Abstract
BACKGROUND:
Stepped wedge cluster randomized trials enable rigorous evaluations of health intervention programs in pragmatic settings. The current study aims to update neurosurgeon scientists on the design of stepped wedge randomized trials.
METHODS:
An overview of recent methodological developments for stepped wedge designs is presented. An update on newer associated methodological tools is included to aid with future study designs.
RESULTS:
The stepped wedge trial design is defined. Indications for the design are reviewed in depth. Key considerations are discussed including mainstream methods of analysis and sample size determination.
COINCLUSIONS:
Stepped wedge designs can be attractive to study intervention programs aiming to improve the delivery of patient care, especially when examining a small number of heterogeneous clusters.
Keywords: Cluster randomized trials, Intraclass correlation coefficient, Sample size determination, Stepped wedge design, Statistical software, Methodological review
INTRODUCTION
Cluster randomized designs are commonly used in pragmatic trial research evaluating the effect of healthcare interventions that are delivered to clinics, hospitals, or nursing homes.1,2 A key feature of these designs is that intact clusters are randomized to each arm, and outcome measurements are typically taken from each participant, just like individual randomized designs. Statistical methods for cluster randomized trials (CRTs) have been extensively studied for decades and have been made accessible in published methodological reviews.3,4 A recent variant of CRTs, called the stepped wedge cluster randomized trials (SW-CRTs), are gaining traction, from just a few published studies two decades ago to more than 40 protocols per year nowadays.5–7 In an SW-CRT, the implementation of intervention is staggered over time such that each cluster is randomized to a time point when the intervention starts to roll out. A typical feature of SW-CRTs is that all clusters will eventually be exposed to the intervention during the study period. Figure 1 provided an illustration of a SW-CRT with the Early Recognition and Response to Increases in Surgical Site Infection (Early 2RIS) Trial.8 Thirteen surgical procedures were classified into six types (e.g., cardiac, spine, etc). Clusters, constructed at each of the 29 study hospitals based on the type of surgical procedure performed, were the units for randomization and analysis. The study randomized 105 clusters over 14 periods to assess the effectiveness of surveillance using optimized statistical process control methods and feedback on rates of surgical site infections compared to traditional infection surveillance methods. In Figure 1, there are 12 randomization sequences; each randomization sequence is determined by the first period a group of clusters crossover from control to intervention. This is in comparison to parallel-arm designs, where usually half of clusters are simultaneously randomized to either intervention or control at baseline.
Figure 1.
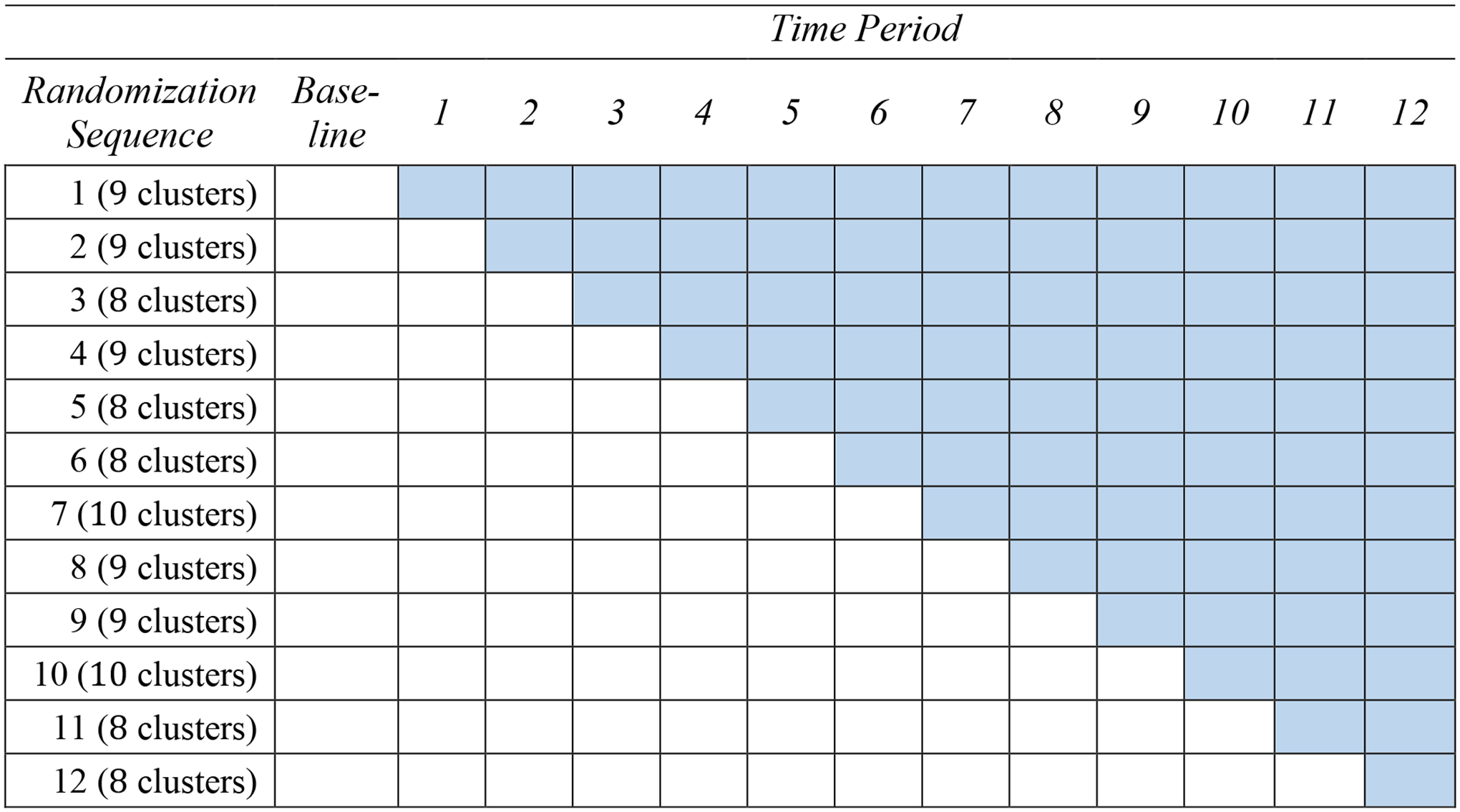
A schematic of stepped wedge design for the Early Recognition and Response to Increases in Surgical Site Infection (Early 2RIS) Trial. The shaded cell indicates treatment status, and a white cell indicates control status. There are 12 randomization sequences (defined by the first time period during which each group of clusters crossover to intervention). There are in total 105 clusters and 13 time periods. In the Early 2RIS trial, the baseline period is one year, and each subsequent period is 3 months.
