Abstract
An efficient method of synthesizing 2-trifluoromethylindoles from indoles with easy-to-handle, cheap and low-toxic CF3SO2Na under metal-free conditions is described, which selectively introduces trifluoromethyl to indoles on the C2 position. The desired product can be obtained in 0.7 g yield. A radical intermediate may be involved in this transformation.
An efficient method of synthesizing 2-trifluoromethylindoles from indoles with easy-to-handle, cheap and low-toxic CF3SO2Na under metal-free conditions is described, which selectively introduces trifluoromethyl to indoles on the C2 position.
Introduction
Indole compounds are widely found in nature and most of them are bioactive and diffusely used in medicine, as food additives and in other fields.1 For example, indometacin is one of the strongest prostaglandins and synthetic inhibitors (Fig. 1a).2 As a special functional group, trifluoromethyl is often applied to materials, pesticides and pharmaceuticals,3 which can enhance the polarity, stability and lipophilicity.4 For instance, Prozac is mainly used for the treatment of mental illness and fludelone can effectively inhibit cancer cells (Fig. 1b and c).5 Given the importance of indoles and trifluoromethyl groups, combining the two into a single entity would be very interesting. In this context, it is of profound significance to study new and potentially physiologically active indoles containing trifluoromethyl from the perspective of synthetic methodology and application prospects.
Fig. 1. Indoles and trifluorides with biological activities.
The classical methods for the synthesis of trifluoromethyl compounds usually use Umemoto,6 Ruppert–Prakash,7 Langlois8 and Togni9 reagents or other reagents.10,12 For the past few decades, exploring effective ways to obtain 2-trifluoromethylindoles from indoles has gained increasing attention. Rey-Rodriguez et al.11 demonstrated the iron(ii) catalyzed trifluoromethylation of indole under mild reaction conditions (Fig. 2a). Unfortunately, it showed low regioselectivity. Furthermore, Choi et al.12 described the platinum(ii) complexes catalyzed trifluoromethylation of indole on the C2 position in the presence of CF3I and visible light; however, CF3I is difficult to preserve and toxic (Fig. 2b). In the recent years, CF3SO2Na has gradually become an environmentally friendly and cheap source of trifluoromethyl in the field of organic synthesis.13 Shi14 offered an efficient copper-catalyzed oxidation method for the trifluoromethylation of C3 position-blocked indoles (Fig. 2c). CF3SO2Na has been widely used as a CF3 source in organic synthesis; however, the metal-free and highly regioselective synthesis of 2-trifluoromethylindoles has rarely been reported. The disadvantages of expensive reagents, poor regioselectivity or difficult-to-handle reagents are often encountered. As part of our ongoing interest in the synthesis of indoles derivatives,15 we reported herein a tert-butyl hydroperoxide (TBHP)-promoted metal-free and highly regio-selective trifluoromethylation of indole on the C2 position using easy-to-handle and low-toxic CF3SO2Na as the trifluoromethyl source.
Fig. 2. Preparation of trifluoromethylindoles from indoles.
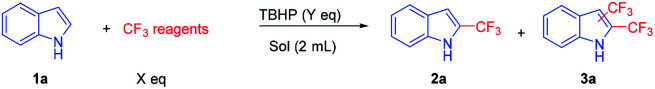
Initially, indole (1a) and CF3SO2Na were selected as model substrates for the optimization of the reaction conditions (Table 1). When 1a, 1 equiv. of CF3SO2Na, 2 equiv. of TBHP and 2 mL CH3CN were added to a sealed Pyrex test tube under room temperature, 2a was obtained in the yield of 15% (entry 1). Subsequently, higher temperature (80 °C and 140 °C) gave the desired product 2a in higher yields (36% and 45%, respectively) (entries 2–3). Next, we switched the ratio of raw materials. It was found that the amount of CF3SO2Na would greatly affect the yield of 2a, which showed that a small loading of CF3SO2Na resulted in less desired product and a higher loading of CF3SO2Na would result in byproduct 3a (entries 5–6). A higher yield of 2a was obtained in the presence of 3 equiv. of TBHP (entries 3–5). A reaction time of 18 hours was found to be enough for this transformation, which gave the desired product 2a in 66% yield (entry 5). Shortening the reaction time to 16 hours or prolonging the reaction time to 20 hours (even 24 hours) afforded 2a in the yield of 53% and 65% (66%), respectively (entries 5–9). When toluene, DMF and H2O were used as solvents, 2a was obtained in the yield of 49%, 41% and 30%, respectively (entries 10–11), while no targeting product 2a was obtained with DMSO or 1,4-dioxane as solvents. Besides, we also investigated other trifluoromethylation reagents such as TMSCF3 (trifluoromethyltrimethylsilane), using which only trace 2a was observed. In brief, CF3SO2Na (2.0 equiv.), TBHP (3.0 equiv.), CH3CN (2 mL), 140 °C and 18 h are the optimized reaction conditions, as shown in entry 5. The two-dimensional NMR (H–H COSEY) plot also supported that trifluoromethylation was on the C2 position of indoles (ESI 5†).
