Abstract
The compartmental pharmacokinetics of anidulafungin (VER-002; formerly LY303366) in plasma were characterized with normal rabbits, and the relationships between drug concentrations and antifungal efficacy were assessed in clinically applicable infection models in persistently neutropenic animals. At intravenous dosages ranging from 0.1 to 20 mg/kg of body weight, anidulafungin demonstrated linear plasma pharmacokinetics that fitted best to a three-compartment open pharmacokinetic model. Following administration over 7 days, the mean (± standard error of the mean) peak plasma concentration (Cmax) increased from 0.46 ± 0.02 μg/ml at 0.1 mg/kg to 63.02 ± 2.93 μg/ml at 20 mg/kg, and the mean area under the concentration-time curve from 0 h to infinity (AUC0–∞) rose from 0.71 ± 0.04 to 208.80 ± 24.21 μg · h/ml. The mean apparent volume of distribution at steady state (Vss) ranged from 0.953 ± 0.05 to 1.636 ± 0.22 liter/kg (nonsignificant [NS]), and clearance ranged from 0.107 ± 0.01 to 0.149 ± 0.00 liter/kg/h (NS). Except for a significant prolongation of the terminal half-life and a trend toward an increased Vss at the higher end of the dosage range after multiple doses, no significant differences in pharmacokinetic parameters were noted in comparison to single-dose administration. Concentrations in tissue at trough after multiple dosing (0.1 to 10 mg/kg/day) were highest in lung and liver (0.85 ± 0.16 to 32.64 ± 2.03 and 0.32 ± 0.05 to 43.76 ± 1.62 μg/g, respectively), followed by spleen and kidney (0.24 ± 0.65 to 21.74 ± 1.86 and <0.20 to 16.92 ± 0.56, respectively). Measurable concentrations in brain tissue were found at dosages of ≥0.5 mg/kg (0.24 ± 0.02 to 3.90 ± 0.25). Implementation of optimal plasma sampling in persistently neutropenic rabbit infection models of disseminated candidiasis and pulmonary aspergillosis based on the Bayesian approach and model parameters from normal animals as priors revealed a significantly slower clearance (P < 0.05 for all dosage groups) with a trend toward higher AUC0–24 values, higher plasma concentrations at the end of the dosing interval, and a smaller volume of distribution (P < 0.05 to 0.193 for the various comparisons among dosage groups). Pharmacodynamic modeling using the residual fungal tissue burden in the main target sites as the primary endpoint and Cmax, AUC0–24, time during the dosing interval of 24 h with plasma drug concentration equaling or exceeding the MIC or the minimum fungicidal concentration for the isolate, and tissue concentrations as pharmacodynamic parameters showed predictable pharmacokinetic-pharmacodynamic relationships in experimental disseminated candidiasis that fitted well with an inhibitory sigmoid maximum effect pharmacodynamic model (r2, 0.492 to 0.819). However, no concentration-effect relationships were observed in experimental pulmonary aspergillosis using the residual fungal burden in lung tissue and survival as parameters of antifungal efficacy. Implementation of optimal plasma sampling in discriminative animal models of invasive fungal infections and pharmacodynamic modeling is a novel approach that holds promise of improving and accelerating our understanding of the action of antifungal compounds in vivo.
Anidulafungin (VER-002 or VER; formerly LY303366) is an investigational, water-soluble, semisynthetic antifungal echinocandin B derivative. It acts by inhibiting the synthesis of β-(1,3)-d-glucan, a major structural cell wall polymer of many opportunistic fungi. Disruption of glucan biosynthesis leads to cell wall damage and organism-dependent cell death (7, 23). Preclinical studies have demonstrated potent and non-cross-resistant activity of VER against medically important fungi both in vitro and in vivo (12, 19).
Little is known, however, about the disposition of this compound in plasma and tissues and the relationships among dosage, concentrations in the body, and antifungal efficacy in vivo. The purpose of our investigations was therefore twofold: first, to characterize the compartmental pharmacokinetics of VER in plasma over a broad dosage range in normal animals and to design an optimal plasma sampling approach, and second, to assess the implementation of optimal plasma sampling in clinically applicable persistently neutropenic infection models of disseminated candidiasis and invasive pulmonary aspergillosis in order to investigate the relationships of drug concentrations in plasma and tissues to antifungal efficacy. In a broader sense, this study should serve to better explain the pharmacokinetic and pharmacodynamic relationships of the current echinocandins in the treatment of invasive candidiasis and aspergillosis in the neutropenic host.
(This work was presented in part at the 38th Interscience, Conference on Antimicrobial Agents and Chemotherapy, San Diego, Calif., 1998, and the 39th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, Calif., 1999.)
MATERIALS AND METHODS
Study drug.
VER was provided by Eli Lilly & Co. (Indianapolis, Ind.) as a 10-mg/ml proprietary solution for parenteral administration and maintained at 4°C. At the 5-, 10-, and 20-mg/kg-of-body-weight dosage levels, undiluted solution was used for dosing. At the 1-mg/kg dosage level, a 2-mg/ml solution was prepared by diluting the 10-mg/ml solution with sterile normal saline (Quality Biologicals Inc., Gaithersburg, Md.). Similarly, at the 0.25- and 0.5-mg/kg dosage levels, a 1-mg/ml solution was prepared, and at the 0.1-mg/kg dosage level, a 0.1-mg/ml solution was prepared. VER was administered at ambient temperature as a slow intravenous bolus over 5 min through the indwelling catheter.
Animals.
Healthy female New Zealand White rabbits (Oryctolagus cuniculus; Hazleton, Denver, Pa.) weighing 2.5 to 3.5 kg were used in all experiments. They were individually housed and maintained with water and standard rabbit feed ad libitum according to National Institutes of Health guidelines for laboratory animal care (3). Vascular access was established in each rabbit prior to experimentation by the surgical placement of a subcutaneous silastic central venous catheter as previously described (33).
Plasma pharmacokinetics in healthy animals.
The single-dose noncompartmental pharmacokinetics of VER have been reported previously (28, 29). In the present study, pharmacokinetic parameters were estimated using pharmacokinetic modeling and include single-dose data as well as data obtained after repeated once-daily dosing over 7 days.
For characterization of the single-dose compartmental pharmacokinetics of VER in plasma, seven groups of three rabbits each were studied. Animals received VER at 0.1, 0.25, 0.5, 1, 5, 10, and 20 mg/kg as a single slow intravenous bolus through the indwelling catheter. Plasma samples were drawn immediately before administration of the compound and then at 0.16, 0.5, 1, 2, 4, 8, 12, 18, 24, 48, 72, and 96 h after dosing. In the multiple-dose study, a different set of animals was studied. Groups of three rabbits each received VER at 0.1, 0.25, 0.5, 1, 5, 10, and 20 mg/kg as a single slow intravenous bolus through the indwelling catheter once a day (QD) for a total of 7 days. On day 7, plasma samples were collected immediately before dosing and then at 0.16, 0.5, 1, 2, 4, 8, 12, 18, 24, 48, 72, and 96 h after administration. Hepatic and renal toxicities were monitored in plasma 24 h after the last of the seven doses and compared to normal values. All animals were clinically evaluated each day and weighed before the first dose and at the end of the study.
Neutropenic rabbit model of subacute disseminated candidiasis.
The efficacy data from these experiments have been reported in detail previously (28). Briefly, a 17-day period of monitored profound (<500/μl) neutropenia was induced and maintained by repeat intravenous administration of 440 mg of cytosine-arabinoside (Ara-C) (Upjohn, Kalamazoo, Mich.) per m2. Intravenous antibacterial prophylaxis with ceftazidime (Glaxo Pharmaceuticals, Research Triangle Park, N.C.) at 75 mg/kg twice a day (BID), gentamicin (Elkins-Sinn Inc., Cherry Hill, N.I.) at 5 mg/kg every other day, and vancomycin (Abbott Laboratories, North Chicago, Ill.) at 15 mg/kg QD was started on day 4 and given throughout the experiment.
On day 6 of the experiment, rabbits were intravenously inoculated with 103 blastoconidia of Candida albicans NIH8621 obtained from a granulocytopenic patient with autopsy-proven disseminated candidiasis. The inoculum was administered in sterile normal saline over 1 min via the indwelling silastic central venous catheter. Using the NCCLS broth microdilution method (26) with antibiotic medium 3 (NIH Media Department, Bethesda, Md.) instead of RPMI, the MIC in vitro of VER for this isolate of C. albicans was 0.015 μg/ml, and the minimum fungicidal concentration (MFC) in vitro was 0.25 μg/ml.
Antifungal treatment was begun 24 h after inoculation and was administered daily for a total of 10 days throughout the experiment. Rabbits received VER at 0.1 mg/kg QD, 0.25 mg/kg QD, 0.5 mg/kg QD, and 1.0 mg/kg QD or normal saline as a slow intravenous bolus. A total of six rabbits were allocated to each treatment group. The normal saline-treated control group consisted of 13 rabbits.
