Abstract
Background
Effective surveillance of antimicrobial resistance (AMR) in Neisseria gonorrhoeae is required for the early detection of resistant strains and to ensure that treatment guidelines are appropriate for the setting in which they are implemented. AMR in N. gonorrhoeae has been identified as a global health threat.
Aim
We performed a systematic review to identify and describe surveillance systems targeting AMR in N. gonorrhoeae.
Methods
We searched Medline, PubMed, Global Health, EMBASE, CINAHL, Web of Science and ProQuest databases and grey literature between 1 January 2012 and 27 September 2020. Surveillance systems were defined as the continuous, systematic collection, analysis and interpretation of N. gonorrhoeae resistance data. The key components of surveillance systems were extracted, categorised, described and summarised.
Results
We found 40 publications reporting on N. gonorrhoeae AMR surveillance systems in 27 countries and 10 multi-country or global surveillance reports. The proportion of countries with surveillance systems in each of the WHO's six regions ranged from one of 22 countries in the Eastern Mediterranean and five of 54 in Africa, to three of 11 countries in South East Asia. Only four countries report systems which are both comprehensive and national. We found no evidence of a current surveillance system in at least 148 countries. Coverage, representativeness, volume, clinical specimen source, type and epidemiological information vary substantially and limit interpretability and comparability of surveillance data for public health action.
Conclusion
Globally, surveillance for N. gonorrhoeae AMR is inadequate and leaves large populations vulnerable to a major public health threat.
Keywords: gonorrhoea, resistance, antimicrobial, surveillance, Neisseria, antibiotic, infection
Introduction
Neisseria gonorrhoeae is one of the most common curable sexually transmitted infections (STI) with an estimated 78 million new gonorrhoea cases worldwide each year [1]. Since the dawn of the antibiotic era, strains of N. gonorrhoeae resistant to guideline-recommended antibiotics have emerged, beginning with penicillin and moving through tetracyclines, spectinomycin, fluoroquinolones and, more recently, treatment failures with macrolides and oral third-generation extended-spectrum cephalosporins [2,3]. Most guidelines now recommend injectable ceftriaxone, sometimes in combination with oral azithromycin [4,5]. However, ceftriaxone-resistant strains have been documented [6-9] and in 2018, the first cases of gonorrhoea with resistance to both ceftriaxone and azithromycin were reported [8,10].
Both gonorrhoea treatment and population exposure to antibiotics select for N. gonorrhoeae resistance [11]. Only surveillance can ensure that clinical guidelines match actual patterns of N. gonorrhoeae antimicrobial resistance (AMR) [12-14]. However, surveillance faces a number of implementation challenges because it relies on bacterial culture of specimens from people with infection. In most low- and middle-income country settings, bacterial culture and nucleic acid amplification testing (NAAT) to identify infection are not available. For this reason, clinicians treat only symptomatic patients based on identification of easily recognised symptoms and signs and without testing, an approach known as syndromic management [15]. This approach limits surveillance because asymptomatic infection cannot be identified [16]. Clinically, it also results in overconsumption of antibiotics because many of these presentations may be due to another pathogen. In high-income countries, culture-based diagnosis has been largely replaced by NAAT testing, which offers higher sensitivity and facilitates asymptomatic testing, but also reduces the availability of culture isolates for resistance testing and surveillance [17].
Tracking N. gonorrhoeae AMR globally is important because there is considerable geographic variation in the epidemiology of AMR, and AMR which arises in one country can become established in another through travel [18]. The World Health Organisation (WHO) Gonococcal Antimicrobial Surveillance Programme (GASP) plays a critical role in global surveillance and informing international collaboration [19,20]. Although there have been many reports of N. gonorrhoeae AMR methods and findings, there does not appear to have been a comprehensive report on the various approaches used for surveillance around the world. With increasing concern about the emergence of resistance, we performed a systematic review of existing surveillance systems for N. gonorrhoeae AMR to describe their characteristics and identify gaps to be addressed at national, regional and global levels.
