Abstract
Objective
To compare the reported efficacy and costs of available interventions used for the management of oral lichen planus (OLP).
Materials and methods
A systematic literature search was performed from database inception until March 2021 in MEDLINE via PubMed and the Cochrane library following PRISMA guidelines. Only randomized controlled trials (RCT) comparing an active intervention with placebo or different active interventions for OLP management were considered.
Results
Seventy (70) RCTs were included. The majority of evidence suggested efficacy of topical steroids (dexamethasone, clobetasol, fluocinonide, triamcinolone), topical calcineurin inhibitors (tacrolimus, pimecrolimus, cyclosporine), topical retinoids, intra-lesional triamcinolone, aloe-vera gel, photodynamic therapy, and low-level laser therapies for OLP management. Based on the estimated cost per month and evidence for efficacy and side-effects, topical steroids (fluocinonide > dexamethasone > clobetasol > triamcinolone) appear to be more cost-effective than topical calcineurin inhibitors (tacrolimus > pimecrolimus > cyclosporine) followed by intra-lesional triamcinolone.
Conclusion
Of common treatment regimens for OLP, topical steroids appear to be the most economical and efficacious option followed by topical calcineurin inhibitors. Large-scale multi-modality, prospective trials in which head-to-head comparisons interventions are compared are required to definitely assess the cost-effectiveness of OLP treatments.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12903-022-02168-4.
Keywords: Oral lichen planus, Treatment, Cost, Efficacy, Critical review
Introduction
Oral lichen planus (OLP) is a chronic, T-cell-mediated inflammatory condition, with a global prevalence between 0.1 and 3.2% [1, 2]. It is most common in the fourth-fifth decade of life and has a female predilection [1]. Clinically, OLP is characterized by white reticulations (Wickham striae), erythema, and/or ulcerations. While there is no consensus on subtypes, OLP is often categorized as reticular/keratotic, erythematous/erosive, or ulcerative. OLP can be either asymptomatic or symptomatic, and when symptomatic, can range from mild sensitivity to significant pain that impacts quality of life. OLP is considered an oral potentially malignant disorder with a malignant transformation rate of 0.4–1.4% [3].
The exact etiology of OLP is unknown, and there is currently no known cure [2]. The primary therapeutic goal is symptom management and current treatment options include corticosteroids, calcineurin inhibitors, retinoids, photodynamic therapy, and natural alternatives, although with varying degrees of efficacy [4, 5]. A recent meta-analysis of 55 RCTs compared different interventions and concluded that topical corticosteroids were the most effective treatment modality [6]. There are, however, multiple classes and preparations of topical corticosteroids, ranging in cost and efficacy. And not all patients respond favorably to steroids making alternative treatment options necessary.
Despite the large number of potential OLP treatment modalities, few comparisons exist relative to their costs, even at a time when the subject of rising healthcare expenses is a concern. Consequently, we thought an appraisal of OLP treatments relative to reported efficacy and costs might be desirable in helping to guide clinical decision-making and innovative management approaches. The aim of this systemic review was to compare the various topical and systemic therapeutic interventions used for the management of oral lichen planus in terms of their reported efficacy and estimated current costs.
Materials and methods
To conduct this systematic review, we followed the steps according to the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA).
Inclusion and exclusion criteria
Included articles were randomized controlled trials (RCTs) that evaluated OLP treatment. RCT eligibility required: (1) studies conducted among adult participants 18 years of age or older; (2) participants with OLP; (3) medication or procedural treatment modalities such as: topical corticosteroids, topical calcineurin inhibitors, systemic therapies, lesion-directed therapy (intra-lesional therapies, phototherapy, laser therapy), natural alternatives, or other topical interventions; (4) measured the treatment efficacy as an outcome, estimated or quantified by various methods of improvement (e.g. different objective and subjective clinical scoring scales/systems). We excluded (1) non-English language papers (2) unavailability of full-text papers; (3) uncontrolled studies without a comparative arm; (4) studies using multiple/combination therapies in single arm, and (5) studies using experimental formulations.
Search strategy
Systematic literature search was performed from database inception until March 2021 in the electronic databases, MEDLINE via PubMed and the Cochrane library. The search was conducted in PubMed on 03/24/2021 using Medical Subject Heading (MeSH) terms, "Lichen Planus, Oral" and "Lichen Planus, Oral/drug therapy". The search strategy was as follows: ("Lichen Planus, Oral" [Mesh] OR "Lichen Planus, Oral/drug therapy"[Mesh] AND “topical corticosteroids”[Mesh] OR dexamethasone[tiab] OR clobetasol[tiab] OR fluocinonide[tiab] OR triamcinolone[tiab] AND “topical calcineurin inhibitors”[tiab] OR tacrolimus[tiab] OR pimecrolimus[tiab] OR cyclosporine[tiab]) AND (“systemic therapies”[Mesh] OR corticosteroids[tiab] OR hydroxychloroquine[tiab] OR dapsone[tiab] OR azathioprine[tiab] OR “mycophenolate mofetil”[tiab] OR levamisole[tiab] OR retinoids[tiab]) AND (“lesion-directed therapy”[Mesh] OR “intra-lesional steroid injections”[tiab] OR “intralesional BCG-PSN"[tiab]) AND (“phototherapy”[Mesh] OR “photodynamic therapy”[tiab] OR “psoralen and ultraviolet A therapy”[tiab]) AND (“laser therapy”[Mesh]) AND (“topical amelaxanox”[tiab]) OR “topical thalidomide”[tiab] OR “topical retinoids”[tiab]) AND (“natural therapies”[Mesh] OR lycopene[tiab] OR Ignatia[tiab] OR curcumin [tiab] OR “aloe-vera”[tiab]).
Study selection
Abstracts of the screened articles were reviewed by two authors for eligibility. Any disagreements were judged by a third author. Full text documents of the articles were retrieved and reviewed for final inclusion in the systematic review.
Data collection and data items
Data extraction was performed independently by eight reviewers. The following information was extracted from each article: author name, publication year, RCT design (single-, double-blind or open-label; parallel or cross-over), treatment modality being studied (strength and preparation, duration, frequency of treatment, treatment outcome and adverse events), sample size (n), therapy assessment (adverse events, relapse rate after successful treatment, follow-up time), cost of therapy and cost of managing the adverse events.
Risk of bias
For the quality assessment of RCTs, we utilized the Revised Cochrane risk-of-bias tool for randomized trials (RoB2) which involves assessment of six domains: 1. randomization process, 2. assignment to intervention, 3. missing outcome data, 4. measurement of the outcome, 5. selection of the reported result, and 6. overall assessment.
Outcome measures
The outcome objective and subjective scoring systems utilized by individual studies were considered for assessing the efficacy of different types of treatment modalities employed. The statistical evidence of efficacy between intervention and control was recognized when p value < 0.05. Costs of the medications and procedures were retrieved and the range of cost per unit of treatment was calculated using information available on various online pharmacies and websites comparing prescription drug prices with discounted prices (i.e., goodrx.com, singlecare.com, pharmacychecker.com, otc-online-store.com, ebay.com, amazon.com, naturallythinking.com, etc.). The cost was estimated for per unit and per month utilization of the generic or branded equivalents of treatments assessed in RCTs. Costs of the interventions not available in the USA were converted into US dollars; all costs in current dollars.
Results
Search results
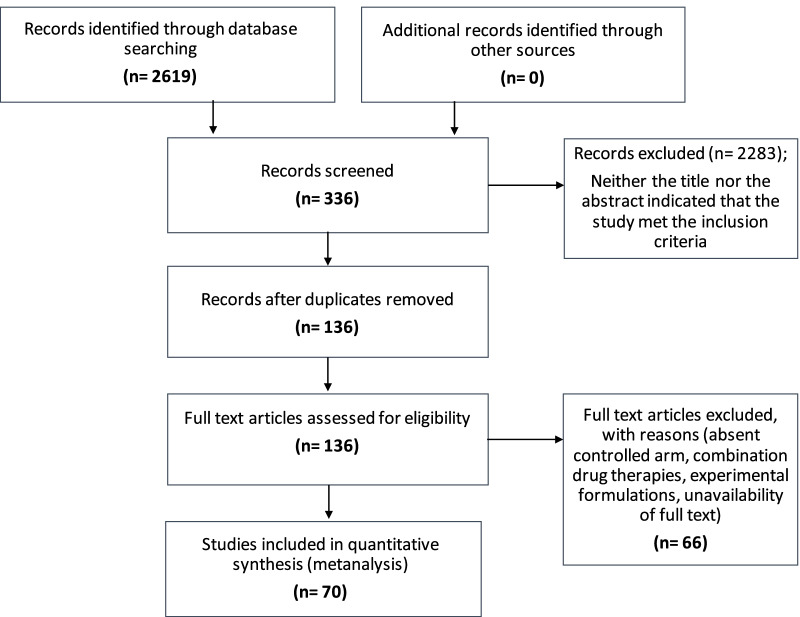
Two-thousand six hundred nineteen (2619) articles were retrieved using the search strategy. Of these, 70 studies were included in the systematic review. Sixty-six full text articles were excluded with reasons {absent controlled arm (35), combination drug therapies (5), experimental formulations (25), unavailability of full text (1)} (Fig. 1).
Fig. 1.
PRISMA flow chart for selection of studies in this systematic review
Study characteristics
70 studies (total of 2612 patients) published between 1977 and 2020 met the inclusion criteria: Four were single-blinded, three were triple-blinded, six were open-label trials, and the remaining were double-blinded. 67 trials had a parallel RCT design and three had a cross-over design. Eighteen RCTs were placebo-controlled, and the remaining 53 trials compared 2–4 treatment modalities. Key characteristics of included studies are listed in Table 1.
Table 1.
