Abstract
Background
Solid-organ transplantation (SOT) from SARS-CoV-2 positive donors could be a life-saving opportunity worth grasping. We perform a systematic review to evaluate the recipient outcomes of SOT from donors with recent or current SARS-CoV-2 infection.
Methods
Search strategy was performed in PubMed, Cochrane COVID-19 Study Register, and Web of Science databases from the 1st of January 2019 to the 31st of December 2021. SOT adult recipients from a donor with past or current SARS-CoV-2 infection were elegible for inclusion. Outcomes were viral transmission, COVID-19 symptoms, mortality, hospital stay, and complications. PROSPERO Register Number: CRD42022303242
Findings
Sixty-nine recipients received 48 kidneys, 18 livers and 3 hearts from 57 donors. Six additional transplants from positive lungs were identified. IgG+ anti-SARS-CoV-2 titers were detected among 10/16 recipients; only 4% (3/69) recipients were vaccinated. Non-lung transplant recipients received organs from 10/57 (17.5%) donors with persistent COVID-19. In 18/57 donors, SARS-CoV-2 RNA was detected (median 32 Cycle threshold [Ct]) at procurement. Among non-lung transplant recipients, SARS-CoV-2 viral transmission was not documented. Four patients presented delayed graft dysfunction, two patients acute rejection, and two patients died of septic shock. The median (IQR) hospital stay was 18 (11–28) days in recipients from symptomatic donors. Viral transmission occurred from three lung donors to their recipients, who developed COVID-19 symptoms. One of the recipients subsequently died.
Conclusion
Use of non-lung (kidney, liver and heart) organs from SARS-CoV-2 positive donors seem to be a safe practice, with a low risk of transmission irrespective of the presence of symptoms at the time of procurement. Low viral replication (Ct > 30) was safe among non-lung donors, even if persistently symptomatic at procurement.
Abbreviations: COVD-19, Coronavirus disease 2019; Ct, Cycle threshold; OPTN, Organ procurement and transplantation network; RNA, Ribonucleic acid; RT-PCR, Real time-polymerase chain reaction; SARS-CoV-2, Severe acute respiratory syndrome coronavirus-2; SOT, Solid-organ transplantation
Keywords: COVID-19, Organ donation, Solid organ transplantation, Viral transmission
Introduction
Solid-organ transplant (SOT) recipients have an increased risk of suffering from respiratory viral infections and are at higher threat for complications from COVID-19, owing to their level of immunosuppression and comorbidities [1], [2]. COVID-19 has also impacted transplant activity, due to concerns of a potential risk of viral transmission from donor to recipient [3], [4]. As the number of persons infected with SARS-CoV-2 is daily increasing, the deferral of all donors who have tested positive for SARS-CoV-2 would result in the loss of a considerable number of medically suitable organs for transplantation.
Given the low number of publications on the subject and the need for information about the safety of the procedure, multiple organisations have released recommendations for clinical practice [5], [6], [7]. These documents emphasise the importance of pre-transplant donor evaluation and testing, considering the disease course and the organ involved in transplantation. Based on existing knowledge about the nature of SARS-CoV-2, lung, intestine, and pancreas transplantation has been not recommended, as they are linked to a higher risk of complications and possible viral transmission [8]. Regarding organs less affected by COVID-19, a waiting period between 21 and 90 days after disease onset has been initially recommended [5], [6], [7]. These recommendations may need to be adapted according to the evolution of the risk for complications in SOT recipients. Factors such as the presence of antibodies against SARS-CoV-2 in the recipient due to vaccination or previous infection, the availability of therapy with anti-spike monoclonal antibodies and direct antivirals, and the potential lower virulence of the Omicron variant of concern, should be taken into consideration [9], [10], [11].
Our hypothesis was that non-lung solid organ transplantations from SARS-CoV-2 positive donors were a safe practice. The aim of this systematic review is to evaluate the recipient outcomes of SOT from donors with persistent, resolved, or asymptomatic SARS-CoV-2 infection.
Material and methods
Registration and protocol
This study was performed following the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines [12]. The protocol was pre-registered on PROSPERO (CRD42022303242). PRISMA checklist is reported in Supplementary Table S1 (Supplementary material).
Data sources
The search strategy was performed in PubMed, Cochrane Library, and Web of Science databases. The Cochrane COVID-19 Study Register was also consulted for other published or ongoing articles. A restriction was applied to the publication time frame from the 1st of January 2019 to the 31st of December 2021 (including ahead of print publication studies). Literature search was limited to human subjects. No language restrictions were applied. Search terms are detailed in Supplementary Table S2 (Supplementary material).
Selection criteria
We included observational studies, case series, and case reports describing adult SOT recipients (≥ 18 years) with a donor with recent or current SARS-CoV-2 infection. Letters from lung transplant were included. Type of organ transplant included lung, liver, kidney, heart, and pancreas. Intestinal and tissue donations were excluded.
Outcomes assessed were: viral transmission, COVID-19 symptoms, mortality, length of hospital stay, and complications after transplantation (acute rejection, delayed graft function, infections, abdominal, coronary and respiratory complications, sepsis, and multisystem organ failure). “Viral transmission” was defined as the presentation of COVID-19 symptomatology with laboratory confirmation, in a previously not symptomatic recipient, after being transplanted from donor with COVID-19.
Definitions
“Persistent” SARS-CoV-2 infection was defined when donor had COVID-19 and symptoms persisted at organ procurement. The clinical severity of COVID-19 (mild or severe) and other definitions were adapted from the Organ Procurement and Transplantation Network (OPTN) [5]. “Mild” infection was defined as detection of SARS-CoV-2 in a respiratory sample in patients with symptoms consistent with COVID-19 infection who did not require oxygen supplementation or inpatient hospitalisation for COVID-19. “Severe” infection was defined as detection of SARS-CoV-2 in a respiratory sample in patients with symptoms consistent with COVID-19 infection who required oxygen supplementation or inpatient hospitalisation for COVID-19. “Resolved” SARS-CoV-2 infection was defined as having a confirmed diagnosis of COVID-19, that has resolved symptoms more than 21 days after symptom onset. “Asymptomatic” SARS-CoV-2 infection was defined as the detection of SARS-CoV-2 in a respiratory sample without current or past symptoms compatible with COVID-19. When it was not reported whether the patients had symptoms or not, the subject was considered as “Unreported”.