Compared to parallel CRTs, SW-CRTs require more sophisticated statistical considerations for design and analysis. For example, the staggered treatment initiation induces confounding by time and unbiased estimation of intervention effect requires statistical adjustment for secular trend; repeated outcome assessment from each cluster also necessitates considerations for complex correlation structures among outcomes.6,9–11 While SW-CRTs have been successfully deployed in many previous trials, with interventions for infectious disease prevention,12 diagnostic imaging,13 geriatric care,14 among others, few neurosurgical studies have adopted the stepped wedge design. In this review, we explore current state of methodological developments for stepped wedge designs and reflect upon potential opportunities for their adoption in neurosurgery trials.
STEPPED WEDGE DESIGNS—DEFINED
Design Variants
In a stepped wedge design, each cluster initially starts from the control period where baseline outcome measures are collected. In each subsequent period, a random selected group of clusters will cross over from control to intervention and the outcome data are collected from each cluster. Depending on how outcomes from participants are collected from each cluster, broadly there are three design variants.15 A cross-sectional design enrolls new participants from each cluster during each period, whereas a closed-cohort design identifies a cohort of participants at the beginning of the study and schedules repeated follow-up outcome assessments for the same cohort over time. An open-cohort design, however, allows the attrition of members and the addition of new members to the existing cohort identified at baseline in each cluster. The choice of design variant is often based on the research question and practical considerations. For example, a closed-cohort design necessitates a longer follow-up time for each participant and can run the risk of informative drop-out, while a cross-sectional design often involves continuous recruitment and only retain each participant for a single period for a short exposure.15
When is a Stepped Wedge Design Appropriate?
The decision to adopt a SW-CRT are based on several considerations.7 First, the successive rollout of intervention to clusters in a SW-CRT ensures all health care units receive intervention before the end of study, and can facilitate cluster recruitment when the intervention is perceived to be effective with minimal harm to providers and patients.16,17 This contrasts with a parallel design, which only allows a subset of the health care units to receive the intervention during the study. Second, the logistical resources and efforts may be less demanding in a SW-CRT if the intervention is rolled out according to a staggered schedule.16,17 Third, a SW-CRT collects outcome data from multiple time periods, and offers the advantage of having each cluster contribute observations under both the intervention and control conditions. As a result, while the treatment effect estimation based on the parallel design only makes use of information from between-cluster comparisons, the stepped wedge design pools information from both within-cluster comparisons and between-cluster comparisons,18,19 and can require fewer clusters to achieve the same amount of statistical power.10 This is especially the case when the intraclass correlation coefficient (ICC) is high or the cluster sizes are large.7
On the other hand, there are also limitations associated with SW-CRTs. For example, SW-CRT involves repeated data collection and can often give rise to a study with a longer duration. There may also be potential for biases due to insufficient control of secular trends (defined as the outcome trajectory in the absence of intervention) affected by a concurrent external intervention program, or unexpected disruption from a pandemic. The decision to adopt a SW-CRT, therefore, should deserve a comprehensive evaluation by weighing the associated strengths against limitations in specific trial contexts.
STATISTICAL CONSIDERATIONS FOR STEPPED WEDGE DESIGNS
Method of Analysis—Conditional Models
To account for clustering between data observations, two mainstream regression models for analyzing SW-CRTs are the conditional (cluster-specific) models and marginal (population-averaged) models. The conditional models refer to the class of mixed-effects models, which specify fixed effects for the secular trend and the intervention effect, and random effects to characterize the correlation among observations collected from the same cluster.11 The widely-used linear mixed model, originally proposed in Hussey and Hughes,18 includes a categorical fixed effect for the secular trend, a common intervention effect and a single cluster-level random intercept. The random intercept implies a common ICC both within the same time period and across any two different time periods. However, this can be a rather strong assumption, especially for trials with a longer duration.20,21
Model extensions have been developed to represent the outcome trajectories and correlations in SW-CRTs in a more flexible fashion. For example, under a cross-sectional design, including a random cluster-by-time interaction accounts for unobserved time-varying factors within each cluster, and differentiates between the so-called within-period and between-period ICCs.22–24 Alternatively, Kasza et al.21 developed the exponential decay model which allows the between-period ICC to decay at an exponential rate over each discrete time period. This model has been generalized to allow for continuous-time correlation decay.25 Besides random deviations across time-periods, model extensions can further accommodate a random-intervention effect to address treatment effect heterogeneity by clusters.26,27 This may be particularly relevant if a study recruits heterogeneous clusters, such as neurosurgeons from multiple hospitals or intensive care units across multiple healthcare systems with diverse health care practice. Under a closed-cohort design, an additional participant-level random effect should be included to adjust for the serial correlation between repeated measurements.22,28 Regardless of design variants, while the most common practice is to assume a categorical secular trend and a common fixed-effect to describe the intervention effect, linear mixed models can be modified with a smooth parametric secular trend (e.g., a linear trend) and delayed intervention effect, which are more appropriate when an intervention requires additional time to become fully embedded and influence the study endpoint.11,26,29
Conditional models have been most frequently used for SW-CRTs, according to recent systematic reviews of published trials.30 They allow flexible specification of complex random effect structures, and have the ability to directly quantify between-cluster heterogeneity through variance component parameters.11 Model parameters are estimated by maximum likelihood or restricted maximum likelihood methods (the latter produces more accurate estimates for the random-effects variance parameters), which now becomes standard in statistical software such as SAS and R.31 Table 1 provides a select summary of model variants and associated software for model fitting. Limitations of the mixed-effects models include that the interpretation of the treatment effect parameter can depend on the specification of random-effects structure.32,33 The validity of hypothesis testing and confidence interval estimation for the treatment effect also critically depends on correct modeling assumptions in these models including the random-effects structure.34
Table 1.
