Abstract
Background:
Triplex vaccine was developed to enhance CMV specific T-cells and prevent CMV reactivation, early after hematopoietic stem cell transplantation (HCT).
Objective:
To determine safety and efficacy of Triplex.
Design:
First-in-patient phase 2 trial (ClinicalTrials.gov: NCT02506933).
Settings:
Three U.S. HCT centers.
Participants:
102 CMV-seropositive HCT recipients, at high-risk for CMV reactivation.
Intervention:
Intramuscular injections on days 28 and 56 post-HCT of Triplex or placebo. Triplex is a recombinant attenuated poxvirus, modified vaccinia Ankara, expressing immunodominant CMV antigens.
Measurements:
The primary outcomes were: CMV events (≥1250 IU/mL; CMV viremia requiring antivirals; or end-organ disease); non-relapse mortality; severe (grade 3, 4) GVHD, all evaluated through 100 days post-HCT, and grade 3-4 adverse events (AEs, probably or definitely attributable to injection) within 2 weeks post-vaccination.
Results:
102 patients received the first vaccination (51/group), 91 (89.2%) received both vaccinations (46 Triplex, 45 placebo). CMV reactivation occurred in 5 Triplex (9.8%), compared to 10 placebo (19.6%) recipients (hazard ratio, 0.46; 95% CI, 0.16 to 1.4; P=0.075). No Triplex recipient experienced non-relapse mortality during the first 100 days or serious AEs, no grade 3-4 AEs related to vaccination within 2 weeks post-vaccination was observed. Incidence of severe acute GVHD was similar between groups, post-injection (hazard ratio, 1.1; 95% CI, 0.53 to 2.4; P=0.23). Significantly higher levels of long lasting pp65-specific T-cells with effector memory phenotype were measured in Triplex compared to placebo recipients.
Limitations:
The lower-than-expected CMV event incidence in the placebo group reduced the power of the trial.
Conclusions:
No vaccine associated safety concerns were identified. Triplex elicited and amplified CMV-specific immune responses, and fewer Triplex vaccinated patients experienced CMV viremia.
Primary Funding Source:
National Cancer Institute and Helocyte Inc.
Reactivation of latent cytomegalovirus (CMV)in CMV seropositive recipients of allogenic stem cell transplant (HCT) heightens risk for CMV complications post-HCT, which are associated with increased morbidity and mortality (1-3). Preemptive therapy (PET) can successfully treat CMV reactivation, although antivirals have significant side effects (4-6). Prophylaxis through day 100 with letermovir effectively suppresses CMV reactivation with a favorable safety profile (7, 8). Nonetheless, when dosing is stopped, ≥30% of high risk patients develop late CMV viremia (8). One cause is mutation-based resistance leading to outcomes such as breakthrough viremia, and CMV disease (9-11). Consequently, there is still an unmet need for a durable approach to suppress both early and late CMV reactivation and its sequelae (4, 12).
This report describes a vaccine to accelerate reconstitution of protective CMV immunity (13). Prior clinical studies have indicated that CMV seropositive HCT recipients respond to subunit CMV vaccines (14-16). Yet, only one phase 3 trial in HCT recipients has been performed (NCT01877655). The results did not demonstrate a significant improvement in overall survival, viremia outcomes and reduction in CMV end-organ disease in vaccine recipients (13).
Triplex [Center for Biomedicine and Genetics, City of Hope National Medical Center, (COH) Duarte CA] is a modified vaccinia Ankara (MVA; viral backbone provided to COH from the National Institute of Allergy and Infectious Diseases, Laboratory of Viral Diseases)-based vaccine encoding three, immunodominant CMV antigens: pp65, IEl-exon4, and IE2-exon5 (17). Their role in protective immunity has been described (18-26). Triplex safely and durably expanded high levels of CMV-specific T-cells, when tested in a phase 1 trial in healthy adults (17). MVA, the attenuated poxvirus vector used in the Triplex vaccine was highly tolerable and immunogenic in HCT recipients (27). These encouraging findings provided the rationale for a first-in-patient phase 2 trial (NCT02506933) to investigate the safety and efficacy of Triplex in CMV seropositive HCT recipients. We report the outcomes of this phase 2 trial.
METHODS
TRIAL DESIGN AND OVERSIGHT
This prospective study was an investigator initiated multicenter, double-blind, randomized, placebo-controlled phase 2 trial conducted in three US cancer centers, specialized in allogeneic HCT. COH (Duarte, CA) was the lead institution; The Dana-Farber Cancer Institute (DFCI; Boston, MA) and The University of Texas MD Anderson Cancer Center (MDA; Houston, TX) were the other participating sites. The trial protocol was approved by the Food and Drug Administration (FDA, investigational new drug BB-15792, held by COH) and institutional regulatory board authorities in each participating site. The trial was undertaken in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki. All participants provided written informed consent (see Supplemental Methods).
PATIENTS
We recruited CMV seropositive patients from among those scheduled to undergo HCT for haematological malignancies. Inclusion and exclusion criteria, and administered therapies are provided in the Supplemental Content. Karnofsky performance score (KPS) and Disease Risk Index (DRI) (28) were assessed for each patient. On day 28 post-HCT, screened patients were excluded from randomization if they had failed to engraft, had grade 3-4 (according to the Keystone Consensus grading system) acute (a)GVHD, received steroids >lmg/kg/day within 7 days, or had any ongoing non-hematologic toxicity ≥grade 3 (according to the Common Terminology Criteria for Adverse Events, CTCAE v4.0).
TRIAL PROCEDURES
Eligible participants were randomly assigned in a 1:1 ratio to the Triplex or placebo groups. The trial used a central registry, with randomization in permuted blocks of size 4, stratified according to CMV donor serostatus and participating site. Only the statistician, registrars and the pharmacists were aware of the trial-group assignments at the level of individual participant data. Participants and their caregivers, physicians, and personnel who assessed the trial outcomes were unaware of the trial-group assignments. After randomization on day 28 post-HCT, participants received the first dose of Triplex or placebo intramuscularly and a second dose on day 56 post-HCT. The patients were clinically and immunologically monitored up to 1 year post-HCT. GVHD and adverse events (AEs) were monitored for all participants as necessary and not less than bi-weekly from day 28 until day 100 post-HCT. Afterwards, GVHD was monitored as necessary or monthly until 6 months, and subsequently as per standard of care. Clinical and immunological monitoring details are provided in Supplemental Methods.
