |
| CAP18-22 | 0.5–32 | 4 | 16 | 4–32 | 1–32 |
| mCRAMP | 1–64 | 32 | 64 | 4–32 | >32 |
| rCRAMP | 8–64 | 32 | 64 | NT | NT |
| SMAP29 | 0.06–8 | 1 | 2 | 1–8 | 1–32 |
| OV-1 | 0.125–16 | 8 | 16 | NT | NT |
| OV-2 | 0.03–16 | 2 | 4 | NT | NT |
| OV-3 | 1–8 | 2 | 4 | NT | NT |
NT, not tested.
Activity of truncated synthetic peptide derivatives against P. aeruginosa
The 18-amino-acid ovispirin OV-1 (MIC50, 8 μg/ml) and the 14-amino-acid derivatives OV-2 (MIC50, 2 μg/ml) and OV-3 (MIC50, 2 μg/ml) had activities comparable to that of the parent compound SMAP29 (Table 3). The shorter CAP18 peptide derivatives had somewhat less activity against P. aeruginosa than did SMAP29 and its derivatives, as the MIC50 of the 18- and 22-amino-acid derivatives CAP18-18 and CAP18-22 were 16 and 4 μg/ml, respectively.
Activities of peptides against B. cepacia, S. maltophilia, and A. xylosoxidans.
The activities of the cathelicidin peptides against other gram-negative, multidrug-resistant pathogens were also evaluated (Table 3). The peptides had some activity against strains of S. maltophilia and A. xylosoxidans (MIC range, 1 to >32 μg/ml), and in general, CAP18 and SMAP29 had the greatest activity. None of the peptides tested in this study had activity against the five strains of B. cepacia (MICs, >128 μg/ml).
Synergy studies. (i) Time-kill studies.
Time-kill studies were done to determine whether the combination of tobramycin and SMAP29 had synergistic activity against P. aeruginosa. This combination of agents reproducibly reduced the CFU of PAO1 ≥100-fold at 4 h compared to either tobramycin or SMAP29 alone (Fig. 1A). However, the effects were reversed by 8 h and full growth occurred by 24 h (data not shown). To determine whether the bacterial population became resistant to the peptide or tobramycin, a PAO1 culture previously exposed to SMAP29 and tobramycin for 24 h was reexposed to each compound alone or in combination (Fig. 1B). Time-kill studies reproducibly demonstrated inhibition by 4 h, suggesting that resistance to these agents did not occur. Time-kill studies performed with PAO1 in the presence of piperacillin and SMAP29 showed indifference, as enhanced killing was not noted (data not shown). Similarly, there was no synergy with tobramycin and SMAP29 against the clinical isolate 50BK (Fig. 1C).
FIG. 1.
Time-kill studies. (A and B) P. aeruginosa PAO1 exposed to tobramycin (0.5 μg/ml), SMAP29 (2 μg/ml), or a combination of the two drugs. (A) Organisms after the first 8 h of exposure to each drug. (B) The organisms exposed to tobramycin and SMAP29 in combination were grown for 24 h and then rediluted and exposed to fresh tobramycin, SMAP29, or a combination of the two drugs (C) P. aeruginosa clinical strain 50BK exposed to tobramycin (1.0 μg/ml), SMAP29 (0.5 μg/ml), or SMAP29* (1.0 μg/ml).
(ii) Checkerboard dilution studies.
Synergy was also evaluated using checkerboard dilution assays. None of the tested combinations of conventional antibiotics paired with peptides had demonstrable synergistic activity against the 24 clinical isolates of P. aeruginosa or the 5 B. cepacia strains tested (data not shown).
Activity of SMAP29 in CF sputum.