Key characteristics of the included randomized clinical trials in this systematic review
| Topical steroids | Reference Study | Intervention | Comparative agent | No. of pts | Indication | Duration | Frequency | Outcome measure | Results | ADRs | Efficacy Comparison | Level of evidence |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dexamethasone | Bakhtiari [27] | Dex solution 0.5 mg/5 mL | PDT | 30; Dex: 15, PDT:15 | Bx-proven clinical OLP | 2 wks | QID | VAS, Thongprasom clinical score, clinical severity index | No significant difference between the two gps in efficacy index, sign score, symptom score or clinical severity on post-treatment days 15, 30, 60 and 90; Decreases in symptoms statistically significant in both (p-value NS) | PDT: 3 pts-pain from manipulation of the probe tip | Dex = PDT | High risk of bias |
| Hambly [28] | Dex solution 0.5 mg/5 mL | Dex solution 0.5 mg/5 mL self-compounded | 9; Dex:4, Dex self-compounded: 5; then cross-over | Symptomatic OLP | 7 wks | TID | VAS, TSQM-9, photos, self-assessment | TSQM-9 revealed the compounded mouth rinse more favorable than the self-formulation rinse, with a mean improv. in convenience of therapy (22.25%), onset of action (8.48%), and attained symptom relief (4.18%) (p-value NS) | None | Commercial dex > self-formulated dex | High risk of bias | |
| Mirza [29] | Dex solution 0.5 mg/5 mL | LLLT vs. PDT | 45; 15 in each group (dex, LLLT, PDT) | Erosive OLP | 4 wks | QID | VAS and clinical score | Significant difference in sign score changes before and after the treatment in the PDT group (p = 0.03), LLLT group (p = 0.04) and in dex group (p = 0.02); statistically significant difference between PDT (p = 0.001) and LLLT (p = 0.001) against dex group before and after treatment. Mean improv. in pain significantly greater in dex group in comparison with the PDT and LLLT gps (p < 0.001). Efficacy index of PDT group improved significantly more than the LLLT (p = 0.001) and corticosteroid gps (p = 0.001) | None | VAS: Dex > LLLT = PDT;Efficacy: PDT > LLLT = Dex | High risk of bias | |
| Clobetasol | Rödström [33] | Clo oint. 0.05% | TA paste 0.1% | 40; 20 in each | Erosive OLP | 9 wks | BIDx3wks, QDx 3wks, once every other dayx3 wks | VAS and 4-point clinical score | Clo more effective than TA at 3 wks (p < 0.05). No significant difference following 6 and 9 wks of treatment | NS | Clo > TA (at 3 wks); Clo = TA (6 & 9 wks) | Low risk of bias |
| Muzio [30] | Clo oint. 0.05% | Clo in analgesic base vs. Clo in denture paste | 24; 8 in each | Bx-proven OLP | 2 wks | TID | VAS | Clo effective in each group (p < 0.05) | candidiasis (number NS) | Clo oint = Clo + analgesic base = Clo + denture paste | Low risk of bias | |
| Carbone [31] | Clo oint. 0.025% | Clo oint. 0.05% | 35; 15 in | Bx- proven symptomatic OLP | 8 wks | BID | VAS and clinical score | VAS improved in both (p = 0.001); clinical score improved (p < 0.05 in both gps). No statistically significant differences b/w gps | None | Clo oint 0.025% = clo oint 0.05% | Low risk of bias | |
| Kaur [32] | Clo oint. 0.025% | TC oint. 0.1% | 40; 20 in each | Bx- proven symptomatic OLP | 4 wks | BID | Symptom and clinical grading score | Improv. in both groups. No statistically significant differences b/w gps | None | Clo oint. 0.025% = TC oint. 0.1% | Low risk of bias | |
| Arduino [8] | Clo gel 0.05% | Placebo | 32; 16 in each group | OLP | 8 wks | BID | VAS and 4-point clinical score | Clo: reduction in VAS and clinical score in tx (p = 0.005) | Clo: 1 pt-GERD; 1pt- mild elevated FBS; placebo: 1pt- skin rxn | Clo > placebo | Low risk of bias | |
| Fluocinonide | Voute [10] | Fluocinonide oint. 0.025% | Placebo | 40; 20 in each group | Bx- proven OLP | 9 wks | 6 × daily | VAS; 4-point clinical score | Statistically significant improv. in fluocinonide group objectively (p = 0.0013) and symptoms (p = 0.008) | None | Flu > placebo | Low risk of bias |
| Carbone [34] | Fluocinonide oint 0.025% | Clo oint. 0.05% vs. placebo | 60 (Flu:25, Clo:24, placebo:11) | Atrophic-erosive symptomatic OLP | 24 wks | TIDx 8wks; BIDx 8wks; QDx 4 wks | Objective and subjective clinical progress score | Clo more effective in atrophic areas (75% vs 25% of total response, respectively) (p = 0.004) | None | Clo > Flu | Low risk of bias | |
| Triamcinolone | Sieg et al. [43] | TA paste 0.1% | Cyclosporin oily liquid preparation | 13; CsA:6, TA:7 | Bx-proven OLP | 6 wks | TID | 7-point mucosal lesion scoring | Clinical improv. in both gps, no statistically significant difference between gps (no p-value) | CsA: precipitation of waxy particles during 'swishing' the oily solution; TA: 3 pts- burning | TA = CsA | Some concerns |
| Ungrouphaiboon et al. [35] | TA paste 0.1% | TA solution 0.1% | 20; TA paste:11, TA rinse:9 | Bx-proven symptomatic OLP | 4 wks | QID | Clinical response: none, partial (1–33% reduction in lesion), good (34–99% lesion reduction, complete response | No statistically significant difference b/w 2 gps | TA paste group: 2 pts- oral candidiasis | TA paste = TA rinse | Some concerns | |
| Laeijendecker et al. [38] | TA oint 0.1% | TC oint. 0.1% | 40; 20 in each | OLP | 6 wks | QID | Reduction in pain | TA: 6 pts healed, 12 showed improv.; TC: 2 pts healed, 7 improved. Initial results better in TC group (p = 0.007) | Temporary pain and burning sensation in both gps | TC > TA | Some concerns | |
| Malhotra et al. [67] | TA paste 0.1% | Oral betamethasone mini pulse (5 mg twice/wk) | 49 (TA: 24, BM: 25) | Bx-proven symptomatic OLP | 24 wks | TA: TID × 12 wks, BID × 4 wks, QD × 4 wks, alternate days × 4 wks; BM: 5 mg × 12 wks, 4 mg × 4 wks; 3 mg x 4wks; 2 mg × 4 wks | Clinical score (based on number of sites and area affected) and change in symptoms | Clinical score: reduction in severity score more in TA group (p = .026); No statistical difference in symptomatic improv. b/w 2 gps | TA group: 5 pts-candidiasis, 1 pt epigastric discomfort; BM group: 7 pts- facial edema, 7 pts epigastric discomfort, 5 pts-fatigue, 4 pts hand/foot edema, 1pt diabetes mellitus | Clinical score: TA > ; Symptoms: TA = BM | High risk of bias | |
| Mansourian et al. [47] | TA paste 0.1% | AV solution | 46; 23 in each | Bx-proven OLP | 4 wks | QID | VAS, Thongprasom score, lesion size (grid) | Both AV and TA significantly reduced VAS, Thongprasom score and lesion size (p < 0.001). No significant difference b/w 2 gps | None | TA = AV | Low risk of bias | |
| Handa [37] | TA paste 0.1% | Fluticasone propionate spray 0.05% | 40; 20 in each group | Symptomatic OLP | 8 wks, 2 wks washout, 8 wks crossover | TA: QID; Fluticasone: 50 μg, 2 dose unit BID | Clinical scoring, VAS, OHIP-14 | No statistically significant difference b/w 2 gps (p value NS) | NS | TA = fluticasone spray | Some concerns | |
| Amanat et al. [54] | TA paste 0.1% in orabase | Cryotherapy (NO) | 30 (one side intervention, the other side control) | Bx-proven, bilateral OLP | 4 wks | TID | Lesion size, RPAE score | Both treatments reduced the sign scores and severity significantly (p < 0.05), no significant differences between gps (p > 0.05) | Cryotherapy: 17 pts- minor swelling. 12 pts- pain in first 7–10 days | TA = cryotherapy | High risk of bias | |
| Kia et al. [48] | TA paste 0.1% | Curcumin paste 5% | 50; 25 in each group | Clinical and bx-proven OLP | 4 wks | TID | VAS and Thongprasom score | No significant difference between the two gps in VAS (VAS at baseline: p = 0.17; VAS two weeks later: p = 0.3; VAS four weeks later: p = 0.46) or Thongprasom score (baseline: p = 0.77, two weeks later: p = 0.92, four weeks later: p = 0.31) | Curcumin: burning sensation, itching, mild swelling and xerostomia, yellow gingiva; TA: 1 burning and 1 mucosal desquamation | TA = Curcumin | Some concerns | |
| Sivaraman et al. [36] | TA paste 0.1% | Clo oint. 0.05%, vs. TC oint. 0.03% | 30; 10 in each of the 3 gps | Atrophic, ulcerative OLP | 6 wks | QID | Reduction in lesion size | TA and Clo: significant reduction in lesion size than Tac gp; overall better results with Clo (p = 0.005) | None | Clo > TA > TC | Some concerns | |
| Thomas et al. [49] | TA paste 0.1% | Curcumin gel 1% TID vs. curcumin gel 6x/d | 75; 25 in each of the 3 gps | Bx-proven symptomatic OLP | 12 wks | TA: TID; curcumin: TID; 6x/d | Numerical Rating Score (burning) and Modified Oral Mucositis Index | Reduction in burning and erythema/ulceration (p < 0.001) in all 3 gps. TA showed max. reduction in burning sensation (77% change) and erythema/ulceration (67% change) (p < 0.001) | None | TA > curcumin gel 1% 6x > curcumin gel 1% TID | High risk of bias | |
| Singh et al. [40] | TA paste 0.1% | Dapsone 100 mg vs. TC oint. 0.1% vs. topical retinoid (type NS) | 40; 10 in each of the 4 gps | Reticular, erosive, atrophic, plaque-like OLP | 12 wks | BID | Symptoms and signs scored according to Raj et al. and Kaliakatsou et al. scales | All clinical improv. (p < 0.05), steroidal and non-steroidal agents had equal efficacy. Of the non-steroidal drugs, oral dapsone had greater efficacy than topical retinoid (p < 0.05); no significant differences between oral dapsone and topical tacrolimus (p > 0.05) or between topical retinoid and TC (p > 0.05) | Mild tingling in the oral cavity in patients treated with topical agents | Dapsone > TA = TC = retinoid | Some concerns | |
| Siponen et al. [39] | TA paste 0.1% | TC oint. 0.1% vs. placebo | 18; TA: 7, TC: 11, placebo: 9 | Bx-proven symptomatic OLP | 9 wks | TID | VAS and clinical score | Reduction in both TC and TA gps as compared to placebo (p = 0.012 and 0.031). No statistically significant difference b/w 2 gps | TA: 3 pts-burning, tingling, gingival tenderness, 2 pts-candidiasis | TA = TC | Low risk of bias | |
| Li et al. [1] | TA paste 0.1% | S. Salivarius K12 lozenge | 40; 20 in each | Symptomatic OLP | 4 wks | TA: TID; Lozenges: BID | Sign scores and VAS | No statistical difference was observed between two gps after 4-week treatment in sign scores (p = 0.063) or VAS (p = 0.698) | None | TA = S. Salivarius K12 | High risk of bias | |
