Abstract
Objectives:
To evaluate the risk of heart failure (HF) linked to human immunodeficiency virus (HIV) infection, how risk varies by demographic characteristics, and whether it is explained by atherosclerotic disease or risk factor treatment.
Patients and Methods:
We performed a retrospective cohort study of persons with HIV (PWHs) from January 1, 2000, through December 31, 2016, frequency-matched 1:10 to persons without HIV on year of entry, age, sex, race/ethnicity, and treating facility. We evaluated the risk of incident HF associated with HIV infection, overall and by left ventricular systolic function, and whether HF risk varied by demographic characteristics.
Results:
Among 38,868 PWHs and 386,586 matched persons without HIV, mean ± SD age was 41.4±10.8 years, with 12.3% female, 21.1% Black, 20.5% Hispanic, and 3.9% Asian/Pacific Islander. During median follow-up of 3.8 years (interquartile range, 1.4-9.0 years), the rate (per 100 person-years) of incident HF was 0.23 in PWHs vs 0.15 in those without HIV (P<.001). The PWHs had a higher adjusted HF rate (adjusted hazard ratio [aHR], 1.73; 95% confidence interval [CI], 1.57 to 1.91), which was only modestly attenuated after accounting for interim acute coronary syndrome events. Results were similar by systolic function category. The adjusted risk of HF in PWHs was more prominent for those 40 years and younger (aHR, 2.45; 95% CI, 1.92 to 3.03), women (aHR, 2.48; 95% CI, 1.90 to 3.26), and Asian/Pacific Islanders (aHR, 2.46; 95% CI, 1.27 to 4.74).
Conclusion:
HIV infection increases the risk of HF, which varied by demographic characteristics and was not primarily mediated through atherosclerotic disease pathways or differential use of cardiopreventive medications.
Because persons living with human immunodeficiency virus (HIV) (PWHs) are living longer, they face an evolving, expanding set of challenges beyond treatment of HIV and acquired immunodeficiency syndrome—related illnesses.1 These challenges include multiple comorbidities, such as atherosclerotic cardiovascular diseases (ASCVDs), cancer, and liver and kidney diseases.1 The risk of ASCVD emerged as a concern early in the antiretroviral therapy (ART) era,2-4 although recent data suggest a lower excess ASCVD risk in PWHs.5
Less is known about other cardiovascular complications, such as heart failure (HF), in PWHs. In the United States, HF affects more than 6 million adults and is a leading cause of hospitalization and death in older persons.6 The epidemiology of HF is also shifting, with greater than 50% of patients having HF with preserved ejection fraction (HFpEF), a syndrome associated with differential but poor outcomes compared with HF with reduced ejection fraction (HFrEF).7 Although common contributors to HF exist in those with and without HIV,8 several potential risk factors unique to PWHs include exposure to chronic immune activation and immunodeficiency4,9,10 and certain ARTs.2,11-16 The Veterans Aging Cohort Study-Virtual Cohort (VACS-VC) suggested a higher risk of HF in PWHs after accounting for cardiovascular risk factors but studied nearly all men, had limited ethnic diversity, and did not account for differential receipt of cardiopreventive therapies.17,18 Furthermore, few data exist about whether risk of HF in PWHs varies by demographic characteristics and potentially contributing modifiable factors.
We addressed these knowledge gaps by evaluating the association between HIV infection and the risk of developing HF overall and by type of HF, contributing risk factors, and whether variation in risk of HF exists across age, sex, and race/ethnicity within a large, multicenter, contemporary, diverse matched population of adults with and without HIV.
METHODS
Source Populations
The source population was derived from 3 integrated health care delivery systems providing comprehensive care tracked through electronic medical record (EMR) systems. Kaiser Permanente Northern California serves more than 4.5 million members, Kaiser Permanente Southern California serves more than 4.6 million members, and Kaiser Permanente Mid-Atlantic States serves more than 800,000 members. The study was approved by participating institutions’ institutional review boards, and a waiver of informed consent was obtained.
Identification of Adults with HIV
Comprehensive HIV registries maintain lists of all members diagnosed as having HIV, HIV transmission risk factors, dates of HIV infection, acquired immunodeficiency syndrome—defining diagnoses, HIV-related laboratory and pharmacy data, and associated EMR data at each site. The HIV registries include all members with HIV since the early 1980s for Kaiser Permanente Northern California, since 1998 for Kaiser Permanente Mid-Atlantic States, and since 2000 for Kaiser Permanente Southern California, with manual confirmation of medical records and case lists. For the present analysis, we included adult (age ≥21 years) members with HIV identified from January 1, 2000, through December 31, 2016.
Identification of Frequency-Matched Adults Without HIV
Adult members without HIV from the same source populations and study period were frequency-matched at a population level up to 10:1 to PWHs based on calendar year (ie, year of the start of follow-up for PWHs), age (±1 year), sex, race, and primary treating facility to account for possible practice differences across sites.
Incident HF and Type
Follow-up occurred through 2016, with censoring at disenrollment, death, or end of follow-up. Deaths were captured comprehensively from EMR data, Social Security Administration files, and state death certificates.
Using a previously validated approach,19-21 we identified newly diagnosed HF based on a primary hospital discharge diagnosis of HF or having 3 or more outpatient visits coded for HF with 1 or more visits to a cardiologist using the International Classification of Diseases, Ninth Revision or the International Classification of Diseases, Tenth Revision (codes available on request). The positive predictive value is greater than 90% for admissions with a primary discharge diagnosis of HF using these codes compared with manual medical record review using Framingham study clinical criteria.22 Our outpatient criteria requiring multiple visits (including ≥ 1 cardiology clinic visit) coded for HF was to enhance specificity. We removed patients with preexisting HF at study entry from the cohort before analysis.
Leveraging previous work,19-21 we ascertained available data on left ventricular ejection fraction (LVEF) from echocardiography, radionuclide scintigraphy, other imaging modalities, and left ventriculography test results using site-specific databases. We categorized patients with HF as having HFpEF, HFrEF, or HF with mid-range ejection fraction (HFmrEF). We defined HFpEF as LVEF of 50% or greater or a physician’s qualitative assessment of preserved or normal systolic function23; HFrEF as LVEF of 40% or less or a physician’s qualitative assessment of moderate, moderate to severe, or severe systolic dysfunction; and HFmrEF as LVEF of 41% to 49% or a physician’s qualitative assessment of mildly reduced systolic function. Although there are other ways of subclassifying HF type, this approach has the most direct clinical implications based on existing randomized trial data for HF therapies and practice guidelines.24
Covariates
We ascertained baseline and updated information on risk factors for HF and comorbidity using International Classification of Diseases, Ninth Revision or International Classification of Diseases, Tenth Revision and Current Procedural Terminology diagnostic or procedure codes, laboratory results, vital signs, or specific therapies received based on validated approaches.21,25,26 These risk factors included demographic characteristics (age, sex, self-reported race/ethnicity), neighborhood-level socioeconomic factors (educational level, income), lifestyle factors (tobacco use, illicit drug and alcohol abuse/dependence), medical history (acute coronary syndrome [ACS], coronary revascularization, atrial fibrillation/flutter, ischemic stroke/transient ischemic attack, peripheral artery disease, valvular heart disease, hypertension, diabetes mellitus, dyslipidemia, HIV-related illnesses, cancer, dementia, depression, lung disease, liver disease, thyroid disease), available laboratory results (low- and high-density lipoprotein cholesterol, estimated glomerular filtration rate, urinary protein excretion), and pharmacy dispensing data (cardiac-related medications, antihypertensive medications, lipid-lowering therapy, diabetes therapy, nonsteroidal anti-inflammatory drugs).