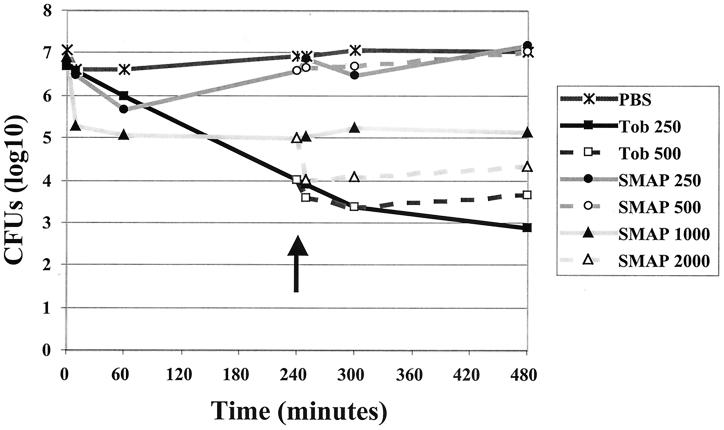
The activities of tobramycin and SMAP29 against P. aeruginosa in CF sputum were compared (Fig. 2). Tobramycin (250 μg/ml) caused a decrease of 103 CFU by 240 min and a further decrease of 101 CFU by 480 min. Increasing the concentration of tobramycin to 500 μg/ml had little effect on bacterial killing. SMAP29 (250 μg/ml) exhibited minimal initial killing in CF sputum, and no long-term effect was noted. Increasing the concentration to 500 μg/ml at 240 min did not enhance killing. However, 1,000 μg of SMAP29 per ml caused a rapid decline of 102 in bacterial counts. Adding additional SMAP29 at 240 min to achieve a final concentration of 2,000 μg/ml led to a further decline of 101 CFU.
FIG. 2.
Activity of SMAP29 against P. aeruginosa in CF sputum. The activities against strains of P. aeruginosa in CF sputum of tobramycin (250 μg/ml), SMAP29 (250 or 1,000 μg/ml), and a PBS control with no antibiotics were compared. CFU/ml were determined after 10, 60, 240, 300, and 480 min of exposure to antibiotics as shown. The effects of the addition of a second dose at 240 min of tobramycin (final concentration, 500 μg/ml) or SMAP29 (final concentration, 500 or 2,000 μg/ml) are also shown. The arrow indicates the timing of the addition of the second dose of antibiotics. The results are representative of three replicate studies.
Effects of cathelicidin peptides on bacterial morphology.
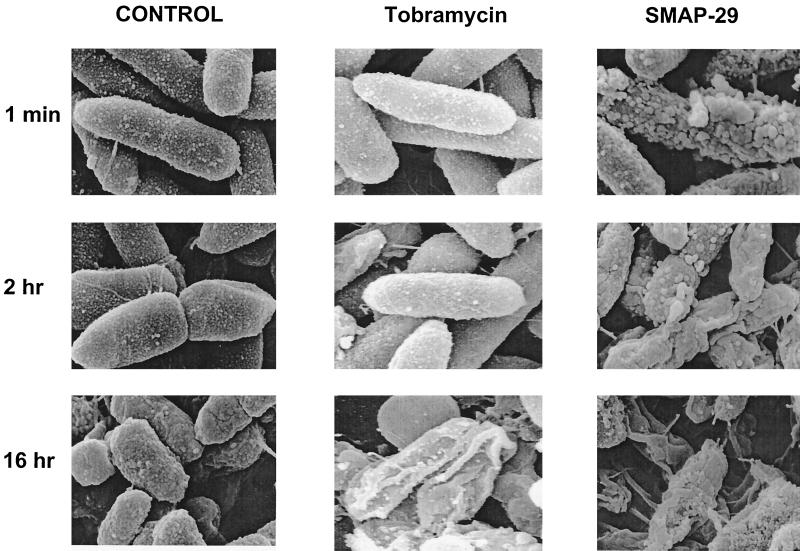
Time-kill studies indicated that SMAP29 had a rapid onset of effect, reducing bacterial CFU within 1 h of treatment (Fig. 1). This rapid onset of effect was further confirmed using electron microscopy. SMAP29 caused dramatic alterations in the cell wall of PAO1 within 1 min (Fig. 3). Numerous blebs or blister-like changes progressed with time. Cell debris was also noted, suggesting that cell destruction had occurred. In contrast, bacteria treated with tobramycin exhibited changes in morphology that occurred at approximately 16 h of incubation. CAP18 induced changes in bacterial cells that were similar in time course and morphology to those elicited by SMAP29 (data not shown).