| Bakshi et al. [27] | TA solution 0.1% | Nanocurcumin gel 1% | 31; 17 in TA + placebo, 14 in TA + NC | Symptomatic OLP | 4 wks | TID | REU score and efficacy index | Both had significant improv. in REU score and efficacy score, TA + NC group significantly better in both measures than TA + placebo (p < 0.001) | NS | Nanocurcumin gel > TA | Low risk of bias | |
| Betamethasone | Tyldesley and Harding[11] | BM valerate aerosol (2 puffs/dose); daily dose: 800/ug | Placebo | 23; BM: 12, placebo: 11 | Symptomatic OLP | 8 wks | QID | Lesion size, discomfort/pain | BM: improv. of lesion size and pain in 8 vs. 2 in placebo (p < 0.05) | BM: 1 pt-oral candidiasis | BM > placebo | Low risk of bias |
| Fluocinolone | Thongprasom et al. [7] | Fluocinolone acetonide 0.025% in orabase | TA 0.1% in orabase | 40; 20 in each | Bx-proven OLP | 4 wks | QID | 5-point Thongprasom clinical score | Fl: lesions in 13/19 pts effectively cured, TA: 8/19 pts cured (p < 0.05) | Oral candidiasis: Fl- 9 pts; TA-4pts | Fluocinolone > TA | High risk of bias |
| Calcineurin inhibitors | ||||||||||||
| Tacrolimus | Radfar et al. [55] | TC oint. 0.1% | Clobetasol gel 0.05% | 29; TC:15, clo:14 | Erosive OLP | 6 wks | QID x 2wk; TID X 2wk; BID X1 wk; QHS × 1 wk | Complete resolution of the clinical signs and symptoms | 82.6% in tacrolimus and 81.6% in the clobetasol group – improv., (p < .0001) | Discomfort, burning and tingling | TC > Clo | Low risk of bias |
| Corrocher et al. [56] | TC oint. 0.1% | Clobetasol oint. 0.05% | 32; 16 in each | OLP | 4 wks | QID | Pain severity, burning sensation, 4-point clinical score | TC group- low median pain score p < 0.001; Clo group- low pain score p < 0.05 but mild increase in the median severity scores | None | TC > Clo | Low risk of bias | |
| Sonthalia and Singal [57] | TC oint. 0.1% | Clobetasol oint. 0.05% | 40; 20 in each | OLP | 8 wks | TID | VAS, Clinical score | VAS and clinical score decreased (p < 0.05) in both gps, but no significant diff b/w 2 gps | Burning and increased sensitivity | TC = Clo | Low risk of bias | |
| Vohra et al. [59] | TC oint. 0.1% | PI cream 1% | 40; 20 in each | Erosive, OLP | 8 wks | BID | Clinical score | Significant reduction in the clinical severity score in both pimecrolimus and tacrolimus (p < 0.05) | None | TC = PI | Low risk of bias | |
| Hettiarachchi et al. [58] | TC cream 0.1% | Clobetasol cream 0.05% | 68; 34 in each | OLP | 3 wks | BID | VAS, Thongprasom clinical response | TC: mean pain score dropped by 1.59 (R) and 1.53 (L), clinical score reduced by 1.18 (R) and 1.0 (L); Clo: VAS drop by 0.94(R) and 0.85 (L) & clinical score reduced by 0.5 (R) and 0.26 (L) (p < 0.05) | None | TC > Clo | Low risk of bas | |
| Pimecrolimus | Swift et al. [12] | PI cream 1% | Placebo | 20; 10 in each | Erosive OLP | 4 wks | BID | Lesion size, VAS | PI more effective; VAS decreased (p = 0.02) | None | PI > Placebo | Low risk of bias |
| Passeron et al. [13] | PI cream 1% | Placebo | 12; 6 in each | Erosive OLP | 4 wks | BID | 12-point clinical score & VAS | PI effective; Mean score 6.83 on day 0 vs. 3.33 on day 28 in PI arm (p = 0.04) | PI: 2 pts transient burning sensation | PI > Placebo | Low risk of bias | |
| Gorouhi et al. [41] | PI cream 1% | TA cream 0.1% | 40; 20 in each | OLp > 8 yrs | 8 wks | QID | VAS, OHIP score & objective clinical score | No significant difference b/w 2 arms in VAS (p = 0.70), OHIP (p = 0.38), clinical score (p = 0.86) | PI: 2pts- transient burning; TA: none | PI = TA | Low risk of bias | |
| Volz et al. [14] | PI cream 1% | Placebo | 20; 10 in each | Erosive OLP | 4 wks | BID | Composite score (mucosal erosions and pain sensation) | Composite score reduced in PI arm (p = 0.025) | PI: 4 pts-burning sensation, 1 pt- mucosal paresthesia; Placebo:1 pt- mucosal paresthesia | PI > Placebo | Low risk of bias | |
| McCaughey et al. [15] | PI cream 1% | Placebo | 21; PI: 10, placebo: 11 | Erosive OLP | 6 wks | BID | Investigator’s Global Assessment of severity, pain, erosion size | PI superior in reducing mean pain and erosion size (mean size 11.10 at baseline vs. 3.70 at week 6) (p = 0.02) | None | PI > Placebo | Low risk of bias | |
| Arduino et al. [9] | PI cream 1% | TC oint. 0.1% | 30; 15 in each | Topical steroid refractory OLP | 8 wks | BID | Symptomatic improv., therapeutic effectiveness | Both effective; no statistically significant difference b/w 2 arms | PI: 2pts- xerostomia, 2pts-GERD, 1pt-herpes labialis; TC: 2pts burning, | PI = TC | Low risk of bias | |
| Arunkumar et al. [46] | PI cream 1% | TA paste 0.1% | 30; 15 in each | Bx-proven symptomatic OLP | 8 wks | QID | VAS, mean clinical score and erythematous area | Reduced clinical score in PI arm (p < 0.01); no statistically significant diff in reduction of VAS (p = 0.18) & erythema (p = 0.07) | None | Clinical score: PI > TA; VAS: PI = TA | Low risk of bias | |
| Pakfetrat et al. [42] | PI cream 1% | TA cream 0.1% | 28; 14 in each | Atrophic-erosive OLP | 8 wks | TID | Thongprasom lesion scoring, VAS | Both effective; No statistically significant difference | None | PI = TA | Low risk of bias | |
| Ezzatt and Helmy [60] | PI cream 1% | Betamethasone valerate cream 0.1% | 30; 15 in each | Atrophic-erosive OLP | 4 wks | QID | Clinical score, VAS | Both showed reduction in clinical score and VAS (p < 0.001) but no statistically significant diff b/w 2 arms in 4 wks; PI: 33% clinical score reduction, 57.5% VAS reduction; BM:13.9% clinical score reduction and 30.6% VAS reduction after 1 wk | PI: 4 pts-burning,2 pts-dysguesia; BM: 2pts-burning, 1pt-dysguesia | PI = BM | Low risk of bias | |
| Cyclosporine | Eisen et al. [16] | CsA solution 100 mg/ml | Placebo | 16; 8 in each | Bx-proven symptomatic OLP | 8 wks | TID | Pain (4-grade scale), erosion (4-grade scale) | CsA: improv. in erythema (p = 0.003), erosion (p = 0.02), reticulation (p = 0.007), pain (p = 0.002) | CsA: transient burning on swishing in all pts | CsA > placebo | Low risk of bias |
| Harpenau et al. [17] | CsA solution 100 mg/ml | Placebo | 14; 7 in each | Bx-proven erosive OLP | 4 wks | QD | VAS; lesion character (ulcer, erythema & reticulation) & size | CsA: significant reduction in erythema, ulceration, and VAS; p-value NS | None | CsA > placebo | Low risk of bias | |
| Lopez [61] | CsA solution 1% | TA solution 0.1% | 20; 10 in each | Bx proven OLP | 8 wks | TID | Symptom, erosion and erythema score | CsA: greater decrease of symptoms (90% vs. 60% in TA), erythema and erosion; p-value NS | NS | CsA > TA | Low risk of bias | |
| Femiano et al. [63] | CsA solution 100 mg/ml | IM sul 600 IU, then oral doses 250 IU | 20; 10 in each | Topical steroid recalcitrant bx-proven OLP | 4 wks | CsA: TID, Sul:BID | Pain relief, clinical resolution of erosion/ulceration | Sulodexide more effective- clinical resolution faster than CsA at a mean of 36 days and pain resolution in 90% by mean 6.4 days (p < 0.004) | CsA: None; Sul: vertigo, vomiting and hot flushes | Sul > CsA | High risk of bias | |
| Yoke et al. [44] | CsA solution 100 mg/ml | TA paste 0.1% | 139; CY: 68; TA:71 | Bx proven OLP | 8 wks | TID | VAS; Thongprasom clinical grading | No statistically significant difference b/w two arms | TA: 3 pts- transient burning; CsA: 14 pts- burning; 4 pts- GI upset; 1pt- lip swelling & itching | CsA = TA | Low risk of bias | |
| Thongprasom et al. [45] | CsA solution 100 mg/ml | TA paste 0.1% | 13; CsA:6, TA:7 | Bx proven symptomatic OLP | 8 wks | TID | VAS, Thongprasom clinical grading (5-point) | No statistically significant differences b/w 2 gps | CsA: 5 pts- burning sensation, itching, swelling lips, petechial hemorrhage; TA: None | CsA = TA | Low risk of bias | |
| Georgaki et al. [62] | CsA solution 100 mg/ml | Dex rinse 0.5 mg/5 ml | 32; 16 in each | Bx proven symptomatic OLP | 4 wks | TID | VAS; Thongprasom clinical grading, dysphagia and speech difficulties | Dex: better in clinical scoring (p = 0.001). No significant diff b/w 2 gps in improv. of pain, dysphagia and speech difficulties | NS | ClinicaL score: Dex > CsA; VAS: Dex = CsA | Low risk of bias | |
| Other topical agents | ||||||||||||
| Amlexanox | Verma [52] | AX paste 5% | TA paste 0.1% | 60; 30 in each | Symptomatic reticular/erosive OLP | 12 wks | QID | VAS; clinical sign stage: erythematous areas, white striae + lesion size | TA more effective > AX. AX: 60% reduction in the clinical sign stage & TA: 98% reduction (p < 0.05); VAS = no significant difference | None | Clinical score: TA > AX; VAS: TA = AX | Low risk of bias |
| Retinoid | Giustina et al. [18] | isotretinoin gel 0.1% | Placebo | 22;11 in each | Ulcerated lichen planus | 8 wks | BID | Reduction in pain and erythema-severity scale (0–5) | Significant improv. in topical retinoid group with statistically significant (p < .002); Reduction in severity scale 3.0 to 1.7 after 8 weeks | Burning and superficial desquamation | Isoretinoin > Placebo | Low risk of bias |
| Petruzzi et al. [20] | Tazarotene cream 0.1% | Placebo | 12; 6 in each | Hyperkeratotic OLP | 8 wks | BID | 6-degree score scale, reduction in lesion | 4 patients healed, 2 patients improved in tazarotene and 5 patients with no improv. and 1 worsening (p = 0.0049) | Burning, taste abnormalities | Tazarotene > Placebo | Low risk of bias | |
| Piattelli et al. [19] | Isotretinoin gel 0.1% | Placebo | 20; 10 in each | Bx proven OLP | 16 wks | TID | Complete healing of the lesions | Isoretinoin: 60% complete healing (p = 0.029) | NA | Isoretinoin > Placebo | Low risk of bias | |
| Tocopherol | Bacci et al. [21] | Tocopherol acetate (gelly formulation) | Placebo | 33; Tocopherol = 17, Placebo = 16; then cross-over | Bx-proven reticular OLP | 4 wks, 2 wk washout, 4 wks crossover | TID | VAS, length of striae, surface area of lesion, Thongprasom score | Significant difference in surface area of lesion (p = 0.0045) and Thongprasom score (p = 0.0052) in tocopherol group | None | Tocopherol > Placebo | Low risk of bias |
| Intralesional | ||||||||||||