Surviving rabbits were euthanatized 24 h after the 10th dose of antifungal treatment or normal saline on the 11th day postinoculation by intravenous pentobarbital injection. The primary endpoint for pharmacodynamic modeling was the residual fungal burden of C. albicans in kidney tissue in log (CFU per gram); secondary endpoints for modeling were the clearance of C. albicans from liver, spleen, lung, and brain tissues (31). The pattern of infection of subacute disseminated candidiasis permitted the survival of nearly all treated rabbits (n = 22 of 24; untreated controls; n = 9 of 13) until the termination of the experiment.
Neutropenic rabbit model of invasive pulmonary aspergillosis.
The efficacy data from these experiments have been reported in detail previously (29). In brief, a 16-day period of monitored profound (<500/μl) neutropenia was induced and maintained by repeat intravenous administration of 525 to 485 mg of Ara-C per m2. Methylprednisolone (Abbott Laboratories) at 5 mg/kg was administered on days 1 and 2 of the experiments to facilitate establishment of infection. Intravenous antibacterial prophylaxis with ceftazidime at 75 mg/kg BID, gentamicin at 5 mg/kg every other day, and vancomycin at 15 mg/kg QD was started on day 4 and given throughout the experiment.
On day 2 of the experiments, rabbits were intratracheally inoculated with a defined 200- to 250-μl inoculum containing 108 conidia of Aspergillus fumigatus NIH4215 obtained from a neutropenic patient with a fatal case of pulmonary aspergillosis. Inoculation was performed under general intravenous anesthesia of a 2:1 (vol/vol) mixture of ketamine (100 mg/ml); Fort Dodge Labs, Fort Dodge, Iowa) and xylazine (20 mg/ml; Mobay Corp., Shawnee, Kans.) using a Flagg O straight-bladed laryngoscope (Welch-Allyn, Skaneateles Falls, N.Y.). Following visualization of the vocal cords, the inoculum was applied into the trachea via a tuberculin syringe attached to a 5.25-in. Teflon catheter (Becton Dickinson, Sandy, Utah). By a standardized microdilution method (10), the MIC of VER for this isolate of A. fumigatus was 0.125 μg/ml. Since the present echinocandins have no apparent fungicidal activity against Aspergillus spp. in vitro (23), an MFC cannot be provided.
Antifungal therapy was begun 24 h after intratracheal inoculation and administered daily throughout the experiments for a maximum of 12 days. Rabbits were grouped to receive VER at 1 mg/kg QD (n = 7), 5 mg/kg QD (n = 7), 10 mg/kg QD (n = 8), and 20 mg/kg QD (n = 7) or normal saline (n = 16) as a slow intravenous bolus. Two additional cohorts of rabbits were treated with VER at dosages of 5 mg/kg BID (n = 7) and 10 mg/kg BID (n = 6).
Surviving rabbits were euthanatized 24 h after the 12th dose of antifungal treatment or normal saline on the 13th day postinoculation by intravenous pentobarbital injection. The primary endpoint for pharmacodynamic modeling was the burden of viable units of A. fumigatus in lung tissue in log (CFU per gram); secondary endpoints for modeling were overall survival in days postinoculation; total lung weight, and the pulmonary infarct score at postmortem (11). The pattern of infection of invasive pulmonary aspergillosis results in the demise of nearly all (n = 15 of 16) untreated controls prior to the termination of the experiments (treated animals, n = 36 of 42).
Plasma pharmacokinetics and tissue disposition in infected animals.
In both infection models, a sparse plasma sampling strategy using optimal sampling theory implemented with the Adapt II computer program (5) was employed in all experimental animals on day 5 of antifungal therapy to obtain key pharmacokinetic parameters in each individual infected animal and to correlate pharmacodynamic parameters with endpoints of antifungal efficacy. For the same purpose, aliquots of tissues were sampled at postmortem and reserved for determination of tissue concentrations.
Processing and extraction of analytical samples.
Blood samples were collected in heparinized syringes. Plasma was separated by centrifugation and stored at −80°C until assay. An aliquot of 300 μl of plasma plus 25 μl of the internal standard LY306168 (20 μg/ml in 50:50 [vol/vol] acetonitrile–50 mM ammonium acetate, pH 4.0) (Eli Lilly) was submitted to extraction.
Tissues were carefully dissected and stored at −80°C. Before assay, tissues were thawed and one aliquot of approximately 1 g was weighed out for each sample. The specimens were thoroughly rinsed with phosphate-buffered saline, pH 7.4. Remaining buffer solution on the tissue surface was blotted with Micro Wipes (Scott Paper Company, Philadelphia, Pa.). Specimens were then reweighed and homogenized in ice-cold normal saline (1:10 [wt/wt]) using a Tissumizer (Tekmar, Cincinnati, Ohio) with a 10N head two times for 30 s each. Quantities of 0.7 ml of the tissue homogenate and 25 μl of internal standard were combined with 0.7 ml of acetonitrile, vortexed, and centrifuged for 10 min at 3,000 × g. Then, 1 ml of the resulting supernatant was added to 4 ml of 50 mM ammonium acetate (pH 4.0), vortexed, and submitted to extraction. Drug concentrations in tissues were calculated to 1 g of tissue.
VER and LY306168, the internal standard, were separated from plasma and tissue homogenates utilizing acetonitrile-ammonium acetate (50 mM, pH 4.0)-based solvents (Fisher Scientific, Fair Lawn, N.J.), C8 bonded phase extraction cartridges (Varian Inc., Harbor City, Calif.), and a vacuum manifold (Supelco Inc., Bellefonte, Pa.). The eluant was dried in an evaporator (Zymark Corp., Hopkinston, Mass.) under a steady stream of nitrogen at 40°C and reconstituted in 50:50 (vol/vol) methanol-ammonium acetate (50 mM, pH 4.0) (Fisher Scientific) for injection. Standards and quality control samples were similarly prepared by adding known amounts of the reference standard of VER (LY303366; Eli Lilly) and internal standard to either normal rabbit serum (Gibco Laboratories, Grand Island, N.Y.), commercially available cerebrospinal fluid standards (Instrumentation Laboratories, Lexington, Mass.), or normal rabbit tissue homogenates. Blank samples of all matrixes also were extracted to ensure the absence of interfering peaks. The average recovery of the extraction procedure in human plasma was >96% compared with unextracted reagent standard.
Analytical method.
Concentrations of VER were determined using a reversed-phase high-performance liquid chromatographic method developed at Lilly Research Laboratories (Indianapolis, Ind.). The mobile phase consisted of acetonitrile-ammonium acetate (50 mM, pH 4.0, 50:50 [vol/vol]), delivered at 0.5 ml/min. The injection volume was 75 μl. VER and LY306168 eluted at approximately 6 and 4 min, respectively, using a 5-μm C8 analytical column (Alltech Inertsil; 150 by 4.6 mm; Alltech, Deerfield, Ill.) maintained at 50°C in conjunction with a precolumn filter containing a 2-μm insert and UV detection at 300 nm. Quantitation was based on the ratio of peak heights of VER and the internal standard LY306168. Ten-point standard curves (20 to 5,120 ng/ml for plasma and 20 to 10,000 ng/ml for tissues) were linear with R2 values of ≥0.999 and ≥0.993, respectively. The lower limit of quantitation (LLQ) was 20 ng/ml for plasma and body fluids and 200 ng/ml for solid tissues. Accuracies in plasma and body fluids were within ±0.4 to 3.2%, and intra- and interday variability (precision) ranged from 1.2 to 4.7% (tissues; ± 1 to 12% and 1.5 to 6.6%, respectively).
Pharmacokinetic modeling.
Pharmacokinetic parameters for VER were determined using compartmental analysis. Experimental plasma concentration-time profiles of healthy animals from the 5-, 10-, and 20-mg/kg dosage cohorts were fitted to a three-compartment open pharmacokinetic model with intravenous bolus input and linear first-order elimination from the central compartment using maximum likelihood implemented with the Adapt II (6) computer program. The parameters obtained in these dosage cohorts were then used as priors to fit the lower dosage levels using Bayesian estimation. Model selection was guided by visual inspection of the plasma profiles and the general information criterion (34), as appropriate. The model fitted the observed data well with a mean r2 value of 0.980 (range, 0.913 to 1.00). The regression lines through the plot of observed versus estimated concentrations did not differ from the line of identity, and no bias was observed. Peak plasma concentration (Cmax) values were determined as model-estimated concentrations immediately after administration, and values for area under the concentration-time curve from 0 h to infinity (AUC0–∞) were calculated from estimated plasma concentration profiles using the trapezoidal rule and extrapolation to infinity by standard techniques. Dose linearity after single and multiple dosing was determined by comparison of the dose-normalized AUC0–∞ across dosage levels by analysis of variance (ANOVA) and linear regression analysis. Accumulation was assessed for each dosage level by comparing the mean AUC between doses after multiple dosing as an approximation of AUC between doses at steady state with the mean AUC0–∞ after single dosing (13).
The time points for sparse plasma sampling in animals infected with C. albicans and A. fumigatus were determined using optimal sampling theory (5) implemented by the Adapt II computer program (6) and full concentration-time plasma profiles derived from the compartmental pharmacokinetic studies with healthy rabbits. The selected time points for sampling were 0.16, 2.5, 8, and 24 h after dosing. Back validation using sparse sampling concentration-time points taken from full single-dose profiles of healthy rabbits and comparison with the complete concentration profiles of the same animals revealed no significant differences between the estimated AUC from 0 to 24 h (AUC0–24) derived by both approaches (r2 = 0.994 by linear regression analysis).