Screening of the reaction conditionsa.
| ||||||
|---|---|---|---|---|---|---|
| Entry | CF3 reagent(X eq.) | Y eq. | Sol (2 mL) | Temp/°C | Time/h | Yieldb2a/(3a) % |
| 1 | CF3SO2Na(2 eq.) | 1 | CH3CN | 25 | 18 | 15 |
| 2 | CF3SO2Na(2 eq.) | 1 | CH3CN | 80 | 18 | 36 |
| 3 | CF3SO2Na(2 eq.) | 1 | CH3CN | 140 | 18 | 45 |
| 4 | CF3SO2Na(2 eq.) | 2 | CH3CN | 140 | 18 | 50 |
| 5 | CF3SO2Na(2 eq.) | 3 | CH3CN | 140 | 18 | 66 |
| 6 | CF3SO2Na(3 eq.) | 3 | CH3CN | 140 | 18 | 56(16) |
| 7 | CF3SO2Na(2 eq.) | 3 | CH3CN | 140 | 16 | 53 |
| 8 | CF3SO2Na(2 eq.) | 3 | CH3CN | 140 | 20 | 65 |
| 9 | CF3SO2Na(2 eq.) | 3 | CH3CN | 140 | 24 | 66 |
| 10 | CF3SO2Na(2 eq.) | 3 | Toluene | 140 | 18 | 49 |
| 11 | CF3SO2Na(2 eq.) | 3 | DMF | 140 | 18 | 41 |
| 12 | CF3SO2Na(2 eq.) | 3 | H2O | 140 | 18 | 30 |
| 13 | CF3SO2Na(2 eq.) | 3 | DMSO | 140 | 18 | nr |
| 14 | CF3SO2Na(2 eq.) | 3 | 1,4-Dioxane | 140 | 18 | nr |
| 15 | TMSCF3(2 eq.) | 3 | CH3CN | 140 | 18 | Trace |
Unless otherwise noted, the reaction was carried out with 1a (0.3 mmol) and solvent CH3CN (2 mL), 140 °C, stirred for 18 h in air.
Isolated yields.
With the optimized reaction conditions in hand, we investigated the substrate scopes with respect to different substituents on indole scaffolds shown in Table 2. To our delight, most of the transformations went smoothly and provided the corresponding products in moderate to good yields. When the hydrogen of NH was replaced by methyl, trifluoromethylation took place and gave 2b in the yield of 56%. It should be noted that when the position of the methyl group on the scaffold of indole was changed from C3 to C7, the corresponding products were obtained with the yield of 73% to 57% (2c, 2d, 2g, 2m, 2t). Indoles with the methyl group substituted at C4 or C6 position successfully converted into corresponding products with the yields of 76% (2d) and 75% (2m), respectively, showing a higher activity. Importantly, this approach was compatible with halogen groups such as bromine (2k) and iodine (2l), which presented corresponding products with the yield of 54% and 59%, respectively. Except for the fluorine-containing substituents, it was found that the yield of electron-donating groups was slightly higher than that of the electron-withdrawing groups (2h, 2n, 2r). For example, 7-azaindole was successfully converted into its corresponding trifluoromethylation product 2s with the yield of 64%.
Trifluoromethylation of indolesa,b.

|
The reaction was carried with 1 (0.3 mmol), CF3SO2Na (2.0 equiv.) and TBHP (3.0 equiv.) were used in CH3CN (2 mL) at 140 °C in air for 18 h.
Isolated yields.