Select summary of conditional and marginal model variants and associated statistical software for estimating model parameters.
| CONDITIONAL MODELS | |||
|---|---|---|---|
| Model (random-effects structure) | Feature | SAS and R Software Package | Reference |
| Random intercept model | One common ICC |
PROC MIXED (SAS)
PROC GLIMMIX (SAS) nlme (R) lme4 (R) |
Hussey and Hughes18 |
| Random cluster-by-time interaction model | Allows for different within-period and between-period ICCs |
PROC MIXED (SAS)
PROC GLIMMIX (SAS) nlme (R) lme4 (R) |
Hooper et al.22 Girling and Hemming24 |
| Random cluster-by-time interaction model (cohort designs) | Allows for different within-period, between-period and repeated measures ICCs |
PROC MIXED (SAS)
PROC GLIMMIX (SAS) nlme (R) lme4 (R) |
Hooper et al.22 Girling and Hemming24 |
| Exponential decay model | Between-period ICC decays exponentially over time |
PROC MIXED (SAS)
PROC HPMIXED (SAS) |
Kasza et al.21 Kasza and Forbes73 |
| Random intervention model | Allows for heterogeneous treatment effect by clusters |
PROC MIXED (SAS)
PROC GLIMMIX (SAS) nlme (R) lme4 (R) |
Hughes et al.26 Hemming et al.27 |
| MARGINAL MODELS | |||
| Model (working correlation structure) | Feature | SAS and R Software Package | Reference |
| Simple exchangeable structure | One common ICC |
PROC GEE (SAS)
PROC GLIMMIX (SAS) gee (R) geepack (R) geesmv (R) |
Hussey and Hughes18 Thompson et al.46 Ford and Westgate47 |
| Nested exchangeable structure | Allows for within-period and between-period ICCs |
%GEECORR (SAS macro)
geepack (R) geeCRT (R) |
Li et al.36,37 |
| Block exchangeable structure (cohort designs) | Allows for within-period, between-period and repeated measures ICCs |
%GEECORR (SAS macro)
geepack (R) geeCRT (R) |
Li et al.36,37 |
| Exponential decay structure | Between-period ICC decays exponentially over time | geeCRT (R) | Li et al.37 |
| Proportional decay structure (cohort designs) | Both the between-period ICC and the repeated measured ICC decay exponentially over time | Available on GitHub and Journal Website | Li41 |
The code for implementing the proportional decay GEE model is available at https://github.com/lifanfrank/Li_Quasi-least-squares_SWD and https://onlinelibrary.wiley.com/doi/10.1002/sim.8415.
Method of Analysis—Marginal Models
Marginal models fitted with the generalized estimating equations (GEE)35 provide a natural approach to analyze SW-CRTs because the population-averaged treatment effect is typically of primary interest. This approach requires the specification of a marginal mean model and a working correlation structure. For SW-CRTs, marginal models are considered more robust because the interpretation of the treatment effect parameter does not depend on the correlation model specification, and that valid estimation and inference of treatment effect does not require the correlation structure to be correctly specified.36,37 This suggests the use of the independence working correlation model coupled with a sandwich standard error as a simple way to analyze SW-CRTs. However, the working independence assumption can lead to loss of statistical power in SW-CRTs, and should be used with caution.34,37–39
In SW-CRTs, the marginal mean model includes a population-averaged secular trend and treatment effect parameter. Because careful modeling of the correlation structure can lead to a more efficient treatment effect estimator, a suitable correlation model often directly describes the within-time and between-time ICC parameters on the natural scale of the outcome measurements.40 Parallel to the development of conditional models, the development of marginal models for SW-CRTs have also investigated different parameterizations of the correlation models, given a categorical secular trend and an average intervention effect parameter. Under a cross-sectional design, Hussey and Hughes18 described the simple exchangeable correlation model, mimicking the linear mixed model with a single cluster-level random intercept. To differentiate the within-period and between-period ICCs, Li et al.36 considered the nested exchangeable correlation structure, which resembles the mixed-effects model with an additional random cluster-by-time interaction. An exponential decay correlation structure has also been studied in the marginal model context with an exponential family type outcome.37 Under a closed-cohort design, the block exchangeable correlation structure and the proportional decay structure have been proposed to accommodate correlation for repeated outcome measurements taken from the same participant.36,41
Compared to mixed-effects models, a caveat of marginal models is that the robust standard errors may exhibit negative bias with a limited number of clusters (often not exceeding 30). In a recent systematic review of SW-CRTs, Grayling et al.5 reported that the median number of clusters in published SW-CRTs is below 25, and small-sample corrections of the sandwich standard error becomes particularly important for marginal model inference in SW-CRTs.42–44 Several simulation studies have reported the performance of small-sample corrections for GEE robust standard errors, and recommended the Kauermann and Carroll, or the Fay and Graubard standard errors for SW-CRTs.36,41,45–47 Finally, while the general method of GEE have been developed decades ago, software that permits the simultaneous estimation of treatment effect and multiple ICC parameters for SW-CRT applications are relatively limited (Table 1). Computational challenges in GEE methods for CRTs with large cluster sizes have also been investigated in Chen et al.48 with a stochastic GEE and Li et al.37 with an efficient cluster-period GEE method.