FIG. 3.
Effects of SMAP29 or tobramycin treatment on the morphology of P. aeruginosa PAO1 evaluated by scanning electron microcopy. PAO1 was treated with media alone, tobramycin (5 μg/ml), or SMAP29 (0.5 μg/ml). At 1 min, 2 h, and 16 h the bacteria were processed for scanning electron microscopy. Within one minute, treatment with SMAP29 resulted in blebbing or blistering of the outer cell wall. The effects of tobramycin were much slower in their onset.
Effects of cathelicidin peptides on transepithelial resistance.
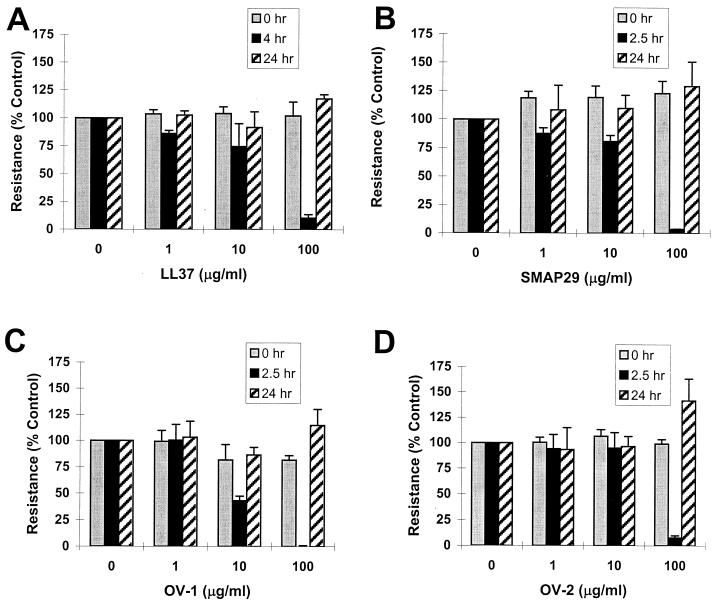
To determine if cathelicidin peptides were toxic to mammalian epithelial cells, epithelial barrier properties were examined in treated and untreated cells. Apical application of cathelicidins to primary cultures of well-differentiated human airway epithelia caused a dose-dependent, reversible decrease in transepithelial resistance (Fig. 4). The drop in resistance was most apparent at the highest peptide concentrations (100 μg/ml). Following removal of the peptide at 2.5 and 4 h, there was complete recovery to baseline transepithelial resistance by 24 h.
FIG. 4.
Effects of cathelicidin peptides on the transepithelial resistance of primary cultures of human airway epithelia. Transepithelial resistance was measured at baseline and after 2.5 h (SMAP29, OV-1, and OV-2) or 4 h (LL37) of exposure to the peptides. The peptides were studied at concentrations of 1, 10, and 100 μg/ml. Transepithelial resistance values shown are normalized to 100% at zero hour. Following the peptide exposure measurement, the solution containing the peptide was removed and replaced with peptide-free solution for measurement of resistance at 24 h. Representative results are shown. The studies were performed three times in quadruplicate except for the LL37 studies, which were performed twice.
DISCUSSION
The cathelicidin antimicrobial peptides possess several unique properties that may make them desirable therapeutic agents (16, 24, 32, 33, 37). Cathelicidin peptides are strongly cationic and have little secondary structure in aqueous solution. These antimicrobial peptides adopt an alpha-helical structure only after binding to negatively charged bacterial cell wall components such as lipopolysaccharide (LPS) or lipoteichoic acid (24, 32, 33). In contrast to most conventional antibiotics, cathelicidin peptides have a rapid effect on bacteria, as confirmed by our electron microscopy studies, which demonstrated profound morphological changes within 1 min of exposure to SMAP29 or CAP18. These changes presumably are associated with permeabilization of the bacterial cell membranes (24, 32). Because the bactericidal activity of peptides involves binding to cell wall components followed by disruption of bacterial membranes, it is unusual for bacteria to develop resistance to cathelicidins. In addition to their antimicrobial effects, there is evidence that the peptides' LPS binding activity reduces the incidence of septic shock in animal models of endotoxemia (14, 17).