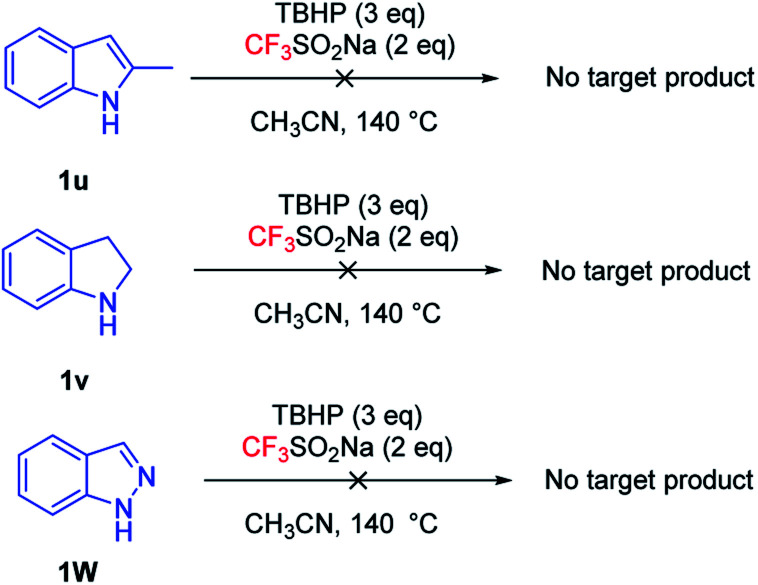
Subsequently, we studied other indole derivatives under optimized conditions (Fig. 3), such as 2-methylindole (1u), indoline (1v) and indazole (1w). The desired product was not obtained, which may indicate that trifluoromethylation occurred on the C2 position and indoline could not be compatible in the present system.
Fig. 3. Trifluoromethylation of other indole derivatives.
Investigations established that a large-scale amplification experiment of 1b (5 mmol) under optimized conditions at 80 °C smoothly gave 2b. We were pleased to observe that 0.507 g of the corresponding product was isolated with the yield being 51% (Fig. 4).
Fig. 4. Gram-scale experiment.
In order to get insights into the reaction mechanism, a series of control experiments were conducted. When 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO) (4 equiv.), butylatedhydroxytoluene (BHT) (4 equiv.) or 1,4-benzoquinone (4 equiv.) was respectively added under the standard reaction conditions (Table 1, entry 6), almost no desired products were detected (Fig. 5). In addition, a radical intermediate (molecular weight: 288) was detected by GC-MS when a radical scavenger (BHT) was used. Based on these results, we proposed herein a plausible reaction pathway (Fig. 6).8a,14 At first, a free radical, tBuO˙ (II) generated from TBHP (I) by heating reacts with a trifluorosulfinate anion to provide the free radical  (III). Subsequently, a free radical
(III). Subsequently, a free radical  was formed by releasing SO2 from the radical
was formed by releasing SO2 from the radical  (III). The intermediate (V) was generated from the radical
(III). The intermediate (V) was generated from the radical  with substrate 1. TBHP accepted one electron from the intermediate (V) to give the intermediate cation (VI) and release OH−. Finally, the product 2 was obtained by releasing a proton from the intermediate cation (VI).
with substrate 1. TBHP accepted one electron from the intermediate (V) to give the intermediate cation (VI) and release OH−. Finally, the product 2 was obtained by releasing a proton from the intermediate cation (VI).
Fig. 5. Control experiments.
Fig. 6. Proposed mechanism for trifluoromethylation of indoles.
Conclusions
In summary, we have developed an original method of synthesizing 2-trifluoromethylindoles from indoles with easy-to-handle, cheap and low-toxic CF3SO2Na under metal-free conditions, which selectively proceeded on the C2 position of indoles. High functional group tolerance and moderate to good yield were presented in this transformation. The control experiments provided evidences that the reaction may undergo a free radical pathway. Further applications of 2-trifluoromethylindoles are currently being carried out in our laboratory.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
We thank the financial support from the National Natural Science Foundation of China (51578224).
Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra07785e
Notes and references
- (a) Zhang B. Studer A. Org. Lett. 2014;16:1216. doi: 10.1021/ol5001395. [DOI] [PubMed] [Google Scholar]; (b) Brand J. P. Charpentier J. Waser J. Angew. Chem., Int. Ed. 2009;48:9346. doi: 10.1002/anie.200905419. [DOI] [PubMed] [Google Scholar]; (c) Wang W. Lian X. Chen D. Liu X. Lin L. Feng X. Chem. Commun. 2011;47:7821. doi: 10.1039/C1CC12306H. [DOI] [PubMed] [Google Scholar]; (d) Han L. Ma X. Liu Y. Yu Z. Liu T. Org. Chem. Front. 2018;5:725. doi: 10.1039/C7QO00911A. [DOI] [Google Scholar]; (e) Yamamoto Y. Ohkubo E. Shibuya M. Adv. Synth. Catal. 2017;359:1747. doi: 10.1002/adsc.201700122. [DOI] [Google Scholar]; (f) Xie E. Rahman A. Lin X. Org. Chem. Front. 2017;4:1407. doi: 10.1039/C7QO00229G. [DOI] [Google Scholar]; (g) Ernst B. Ruehling A. Wibbeling B. Glorius F. Chem.–Eur. J. 2016;22:4400. doi: 10.1002/chem.201600377. [DOI] [PubMed] [Google Scholar]
- (a) Tu D. Luo J. Jiang C. Chem. Commun. 2018;54:2514. doi: 10.1039/C8CC00267C. [DOI] [PubMed] [Google Scholar]; (b) Jana S. Verma A. Kadu R. Kumar S. Chem. Sci. 2017;8:6633. doi: 10.1039/C7SC02556D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Gao G. L. Yang C. Xia W. Chem. Commun. 2017;53:1041. doi: 10.1039/C6CC08975E. [DOI] [PubMed] [Google Scholar]; (b) Ye Y. Cheung K. P. S. He L. Tsui G. C. Org. Chem. Front. 2018;5:1511. doi: 10.1039/C8QO00191J. [DOI] [Google Scholar]; (c) Blay G. Fernandez I. Carmen Munoz M. Pedro J. R. Vila C. Chem.–Eur. J. 2010;16:9117. doi: 10.1002/chem.201000568. [DOI] [PubMed] [Google Scholar]; (d) Jiang L. Yi W. Liu Q. Adv. Synth. Catal. 2016;358:3700. doi: 10.1002/adsc.201600651. [DOI] [Google Scholar]
- (a) Chaudhary B. Diwaker M. Sharma S. Org. Chem. Front. 2018;5:3133. doi: 10.1039/C8QO00932E. [DOI] [Google Scholar]; (b) Nagib D. A. MacMillan D. W. C. Nature. 2011;480:224. doi: 10.1038/nature10647. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Borkin D. A. Landge S. M. Toeroek B. Chirality. 2011;23:612. doi: 10.1002/chir.20982. [DOI] [PubMed] [Google Scholar]; (d) Lou Q. Ding Y. Xu D. Liu G. Zhao J. Adv. Synth. Catal. 2017;359:2557. doi: 10.1002/adsc.201700465. [DOI] [Google Scholar]
- (a) Zhang C. P. Wang Z. L. Chen Q. Y. Zhang C. T. Gu Y. C. Xiao J. C. Angew. Chem., Int. Ed. 2011;50:1896. doi: 10.1002/anie.201006823. [DOI] [PubMed] [Google Scholar]; (b) Wiehn M. S. Vinogradova E. V. Togni A. J. Fluorine Chem. 2010;131:951. doi: 10.1016/j.jfluchem.2010.06.020. [DOI] [Google Scholar]; (c) Huang Y. Suzuki S. Liu G. Tokunaga E. Shiro M. Shibata N. New J. Chem. 2011;35:2614. doi: 10.1039/C1NJ20550A. [DOI] [Google Scholar]
- Xu C. Liu J. Ming W. Liu Y. Liu J. Wang M. Chem.–Eur. J. 2013;19:9104. doi: 10.1002/chem.201301585. [DOI] [PubMed] [Google Scholar]
- (a) Seo S. Taylor J. B. Greaney M. F. Chem. Commun. 2013;49:6385. doi: 10.1039/C3CC41829D. [DOI] [PubMed] [Google Scholar]; (b) Mu X. Chen S. Zhen X. Liu G. Chem.–Eur. J. 2011;17:6039. doi: 10.1002/chem.201100283. [DOI] [PubMed] [Google Scholar]