Method of Analysis—Randomization-Based Inference
Randomization-based inference provides a flexible alternative to analyze SW-CRTs. In this approach, the outcome data in an SW-CRT are first analyzed based on the actual randomized allocation. Then the observed statistics is referenced against the exact randomization distribution obtained by permuting the time points clusters crossover to intervention status.49 Under the strong null hypothesis, the randomization test can preserve the type I error rate without requiring a correctly-specified correlation structure in a linear mixed model.50,51 Thompson et al.52 and Kennedy-Shaffer et al.53 developed test statistics that are more robust to modeling assumptions on secular trend by leveraging the between-cluster contrasts in outcomes. To alleviate the computational burden associated with enumerating all possible randomized allocations, Hughes et al.54 derived the closed-form permutation variances of the test statistic and showed that the resulting randomization test preserved the valid type I error even when both the mean and covariance structures are incorrectly specified. Beyond testing, Rabideau and Wang55,56 developed a computationally efficient method to estimate randomization-based confidence intervals for the treatment effect in SW-CRTs.
Sample Size Determination
For a continuous outcome, the required number of clusters in an SW-CRT for a desired level of power depends on the number of time periods, number of participants per period, effect size and ICC parameters.9,10,18,57 An analytical sample size formula was developed in Hussey and Hughes for the random intercept model,18 and has been extended by a number of authors to address more complex random-effects structures.11,21,22,25,58 Table 2 provides a select summary of sample size procedures and the associated statistical software. A tutorial and R Shiny App can be found in Hemming et al.59 In the design stage, sample size estimates can be sensitive to the choice of random-effect structure. While historical or routinely collected data can be useful for drawing assumptions on the random-effects or correlation structures, in the absence of such information, sensitivity analysis is recommended to obtain more robust sample size estimates.21 In simple cases, the relationship between sample size and ICC parameters have been studied, which facilitates the choice of design parameters. For example, under the cross-sectional design, the required sample size increases with larger within-period ICC but decreases with larger between-period ICC, suggesting that a conservative between-period ICC value is unlikely to result in an underpowered study.36 Table 2 also reveals that there are relatively more methods devoted to estimating the sample size in SW-CRTs with a continuous outcome, whereas software for binary and count outcomes (except for those based on linear mixed model approximation) is limited, even though they are common especially for patient-reported outcomes in clinical research. Zhou et al.60 developed a maximum likelihood procedure to obtain the sample size for cross-sectional SW-CRTs with a binary outcome, and found inadequacy from the conventional linear mixed model approximation.18 Li et al.36 developed a GEE approach to obtain the sample size with binary outcomes. These sample size methods for binary outcomes have been implemented in an R package swdpwr.61
Table 2.
Select summary of sample size methods for stepped wedge cluster randomized trials and related software.
| Outcome Type | Design | Analysis | Software | Reference |
|---|---|---|---|---|
| Continuous | Cross-sectional | Linear mixed model (random intercept) |
swCRTdesign (R)
74
swdpwr (R) 61 SWSamp (R) 75 SteppedPower (R) * %swdpwr (SAS macro) 61 steppedwedge (Stata) 76 Shiny CRT Calculator (Web-based) 59 |
Hussey and Hughes18 |
| Linear mixed model (random cluster-by-time interaction) |
swCRTdesign (R)
74
swdpwr (R) 61 SteppedPower (R) * %swdpwr (SAS macro) 61 Shiny CRT Calculator (Web-based) 59 |
Hooper et al.22 | ||
| Linear mixed model (exponential decay) | Shiny CRT Calculator (Web-based) 59 | Kasza et al.21 | ||
| Linear mixed model (random intervention) | swCRTdesign (R) 74 | Hughes et al.26 Hemming et al.27 |
||
| GEE (nested exchangeable correlation structure) |
swdpwr (R)
61
%swdpwr (SAS macro) 61 |
Li et al.36 | ||
| Closed-cohort | Linear mixed model (random cluster-by-time interaction) |
swdpwr (R)
61
%swdpwr (SAS macro) 61 Shiny CRT Calculator (Web-based) 59 |
Hooper et al.22 | |
| GEE (block exchangeable correlation structure) |
swdpwr (R)
61
%swdpwr (SAS macro) 61 |
Li et al.36 | ||
| GEE (proportional decay correlation structure) | SteppedPower (R) * | Li41 | ||
| Open-cohort | Linear mixed model (random cluster-by-time interaction or exponential decay) | SteppedPower (R) * | Kasza et al.58 | |
| Binary | Cross-sectional | Linear mixed model approximation |
swCRTdesign (R)
74
SWSamp (R) 75 steppedwedge (Stata) 76 Shiny CRT Calculator (Web-based) 59 |
Hussey and Hughes18 |
| Linear probability mixed model (random intercept) |
swdpwr (R)
61
%swdpwr (SAS macro) 61 |
Zhou et al.60 | ||
| GEE (nested exchangeable correlation structure) |
swdpwr (R)
61
%swdpwr (SAS macro) 61 |
Li et al.36 | ||
| GEE (exponential decay correlation structure) | Available on GitHub or Journal website † | Harrison and Wang38 Tian et al.39 |
||
| Closed-cohort | GEE (block exchangeable correlation structure) |
swdpwr (R)
61
%swdpwr (SAS macro) 61 |
Li et al.36 |
The SteppedPower R package is available on The Comprehensive R Archive Network (CRAN) at https://cran.r-project.org/web/packages/SteppedPower/index.html
The code for sample size estimation with exponential decay correlation structure can be found in https://github.com/lindajaneharrison/SW-CRTs/releases/tag/v2.0 for Harrison and Wang38, and in https://github.com/Zebedial/SWD-variable-cluster-sizes or https://onlinelibrary.wiley.com/doi/full/10.1002/bimj.202100112 for Tian et al.39