Data extraction and study selection process
Two authors (RMR and HNK) independently analysed all articles that were retrieved by reading and assessing their titles, abstracts, and full texts. In case of disagreement, a third reviewer (ST) was consulted to determine eligibility. Literature search results where uploaded to Rayyan, a program that facilitates management of studies and the study selection process. A predesigned Excel spreadsheet to collect study data in a standardised way was used.
Data extracted consisted in country, type of study design, organ transplant, patients’ characteristics (number, age, sex, medical/social history), time from SARS-CoV-2 infection to transplant, viral load of SARS-CoV-2 in a laboratory test at transplantation, measured by the Cycle Threshold (Ct) value of the real time-polymerase chain reaction (RT-PCR), SARS-CoV-2 vaccination in the recipient, and follow-up after transplant. The analysis was split in lung and non-lung organs, in two different groups.
Quality assessment
Two investigators (RMR and HNK) independently assessed the risk of bias in the included studies. Disagreement regarding quality assessment was resolved by a third author (ST). Quality assessment was performed for observational studies, case reports, and case series. The Newcastle-Ottawa Scale [13] was used to assess the quality of the observational studies. The proposed tool specified by Murad et al. [14] was used to assess the quality of case reports and case series. The scale is based on convergence of previous criteria from Pierson [15], Bradford Hill [16], and Newcastle-Ottawa [13] scale modification.
Statistical analysis
We used descriptive statistics to characterise the study population, separately for donors and recipients. Continuous variables were described as medians with their interquartile ranges (IQR) and categorical variables were presented as counts and percentages. Pairwise comparisons for categorical variables were performed by using a χ2 test. P-values less than 0.05 were considered to be statistically significant. Heterogeneity was investigated by ordering tables, showing donor classification (persistent, resolved, asymptomatic, and unreported), characteristics (presence of SARS-CoV-2 antibodies, severity of illness, Ct, and days from symptoms to organ recovery) and outcomes. When relevant information was not reported, study’s authors were contacted to obtain missing data from included articles. Statistical analyses were performed with SPSS Statistics version 25.0 software.
Results
Study selection
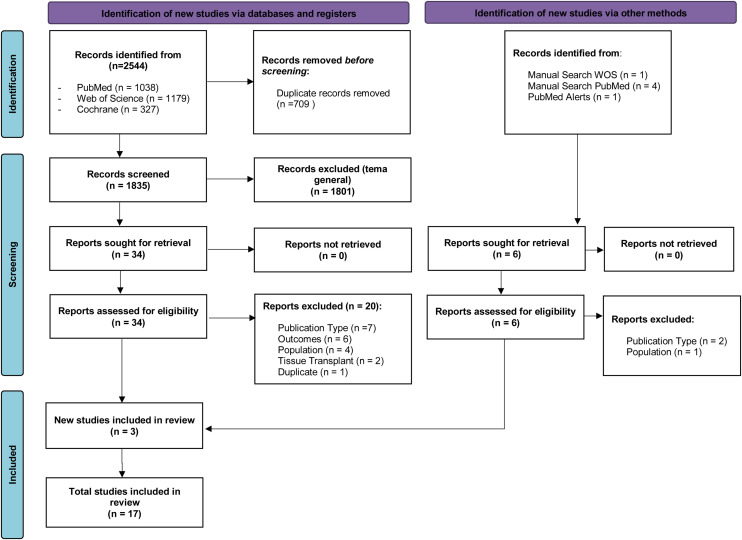
A total of 2544 studies were identified. After screening by inclusion and exclusion criteria, 17 studies were included in this systematic review [9], [10], [11], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30]. The PRISMA flow diagram process is shown in Fig. 1 .
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) flow diagram of the study selection.
Population characteristics
Eleven case reports, three case series, and one retrospective cohort study met the eligibility criteria. A letter [29] and an editorial [30] including lung transplant recipients were also included. Overall, the present study includes 74 solid organ transplant recipients (48 kidneys, 18 livers, 3 hearts, and 5 lungs) from donors who had resolved, persistent or asymptomatic SARS-CoV-2 infection. To this date, cases of pancreas or intestine transplantation from a COVID-19 positive donor have not been reported. Population characteristics are detailed in Supplementary Table S3 (Supplementary material).
Non-lung transplantation
Donors
Overall, 57 donors of non-lung organs were included (Table 1 ). Ten of them (17.5%) presented a persistent COVID-19 at the time of procurement. Symptoms were mild non-respiratory in 1/10 (10%), severe respiratory in 4/10 (40%), and unknown severity in 5/10 (50%) of patients.
Table 1.
Summary of 57 non-lung donors with (A) persistent, (B) resolved, (C) asymptomatic SARS-CoV-2 infection and (D) unreported symptoms at transplantation and corresponding recipients.