Statistical Approach
Analyses were conducted using a statistical software program (SAS 9.4; SAS Institute Inc). We compared baseline characteristics between PWHs and adults without HIV using t tests for continuous variables and χ2 tests for categorical variables. We calculated annualized incidence rates (per 100 person-years) of incident HF by HIV status and compared Kaplan-Meier curves of survival free of HF using a log-rank test.
To evaluate the association of HIV infection with incident HF and potential explanatory factors, we performed Cox proportional hazards regression models that serially adjusted for the following explanatory variables: site and calendar era, demographic characteristics, lifestyle factors, cardiovascular history, medical history, and receipt of cardiopreventive and other medications. In the final model, we additionally adjusted for interim hospitalizations for ACS during follow-up as a potential explanation for excess HF risk. We also evaluated separate interaction terms for HIV status and age, sex, and race/ethnicity.
Based on a priori hypotheses, we examined whether the association of HIV infection with incident HF varied by demographic characteristics using stratified analyses by age group (21-40, 41-50, ≥51 years old), sex (male, female), and self-reported race/ethnicity (White, Black, Asian/Pacific Islander, Hispanic). Finally, we conducted separate analyses for HFrEF, HFpEF, and HFmrEF.
RESULTS
Baseline Characteristics
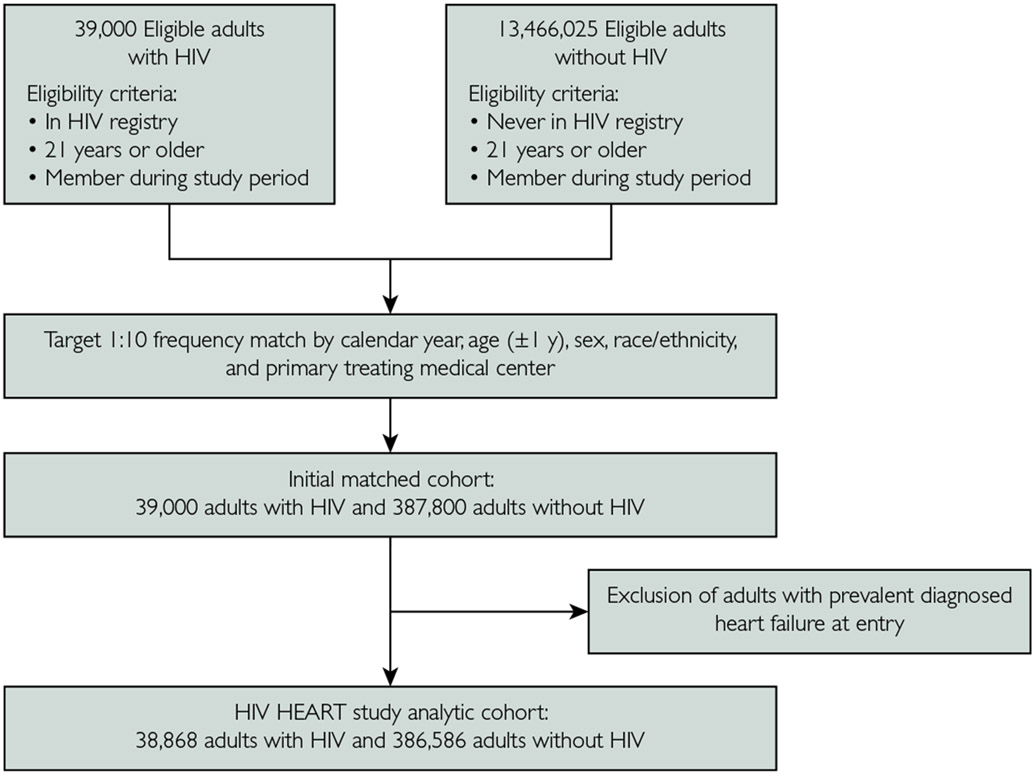
We identified 38,868 PWHs and 386,586 frequency-matched adults without HIV (Figure 1). Mean participant age was 41.4 years, 12.3% were women, and there was broad racial/ethnic diversity (21.1% Black, 20.5% Hispanic, and 3.9% Asian/Pacific Islander). Compared with adults without HIV, PWHs were more likely to have liver disease, depression, proteinuria, and previous illicit drug use or alcohol abuse. The PWHs were less likely than those without HIV to have known cardiovascular or metabolic conditions, including atrial fibrillation/flutter, coronary revascularization, valvular disease, hypertension, diabetes, and dyslipidemia (Table).
FIGURE 1.
Assembly of matched adults with and without human immunodeficiency virus (HIV) infection, 2000-2016.
TABLE.
Baseline Characteristics of Persons Living With HIV and Matched Persons Without HIV Identified From January 1, 2000, Through December 31, 2016
| Characteristic | Overall (N=425,454) |
Persons with HIV (n=38,868) | Persons without HIV (n=386,586) | P value |
|---|---|---|---|---|
| Sociodemographic characteristics | ||||
| Age (y), mean ± SD | 41.4±10.8 | 41.4±10.8 | 41.4±10.8 | .87 |
| Age category, y (No. [%]) | >.99 | |||
| 21-30 | 77,332 (18.2) | 7043 (18.1) | 70,289 (18.2) | |
| 31-40 | 135,179 (31.8) | 12,341 (31.8) | 122,838 (31.8) | |
| 41-50 | 133,169 (31.3) | 12,189 (31.4) | 120,980 (31.3) | |
| 51-60 | 62,310 (14.6) | 5691 (14.6) | 56,619 (14.6) | |
| 61-70 | 15,073 (3.5) | 1382 (3.6) | 13,691 (3.5) | |
| ≥71 | 2391 (0.6) | 222 (0.6) | 2169 (0.6) | |
| Sex (No. [%]) | .68 | |||
| Female | 52,194 (12.3) | 4743 (12.2) | 47,451 (12.3) | |
| Male | 373,260 (87.7) | 34,125 (87.8) | 339,135 (87.7) | |
| Race (No. [%]) | .98 | |||
| White | 161,351 (37.9) | 14,796 (38.1) | 146,555 (37.9) | |
| Black/African American | 89,680 (21.1) | 8161 (21.0) | 81,519 (21.1) | |
| Hispanic | 87,032 (20.5) | 7928 (20.4) | 79,104 (20.5) | |
| Asian/Pacific Islander | 16,489 (3.9) | 1506 (3.9) | 14,983 (3.9) | |
| Other/unknown | 70,902 (16.7) | 6477 (16.7) | 64,425 (16.7) | |
| Low educational attainment (No. [%]) | <.001 | |||
| No | 261,644 (61.5) | 21,817 (56.1) | 239,827 (62.0) | |
| Yes | 83,709 (19.7) | 7159 (18.4) | 76,550 (19.8) | |
| Missing | 80,101 (18.8) | 9892 (25.5) | 70,209 (18.2) | |
| Low median household income (No. [%]) | <.001 | |||
| No | 308,141 (72.4) | 24,748 (63.7) | 283,393 (73.3) | |
| Yes | 37,078 (8.7) | 4213 (10.8) | 32,865 (8.5) | |
| Missing | 80,235 (18.9) | 9907 (25.5) | 70,328 (18.2) | |
| Baseline ART use (No. [%]) | ||||
| HIV treatment | 6637 (1.6) | 6637 (17.1) | 0 | |
| Preexposure prophylaxis | 63 (0.0) | 0 | 63 (0.0) | |
| Postexposure prophylaxis | 95 (0.0) | 0 | 95 (0.0) | |
| Hepatitis B infection | 86 (0.0) | 0 | 86 (0.0) | |
| Other | 8 (0.0) | 0 | 8 (0.0) | |
| HIV infection route (No. [%]) | ||||
| Heterosexual | 5989 (1.4) | 5989 (15.4) | NA | |
| Intravenous drug use | 2495 (0.6) | 2495 (6.4) | NA | |
| Men who have sex with men | 20,736 (4.9) | 20,736 (53.3) | NA | |
| Other | 520 (0.1) | 520 (1.3) | NA | |
| Unknown | 9128 (2.1) | 9128 (23.5) | NA | |
| Baseline HIV severity | ||||
| CD4 count (cells/mm3) | ||||
| Median (IQR) | 406 (222-610) | 406 (222-610) | NA | |
| Missing (No. [%]) | 16,781 (3.9) | 16,781 (43.2) | NA | |
| <200 | 4928 (1.2) | 4928 (12.7) | NA | |
| 200-499 | 8941 (2.1) | 8941 (23.0) | NA | |
| ≥500 | 8218 (1.9) | 8218 (21.1) | NA | |
| Unknown | 16,781 (3.9) | 16,781 (43.2) | NA | |
| HIV RNA copies (/mL) | ||||
| Median (IQR) | 3452 (49-47,152) | 3452 (49-47,152) | NA | |
| Missing (No. [%]) | 19,462 (4.6) | 19,462 (50.0) | NA | |
| <75 | 6537 (1.5) | 6537 (16.8) | NA | |