OUTCOMES
The primary outcomes included one measure of efficacy and three measures of safety, evaluated in the first 100 days post-HCT. For the primary efficacy outcome, CMV events were defined as reactivation (≥1250 CMV DNA IU/mL); viremia requiring treatment; or end-organ disease (29). Primary safety outcomes included non-relapse mortality (NRM), severe (grade 3, 4) aGVHD, and grade 3-4 AEs within 2 weeks post vaccination that were probably or definitely attributable to the injection. The secondary outcomes included duration of CMV viremia and antiviral treatment; incidence of adverse transplant-related events; induction of CMV-specific cellular immunity, with focus on functional levels and memory phenotypes of CMV-specific T-cells.
STATISTICAL ANALYSIS
The study was designed to have 90% power to detect a reduction in CMV events in the vaccine group from 40% to 15% or 30% to 10%, at one-sided 0.10 level of significance (appropriate for a phase 2 trial (30)), with a sample size of 102 patients. The primary efficacy outcome (CMV events to day 100) was compared between groups using a one sided logrank test with estimated hazard ratio. No adjustment for the randomization strata was used in the primary analysis, per protocol, but robustness to such adjustments is reported. The Andersen-Gill approach (31) was used for inference about the hazard of recurrent CMV events throughout the year of follow-up, however no CMV events recurred prior to day 100. The PH model, without repeated events, was also used with the full follow-up data to model the joint effects of vaccine group, donor CMV serostatus (3, 32), and use of prednisone (33) at the first injection (within one day), as baseline covariates, with aGVHD requiring prednisone as a time-dependent covariate. The three primary safety outcomes (NRM by day 100, severe aGVHD, and grade 3-4 probably or definitely related AEs occurring within 2 weeks after the vaccination) were summarized as binomial counts, with confidence intervals for the difference of incidence. Kaplan-Meier plots and hazard ratios (from a simple Cox model) were generated to visualize differences in times-to-event between groups for NRM, CMV events, acute and chronic GVHD, and all-cause mortality. The PH assumption was assessed using the weighted residuals test (R survival package), as well as by visual examination of residual plots. No violations of the PH assumption were indicated. Patients were censored at loss to follow-up (death, relapse, or withdrawal) or end of follow-up (day 100 for the primary outcomes, or final follow-up visit).
Generalized estimating equations (GEE) were used to estimate the effect of Triplex on loglO transformed concentration of CMV specific T-cells at Days 28, 42, 56, 70, 84, 100, 140, 180, 270, and 365. The regression model included an indicator variable for Triplex vaccination, study day as a categorical variable, and baseline (pre-injection Day 28) immune response as a three-level categorical variable based on the tertiles of the Day 28 data. No interaction term between Triplex and study day was included. Thus, the estimated effect of Triplex represents the average effect over the specified post-vaccination follow-up period. Point estimates represent average fold-increase in T-cell concentrations. Further details of the GEE analysis and statistical methods for secondary outcomes are provided in the Supplemental Methods (34). All analyses were done as randomized (Intention to Treat principle), using all available data. Analyses were performed using R software, version 3.5.1 (https://www.R-project.org. R Foundation for Statistical Computing, Vienna, Austria), including the survival package version 2.42-3 (https://CRAN.R-project.org/package=survival: A Package for Survival Analysis, Therneau and Lumley) and gee package (version 4.13-19, Carey, Lumley and Ripley) (32).
ROLE OF THE FUNDING SOURCE
This clinical study was designed and the data analyzed by the senior authors and biostatisticians, and was supported by grants from the National Cancer Institute and Helocyte Inc. The funders had no influence on the design or conduct of the trial and were not involved in data collection or analysis, in the writing of the manuscript, or in the decision to submit it for publication.
RESULTS
CHARACTERISTICS OF THE PATIENTS
From December 21, 2015, to November 13, 2017, 135 patients were approached for study participation around the time of initiating HCT at 3 centers. Thirty-three patients were deemed ineligible for randomization per protocol or declined interest in participating by day 28 and were not randomized. Of the remaining 102 patients, half were randomized to the Triplex group and half to the placebo group (Fig. 1). There were more female patients in the Triplex compared to the placebo group. Primary diagnoses varied among participants; 75% of the vaccine group and 90% of the placebo group had KPS 80-100. HCT parameters such as conditioning regimen, and the overall DRI, the strongest determinant of survival after HCT were closely balanced between study groups (Table 1).
Figure 1. Enrollment and Randomization of Patients.
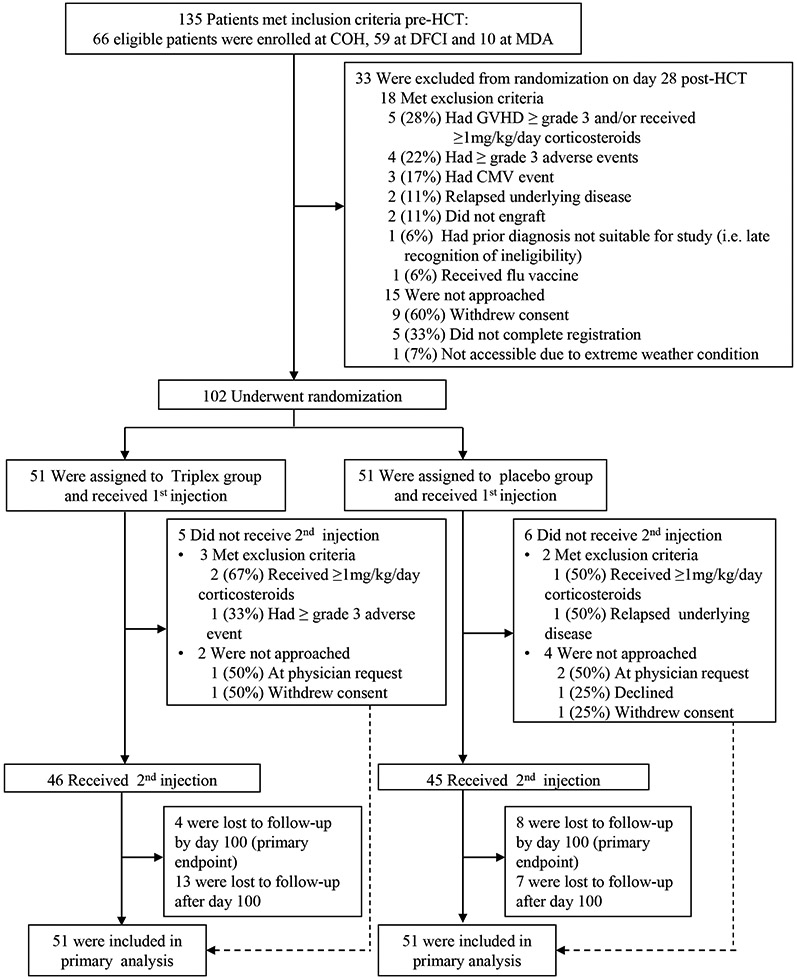
Details regarding eligibility and exclusion criteria are provided in the Methods. Reasons for patients lost to follow up (boxes after number received 2nd injection) are detailed in Table S1. COH denotes City of Hope, DFCI The Dana-Farber Cancer Center Institute, MDA The University of Texas MD Anderson Cancer Center, HCT hematopoietic cell transplantation, GVHD graft-versus-host disease.