Methods
Literature sources and search strategy
We performed a search of published literature from 1 January 2012 to 27 September 2020 using seven electronic databases (Medline, PubMed, Global Health, EMBASE, CINAHL, Web of Science and ProQuest) with search terms and variations of: gonorrhoea, Neisseria gonorrhoea, resistance, surveillance, sentinel, syndromic and programme. We searched records in all languages, including those other than English. The restriction on records before 2012 was to focus the review on currently active surveillance. Reference lists were hand-searched for additional records. Non peer-reviewed records were identified by searching Google and the websites of the Australian Department of Health, European Centre for Disease Prevention and Control, National Institute for Communicable Diseases (South Africa), New Zealand Ministry of Health, Public Health Agency of Canada, Public Health England (now known as United Kingdom (UK) Health Security Agency), United States Centers for Disease Control and Prevention (CDC), and the World Health Organization (WHO) using the same search string. These websites were also used to provide additional information on gonorrhoea cases by country. No restriction was made on language of publication.
Definition of a surveillance system
We used the WHO definition of a surveillance system as ’the continuous, systematic collection, analysis and interpretation of health-related data needed for the planning, implementation, and evaluation of public health practice’ [21]. We interpreted the ‘continuous, systematic’ aspect broadly, to include ongoing data collection repeated at regular intervals but not including one-off publication of data.
Eligibility criteria
Inclusion criteria were: (i) the publication had sufficient detail about methods to determine that the system described met the definition of surveillance system, (ii) detection of AMR in N. gonorrhoeae was reported as the outcome of interest and (iii) the findings were published on or after 1 January 2012. Exclusion criteria were: (i) one-off studies without an ongoing data collection component described in the methods or presented in the results and (ii) studies concerned with specific clinical or microbiological features of N. gonorrhoeae AMR, rather than surveillance. Where more than one article comprehensively described a particular surveillance system, only the most recent was included. Where two or more articles described non-overlapping components of a surveillance system, both were included. Although surveillance systems, as defined above, were the focus of the review, we also included reports from WHO GASP (referred to as GASP reports) for reference and comparison.
Study selection
Articles retrieved from database searches were imported into EndNote and duplicates removed. Screening was performed by applying exclusion criteria to the title and full abstracts. Full-text articles were then downloaded for eligibility assessment. Screening was performed in parallel and independently by NM and PG, or NM or YZ, with disagreements discussed and resolved by consensus.
Data extraction and analysis
We developed, piloted and applied a standardised extraction tool based on WHO recommendations for necessary components of national antimicrobial resistance surveillance systems [22]. These components included the type of surveillance and the elements of the surveillance system such as: antimicrobial agents chosen for susceptibility testing, testing method, number of samples, timeframe, population, location and type of collection site (See Supplementary Table S1: Antimicrobial resistance surveillance system core components checklist). Extracted data were stored in an Excel (Microsoft Corp.) database.
Surveillance systems were categorised as comprehensive if they include all types of clinical sites and laboratories or sentinel if they include specific selected clinical or laboratory sites based on specified criteria. Surveillance systems were categorised as national if they covered the entire country or greater than 50% of states or provinces, or subnational with less than 50% of states or provinces.
Some records described multi-country programmes but did not contain enough detail on individual country systems to meet eligibility criteria, most notably the GASP reports. These were included for reference but not in the descriptive analysis.
The findings of this review are reported in accordance with the PRISMA Statement [23].