A typical assumption made in many sample size formulas is that the same number of participants are recruited in each cluster per period. This assumption, while convenient for deriving sample size formulas, is often violated, for example, when each intensive care unit in a SW-CRT has different patient volumes or each surgeon corresponds to a different patient panel size.62 Martin et al.63 studied the implication of unequal cluster sizes in SW-CRTs and conclude that the average power is less affected in SW-CRTs compared to a parallel design. Girling64 suggested variance inflation expressions between unequal versus equal cluster sizes assuming a linear mixed model analysis of SW-CRTs. Harrison et al.65 provided a set of sample size expressions which specifically includes the coefficient of variation of cluster sizes. Methods for addressing unequal cluster sizes were also extended to accommodate SW-CRTs with a binary outcome.38,39
OPPORTUNITIES FOR CONSIDERING STEPPED WEDGE DESIGNS IN FUTURE NEUROSURGERY TRIALS
Stepped wedge designs are relatively uncommon in existing neurosurgical trials, but they can provide a robust design option for future studies. Besides the Early 2RIS trial (Figure 1), we identified four additional SW-CRT examples related to neurosurgery66–69 and summarized them in Table 3. In these trials, a neurosurgeon or a service unit performing neurosurgical operations was the unit of randomization, and the study intervention program often aimed at improving surgery-related patient care. Furthermore, the advantage of rolling out the intervention to all clusters and logistical convenience in staggered implementation were the primary reasons for choosing a stepped wedge design. Following these published trials, when limited resource or capacity is available to simultaneously roll out the program in a future neurosurgery trial, a SW-CRT may be considered as a robust design to effectively study intervention programs. In addition, three trials in Table 3 included fewer than 10 clusters, in which case a stepped wedge design can be more powerful than the parallel-arm design by leveraging both within-cluster and between-cluster comparisons. Finally, three out of four SW-CRTs in Table 3 adopted the linear or generalized linear mixed models for the design and analysis, whereas our methodological review suggests that marginal models are viable alternatives for designing and analyzing SW-CRTs. Application of marginal models to future neurosurgical SW-CRTs can be based on software tools listed in Table 1 and 2.
Table 3.
Examples of stepped wedge cluster randomized trials related to neurosurgery.
| Publication | Description | Reasons for SW-CRT | Relevance |
|---|---|---|---|
| Palmay et al.66 | Rolling out an intensive care unit (ICU) audit-and-feedback program to 6 non-ICU services, and evaluating targeted antimicrobial utilization. Location: Toronto, Canada. |
1. All participating clusters receive the intervention during the study. 2. Overcomes the financial or workload difficulties in concurrent roll-out. |
One of the inpatient services receiving intervention is a neurosurgery unit. |
| Haugen et al.67 | Rolling out the WHO Surgical Safety Checklist intervention to 5 surgical specialties (clusters), and evaluating outcomes including morbidity, mortality and length of hospital stay. Location: Norway |
1. Unethical not to deliver or retract intervention with perceived benefit. 2. Logistical and financial reasons to stagger the intervention delivery |
One of the surgical specialties is neurosurgery. |
| Schwarze et al.68 | Rolling out the question prompt list intervention to 40 surgeons and assessing its effectiveness on patient engagement and well-being among patients considering major surgery. Location: United States. |
1. Allows all surgeons to have access to the intervention during the study, and avoided contamination between study participants. | The participating surgeons include neurosurgeons performing high-risk neurosurgical operations. |
| Malone et al.69 | Assessing the impact of two de-implementation strategies, order set change and facilitation training, across 9 Children’s Hospitals. Location: United States |
1. Allows for phased implementation of intervention with logistical convenience. 2. Permits all clusters to receive intervention and therefore increases participation. |
The intervention aims to reduce unnecessary post-operative antibiotics in surgical procedures performed by surgeons, including neurosurgeons. |
CONCLUSION
The review in this article provides an accessible entry point to the recent developments in methods for SW-CRTs, particularly on available tools to assist design and analysis. A critical consideration in using existing tools for sample size calculation is the assumptions on unknown ICCs. It has been encouraged in the CRT and SW-CRT literature70 71 to report ICCs to facilitate the design of future trials with similar endpoints. Beyond reporting ICCs, a high-quality SW-CRT should also clearly describe the modeling assumptions for secular trend, and the random-effects or correlation structures to ensure reproducible sample size calculation.11 The CONSORT extension to SW-CRTs72 is devoted to developing the recommended practices for conduct and reporting, which serve as a principal guidance for researchers working with SW-CRTs. By providing key conceptual and analytical considerations, we aspire to encourage researchers to evaluate the potential for adopting a stepped wedge design in their study and thereby help with generating high-quality treatment effect evidence for patient care.
ACKNOWLEDGEMENT
We thank the Editor, the Section Editor, and two Reviewers for their comments and suggestions, which led to an improved paper. This work was in part supported by R01 AI136947 from the National Institute of Allergy and Infectious Diseases.