| (A) Persistent SARS-CoV-2 infection | ||||||||
|---|---|---|---|---|---|---|---|---|
| Author, year [ref] | Donors |
Recipients |
||||||
| N = 10 | Symptoms severity | RT-PCR at organ procurement (days)a | Serostatus | Organ Transplant | N = 11 | Serostatus | Vaccine | |
| Hong, 2020 [19] | 1 | Mild | Positive | – | Liver | 1 | – | No |
| Puodziukaite, 2021 [10] | 1 | Severe | Positive | IgG+ | Kidney | 2 | IgG+ | No |
| Romagnoli, 2021 [9] | 1 | Severe | Positive | IgG- | Liver | 1 | IgG+ | No |
| 1 | Severe | Negative (3) | IgG- | Liver | 1 | IgG+ | No | |
| 1 | Severe | Positive | IgG+ | Liver | 1 | IgG+ | No | |
| 1 | – | Positive | IgG- | Liver | 1 | IgG+ | No | |
| 1 | – | Positive | IgG- | Liver | 1 | IgG+ | No | |
| 1 | – | Positive | IgG- | Liver | 1 | IgG+ | No | |
| 1 | – | Positive | – | Liver | 1 | IgG+ | No | |
| 1 | – | Positive | IgG- | Liver | 1 | IgG+ | No | |
| (B) Resolved SARS-CoV-2 infection | ||||||||
|---|---|---|---|---|---|---|---|---|
| Author, year [ref] | Donors |
Recipients |
||||||
| N = 13 | Symptoms severity | RT-PCR at organ procurement (days)a | Serostatus | Organ Transplant | N = 18 | Serostatus | Vaccine | |
| Niedlinger, 2021 [28] | 1 | Severe | Negative | IgG+ | Kidney | 2 | – | No |
| 1 | Mild | Negative (98) | IgG+ | Liver/Heart/ Kidney | 4 | – | No | |
| 1 | Mild | Negative (38) | IgG+ | Liver /Heart | 2 | IgG- (n = 1) | No | |
| Kucuk, 2021 [21] | 1 | Mild | Negative (28) | IgG- | Kidney | 1 | IgG- | No |
| Kute, 2021 [22] | 9 | Mild | Negative | – | Kidney | 9 | – | No |
| (C) Asymptomatic SARS-CoV-2 infection | ||||||||
|---|---|---|---|---|---|---|---|---|
| Author, year [ref] | Donors |
Recipients |
||||||
| N = 25 | Symptoms severity | RT-PCR at organ procurement (days)a | Serostatus | Organ Transplant | N = 25 | Serostatus | Vaccine | |
| Kute, 2021 [22] | 22 | No | Negative | – | Kidney | 22 | – | No |
| Nguyen, 2021 [17] | 1 | No | Negative* | – | Liver | 1 | IgG- | No |
| Niedlinger, 2021 [28] | 1 | No | Positive | IgG+ | Liver | 1 | – | No |
| 1 | No | Negative (48) | IgG+ | Liver | 1 | – | No | |
| (D) SARS-CoV-2 infection with unreported symptoms | ||||||||
|---|---|---|---|---|---|---|---|---|
| Author, year [ref] | Donors |
Recipients |
||||||
| N = 9 | Symptoms severity | RT-PCR at organ procurement (days)a | Serostatus | Organ Transplant | N = 15 | Serostatus | Vaccine | |
| Manzia, 2021 [11] | 1 | – | Positive | – | Liver | 1 | IgG+ | No |
| La Hoz, 2022 [20] | 1 | – | Positive | – | Liver | 1 | – | No |
| 1 | – | Positive | – | Liver | 1 | – | Yes | |
| Perlin, 2021 [24] | 1 | – | Positive | – | Kidney | 2 | IgG- | No |
| Niedlinger, 2021 [28] | 1 | – | Positiveb | – | Heart/Kidney | 3 | – | No |
| Tuncer, 2021 [18] | 1 | – | Negative (11) | – | Liver | 1 | – | No |
| Koval, 2021 [23] | 1 | – | Positive | – | Kidney | 2 | – | No |
| 1 | – | Positive | – | Kidney | 2 | – | Yes (n = 1) | |
| 1 | – | Positive | – | Kidney | 2 | – | Yes (n = 1) | |
Ct: Cycle threshold values indicate the number of amplification cycles needed to achieve a positive result from a RT-PCR test and is a surrogate marker for viral load with an inverse correlation.
Living donor with a negative RT-PCR nasopharyngeal swab (Ct not reported) three days prior to procurement. On postoperative day three, SARS-CoV-2 RNA detected in a nasopharyngeal swab with when developed COVID-19 with oxygen requirement.
Time to the last positive RT-PCR (days before organ donation).
Positive stool RT-PCR.
Thirteen donors out of 57 (22.8%) had a resolved COVID-19 disease (Table 1). The latest positive RT-PCR among recovered donors, when reported, was estimated to be median 38 days.
Twenty-five out of 57 (43.9%) were classified as asymptomatic according to the OPTN classification [5]. One of them was a living donor with a negative RT-PCR nasopharyngeal swab (Ct not reported) three days prior to procurement [17]. On postoperative day three, SARS-CoV-2 RNA was detected in a nasopharyngeal swab of the donor, who developed COVID-19 with oxygen requirement (Table 1). Symptoms were not reported in the additional 9/57 (15.7%) donors.
Eighteen non-lung (12 liver) donors reported positive nasopharyngeal swab RT-PCT test at procurement. Six reported median Ct values of 32 (IQR 29-37) and only one was below 30 cycles. Details of these donors and respective recipients are reported in Table 2 . No viral transmission was documented.
Table 2.
Summary of 18 non-lung donors with positive SARS-CoV-2 test at procurement and corresponding recipients.