Estimation of compartmental pharmacokinetic parameters in infected animals was based on the observed plasma concentration data at the time points of the dosing interval selected by optimal sampling and Bayesian estimation using the known model parameters from healthy rabbits as priors. The three-compartment open model fitted the data well with a mean r2 value of 0.996 (range, 0.992 to 1.000) for the disseminated candidiasis model and 0.995 (range, 0.977 of 1.000) for the pulmonary aspergillosis model.
Pharmacodynamic modeling.
Pharmacodynamic parameters studied in the disseminated candidiasis model included Cmax; AUC0–24; Tτ≥MFC (time during the dosing interval τ with plasma concentrations above the MFC for the infecting isolate); and drug concentrations in kidney, liver, spleen, lung, and brain. Pharmacodynamic parameters studied in the pulmonary aspergillosis model included Cmax, AUC0–24, Tτ≥(3× MIC) (since the Tτ≥MIC was 24 h in all animals), and drug concentrations in the lung. Because only one infecting strain was used in each of the two infection models, Cmax/MIC, Cmax/MFC, AUC0–24/MIC, and AUC0–24/MFC were not separately evaluated. The MFC was used for the candidiasis model and the MIC was used for the aspergillosis model, since the current echinocandins are fungicidal against Candida spp. but not against Aspergillus spp. (23). The described pharmacodynamic parameters were related to the residual fungal burden in tissues in log(CFU per gram) at postmortem (disseminated candidiasis and pulmonary aspergillosis) and survival and endpoints of pulmonary injury (pulmonary aspergillosis) using inhibitory maximum effect pharmacodynamic models with the WinNonlin computer program (Scientific Consultants, Lexington, Ky.).
Statistical analysis.
Differences between the means of pharmacokinetic parameters across the dosage levels were evaluated by the Kruskal-Wallis nonparametric ANOVA test and Dunn's correction for multiple comparisons. For comparison of two dosage levels, the Mann-Whitney U test was used additionally, as appropriate. A two-tailed P value of <0.05 was considered statistically significant.
RESULTS
Single-dose pharmacokinetics of VER in plasma in healthy animals.
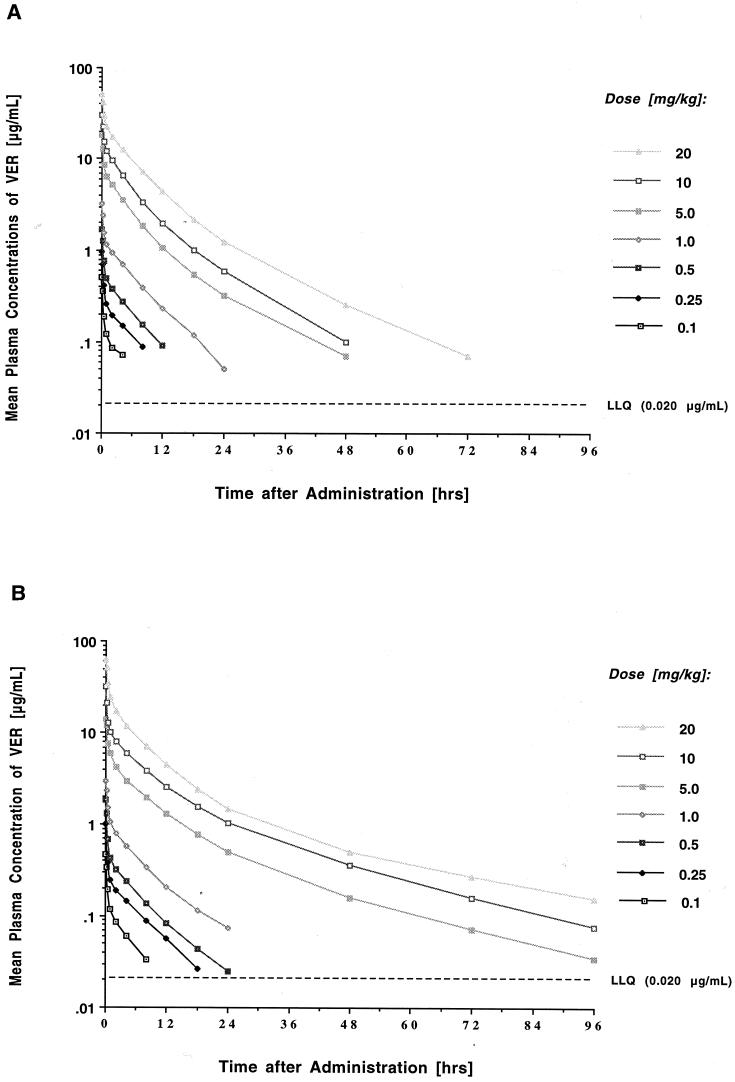
The estimated plasma concentration-time profiles of VER following administration of a single dose are shown in Fig. 1A, and the corresponding compartmental pharmacokinetic parameters are listed in Table 1. Intravenous bolus administration of VER at dosages of 0.1 to 20 mg/kg resulted in peak levels in plasma that ranged from 0.515 ± 0.08 to 51.06 ± 2.94 μg/ml. The concentration profiles in plasma exhibited a rapid initial distribution phase; a second, somewhat slower, distribution-elimination phase; and a prolonged terminal elimination phase with a mean terminal half-life ranging from 10 to 18 h. Mean levels in plasma fell below the LLQ in a dose-dependent manner at 8, 12, 18, 48, 72, 72, and 96 h postdosing for the seven groups. VER demonstrated overall linear plasma pharmacokinetics after single dosing with dose-proportional increases in the mean AUC0–∞ between the 1- and 20-mg/kg dosage levels (P = 0.67 by ANOVA) and no significant changes in total clearance. The apparent volume of distribution at steady state (Vss) ranged from 0.93 to 1.15 liter/kg and was independent of the dosage.
FIG. 1.
Concentration-time plasma profiles after single dosing (A) and after multiple daily dosing over 7 days (B) with 0.1, 0.25, 0.5, 1, 5, 10, and 20 mg of VER-002 per kg. Each point plots the mean concentration of three rabbits each at that time.
TABLE 1.
Single- and multiple-dose compartmental pharmacokinetic parameters of VER in plasmaa
| Dose type | Dose (mg/kg) | Cmax (μg/ml) | Cmin (μg/ml) | AUC0–∞ (μg · h/ml) | V1 (liters/kg) | V2 (liters/kg) | V3 (liters/kg) |
|---|---|---|---|---|---|---|---|
| Single | 0.10 | 0.515 ± 0.08 | <LLQ | 0.477 ± 0.032 | 0.215 ± 0.030 | 0.672 ± 0.102 | 0.270 ± 0.035 |
| 0.25 | 1.486 ± 0.514 | <LLQ | 1.499 ± 0.071 | 0.260 ± 0.029 | 0.515 ± 0.076 | 0.378 ± 0.000 | |
| 0.50 | 1.704 ± 0.087 | <LLQ | 3.224 ± 0.280 | 0.295 ± 0.015 | 0.495 ± 0.036 | 0.441 ± 0.037 | |
| 1 | 3.209 ± 0.366 | 0.050 ± 0.025 | 9.153 ± 0.666 | 0.320 ± 0.034 | 0.374 ± 0.034 | 0.350 ± 0.033 | |
| 5 | 18.091 ± 2.542 | 0.317 ± 0.043 | 50.573 ± 3.842 | 0.289 ± 0.045 | 0.316 ± 0.018 | 0.335 ± 0.032 | |
| 10 | 30.053 ± 3.089 | 0.594 ± 0.051 | 92.086 ± 2.806 | 0.340 ± 0.038 | 0.310 ± 0.028 | 0.284 ± 0.020 | |
| 20 | 51.066 ± 2.943 | 1.233 ± 0.167 | 187.066 ± 7.910 | 0.397 ± 0.023 | 0.374 ± 0.030 | 0.275 ± 0.020 | |
| P | 0.004 | ND | 0.004 | 0.116 | 0.020 | 0.054 | |
| Multiple | 0.10 | 0.466 ± 0.027 | <LLQ | 0.712 ± 0.049 | 0.219 ± 0.013 | 0.439 ± 0.027 | 0.295 ± 0.019 |
| 0.25 | 0.986 ± 0.074 | <LLQ | 2.014 ± 0.446 | 0.262 ± 0.020 | 0.588 ± 0.037 | 0.382 ± 0.056 | |
| 0.50 | 1.864 ± 0.037 | 0.025 ± 0.003 | 3.420 ± 0.107 | 0.272 ± 0.006 | 0.670 ± 0.072 | 0.378 ± 0.009 | |
| 1 | 2.982 ± 0.398 | 0.074 ± 0.004 | 7.973 ± 0.433 | 0.358 ± 0.045 | 0.468 ± 0.008 | 0.605 ± 0.088 | |
| 5 | 14.183 ± 2.262 | 0.492 ± 0.081 | 52.033 ± 5.184 | 0.392 ± 0.071 | 0.350 ± 0.046 | 0.592 ± 0.041 | |
| 10 | 32.240 ± 10.886 | 1.036 ± 0.139 | 112.60 ± 14.860 | 0.390 ± 0.103 | 0.602 ± 0.067 | 0.644 ± 0.116 | |
| 20 | 63.026 ± 2.939 | 1.446 ± 0.118 | 208.80 ± 24.215 | 0.328 ± 0.015 | 0.502 ± 0.095 | 0.604 ± 0.131 | |
| P | 0.003 | ND | 0.003 | 0.138 | 0.039 | 0.047 |
| Vss (liters/kg) | CL2 (liters/h/kg) | CL3 (liters/h/kg) | CLt (liters/h/kg) | t1/2α (h) | t1/2β (h) | t1/2γ (h) |
|---|---|---|---|---|---|---|
| 1.157 ± 0.041 | 0.445 ± 0.003 | 0.016 ± 0.002 | 0.070 ± 0.009 | 0.221 ± 0.013 | 6.558 ± 0.935 | 17.60 ± 1.025 |
| 1.153 ± 0.046 | 0.440 ± 0.002 | 0.021 ± 0.001 | 0.103 ± 0.005 | 0.237 ± 0.011 | 4.498 ± 0.254 | 16.150 ± 0.320 |
| 1.232 ± 0.085 | 0.488 ± 0.035 | 0.024 ± 0.002 | 0.121 ± 0.012 | 0.235 ± 0.005 | 4.018 ± 0.156 | 16.293 ± 0.075 |
| 1.043 ± 0.055 | 0.528 ± 0.030 | 0.020 ± 0.002 | 0.097 ± 0.006 | 0.209 ± 0.012 | 4.071 ± 0.242 | 16.017 ± 0.876 |