Abbreviations and Acronyms:
- CRT
cluster randomized trial
- ICC
intraclass correlation coefficient
- SW-CRT
stepped wedge cluster randomized trials
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
None.
CREDIT AUTHOR STATEMENT
Both authors contributed to the conceptualization of, drafting, reviewing and editing the manuscript.
REFERENCES
- 1.Cook AJ, Delong E, Murray DM, Vollmer WM, Heagerty PJ. Statistical lessons learned for designing cluster randomized pragmatic clinical trials from the NIH Health Care Systems Collaboratory Biostatistics and Design Core. Clin Trials. 2016;13(5):504–512. doi: 10.1177/1740774516646578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weinfurt KP, Hernandez AF, Coronado GD, et al. Pragmatic clinical trials embedded in healthcare systems: generalizable lessons from the NIH Collaboratory. BMC Med Res Methodol. 2017;17(1):144. doi: 10.1186/s12874-017-0420-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turner EL, Li F, Gallis JA, Prague M, Murray DM. Review of Recent Methodological Developments in Group-Randomized Trials: Part 1—Design. Am J Public Health. 2017;107(6):907–915. doi: 10.2105/AJPH.2017.303706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turner EL, Prague M, Gallis JA, Li F, Murray DM. Review of Recent Methodological Developments in Group-Randomized Trials: Part 2—Analysis. Am J Public Health. 2017;107(7):1078–1086. doi: 10.2105/AJPH.2017.303707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grayling MJ, Wason JM, Mander AP. Stepped wedge cluster randomized controlled trial designs: a review of reporting quality and design features. Trials. 2017;18(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taljaard M, Hemming K, Shah L, Giraudeau B, Grimshaw JM, Weijer C. Inadequacy of ethical conduct and reporting of stepped wedge cluster randomized trials: Results from a systematic review. Clinical Trials. 2017;14(4):333–341. [DOI] [PubMed] [Google Scholar]
- 7.Hemming K, Taljaard M. Reflection on modern methods: when is a stepped-wedge cluster randomized trial a good study design choice? International Journal of Epidemiology. 2020;49(3):1043–1052. doi: 10.1093/ije/dyaa077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson DJ, Ilieş I, Foy K, et al. Early recognition and response to increases in surgical site infections using optimized statistical process control charts—the Early 2RIS Trial: a multicenter cluster randomized controlled trial with stepped wedge design. Trials. 2020;21(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hemming K, Lilford R, Girling AJ. Stepped-wedge cluster randomised controlled trials: a generic framework including parallel and multiple-level designs. Statistics in Medicine. 2015;34(2):181–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hemming K, Taljaard M. Sample size calculations for stepped wedge and cluster randomised trials: a unified approach. Journal of Clinical Epidemiology. 2016;69:137–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li F, Hughes JP, Hemming K, Taljaard M, Melnick ER, Heagerty PJ. Mixed-effects models for the design and analysis of stepped wedge cluster randomized trials: An overview. Statistical Methods in Medical Research. 2021;30(2):612–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Golden MR, Kerani RP, Stenger M, et al. Uptake and population-level impact of expedited partner therapy (EPT) on Chlamydia trachomatis and Neisseria gonorrhoeae: the Washington State community-level randomized trial of EPT. PLoS Med. 2015;12(1):e1001777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jarvik JG, Meier EN, James KT, et al. The effect of including benchmark prevalence data of common imaging findings in spine image reports on health care utilization among adults undergoing spine imaging: a stepped-wedge randomized clinical trial. JAMA Network Open. 2020;3(9):e2015713–e2015713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoogendijk EO, van der Horst HE, van de Ven PM, et al. Effectiveness of a Geriatric Care Model for frail older adults in primary care: Results from a stepped wedge cluster randomized trial. Eur J Intern Med. 2016;28:43–51. doi: 10.1016/j.ejim.2015.10.023 [DOI] [PubMed] [Google Scholar]
- 15.Copas AJ, Lewis JJ, Thompson JA, Davey C, Baio G, Hargreaves JR. Designing a stepped wedge trial: three main designs, carry-over effects and randomisation approaches. Trials. 2015;16(1):352. doi: 10.1186/s13063-015-0842-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hargreaves JR, Copas AJ, Beard E, et al. Five questions to consider before conducting a stepped wedge trial. Trials. 2015;16(1):1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prost A, Binik A, Abubakar I, et al. Logistic, ethical, and political dimensions of stepped wedge trials: critical review and case studies. Trials. 2015;16(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hussey MA, Hughes JP. Design and analysis of stepped wedge cluster randomized trials. Contemporary Clinical Trials. 2007;28(2):182–191. [DOI] [PubMed] [Google Scholar]
- 19.Matthews JNS, Forbes AB. Stepped wedge designs: insights from a design of experiments perspective. Statistics in Medicine. 2017;36(24):3772–3790. [DOI] [PubMed] [Google Scholar]
- 20.Taljaard M, Teerenstra S, Ivers NM, Fergusson DA. Substantial risks associated with few clusters in cluster randomized and stepped wedge designs. Clinical Trials. 2016;13(4):459–463. [DOI] [PubMed] [Google Scholar]
- 21.Kasza J, Hemming K, Hooper R, Matthews JNS, Forbes AB. Impact of non-uniform correlation structure on sample size and power in multiple-period cluster randomised trials. Statistical Methods in Medical Research. 2019;28(3):703–716. [DOI] [PubMed] [Google Scholar]
- 22.Hooper R, Teerenstra S, de Hoop E, Eldridge S. Sample size calculation for stepped wedge and other longitudinal cluster randomised trials. Statistics in Medicine. 2016;35(26):4718–4728. [DOI] [PubMed] [Google Scholar]