| Author, year [ref] | Donors |
Recipients |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| N = 18 | Symptoms classification | RT-PCR test | Cycle Threshold | Serostatus | Organ Transplant | N = 25 | Serostatus | Vaccine | |
| Hong, 2020 [19] | 1 | Symptomatic | Nasopharyngeal swab, Oropharyngeal Swab | – | – | Liver | 1 | – | No |
| Puodziukaite, 2021 [10] | 1 | Symptomatic | Nasopharyngeal swab | 32 | IgG+ | Kidney | 2 | IgG+ | No |
| Romagnoli, 2021 [9] | 1 | Symptomatic | Bronchoalveolar lavage | – | IgG- | Liver | 1 | IgG+ | No |
| 1 | Symptomatic | Nasopharyngeal swab | – | IgG+ | Liver | 1 | IgG+ | No | |
| 1 | Symptomatic | Nasopharyngeal swab | – | IgG- | Liver | 1 | IgG+ | No | |
| 1 | Symptomatic | Nasopharyngeal swab | – | IgG- | Liver | 1 | IgG+ | No | |
| 1 | Symptomatic | Nasopharyngeal swab | – | IgG- | Liver | 1 | IgG+ | No | |
| 1 | Symptomatic | Nasopharyngeal swab, Bronchoalveolar lavage | – | – | Liver | 1 | IgG+ | No | |
| 1 | Symptomatic | Nasopharyngeal swab | – | IgG- | Liver | 1 | IgG+ | No | |
| Niedlinger, 2021 [28] | 1 | Asymptomatic | Nasopharyngeal swab | – | IgG+ | Liver | 1 | – | No |
| 1 | – | Stool RT-PCR | – | – | Heart/Kidney | 3 | – | No | |
| Manzia, 2021 [11] | 1 | – | Bronchoalveolar lavage | 24 | – | Liver | 1 | IgG+ | No |
| La Hoz, 2022 [20] | 1 | – | Lower respiratory tract nucleic acid test | 37.8 | – | Liver | 1 | – | No |
| 1 | – | Lower respiratory tract nucleic acid test | 32.8 | – | Liver | 1 | – | Yes | |
| Perlin, 2021 [24] | 1 | – | Nasopharyngeal swab | – | – | Kidney | 2 | IgG- | No |
| Koval, 2021 [23] | 1 | – | Nasopharyngeal swab | 38 | – | Kidney | 2 | – | No |
| 1 | – | Nasopharyngeal swab | – | – | Kidney | 2 | – | Yes (n = 1) | |
| 1 | – | Nasopharyngeal swab | 31 | – | Kidney | 2 | – | Yes (n = 1) | |
Fourteen out of 57 (24.5%) donors had anti-SARS-CoV-2 IgG evaluated at the time of transplant, and 7/14 (50%) were detectable. Details are shown in Tables 1 & 2.
Recipients
Sixty-nine recipients were included (Table 1). Eleven out of sixty-nine (15.9%) were transplanted from a donor with persistent SARS-CoV-2 infection. Eighteen (26.1%) received an organ from an already recovered donor. Twenty-five (36.2%) organs were obtained from donors classified as asymptomatic. Fifteen patients (21.7%) were transplanted from a donor with unreported symptomatology. Three (4%) recipients, from donors with unreported symptoms, were vaccinated before transplantation. Sixteen out of sixty-nine (23.1%) organ recipients had anti-SARS-CoV-2 IgG measured and 11/16 (68.7%) recipients had detectable titres.
Ten recipients from non-lung persistent COVID-19 donors reported serostatus; positive IgG titres were detected in all of them. They presented more frequently IgG titres than recipients from non-symptomatic donors (100% vs. 16.6%, p < 0.05). Six recipients from donors with persistent COVID-19 received steroid pulses; none was treated with anti-Spike monoclonal antibodies or remdesivir. Symptoms and laboratory findings among recipients are detailed in Table 1 and Supplementary Table S4 (Supplementary material).
Outcomes
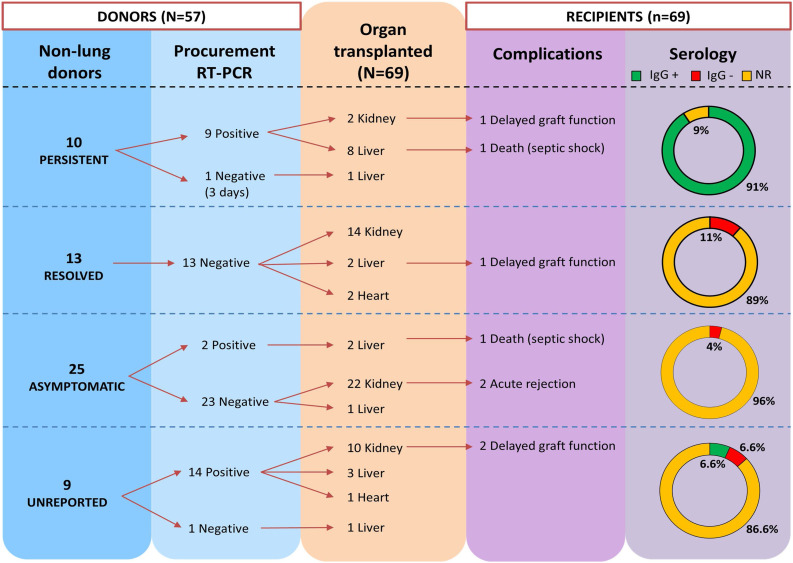
None of the non-lung transplants recipients developed COVID-19 symptomatology after transplantation, so no cases of new viral transmission were reported. Even the liver recipient from the living donor who developed severe COVID-19 three days after transplantation remained uninfected and survived. Only two [9] recipients tested SARS-CoV-2 positive after transplantation, being infected a month before transplantation and having detectable SARS-CoV-2 IgG titres. Twelve (17.3%) non-lung transplant recipients reported fifteen complications post-transplant (Table 3). Delayed graft function was reported in four recipients (kidney and liver transplant). Acute rejection episode was reported in two kidney transplant recipients. The median (IQR) hospital stay was 18 days (11–28) in recipients from persistent SARS-CoV-2 donors. Two liver transplant recipients died due to septic shock and multisystem organ failure, respectively. The outcomes in SOT recipients are detailed in Table 3 and Supplementary Table S4 (Supplementary material). Summary of non-lung transplantation from 57 donors with SARS-CoV-2 infection is reported in Fig. 2 .
Table 3.