| 75-199 | 683 (0.2) | 683 (1.8) | NA | |
| 200-499 | 753 (0.2) | 753 (1.9) | NA | |
| ≥500 | 11,433 (2.7) | 11,433 (29.4) | NA | |
| Unknown | 19,462 (4.6) | 19,462 (50.0) | NA | |
| Medical history (No. [%]) | ||||
| Atrial fibrillation or flutter | 1753 (0.4) | 84 (0.2) | 1669 (0.4) | <.001 |
| Ischemic stroke/transient ischemic attack | 894 (0.2) | 79 (0.2) | 815 (0.2) | .76 |
| Acute myocardial infarction | 1111 (0.3) | 96 (0.2) | 1015 (0.3) | .57 |
| Coronary artery bypass graft | 489 (0.1) | 26 (0.1) | 463 (0.1) | .003 |
| Percutaneous coronary intervention | 1223 (0.3) | 50 (0.1) | 1173 (0.3) | <.001 |
| Mitral or aortic valvular disease | 1233 (0.3) | 80 (0.2) | 1153 (0.3) | .001 |
| Peripheral artery disease | 529 (0.1) | 34 (0.1) | 495 (0.1) | .03 |
| Hypertension | 47,900 (11.3) | 2678 (6.9) | 45,222 (11.7) | <.001 |
| Dyslipidemia | 61,774 (14.5) | 3135 (8.1) | 58,639 (15.2) | <.001 |
| Diabetes mellitus | 21,317 (5.0) | 1277 (3.3) | 20,040 (5.2) | <.001 |
| Hyperthyroidism | 2004 (0.5) | 96 (0.2) | 1908 (0.5) | <.001 |
| Hypothyroidism | 6912 (1.6) | 381 (1.0) | 6531 (1.7) | <.001 |
| Chronic liver disease | 6576 (1.5) | 876 (2.3) | 5700 (1.5) | <.001 |
| Chronic lung disease | 36,538 (8.6) | 3380 (8.7) | 33,158 (8.6) | .42 |
| Diagnosed dementia | 983 (0.2) | 195 (0.5) | 788 (0.2) | <.001 |
| Diagnosed depression | 22,989 (5.4) | 2966 (7.6) | 20,023 (5.2) | <.001 |
| Hospitalized bleeding | 1362 (0.3) | 137 (0.4) | 1225 (0.3) | .24 |
| Systemic cancer | 7003 (1.6) | 968 (2.5) | 6035 (1.6) | <.001 |
| Tobacco use (No. [%]) | <.001 | |||
| None | 366,991 (86.3) | 33,491 (86.2) | 333,500 (86.3) | |
| Passive smoker | 2190 (0.5) | 63 (0.4) | 2,027 (0.5) | |
| Former smoker | 26,213 (6.2) | 1904 (4.9) | 24,309 (6.3) | |
| Current smoker | 30,060 (7.1) | 3310 (8.5) | 26,750 (6.9) | |
| Baseline medication (No. [%]) | ||||
| ACE inhibitor | 22,526 (5.3) | 1001 (2.6) | 21,525 (5.6) | <.001 |
| Angiotensin II receptor blocker | 3794 (0.9) | 127 (0.3) | 3667 (0.9) | <.001 |
| β-Blocker | 16,529 (3.9) | 821 (2.1) | 15,708 (4.1) | <.001 |
| Calcium channel blocker | 9582 (2.3) | 471 (1.2) | 9111 (2.4) | <.001 |
| Diuretic | 14,703 (3.5) | 691 (1.8) | 14,012 (3.6) | <.001 |
| Aldosterone receptor antagonist | 440 (0.1) | 50 (0.1) | 390 (0.1) | .10 |
| α-Blocker | 4362 (1.0) | 278 (0.7) | 4084 (1.1) | <.001 |
| Statin | 23,591 (5.5) | 905 (2.3) | 22,686 (5.9) | <.001 |
| Other lipid-lowering agent | 3254 (0.8) | 322 (0.8) | 2,932 (0.8) | .13 |
| Nonaspirin antiplatelet agent | 2,072 (0.5) | 151 (0.4) | 1921 (0.5) | .003 |
| Anticoagulant | 1640 (0.4) | 124 (0.3) | 1516 (0.4) | .03 |
| Diabetic therapy | 14,896 (3.5) | 673 (1.7) | 14,223 (3.7) | <.001 |
| Nonsteroidal anti-inflammatory drug | 33,764 (7.9) | 2613 (6.7) | 31.151 (8.1) | <.001 |
| Vital signs | ||||
| Systolic blood pressure (mm Hg) | ||||
| Mean ±SD | 123.8±13.6 | 121.9±14.4 | 124.0±13.5 | <.001 |
| Missing (No. [%]) | 276,536 (65.0) | 27,536 (70.8) | 249,000 (64.4) | |
| Diastolic blood pressure (mm Hg) | ||||
| Mean ± SD | 74.4±10.2 | 73.6±10.7 | 74.5±10.2 | <.001 |
| Missing (No. [%]) | 276,535 (65.0) | 27,536 (70.8) | 248,999 (64.4) | |
| Body mass index | ||||
| Mean ± SD | 29.0±6.2 | 26.4±5.5 | 29.2±6.2 | <.001 |
| Missing (No. [%]) | 285,853 (67.2) | 28,599 (73.6) | 257,254 (66.5) | |
| Laboratory results | ||||
| Estimated glomerular filtration rate, mL/min/1.73 m2 (No. [%]) | ||||
| >150 | 436 (0.1) | 71 (0.2) | 365 (0.1) | <.001 |
| 90-150 | 86,679 (20.4) | 9017 (23.2) | 77,662 (20.1) | |
| 60-89 | 48,906 (11.5) | 3748 (9.6) | 45,158 (11.7) | |
| 45-59 | 2851 (0.7) | 315 (0.8) | 2536 (0.7) | |
| 30-44 | 604 (0.1) | 90 (0.2) | 514 (0.1) | |
| 15-29 | 224 (0.1) | 34 (0.1) | 190 (0.0) | |
| <15 | 104 (0.0) | 19 (0.0) | 85 (0.0) | |
| Long-term dialysis | 201 (0.0) | 21 (0.1) | 180 (0.0) | |
| Previous renal transplant | 211 (0.0) | 8 (0.0) | 203 (0.1) | |
| Unknown | 285,238 (67.0) | 25,545 (65.7) | 259,693 (67.2) | |
| Documented proteinuria (No. [%]) | <.001 | |||
| No | 410,604 (96.5) | 36,720 (94.5) | 373,884 (96.7) | |
| Yes | 14,850 (3.5) | 2148 (5.5) | 12,702 (3.3) | |
| High-density lipoprotein cholesterol, mg/dL (No. [%]) | <.001 | |||
| ≥60 | 24,098 (5.7) | 1076 (2.8) | 23,022 (6.0) | |
| 50–59 | 31,010 (7.3) | 1569 (4.0) | 29,441 (7.6) | |
| 40-49 | 46,875 (11.0) | 2815 (7.2) | 44,060 (11.4) | |
| 35-39 | 20,442 (4.8) | 1811 (4.7) | 18,631 (4.8) | |
| <35 | 16,802 (3.9) | 2861 (7.4) | 13,941 (3.6) | |
| Unknown | 286,227 (67.3) | 28,736 (73.9) | 257,491 (66.6) | |
| Low-density lipoprotein cholesterol, mg/dL (No. [%]) | ||||
| ≥200 | 2171 (0.5) | 105 (0.3) | 2066 (0.5) | <.001 |
| 160-199 | 12,138 (2.9) | 536 (1.4) | 11,602 (3.0) | |
| 130-159 | 28,654 (6.7) | 1412 (3.6) | 27,242 (7.0) | |
| 100-129 | 43,066 (10.1) | 2857 (7.4) | 40,209 (10.4) | |
| 70-99 | 32,039 (7.5) | 2996 (7.7) | 29,043 (7.5) | |
| <70 | 9874 (2.3) | 1299 (3.3) | 8575 (2.2) | |
| unknown | 297,512 (69.9) | 29,663 (76.3) | 267,849 (69.3) |
ACE, angiotensin-converting enzyme; ART, antiretroviral therapy; HIV, human immunodeficiency virus; IQR, interquartile range; NA, not applicable.