| Triamcinolone | Ahuja et al. [65] | Intralesional triamcinolone (10 mg/ml) | PRP 0.5 ml | 20; 10 in each | Erosive OLP | 8 wks | Weekly injection—for 2 to 4 months | VAS, reduction in erythema and size of the lesions | Statistically significant reduction in both gps (p < 0.005); no significant difference b/w 2 gps | Intralesional TA: Erythema in 1 pt; PRP: increased VAS score in 1 pt | TA = PRP | Low risk of bias |
| BCG-PSN | Xiong et al. [64] | Bacillus Calmette–Guerin polysaccharide nucleic acid (BCG‐PSN) | Intralesional triamcinolone (10 mg/ml) | 56; BCG-PSN = 31 & TA = 25 | Bx-proven erosive OLP | 2 wks | BCG-PSN: every other day. TA: every week | VAS & measured erosive areas | No statistical differences b/w 2 gps in erosive areas (p = 0.801) and VAS scores (p = 0.946) | Burning/swelling at injection site in 9.7% of BCG-PSN group and 8% in TA group | BCG = TA | Low risk of bias |
| Systemic Therapies | ||||||||||||
| Systemic retinoids | Hersle et al. [22] | Etretinate 25 mg | Placebo | 28; 14 in each | Bx-proven OLP for atleast 6 mths | 8 wks | TID | 4-point clinical scoring | Etretinate: 93% improv. vs. 5% in placebo (p < 0.001) | Etretinate: all pts- skin and mucosa dryness; 6 pts-keratoconjunctivitis, rash, headache, itchiness & hair loss | Etretinate > Placebo | Some concerns |
| Levamisole | Lin et al. [66] | Levamisole 50 mg | (Levamisole + vit B12) and (Vit B12 only) | 147; 100 in L + B12 gp, 37 in L gp, & 10 in B12 gp | OLP | 2–50 months (mean = 14) | BID if 30–50 kg or TID if 50–70 kg, for 3 days at 2 wk interval | Size & distribution of lesions, pain & burning symptoms | L only group & L + B12 group: 100% objective & subjective improv.; Vit B12 alone: 13% improv. in symptoms and 20% improv. in signs (p-value NS) | None | Levamisole = Levamisole + B12 > B12 only | High risk of bias |
| Natural alternative | ||||||||||||
| Lycopene (systemic) | Saawaran et al. [23] | Lycopene 4 mg | Placebo | 30; 15 in each | Bx proven symptomatic OLP | 8 wks | BID | VAS; Tel Aviv-San Francisco scale | Lycopene: 84% VAS reduction, 100% showed > 50% benefit; Placebo: 67% VAS reduction, 66.6% showed > 50% benefit (p < 0.05) | None | Lycopene > Placebo | Low risk of bias |
| Ignatia (topical) | Mousavi et al. [24] | Ignatia 30C liquid | Placebo | 30; 15 in each | Bx proven atrophic/erosive OLP | 12 wks | QD | VAS and mean lesion size (cm) | Ignatia more effective; Ignatia: mean lesion size- 2.2 cm, VAS-13 mm; Placebo: mean lesion size-4 cm, VAS-40 mm (p < 0.05) | None | Ignatia > Placebo | Low risk of bias |
| Aloe Vera (topical) | Choonhakarn et al. [25] | AV gel 70% | Placebo | 54; 27 in each | Bx proven OLP | 8 wks | BID | VAS and Thongprasom clinical scale | AV: improved clinical response in 88% and improved burning in 33% vs. 4% in placebo group (p < 0.001) | None | AV > Placebo | Low risk of bias |
| Salazar-Sánchez et al. [26] | AV gel 70%- 0.4 ml/dose | Placebo | 64; 32 in each | Bx proven OLP | 12 wks | TID | VAS, Thongprasom clinical scale, OHIP-49 | No statistically significant diff in VAS and clinical score at 12 wk; AV showed improv. in total OHIP score (p = 0.046) | None | AV = Placebo | Low risk of bias | |
| Reddy et al. [51] | AV gel 70% | TA 0.1% paste | 40; 20 in each | Erosive & atrophic OLP | 8 wks | TID | VAS & clinical score | AV: clinical score and VAS significantly better than TA (p < 0.05) | None | AV > TA | Low risk of bias | |
| Other procedural modalities | ||||||||||||
| LLLT | Jajarm et al. [68] | Low intensity laser therapy (LILT) 630 nm diode laser | Dexamethasone solution 0.5 mg/5 ml | 30 (one side intervention, the other side control) | Erosive-atrophic OLP | 4 wks | LILT: BID; Dex: QID | Thongprasom clinical scale, VAS, RAE | Appearance score, pain score, and lesion severity was reduced in both gps (p value NS). No significant differences b/w the treatment gps regarding the response rate and relapse | None | LLLT = Dex | Some concerns |
| Laser | Agha-Hosseini et al. [72] | CO2 laser irradiation | low-level laser therapy (LLLT) | 28 (one side intervention, the other side control) | Oral lichen planus | 2 wks | CO2 laser: 1 session; LLLT: 5 sessions | Thongprasom clinical scale, VAS, size of lesions | Lesion size reduction significantly higher in LLLT compared to CO2 (p < 0.05). Improv. in clinical signs significantly higher in LLLT (p < 0.05). Symptom reduction was significantly higher in LLLT group (p < 0.05) | NS | LLLT > CO2 laser | High risk of bias |
| LLLT | Dillenburg et al. [70] | Laser phototherapy (LPT) 660 nm diode laser | Clobetasol gel 0.05% | 42 (one side intervention, the other side control) | Atrophic/erosive OLP | 4 wks | LPT- 3x/wk; Clo: TID | Clinical, symptoms, and functional scores | The LPT group had significantly lower clinical scores compared to clobetasol group (p = 0.001). Symptom score was maintained at a stable level for the LPT group in the follow up period, whereas a significant increase was found in the clobetasol group (p = 0.05) | Clo: 3 pts- Transient burning sensation; LPT: None | LPT > Clo | Low risk of bias |
| PDT | Jajarm et al. [68] | Toluidine blue for 10 min followed by photodynamic therapy | Dexamethasone rinse 0.5 mg/5 ml | 25 (one side intervention, the other side control) | Erosive/atrophic OLP | 4 wks | PDT:2x/wk; Dex:QID | Thongprasom clinical scale, efficacy indices, and experienced pain | Statistically significant reduction in sign score for the experimental (p = 0.021) and control (p = 0.002) gps. Efficacy index of the control group improved significantly more than the experimental group (p = 0.001) | None | Dex > PDT | High risk of bias |
| Laser | Kazancioglu (2015) | A diode laser 808 | Ozone vs. dex rinse vs. placebo | 120; 30 in each gp | atrophic-erosive OLP | 4 wks | Laser:2x/wk; Ozone:2x/wk; Dex: QID | Thongprasom clinical scale, VAS, RAE score | Improv. in all gps but significantly better in Ozone and steroid gps (p < 0.05) as compared to laser and placebo | None | Ozone = Dex > Laser > placebo | Some concerns |
| Laser | Othman et al. [74] | A diode laser 970 | TA 0.1% orabase | 24 (one side intervention, the other side control) | Erosive-atrophic Reticular | 4–5 wks | Laser: 2x/wk; TA: QID | Thongprasom clinical scale, RAE score, TNF α level | TA group showed statistically significantly lower mean RAE score than Laser group (p = 0.02) as well as lower TNF-α level | None | TA > laser | Some concerns |
| Laser | El Shenawy et al. [75] | A diode laser 970 | TA 0.1% orabase | 24; 12 in each | Erosive-atrophic | Laser: 8 wks; TA: 4 wks | Laser: 2x/wk; TA: QID | VAS, RAE score | Significant improv. in TA group than laser group (p < 0.05) | NS | TA > laser | Some concerns |
| PDT | Lavaee and Shadmanpour [69] | 660-nm diode laser for 10 min | Topical TA 0.1% | 8 (one side intervention, the other side control) | Atrophic/erosive OLP | PDT: 3 wks; TA: 4 wks | PDT: 1x/wk; TA: TID | Thongprasom clinical scale, VAS, size of lesions | Significant difference in all scores between session 0 and 4 in both gps (p < 0.05). Changes in scores between the intervention and comparative gps were not statistically significant (p = 0.340) | None | PDT = TA | Low risk of bias |
| LLLT | Ferri et al. [71] | Clo gel 0.05% | Photobiomodulation (PBM) | 34; 17 in each group | Reticular,atrophic, and erosive OLP | 4 wks | Clo: TID; PBM: 2x/wk | VAS; Thongprasom clinical score | Decreased pain in both; clinical resolution: clo- 79.4%, PBM- 64.7% (p < 0.05) | None | Clo > PBM | Low risk of bias |
AV: aloe-vera, BM: betamethasone, Bx: biopsy, b/w: between, BCG‐PSN: Bacillus Calmette–Guerin polysaccharide nucleic acid, Clo: clobetasol, CsA: cyclosporine, Dex: dexamethasone, Flu: fluocinonide, FBS: fasting blood sugar, Gp: group, Improv.: improvement, LLLT: low level laser therapy, LPT: laser phototherapy, Mins: minutes, NS: not stated, NC: nanocurcumin, OLP: oral lichen planus, OHIP: Oral Health Impact Profile, Oint.: ointment, PDT: photodynamic therapy, PBM: photobiomodulation, PI: pimecrolimus, Pt: patient, RAE: reticulation, atrophy, erosion score; RPAE: reticular, white plaque, atrophy, erosion and ulceration clinical score, Rxn: reaction, TA: triamcinolone, TC: tacrolimus, TSQM: Treatment Satisfaction Questionnaire for Medication, Tx: treatment, VAS: visual analog scale, wk: week
Treatment modalities
The treatment modalities investigated in eligible studies included: topical therapies {dexamethasone (n = 3), clobetasol (n = 6), fluocinonide (n = 2), triamcinolone (n = 14), betamethasone (1), fluocinolone (1), tacrolimus (5), pimecrolimus (9), cyclosporine (7), amlexanox (1), retinoids (3), tocopherol (1)}; systemic therapies {retinoids (1), levamisole (1)}; intra-lesional therapies {triamcinolone (1), Bacillus Calmette-Guerin polysaccharide nucleic acid (1)}; natural alternatives {aloe-vera (3), Ignatia (1), lycopene (1)}; laser (6) and photodynamic therapy (2).
Outcome measures
For assessing the subjective treatment response, the majority of RCTs (57%) used a visual analog scale (VAS) [7–10, 12, 13, 17, 21, 23–31, 33, 37, 39, 41, 42, 45–48, 51, 53, 57, 58, 60, 62, 64, 65, 68, 69, 71, 73, 75, 76]. While there was significant heterogeneity in the clinical scoring scales used to measure treatment response among studies, the Thongprasom scoring system was used most often (19 RCTs; 27%) [7, 21, 25–27, 42, 44, 45, 47, 48, 58, 62, 68, 69, 71–74, 76]. Alternatively, other scales included the Modified Oral Mucositis Index, the Tel Aviv-San Francisco scale, RAE score (reticulation, atrophy, erosion), RPAE score (reticular, white plaque, atrophy, erosion and ulceration), and the REU (reticulation, erosion, ulceration) score [23, 49, 50, 54, 73–75].
Efficacy (objective and subjective improvement)
The two primary efficacy endpoints reported in the RCTs were objective improvement (reduction in the clinical score or severity) and subjective improvement (reduction in pain/VAS). Most studies (57%) showed statistically significant results (p < 0.05) supporting the effectiveness of their respective interventions. Based on the RCTs results, we created a consensus list reflecting the level of efficacy from most efficacious to the least for steroidal and non-steroidal modalities (Additional file 1: Table S1).