| 0.939 ± 0.089 | 0.549 ± 0.081 | 0.028 ± 0.009 | 0.099 ± 0.008 | 0.184 ± 0.027 | 3.199 ± 0.426 | 13.573 ± 2.411 |
| 0.934 ± 0.043 | 0.598 ± 0.067 | 0.030 ± 0.001 | 0.109 ± 0.003 | 0.179 ± 0.013 | 2.914 ± 0.165 | 9.764 ± 0.388 |
| 1.045 ± 0.17 | 0.471 ± 0.085 | 0.018 ± 0.002 | 0.109 ± 0.005 | 0.287 ± 0.069 | 4.176 ± 0.385 | 13.620 ± 1.107 |
| 0.052 | 0.393 | 0.104 | 0.071 | 0.160 | 0.026 | 0.074 |
| 0.953 ± 0.053 | 0.346 ± 0.020 | 0.017 ± 0.001 | 0.117 ± 0.004 | 0.247 ± 0.007 | 3.863 ± 0.180 | 14.663 ± 0.203 |
| 1.231 ± 0.048 | 0.504 ± 0.029 | 0.021 ± 0.002 | 0.127 ± 0.021 | 0.214 ± 0.007 | 4.376 ± 0.323 | 16.013 ± 1.644 |
| 1.319 ± 0.062 | 0.544 ± 0.031 | 0.021 ± 0.000 | 0.149 ± 0.005 | 0.211 ± 0.008 | 4.266 ± 0.351 | 15.063 ± 0.222 |
| 1.430 ± 0.138 | 0.486 ± 0.035 | 0.030 ± 0.004 | 0.126 ± 0.009 | 0.258 ± 0.005 | 3.861 ± 0.165 | 18.343 ± 0.364 |
| 1.334 ± 0.084 | 0.295 ± 0.037 | 0.027 ± 0.004 | 0.107 ± 0.011 | 0.412 ± 0.112 | 4.093 ± 0.700 | 20.897 ± 1.161 |
| 1.636 ± 0.224 | 0.766 ± 0.281 | 0.029 ± 0.009 | 0.118 ± 0.018 | 0.285 ± 0.151 | 4.920 ± 0.744 | 22.437 ± 2.596 |
| 1.434 ± 0.233 | 0.382 ± 0.122 | 0.017 ± 0.005 | 0.119 ± 0.014 | 0.3870 ± 0.126 | 4.802 ± 0.235 | 30.230 ± 3.737 |
| 0.116 | 0.134 | 0.223 | 0.335 | 0.049 | 0.354 | 0.014 |
All values represent the means ± standard errors of the means of three rabbits each. Cmin, concentration in plasma at the end of the recommended dosing interval (24 h); V1, V2, and V3, volume of distribution of the first, second, and third compartment, respectively; CL2 and CL3, distributional clearance; CLt, total plasma clearance; t1/2α, distributional half-life; t1/2β, apparent elimination half-life; t1/2γ, terminal elimination half-life. The LLQ was 0.020 μg/ml. P values were determined for the comparison among dosage groups by Kruskal-Wallis nonparametric ANOVA. ND, not determined.
Multiple-dose pharmacokinetics of VER in plasma in healthy animals.
The estimated plasma concentration-time profiles of VER following daily administration over 7 days are shown in Fig. 1B, and the corresponding mean pharmacokinetic parameters are listed in Table 1. With the exception of the 20-mg/kg dosage level (63.02 versus 51.06 μg/ml; P = 0.045), mean peak plasma concentrations immediately after dosing were not significantly different from those observed after administration of a single dose. Mean plasma levels fell below the LLQ in a dose-dependent manner at 12, 24, 48, and 48 h at the 0.1-, 0.25-, 0.5-, and 1.0-mg/kg dosage levels, respectively, and remained above the LLQ until 96 h at the 5-, 10-, and 20-mg/kg dosage levels. There were no significant differences in AUC and total clearance from those with single dosing. There was, however, a significant prolongation of the terminal (γ) elimination half-life at the higher dosage levels (i.e., 5, 10, and 20 mg/kg; P ≤ 0.05 for the comparison between single- and multiple-dose values) along with a trend toward a larger Vss after multiple dosing (P ≤ 0.05 for the 1-, 5-, and 10-mg/kg dosages and P = 0.1714 for the 20-mg/kg dosage). This difference was related to an increase in the volume of distribution in the third compartment (P ≤ 0.05). Consistent with the unchanged clearance, linear regression of dosage-normalized AUC0–∞ versus dosage for the 1-, 5-, 10-, and 20-mg/kg dosages revealed no statistically significant departure from linearity (P = 0.11), indicating linear pharmacokinetics of VER across the investigated dosage range after multiple dosing also.
Bilirubin, alkaline phosphatase, and alanine and aspartate aminotransferase levels as well as serum creatinine and blood urea nitrogen values determined after 7 days of treatment with VER were within the range of normal values determined in 24 healthy and drug-naive animals (data not shown). Infusion-related toxicity or other clinical abnormalities including abnormal weight changes were not observed.
Optimal sampling-derived pharmacokinetics of VER in plasma in infected animals.
In comparison to healthy rabbits treated with multiple doses of VER, severely ill animals with a life-threatening invasive fungal infection displayed a significantly lower mean clearance rate than their uninfected counterparts (range, 0.050 to 0.081 liter/h/kg; P < 0.05 for all comparisons among dosage groups), accompanied by an overall trend toward higher AUC0–24 values, higher concentrations in plasma at the end of the dosing interval of 24 h and a smaller volume of distribution (P < 0.05 to 0.193 for the various comparisons among dosage groups).
In rabbits with disseminated candidiasis treated with dosages ranging from 0.1 to 1 mg/kg, VER had apparently nonlinear kinetics (pseudononlinearity) with significant differences in the mean clearance (P < 0.05) and the dose-normalized AUC0–∞ (P < 0.05). However, the disposition of the compound was linear over the investigated dosage range of 1 to 20 mg/kg in animals with invasive pulmonary aspergillosis (P = 0.666 and 0.5929, respectively). In the latter model, splitting daily dosages of 10 and 20 mg/kg into two equally divided (BID) dosages had no impact on the 24-h plasma AUC and clearance of VER. Other than the above-described pseudononlinearity, no differences in pharmacokinetics were noted between the two infection models.
Concentrations of VER in tissue in infected animals.
Tissue concentrations at the end of the 24 h dosing interval after daily dosing over 10 days in animals systemically infected with C. albicans revealed preferential accumulation of the compound in the lung and liver and, to a somewhat lesser extent, the spleen and kidneys (Table 2). Measurable concentrations in brain tissue were noted at dosages of 0.5 mg/kg and higher; in choroid and vitreous humor, VER was detectable after administration of 5 mg/kg and higher. Accumulation in aqueous humor was minimal. Mean tissue concentrations above the MFC for the experimental isolate in lung, liver, and spleen, as well as the kidney, were found at dosages of 0.25 mg/kg and higher. Mean tissue concentrations in the brain began to exceed this MFC at a dosage of 1 mg/kg.
TABLE 2.