Follow-up and Rates of Incident HF
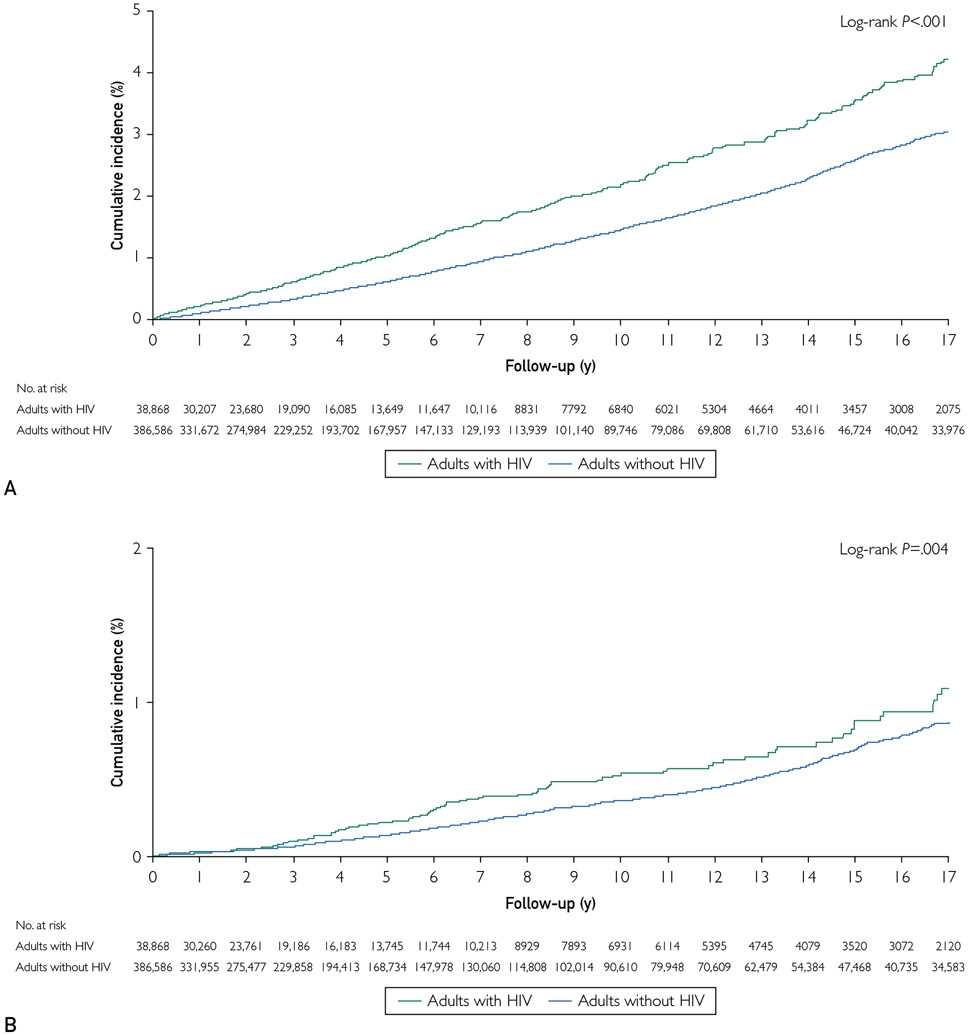
During median follow-up of 3.8 years (interquartile range, 1.4-9.0 years), PWHs had a rate (per 100 person-years) of incident HF of 0.23 (95% CI, 0.21 to 0.25) vs 0.15 (95% CI, 0.15 to 0.16) in adults without HIV (P<.001). Crude rates of HFpEF, HFrEF, and HFmrEF were all higher in PWHs (Supplemental Table 1, available online at http://www.mayoclinicproceedings.org, and Figure 2).
FIGURE 2.
Incidence of incident heart failure overall (A), with reduced ejection fraction (B), and with preserved ejection fraction (C) by human immunodeficiency virus (HIV) status, 2000-2016.
Multivariable Association of HIV Status With HF
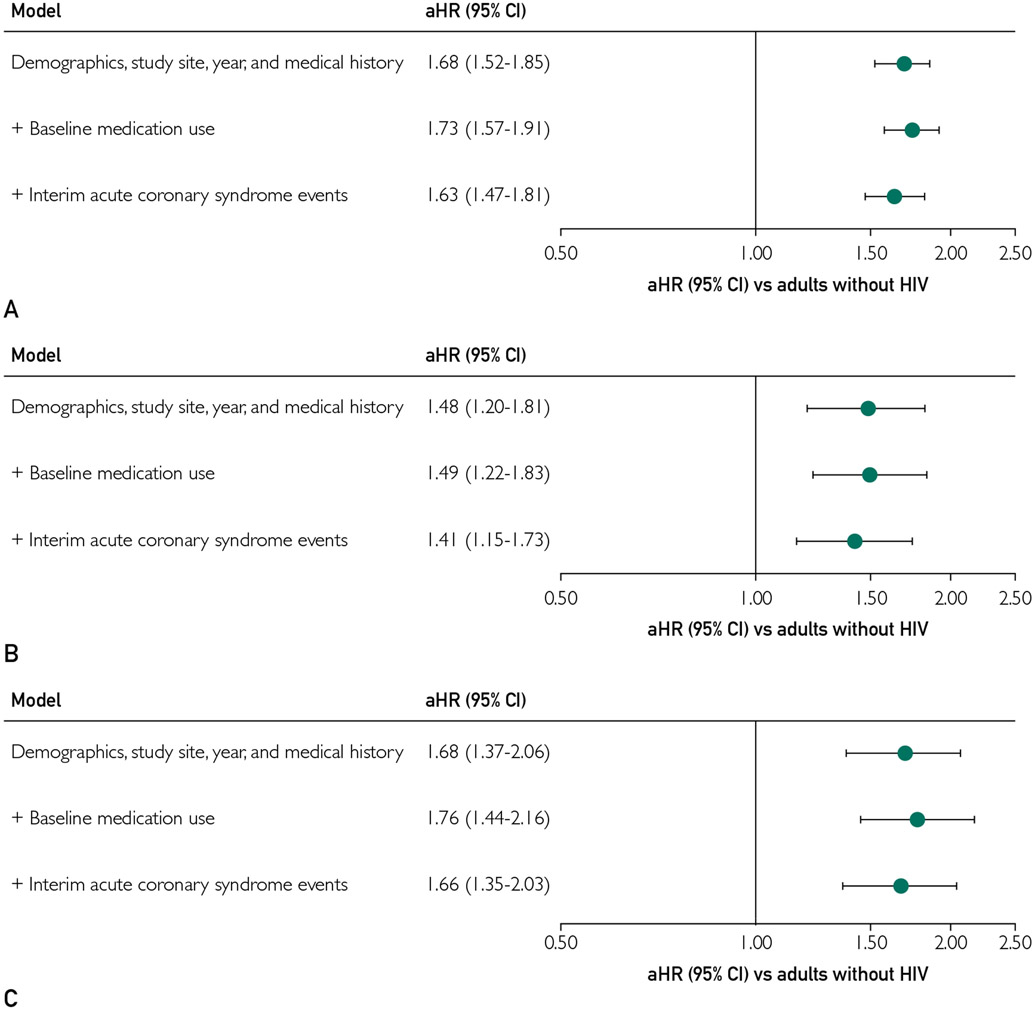
After adjustment for health system, entry year, demographic characteristics, and cardiovascular and medical history, PWHs had an increased rate of incident HF compared with matched controls (adjusted hazard ratio [aHR], 1.68; 95% CI, 1.52 to 1.85) (Figure 3). This excess risk did not materially change after further adjustment for cardiovascular-related medication use (aHR, 1.73; 95% CI, 1.57 to 1.91). Additional adjustment for hospitalizations for ACS during follow-up only modestly attenuated the excess risk of HF in PWHs (aHR, 1.63; 95% CI, 1.47 to 1.81) (Figure 3).