Placebo-controlled trials (18)
Of the 70 trials, 18 compared an intervention to placebo. The following were associated with statistically significant improvements in pain and lesion response compared to placebo: clobetasol gel 0.05% [8], fluocinonide ointment 0.025% [10], betamethasone valerate aerosol [11], pimecrolimus cream 1% [12–15], cyclosporine solution 100 mg/ml [16, 17], isoretinoin gel 0.1% [18, 19], tazarotene cream 0.1% [20], tocopherol gel [21], systemic retinoid [22] and the three natural alternatives (oral lycopene 4 mg, Ignatia 30 C liquid and aloe-vera gel 70% [23–25]. There was a single placebo-controlled trial (n = 4) comparing aloe-vera gel 70% with placebo that did not demonstrate statistically significant superiority of the intervention [26].
RCTs comparing interventions
Topical Dexamethasone (Dex)
Commercially available dexamethasone solutions 0.5 mg/5 ml were associated with better clinical outcomes than self-compounded dex [27]. One study comparing dex to photodynamic therapy (PDT) found no difference in efficacy [28], while another comparing dexamethasone, PDT, and low-level laser therapy (LLLT) found dex to be most effective in reducing the pain score and PDT to be most effective in improving the clinical lesions [29].
Topical Clobetasol (Clo)
Studies comparing delivery methods of clobetasol 0.05%- clo ointment vs. clo in oral analgesic base vs. clo in denture paste (n = 24) and concentrations of clo (0.025% vs. 0.05%) found each to be effective in reducing pain with additional improvement in clinical scores in the latter (n = 35) [30, 31]. Clo ointment 0.025% was also shown to be comparable to tacrolimus ointment 0.1% (n = 40) [32].
In comparison to triamcinolone paste 0.1%, clo ointment 0.05% showed greater efficacy at 3 weeks of treatment, however, at 6 and 9 weeks of treatment, there was no significant difference between the two (n = 40) [33]. Clo ointment 0.05% demonstrated greater efficacy in reducing objective scores than fluocinonide ointment 0.05% and placebo (n = 60) [34].
Topical Triamcinolone (TA)
Over a third of the RCTs (26/70; 37%) studied the efficacy of TA paste 0.1%. The two formulations of TA paste and TA solution were determined to be equally efficacious [35]. Three RCTs (n = 30, 40 and 40) comparing TA paste 0.1% with other topical steroids found that clobetasol 0.05% ointment and fluocinolone acetonide 0.025% in orabase were more efficacious than TA [7, 36] but fluticasone spray 0.05% was equally efficacious to TA [37].
In comparison to tacrolimus (TC) ointment, four RCTs (n = 40, 30, 18 and 40) found different results, with TA paste 0.1% shown to be inferior to TC ointment 0.1% [38], superior to TC ointment 0.03% [36] and equal to TC ointment 0.1% [39, 40] in terms of clinical improvement. Two RCTs (n = 40 and 28) comparing pimecrolimus cream 1% with TA cream 0.1% [41, 42], and three RCTs (n = 13, 139 and 13) comparing cyclosporine solution with TA paste 0.1% found no statistically significant difference between these therapies [43–45]. A double-blind RCT (n = 30) comparing pimecrolimus cream 1% with TA paste 0.1% showed a mixed outcome, with TA showing equal efficacy in reducing VAS but reduced efficacy in reducing the clinical score at 8 weeks of treatment [46].
In comparison to natural alternatives, the results were mixed. While two RCTs (n = 46 and 50) found TA paste 0.1% to be equally efficacious to aloe-vera (AV) solution and curcumin paste 5% respectively [47, 48]; one study (n = 75) showed that TA paste 0.1% was better than curcumin gel 1% [49] and another study (n = 31) showed nanocurcumin gel 1% was better than TA solution [50]. A double blind RCT (n = 40) comparing AV gel 70% to TA paste 0.1% for 8 weeks showed that OLP clinical score and VAS was statistically significantly better in the AV arm [51].
A trial (n = 60) showed that TA paste 0.1% was more effective than amlexanox paste 5% (anti-inflammatory agent) in improving clinical signs but there was statistically insignificant different between the two in terms of reduction of VAS [52]. No statistically significant difference was observed between TA paste 0.1% vs. S. salivarius K12 probiotic lozenge (n = 30) [53] or between TA paste 0.1% and cryotherapy with nitrous oxide (n = 40) with respect to VAS and objective scores [54].
Topical Tacrolimus (TC)
Four trials compared different topical formulations of clobetasol and TC. Two trials (n = 29 and 32), showed TC ointment 0.1% was superior to clobetasol gel 0.05% and clobetasol ointment 0.05%, respectively [55, 56]; however, the third RCT (n = 40) demonstrated no significant difference between TC ointment 0.1% and clobetasol ointment 0.05% [57]. The fourth RCT compared TC cream 0.1% (compounded) and clobetasol cream 0.05% (n = 68) and found TC cream to be more effective in reducing VAS and clinical response score [58].
Topical Pimecrolimus (PI)
Two RCTs (n = 40 and 30) compared PI cream 1% and tacrolimus ointment 0.1% and showed no statistically significant difference between the two in therapeutic effectiveness [9, 59]. Additionally, the efficacy of PI cream 1% was found to be equal to betamethasone valerate cream 0.1% in reducing clinical score and VAS (n = 30) [60].
Topical Cyclosporine (CsA)
When CsA solution 100 mg/ml (with a 10% dilution in olive oil) was compared with triamcinolone solution 0.1% (n = 20), there was greater symptomatic and clinical improvement in the CsA group after 8 weeks, although, p-value was not stated [61]. On the other hand, dexamethasone solution 0.5 mg/5 ml was found to be significantly better than CsA solution 100 mg/ml (n = 32) in reducing the clinical score (although both were equally effective in improving VAS) [62].
An open-label trial (n = 20) comparing sulodexide, a systemic heparinoid, with topical CsA (100 mg/ml solution) showed that sulodexide (one dose of I/M followed by oral doses) led to a faster clinical resolution [63].
Intralesional therapies
The two RCTs included in this systematic review that evaluated intralesional therapies compared intralesional triamcinolone (TA) 10 mg/ml with Bacillus Calmette-Guérin polysaccharide nucleic acid (BCG-PSN) and autologous platelet rich plasma (PRP). Intralesional injection of the immunomodulatory extract of BCG administered every other day was found to be equally effective as weekly administration of intralesional TA (n = 56) in reducing lesion size and VAS in OLP [64]. Similarly, the RCT comparing intralesional TA and PRP (n = 20) did not find any significant difference between the two arms [65].
Systemic therapies
An anti-helminthic and immunomodulatory agent, levamisole (not available in US), was studied in a triple arm open label RCT (n = 147) comparing levamisole 50 mg vs. vitamin B12 vs. combination of levamisole + B12 [66]. The results showed clinical and symptomatic improvement in all patients in both the levamisole arm and the levamisole + vitamin B12 arm, but the p-value was not-stated.
Dapsone, another immunomodulatory agent, showed the highest clinical and symptomatic improvement in a four-arm open-label RCT (n = 40) comparing oral dapsone 100 mg vs. TA paste 0.1% vs. TC ointment 0.1% vs. topical retinoid (type not stated in the study) after 12 weeks [40]. Another open-label trial (n = 49) comparing TA paste 0.1% with systemic betamethasone (mini-pulse therapy with oral betamethasone 5 mg on 2 consecutive days/week) for 24 weeks, found significant reduction in clinical severity score in the TA group but no difference in the symptomatic improvement between the two groups [67].
Laser and Photodynamic therapies
Eleven RCTs studying laser and photodynamic therapies (PDT) met the inclusion criteria. When comparing PDT with topical steroids, the studies indicated mixed results- one study (n = 45) showed superiority of PDT over dexamethasone [29], another (n = 25) showed inferiority to dexamethasone [68], and two studies (n = 30 and 8) showed equal efficacy (PDT = dex and PDT = triamcinolone paste 0.1%) [27, 69]. Similar mixed results were seen with LLLT, and topical steroids- one study (n = 42) showed increased efficacy (LLLT > clobetasol gel 0.05%), another (n = 34) showed reduced efficacy (clobetasol gel 0.05% > LLLT) and the third (n = 30) showed equal efficacy (LLLT = dexamethasone) [70–72].
Dexamethasone solution and triamcinolone paste 0.1% showed higher efficacy than laser therapies (n = 120, 24 and 24) [73–75]. In comparing the clinical efficacy of the three phototherapies, a direct comparison trial (n = 45) showed PDT to be more efficacious than LLLT [29] and the second (n = 28) showed superior results with LLLT than carbon dioxide laser [76].
Adverse reactions
Twenty-six studies reported adverse drug reactions (ADRs) (Additional file 2: Table S2). Most topical interventions were associated with mild, local ADRs. Oral candidiasis was a common documented ADR of topical corticosteroids (clobetasol, triamcinolone, betamethasone and fluocinolone) [7, 11, 30, 35, 67]. Oral burning sensation was associated with topical agents- tacrolimus, pimecrolimus, cyclosporine, triamcinolone, retinoids, and curcumin [9, 13, 14, 16, 18, 20, 38–41, 43–45, 48, 55, 57, 60, 68]. Overall, topical regimens were well-tolerated without evidence of systemic ADRs.
While patients treated with systemic therapies such as levamisole and lycopene did not experience any local or systemic side-effects, significant systemic side effects including skin dryness, keratoconjunctivitis, rash, headache, itchiness, and hair loss were reported in patients treated with etretinate, a systemic retinoid [22]. ADRs such as vertigo, vomiting and hot flushes were documented in patients treated with sulodexide [63]. Intralesional therapies were associated with local erythema (TA), increased pain (PRP) and burning/swelling at injection site (BCG-PSN and TA) in a subset of patients [64, 65].
Among patients treated with cryotherapy using nitrous oxide, the majority experienced local swelling at the treatment side [54]. None of the studies reported any side effects associated with laser therapy; only one study on PDT reported pain upon manipulation with probe tip [27].
Assessment of risk of bias
At the individual study level, most of the domains were with low risk of bias. The overall assessment of the risk of bias showed that 49 (70%) studies had low risk of bias, 11 (15.7%) studies had high risk of bias, and 10 (14.2%) studies had some concern.
Cost of therapeutics
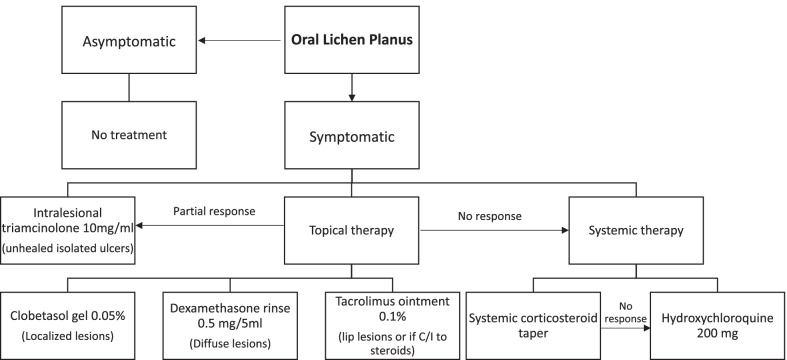
Table 2 presents the estimated costs (U.S. dollars) for the studied interventions. The costs range of topical steroids and topical calcineurin is from $0.04–14.13/unit and $1.13–10.16/unit respectively. The cost of commonly used and commercially available topical therapies is as follows (from highest to lowest): cyclosporine solution > pimecrolimus cream > tacrolimus ointment > clobetasol gel > clobetasol ointment > dexamethasone solution > fluocinonide ointment > betamethasone cream > triamcinolone paste. The cost of intralesional triamcinolone (10 mg/ml) ranges from $10.24–17.00 per ml, but this excludes the procedural cost. Among the systemic medications, the cost of betamethasone was the lowest and oral dapsone was the highest. Considering the costs of different therapeutics and their efficacies, treatment recommendations for OLP have been made based on expert opinion (Fig. 2).