The studies presented here demonstrate the potential clinical applications of both naturally occurring and synthetic antimicrobial peptides for CF patients. We showed that the sheep and rabbit cathelicidin-derived peptides exhibit potent activities against a large number of CF clinical isolates of multiply antibiotic-resistant pathogens, including nonmucoid and mucoid strains of P. aeruginosa and some strains of S. maltophilia, and A. xylosoxidans. The 14- to 18-amino-acid ovispirins and the CAP18 derivatives also demonstrated significant activities. These shorter peptides may be better suited to pharmaceutical scale production than the longer parent compounds. Consistent with previous observations, the peptides demonstrated no activity against B. cepacia (38).
However, other studies have shown synergy between antibacterial peptides and conventional antibiotics (38). Our results are more variable and suggest less benefit. SMAP29 showed evidence of synergy with conventional antibiotics in time-kill studies against P. aeruginosa strain PAO1, but not the CF clinical isolate 50BK. Similarly, no evidence of synergy between peptides and conventional agents was observed in checkerboard dilution studies of 24 clinical P. aeruginosa isolates and 5 B. cepacia isolates. These results suggest that different test conditions and strains, including virulence properties and antibiotic susceptibility of the isolates tested, may account for the variable response to antibiotic combinations.
While several of the cathelicidin peptides demonstrated activity against CF strains of P. aeruginosa grown in microtiter wells, the activity of SMAP29 against P. aeruginosa in CF sputum was markedly reduced. CF sputum can reduce the activity of tobramycin 10- to 100-fold due to binding to sputum mucins (19). The observed reduction in peptide antimicrobial activity in the presence of sputum may be secondary to the presence of anionic substances including DNA and mucins that bind the cationic peptides and reduce bactericidal activity (19) or may be secondary to poor growth of the bacteria in sputum as demonstrated by the PBS control. Furthermore, P. aeruginosa organisms exist as a biofilm within the CF lung (6). Bacterial biofilms are structured communities of bacteria encased in a self-produced polymeric matrix. These organisms are more resistant to antimicrobial treatment and are known to express genes different from those of planktonically grown bacteria (5, 6). As recently demonstrated, P. aeruginosa isolates grown in a biofilm have altered expression of genes that control LPS synthesis, which results in specific lipid A modifications (10). Such changes could alter the binding of cationic antimicrobial peptides. The results of the present study and previous observations indicate that cationic peptides may be more useful as agents for early prophylactic treatment of CF lung disease rather than as late interventions for treatment of established bacterial biofilms.
While morphological effects were noted in the bacterial cell membrane within 1 min of exposure to the cathelicidin peptides studied, such rapid effects were not noted when airway epithelia were exposed to the same peptide. Reversible changes in transepithelial resistance occurred after 2.5 to 4 h of exposure. Furthermore, the concentrations at which these changes occurred were 50- to 100-fold greater than the doses associated with killing of planktonically grown clinical strains of P. aeruginosa, although similar to those needed for killing in CF sputum. A similar lack of toxicity to mammalian cells has been noted by other investigators (24, 32, 33). These findings suggest that the effective bactericidal concentrations for the peptides studied may be below the range at which toxicity to host cell membranes is detected. Thus, these peptides may be safely used as aerosolized agents.
There has been a renewed interest in the use of aerosolized antibiotics in the management of CF lung disease (4, 28). Aerosolization has the advantage of minimizing systemic absorption and therefore toxicity by allowing increased concentrations of an antimicrobial agent to be delivered directly to the endobronchial site of infection. Numerous agents have been aerosolized for use as suppressive therapy in patients infected with P. aeruginosa and to prevent infection and colonization (4). Most recently, aerosolized tobramycin has been shown to improve pulmonary function in patients chronically infected with P. aeruginosa and is the first agent to be approved for suppressive use (28).