Table 1.
Characteristics of the Patients at Baseline
| Characteristics | Vaccine (N=51) | Placebo (N=51) |
|---|---|---|
| Median age (IQR) –yr | 60 (47-66) | 57 (46-66) |
| Female sex– no. (%) | 24 (47) | 13 (25) |
| Primary diagnosis – no. (%) | 24 (47) | 13 (25) |
| Acute Myelogenous Leukemia | 19 (37) | 14 (27) |
| Acute Lymphoblastic Leukemia | 11 (21) | 6 (12) |
| Myelodysplastic Syndrome | 8 (16) | 11 (21) |
| Non-Hodgkin Lymphoma | 6 (12) | 10 (20) |
| Myeloproliferative Disease | 5 (10) | 5 (10) |
| Acute Leukemia NOS | 1 (2) | 3 (6) |
| Hodgkin Lymphoma | 1 (2) | 2 (4) |
| Disease Risk Index - no. (%) | ||
| Low | 3(6) | 5(10) |
| Intermediate | 40 (78) | 37(72) |
| High | 7(14) | 9(18) |
| Very High | 1(2) | 0(0) |
| Karnofsky performance score (at conditioning for HCT) – no. (%) | ||
| 60 | 2 (4) | 0 (0) |
| 70 | 11 (21) | 5 (10) |
| 80 | 8 (16) | 23 (45) |
| 90 | 27 (53) | 15 (29) |
| 100 | 3 (6) | 8 (16) |
| Conditioning regimen – no. (%) | ||
| Fully ablative | 20 (39) | 17 (33) |
| Reduced intensity | 31 (61) | 34 (67) |
| Donor/recipient CMV status – no. (%) | ||
| −/+ | 24 (47) | 25 (49) |
| +/+ | 27 (53) | 26 (51) |
IQR denotes interquartile range, NOS not otherwise specified, HCT hematopoietic cell transplantation, HLA human leukocyte antigen, CMV cytomegalovirus.
INTERVENTION AND FOLLOW-UP
All randomized patients received their first injection on day 28 post-HCT; 46 patients (90%) assigned to the Triplex group, and 45 patients (88%) assigned to the placebo group received the second injection on day 56 post-HCT, completing treatment (Figure 1). Follow-up to day 100 (primary endpoint) was completed for 47 patients (92%; 2 relapsed their underlying disease and 2 withdrew consent) in the Triplex group, and for 43 patients (84%; 2 experienced NRM; 5 relapsed their underlying disease; and 1 withdrew consent) in the placebo group. Follow up to study end was completed for 34 patients (67%, median days=356, IQR 213-364) in the Triplex group and for 36 patients (71%, median days=356, IQR 246-365) in the placebo group. Reasons for patients to be lost to follow up are detailed in Table S1. All randomized patients were included in the primary analysis.
SAFETY
No patient in the Triplex group experienced NRM during the first 100 days (primary outcome) versus 2 patients in the placebo group (P=0.50); the hazard ratio (HR) for post-injection severe aGVHD was 1.1 as compared with the placebo group (95% Cl, 0.53–2.4; P=0.23; Table 2 and Figure 2A). No serious AEs or grade 3-4 AEs probably or definitely related to vaccination within 2 weeks from first and second injection were observed in the Triplex group (Table 2). Additionally, the incidence of grade 3-4 AEs occurring within 2 weeks of the injections and considered possibly related to the vaccination were low for both Triplex and placebo recipients (see Table S2). No patient assigned to the Triplex group met predefined stopping rules after the first or second injection; and none of the participants discontinued participation because of a drug-related toxicity. No Triplex-related infections or deaths occurred. MVA viral DNA was undetectable at all tested time points (data not shown). The secondary clinical outcomes (Table 2) of grade 2-4 acute and chronic GVHD (Figure 2B), relapse, NRM, all-cause mortality and infections had similar incidence in both study groups.
Table 2.