Table 2.
Estimated cost per unit and per month of common commercially available oral lichen planus interventions studied in the included randomized controlled trials
| Intervention | Estimated cost per unit* | Estimated cost per month** |
|---|---|---|
| Topical steroids | ||
| 1. Dexamethasone solution | $0.04–0.27 per ml | $77.50 |
| 2. Clobetasol ointment | $0.44–7.8 per g | $123.60 |
| 3. Clobetasol gel | $0.76–8.33 per g | $136.35 |
| 4. Fluocinonide ointment | $0.39–2.68 per g | $46.05 |
| 5. Fluocinonide gel | $1.19–3.45 per g | $69.60 |
| 6. Triamcinolone ointment | $0.17–0.44 per g | $9.15 |
| 7. Triamcinolone paste | $0.30–1.06 per g | $20.40 |
| 8. Betamethasone valerate cream | $0.43–0.96 per g | $20.85 |
| 9. Fluocinolone acetonide ointment | $0.52–2.91 per g | $51.45 |
| Topical calcineurin inhibitors | ||
| 1. Tacrolimus ointment | $1.26–8.66 per g | $148.80 |
| 2. Tacrolimus cream | $1.13–3.39 per g | $67.80 |
| 3. Pimecrolimus cream | $2.06–10.16 per g | $183.30 |
| 4. Cyclosporine rinse | $1.83–8.66 per ml | $2622.50 |
| Other topical agents | ||
| 1. Amlexanox paste (not available in US) | $0.28–1.38 per g | $24.90 |
| 2. Isotretinoin gel | $0.63–1.34 per g | $29.55 |
| 3. Tazarotene cream | $1.28–3.66 per g | $74.10 |
| 4. Tocopherol acetate gel | $0.15–0.27 per g | $6.30 |
| 5. Ignatia liquid | $0.14–0.64 per ml | $195.00 |
| 6. Aloe vera gel | $0.02–0.06 per g | $1.20 |
| 7. Curcumin gel | $1.48–3.18 per g | $69.9 |
| 8. Nanocurcumin gel | $0.27–0.45 per g | $10.8 |
| 9. S. salivarius K12 lozenge | $0.59–1.13 per lozenge | $51.60 |
| Intralesional therapies | ||
| 1. Triamcinolone | $10.24–17.00 per ml | $54.48 |
| 2. BCG-PSN | $2.67–3.26 per ml | $44.48 |
| Systemic therapies | ||
| 1. Lycopene | $0.07–0.19 per capsule (4 mg) | $7.80 |
| 2. Oral dapsone | $0.73–3.12 per tablet (100 mg) | $115.50 |
| 3. Oral betamethasone | $0.52–0.64 per tablet (0.5 mg) | $46.60 |
| Other procedure-directed therapies | ||
| 1. Photodynamic therapy | $100–4000 per treatment | $16,400.00 |
| 2. Low level laser therapy | $30–200 per treatment | $920.00 |
| 3. CO2 laser | $450–1450 per treatment | $7600 |
*Based on information available on websites- goodrx.com, singlecare.com, pharmacychecker.com, otc-online-store.com, rupills.com, usaherbalmart.com, ebay.com, amazon.com, adooq.com, sastasundar.com, naturallythinking.com, aaos.org, plasticsurgery.org
**Calculated based on mean price per unit and the following amounts dispensed: 500 mL for solutions; 30 g for ointments, gels, and creams; BID for lozenges; 1 mL weekly for intralesional triamcinolone; 1 mL every other day for intralesional BCG-PSN; BID for lycopene and dapsone; 5 mg twice weekly for betamethasone; twice weekly photodynamic therapy, low level laser therapy, and CO2 laser sessions
Fig. 2.
Treatment recommendations for treatment of OLP based on expert opinion
Discussion
Ideal therapies are cost-effective, efficacious, and carry a low risk of local or systemic toxicity. The preferred modality for treating OLP is topical therapy due to ease of application, liberty to modify the frequency and duration of treatment and lack of systemic side-effects [5]. Important considerations in choosing a topical regimen include the location, extent of the lesions, and patient tolerability. Gels, ointments, and pastes are best used for focal lesions. For lesions that are more diffuse and/or difficult to access, solutions are preferable, though adequate contact time (3–5 min) must be ensured.
Consistent with other reviews, we found that OLP responds to a wide range of topically delivered medications and procedures including topical steroids (dexamethasone, clobetasol, fluocinonide, triamcinolone), topical calcineurin inhibitors (tacrolimus, pimecrolimus, cyclosporine), topical retinoids, intra-lesional triamcinolone, aloe-vera gel, photodynamic therapy and low-level laser therapies in OLP management.
Comparatively, the high potency topical steroid, clobetasol with an average cost of ~ $4.12/g for the ointment formulation and $4.54/g for the gel formulation, was found to be efficacious compared to topical fluocinonide, triamcinolone and tacrolimus [34, 36]. Contrastingly, three RCTs demonstrated higher efficacy of topical tacrolimus over topical clobetasol, with the average cost of tacrolimus being about $4.96/g [55, 56, 58]. Triamcinolone paste 0.1%, a low potency steroid, costs the least (average cost $0.68/g) among the topical steroids and calcineurin inhibitors. Topical pimecrolimus was comparable to topical triamcinolone, topical betamethasone, and topical tacrolimus [9, 42, 60], but the average cost of pimecrolimus ($6.11/g) was comparatively higher. The higher cost of topical calcineurin inhibitors discourages their use as first-line therapy in OLP management.
Intralesional steroid therapy has been shown to be efficacious but can be deemed invasive, technique sensitive with need for repeated procedures [64, 65]. While the average cost of triamcinolone solution (10 mg/ml) is roughly $13.62/ml, the total cost would also include the procedural cost of the injection itself. Although PDT and laser therapy were shown to be efficacious lesion-directed therapies without significant side-effects [70, 72, 76], the range of cost per treatment session was highest among all the treatment modalities. Among natural alternatives, aloe-vera gel was shown to be comparable to triamcinolone paste 0.1% [51], with the most modest price of $0.04/g. Based on the estimated cost/month and the evidence for efficacy and side-effects, topical steroids (fluocinonide > dexamethasone > clobetasol > triamcinolone) appear to be more cost-effective than topical calcineurin inhibitors (tacrolimus > pimecrolimus > cyclosporine) followed by intra-lesional triamcinolone.
Systemic steroids can require complex dosing schedules and carry an increased risk of side effects. They are most used short-term to treat severe flare-ups, and while low cost, monitoring and treating side effects when used longer term can significantly alter the cost-to-benefit ratio. Surprisingly, few trials have studied the use of systemic steroids in OLP, and only one comparing short-term betamethasone pulse therapy to topical triamcinolone met the inclusion criteria [67]. The average price of betamethasone 0.5 mg tablet is $0.58/tablet, but the total cost would vary according to the frequency and duration of the steroid pulse. Another systemic agent, dapsone which costs about $1.92/100 mg tablet was demonstrated to have increased efficacy over topical triamcinolone, tacrolimus, and retinoids [40].
There are several limitations to our study. There was significant heterogeneity in inclusion criteria and outcome measures of the RCTs included in this systematic review. Inclusion criteria of some trials required only a clinical diagnosis of OLP, while others required biopsy proven or symptomatic OLP. Furthermore, variable outcome measures, different trial durations, dosing regimens, and small sample sizes limited objective comparison of treatment outcomes. This heterogeneity underscores the necessity of developing consensus outcome measurements in the treatment of OLP to reduce study biases and allow for meta-analyses.
Conclusion
Various therapeutics have been used for the treatment of OLP over the past five decades, but a consensus treatment guideline is still lacking. In this systematic review, topical steroids were found to be potentially the most economical and efficacious treatment modality followed by topical calcineurin inhibitors supporting the use of topical steroids as the first-line treatment with escalation to other treatment modalities only as needed. Future standardized RCTs and meta-analyses are required to assess the efficacy of additional therapeutics, especially systemic therapies.
Supplementary Information
Additional file 1: Table S1. Consensus efficacy list of topical steroid and non-steroidal therapies.
Additional file 2: Table S2. Reported adverse reactions to oral lichen planus interventions.
Acknowledgements
Not applicable.
Author contributions
SS, BAK, MA-H, PC, AB, YX, RI, MH and SS contributed to the conception and design of the study, acquisition and interpretation of data, drafting of the article, revising it critically for important intellectual content, and the final approval of the version to be submitted. PV, HS, and NT contributed to drafting of the article, revising it critically for important intellectual content, and All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
All data generated during this study are included in this published article (Table 1).
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Li C, Tang X, Zheng X, Ge S, Wen H, Lin X, Chen Z, Lu L. Global prevalence and incidence estimates of oral lichen planus: a systematic review and meta-analysis. JAMA Dermatol. 2020 doi: 10.1001/jamadermatol.2019.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou XJ, Sugerman PB, Savage NW, Walsh LJ, Seymour GJ. Intra-epithelial CD8+ T cells and basement membrane disruption in oral lichen planus. J Oral Pathol Med. 2002 doi: 10.1046/j.0904-2512.2001.10063.x. [DOI] [PubMed] [Google Scholar]
- 3.Wang D, Sandhu S, Woo SB. A guide for dental practitioners of common oral potentially malignant disorders. CDA J 2021;49.