These studies suggest that cathelicidin peptides have substantial in vitro activity against P. aeruginosa isolates from CF patients. At concentrations shown to have antimicrobial activity, little effect was seen on an in vitro model of polarized human airway epithelia. However, further studies in animal models are needed to understand whether these in vitro results will translate into favorable in vivo efficacy and safety profiles.
ACKNOWLEDGMENTS
We thank Elena Rus and Brian Morrison of the Protein Structure Facility at the University of Iowa for peptide synthesis. We thank Phil Karp and Pary Weber for preparation of the human airway cell cultures and Randy Nessler for technical assistance with scanning electron microscopy.
This work was supported in part by the Cystic Fibrosis Foundation (P.B.M.; 97ZO) and NIH HL-61234 (P.B.M. and B.F.T.). P.L.W. was supported by a Merit Review grant from the Veterans Administration. We acknowledge the support of the Morphology Core and Cell Culture Core, partially supported by the Cystic Fibrosis Foundation, NHLBI (PPG HL51670-05), the Carver Foundation, and the Center for Gene Therapy for Cystic Fibrosis (NIH P30 DK-97-010).
REFERENCES
- 1.Agerberth B, Gunne H, Odeberg J, Kogner P, Boman H G, Gudmundsson G H. FALL-39, a putative human peptide antibiotic, is cysteine-free and expressed in bone marrow and testis. Proc Natl Acad Sci USA. 1995;92:195–199. doi: 10.1073/pnas.92.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bagella L, Scocchi M, Zanetti M. cDNA sequences of three sheep myeloid cathelicidins. FEBS Lett. 1995;376:225–228. doi: 10.1016/0014-5793(95)01285-3. [DOI] [PubMed] [Google Scholar]
- 3.Burns J L, Emerson J, Stapp J R, Yim D L, Krzewinski J, Louden L, et al. Microbiology of sputum from patients at cystic fibrosis centers in the United States. Clin Infect Dis. 1998;27:158–163. doi: 10.1086/514631. [DOI] [PubMed] [Google Scholar]
- 4.Campbell P W, III, Saiman L. Use of aerosolized antibiotics in patients with cystic fibrosis. Chest. 1999;116:775–788. doi: 10.1378/chest.116.3.775. [DOI] [PubMed] [Google Scholar]
- 5.Costerton J W, Lewandowski Z, Caldwell D E, Korber D R, Lappin-Scott H M. Microbial biofilms. Annu Rev Microbiol. 1995;49:711–745. doi: 10.1146/annurev.mi.49.100195.003431. [DOI] [PubMed] [Google Scholar]
- 6.Costerton J W, Stewart P S, Greenberg E P. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 7.Demko C A, Byard P J, Davis P B. Gender differences in cystic fibrosis: Pseudomonas aeruginosa infection. J Clin Epidemiol. 1995;48:1041–1049. doi: 10.1016/0895-4356(94)00230-n. [DOI] [PubMed] [Google Scholar]
- 8.Demko C A, Stern R C, Doershuk C F. Stenotrophomonas maltophilia in cystic fibrosis: incidence and prevalence. Pediatr Pulmonol. 1998;25:304–308. doi: 10.1002/(sici)1099-0496(199805)25:5<304::aid-ppul3>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 9.Eliopolous G M, Moellering R C. Antimicrobial combinations. In: Lorian V, editor. Antibiotics in laboratory medicine. 4th ed. Baltimore, Md: Williams & Wilkins Co.; 1996. pp. 330–396. [Google Scholar]
- 10.Ernst R K, Yi E C, Guo L, Lim K B, Burns J L, Hackett M, Miller S I. Specific lipopolysaccharide found in cystic fibrosis airway Pseudomonas aeruginosa. Science. 1999;286:1561–1565. doi: 10.1126/science.286.5444.1561. [DOI] [PubMed] [Google Scholar]