Outcomes, by Assigned Treatment Group and Treatment Group Differences (95% Confidence Intervals)
| Outcomes | Triplex Group (N=51) |
Placebo Group (N=51) |
Hazard Ratio or Risk Difference (95% Cl) |
P Value |
|---|---|---|---|---|
| Primary Outcomes | ||||
| Non-relapse mortality at 100 days post-HCT– no. pts. (%) | 0 (0) | 2(3.9) | RD: −3.9 (−11, 3.4) | 0.50 |
| Severe (grade 3-4) acute GVHD – no. pts. (%) | 8(15.7) | 4(7.8) | RD: 7.8 (−6.5, 22) | 0.23 |
| Grade 3-4 adverse events1 – no. pts. (%) | 0 (0) | 1 (2.0) | RD: −2.0 (−7.7, 3.8) | 1.0 |
| CMV events2 through day 100 post-HCT – no. pts. (%) | 5 (9.8) | 10 (19.6) | HR: 0.46 (0.16, 1.4) | 0.075 |
| Secondary Outcomes | ||||
| Any CMV event from injection to study end – no. pts. (%) | 10 (19.6) | 14 (27.5) | HR: 0.63 (0.28, 1.4) | 0.26 |
| Recurrence of CMV events through study end3 – no. events | 21 | 17 | HR: 1.2 (0.64,2.28) | 0.57 |
| Duration of viremia4 – mean days (range) | 11.8 (0, 154) | 7.8 (0, 75) | NA | 0.49 |
| Use of antivirals – no. pts. (%) | 10(19.6) | 13 (25.5) | RD −5.9 (−24, 12) | 0.64 |
| Grade 2-4 acute GVHD – no. pts. (%) | 16 (31.4) | 15 (29.4) | HR 1.05 (0.52, 2.13) | 0.88 |
| Chronic GVHD - no. pts. (%) | 23 (45.1) | 26 (51.0) | RD −5.9 (−27, 15) | 0.27 |
| Relapse – no. pts. (%) | 11 (21.6) | 8 (15.7) | HR 1.36 (0.55, 3.38) | 0.51 |
| Non-relapse mortality – no. pts. (%) | 4 (7.8) | 5 (9.8) | HR 0.80 (0.22,2.93) | 0.73 |
| All-cause mortality – no. pts. (%) | 8 (15.7) | 8 (15.7) | HR 0.96 (0.36,2.56) | 0.93 |
| Infections – no. pts. (%) | 7 (13.7) | 7 (13.7) | RD 0 (−13, 13) | 1.0 |
| Grade 3-4 adverse events5 – no. pts. (%) | 2(3.9) | 4 (7.8) | RD −3.9 (−7.1, 15) | 0.68 |
| Cellular immunity (see Figure 3) | ||||
Based on Common Terminology Criteria for Adverse Events (CTCAE, v4.3), at least probably or definitely related (detail are found in Table S2) to vaccination within 2 weeks from first and second injection
CMV reactivation >1250 CMV DNA IU/mL; CMV viremia prompting antiviral therapy, or CMV disease prior to day 100 post-HCT
Andersen-Gill method (30) was used to compare hazard of initial and recurrent CMV events in patients receiving Triplex or placebo over the entire study period
Medians are zero, p-value from rank-sum test
AEs (CTCAE, version 4.3) at least possibly related to vaccination (details are found in Table S2) within 2 weeks from first and second injection; RD denotes Risk Difference, GVHD graft-versus-host disease, HR hazard ratio, pts. Patients, NA not applicable.
Figure 2. Time-to-Event Curves, Acute GVHD (Grade 3-4), Chronic GVHD, and CMV events.
Kaplan-Meier estimates are shown, with censoring times indicated. Note that for severe acute GVHD, half of the events on each arm, hence half of the separation, was for diagnoses made before the first injection on day 28. These events are not included in estimation of the hazard ratio. CI denotes Confidence Interval, GVHD graft-versus-host disease, CMV cytomegalovirus.
CMV-RELATED OUTCOMES
The primary outcome, CMV events through day 100 post-HCT, occurred in 5 patients (9.8%) in the Triplex group and in 10 patients (19.6%) in the placebo group (Figure 2C and Table 2). The HR for CMV events by day 100 post-HCT was 0.46 (95% Cl, 0.16–1.4; one-sided P=0.075). No patient developed a recurrent CMV event before day 100, and the vaccine effect estimates were robust to inclusion of donor stratum and center as factors in PH models (Cl within 0.15 to 1.44, one-sided P within 0.065 to 0.09). A post-hoc PH model (Table S3) fit to the first occurrences of CMV events over the full year of follow-up, indicated an increased risk for a CMV event after prednisone administration for GVHD (HR 12; CI, 4.9–31; P<0.001). In contrast, when prednisone was not administered at first injection (HR 0.22; CI, 0.081–0.62; P=0.004), or the patients had a CMV seropositive donor (HR 0.19; CI, 0.072–0.50; P<0.001), the hazard was lower. The estimated full-year effect of Triplex vaccination was robust to the adjustment for these risk factors (HR 0.5; CI, 0.21–1.2; P=0.120). The secondary outcome of late CMV events occurred with similar frequency in the two groups, 5 CMV events in the Triplex group vs. 4 events in the placebo group. No cases of CMV disease were observed; all patients who had a CMV event received antiviral treatment, except for one COH patient from the placebo group, whose viremia self-resolved. The Triplex group did not significantly differ from the placebo group in CMV viremia duration, recurrence and usage of antivirals (Table 2).
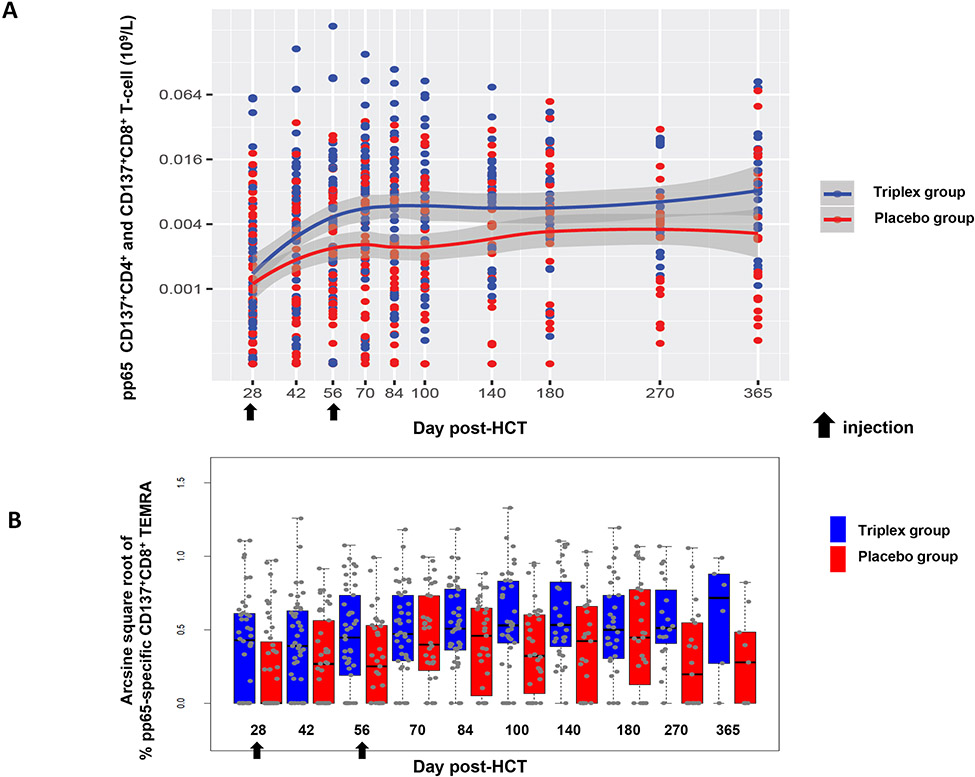
Results of the immunogenicity analyses are shown in Figures 3 and S1, and Tables S4 and S5. Concentrations of pre-vaccination CMV-specific CD137+ CD4+ and CD8+ T-cells varied widely among patients, with higher levels more likely to be observed when the donor was CMV seropositive. Concentrations increased longitudinally in the placebo group as immune reconstitution occurred with levels plateauing beyond day 100 post-HCT. Among vaccinated patients, concentrations of pp65-specific CD137+ CD4+ T-cells were significantly higher compared to placebo between Day 42 and Day 100 by a multiplicative factor of 1.98 (CI: 1.26–3.12; P=0.003), and CD8+ T-cells were increased by a multiplicative factor of 1.71 (CI: 1.05–2.80; P=0.030) (Tables S4). The combined concentration of CD4+ and CD8+ T-cells showed a multiplicative effect of vaccination of 1.97 (CI: 1.27–3.03; P=0.003). Similar results were found among patients with a CMV seropositive donor, in whom point estimates for the vaccine effect were greater than 1.0, but intervals included 1.0 (Table S4, lower panel). While the effect of vaccination on immunogenicity was less among patients with a CMV negative donor, the test of interaction was non-significant. However, the study was not powered to detect interaction differences. No effect of vaccination was found for CD137+ CD4+ and CD8+ T-cells specific for IE1 and IE2. Effector “revertant” T-cells (CD45RA+ CD28−, re-expressing the RA isoform of the CD45 surface marker, TEMRA) and naive phenotypes (CD45RA+ CD28+) of CD137+ CD3+ CD8+ T-cells specific for pp65 (Figure 3B and Table S5) were more frequently detected in the Triplex group compared to the placebo group (P=0.020 for TEMRA and P<0.001 for naive, respectively). The memory phenotype of CMV-specific CD137+ CD3+ CD4+ T-cells was largely composed of central memory T-cell subsets (CD45RA− CD28+, TCM; all median >90%), with similar frequencies in both groups (data not shown).