FIGURE 3.
Multivariable association of human immunodeficiency virus (HIV) status with incident heart failure overall (A), with reduced ejection fraction (B), and with preserved ejection fraction (C). Baseline medication use includes all cardiovascular-related medications. Interim acute coronary events include hospitalized acute myocardial infarction or unstable angina after study entry and occurrence of either newly diagnosed heart failure or a censoring event. Demographic characteristics include index age, sex, race/ethnicity, low income by census block, and low educational level by census block. Medical history includes baseline smoking status, acute coronary syndrome, coronary revascularization, atrial fibrillation, mitral or aortic valvular disease, peripheral artery disease, diabetes, hypertension, dyslipidemia, depression, chronic liver disease, hyperthyroidism, hypothyroidism, proteinuria, alcohol abuse, drug use, and estimated glomerular filtration rate. Medications include angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, β-blockers, calcium channel blockers, diuretics, α-adrenergic receptor agonists, aldosterone receptor antagonists, statins, nonstatin lipid therapies, anticoagulants, diabetic therapies, and nonsteroidal anti-inflammatory agents. aHR = adjusted hazard ratio; CI = confidence interval.
Variation of HF Risk in PWHs by Demographic Characteristics
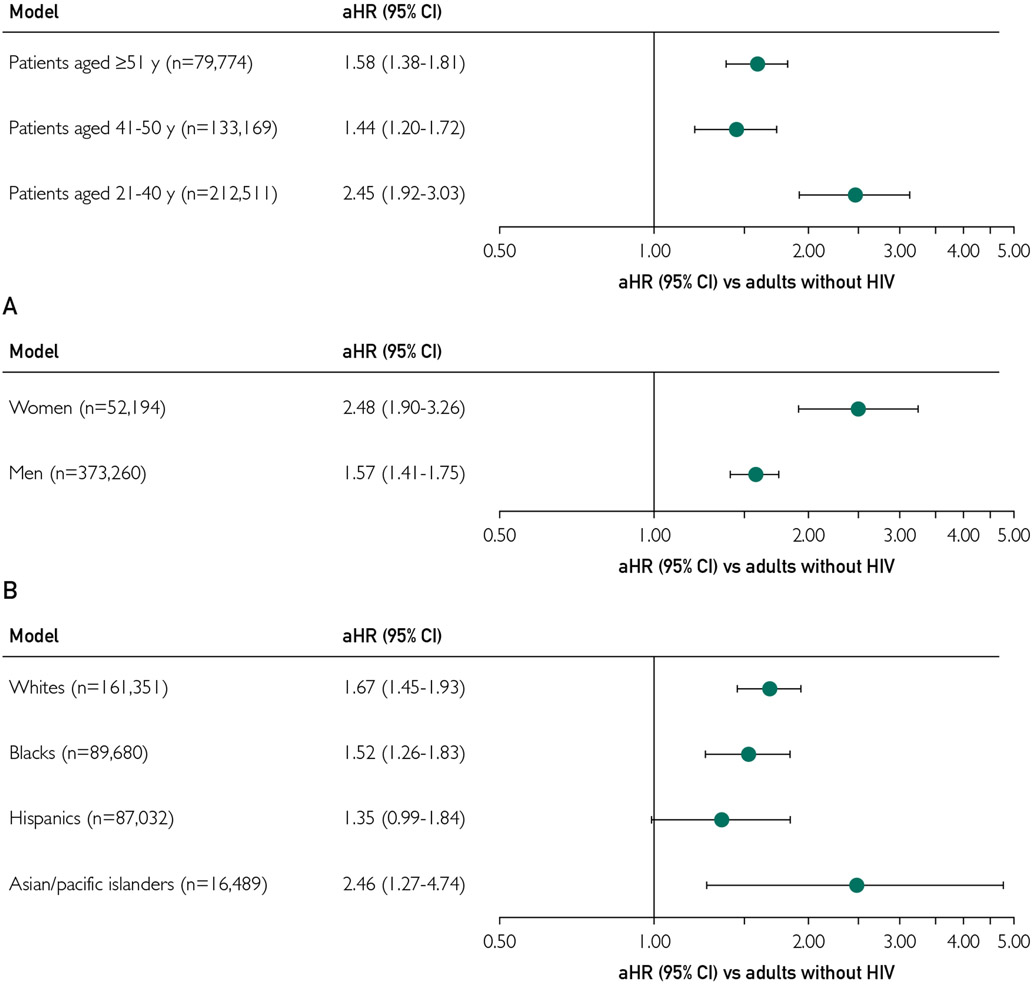
Crude rates of incident HF by prespecified demographic subgroups (age, sex, race/ethnicity) are shown in Supplemental Tables 2, 3, and 4 (available online at http://www.mayoclinicproceedings.org). In multivariable models stratified by age, a stronger association of positive HIV status with incident HF was observed with younger age. Among persons aged 21 to 40 years, PWHs had a nearly 2.5-fold increased rate of incident HF (aHR, 2.45; 95% CI, 1.92 to 3.03) compared with adults without HIV, which was stronger than in those aged 41 to 50 years (aHR, 1.44; 95% CI, 1.20 to 1.72) and those 51 years and older (aHR, 1.58; 95% CI, 1.38 to 1.81) (Figure 4). Statistically significant interactions were noted between HIV status and age (P=.009 for 41-50 years old and P<.001 for ≥51 years old). Also, PWHs had a stronger association with incident HF in women (aHR, 2.48; 95% CI, 1.90 to 3.26) than in men (aHR, 1.57; 95% CI, 1.41 to 1.75) (Figure 4), with a significant interaction (P<.001). Finally, PWHs had an increased adjusted rate of incident HF in White, Black, and Asian/Pacific Islander adults, with the point estimate being highest in Asian/Pacific Islander adults (aHR, 2.46; 95% CI, 1.27 to 4.74) compared with White adults (aHR, 1.67; 95% CI, 1.45 to 1.93) and Black adults (aHR, 1.52; 95% CI, 1.26 to 1.83) (Figure 4). Of note, among Hispanic adults, the adjusted risk of incident HF in PWHs was 1.35 (95% CI, 0.99 to 1.84). We found no significant interactions between HIV status and race/ethnicity (P=.44 for Black adults, P=.19 for Hispanic adults, and P=.18 for Asian/Pacific Islander adults).