Results
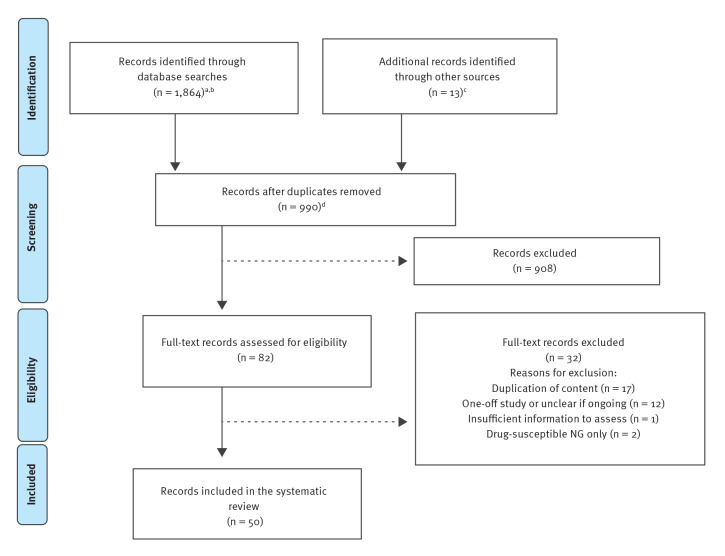
A total of 1,864 records were identified through database searches and 13 through a grey literature search. Screening after removal of 990 duplicates identified 82 records for full-text assessment, of which 50 were eligible for inclusion (Figure 1). Forty records described 32 country-level N. gonorrhoeae AMR surveillance systems in 27 countries, while 10 records described four regional WHO GASP reports and two global WHO GASP reports (Table 1).
Figure 1.
PRISMA flowchart for search strategy and literature review of surveillance systems, worldwide, to monitor antimicrobial resistance in Neisseria gonorrhoeae, 1 January 2012–27 September 2020
NG: Neisseria gonorrhoea.
a Medline, PubMed, Global Health, EMBASE, CINAHL, Web of Science, ProQuest
b Search keywords were ‘gonorrhea’ OR ‘gonorrhoea’ OR ‘Neisseria gonorrhoeae’ AND ‘drug resistance’ OR ‘drug resistance, microbial’ OR ‘drug resistance, bacterial’ OR ‘multidrug resistance’ OR ‘antibiotic resistance’ AND ‘population surveillance’ OR ‘surveillance’ OR ‘sentinel surveillance’ OR ‘syndromic surveillance’ OR ‘drug surveillance programme’.
c Reference lists and grey literature.
d No restriction was made on language of publication. All records identified were screened using an English language title and abstract, which was available in all cases. All records assessed for eligibility were in English.
Table 1. Characteristics of Neisseria gonorrhoeae antimicrobial resistance surveillance systems, worldwide, 1 January 2012−27 September 2020 (n = 32).
| Country | Included report(s) | Surveillance system namea | Est. | System typeb | Coveragec | Data sourcesd | Number of isolates | Timeframe |
|---|---|---|---|---|---|---|---|---|
| WHO African Region | ||||||||
| Ghana | [24] | (United States) Armed Forces Health Surveillance Centre (AFHSC) Network | NA | Other | Subnational | Military clinics, hospitals | 13 | 2012–13 |
| Kenya | [25] | NA | NA | Other | Subnational | Sex worker outreach | 238 | 2012–15 |
| South Africa | [26,27] | NA | 2005 | Sentinel | Subnational | Primary care | 4,224e | 2008–15 |
| Côte d'Ivoire | [29] | NA | NA | Sentinel | National | Sexual health | 212 | 2014–17 |
| Zimbabwe | [28] | NA | NA | Sentinel | Subnational | Primary care | 102 | 2015–16 |
| WHO Region of the Americas | ||||||||
| Argentina | [30] | Gonococcal antimicrobial surveillance system | 1983 | Other | National | Hospitals | 1,987 | 2009–13 |
| Brazil | [31] | NA | NA | Sentinel | National | Sexual health, hospitals | 550 | 2015–16 |
| Canada | [32,33,39] | National Surveillance of Antimicrobial Susceptibilities of N. gonorrhoeae | 1985 | Comprehensive | National | NA | 4,538 | 2016 |
| United States | [34-37] | Gonococcal Isolate Surveillance Project (GISP) | 1986 | Sentinel | National | Sexual health | 5,160 | 2018 |
| [37,38] | Enhanced Gonococcal Isolate Surveillance Project (eGISP) | 2017 | Sentinel | Subnational | Sexual health | 16,842f | 2017–18 | |
| [37] | Strengthening the US. Response to Resistant Gonorrhoea (SURRG) | 2016 | Sentinel | Subnational | Sexual health | |||
| WHO Eastern Mediterranean Region | ||||||||
| Morocco | [40] | NA | 1998 | Sentinel | National | Primary care | 72 | 2009 |
| WHO European Region | ||||||||
| Austria | [41] | NA | NA | Other | National | Sexual health | 3,584 | 2010–14 |
| Belarus | [42] | NA | 2009 | Sentinel | Subnational | Sexual health | 193 | 2010–13 |
| United Kingdom | [43,44] | Gonococcal Resistance to Antimicrobials Surveillance Programme (England and Wales) | 2000 | Sentinel | Subnational | Sexual health | 1,284 | 2016 |