Levels of VER in tissue after multiple dosing in animals systemically infected with C. albicansa
| Dose (mg/kg · day) | Brain (μg/g) | Aqueous humor (μg/ml) | Vitreous humor (μg/ml) | Choroid (μg/ml) | Lung (μg/g) | Liver (μg/g) | Spleen (μg/g) | Kidney (μg/g) |
|---|---|---|---|---|---|---|---|---|
| 0.1 | <LLQ | <LLQ | <LLQ | <LLQ | 0.851 ± 0.16 | 0.327 ± 0.05 | 0.245 ± 0.65 | <LLQ |
| 0.25 | <LLQ | <LLQ | <LLQ | <LLQ | 1.311 ± 0.15 | 0.621 ± 0.17 | 0.443 ± 0.11 | 0.360 ± 0.16 |
| 0.5 | 0.247 ± 0.02 | <LLQ | <LLQ | <LLQ | 2.520 ± 0.35 | 1.236 ± 0.16 | 0.892 ± 0.13 | 0.701 ± 0.10 |
| 1 | 0.415 ± 0.02 | <LLQ | <LLQ | <LLQ | 4.196 ± 0.46 | 2.808 ± 0.22 | 1.549 ± 0.02 | 1.332 ± 0.08 |
| 5 | 1.580 ± 0.07 | <LLQ | 0.098 ± 0.15 | 0.299 ± 0.07 | 17.93 ± 0.90 | 16.82 ± 0.84 | 9.820 ± 0.81 | 6.870 ± 0.65 |
| 10 | 3.907 ± 0.25 | 0.064 ± 0.006 | 0.184 ± 0.06 | 1.469 ± 0.21 | 32.64 ± 2.03 | 43.76 ± 1.62 | 21.74 ± 1.86 | 16.92 ± 0.56 |
Values represent the means ± standard errors of the means of six rabbits each except for the 10-mg/kg dosage cohort (three rabbits). Tissues were obtained 24 h after the last of 10 daily dosages. The LLQ of the analytical assay was <0.020 μg/ml for aqueous and vitreous humor and choroid and <0.200 μg/g for all solid tissues.
Mean tissue concentrations in the lungs of animals with invasive pulmonary aspergillosis that succumbed to their infection at various time points during the experiment (and the dosing interval) ranged from 5.18 to 9.18 μg/g at the 1-mg/kg dosage level to 68.20 to 170.06 μg/kg at the 20-mg/kg dosage level. There was no apparent trend toward increased accumulation in lung tissue of rabbits receiving BID dosing rather than QD dosing of total daily dosages of 10 and 20 mg/kg.
Pharmacodynamics of VER in neutropenic rabbits with disseminated candidiasis.
Rabbits treated with VER demonstrated a dose-dependent clearance of C. albicans from kidney tissue with 100% eradication of the organism at dosages of ≥0.5 mg/kg/day (P < 0.001 by ANOVA). This eradication corresponded to the achievement of a mean Cmax of VER of ≥1.95 ± 0.12 μg/ml, an AUC0–24 of ≥8.25 ± 0.65 μg · h/ml, ≥11.5 ± 1 h with plasma concentrations exceeding the MFC for the test organism (Tτ≥MFC), and a mean tissue concentration at trough of ≥0.700 ± 0.106 μg/g (Table 3). Notably, similar potent antifungal activity with complete clearance of the organism at dosages of 0.5 mg/kg and higher was also observed previously for the liver, spleen, lung, and brain (28).
TABLE 3.
Effect of VER on residual fungal burden in kidney tissue and dosage-related pharmacodynamic parameters in persistently neutropenic rabbits with subacute disseminated candidiasisa
| Dosage group | C. albicans in kidney tissue [log(CFU/g)] | Cmax (μg/ml) | Cmin (μg/ml) | AUC0–24 (μg · h/ml) | Tτ≥MFC (h) | Ckidney tissue (μg/g) |
|---|---|---|---|---|---|---|
| 0.1 mg/kg QD | 5.678 ± 0.637 | 0.655 ± 0.119 | 0.028 ± 0.009 | 2.283 ± 0.443 | 0.904 ± 0.202 | 0.166 ± 0.034 |
| 0.25 mg/kg QD | 2.575 ± 1.197 | 1.412 ± 0.155 | 0.037 ± 0.006 | 4.390 ± 0.473 | 3.500 ± 0.404 | 0.360 ± 0.165 |
| 0.5 mg/kg QD | 0.000 ± 0.000 | 1.951 ± 0.122 | 0.088 ± 0.013 | 8.251 ± 0.658 | 11.580 ± 1.020 | 0.700 ± 0.106 |
| 1.0 mg/kg QD | 0.000 ± 0.000 | 3.290 ± 0.216 | 0.129 ± 0.007 | 12.466 ± 0.568 | 16.13 ± 1.040 | 1.333 ± 0.086 |
| Untreated controls | 6.225 ± 0.479 | NA | NA | NA | NA | NA |
All parameters are provided as means ± standard errors of the means. Cmin, concentration in plasma 24 h after dosing; Ckidney tissue, concentration of VER in kidney tissue 24 h after the last of 10 daily dosages; NA, not applicable.
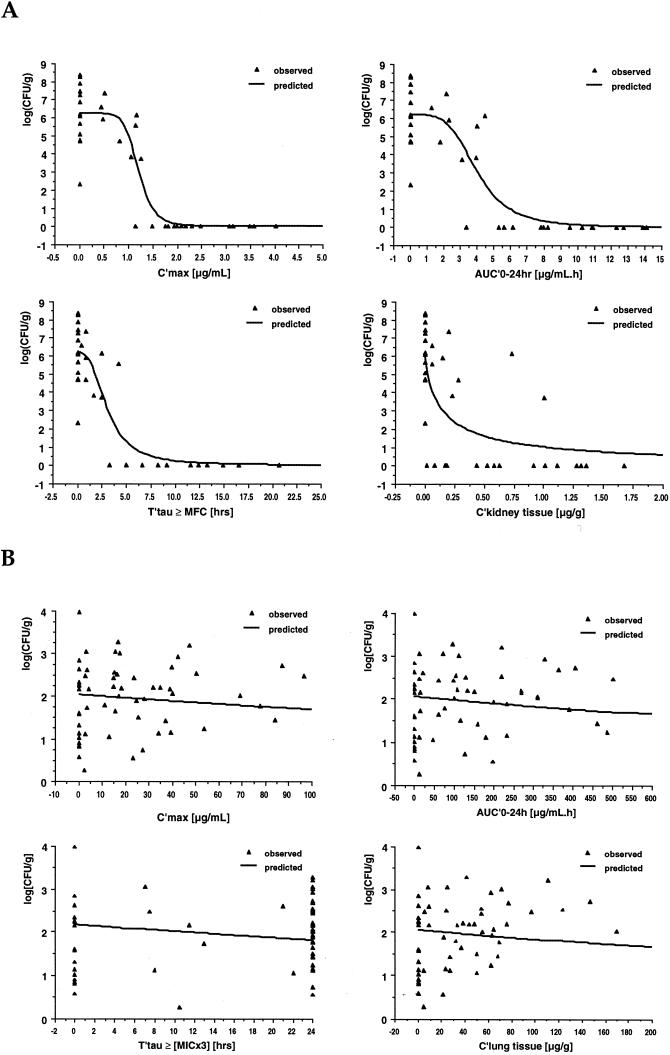
For all sites, the relationships between pharmacodynamic parameters and the clearance of C. albicans from infected tissues was best described by an inhibitory effect sigmoid Emax pharmacodynamic model with 100% efficacy at infinity. Figure 2A graphically depicts the pharmacodynamic relationships between Cmax AUC0–24, Tτ≥MFC, and VER kidney tissue concentrations and the residual fungal burden in kidney tissue; Table 4 lists the model equation, the respective estimated parameters, and the goodness of fit.
FIG. 2.
Plot of the relationships between pharmacodynamic parameters and the residual fungal burden in kidney tissue in log(CFU per gram) in persistently neutropenic rabbits systemically infected with C. albicans (A) and plot of the relationships between pharmacodynamic parameters and the residual fungal burden in lung tissue in log(CFU per gram) in persistently neutropenic rabbits with pulmonary aspergillosis (B). The triangles represent the relationship for each individual animal, while the line describes the relationship predicted by the inhibitory sigmoid maximum effect pharmacodynamic model on the basis of the sum of observed values. Parameter abbreviations: Ckidney tissue, concentration of VER in kidney tissue 24 h after the last of 10 daily dosages; Clung tissue, concentration of VER in lung tissue at autopsy.
TABLE 4.
Relationship between pharmacodynamic parameters of VER and residual fungal burden of C. albicans in kidney tissue at postmortema
| Parameter compared with fungal burden | Value with inhibitory effect sigmoidal Emax pharmacodynamic model
|
|||
|---|---|---|---|---|
| Emax | EP50 | γ | r2 (obs/pred) | |
| Cmax | 6.233 ± 0.34 | 1.204 ± 0.06 | 7.89 ± 4.19 | 0.8195b |
| AUC0–24 | 6.203 ± 0.41 | 4.004 ± 0.45 | 4.13 ± 1.76 | 0.7698b |
| Tτ≥MFC | 6.206 ± 0.36 | 3.069 ± 0.53 | 2.73 ± 1.18 | 0.8055b |
| C24 in kidney tissue | 5.955 ± 0.64 | 0.157 ± 0.10 | 0.85 ± 0.84 | 0.4922b |
All values, except r2, are expressed as means ± standard deviations. C24 in kidney tissue, concentration of VER in kidney tissue 24 h after the last of 10 daily dosages. The equation for the model is E = Emax ∗ {1 − [Pγ/(Pγ + EP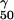 )]}, where Emax is maximum log(CFU per gram) of C. albicans in kidney tissue, EP50 is the magnitude of the assessed parameter that is associated with a 50% reduction in log(CFU per gram) in kidney tissue, and γ is the equation constant that describes the maximum slope of the function describing the investigated pharmacodynamic relationship. obs/pred, observed/predicted.