FIGURE 4.
Multivariable association of human immunodeficiency virus (HIV) status with incident heart failure stratified by age (A), sex (B), and race/ethnicity (C). All models are adjusted for HIV status, study site, index year, index age, sex, race/ethnicity, low income by census block, low educational level by census block, baseline smoking status, coronary revascularization, atrial fibrillation, mitral or aortic valvular disease, peripheral artery disease, diabetes, hypertension, dyslipidemia, depression, chronic liver disease, hyperthyroidism, hypothyroidism, proteinuria, alcohol abuse, drug use, estimated glomerular filtration rate, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, β-blockers, calcium channel blockers, diuretics, α-adrenergic receptor agonists, aldosterone receptor antagonists, statins, nonstatin lipid therapies, anticoagulants, diabetic therapies, nonsteroidal anti-inflammatory agents, and time-updated acute coronary syndrome events. aHR = adjusted hazard ratio; CI = confidence interval.
HIV Status and HF Subtype
Finally, we examined the association of HIV status with incident HF type. After adjustment for health system, entry year, demographic characteristics, and medical history, PWHs had a higher rate of HFpEF (aHR, 1.68; 95% CI, 1.37 to 2.06), which strengthened after further adjustment for baseline cardiovascular-related medication use (aHR, 1.76; 95% CI, 1.44 to 2.16) and persisted after additional adjustment for interim ACS events (aHR, 1.66; 95% CI, 1.35 to 2.03). Similarly, PWHs had an increased rate of HFrEF after adjustment for health system, entry year, patient demographic characteristics, and medical history (aHR, 1.48; 95% CI, 1.20 to 1.81), which was not changed after additional adjustment for baseline medication use (aHR, 1.49; 95% CI, 1.22 to 1.83) or further accounting for interim ACS events (aHR, 1.41; 95% CI, 1.15 to 1.73). Finally, PWHs had a 2-fold higher rate of HFmrEF after accounting for differences in baseline characteristics (aHR, 2.06; 95% CI, 1.50 to 2.84), which increased after further adjustment for baseline cardiovascular-related medication use (aHR, 2.16; 95% CI, 1.57 to 2.99) and was only modestly attenuated after additional adjustment for interim ACS events (aHR, 1.97; 95% CI, 1.42 to 2.73).
DISCUSSION
In a large, ethnically and geographically diverse, multi-institutional cohort of adults with and without HIV, HIV infection was associated with an increased risk of HF even after adjustment for potential explanatory effects of sociodemographic and clinical risk factors, cardiovascular-related medication use, and interim ACS events. This relationship was present for all HF subtypes and was strongest for HFpEF and HFmrEF. Furthermore, the adjusted excess risk of HF in PWHs seemed stronger in younger (age 21-40 years) patients, women, and Asian/Pacific Islander adults.
The present study represents one of the largest, most comprehensive investigations evaluating the risk of HF associated with HIV infection, overall, by HF type (HFpEF, HFmrEF, and HFrEF), and across demographic subgroups. A VACS-VC analysis primarily in the pre-ART era reported an 81% higher adjusted risk of HF in 2391 veterans with HIV compared with 6095 matched veterans without HIV,17 with a subsequent larger analysis in predominantly White or Black male veterans observing a 41% higher adjusted risk of HF in PWHs.18 In the present, substantially larger and more diverse cohort of both women and men, we found a 65% higher adjusted risk of HF in PWHs not explained by known confounders, receipt of cardiopreventive therapies, or interim ACS events, which materially expands beyond results of other cohorts within and outside the United States.17,18,27,28 The present study also provides important insights into higher rates of HFpEF and HFmrEF in PWHs. Although PWHs experienced higher adjusted rates of HFrEF in this cohort consistent with that observed in the VACS-VC,18 there were stronger associations for HFpEF and HFmrEF.
The present finding that the excess adjusted rate of incident HF in PWHs was more prominent in younger persons (age 21-40 years) supports and extends results from previous studies.18,29 In an analysis of billing claims data among 19,798 PWHs and 59,302 age- and sex-matched persons without HIV, Alonso et al29 reported a higher adjusted risk of diagnosed HF in those younger than 50 years (aHR, 5.9; 95% CI, 3.4 to 10.1) than in those 50 years and older (aHR, 2.5; 95% CI, 1.8 to 3.5), but only limited adjustment for potential confounders was performed and type of HF was not addressed. In the primarily White male veteran VACS-VC sample, there was a greater than 3.5-fold higher rate of incident HF in PWHs compared with persons without HIV (aHR, 3.59; 95% CI, 1.95 to 6.58) for those younger than 40 years,18 and HF occurred at a younger age in veterans with HIV compared with those without HIV. Specific reasons for why HIV infection can be more harmful in younger persons remain unclear.
Concern exists that women living with HIV might be at higher risk for HF with associated potential sex-specific risk factors and mechanisms30 that can differ than for complications such as myocardial infarction.8 Importantly, existing studies have primarily included modest sample sizes and yielded conflicting results. For example, in 26,272 PWHs in Taiwan, Lai et al31 reported a higher age- and calendar period—standardized incidence of HF in women (2.51; 95% CI, 1.71 to 3.56) than in men (1.41; 95% CI, 1.22 to 1.62), whereas no interaction by sex was observed in the excess risk of incident HF in PWHs in the HIV Electronic Comprehensive Cohort of CVD Complications (HIVE-4CVD) involving 4640 PWHs and 4250 persons without HIV frequency-matched on age, sex, race/ethnicity, zip code, and clinic location.27 In contrast, after adjustment for a wide range of confounders, receipt of cardiovascular-related medications, and interim ACSs, we found that the excess risk of incident HF in PWHs was more prominent in women (aHR, 2.48; 95% CI, 1.90 to 3.26) than in men (aHR, 1.57; 95% CI, 1.41 to 1.75). The HIV infection is associated with increased myocardial fibrosis, intramyocardial triglyceride deposition, and impaired diastolic function, as well as immune activation and dysregulation of selected metabolic pathways in women, but it remains unclear whether HIV has direct negative effects or works through promoting existing aging-related mechanisms differentially in women than in men.32,33
Even less is known about how race and ethnicity influence risk of incident HF in PWHs. In HIVE-4CVD, no significant racial difference was reported in the excess risk of developing HF in PWHs, but relatively few cases of HF were observed and they were unable to examine racial/ethnic groups beyond White and Black.27 Within the VACS-VC, similar associations were observed for White (aHR, 1.31; 95% CI, 1.12 to 1.52) and Black (aHR, 1.41; 95% CI, 1.26 to 1.59) adults, but they also were not able to evaluate other racial/ethnic groups.18 Importantly, although crude rates of HF were highest in Black adults overall, we observed that the multivariable point estimate for HIV-associated incident HF was highest in Asian/Pacific Islander adults (aHR, 2.46; 95% CI, 1.27-4.74), was similar in White adults (aHR, 1.67; 95% CI, 1.45 to 1.93) and Black adults (aHR, 1.52; 95% CI, 1.26 to 1.83), but was not statistically significant in Hispanic adults. Little is known about the risk and mechanisms of HF associated with HIV infection in Asian/Pacific Islander adults, especially given the many different subgroups, with one small study in Chinese patients reporting that PWHs were more likely to have diastolic dysfunction, mildly reduced left ventricular systolic dysfunction, and greater left ventricular mass.34
Beyond larger sample size, we had significantly greater representation of Hispanic adults (20.4%), Asian/Pacific Islander adults (3.9%), and women (12.2%) compared with previous studies,17,18 with results that are likely more generalizable to contemporary US PWHs (26% Hispanic, 2% Asian/Pacific Islander, and 19% women).35 We also accounted for potential influences of lifestyle factors, socioeconomic status, HIV infection route, and use of cardiovascular-related medications, in addition to controlling for baseline ART use on the risk of incident HF. Participating institutions employ comprehensive HIV registries and validated algorithms to systematically capture HF events, potential confounders, and mediating factors based on EMR data. The present findings also reflect the most contemporary data of incident HF and HF type among adults with and without HIV.