- 11.FitzSimmons S C. The changing epidemiology of cystic fibrosis. J Pediatr. 1993;122:1–9. doi: 10.1016/s0022-3476(05)83478-x. [DOI] [PubMed] [Google Scholar]
- 12.FitzSimmons S C. Cystic Fibrosis Foundation patient registry annual data report. Bethesda, Md: Cystic Fibrosis Foundation; 1997. [Google Scholar]
- 13.Gallo R L, Kim K J, Bernfield M, Kozak C A, Zanetti M, Merluzzi L, Gennaro R. Identification of CRAMP, a cathelin-related antimicrobial peptide expressed in the embryonic and adult mouse. J Biol Chem. 1997;272:13088–13093. doi: 10.1074/jbc.272.20.13088. [DOI] [PubMed] [Google Scholar]
- 14.Gough H, Hancock R E W, Kelly N M. Antiendotoxin activity of cationic peptide antimicrobial agents. Infect Immun. 1996;64:4922–4927. doi: 10.1128/iai.64.12.4922-4927.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamosh A, FitzSimmons S C, Macek M, Jr, Knowles M R, Rosenstein B J, Cutting G R. Comparison of the clinical manifestations of cystic fibrosis in black and white patients. J Pediatr. 1998;132:255–259. doi: 10.1016/s0022-3476(98)70441-x. [DOI] [PubMed] [Google Scholar]
- 16.Hancock R E W, Lehrer R. Cationic peptides: a new source of antibiotics. Trends Biotechnol. 1998;16:82–88. doi: 10.1016/s0167-7799(97)01156-6. [DOI] [PubMed] [Google Scholar]
- 17.Hancock R E W. Peptide antibiotics. Lancet. 1997;349:418–422. doi: 10.1016/S0140-6736(97)80051-7. [DOI] [PubMed] [Google Scholar]
- 18.Holloway B W, Krishnapillai V, Morgan A F. Chromosomal genetics of Pseudomonas. Microbiol Rev. 1979;43:73–102. doi: 10.1128/mr.43.1.73-102.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hunt B E, Weber A, Berger A, Ramsey B, Smith A L. Macromolecular mechanisms of sputum inhibition of tobramycin activity. Antimicrob Agents Chemother. 1995;39:34–39. doi: 10.1128/aac.39.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huttner K M, Lambeth M R, Burkin H R, Burkin D J, Broad T E. Localization and genomic organization of sheep antimicrobial peptide genes. Gene. 1998;206:85–91. doi: 10.1016/s0378-1119(97)00569-6. [DOI] [PubMed] [Google Scholar]
- 21.Kerem E, Corey M, Gold R, Levison H. Pulmonary function and clinical course in patients with cystic fibrosis after pulmonary colonization with Pseudomonas aeruginosa. J Pediatr. 1990;116:714–719. doi: 10.1016/s0022-3476(05)82653-8. [DOI] [PubMed] [Google Scholar]
- 22.Larrick J W, Hirata M, Balint R F, Lee J, Zhong J, Wright S C. Human CAP18: a novel antimicrobial lipopolysaccharide-binding protein. Infect Immun. 1995;63:1291–1297. doi: 10.1128/iai.63.4.1291-1297.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Larrick J W, Morgan J G, Palings I, Hirata M, Yen M H. Complementary DNA sequence of rabbit CAP18 B a unique lipopolysaccharide binding protein. Biochem Biophys Res Commun. 1991;179:170–175. doi: 10.1016/0006-291x(91)91350-l. [DOI] [PubMed] [Google Scholar]
- 24.Lehrer R I, Barton A, Ganz T. Concurrent assessment of inner and outer membrane permeabilization and bacteriolysis in E. coli by multiple-wavelength spectrophotometry. J Immunol Methods. 1988;108:153–158. doi: 10.1016/0022-1759(88)90414-0. [DOI] [PubMed] [Google Scholar]
- 25.LiPuma J J, Dulaney B J, McMenamin J D, Whitby P W, Stull T L, Coenye T, Vandamme P. Development of rRNA-based PCR assays for identification of Burkholderia cepacia complex isolates recovered from cystic fibrosis patients. J Clin Microbiol. 1999;37:3167–3170. doi: 10.1128/jcm.37.10.3167-3170.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahoney M M, Lee A Y, Brezinski-Caliguri D J, Huttner K M. Molecular analysis of the sheep cathelin family reveals a novel antimicrobial peptide. FEBS Lett. 1995;377:519–522. doi: 10.1016/0014-5793(95)01390-3. [DOI] [PubMed] [Google Scholar]