- 23.Hemming K, Taljaard M, Forbes A. Analysis of cluster randomised stepped wedge trials with repeated cross-sectional samples. Trials. 2017;18(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Girling AJ, Hemming K. Statistical efficiency and optimal design for stepped cluster studies under linear mixed effects models. Statistics in Medicine. 2016;35(13):2149–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grantham KL, Kasza J, Heritier S, Hemming K, Forbes AB. Accounting for a decaying correlation structure in cluster randomized trials with continuous recruitment. Statistics in Medicine. 2019;38(11):1918–1934. [DOI] [PubMed] [Google Scholar]
- 26.Hughes JP, Granston TS, Heagerty PJ. Current issues in the design and analysis of stepped wedge trials. Contemporary Clinical Trials. 2015;45:55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hemming K, Taljaard M, Forbes A. Modeling clustering and treatment effect heterogeneity in parallel and stepped-wedge cluster randomized trials. Statistics in Medicine. 2018;37(6):883–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li F, Turner EL, Preisser JS. Optimal allocation of clusters in cohort stepped wedge designs. Statistics & Probability Letters. 2018;137:257–263. [Google Scholar]
- 29.Nickless A, Voysey M, Geddes J, Yu L-M, Fanshawe TR. Mixed effects approach to the analysis of the stepped wedge cluster randomised trial—Investigating the confounding effect of time through simulation. PloS one. 2018;13(12):e0208876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barker D, McElduff P, D’Este C, Campbell MJ. Stepped wedge cluster randomised trials: a review of the statistical methodology used and available. BMC Medical Research Methodology. 2016;16(1):1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pinheiro J, Bates D. Mixed-Effects Models in S and S-PLUS. Springer Science & Business Media; 2006. [Google Scholar]
- 32.Drum M, McCullagh P. [Regression Models for Discrete Longitudinal Responses]: Comment. Statistical Science. 1993;8(3):300–301. [Google Scholar]
- 33.Heagerty PJ, Zeger SL. Marginalized multilevel models and likelihood inference (with comments and a rejoinder by the authors). Statistical Science. 2000;15(1):1–26. [Google Scholar]
- 34.Thompson JA, Fielding KL, Davey C, Aiken AM, Hargreaves JR, Hayes RJ. Bias and inference from misspecified mixed-effect models in stepped wedge trial analysis. Statistics in Medicine. 2017;36(23):3670–3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liang K-Y, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73(1):13–22. [Google Scholar]
- 36.Li F, Turner EL, Preisser JS. Sample size determination for GEE analyses of stepped wedge cluster randomized trials. Biometrics. 2018;74(4):1450–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li F, Yu H, Rathouz PJ, Turner EL, Preisser JS. Marginal modeling of cluster-period means and intraclass correlations in stepped wedge designs with binary outcomes. Biostatistics. Published online 2021. doi: 10.1093/biostatistics/kxaa056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harrison LJ, Wang R. Power calculation for cross-sectional stepped-wedge cluster randomized trials with binary outcomes. Statistics in Medicine. Published online 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tian Z, Preisser J, Esserman D, Turner E, Rathouz P, Li F. Impact of unequal cluster sizes for GEE analyses of stepped wedge cluster randomized trials with binary outcomes. Biometrical Journal. Published online 2021. doi: 10.1101/2021.04.07.21255090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Preisser JS, Young ML, Zaccaro DJ, Wolfson M. An integrated population-averaged approach to the design, analysis and sample size determination of cluster-unit trials. Statistics in Medicine. 2003;22(8):1235–1254. [DOI] [PubMed] [Google Scholar]
- 41.Li F. Design and analysis considerations for cohort stepped wedge cluster randomized trials with a decay correlation structure. Statistics in Medicine. 2020;39(4):438–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mancl LA, DeRouen TA. A covariance estimator for GEE with improved small-sample properties. Biometrics. 2001;57(1):126–134. [DOI] [PubMed] [Google Scholar]
- 43.Kauermann G, Carroll RJ. A note on the efficiency of sandwich covariance matrix estimation. Journal of the American Statistical Association. 2001;96(456):1387–1396. [Google Scholar]
- 44.Lu B, Preisser JS, Qaqish BF, Suchindran C, Bangdiwala SI, Wolfson M. A comparison of two bias-corrected covariance estimators for generalized estimating equations. Biometrics. 2007;63(3):935–941. [DOI] [PubMed] [Google Scholar]
- 45.Scott JM, deCamp A, Juraska M, Fay MP, Gilbert PB. Finite-sample corrected generalized estimating equation of population average treatment effects in stepped wedge cluster randomized trials. Statistical Methods in Medical Research. 2017;26(2):583–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thompson JA, Hemming K, Forbes A, Fielding K, Hayes R. Comparison of small-sample standard-error corrections for generalised estimating equations in stepped wedge cluster randomised trials with a binary outcome: A simulation study. Statistical Methods in Medical Research. Published online 2020:0962280220958735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ford WP, Westgate PM. Maintaining the validity of inference in small-sample stepped wedge cluster randomized trials with binary outcomes when using generalized estimating equations. Statistics in Medicine. 2020;39(21):2779–2792. [DOI] [PubMed] [Google Scholar]
- 48.Chen T, Tchetgen ET, Wang R. A stochastic second-order generalized estimating equations approach for estimating intraclass correlation coefficient in the presence of informative missing data. Journal of Computational and Graphical Statistics. 2020;29(3):547–561. doi: 10.1080/10618600.2019.1710156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Good P Permutation Tests: A Practical Guide to Resampling Methods for Testing Hypotheses. Springer Science & Business Media; 2013. [Google Scholar]