Outcomes for recipients from donors with (A) persistent, (B) non-persistent SARS-CoV-2 infection at transplantation, and (C) only from lung transplant donors.
| (A) Persistent SARS-CoV-2 infection | |||||||
|---|---|---|---|---|---|---|---|
| Author, year [ref] | N recipients | Organ Transplanted (n) | Hospitalisation, days | Complications (n) |
Symptoms (Post operation) |
Mortality (n) |
Viral transmission |
| Hong, 2020 [19] | 1 | Liver (1) | 69a | Peritonitis, coronary occlusion | No | Alive | No |
| Puodziukaite, 2021 [10] | 2 | Kidney (2) | 18 | Delayed graft function (1) | No | Alive | No |
| Romagnoli, 2021 [9] | 8 | Liver (8) | 14.5 (10.2−25.5)* | Septic shock (1) | No | Death (1)/Alive (7) | No |
| (B) Non-Persistent SARS-CoV-2 infection | |||||||
|---|---|---|---|---|---|---|---|
| Author, year [ref] | N recipients | Organ Transplanted (n) | Hospitalisation, days | Complications (n) |
Symptoms (Post operation) |
Mortality (n) |
Viral transmission |
| Neidlinger, 2021 [28] | 13 | Liver (4), Heart (3), Kidney (6) | – | Multisystem organ failure (1) Delayed graft function (1) |
No | Death (1)/Alive (12) | No |
| Kucuk, 2021 [21] | 1 | Kidney (1) | 15 | No | No | Alive | No |
| Nguyen, 2021 [17] | 1 | Liver (1) | 13 | No | No | Alive | No |
| Kute, 2021 [22] | 31 | Kidney (31) | – | Acute rejection (2) | No | Alive | No |
| Manzia, 2021 [11] | 1 | Liver (1) | 56a | Obstructive jaundice Renal impairment |
No | Alive | No |
| La Hoz, 2022 [20] | 2 | Liver (2) | – | Biliary stricture (1) | No | Alive | No |
| Perlin, 2021 [24] | 2 | Kidney (2) | 35 | Delayed graft function (1) Genital herpes (1) |
No | Alive | No |
| Tuncer, 2021 [18] | 1 | Liver (1) | 16a | No | No | Alive | No |
| Koval, 2021 [23] | 6 | Kidney (6) | – | Delayed graft function (1) Ileus (1) |
No | Alive | No |
| (C) Lung transplant donors | |||||||
|---|---|---|---|---|---|---|---|
| Author, year [ref] | N recipients | Organ Transplanted (n) | Hospitalisation, days | Complications (n) |
Symptoms (Post operation) |
Mortality (n) |
Viral transmission |
| Querrey, 2021 [25] | 1 | Lung | 40ª | Pleural effusions | No | Alive | No |
| Ceulemans, 2021 [27] | 1 | Lung | 35 | Acute rejection Pneumothorax (dislocated tube) | No | Alive | No |
| Kaul, 2021 [26] | 1 | Lung | 61 | Septic shock Multisystem organ failure |
Yes | Death | Yes |
| Kumar, 2021 [29] | 1 | Lung | 25ª | Bilateral airspace disease | Yes | Alive | Yes |
| Eichenberger, 2021 [30] | 1 | Lung | – | – | – | Alive | Yes |
Data reported as median (interquartile range).
Last follow update reported. To this day, the patient was still hospitalised.
Fig. 2.
Summary of non-lung transplantation from 57 donors with SARS-CoV-2 infection.
Lung transplantation
At the end of our search period, six positive SARS-CoV-2 lung donors were identified, from which five lungs were transplanted. Details from positive lung donors are reported in Table 4 . Two and one lung-transplants reported multilobar and lobar lung CT scans opacities, respectively. Three out of five lung recipients (all with negative SARS-CoV-2 nasal swab at procurement time, but subsequent SARS-CoV-2 recovering from lower respiratory samples) developed critical COVID-19 and one of them died. Two lung recipients received SOT from SARS-CoV-2 positive donors at procurement (bronchoalveolar lavage Ct 8.5/9.5; bronchial wash Ct 26.6) and viral transmission was documented in both transplants. One double-lung recipient from symptomatic donor developed COVID-19 symptoms and subsequently died. He was treated with remdesivir (two five-day courses), convalescent plasma on two occasions, and rescue pulse steroids. Immunosuppression with methylprednisolone, tacrolimus, and mycophenolate mofetil was maintained. Disease progressed with worsening respiratory distress and required veno-venous extracorporeal membrane oxygenation. The death occurred after 61 days of worsening condition.
Table 4.
Results for recipients from lung transplant donors with SARS-CoV-2 positive infection.
| Author, year [ref] | Donor |
Recipient |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Infection Status | Severity | Procurement RT-PCR |
Procurement RT-PCR |
Organ | Immunosuppression | Therapy | Viral transmission | Death | |
| Querrey, 2021 [25] | Recovered (7 weeks)c |
Mild | Nasopharyngeal Swab (−) Bronchoalveolar Lavage (−) |
Bronchoalveolar Lavage (−) | Lung | Maintain: Tacrolimus, mycophenolate mofetil, prednisone Induction: Rituximab, basiliximab, methylprednisolone |
Plasmapheresis, eculizumab for acute graft disfunction | No | No |
| Ceulemans, 2021 [27] | Recovered (3 months)c |
Mild | Nasopharyngeal Swab (−) Bronchoalveolar Lavage (−) Lung biopsy (Ct 35) Serostatus: IgG+ |
Nasopharyngeal Swab (−) Bronchoalveolar Lavage (−) Serostatus: IgG- |
Lung | Antithymocyte globulin, tacrolimus, mycophenolate mofetil, methylprednisolone | Azithromycin, acyclovir | No | No |
| Kaul, 2021 [26] | Persistent | Mild | Nasopharyngeal Swab (−) Bronchoalveolar Lavage (Ct 8.5/9.5) |
Pre-operation Nasopharyngeal Swab (−) Post-operation Bronchoalveolar Lavage (Ct 8.1/9.2) |
Lung | Methylprednisolone, tacrolimus, mycophenolate mofetil | Remdesivir, convalescent plasma, steroid pulse, veno-venous ECMO | Yes | Yes |
| Kumar, 2021 [29] | Asymptomatic | Noa | Nasopharyngeal Swab (−) Bronchial Wash (Ct 26.4)b |
Pre-operation Bronchial Wash (−) Post-operation Nasopharyngeal Swab (Ct 10.7/13) |
Lung | Methylprednisolone | Remdesivir | Yes | No |
| – | Kidney (2), Liver | – | – | No | Yes (1 kidney) |
||||
| Persistent | Mild | Nasopharyngeal Swab (−) Bronchoalveolar Lavage (+) |
– | Kidney | – | No | No | ||
| Eichenberger, 2021 [30] | – | – | – | – | Lung | – | – | Yes | No |
Ct: Cycle threshold values indicate the number of amplification cycles needed to achieve a positive result from a RT-PCR test and is a surrogate marker for viral load with an inverse correlation; (−) negative SARS-CoV-2; (+) positive SARS-CoV-2.