)]}, where Emax is maximum log(CFU per gram) of C. albicans in kidney tissue, EP50 is the magnitude of the assessed parameter that is associated with a 50% reduction in log(CFU per gram) in kidney tissue, and γ is the equation constant that describes the maximum slope of the function describing the investigated pharmacodynamic relationship. obs/pred, observed/predicted.
P < 0.05.
There was an extremely good fit between observed data and the model equation for plasma concentration-based parameters such as Cmax, AUC0–24, and Tτ≥MFC, as evidenced by a coefficient of determination r2 that ranged from 0.819 to 0.769. Similar fits were also observed for the relationships of pharmacodynamic parameters and the clearance of C. albicans from liver and lung. However, for spleen and brain tissue, coefficients of determination were between 0.061 and 0.062 and between 0.188 and 0.214, respectively, possibly reflecting differences in local host factors at these sites (e.g., the presence of a large number of phagocytic cells in the spleen and the blood-brain barrier in brain tissue).
Pharmacodynamics of VER in neutropenic rabbits with pulmonary aspergillosis.
Despite dose escalation up to 20 mg/kg, treatment with VER had no effect on the residual fungal burden of A. fumigatus in lung tissue (P = 0.3502 by ANOVA) (Table 5). There was, however, a significant improvement in survival (P < 0.05) and a significant reduction in pulmonary tissue injury, as measured by the mean pulmonary infarct scores (P < 0.005) and the mean lung weights (P = 0.078) at autopsy (29). Nevertheless, these beneficial effects were independent of the dosage. Computation of key pharmacodynamic parameters in infected animals revealed escalating mean peak concentrations of VER in plasma and lung tissue that were at least 20- and 50-fold, respectively, in excess of the MIC of VER for the experimental isolate, and in all animals, plasma drug concentrations were above this MIC throughout the entire dosing interval.
TABLE 5.
Effect of VER on residual fungal burden in lung tissue and dosage-related pharmacodynamic parameters in persistently neutropenic rabbits with invasive pulmonary aspergillosisa
| Dosage group | A. fumigatus in lung tissue [log(CFU/g)] | Cmax (μg/ml) | Cmin (μg/ml) | AUC0–24 (μg · h/ml) | Tτ≥(3× MIC) (h) | Clung tissue (μg/g) |
|---|---|---|---|---|---|---|
| 1 mg/kg QD | 1.919 ± 0.362 | 3.260 ± 0.200 | 0.171 ± 0.033 | 13.877 ± 1.386 | 11.21 ± 1.83 | 6.690 ± 0.925 |
| 5 mg/kg QD | 2.356 ± 0.311 | 15.764 ± 0.578 | 1.168 ± 0.278 | 78.000 ± 9.261 | 23.71 ± 0.28 | 34.605 ± 4.722 |
| 10 mg/kg QD | 1.598 ± 0.216 | 32.251 ± 2.222 | 1.965 ± 0.409 | 164.22 ± 21.26 | 24.00 ± 0.00 | 47.737 ± 5.637 |
| 5 mg/kg BID | 1.960 ± 0.257 | 17.838 ± 2.601 | 2.732 ± 0.522 | 142.21 ± 21.01 | 24.00 ± 0.00 | 33.647 ± 4.690 |
| 20 mg/kg QD | 2.310 ± 0.226 | 73.279 ± 7.087 | 3.190 ± 0.406 | 359.03 ± 42.32 | 24.00 ± 0.00 | 112.430 ± 14.421 |
| 10 mg/kg BID | 2.175 ± 0.244 | 39.908 ± 3.471 | 6.652 ± 1.476 | 326.70 ± 39.27 | 24.00 ± 0.00 | 67.670 ± 2.406 |
| Untreated controls | 1.768 ± 0.231 | NA | NA | NA | NA | NA |
All parameters are provided as means ± standard errors of the means. Cmin, concentration in plasma 24 h after dosing; Clung tissue, concentration of VER in lung tissue at autopsy; NA, not applicable.
Pharmacodynamic modeling as well as linear regression analysis revealed the absence of statistically significant relationships between pharmacokinetic parameters such as Cmax, AUC0–24, and Tτ≥(3× MIC) and the residual fungal burden of A. fumigatus (Table 6 and Fig. 2B). Similarly, no correlations were found between Cmax, AUC0–24, Tτ≥(3× MIC), and concentrations of VER in tissue and survival and residual lung weight. There was, however, a weak correlation between these parameters and the residual pulmonary infarct score (P < 0.05 by pharmacodynamic modeling using the inhibitory effect Emax model; P = 0.0915 to P < 0.05 by linear regression.
TABLE 6.
Relationships between pharmacodynamic parameters of VER-002 and the residual fungal burden of A. fumigatus in lung tissue at postmortema
| Parameter compared with fungal burden | Value with inhibitory effect Emax pharmacodynamic Model
|
Linear regression (r) | ||
|---|---|---|---|---|
| Emax | EP50 | r2 (obs/pred) | ||
| Cmax | 2.038 ± 0.148 | 448 ± 632 | 0.0142 | 0.0436 |
| AUC0–24 | 2.049 ± 0.148 | 2,508 ± 2,941 | 0.0115 | 0.0248 |
| Tτ≥(3× MIC) | 2.180 ± 0.200 | 119 ± 88 | 0.0298 | 0.0616 |
| Clung tissue | 2.049 ± 0.158 | 848 ± 1,245 | 0.0851b | 0.2446 |
Values for Emax and EP50 are expressed as means ± standard deviations. Clung tissue, concentration of VER in lung tissue at autopsy. The equation for the pharmacodynamic model is E = Emax ∗ {1 − [C/(C + EP50)]}, where Emax is maximum log(CFU per gram) of A. Fumigatus in lung tissue and EP50 is the magnitude of the assessed parameter that would be associated with a 50% reduction in log(CFU per gram) in lung tissue. obs/pred, observed/predicted.
P < 0.05.
DISCUSSION
The results of our studies demonstrate linear pharmacokinetics of VER in plasma over a broad dosage range of 0.1 to 20 mg/kg with dose-independent plasma clearance and dose-proportional increases in AUC. Plasma concentration data fitted into a three-compartment open pharmacokinetic model that revealed a terminal elimination half-life of up to 30 h. In comparison to single dosing, multiple daily dosing over 7 days led to a significant prolongation of the terminal elimination half-life at the higher end of the dosage range and a trend toward a larger Vss. However, no changes were noted in the mean plasma AUC and in total plasma clearance. Tissue concentrations at trough after multiple dosing were highest in lung and liver, followed by spleen and kidney. Measurable concentrations in brain tissue were noted at dosages of ≥0.5 mg/kg. Implementation of optimal plasma sampling in rabbits with experimental invasive fungal infections by using the Bayesian approach and model parameters from healthy rabbits as priors revealed a significantly slower mean plasma clearance in infected animals along with a trend toward higher AUC values, higher plasma drug concentrations at the end of the dosing interval, and a smaller volume of distribution. Pharmacodynamic modeling in persistently neutropenic rabbits using the residual fungal tissue burden in the main target sites as the primary endpoint and Cmax, AUC0–24, Tτ≥MIC or Tτ≥MFC, and tissue concentrations as pharmacodynamic parameters showed predictable pharmacokinetic and pharmacodynamic relationships in experimental subacute disseminated candidiasis that fitted well with an inhibitory sigmoid maximum effect pharmacodynamic model. In contrast, no concentration-effect relationships were observed in experimental pulmonary aspergillosis using the residual fungal burden in lung tissue and survival as parameters of antifungal efficacy.
The favorable pharmacokinetic properties of VER are in sharp contrast to those of its predecessor, the semisynthetic 4-n-octyloxybenzoyl echinocandin B analogue cilofungin, whose clinical development was discontinued due to toxicity associated with its intravenous vehicle, polyethylene glycol (20). Across species, the disposition of cilofungin was characterized by a very short half-life, a small V, negligible tissue accumulation, and inconsistent efficacy in animal models of susceptible opportunistic mycoses after single-dose administration (18). Increased antifungal efficacy, particularly in the brain, could be achieved only through intermittent and continuous administration of high daily dosages that elicited nonlinear saturable plasma pharmacokinetics with sustained plasma and tissue drug concentrations (2, 25, 30, 32).