| [45,46] | Second Generation Surveillance System (England and Wales) | NA | Comprehensive | National | NA | 17,099 | 2016 | |
| Scotland | [47,54] | Gonococcal Antibiotic Surveillance in Scotland (GASS) | NA | Comprehensive | National | Primary care, Sexual health | 3,168 | 2018 |
| France | [48] | Rénago - National Gonorrhoea Network | NA | Sentinel | National | NA | 8,649 | 2001–12 |
| Germany | [49] | Gonococcal Resistance Network (GORENET) | 2014 | Sentinel | National | NA | 1,654 | 2014–15 |
| Italy | [50] | NA | NA | NA | National | Sexual health | 1,688 | 2009–16 |
| Netherlands | [51] | NA | 2007 | Other | Subnational | Sexual health | 11,678 | 2007–15 |
| Russia | [52] | Russian Gonococcal Antimicrobial Surveillance Programme (RU-GASP) | 2004 | Other | National | Sexual health | 5,038 | 2005–16 |
| Switzerland | [53] | NA | NA | Other | Subnational | NA | 318 | 1990, 2000–12 |
| WHO South-East Asia Region | ||||||||
| India | [55] | NA | NA | Other | Subnational | Sexual health | 124 | 2013–16 |
| Nepal | [56] | NA | 1998 | Other | National | Not specified | 181 | 1999–2012 |
| Thailand | [57] | Enhanced Gonococcal Antimicrobial Surveillance Programme (E-GASP) | 2015 | Sentinel | Subnational | Sexual health | 590 | 2015–16 |
| WHO Western Pacific Region | ||||||||
| Australia | [64] | Australian Gonococcal Surveillance Programme | 1979 | Comprehensive | National | All sites | 9,668 | 2019 |
| China | [58] | China Gonococcal Resistance Surveillance Programme (Mainland China) | 1987 | Sentinel | Subnational | Sexual health | 3,849g | 2013–16 |
| [59] | Hong Kong SAR | NA | Other | National | Sexual health | 947 | 2010 | |
| Japan | [60] | NA | NA | Other | National | Medical institutions | 2,471 | 2000–15 |
| Korea | [61] | NA | NA | Other | National | All sites | 210 | 2011–13 |
| New Zealand | [62,63] | NA | NA | Comprehensive | National | NA | 667 | 2014–15 |
| Multi-country Gonococcal Antimicrobial Surveillance Programmes | ||||||||
| Europe | [65-68,70] | Euro-GASP | 2009 | Sentinel | Supp.h | Supp.h | NA | 2010–18 |
| Latin America and the Caribbean | [71] | LAC-GASP | 1990 | Other | Supp.h | Supp.h | NA | 1990–2011 |
| South-East Asia | [72] | SEAR-GASP | 1997 | Other | Supp.h | Supp.h | NA | 2009–12, 2016 |
| Western Pacific | [73] | WPR-GASP | 1992 | Other | Supp.h | Supp.h | NA | 2016 |
| Global GASP | [88,89] | NA | 1990 | Mixed | Supp.h | Supp.h | NA | 2009–14, 2016 |
Est.: year established; GASP: gonococcal antimicrobial surveillance programmes; NA: not available in cited publication(s); SAR: special administrative region; Supp.: supplementary materials.
a The names of surveillance systems were listed if available.
b System type: comprehensive: includes all healthcare providers and laboratories; sentinel: selected sites chosen on specified criteria; other: selected sites by criteria not specified.
c Coverage: national: country-wide of greater than 50% of all states or provinces; subnational: less than 50% of states and provinces.
d Data sources: primary care including general practice, community health centres and clinics, sexual health services including clinics and networks for diagnosis and treatment of sexually transmissible infections, genitourinary medicine departments, dermato-venereology services.
e From two geographic areas only.
f Samples across Gonococcal Isolate Surveillance Project (GISP), Enhanced Gonococcal Isolate Surveillance Project (eGISP) and Strengthening the US. Response to Resistant Gonorrhoea (SURRG) [37].
g From seven (of nine) provinces only.
h See Supplementary materials for description of individual country components of multi-country surveillance reports.