Normal CT chest.
Post implantation bronchial wash (performed during the transplant surgery immediately after implantation of the first lung.
Time to the last positive RT-PCR (days before organ donation).
Two recovered lung donors were also identified. Both had resolved mild COVID-19 (seven weeks and three months pre-transplantation). No viral transmission was reported in the recipients. One of them developed acute rejection. Patients recovered after hospital stay of 40 and 35 days, respectively.
From the lung donors, in addition to the five lungs, three kidneys and one liver were transplanted. No viral transmission occurred in the transplantation of the non-lung organs.
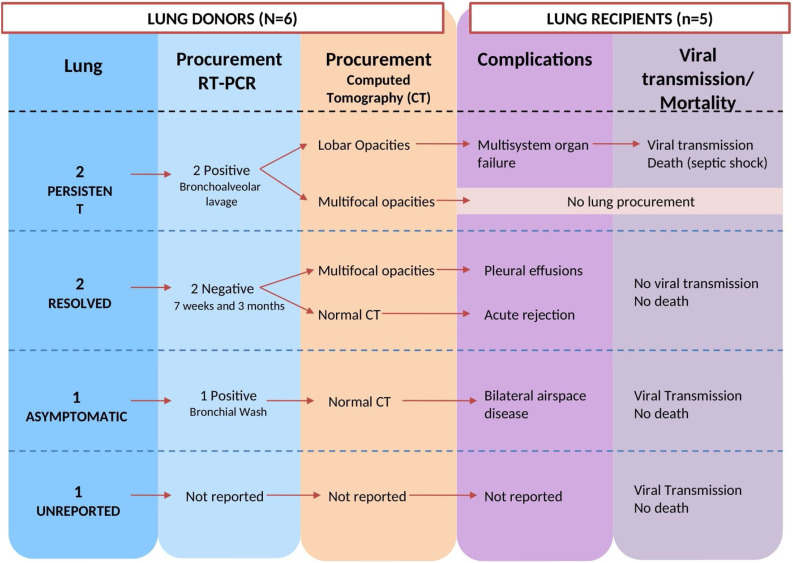
Summary of transplantation from six lung donors with SARS-CoV-2 infection is reported in Fig. 3 .
Fig. 3.
Summary of lung transplantation from six donors with SARS-CoV-2 infection.
Quality assessment
Risk of bias of observational study was assessed by the Newcastle Ottawa Scale [13] tool (Supplementary Table S5A in Supplementary material). The study was resulting in moderate quality of evidence, mainly due to issues in “comparability of study groups”. Risk of bias of case reports and case series were assessed by Murad et al. [14] (Supplementary Table S5B in Supplementary material). Eight studies had moderate quality, while six had a low quality, mainly due to issues of “causality”. The best score was reporting for “ascertainment” followed by “reporting”.
Discussion
To our knowledge, this is the first systematic review looking at the risk of transmission of COVID-19 in solid organ transplantation from donors with a past or current SARS-CoV-2 infection, focusing on short-term outcomes. We analysed 74 solid organ transplantations from SARS-CoV-2 positive donors with resolved, asymptomatic or persistent infection, in studies reported until the 31th of December 2021. Eighteen donors had positive RT-PCR at procurement. Among non-lung transplant recipients, 11 recipients received organs from donors with persistent symptoms and one from a living donor with a pre-symptomatic infection. We found no documented cases of donor-derived infection among kidney, liver, and heart transplant recipients. COVID-19 developed in lung transplant recipients from three donors [26], [29], [30] with negative nasopharyngeal swab at procurement, and one of them died. Although more data are urgently needed, our findings question the safety of lung transplantation for persistent or asymptomatic lung donors, even in presence of negative nasopharyngeal or bronchial swabs. For respiratory assessment, a bronchoalveolar lavage is strongly recommended.
The main results of our systematic review are the low transmission risk of COVID-19 when using organs from donors with recent or current SARS-CoV-2 infection to non-lung recipients, even if persistently symptomatic at procurement. This is probably related with the mechanism of viral transmission of SARS-CoV-2. SARS-CoV-2 spreads during close contact by airborne transmission, infectiousness peaks between two days before and one day after symptom onset and declines within seven days. The risk of donor-recipient transmission relates to the presence of transmissible virus in organs; in non-lung organs, this is mainly due to ongoing viraemia at the time or procurement, resulting in sustained viral replication in the presence of cellular entry factor [31]. In early studies, only 15% of patients with COVID-19 had SARS-CoV-2 viraemia [32]. Further, there is no reported haematogenous transmission to date, and data suggests the level of SARS-CoV-2 viraemia is low, with high Ct values of RT-PCR amplification [33]. None non-lung transplant recipient developed COVID-19 infection during the follow-up period. This outcome was expected, as a Ct value of > 35 represents residual RNA detection [34]. In our series, only three recipients were vaccinated. However, when reported, most recipients documented positive IgG titres anti-SARS-CoV-2, reflecting a probable previous infection. Four patients received organs from donors who presented severe symptomatic disease without documented viral transmission.
In clinical practice, it is important to differentiate between current vs. recent infection, with decisions taken not only based on the qualitative interpretation of PCR. Symptoms, timing, specimen sample and Ct should be considered among donors for a personalised interpretation. SARS-CoV-2 serostatus in recipients needs to be assessed. Our findings report that negative SARS-CoV-2 PCR amplification at procurement among 39 non-lung donors with a recent history of COVID-19 seems safe for transplantation (even if persistently symptomatic). In addition, only 18 donors were RT-PCR positive at the time of procurement (Table 2). Only 1/18 SARS-CoV-2 positive non-lung donor with low Ct (and high replication) was identified [11] for a seropositive recipient. SARS-CoV-2 positive with high Ct (and low replication) was also safe (even if persistently symptomatic at procurement) among 17/18 non-lung donors. Our data emphasise the importance of interpreting the Ct as a surrogate of replication, with low replication (high Ct) probably safe for non-lung donors, even if persistently symptomatic at procurement. Given the mechanism of transmission of SARS-CoV-2, it is highly unlikely that organs from donors even with high viral loads could lead to viral transmission, but data on the use of these organs are still needed.