- 27.Popsueva A E, Zinovjeva M V, Visser J W M, Zijlmans J M, Fibbe W E, Belyavsky A V. A novel murine cathelin-like protein expressed in bone marrow. FEBS Lett. 1996;391:5–8. doi: 10.1016/0014-5793(96)00692-8. [DOI] [PubMed] [Google Scholar]
- 28.Ramsey B W, Dorkin H L, Eisenberg J D, Gibson R L, Harwood I R, Kravitz R M, Schidlow D V, Wilmott R W, Astley S J, McBurnie M A. Efficacy of aerosolized tobramycin in patients with cystic fibrosis. N Engl J Med. 1993;328:1740–1746. doi: 10.1056/NEJM199306173282403. [DOI] [PubMed] [Google Scholar]
- 29.Rosenstein B J, Zeitlin P L. Cystic fibrosis. Lancet. 1998;351:277–282. doi: 10.1016/S0140-6736(97)09174-5. [DOI] [PubMed] [Google Scholar]
- 30.Saiman L, Mehar F, Niu W W, Neu H G, Shaw K J, Miller G, Prince A. Antibiotic susceptibility of multiply resistant Pseudomonas aeruginosa isolated from cystic fibrosis patients, including transplant candidates. Clin Infect Dis. 1996;23:532–537. doi: 10.1093/clinids/23.3.532. [DOI] [PubMed] [Google Scholar]
- 31.Saiman L, Burns J L, Whittier S, Krzewinski S, Marshall S A, Jones R A. Evaluation of reference dilution test methods for antimicrobial susceptibility testing of Pseudomonas aeruginosa isolated from patients with cystic fibrosis. J Clin Microbiol. 1999;37:2987–2991. doi: 10.1128/jcm.37.9.2987-2991.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Skerlavaj B, Benincasa M, Risso A, Zanetti M, Gennaro R. SMAP29: a potent antibacterial and antifungal peptide from sheep leukocytes. FEBS Lett. 1999;463:58–62. doi: 10.1016/s0014-5793(99)01600-2. [DOI] [PubMed] [Google Scholar]
- 33.Travis S M, Anderson N, Forsyth W R, Espiritu C, Conway B D, Greenberg E P G, McCray P B, et al. Bactericidal activity of mammalian cathelicidin peptides. Infect Immun. 2000;68:2748–2755. doi: 10.1128/iai.68.5.2748-2755.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vu-Thien H, Moissenet D, Valcin M, Dulot C, Tournier G, Garbarg-Chenon Molecular epidemiology of Burkholderia cepacia, Stenotrophomonas maltophilia, and Alcaligenes xylosoxidans in cystic fibrosis center. Eur J Clin Microbiol Infect Dis. 1996;15:876–879. doi: 10.1007/BF01691221. [DOI] [PubMed] [Google Scholar]
- 35.Yamaya M, Finkbeiner W E, Chun S Y, Widdecombe J H. Differentiated structure and function of cultures from human tracheal epithelium. Am J Physiol (Lung Cell Mol Physiol) 1992;262:L713–L724. doi: 10.1152/ajplung.1992.262.6.L713. [DOI] [PubMed] [Google Scholar]
- 36.Zabner J, Zeiher B G, Friedman E, Welsh M J. Adenovirus-mediated gene transfer to ciliated airway epithelia requires prolonged incubation time. J Virol. 1996;70:6994–7003. doi: 10.1128/jvi.70.10.6994-7003.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zannetti M, Gennaro R, Romeo D. Cathelicidins; a novel protein family with a common proregins and a variable C-terminal antimicrobial domain. FEBS Lett. 1995;374:1–5. doi: 10.1016/0014-5793(95)01050-o. [DOI] [PubMed] [Google Scholar]
- 38.Zhang L, Benz R, Hancock R E W. Influence of proline residues on the antibacterial and synergistic activities of alpha-helical peptides. Biochemistry. 1999;38:8102–8111. doi: 10.1021/bi9904104. [DOI] [PubMed] [Google Scholar]