- (a) Langlois B. R. Laurent E. Roidot N. Tetrahedron Lett. 1991;32:7525. doi: 10.1016/0040-4039(91)80524-A. [DOI] [Google Scholar]; (b) Miller S. A. van Beek B. Hamlin T. A. Bickelhaupt F. M. Leadbeater N. E. J. Fluorine Chem. 2018;214:94. doi: 10.1016/j.jfluchem.2018.08.005. [DOI] [Google Scholar]; (c) Ji Y. Brueckl T. Baxter R. D. Fujiwara Y. Seiple I. B. Su S. Blackmond D. G. Baran P. S. Proc. Natl. Acad. Sci. U. S. A. 2011;108:14411. doi: 10.1073/pnas.1109059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Mejía E. Togni A. ACS Catal. 2012;2:521. doi: 10.1021/cs300089y. [DOI] [Google Scholar]; (b) Shimizu R. Egami H. Nagi T. Chae J. Hamashima Y. Sodeoka M. Tetrahedron Lett. 2010;51:5947. doi: 10.1016/j.tetlet.2010.09.027. [DOI] [Google Scholar]; (c) Hu L. Chen X. Zhu G. Chem. Commun. 2016;52:6845. doi: 10.1039/C6CC02412B. [DOI] [PubMed] [Google Scholar]; (d) Pitre S. P. McTiernan C. D. Ismaili H. Scaiano J. C. ACS Catal. 2014;4:2530. doi: 10.1021/cs5005823. [DOI] [Google Scholar]; (e) Liu J. Li L. Yu L. Tang L. Chen Q. Shi M. Adv. Synth. Catal. 2018;360:2959. doi: 10.1002/adsc.201800568. [DOI] [Google Scholar]; (f) He Y.-T. Wang Q. Zhao J. Wang X.-Z. Qiu Y.-F. Yang Y.-C. Hu J.-Y. Liu X.-Y. Liang Y.-M. Adv. Synth. Catal. 2015;357:3069. doi: 10.1002/adsc.201500510. [DOI] [Google Scholar]
- (a) Wang Z. Ge F. Wan W. Jiang H. Hao J. J. Fluorine Chem. 2007;128:1143. doi: 10.1016/j.jfluchem.2007.04.023. [DOI] [Google Scholar]; (b) Iqbal N. Choi S. Ko E. Cho E. J. Tetrahedron Lett. 2012;53:2005. doi: 10.1016/j.tetlet.2012.02.032. [DOI] [Google Scholar]; (c) Chen M. Huang Z.-T. Zheng Q.-Y. Chem. Commun. 2012;48:11686. doi: 10.1039/C2CC36866H. [DOI] [PubMed] [Google Scholar]; (d) Torok B. Abid M. London G. Esquibel J. Torok M. Mhadgut S. C. Yan P. Prakash G. K. S. Angew. Chem., Int. Ed. 2005;44:3086. doi: 10.1002/anie.200462877. [DOI] [PubMed] [Google Scholar]; (e) Li P. Zhao G. Zhu S. Chin. J. Chem. 2011;29:2749. doi: 10.1002/cjoc.201100034. [DOI] [Google Scholar]; (f) Hui Y. Chen W. Wang W. Jiang J. Cai Y. Lin L. Liu X. Feng X. Adv. Synth. Catal. 2010;352:3174. doi: 10.1002/adsc.201000643. [DOI] [Google Scholar]
- Rey-Rodriguez R. Retailleau P. Bonnet andI.Gillaizeau P. Chem.–Eur. J. 2015;21:3572. doi: 10.1002/chem.201406333. [DOI] [PubMed] [Google Scholar]
- Choi W. J. Choi S. Ohkubo K. Fukuzumi S. Cho E. J. You Y. Chem. Sci. 2015;6:145. doi: 10.1039/c4sc02537g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Chang B. Shao H. Yan P. Qiu W. Weng Z. Yuan R. ACS Sustainable Chem. Eng. 2016;5:334. doi: 10.1021/acssuschemeng.6b01682. [DOI] [Google Scholar]; (b) Zhang Y. Han X. Zhao J. Qian Z. Li T. Tang Y. Adv. Synth. Catal. 2018;360:2659. doi: 10.1002/adsc.201800488. [DOI] [Google Scholar]; (c) Jiang L. Qian J. Yi W. Lu G. Cai C. Zhang W. Angew. Chem., Int. Ed. 2015;54:14965. doi: 10.1002/anie.201508495. [DOI] [PubMed] [Google Scholar]
- Shi X. Li X. Ma L. Shi D. Catalysts. 2019;9:278. doi: 10.3390/catal9030278. [DOI] [Google Scholar]
- Wang C. P. Jiang G. F. Tetrahedron Lett. 2017;58:1747. doi: 10.1016/j.tetlet.2017.03.060. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.