Different Transplantation Societies recommended a recovery period of 21–90 days before transplantation donation [5], [6], [7]. Still, in our series, even when this recommended timeframe was not followed, there were no cases of infection transmission after non-lung transplantation. Considering this evidence, in non-lung transplantation, the stratification of donors in high and low risk of transmission, according to the severity and duration of symptoms, as well as RT-PCR viral load (Ct value as surrogate), could represent a pragmatic strategy. Furthermore, vaccination status and serological response of the recipient should be taken into account, with a theoretical safer utilisation of organs from high-risk infected donors in patients with appropriate immune response to vaccination. Thus, more extensive case series and stratified evaluation of transplant outcomes by vaccination and serologic status are requested.
Concerning lung transplantation, as described with other respiratory viruses, high risk of donor-derived SARS-CoV-2 transmission is expected. Three viral transmissions were documented among six lung transplant donors. It is relevant from these reports that a negative nasopharyngeal swab did not exclude infection and viral transmission from the lung may be present to lung recipients. In this cohort, viral transmission to non-lung transplant recipients was not documented.
The main limitation of this systematic review is the sample size, consisting of case reports and case series. However, it is important to report it due to the scarce current information and lack of evidence in the recommendations. In addition, the heterogeneity of the information included important missing data, such as viral load (Ct), time of symptoms evolution, and immunological status among many donors and recipients, with important differences in the follow-up period and the capturing outcomes. Donors classified as asymptomatic accordingly to OPTN, merged positive and negative RT-PCR amplification tests at procurement. This is why we focused on the 18 positive RT-PCR donors at procurement. Because search was limited to the end of 2021, our findings should be extrapolated with caution when other variants, such as Omicron or other subvariants such as BA.2, would be prevalent. Likewise, the effect on long-term follow-up remains also unknown.The reported studies mostly had moderate quality of evidence. Another limitation is a possible publication bias, which conditioned the search results. Although our data seems encouraging in non-lung transplant, more studies with larger series are still required to confirm these results. Further, the role of post-exposure prophylaxis using pre-emptive boosting the humoral response with a long-acting monoclonal antibody after the graft is implanted and the role of empiric antiviral pre-emptive treatment regimens in this setting are still to define. Thus, authors are commended to share other experiences because expanding literature on COVID-19 positive donors is an unmet clinical need. Lastly, further studies are still required to assess the long-term outcomes, including allograft dysfunction, post-acute COVID-19 syndrome and long-term mortality among transplant recipients receiving organs from SARS-CoV-2 positive donors.
Conclusion
Use of non-lung (kidney, liver and heart) organs from SARS-CoV-2 positive donors seems to be a safe practice, with a low risk of transmission irrespective of the presence of symptoms at the time of procurement. Furthermore, these reports emphasise the importance of interpreting the Ct as a surrogate of replication, with low replication (high Ct) probably safe for non-lung donors, even if persistently symptomatic at procurement. This practice may reduce the transplant waiting list in hospitals.
Disclosure of interest
Oriol Manuel served as a consultant for MSD, Gilead and Takeda Advisory Boards. The other authors declare that they have no conflict of interest.
Author contributions
Raquel Martinez-Reviejo: Methodology, software, formal analysis, and writing original draft. Sofia Tejada: Methodology, validation, formal analysis, and writing original draft. Ana Sobral-Cipriano: Writing original draft, writing - review & editing. Hanife Nur Karakoc: Methodology, software. Oriol Manuel: Conceptualisation, writing - review & editing. Jordi Rello: Conceptualisation, writing - review & editing, supervision. All authors read and approved the final manuscript.
Funding source
This work was funded by CIBERES, Instituto de Salud Carlos III, Madrid, Spain (Fondos FEDER) (CB06-06-036).
Compliance with ethical standards
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.accpm.2022.101098.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Akalin E., Azzi Y., Bartash R., Seethamraju H., Parides M., Hemmige V., et al. Covid-19 and kidney transplantation. N Engl J Med. 2020;382:2475–2477. doi: 10.1056/NEJMc2011117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chandorkar A., Coro A., Natori Y., Anjan S., Abbo L.M., Guerra G., et al. Kidney transplantation during coronavirus 2019 pandemic at a large hospital in Miami. Transpl Infect Dis. 2020;22 doi: 10.1111/tid.13416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bordes S.J., Montorfano L., West-Ortiz W., Valera R., Cracco A., Alonso M., et al. Trends in US kidney transplantation during the COVID-19 pandemic. Cureus. 2020;12 doi: 10.7759/cureus.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Maira T., Berenguer M. COVID-19 and liver transplantation. Nat Rev Gastroenterol Hepatol. 2020;17:1–3. doi: 10.1038/s41575-020-0347-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Organ Procurement and Transplantation Network (OPTN) COVID-19. Organ procurement and transplantation network. 2022. https://optn.transplant.hrsa.gov/covid-19 (Accessed 21 March 2022)
- 6.American Society of Transplantation (AST) American Society of Transplantation; 2021. Recommendations and guidance for organ donor testing. https://www.myast.org/recommendations-and-guidance-organ-donor-testing (Accessed 28 January 2022)
- 7.Kute V., Guleria S., Bhalla A., Sharma A., Agarwal S., Sahay M., et al. ISOT consensus statement for the kidney transplant recipient and living donor with a previous diagnosis of COVID-19. Indian J Transplant. 2021;15:131. doi: 10.4103/ijot.ijot_26_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kates O.S., Fisher C.E., Rakita R.M., Reyes J.D., Limaye A.P. Use of SARS-CoV-2 infected deceased organ donors: should we always “just say no?”. Am J Transplant. 2020;20:1787–1794. doi: 10.1111/ajt.16000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Romagnoli R., Gruttadauria S., Tisone G., Ettorre G.M., Carlis L.D., Martini S., et al. Liver transplantation from active COVID-19 donors: a lifesaving opportunity worth grasping? Am J Transplant. 2021;21 doi: 10.1111/ajt.16823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Puodziukaite L., Serpytis M., Kundrotaite A., Sipylaite J., Miglinas M., Janusaite M.M., et al. Kidney transplantation from a SARS-CoV-2 positive donor for the recipients with immunity after COVID-19. Transpl Infect Dis. 2021;23 doi: 10.1111/tid.13666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manzia T.M., Gazia C., Lenci I., Angelico R., Toti L., Monaco A., et al. Liver transplantation performed in a SARS-CoV-2 positive hospitalized recipient using a SARS-CoV-2 infected donor. Am J Transplant. 2021;21:2600–2604. doi: 10.1111/ajt.16548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa Hospital Research Institute, n.d. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (Accessed 28 February 2022).