The present findings carry important implications. Despite recommendations about preventing coronary disease in PWHs, current guidelines do not address screening for or prevention of HF.36 Implementing validated HF screening methods, including a history and physical examination22 supported by selected biomarker testing (N-terminal pro—B-type natriuretic peptide or B-type natriuretic peptide) and echocardiography, in PWHs can expedite time to diagnosis and treatment of ventricular dysfunction and possibly prevention of clinical HF.37 The present findings also reinforce the importance of preventing or aggressively treating vascular risk factors and disease and other associated HF-related risk factors (eg, anemia, sleep-disordered breathing). Additional efforts to prevent HIV infection and to detect HIV infection early should also be prioritized to reduce excess population burden of HF, among other HIV-associated complications.
This study also had limitations. Data were not systematically available for certain risk factors and laboratory tests (eg, kidney function, lipoproteins, natriuretic peptides, troponin, HIV genotype, and non-HIV viral serologies) that can be associated with HF risk. Although we subtyped most HF cases, information on left ventricular systolic function or cardiac structure was not universally available due to limitations in available echocardiographic data primarily from earlier study years, which would be a nondifferential type of bias, and this was supported by the excess risk of each HF type in PWHs. We were unable to delineate specific mechanisms by which HIV infection could increase risk of HF separate from ASCVD-related pathways. The involved pathways are likely multifactorial, with contributions from chronic immune activation on myocardial function and fibrosis,38 direct myocyte invasion, and induction of mitochondrial dysfunction and adverse cellular signaling pathways by HIV,39 co-infection with other viruses (eg, Coxsackie virus B3, cytomegalovirus), impaired nutritional status, and possible adverse effects of different ART regimens on cardiac function.40 We studied insured adults receiving care in integrated health care delivery systems, so the results might not be completely generalizable to all geographic areas or settings.
CONCLUSION
Compared with persons without HIV, PWHs had a higher risk of HF, overall and for each HF type, that was not primarily explained through atherosclerotic pathways or differential use of cardiopreventive medications. Excess HF risk varied significantly by demographic characteristics, which supports improved risk stratification and systematic surveillance for HF and delineation of HIV-specific mechanisms that could serve as therapeutic targets to prevent HIV-associated HF.
Supplementary Material
ACKNOWLEDGMENTS
We thank Elisha Garcia, Richard Contreras, and Haihong Hu for their technical assistance with this study; the people living with and without HIV who made this study possible; and the physicians and staff who provide care for them in the participating Kaiser Permanente health care delivery systems.
Grant Support:
This study was supported by grant R01 HL132640 from the National Heart, Lung, and Blood Institute of the National Institutes of Health.
Abbreviations and Acronyms:
- ACS
acute coronary syndrome
- aHR
adjusted hazard ratio
- ART
antiretroviral therapy
- ASCVD
atherosclerotic cardiovascular disease
- EMR
electronic medical record
- HF
heart failure
- HFmrEF
heart failure with mid-range ejection fraction
- HFpEF
heart failure with preserved ejection fraction
- HFrEF
heart failure with reduced ejection fraction
- HIV
human immunodeficiency virus
- LVEF
left ventricular ejection fraction
- PWH
person living with HIV
- VACS-VC
Veterans Aging Cohort Study-Virtual Cohort
Footnotes
SUPPLEMENTAL ONLINE MATERIAL
Supplemental material can be found online at http://www.mayoclinicproceedings.org. Supplemental material attached to journal articles has not been edited, and the authors take responsibility for the accuracy of all data.
Potential Competing Interests: The authors report no competing interests.
Contributor Information
Alan S. Go, Division of Research, Kaiser Permanente Northern California, Oakland; Department of Health Systems Science, Kaiser Permanente Bernard J. Tyson School of Medicine, Pasadena, CA; Departments of Epidemiology, Biostatistics, and Medicine, University of California, San Francisco, San Francisco; Department of Medicine, Stanford University, Palo Alto, CA.
Kristi Reynolds, Department of Health Systems Science, Kaiser Permanente Bernard J. Tyson School of Medicine, Pasadena, CA; Department of Research and Evaluation, Kaiser Permanente Southern California, Pasadena.
Harshith R. Avula, Department of Cardiology, Kaiser Permanente Walnut Creek Medical Center, Walnut Creek, CA.
William J. Towner, Department of Clinical Science, Kaiser Permanente Bernard J. Tyson School of Medicine, Pasadena, CA; Department of Infectious Disease, Kaiser Permanente Los Angeles Medical Center, Los Angeles, CA.
Rulin C. Hechter, Department of Health Systems Science, Kaiser Permanente Bernard J. Tyson School of Medicine, Pasadena, CA; Department of Research and Evaluation, Kaiser Permanente Southern California, Pasadena.
Michael A. Horberg, Department of Health Systems Science, Kaiser Permanente Bernard J. Tyson School of Medicine, Pasadena, CA; Mid-Atlantic Permanente Research Institute, Kaiser Permanente Mid-Atlantic States, Rockville, MD.
Suma Vupputuri, Mid-Atlantic Permanente Research Institute, Kaiser Permanente Mid-Atlantic States, Rockville, MD.
Thomas K. Leong, Division of Research, Kaiser Permanente Northern California, Oakland.
Wendy A. Leyden, Division of Research, Kaiser Permanente Northern California, Oakland.
Teresa N. Harrison, Department of Research and Evaluation, Kaiser Permanente Southern California, Pasadena.
Keane K. Lee, Division of Research, Kaiser Permanente Northern California, Oakland; Department of Cardiology, Kaiser Permanente Santa Clara Medical Center, Santa Clara, CA.
Sue Hee Sung, Division of Research, Kaiser Permanente Northern California, Oakland.
Michael J. Silverberg, Division of Research, Kaiser Permanente Northern California, Oakland.