Figure 3.
(A) Longitudinal levels of combined pp65-specific CD137+CD4+ and CD137+CD8+ T-cells (109/L, logarithms of the concentrations). T-cell concentrations prior to protocol-defined CMV event were used. The band shown was computed using the loess scatterplot smoother providing the marginal geometric mean concentrations through time for each group. A 95% confidence band is shown in gray and individual measurement trajectories are shown for each study subject, up to 7 days prior to reactivation. Logarithmic spacing of both scales is used to aid visualization. (B) Boxplots showing percentages on arcsine scale of pp65-specific CD137+CD8+ T effector memory re-expressing the RA isoform (TEMRA) post-HCT. Box spans the interquartile range (IQR), the central bar shows median and the whiskers extend to 1.5 times the IQR.
DISCUSSION
The MVA vectored CMV vaccine (Triplex) was well tolerated when administered early post-transplant to HCT recipients. Transplant outcomes and treatment-emergent adverse events did not significantly differ between the Triplex and placebo groups (Table 2).
The risk of having a clinically relevant CMV event during the first 100 days posttransplant was halved in patients vaccinated with Triplex (Figure 2C). Consequently, Triplex met the per protocol primary design criterion of reducing CMV event incidence. Nonetheless, the lower-than-expected CMV event incidence observed in the placebo group (Table 2) reduced the power of the trial (35). Protocol exclusion criteria related to GVHD and corticosteroids are likely to have impacted the CMV event incidence. Post-hoc multivariate analysis of risk factors for CMV events (Table S3) demonstrated significant risk when GVHD occurred and prednisone (allowed per protocol) was administered, thus supporting our interpretation and previous findings (33, 35). Acute GVHD grade 3-4 was higher in the Triplex group, before and after vaccination: 4 patients were diagnosed prior to the first injection compared to 2 in the placebo group. However, the difference was not beyond expected variation (Figure 2A). Nonetheless in the Triplex group, in which more patients were treated with steroids for high grade aGVHD, the hazard of CMV reactivation (defined as the protocol-specified primary endpoint) was still 50% less than in the placebo group (Figure 2C and Table S3).
Triplex vaccinated patients had reduced CMV reactivation and higher levels of CMV specific T-cells compared to the placebo group in the early post-HCT time frame. In particular, immune reconstitution of functionally activated CD137+ CD4+ and CD8+ pp65-specific T-cells were significantly enhanced in the Triplex group through day 100, as well as one year post-HCT (Figure 3A). Both clinical and subclinical CMV viremia has the potential of triggering CMV specific T-cells in immune reconstituting HCT recipients (36). Levels, dynamics and timing of CMV specific T-cell induction varies greatly among viremic patients (37, 38). To minimize the impact of viremia-stimulated CMV-specific T-cells in the immune analysis of the randomized patients, all data points after CMV reactivation were excluded from both study groups (15, 16). Due to the limited number of the CMV viremic patients, the high variability of the responses, and the lack of information regarding the exact start time of CMV viremia for each patient, correlative analyses of CMV specific T-cells and viremia were infeasible.
Protocol-specified secondary outcomes confirm previous prospective analysis and retrospective reports on the critical role of pp65-specific T-cells, in protecting HCT recipients from CMV viremia, early post-HCT (14, 15, 32, 39). The findings here and elsewhere that transplant from a CMV seropositive donor has a significantly decreased risk for a CMV event (Table S3) (20, 40), support the protective role of CMV cellular immunity (3, 32). However, the effect of Triplex vaccination in HCT recipients was observed independent of donor CMV serostatus (17). To our knowledge, this is the first evidence of a universal (non-HLA-specific) (15) viral-based vectored CMV vaccine, which safely induces a robust and durable CMV-specific T-cell response in immune suppressed patients, starting early post-HCT.
Similar to what was found in healthy adults vaccinated with Triplex, levels of CD137+ CD4+ and CD8+ IE1 and IE2-specific T-cells did not significantly differ between groups (17). Both IE1 and IE2 CMV antigens were frequently recognized by the study recipients post-HCT, as previously reported in transplant patients (18-26). In solid organ transplant patients, protection against CMV disease was associated with IE1-specific CD8+ T-cells (21, 41), whereas other reports showed a positive correlation between pp65 T-cell responses and CMV viremia (42). As for HCT, reconstitution of pp65-specific CD8+ T-cells correlates with decreased frequency of early CMV reactivation and improved outcomes of CMV disease (38, 43-46). Recently, pp65-specific CD8+ T-cell functional signatures with robust predictive value for risk of clinically significant CMV infection following HCT have been identified (39). Thus, our findings of increased levels of pp65-specitic T-cell responses and reduced viremia in Triplex vaccinated patients appear to confirm the critical role of pp65 for HCT recipients. Future studies of Triplex vaccine in the context of solid organ transplantation may shed light on the controversial issue regarding the protective role of IE1 T-cells against CMV reactivation.