While the favorable pharmacokinetic profile of VER is in principle shared by the two other current echinocandins, caspofungin (formerly MK-0991) and micafungin (formerly FK463) (16, 17), differences can nevertheless be observed. In healthy rabbits receiving similar dosages of 1 mg/kg, VER exhibited approximately sixfold-lower mean peak concentrations in plasma, twofold-lower AUC values, and a fourfold-larger V compared to values for caspofungin and micafungin (15; A. H. Groll, D. Mickiene, V. Petraitis, R. Petraitiene, R. Alfaro, K. H. Ibrahim, A. Kalim, I. Bekersky, S. C. Piscitelli, and T. J. Walsh, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1688, 2000). At the present time, however, it remains open whether these differences in the pharmacokinetics in plasma of the current echinocandins are associated with different pharmacodynamics.
The pharmacokinetics of VER in plasma in rabbits after single intravenous dosing are consistent with those obtained in Harlan Lewis rats and beagle dogs. After a single intravenous bolus of 5 mg/kg, the mean Cmax was 5.91 and 6.16 μg/ml, the mean AUC0–∞ was 78 and 49 μg · h/ml, mean V was 1.7 and 1.7 liter/kg, mean clearance was 0.066 and 0.100 liter/h/kg, and the elimination half-life was 18 and 15 h, respectively (L. Zornes, R. Stratford, M. Novilla, D. Turner, C. Boylan, B. Boyll, T. Butler, Y. Lin, D. Zeckner, W. Turner, and W. Current, Program Abstr. 33rd Intersci. Conf. Antimicrob. Agents Chemother., abstr. 370, 1993). In human subjects, at escalating dosages of 35, 50, 70, and 100 mg given by the intravenous route to healthy volunteers, VER exhibited linear pharmacokinetics with mean peak plasma levels ranging from 1.71 to 3.82 μg/ml and mean AUC0–∞ values ranging from 37.46 to 104.81 μg · h/ml. The mean volume of distribution was between 0.72 and 0.90 liter/kg, and the terminal half-life was approximately 40 h (I. Rajman, K. Desante, B. Hatcher, J. Hemingway, R. Lachno, S. Brooks, and M. Turik, Abstr. 37th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-74, 1997). These findings indicate a slower clearance of the compound in humans than in rats, rabbits, and beagle dogs.
As the vast majority of invasive mycoses reside in tissues, data on tissue concentrations may provide guidance for the selection of the appropriate antifungal agents. Assessment of concentrations of VER in tissue after chronic dosing over 10 days demonstrated potentially therapeutic trough concentrations in lung, liver, spleen, and kidney at a dosage as low as 0.25 mg/kg and substantial accumulation in brain tissue at dosages of ≥0.5 mg/kg/day. Although these concentrations represent only one time point during the dosing interval and are a mixture of drug concentrations in the intravascular, the interstitial, and the intracellular tissue compartments (1), they can be useful for assessing antifungal effects, as evidenced by a statistically significant pharmacodynamic relationship with antifungal efficacy in the model of subacute disseminated candidiasis.
One important goal in the development of antimicrobial agents is to understand the relationships among antimicrobial in vitro susceptibility, dosage, drug concentrations in the body, and antimicrobial or toxicological effects. Linking pharmacokinetic and pharmacodynamic information provides critical information for interspecies scaling, definition of breakpoints for in vitro susceptibility testing, and the selection of appropriate dosing regimens (4). In an iterative process, hypotheses generated by in vitro investigations of potency, mode, and time course of antimicrobial activity are tested in discriminative animal models that allow for the assessment of pharmacokinetics and true outcome measurement and, ultimately, translation into clinical studies.
Implementation of an optimal sampling strategy is a novel approach in the experimental investigation of pharmacokinetic and pharmacodynamic relationships. It allows for direct correlation of pharmacokinetic data obtained near steady state with endpoints of antifungal efficacy in each individual infected animal. Comparison of pharmacokinetic parameters derived from plasma concentration data from optimal sampling time points and from the full profiles in 21 subjects revealed excellent congruence by linear regression analysis. An important finding was that, in comparison to healthy animals, rabbits severely compromised by a life-threatening invasive fungal infection displayed a significantly slower clearance of VER that was associated with a trend toward higher AUC0–24 values, higher plasma concentrations at the end of the dosing interval, and a smaller volume of distribution. Although speculative, it appears conceivable that alterations in the composition of the blood and fluid compartments (i.e., changes in blood volume, hematocrit, and/or protein binding) as well as alterations in metabolism and excretion are responsible for this observation.
Similar to cilofungin (32), caspofungin (16), and micafungin (17), VER is fungicidal against most Candida spp. in vitro with predominantly concentration-dependent activity in concentration response and time course experiments (9, 14, 21, 22, 28, 35). In addition, following exposure of C. albicans for 1 h at concentrations of 0.25 to 4 times the MIC, VER displayed a prolonged postantifungal effect that exceeded 12 h (8). In our infection model, achievement of a peak plasma level of approximately 2 μg/ml, an AUC0–24 of 8 μg · h/ml, and a time of 12 h with plasma concentrations above the MFC for the test organism were associated with 100% efficacy. While pharmacodynamic modeling very efficiently described the character and magnitude of these parameter-response relationships, however, it did not allow for an assessment of which of these pharmacodynamic parameters is most closely associated with optimal antifungal activity. The documentation of a prolonged postantifungal effect and the fact that a Tτ≥MFC of 12 h was associated with 100% efficacy suggest that a once-daily dosing regimen could be appropriate.
In the setting of persistent and profound neutropenia, VER had no effect on the residual fungal burden of A. fumigatus in rabbits with invasive pulmonary aspergillosis, despite dose escalation to as much as 20 mg/kg and achievement of plasma and lung drug concentrations that were more than 20- and 50-fold, respectively, in excess of the MIC of VER for the experimental isolate, as well as maintenance of plasma drug concentrations above this MIC throughout the entire dosing interval. Pharmacodynamic modeling revealed no correlation between exposure to VER and residual fungal burden and survival but did demonstrate a weak reciprocal correlation with pulmonary injury as measured by the pulmonary infarct score. While VER has at least similar potencies for inhibition of glucan synthesis in membrane preparations of A. fumigatus and C. albicans (J. Tang, T. R. Parr, W. Turner, M. Debono, L. Lagrandeur, F. Burkhard, M. Rodriguez, M. Zweifel, J. Nissen, and K. Clingerman, Program Abstr. 33rd Intersci. Conf. Antimicrob. Agents Chemother., abstr. 367, 1993), the effects of VER and other current echinocandins on A. fumigatus in vitro are not uniformly fungicidal (24, 27, 29; R. Rennie, C. Sand, and R. Sherburne, Abstr. 13th Meet. Int. Soc. Hum. Anim. Mycol., abstr. P451, 1997). In persistently neutropenic animals, VER leads to a dose-dependent morphological damage of hyphal structures, which results in a lessened ability to invade blood vessels and to cause pulmonary infarction and death (29). However, in the absence of host effector cells, the damaged hyphal elements are still viable and able to grow with normal morphology when not exposed to the drug.
In conclusion, VER demonstrated linear plasma pharmacokinetics that fitted with an open three-compartment pharmacokinetic model, and it achieved substantial penetration in tissue sites relevant for treatment of invasive opportunistic fungal infections. Pharmacodynamic modeling using the residual fungal tissue burden in the kidney as the primary endpoint and Cmax AUC0–24, Tτ≥MIC or Tτ≥MFC, and tissue concentrations as pharmacodynamic parameters showed predictable pharmacodynamic relationships in experimental subacute disseminated candidiasis that were well described by an inhibitory sigmoid maximum effect model. In contrast, no concentration effect and exposure effect relationships for the residual fungal burden in lung tissue and survival were observed in experimental pulmonary aspergillosis. Implementation of optimal sampling in discriminative animal models of invasive fungal infections and pharmacodynamic modeling is a novel approach that holds promise in improving and accelerating the understanding of the action of antifungal compounds in vivo.
ACKNOWLEDGMENTS
We thank Carl L. McMillian at Eli Lilly & Co., Indianapolis, Ind., for assistance with the analytical assay of VER-002 and our colleagues Aaron Bell, Diana Callender, Myrna Candelario, Aida Field-Ridley, Joanne Peter, Tin Sein, and Robert Schaufele for expert technical support in the conduct of the experiments.