REFERENCES
- 1.Phillips AN, Neaton J, Lundgren JD. The role of HIV in serious diseases other than AIDS. AIDS. 2008;22(18):2409–2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klein D, Hurley LB, Quesenberry CP Jr, Sidney S. Do protease inhibitors increase the risk for coronary heart disease in patients with HIV-1 infection? J Acquir Immune Defic Syndr. 2002;30(5):471–477. [DOI] [PubMed] [Google Scholar]
- 3.Freiberg MS, Chang CC, Kuller LH, et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med. 2013;173(8):614–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Silverberg MJ, Leyden WA, Xu L, et al. Immunodeficiency and risk of myocardial infarction among HIV-positive individuals with access to care. J Acquir Immune Defic Syndr. 2014;65(2):160–166. [DOI] [PubMed] [Google Scholar]
- 5.Klein DB, Leyden WA, Xu L, et al. Declining relative risk for myocardial infarction among HIV-positive compared with HIV-negative individuals with access to care. Clin Infect Dis. 2015;60(8):1278–1280. [DOI] [PubMed] [Google Scholar]
- 6.Heidenreich PA, Albert NM, Allen LA, et al. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6(3):606–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Avula HR, Leong TK, Lee KK, Sung SH, Go AS. Long-term outcomes of adults with heart failure by left ventricular systolic function status. Am J Cardiol. 2018;122(6):1008–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92(7):2506–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lang S, Mary-Krause M, Simon A, et al. HIV replication and immune status are independent predictors of the risk of myocardial infarction in HIV-infected individuals. Clin Infect Dis. 2012;55(4):600–607. [DOI] [PubMed] [Google Scholar]
- 10.Lichtenstein KA, Armon C, Buchacz K, et al. Low CD4+ T cell count is a risk factor for cardiovascular disease events in the HIV outpatient study. Clin Infect Dis. 2010;51(4):435–447. [DOI] [PubMed] [Google Scholar]
- 11.Friis-Moller N, Reiss P, Sabin CA, et al. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med. 2007;356(17):1723–1735. [DOI] [PubMed] [Google Scholar]
- 12.Holmberg SD, Moorman AC, Greenberg AE. Trends in rates of myocardial infarction among patients with HIV. N Engl J Med. 2004;350(7):730–732. [DOI] [PubMed] [Google Scholar]
- 13.Mary-Krause M, Cotte L, Simon A, Partisani M, Costagliola D. Increased risk of myocardial infarction with duration of protease inhibitor therapy in HIV-infected men. AIDS. 2003;17(17):2479–2486. [DOI] [PubMed] [Google Scholar]
- 14.Worm SW, Sabin C, Weber R, et al. Risk of myocardial infarction in patients with HIV infection exposed to specific individual antiretroviral drugs from the 3 major drug classes: the data collection on adverse events of anti-HIV drugs (D:A:D) study. J Infect Dis. 2010;201(3):318–330. [DOI] [PubMed] [Google Scholar]
- 15.Lang S, Mary-Krause M, Cotte L, et al. Impact of individual antiretroviral drugs on the risk of myocardial infarction in human immunodeficiency virus-infected patients: a case-control study nested within the French Hospital Database on HIV ANRS cohort CO4. Arch Intern Med. 2010;170(14):1228–1238. [DOI] [PubMed] [Google Scholar]
- 16.Sabin CA, Worm SW, Weber R, et al. Use of nucleoside reverse transcriptase inhibitors and risk of myocardial infarction in HIV-infected patients enrolled in the D:A:D study: a multi-cohort collaboration. Lancet. 2008;371(9622):1417–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Butt AA, Chang CC, Kuller L, et al. Risk of heart failure with human immunodeficiency virus in the absence of prior diagnosis of coronary heart disease. Arch Interl Med. 2011;171(8):737–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freiberg MS, Chang CH, Skanderson M, et al. Association between HIV infection and the risk of heart failure with reduced ejection fraction and preserved ejection fraction in the antiretroviral therapy era: results from the Veterans Aging Cohort Study. JAMA Cardiol. 2017;2(5):536–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allen LA, Magid DJ, Gurwitz JH, et al. Risk factors for adverse outcomes by left ventricular ejection fraction in a contemporary heart failure population. Circ Heart Fail. 2013;6(4):635–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saczynski JS, Go AS, Magid DJ, et al. Patterns of comorbidity in older adults with heart failure: the Cardiovascular Research Network PRESERVE study. J Am Geriatr Soc. 2013;61(1):26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith DH, Thorp ML, Guwitz JH, et al. Chronic kidney disease and outcomes in heart failure with preserved versus reduced ejection fraction: the Cardiovascular Research Network PRESERVE Study. Circ Cardiovasc Qual Outcomes. 2013;6(3):333–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971;285(26):1441–1446. [DOI] [PubMed] [Google Scholar]
- 23.Redfield MM, Jacobsen SJ, Burnett JC Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289(2):194–202. [DOI] [PubMed] [Google Scholar]
- 24.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62(16):e147–e239. [DOI] [PubMed] [Google Scholar]
- 25.Go AS, Yang J, Gurwitz JH, Hsu J, Lane K, Platt R. Comparative effectiveness of different beta-adrenergic antagonists on mortality among adults with heart failure in clinical practice. Arch Intern Med. 2008;168(22):2415–2421. [DOI] [PubMed] [Google Scholar]
- 26.Go AS, Lee WY, Yang J, Lo JC, Gurwitz JH. Statin therapy and risks for death and hospitalization in chronic heart failure. JAMA. 2006;296(17):2105–2111. [DOI] [PubMed] [Google Scholar]
- 27.Feinstein MJ, Steverson AB, Ning H, et al. Adjudicated heart failure in HIV-infected and uninfected men and women. J Am Heart Assoc. 2018;7(21):e009985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yen YF, Ko MC, Yen MY, et al. Human immunodeficiency virus increases the risk of incident heart failure. J Acquir Immune Defic Syndr. 2019;80(3):255–263. [DOI] [PubMed] [Google Scholar]
- 29.Alonso A, Barnes AE, Guest JL, Shah A, Shao IY, Marconi V. HIV infection and incidence of cardiovascular diseases: an analysis of a large healthcare database. J Am Heart Assoc. 2019;8(14):e012241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abelman RA, Mugo BM, Zanni MV. Conceptualizing the risks of coronary heart disease and heart failure among people aging with HIV: sex-specific considerations. Curr Treat Options Cardiovasc Med. 2019;21(8):41. [DOI] [PubMed] [Google Scholar]
- 31.Lai YJ, Chen YY, Huang HH, Ko MC, Chen CC, Yen YF. Incidence of cardiovascular diseases in a nationwide HIV/AIDS patient cohort in Taiwan from 2000 to 2014. Epidemiol Infect. 2018;146(16):2066–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zanni MV, Awadalla M, Toribio M, et al. Immune correlates of diffuse myocardial fibrosis and diastolic dysfunction among aging women with human immunodeficiency virus. J Infect Dis. 2020;221(8):1315–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Toribio M, Neilan TG, Awadalla M, et al. Intramyocardial triglycerides among women with vs without HIV: hormonal correlates and functional consequences. J Clin Endocrinol Metab. 2019;104(12):6090–6100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luo L, Zeng Y, Li T, et al. Prospective echocardiographic assessment of cardiac structure and function in Chinese persons living with HIV. Clin Infect Dis. 2014;58(10):1459–1466. [DOI] [PubMed] [Google Scholar]
- 35.Centers for Disease Control and Prevention. HIV Surveillance Report, 2016. Vol 28. US Department of Health and Human Services; 2017. [Google Scholar]
- 36.Thompson MA, Horberg MA, Agwu AL, et al. Primary care guidance for persons with human immunodeficiency virus: 2020 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis. 2020:ciaa1391. [DOI] [PubMed] [Google Scholar]
- 37.Ledwidge M, Gallagher J, Conlon C, et al. Natriuretic peptide-based screening and collaborative care for heart failure: the STOP-HF randomized trial. JAMA. 2013;310(1):66–74. [DOI] [PubMed] [Google Scholar]
- 38.Ntusi N, O’Dwyer E, Dorrell L, et al. HIV-1-related cardiovascular disease is associated with chronic inflammation, frequent pericardial effusions, and probable myocardial edema. Circ Cardiovasc Imaging. 2016;9(3):e004430. [DOI] [PubMed] [Google Scholar]
- 39.Cheung JY, Gordon J, Wang J, et al. Mitochondrial dysfunction in human immunodeficiency virus-1 transgenic mouse cardiac myocytes. J Cell Physiol. 2019;234(4):4432–4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alvi RM, Neilan AM, Tariq N, et al. Protease inhibitors and cardiovascular outcomes in patients with HIV and heart failure. J Am Coll Cardiol. 2018;72(5):518–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.