We found that patients in the Triplex group had larger proportions of pp65-specific CD8+ T-cells, displaying the highly functional and long-lasting TEMRA effector memory phenotype (Figure 3B) (47) in Triplex compared to the placebo group. In healthy CMV seropositives, high frequencies of activated CMV-specific TEM and TEMRA cells (16, 48) were associated with a lack of virus detection in the blood, suggesting that activated T-cells may play a role in limiting viremia (49). In particular, CMV-specific TEMRA are subsets of persistently activated effector memory T-cells, which re-express CD45RA after antigenic stimulation (49). The expression of CD45RA is often associated with naive T-cells, from which TEMRA are generated (50). Interestingly, frequencies of naive pp65-specific CD8+ T-cells were also much higher in the Triplex compared to the placebo group (Table S5). Altogether, these results suggest that the antigenic stimulation driven by the Triplex vaccination may potentiate the recovery of the T-cell compartment, by increasing the production of naive T-cells in the thymus (48).
Despite these promising attributes, this first-in-patient phase 2 trial has several design limitations. First, two injections of Triplex were the maximum number given to patients on days 28 and 56 post-HCT, since Triplex tolerability in an immunocompromised population was unknown. This may have resulted in the lack of protective effect beyond day 100 post-HCT observed in some vaccinated patients. Additional Triplex injections are proposed in a planned phase 3 trial, to potentially lengthen the vaccine protective and immune stimulating effect, especially in patients who develop high grade aGVHD, and are at higher risk for CMV reactivation. MVA-based vaccines for cancer treatment are safely administered multiple times, without reduction of efficacy (51). Second, the viremia threshold (>1250 CMV DNA IU/mL) recommended by the lead institution (COH) for PET initiation was included in the composite primary endpoint. However, CMV viremia above the pre-defined threshold was not uniformly treated at COH. In contrast, physicians at DFCI and MDA promptly treated patients when >400 CMV DNA IU/ml were detected. The variable initiation of PET among the participating institutions might have affected the possibility of observing a vaccine effect for viremia related secondary outcomes, including reduction of CMV viremia duration and antiviral usage. Finally, the study was designed expecting at least 30% of patients in the placebo group to have CMV events. Since the actual CMV event incidence was close to 20% in this group, the reduced number of events contributing to the primary endpoint prevented conclusive statistical analyses and estimates on the possible clinical and immunological factors for Triplex vaccine failures.
In conclusion, Triplex is an attractive immunotherapeutic to elicit and enhance protective CMV immunity. Its favorable outcomes and multiplicity of clinical applications (see Supplemental Appendix) are of special interest for infectious disease and transplant physicians, eagerly awaiting new treatments for reducing the burden of CMV morbidity in both the HCT and solid organ transplant settings.
Supplementary Material
Acknowledgments
Supported by grants from the National Cancer Institute (NCI) 5R01 CA077544; NCI P30 CA033572; NCI-SAIC-Frederick 28XS061 and Helocyte Inc. MVA was provided under a Material Transfer Agreement to COH from the National Institute of Allergy and Infectious Diseases, Laboratory of Viral Diseases (Dr. Bernard Moss, Director). The following reagent was obtained through the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH: HCMV pp65 Peptide Pool (referred as pp65 library in the manuscript).
Dr. La Rosa, reports receiving consulting fees and research funding from Helocyte Inc.; Dr. Aldoss consulting fees from Helocyte Inc.; Dr. Rida consulting fees from Helocyte Inc.; Dr. Diamond fees for royalties, research funding, and fees for serving on the advisory board of Helocyte Inc. No other potential conflict of interest relevant to this article was reported. Disclosure forms provided by the authors are available with the full text of this article at rmed.acponline.org.
We thank the patients, nurses, technicians, research coordinators and clinicians at all participating sites, and the staff at the data safety monitoring and coordinating center without whose support this trial could not have been conducted.
Footnotes
A complete list of team members in the TRIPLEX VACCINE Study group to Assess the Efficacy of Triplex versus Placebo for the Prevention of Cytomegalovirus viremia in allogeneic stem cell transplantation is provided in the Supplemental Appendix, available at AIM.org.