- 4.Oberti L, Gabrione F, Lucchese A, Di Stasio D, Carinci F, Lauritano D. Treatment of oral lichen planus: a narrative review. Front Physiol. 2019 doi: 10.3389/conf.fphys.2019.27.00004. [DOI] [Google Scholar]
- 5.Lodi G, Manfredi M, Mercadante V, Murphy R, Carrozzo M. Interventions for treating oral lichen planus: corticosteroid therapies. Cochrane Database Syst Rev. 2020 doi: 10.1002/14651858.CD001168.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sridharan K, Sivaramakrishnan G. Interventions for oral lichen planus: a systematic review and network meta-analysis of randomized clinical trials. Aust Dent J. 2021 doi: 10.1111/adj.12835. [DOI] [PubMed] [Google Scholar]
- 7.Thongprasom K, Luangjarmekorn L, Sererat T, Taweesap W. Relative efficacy of fluocinolone acetonide compared with triamcinolone acetonide in treatment of oral lichen planus. J Oral Pathol Med. 1992 doi: 10.1111/j.1600-0714.1992.tb00974.x. [DOI] [PubMed] [Google Scholar]
- 8.Arduino PG, Campolongo MG, Sciannameo V, Conrotto D, Gambino A, Cabras M, Ricceri F, Carossa S, Broccoletti R, Carbone M. Randomized, placebo-controlled, double-blind trial of clobetasol propionate 0.05% in the treatment of oral lichen planus. Oral Dis. 2018 doi: 10.1111/odi.12821. [DOI] [PubMed] [Google Scholar]
- 9.Arduino PG, Carbone M, Della Ferrera F, Elia A, Conrotto D, Gambino A, Comba A, Calogiuri PL, Broccoletti R. Pimecrolimus vs. tacrolimus for the topical treatment of unresponsive oral erosive lichen planus: a 8 week randomized double-blind controlled study. J Eur Acad Dermatol Venereol. 2014 doi: 10.1111/jdv.12128. [DOI] [PubMed] [Google Scholar]
- 10.Voûte ABE, Schulten EAJM, Langendijk PNJ, Kostense PJ, van der Waal I. Fluocinonide in an adhesive base for treatment of oral lichen planus. A double-blind, placebo-controlled clinical study. Oral Surg Oral Med Oral Pathol. 1993 doi: 10.1016/0030-4220(93)90091-H. [DOI] [PubMed] [Google Scholar]
- 11.Tyldesley WR, Harding SM. Betamethasone valerate aerosol in the treatment of oral lichen planus. Br J Dermatol. 1977 doi: 10.1111/j.1365-2133.1977.tb05211.x. [DOI] [PubMed] [Google Scholar]
- 12.Swift JC, Rees TD, Plemons JM, Hallmon WW, Wright JC. The Effectiveness of 1% pimecrolimus cream in the treatment of oral erosive lichen planus. J Periodontol. 2005 doi: 10.1902/jop.2005.76.4.627. [DOI] [PubMed] [Google Scholar]
- 13.Passeron T, Lacour JP, Fontas E, Ortonne JP. Treatment of oral erosive lichen planus with 1% pimecrolimus cream: a double-blind, randomized, prospective trial with measurement of pimecrolimus levels in the blood. Arch Dermatol. 2007 doi: 10.1001/archderm.143.4.472. [DOI] [PubMed] [Google Scholar]
- 14.Volz T, Caroli U, Lüdtke H, Bräutigam M, Kohler-Späth H, Röcken M, Biedermann T. Pimecrolimus cream 1% in erosive oral lichen planus—a prospective randomized double-blind vehicle-controlled study. Br J Dermatol. 2008 doi: 10.1111/j.1365-2133.2008.08726.x. [DOI] [PubMed] [Google Scholar]
- 15.McCaughey C, MacHan M, Bennett R, Zone JJ, Hull CM. Pimecrolimus 1% cream for oral erosive lichen planus: a 6-week randomized, double-blind, vehicle-controlled study with a 6-week open-label extension to assess efficacy and safety. J Eur Acad Dermatol Venereol. 2011 doi: 10.1111/j.1468-3083.2010.03923.x. [DOI] [PubMed] [Google Scholar]
- 16.Eisen D, Ellis CN, Duell EA, Griffiths CE, Voorhees JJ. Effect of topical cyclosporine solution on oral lichen planus. A double-blind analysis. N Engl J Med. 1990. [DOI] [PubMed]
- 17.Harpenau LA, Plemons JM, Rees TD. Effectiveness of a low dose of cyclosporine in the management of patients with oral erosive lichen planus. Oral Surg Oral Med Oral Pathol Oral Radiol. 1995 doi: 10.1016/S1079-2104(05)80195-7. [DOI] [PubMed] [Google Scholar]
- 18.Giustina TA, Stewart JCB, Ellis CN, Regezi JA, Annesley T, Woo TY, Voorhees JJ. Topical application of isotretinoin gel improves oral lichen planus: a double-blind study. Arch Dermatol. 1986 doi: 10.1001/archderm.1986.01660170064021. [DOI] [PubMed] [Google Scholar]
- 19.Piattelli A, Carinci F, Iezzi G, Perrotti V, Goteri G, Fioroni M, Rubini C. Oral lichen planus treated with 13-cis-retinoic acid (isotretinoin): effects on the apoptotic process. Clin Oral Invest. 2007 doi: 10.1007/s00784-007-0117-0. [DOI] [PubMed] [Google Scholar]
- 20.Petruzzi M, De Benedittis M, Grassi R, Cassano N, Vena G, Serpico R. Oral lichen planus: a preliminary clinical study on treatment with tazarotene. Oral Dis. 2002 doi: 10.1034/j.1601-0825.2002.02833.x. [DOI] [PubMed] [Google Scholar]
- 21.Bacci C, Vanzo V, Frigo AC, Stellini E, Sbricoli L, Valente M. Topical tocopherol for treatment of reticular oral lichen planus: a randomized, double-blind, crossover study. Oral Dis. 2017 doi: 10.1111/odi.12573. [DOI] [PubMed] [Google Scholar]
- 22.Hersle K, Mobacken H, Sloberg K, Thilander H. Severe oral lichen planus: treatment with an aromatic retinoid (etretinate) Br J Dermatol. 1982 doi: 10.1111/j.1365-2133.1982.tb00904.x. [DOI] [PubMed] [Google Scholar]
- 23.Saawarn N, Shashikanth M, Saawarn S, Jirge V, Chaitanya N, Pinakapani R. Lycopene in the management of oral lichen planus: a placebo-controlled study. Indian J Dent Res. 2011 doi: 10.4103/0970-9290.93448. [DOI] [PubMed] [Google Scholar]
- 24.Mousavi F, Sherafati S, Nozad Mojaver Y. Ignatia in the treatment of oral lichen planus. Homeopathy. 2009 doi: 10.1016/j.homp.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 25.Choonhakarn C, Busaracome P, Sripanidkulchai B, Sarakarn P. The efficacy of aloe vera gel in the treatment of oral lichen planus: a randomized controlled trial. Br J Dermatol. 2008 doi: 10.1111/j.1365-2133.2007.08370.x. [DOI] [PubMed] [Google Scholar]
- 26.Salazar-Sánchez N, López-Jornet P, Camacho-Alonso F, Sánchez-Siles M. Efficacy of topical Aloe vera in patients with oral lichen planus: a randomized double-blind study. J Oral Pathol Med. 2010 doi: 10.1111/j.1600-0714.2010.00947.x. [DOI] [PubMed] [Google Scholar]
- 27.Bakhtiari S, Azari-Marhabi S, Mojahedi SM, Namdari M, Rankohi ZE, Jafari S. Comparing clinical effects of photodynamic therapy as a novel method with topical corticosteroid for treatment of Oral Lichen Planus. Photodiagn Photodyn Ther. 2017 doi: 10.1016/j.pdpdt.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 28.Hambly JL, Haywood A, Hattingh L, Nair RG. Comparison between self-formulation and compounded-formulation dexamethasone mouth solution for oral lichen planus: a pilot, randomized, cross-over trial. J Investig Clin Dent. 2017 doi: 10.1111/jicd.12225. [DOI] [PubMed] [Google Scholar]
- 29.Mirza S, Rehman N, Alrahlah A, Alamri WR, Vohra F. Efficacy of photodynamic therapy or low level laser therapy against steroid therapy in the treatment of erosive-atrophic oral lichen planus. Photodiagn Photodyn Ther. 2018 doi: 10.1016/j.pdpdt.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 30.Muzio LL, Della Valle A, Mignogna MD, Pannone G, Bucci P, Bucci E, Sciubba J. The treatment of oral aphthous ulceration or erosive lichen planus with topical clobetasol propionate in three preparations: a clinical and pilot study on 54 patients. J Oral Pathol Med. 2001 doi: 10.1034/j.1600-0714.2001.301006.x. [DOI] [PubMed] [Google Scholar]
- 31.Carbone M, Arduino PG, Carrozzo M, Caiazzo G, Broccoletti R, Conrotto D, Bezzo C, Gandolfo S. Topical clobetasol in the treatment of atrophic-erosive oral lichen planus: a randomized controlled trial to compare two preparations with different concentrations. J Oral Pathol Med. 2009 doi: 10.1111/j.1600-0714.2008.00688.x. [DOI] [PubMed] [Google Scholar]
- 32.Kaur M, Kathariya R, Bontha SC, Chavva SC, Krishna MB. Topical clobetasol (0.025%) and tacrolimus (0.1%) in the management of Oral lichen planus: a comparative study. Research J Pharmaceut Biol Chem Sci. 2016.
- 33.Rödström PO, Hakeberg M, Jontell M, Nordin P. Erosive oral lichen planus treated with clobetasol propionate and triamcinolone acetonide in orabase: a double-blind clinical trial. J Dermatol Treat. 1994 doi: 10.3109/09546639409081837. [DOI] [Google Scholar]
- 34.Carbone M, Conrotto D, Carrozzo M, Broccoletti R, Gandolfo S, Scully C. Topical corticosteroids in association with miconazole and chlorhexidine in the long-term management of atrophic-erosive oral lichen planus: a placebo-controlled and comparative study between clobetasol and fluocinonide. Oral Dis. 1999 doi: 10.1111/j.1601-0825.1999.tb00063.x. [DOI] [PubMed] [Google Scholar]
- 35.Ungphaiboon S, Nittayananta W, Vuddhakul V, Maneenuan D, Kietthubthew S, Wongpoowarak W, Phadoongsombat N. Formulation and efficacy of triamcinolone acetonide mouthwash for treating oral lichen planus. Am J Health Syst Pharm. 2005 doi: 10.1093/ajhp/62.5.485. [DOI] [PubMed] [Google Scholar]
- 36.Sivaraman S, Santham K, Nelson A, Laliytha B, Azhalvel P, Deepak J. A randomized triple-blind clinical trial to compare the effectiveness of topical triamcinolone acetonate (0.1%), clobetasol propionate (0.05%), and tacrolimus orabase (0.03%) in the management of oral lichen planus. J Pharmacy Bioallied Sci. 2016 doi: 10.4103/0975-7406.191976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Handa, Comparison of efficacy and safety of topical triamcinolone acetonide paste 0.1% and fluticasone propionate spray 0.05% in the treatment of symptomatic oral lichen planus and their influence on quality of life. J Am Acad Dermatol. 2012 doi: 10.1016/j.jaad.2011.11.792. [DOI] [Google Scholar]
- 38.Laeijendecker R, Tank B, Dekker SK, Neumann HAM. A comparison of treatment of oral lichen planus with topical tacrolimus and triamcinolone acetonide ointment. Acta Derm Venereol. 2006 doi: 10.2340/00015555-0070. [DOI] [PubMed] [Google Scholar]
- 39.Siponen M, Huuskonen L, Kallio-Pulkkinen S, Nieminen P, Salo T. Topical tacrolimus, triamcinolone acetonide, and placebo in oral lichen planus: a pilot randomized controlled trial. Oral Dis. 2017 doi: 10.1111/odi.12653. [DOI] [PubMed] [Google Scholar]
- 40.Singh AR, Rai A, Aftab M, Jain S, Singh M. Efficacy of steroidal vs non-steroidal agents in oral lichen planus: a randomised, open-label study. J Laryngol Otol. 2017 doi: 10.1017/S0022215116009658. [DOI] [PubMed] [Google Scholar]
- 41.Gorouhi F, Solhpour A, Beitollahi JM, Afshar S, Davari P, Hashemi P, Nassiri Kashani M, Firooz A. Randomized trial of pimecrolimus cream versus triamcinolone acetonide paste in the treatment of oral lichen planus. J Am Acad Dermatol. 2007 doi: 10.1016/j.jaad.2007.06.022. [DOI] [PubMed] [Google Scholar]
- 42.Pakfetrat A, Delavarian Z, Falaki F, Khorashadizadeh M, Saba M. The effect of pimecrolimus cream 1% compared with triamcinolone acetonide paste in treatment of atrophic-erosive oral lichen planus. Iran J Otorhinolaryngol. 2015 doi: 10.22038/ijorl.2015.3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sieg P, Von Domarus H, Von Zitzewitz V, Iven H, Farber L. Topical cyclosporin in oral lichen planus: a controlled, randomized, prospective trial. Br J Dermatol. 1995 doi: 10.1111/j.1365-2133.1995.tb00728.x. [DOI] [PubMed] [Google Scholar]
- 44.Yoke PC, Tin GB, Kim MJ, Rajaseharan A, Ahmed S, Thongprasom K, Chaimusik M, Suresh S, Machin D, Bee WH, Seldrup J. A randomized controlled trial to compare steroid with cyclosporine for the topical treatment of oral lichen planus. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol. 2006 doi: 10.1016/j.tripleo.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 45.Thongprasom K, Chaimusig M, Korkij W, Sererat T, Luangjarmekorn L, Rojwattanasirivej S. A randomized-controlled trial to compare topical cyclosporin with triamcinolone acetonide for the treatment of oral lichen planus. J Oral Pathol Med. 2007 doi: 10.1111/j.1600-0714.2007.00510.x. [DOI] [PubMed] [Google Scholar]
- 46.Arunkumar S, Kalappa S, Kalappanavar A, Annigeri R. Relative efficacy of pimecrolimus cream and triamcinolone acetonide paste in the treatment of symptomatic oral lichen planus. Indian J Dent. 2015 doi: 10.4103/0975-962x.151692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mansourian A, Momen-Heravi F, Saheb-Jamee M, Esfehani M, Khalilzadeh O, Momen-Beitollahi J. Comparison of aloe vera mouthwash with triamcinolone acetonide 0.1% on oral lichen planus: a randomized double-blinded clinical trial. Am J Med Sci. 2011 doi: 10.1097/MAJ.0b013e3182171164. [DOI] [PubMed] [Google Scholar]
- 48.Kia SJ, Shirazian S, Mansourian A, Khodadadi Fard L, Ashnagar S. Comparative efficacy of topical curcumin and triamcinolone for oral lichen planus: a randomized, controlled clinical trial. J Dent (Tehran, Iran). 2015. [PMC free article] [PubMed]
- 49.Thomas AE, Varma B, Kurup S, Jose R, Chandy ML, Kumar SP, Aravind MS, Ramadas AA. Evaluation of efficacy of 1% curcuminoids as local application in management of oral lichen planus—interventional study. J Clin Diagn Res. 2017 doi: 10.7860/JCDR/2017/20898.9715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bakhshi M, Gholami S, Mahboubi A, Jaafari MR, Namdari M. Combination therapy with 1% nanocurcumin gel and 0.1% triamcinolone acetonide mouth solution for oral lichen planus: A randomized double-blind placebo controlled clinical trial. Dermatol Res Pract. 2020 doi: 10.1155/2020/4298193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reddy RL, Reddy RS, Ramesh T, Singh TR, Swapna LA, Laxmi NV. Randomized trial of aloe vera gel vs triamcinolone acetonide ointment in the treatment of oral lichen planus. Quintessence Int (Berlin, Germany:1985). 2012. [PubMed]
- 52.Verma S, Sonali P, Chaudhary A. Evaluation of efficacy of topical application of 5% amlexanox oral paste and 0.1% triamcinolone acetonide oro-mucosal paste in the treatment of oral lichen planus. Global J Res Anal 2020;9.