- 14.Murad M.H., Sultan S., Haffar S., Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med. 2018;23:60–63. doi: 10.1136/bmjebm-2017-110853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pierson D.J. How to read a case report (or teaching case of the month) Respir Care. 2009;54:1372–1378. [PubMed] [Google Scholar]
- 16.Hill A.B. The environment and disease: association or causation? Proc R Soc Med. 1965;58:295–300. doi: 10.1177/0141076814562718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nguyen M.C., Lee E.J., Avery R.K., Dioverti-Prono M.V., Shoham S., Tobian A.A.R., et al. Transplant of SARS-CoV-2–infected living donor liver: case report. Transplant Direct. 2021;7 doi: 10.1097/TXD.0000000000001178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tuncer A., Akbulut S., Baskiran A., Karakas E.E., Baskiran D.Y., Carr B., et al. A recipient and donor both have COVID-19 disease. Should we perform a liver transplant? J Gastrointest Cancer. 2021;52:1143–1147. doi: 10.1007/s12029-021-00590-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hong H., Kim S., Choi D.L., Kwon H.H. A case of coronavirus disease 2019–infected liver transplant donor. Am J Transplant. 2020;20:2938–2941. doi: 10.1111/ajt.15997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.La Hoz R.M., Mufti A.R., Vagefi P.A. Short-term liver transplant outcomes from SARS-CoV-2 lower respiratory tract NAT positive donors. Transpl Infect Dis. 2022;24 doi: 10.1111/tid.13757. [DOI] [PubMed] [Google Scholar]
- 21.Kucuk E.V., Sit D., Kayabasi H., Tahra A., Sobay R., Yilmaz S.N.G., et al. The first kidney transplantation after recipient and living donor recovered from COVID-19. North Clin Istanb. 2021;8:187–189. doi: 10.14744/nci.2021.70457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kute V.B., Godara S., Guleria S., Ray D.S., Aziz F., Hegde U., et al. Is it safe to Be transplanted from living donors who recovered from COVID-19? Experience of 31 kidney transplants in a multicenter cohort study from India. Transplantation. 2020;105:842–850. doi: 10.1097/TP.0000000000003609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koval C.E., Poggio E.D., Lin Y., Kerr H., Eltemamy M., Wee A. Early success transplanting kidneys from donors with new SARS-CoV-2 RNA positivity: a report of 10 cases. Am J Transplant. 2021;21 doi: 10.1111/ajt.16765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perlin D.V., Dymkov I.N., Terentiev A.V., Perlina A.V. Is kidney transplantation from a COVID-19–Positive deceased donor safe for the recipient? Transplant Proc. 2021;53:1138–1142. doi: 10.1016/j.transproceed.2021.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Querrey M., Kurihara C., Manerikar A., Garza-Castillon R., Lysne J., Tomic R., et al. Lung donation following SARS-CoV-2 infection. Am J Transplant. 2021;21:4073–4078. doi: 10.1111/ajt.16777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaul D.R., Valesano A.L., Petrie J.G., Sagana R., Lyu D., Lin J., et al. Donor to recipient transmission of SARS-CoV-2 by lung transplantation despite negative donor upper respiratory tract testing. Am J Transplant. 2021;21:2885–2889. doi: 10.1111/ajt.16532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ceulemans L.J., Van Slambrouck J., De Leyn P., Decaluwé H., Van Veer H., Depypere L., et al. Successful double-lung transplantation from a donor previously infected with SARS-CoV-2. Lancet Respir Med. 2021;9:315–318. doi: 10.1016/S2213-2600(20)30524-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neidlinger N.A., Smith J.A., D’Alessandro A.M., Roe D., Taber T.E., Pereira M.R., et al. Organ recovery from deceased donors with prior COVID-19: a case series. Transpl Infect Dis. 2021;23 doi: 10.1111/tid.13503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar D., Humar A., Keshavjee S., Cypel M. A call to routinely test lower respiratory tract samples for SARS-CoV-2 in lung donors. Am J Transplant. 2021;21:2623–2624. doi: 10.1111/ajt.16576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eichenberger E.M., Kaul D.R., Wolfe C.R. The pandemic provides a pathway: what we know and what we need to know about using COVID positive donors. Transpl Infect Dis. 2021;23 doi: 10.1111/tid.13727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gaussen A., Hornby L., Rockl G., O’Brien S., Delage G., Sapir-Pichhadze R., et al. Evidence of SARS-CoV-2 infection in cells, tissues, and organs and the risk of transmission through transplantation. Transplantation. 2021;105:1405–1422. doi: 10.1097/TP.0000000000003744. [DOI] [PubMed] [Google Scholar]
- 32.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leblanc J.-F., Germain M., Delage G., OʼBrien S., Drews S.J., Lewin A. Risk of transmission of severe acute respiratory syndrome coronavirus 2 by transfusion: a literature review. Transfusion. 2020;60:3046–3054. doi: 10.1111/trf.16056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singanayagam A., Patel M., Charlett A., Bernal J.L., Saliba V., Ellis J., et al. Duration of infectiousness and correlation with RT-PCR cycle threshold values in cases of COVID-19, England, January to May 2020. Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.32.2001483. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.