References
- 1.Griffiths P, Baraniak I, Reeves M. The pathogenesis of human cytomegalovirus. J Pathol. 2015;235(2):288–97. [DOI] [PubMed] [Google Scholar]
- 2.Ljungman P The role of cytomegalovirus serostatus on outcome of hematopoietic stem cell transplantation. Curr Opin Hematol. 2014;21(6):466–9. [DOI] [PubMed] [Google Scholar]
- 3.Teira P, Battiwalla M, Ramanathan M, Barrett AJ, Ahn KW, Chen M, et al. Early cytomegalovirus reactivation remains associated with increased transplant-related mortality in the current era: a CIBMTR analysis. Blood. 2016;127(20):2427–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.El Chaer F, Shah DP, Chemaly RF. How I treat resistant cytomegalovirus infection in hematopoietic cell transplantation recipients. Blood. 2016;128(23):2624–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Camargo JF, Komanduri KV. Emerging concepts in cytomegalovirus infection following hematopoietic stem cell transplantation. Hematol Oncol Stem Cell Ther. 2017;10(4):233–8. [DOI] [PubMed] [Google Scholar]
- 6.Tomblyn M, Chiller T, Einsele H, Gress R, Sepkowitz K, Storek J, et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant. 2009;15(10):1143–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Green ML, Leisenring W, Stachel D, Pergam SA, Sandmaier BM, Wald A, et al. Efficacy of a viral load-based, risk-adapted, preemptive treatment strategy for prevention of cytomegalovirus disease after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012;18(11):1687–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marty FM, Ljungman P, Chemaly RF, Maertens J, Dadwal SS, Duarte RF, et al. Letermovir Prophylaxis for Cytomegalovirus in Hematopoietic-Cell Transplantation. N Engl J Med. 2017;377(25):2433–44 [DOI] [PubMed] [Google Scholar]
- 9.Chou S Rapid In Vitro Evolution of Human Cytomegalovirus UL56 Mutations That Confer Letermovir Resistance. Antimicrob Agents Chemother. 2015;59(10):6588–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frietsch JJ, Michel D, Stamminger T, Hunstig F, Birndt S, Schnetzke U, et al. In Vivo Emergence of UL56 C325Y Cytomegalovirus Resistance to Letermovir in a Patient with Acute Myeloid Leukemia after Hematopoietic Cell Transplantation. Mediterr J Hematol Infect Dis. 2019;11(1):e2019001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knoll BM, Seiter K, Phillips A, Soave R. Breakthrough cytomegalovirus pneumonia in hematopoietic stem cell transplant recipient on letermovir prophylaxis. Bone Marrow Transplant. 2019;54(6):911–2. [DOI] [PubMed] [Google Scholar]
- 12.Elliot K, Tooze JA, Geller R, Powell BL, Pardee TS, Ritchie E, et al. The prognostic importance of polypharmacy in older adults treated for acute myelogenous leukemia (AML). Leuk Res. 2014;38(10):1184–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diamond DJ, La Rosa C, Chiuppesi F, Contreras H, Dadwal S, Wussow F, et al. A fifty-year odyssey: prospects for a cytomegalovirus vaccine in transplant and congenital infection. Expert Rev Vaccines. 2018;17(10):889–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kharfan-Dabaja MA, Boeckh M, Wilck MB, Langston AA, Chu AH, Wloch MK, et al. A novel therapeutic cytomegalovirus DNA vaccine in allogeneic haemopoietic stem-cell transplantation: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Infect Dis. 2012;12(4):290–9. [DOI] [PubMed] [Google Scholar]
- 15.Nakamura R, La Rosa C, Longmate J, Drake J, Slape C, Zhou Q, et al. Viraemia, immunogenicity, and survival outcomes of cytomegalovirus chimeric epitope vaccine supplemented with PF03512676 (CMVPepVax) in allogeneic haemopoietic stem-cell transplantation: randomised phase 1b trial. Lancet Haematol. 2016;3(2):e87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.La Rosa C, Longmate J, Lingaraju CR, Zhou Q, Kaltcheva T, Hardwick N, et al. Rapid Acquisition of Cytomegalovirus-Specific T Cells with a Differentiated Phenotype, in Nonviremic Hematopoietic Stem Transplant Recipients Vaccinated with CMVPepVax. Biol Blood Marrow Transplant. 2019;25(4):771–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.La Rosa C, Longmate J, Martinez J, Zhou Q, Kaltcheva TI, Tsai W, et al. MVA vaccine encoding CMV antigens safely induces durable expansion of CMV-specific T cells in healthy adults. Blood. 2017;129(1):114–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Z, Zhou W, Srivastava T, La Rosa C, Mandarino A, Forman SJ, et al. A fusion protein of HCMV IE1 exon4 and IE2 exon5 stimulates potent cellular immunity in an MVA vaccine vector. Virology. 2008;377(2):379–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gallez-Hawkins G, Thao L, Lacey SF, Martinez J, Li X, Franck AE, et al. Cytomegalovirus immune reconstitution occurs in recipients of allogeneic hematopoietic cell transplants irrespective of detectable cytomegalovirus infection. Biol Blood Marrow Transplant. 2005;11(11):890–902. [DOI] [PubMed] [Google Scholar]
- 20.Sylwester AW, Mitchell BL, Edgar JB, Taormina C, Pelte C, Ruchti F, et al. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J Exp Med. 2005;202(5):673–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bunde T, Kirchner A, Hoffmeister B, Habedank D, Hetzer R, Cherepnev G, et al. Protection from cytomegalovirus after transplantation is correlated with immediate early 1-specific CD8 T cells. J Exp Med. 2005;201(7):1031–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lacey SF, La Rosa C, Zhou W, Sharma MC, Martinez J, Krishnan A, et al. Functional comparison of T cells recognizing cytomegalovirus pp65 and intermediate-early antigen polypeptides in hematopoietic stem-cell transplant and solid organ transplant recipients. J Infect Dis. 2006;194(10):1410–21. [DOI] [PubMed] [Google Scholar]
- 23.Gabanti E, Lilleri D, Ripamonti F, Bruno F, Zelini P, Furione M, et al. Reconstitution of Human Cytomegalovirus-Specific CD4+ T Cells is Critical for Control of Virus Reactivation in Hematopoietic Stem Cell Transplant Recipients but Does Not Prevent Organ Infection. Biol Blood Marrow Transplant. 2015;21(12):2192–202. [DOI] [PubMed] [Google Scholar]
- 24.Gimenez E, Munoz-Cobo B, Solano C, Amat P, de la Camara R, Nieto J, et al. Functional patterns of cytomegalovirus (CMV) pp65 and immediate early-1-specific CD8(+) T cells that are associated with protection from and control of CMV DNAemia after allogeneic stem cell transplantation. Transpl Infect Dis. 2015;17(3):361–70. [DOI] [PubMed] [Google Scholar]
- 25.Tormo N, Solano C, Benet I, Nieto J, de la Camara R, Lopez J, et al. Reconstitution of CMV pp65 and IE-1-specific IFN-gamma CD8(+) and CD4(+) T-cell responses affording protection from CMV DNAemia following allogeneic hematopoietic SCT. Bone Marrow Transplant. 2011;46(11): 1437–43. [DOI] [PubMed] [Google Scholar]