Of 27 individual countries with included surveillance systems, five were in the WHO African Region (n = 54 countries) [24-29], four in WHO Region of the Americas (n = 41 countries) [30-39]; one in the WHO Eastern Mediterranean Region (n = 22 countries) [40], nine in the WHO European Region (n = 53 countries) [41-54], three in the WHO South-East Asia Region (n = 11 countries) [55-57], and five in the WHO Western Pacific Region (n = 27 countries) [58-64]. See Table 1 and Supplementary Table S2a and Table S2b, which provide an overview of surveillance systems.
WHO GASP and the European Centre for Disease Prevention and Control have published reports which include data from 27 countries in the European Union/European Economic Area [65-70]. Although these may be considered ongoing surveillance systems, the description of each individual country level system did not meet inclusion criteria. In addition, WHO GASP reports included 23 Latin American and Caribbean countries [71], six South-East Asian Countries [72] and 12 Western Pacific countries [73]. See Table 1 and Supplementary Table S2 for additional surveillance details.
Comprehensiveness and national coverage
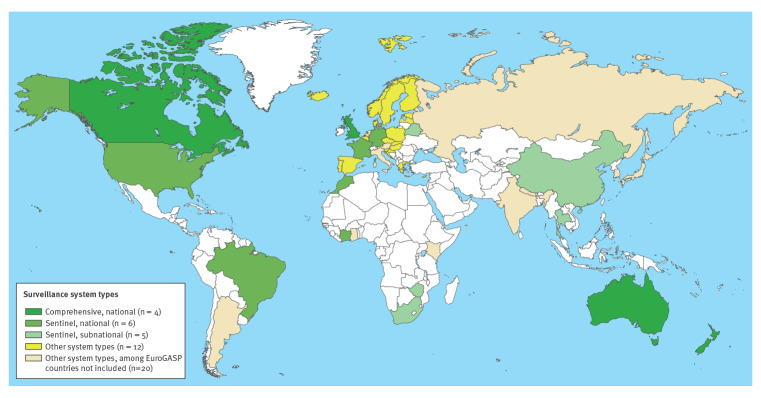
Globally, four countries had gonorrhoea AMR surveillance systems which were both comprehensive and national in that they aimed to cover all culture-evaluable gonorrhoea diagnoses and covered more than 50% of jurisdictions: Australia, Canada, New Zealand and the UK. Of the remaining 23 countries, coverage was national in 13 and subnational in 10, in that they reported data from less than 50% of states or provinces. Among those 23 countries, the selection criteria for sentinel sites were reported in 12 and not reported in the remaining 11. See Table 1 and Figure 2.
Figure 2.
Types of country-level Neisseria gonorrhoeae antimicrobial resistance surveillance systems, worldwide, 1 January 2012−27 September 2020 (n = 47 countries)
GASP: gonococcal antimicrobial surveillance programmes.
Of 27 countries in the Euro-GASP report [66], seven are included in this review (see Table 1) and marked here by system type. Although the remaining Euro-GASP 20 countries (beige) could be considered to have ongoing surveillance, the description of each individual country level system did not meet inclusion criteria for separate inclusion in this review.
Altogether, four countries have N. gonorrhoeae AMR surveillance systems which are national and comprehensive, six countries have national, sentinel surveillance systems, five countries have subnational sentinel surveillance systems, seven countries have systems which are national but neither comprehensive nor sentinel, five countries have other systems which are neither national nor comprehensive nor sentinel; at least 148 countries did not have evidence of systematic, ongoing N. gonorrhoeae AMR surveillance. See Table 1 and Figure 2.