- 50.Wang R, De Gruttola V. The use of permutation tests for the analysis of parallel and stepped-wedge cluster-randomized trials. Statistics in Medicine. 2017;36(18):2831–2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ji X, Fink G, Robyn PJ, Small DS. Randomization inference for stepped-wedge cluster-randomized trials: an application to community-based health insurance. The Annals of Applied Statistics. 2017;11(1):1–20. [Google Scholar]
- 52.Thompson JA, Davey C, Fielding K, Hargreaves JR, Hayes RJ. Robust analysis of stepped wedge trials using cluster-level summaries within periods. Statistics in Medicine. 2018;37(16):2487–2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kennedy-Shaffer L, De Gruttola V, Lipsitch M. Novel methods for the analysis of stepped wedge cluster randomized trials. Statistics in Medicine. 2020;39(7):815–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hughes JP, Heagerty PJ, Xia F, Ren Y. Robust inference for the stepped wedge design. Biometrics. 2020;76(1):119–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rabideau DJ, Wang R. Randomization-based confidence intervals for cluster randomized trials. Biostatistics. 2021;22(4):913–927. doi: 10.1093/biostatistics/kxaa007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rabideau DJ, Wang R. Randomization-based inference for a marginal treatment effect in stepped wedge cluster randomized trials. Statistics in Medicine. Published online 2021. doi: 10.1002/sim.9040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rhoda DA, Murray DM, Andridge RR, Pennell ML, Hade EM. Studies with staggered starts: multiple baseline designs and group-randomized trials. American Journal of Public Health. 2011;101(11):2164–2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kasza J, Hooper R, Copas A, Forbes AB. Sample size and power calculations for open cohort longitudinal cluster randomized trials. Statistics in Medicine. 2020;39(13):1871–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hemming K, Kasza J, Hooper R, Forbes A, Taljaard M. A tutorial on sample size calculation for multiple-period cluster randomized parallel, cross-over and stepped-wedge trials using the Shiny CRT Calculator. International Journal of Epidemiology. 2020;49(3):979–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhou X, Liao X, Kunz LM, Normand S-LT, Wang M, Spiegelman D. A maximum likelihood approach to power calculations for stepped wedge designs of binary outcomes. Biostatistics. 2020;21(1):102–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen J, Zhou X, Li F, Spiegelman D. swdpwr: A SAS Macro and An R Package for Power Calculation in Stepped Wedge Cluster Randomized Trials. arXiv preprint arXiv:201106031. Published online 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kristunas C, Morris T, Gray L. Unequal cluster sizes in stepped-wedge cluster randomised trials: a systematic review. BMJ open. 2017;7(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Martin JT, Hemming K, Girling A. The impact of varying cluster size in cross-sectional stepped-wedge cluster randomised trials. BMC Medical Research Methodology. 2019;19(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Girling AJ. Relative efficiency of unequal cluster sizes in stepped wedge and other trial designs under longitudinal or cross-sectional sampling. Statistics in Medicine. 2018;37(30):4652–4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Harrison LJ, Chen T, Wang R. Power calculation for cross-sectional stepped wedge cluster randomized trials with variable cluster sizes. Biometrics. 2020;76(3):951–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Palmay L, Elligsen M, Walker SA, et al. Hospital-wide rollout of antimicrobial stewardship: a stepped-wedge randomized trial. Clinical infectious diseases. 2014;59(6):867–874. [DOI] [PubMed] [Google Scholar]
- 67.Haugen AS, Søfteland E, Almeland SK, et al. Effect of the World Health Organization checklist on patient outcomes: a stepped wedge cluster randomized controlled trial. Annals of surgery. 2015;261(5):821–828. [DOI] [PubMed] [Google Scholar]
- 68.Schwarze ML, Buffington A, Tucholka JL, et al. Effectiveness of a question prompt list intervention for older patients considering major surgery: a multisite randomized clinical trial. JAMA surgery. 2020;155(1):6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Malone S, McKay VR, Krucylak C, et al. A cluster randomized stepped-wedge trial to de-implement unnecessary post-operative antibiotics in children: the optimizing perioperative antibiotic in children (OPerAtiC) trial. Implementation Science. 2021;16(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Campbell MK, Grimshaw JM, Elbourne DR. Intracluster correlation coefficients in cluster randomized trials: empirical insights into how should they be reported. BMC Medical Research Methodology. 2004;4(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Martin J, Taljaard M, Girling A, Hemming K. Systematic review finds major deficiencies in sample size methodology and reporting for stepped-wedge cluster randomised trials. BMJ open. 2016;6(2):e010166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hemming K, Taljaard M, McKenzie JE, et al. Reporting of stepped wedge cluster randomised trials: extension of the CONSORT 2010 statement with explanation and elaboration. BMJ. 2018;363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kasza J, Forbes AB. Inference for the treatment effect in multiple-period cluster randomised trials when random effect correlation structure is misspecified. Statistical Methods in Medical Research. 2019;28(10–11):3112–3122. [DOI] [PubMed] [Google Scholar]
- 74.Voldal EC, Hakhu NR, Xia F, Heagerty PJ, Hughes JP. swCRTdesign: An R Package for Stepped Wedge Trial Design and Analysis. Computer Methods and Programs in Biomedicine. 2020;196:105514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Baio G, Copas A, Ambler G, Hargreaves J, Beard E, Omar RZ. Sample size calculation for a stepped wedge trial. Trials. 2015;16(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hemming K, Girling A. A menu-driven facility for power and detectable-difference calculations in stepped-wedge cluster-randomized trials. The Stata Journal. 2014;14(2):363–380. [Google Scholar]