- 26.Gratama JW, Brooimans RA, van der Holt B, Sintnicolaas K, van Doornum G, Niesters HG, et al. Monitoring cytomegalovirus IE-1 and pp65-specific CD4+ and CD8+ T-cell responses after allogeneic stem cell transplantation may identify patients at risk for recurrent CMV reactivations. Cytometry B Clin Cytom. 2008;74(4):211–20. [DOI] [PubMed] [Google Scholar]
- 27.Walsh SR, Wilck MB, Dominguez DJ, Zablowsky E, Bajimaya S, Gagne LS, et al. Safety and immunogenicity of modified vaccinia Ankara in hematopoietic stem cell transplant recipients: a randomized, controlled trial. J Infect Dis. 2013;207(12):1888–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Armand P, Kim HT, Logan BR, Wang Z, Alyea EP, Kalaycio ME, et al. Validation and refinement of the Disease Risk Index for allogeneic stem cell transplantation. Blood. 2014;123(23):3664–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ljungman P, Boeckh M, Hirsch HH, Josephson F, Lundgren J, Nichols G, et al. Definitions of Cytomegalovirus Infection and Disease in Transplant Patients for Use in Clinical Trials. Clin Infect Dis. 2017;64(1):87–91. [DOI] [PubMed] [Google Scholar]
- 30.Rubinstein LV, Korn EL, Freidlin B, Hunsberger S, Ivy SP, Smith MA. Design issues of randomized phase II trials and a proposal for phase II screening trials. J Clin Oncol. 2005;23(28):7199–206. [DOI] [PubMed] [Google Scholar]
- 31.Andersen PK, Gill RD. Cox's regression model for counting processes: a large sample study. Ann. Statist 1982;10(4):1100–20. [Google Scholar]
- 32.Zhou W, Longmate J, Lacey SF, Palmer JM, Gallez-Hawkins G, Thao L, et al. Impact of donor CMV status on viral infection and reconstitution of multifunction CMV-specific T cells in CMV-positive transplant recipients. Blood. 2009;113(25):6465–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ljungman P, Hakki M, Boeckh M. Cytomegalovirus in hematopoietic stem cell transplant recipients. Hematol Oncol Clin North Am. 2011;25(1):151–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wickham H. Ggplot2 : elegant graphics for data analysis. New York: Springer; 2016. [Google Scholar]
- 35.Green ML, Leisenring W, Xie H, Mast TC, Cui Y, Sandmaier BM, et al. Cytomegalovirus viral load and mortality after haemopoietic stem cell transplantation in the era of pre-emptive therapy: a retrospective cohort study. Lancet Haematol. 2016;3(3):e119–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gratama JW, Boeckh M, Nakamura R, Cornelissen JJ, Brooimans RA, Zaia JA, et al. Immune monitoring with iTAg MHC Tetramers for prediction of recurrent or persistent cytomegalovirus infection or disease in allogeneic hematopoietic stem cell transplant recipients: a prospective multicenter study. Blood. 2010;116(10):1655–62. [DOI] [PubMed] [Google Scholar]
- 37.Widmann T, Sester U, Gartner BC, Schubert J, Pfreundschuh M, Kohler H, et al. Levels of CMV specific CD4 T cells are dynamic and correlate with CMV viremia after allogeneic stem cell transplantation. PLoS One. 2008;3(11):e3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lilleri D, Gerna G, Fornara C, Lozza L, Maccario R, Locatelli F. Prospective simultaneous quantification of human cytomegalovirus-specific CD4+ and CD8+ T-cell reconstitution in young recipients of allogeneic hematopoietic stem cell transplants. Blood. 2006;108(4):1406–12. [DOI] [PubMed] [Google Scholar]
- 39.Camargo JF, Wieder ED, Kimble E, Benjamin CL, Kolonias DS, Kwon D, et al. Deep functional immunophenotyping predicts risk of cytomegalovirus reactivation after hematopoietic cell transplantation. Blood. 2019;133(8):867–77. [DOI] [PubMed] [Google Scholar]
- 40.La Rosa C, Longmate J, Lacey SF, Kaltcheva T, Sharan R, Marsano D, et al. Clinical evaluation of safety and immunogenicity of PADRE-cytomegalovirus (CMV) and tetanus-CMV fusion peptide vaccines with or without PF03512676 adjuvant. J Infect Dis. 2012;205(8):1294–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nickel P, Bold G, Presber F, Biti D, Babel N, Kreutzer S, et al. High levels of CMV-IE-1-specific memory T cells are associated with less alloimmunity and improved renal allograft function. Transpl Immunol. 2009;20(4):238–42. [DOI] [PubMed] [Google Scholar]
- 42.Pipeling MR, John ER, Orens JB, Lechtzin N, McDyer JF. Primary cytomegalovirus phosphoprotein 65-specific CD8+ T-cell responses and T-bet levels predict immune control during early chronic infection in lung transplant recipients. J Infect Dis. 2011;204(11): 1663–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bosch M, Khan FM, Storek J. Immune reconstitution after hematopoietic cell transplantation. Current Opinion in Hematology. 2012;19(4):324–35. [DOI] [PubMed] [Google Scholar]
- 44.Gratama JW, van Esser JW, Lamers CH, Tournay C, Lowenberg B, Bolhuis RL, et al. Tetramer-based quantification of cytomegalovirus (CMV)-specific CD8+ T lymphocytes in T-cell-depleted stem cell grafts and after transplantation may identify patients at risk for progressive CMV infection. Blood. 2001;98(5):1358–64. [DOI] [PubMed] [Google Scholar]
- 45.Einsele H, Rauser G, Grigoleit U, Hebart H, Sinzger C, Riegler S, et al. Induction of CMV-specific T-cell lines using Ag-presenting cells pulsed with CMV protein or peptide. Cytotherapy. 2002;4(1):49–54. [DOI] [PubMed] [Google Scholar]
- 46.Cwynarski K, Ainsworth J, Cobbold M, Wagner S, Mahendra P, Apperley J, et al. Direct visualization of cytomegalovirus-specific T-cell reconstitution after allogeneic stem cell transplantation. Blood. 2001;97(5):1232–40. [DOI] [PubMed] [Google Scholar]
- 47.Lilleri D, Fornara C, Revello MG, Gerna G. Human cytomegalovirus-specific memory CD8+ and CD4+ T cell differentiation after primary infection. J Infect Dis. 2008;198(4):536–43. [DOI] [PubMed] [Google Scholar]
- 48.Ogonek J, Kralj Juric M, Ghimire S, Varanasi PR, Holler E, Greinix H, et al. Immune Reconstitution after Allogeneic Hematopoietic Stem Cell Transplantation. Front Immunol. 2016;7:507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gordon CL, Miron M, Thome JJ, Matsuoka N, Weiner J, Rak MA, et al. Tissue reservoirs of antiviral T cell immunity in persistent human CMV infection. J Exp Med. 2017;214(3):651–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thome JJ, Yudanin N, Ohmura Y, Kubota M, Grinshpun B, Sathaliyawala T, et al. Spatial map of human T cell compartmentalization and maintenance over decades of life. Cell. 2014;159(4):814–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Heery CR, Palena C, McMahon S, Donahue RN, Lepone LM, Grenga I, et al. Phase I Study of a Poxviral TRICOM-Based Vaccine Directed Against the Transcription Factor Brachyury. Clin Cancer Res. 2017;23(22):6833–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.