REFERENCES
- 1.Cars O. Pharmacokinetics of antibiotics in tissues and tissue fluids: a review. Scand J Infect Dis. 1991;74(Suppl.):23–33. [PubMed] [Google Scholar]
- 2.Cole G T, Lynn K T, Seshan K R. Evaluation of a murine model of hepatic candidiasis. J Clin Microbiol. 1990;28:1838–1841. doi: 10.1128/jcm.28.8.1828-1841.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Committee on the Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources; Commission on Life Sciences, National Research Council. Guide for the care and use of laboratory animals. Washington, D.C.: National Academy Press; 1996. [Google Scholar]
- 4.Craig W A. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis. 1998;26:1–10. doi: 10.1086/516284. [DOI] [PubMed] [Google Scholar]
- 5.D'Argenio D Z. Optimal sampling times for pharmacokinetic experiments. J Pharmacokinet Biopharm. 1981;9:739–756. doi: 10.1007/BF01070904. [DOI] [PubMed] [Google Scholar]
- 6.D'Argenio D Z, Schumitzky A. Adapt II user's guide. University of Southern CaliforniaLos Angeles: Biomedical Simulations Resource; 1990. [Google Scholar]
- 7.Debono M, Gordee R S. Antibiotics that inhibit fungal cell wall development. Annu Rev Microbiol. 1994;48:471–497. doi: 10.1146/annurev.mi.48.100194.002351. [DOI] [PubMed] [Google Scholar]
- 8.Ernst E J, Klepser M E, Pfaller M A. Postantifungal effects of echinocandin, azole, and polyene antifungal agents against Candida albicans and Cryptococcus neoformans. Antimicrob Agents Chemother. 2000;44:1108–1111. doi: 10.1128/aac.44.4.1108-1111.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ernst M E, Klepser M E, Wolfe E J, Pfaller A. Antifungal dynamics of LY303366, an investigational echinocandin B analog, against Candida spp. Diagn Microbiol Infect Dis. 1996;26:125–131. doi: 10.1016/s0732-8893(96)00202-7. [DOI] [PubMed] [Google Scholar]
- 10.Espinel-Ingroff A, Bartlett M, Bowden R, Chin N X, Cooper C, Jr, Fothergill A, McGinnis M R, Menezes P, Messer S A, Nelson P W, Odds F C, Pasarell L, Peter J, Pfaller M A, Rex J H, Rinaldi M G, Shankland G S, Walsh T J, Weitzman I. Multicenter evaluation of proposed standardized procedure for antifungal susceptibility testing of filamentous fungi. J Clin Microbiol. 1997;35:139–143. doi: 10.1128/jcm.35.1.139-143.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Francis P, Lee J W, Hoffman A, Peter J, Francesconi A, Bacher J S, Shelhamer J, Pizzo P A, Walsh T J. Efficacy of unilamellar liposomal amphotericin B in treatment of pulmonary aspergillosis in persistently granulocytopenic rabbits: the potential role of bronchoalveolar D-mannitol and serum galactomannan as markers of infection. J Infect Dis. 1994;169:356–368. doi: 10.1093/infdis/169.2.356. [DOI] [PubMed] [Google Scholar]
- 12.Fromtling R A, Castaner J. LY-303366. Drugs Future. 1994;19:338–342. [Google Scholar]
- 13.Gibaldi M, Perrier D. Pharmacokinetics. 2nd ed. New York, N.Y: Marcel Dekker, Inc; 1982. pp. 455–459. [Google Scholar]
- 14.Green L J, Marder P, Mann L L, Chio L C, Current W. LY303366 exhibits rapid and potent fungicidal activity in flow cytometric assays of yeast viability. Antimicrob Agents Chemother. 1999;43:830–835. doi: 10.1128/aac.43.4.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Groll A H, Gullick B M, Petraitiene R, Petraitis V, Candelario M, Piscitelli S C, Walsh T J. Compartmental plasma pharmacokinetics of the antifungal echinocandin caspofungin (MK-0991) in rabbits. Antimicrob Agents Chemother. 2001;45:596–600. doi: 10.1128/AAC.45.2.596-600.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Groll, A. H., and T. J. Walsh. Caspofungin: pharmacology, safety, and therapeutic potential in superficial and invasive fungal infections. Expert Opin. Investig. Drugs, in press. [DOI] [PubMed]
- 17.Groll A H, Walsh T J. FK-463. Curr Opin Anti-Infect Investig Drugs. 2000;2:405–412. [Google Scholar]
- 18.Groll A H, Piscitelli S C, Walsh T J. Clinical pharmacology of systemic antifungal agents: a comprehensive review of agents in clinical use, current investigational compounds, and putative targets for antifungal drug development. Adv Pharmacol. 1998;44:343–500. doi: 10.1016/s1054-3589(08)60129-5. [DOI] [PubMed] [Google Scholar]
- 19.Hawser S. LY-303366. Curr Opin Anti-Infect Investig Drugs. 1999;1:353–360. [Google Scholar]
- 20.Hector R F. Compounds active against cell walls of medically important fungi. Clin Microbiol Rev. 1993;6:1–21. doi: 10.1128/cmr.6.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karlowsky J A, Harding G A, Zelenitsky S A, Hoban D J, Kabani A, Balko T V, Turik M, Zhanel G G. In vitro kill curves of a new semisynthetic echinocandin, LY-303366, against fluconazole-sensitive and -resistant Candida species. Antimicrob Agents Chemother. 1997;41:2576–2578. doi: 10.1128/aac.41.11.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klepser M E, Ernst E J, Ernst M E, Pfaller M A. Growth medium effect on the antifungal activity of LY 303366. Diagn Microbiol Infect Dis. 1997;29:227–231. doi: 10.1016/s0732-8893(97)00144-2. [DOI] [PubMed] [Google Scholar]
- 23.Kurtz M B, Douglas C M. Lipopeptide inhibitors of fungal glucan synthase. J Med Vet Mycol. 1997;35:79–86. doi: 10.1080/02681219780000961. [DOI] [PubMed] [Google Scholar]
- 24.Kurtz M B, Heath I B, Marrinan J, Dreikorn S, Onishi J, Douglas C. Morphological effects of lipopeptides against Aspergillus fumigatus correlate with activities against (1,3)-β-d-glucan synthase. Antimicrob Agents Chemother. 1994;38:1480–1489. doi: 10.1128/aac.38.7.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee J W, Kelly P, Lecciones J, Coleman D, Gordee R, Pizzo P A, Walsh T J. Cilofungin ( LY121019) shows nonlinear plasma pharmacokinetics and tissue penetration in rabbits. Antimicrob Agents Chemother. 1990;34:2240–2245. doi: 10.1128/aac.34.11.2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.NCCLS. Reference method for broth dilution antifungal susceptibility testing of yeasts. Tentative standard. NCCLS document M27-T. Wayne, Pa: NCCLS; 1997. [Google Scholar]
- 27.Oakley K L, Moore C B, Denning D W. In vitro activity of the echinocandin antifungal agent LY303, 366 in comparison with itraconazole and amphotericin B against Aspergillus spp. Antimicrob Agents Chemother. 1998;42:2726–2730. doi: 10.1128/aac.42.10.2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petraitiene R, Petraitis V, Groll A H, Candelario M, Sein T, Bell A, Lyman C A, McMillian C L, Bacher J, Walsh T J. Antifungal activity of LY303366, a novel echinocandin B, in experimental disseminated candidiasis in rabbits. Antimicrob Agents Chemother. 1999;43:2148–2155. doi: 10.1128/aac.43.9.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petraitis V, Petraitiene R, Groll A H, Bell A, Callender D P, Candelario M, Lyman C A, Bacher J, Walsh T J. Efficacy of LY-303366 against invasive pulmonary aspergillosis. Antimicrob Agents Chemother. 1998;42:2898–2905. doi: 10.1128/aac.42.11.2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rouse M S, Tallan B M, Steckelnberg J M, Henry N K, Wilson W R. Efficacy of cilofungin therapy administered by continuous intravenous infusion for experimental disseminated candidiasis in rabbits. Antimicrob Agents Chemother. 1992;36:56–58. doi: 10.1128/aac.36.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walsh T J, Lee J W, Roilides E, Francis P, Backer J, Lyman C A, Pizzo P A. Experimental antifungal chemotherapy in granulocytopenic animal models of disseminated candidiasis: approaches to understanding investigational antifungal compounds for patients with neoplastic diseases. Clin Infect Dis. 1992;14(Suppl. 1):139–145. doi: 10.1093/clinids/14.supplement_1.s139. [DOI] [PubMed] [Google Scholar]
- 32.Walsh T J, Lee J W, Kelly P, Bacher J, Lecciones J, Thomas V, Lyman C, Coleman D, Gordee R, Pizzo P A. Antifungal effects of the nonlinear pharmacokinetics of cilofungin, a 1,3-β-glucan synthetase inhibitor, during continuous and intermittent intravenous infusions in treatment of experimental disseminated candidiasis. Antimicrob Agents Chemother. 1991;35:1321–1328. doi: 10.1128/aac.35.7.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walsh T J, Bacher P, Pizzo P A. Chronic silastic central venous catheterization for induction, maintenance, and support of persistent granulocytopenia in rabbits. Lab Anim Med. 1988;38:467–470. [PubMed] [Google Scholar]
- 34.Yamaoka K, Nakagawa T, Uno T. Application of Akaike's information criterion in the evaluation of linear pharmacokinetic equations. J Pharmacokinet Biopharm. 1978;6:165–175. doi: 10.1007/BF01117450. [DOI] [PubMed] [Google Scholar]
- 35.Zhanel G G, Karlowsky J A, Harding G A, Balko T V, Zelenitsky S A, Friesen M, Kabani A, Turik M, Hoban D J. In vitro activity of a new semisynthetic echinocandin, LY-303366, against systemic isolates of Candida species, Cryptococcus neoformans, Blastomyces dermatitidis, and Aspergillus species. Antimicrob Agents Chemother. 1997;41:863–865. doi: 10.1128/aac.41.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]