- 53.Li Y, Shao F, Zheng S, Tan Z, He Y. Alteration of Streptococcus salivarius in buccal mucosa of oral lichen planus and controlled clinical trial in OLP treatment. Probiotics Antimicrobial Proteins. 2020 doi: 10.1007/s12602-020-09664-5. [DOI] [PubMed] [Google Scholar]
- 54.Amanat D, Ebrahimi H, Zahedani MZ, Zeini N, Pourshahidi S, Ranjbar Z. Comparing the effects of cryotherapy with nitrous oxide gas versus topical corticosteroids in the treatment of oral lichen planus. Indian J Dent Res. 2014 doi: 10.4103/0970-9290.152166. [DOI] [PubMed] [Google Scholar]
- 55.Radfar L, Wild RC, Suresh L. A comparative treatment study of topical tacrolimus and clobetasol in oral lichen planus. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol. 2008 doi: 10.1016/j.tripleo.2007.07.029. [DOI] [PubMed] [Google Scholar]
- 56.Corrocher G, Di Lorenzo G, Martinelli N, Mansueto P, Biasi D, Nocini PF, Lombardo G, Fior A, Corrocher R, Bambara LM, Gelio S, Pacor ML. Comparative effect of tacrolimus 0.1% ointment and clobetasol 0..05% ointment in patients with oral lichen planus. J Clin Periodontol. 2008 doi: 10.1111/j.1600-051X.2007.01191.x. [DOI] [PubMed] [Google Scholar]
- 57.Sonthalia S, Singal A. Comparative efficacy of tacrolimus 0.1% ointment and clobetasol propionate 0.05% ointment in oral lichen planus: a randomized double-blind trial. Int J Dermatol. 2012 doi: 10.1111/j.1365-4632.2012.05459.x. [DOI] [PubMed] [Google Scholar]
- 58.Hettiarachchi PVKS, Hettiarachchi RM, Jayasinghe RD, Sitheeque M. Comparison of topical tacrolimus and clobetasol in the management of symptomatic oral lichen planus: a double-blinded, randomized clinical trial in Sri Lanka. J Investig Clin Dent. 2017 doi: 10.1111/jicd.12237. [DOI] [PubMed] [Google Scholar]
- 59.Vohra S, Singal A, Sharma SB. Clinical and serological efficacy of topical calcineurin inhibitors in oral lichen planus: a prospective randomized controlled trial. Int J Dermatol. 2016 doi: 10.1111/ijd.12887. [DOI] [PubMed] [Google Scholar]
- 60.Ezzatt OM, Helmy IM. Topical pimecrolimus versus betamethasone for oral lichen planus: a randomized clinical trial. Clin Oral Invest. 2019 doi: 10.1007/s00784-018-2519-6. [DOI] [PubMed] [Google Scholar]
- 61.López J, Roselló Llabrés X. Cyclosporine A, an alternative to the oral lichen planus erosive treatment. Bulletin Du Groupement International Pour La Recherche Scientifique En Stomatologie & Odontologie; 1995. [PubMed]
- 62.Georgaki M, Nikitakis N, Diamanti S, Sklavounou-Andrikopoulou A. Long-term effectiveness of dexamethasone vs cyclosporine for oral lichen planus. Oral Dis. 2014.
- 63.Femiano F, Gombos F, Scully C. Oral erosive/ulcerative lichen planus: Preliminary findings in an open trial of sulodexide compared with cyclosporine (ciclosporin) therapy. Int J Dermatol. 2003 doi: 10.1046/j.1365-4362.2003.01770.x. [DOI] [PubMed] [Google Scholar]
- 64.Xiong C, Li Q, Lin M, Li X, Meng W, Wu Y, Zeng X, Zhou H, Zhou G. The efficacy of topical intralesional BCG-PSN injection in the treatment of erosive oral lichen planus: a randomized controlled trial. J Oral Pathol Med. 2009 doi: 10.1111/j.1600-0714.2009.00796.x. [DOI] [PubMed] [Google Scholar]
- 65.Ahuja US, Puri N, More CB, Gupta R, Gupta D. Comparative evaluation of effectiveness of autologous platelet rich plasma and intralesional corticosteroids in the management of erosive oral Lichen planus- a clinical study. J Oral Biol Craniofac Res. 2020 doi: 10.1016/j.jobcr.2020.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lin HP, Wang YP, Chia JS, Chiang CP, Sun A. Modulation of serum gastric parietal cell antibody level by levamisole and vitamin B12 in oral lichen planus. Oral Dis. 2011 doi: 10.1111/j.1601-0825.2010.01711.x. [DOI] [PubMed] [Google Scholar]
- 67.Malhotra AK, Khaitan BK, Sethuraman G, Sharma VK. Betamethasone oral mini-pulse therapy compared with topical triamcinolone acetonide (0.1%) paste in oral lichen planus: a randomized comparative study. J Am Acad Dermatol. 2008 doi: 10.1016/j.jaad.2007.11.022. [DOI] [PubMed] [Google Scholar]
- 68.Jajarm HH, Falaki F, Sanatkhani M, Ahmadzadeh M, Ahrari F, Shafaee H. A comparative study of toluidine blue-mediated photodynamic therapy versus topical corticosteroids in the treatment of erosive-atrophic oral lichen planus: a randomized clinical controlled trial. Lasers Med Sci. 2015 doi: 10.1007/s10103-014-1694-1. [DOI] [PubMed] [Google Scholar]
- 69.Lavaee F, Shadmanpour M. Comparison of the effect of photodynamic therapy and topical corticosteroid on oral lichen planus lesions. Oral Dis. 2019 doi: 10.1111/odi.13188. [DOI] [PubMed] [Google Scholar]
- 70.Dillenburg CS, Martins MAT, Munerato MC, Marques MM, Carrard VC, Filho MS, Castilho RM, Martins MD. Efficacy of laser phototherapy in comparison to topical clobetasol for the treatment of oral lichen planus: a randomized controlled trial. J Biomed Opt. 2014 doi: 10.1117/1.jbo.19.6.068002. [DOI] [PubMed] [Google Scholar]
- 71.Ferri EP, Gallo CDB, Abboud CS, Yanaguizawa WH, Horliana ACRT, De Fatima Teixeira Da Silva D, Pavani C, Bussadori SK, Nunes FD, Mesquita-Ferrari RA, Fernandes KPS, Rodrigues MFSD. Efficacy of photobiomodulation on oral lichen planus: A protocol study for a double-blind, randomised controlled clinical trial. BMJ Open. 2018 doi: 10.1136/bmjopen-2018-024083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jajarm HH, Falaki F, Mahdavi O. A comparative pilot study of low intensity laser versus topical corticosteroids in the treatment of erosive-atrophic oral lichen planus. Photomed Laser Surg. 2011 doi: 10.1089/pho.2010.2876. [DOI] [PubMed] [Google Scholar]
- 73.Kazancioglu HO, Erisen M. Comparison of low-level laser therapy versus ozone therapy in the treatment of oral lichen planus. Ann Dermatol. 2015 doi: 10.5021/ad.2015.27.5.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Othman NA, Shaker OG, Elshenawy HM, Abd-Elmoniem W, Eldin AM, Fakhr MY. The effect of diode laser and topical steroid on serum level of TNF-alpha in oral lichen planus patients. J Clin Exp Dent. 2016 doi: 10.4317/jced.52665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.El Shenawy HM, Eldin AM, Nasry SA. Management of pain in oral lichen planus patients: a comparative pilot study. Bull Natl Res Centre. 2018 doi: 10.1186/s42269-018-0014-5. [DOI] [Google Scholar]
- 76.Agha-Hosseini F, Moslemi E, Mirzaii-Dizgah I. Comparative evaluation of low-level laser and CO2 laser in treatment of patients with oral lichen planus. Int J Oral Maxillofac Surg. 2012;41(10):1265–1269. doi: 10.1016/j.ijom.2012.06.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Consensus efficacy list of topical steroid and non-steroidal therapies.
Additional file 2: Table S2. Reported adverse reactions to oral lichen planus interventions.
Data Availability Statement
All data generated during this study are included in this published article (Table 1).