Population, sampling methods and clinical site
Ten of the country-level systems reported data from symptomatic patients alone, including five from males only and one from female sex workers only. Nine country-level systems used protocols requiring antimicrobial susceptibility testing of all culture-positive cases presenting within a specified time frame; three used convenience sampling, and 19 did not specify the sampling method. Nine country-level systems specified that sample collection took place in STI clinics alone, while four also included other health services such as hospitals, 10 used other health services alone, and nine did not specify (Table 2 and Supplementary Table S2: country surveillance systems). Data on population, sampling and clinical site collection from GASP reports, where available, are also included in Supplementary Table S2.
Table 2. Attributes of country-level Neisseria gonorrhoeae antimicrobial resistance surveillance systems (aggregated), worldwide, 1 January 2012−27 September 2020 (n = 32 systems in 27 countries).
| Attributes | Country-level systems (n = 32) | |||
|---|---|---|---|---|
| System typea | Comprehensive (n = 5) | Sentinel (n = 14) | Other (n = 12) | NS (n = 1) |
| Geographical coverageb | National (n = 19) | Subnational (n = 12) | NS (n = 1) | |
| Populationc | Symptomatic (n = 10) | Laboratory (n = 14) | NS (n = 8) | |
| Sampling | Consecutive (n = 9) | Convenience (n = 3) | Mixed (n = 1) | NS (n = 19) |
| Where isolates are collected | STI clinics (n = 9) | Other services (n = 10) | Both (n = 4) | NS (n = 9) |
| Anatomical sitesd | Yes (n) | No (n) | NS (n) | |
| Male anogenital | 25 | 1 | 6 | |
| Male non-anogenital | 13 | 13 | 6 | |
| Female anogenital | 21 | 5 | 6 | |
| Female non-anogenital | 13 | 12 | 7 | |
| Patient characteristicsd,e | Yes (n) | No (n) | NS (n) | |
| Age | 21 | 3 | 8 | |
| Sex | 21 | 4 | 7 | |
| Site of infection | 11 | 11 | 10 | |
| Other demographic informationf | 8 | 15 | 9 | |
| Behavioural | 13 | 12 | 7 | |
| Clinical | 12 | 13 | 7 | |
NS: Not specified.
a System type: comprehensive: includes all healthcare providers and laboratories; sentinel: selected sites chosen on specified criteria; other: selected sites by criteria not specified.
b Coverage: national: country-wide of greater than 50% of all jurisdictions; subnational: less than 50% of states and provinces.
c Population: symptomatic: surveillance specimens collected from patients presenting with gonorrhoea infection syndromes; laboratory: specimens collected from laboratory confirmed diagnoses.
d For attributes of anatomical sites and patient characteristics, the n number specified under categories Yes or No refers to the number of surveillance systems of the total 32 that do or do not collect data on respective variables. NS indicates that the system does not specify collection of data.
e Patient characteristics: patient characteristics information could include sociodemographic characteristics (age, sex and other), site of infection, behavioural information (e.g. sexual history), and clinical information (e.g. co-infections).
f Other demographic information: other demographic information could include area of residence and sexual orientation.
Anatomical site of collection and patient characteristics
Male anogenital specimens were collected by 25 country-level systems, female anogenital specimens by 21 and 14 collected both. Collection of non-anogenital (pharyngeal) specimens was reported by 13 (Table 2 and Supplementary Table S3: anatomical site, reported cases and data collection variables, along with data from GASP reports, where available).
Most country-level systems reported data by age (n = 21) and sex (n = 21), while a third reported by site of infection (n = 11), other demographic information such as area of residence (n = 8), behavioural information such as sexual history (n = 13) and 12 reported clinical information such as co-infections. For six country-level systems, information was insufficient to ascertain what characteristics were collected (Table 2 and Supplementary Table S3). Data on patient characteristics from GASP reports, where available, are also included in Supplementary Table S3.
Numbers of isolates tested
The time period over which reported numbers of isolates were tested varied from less than 1 up to 12 years and the total number of isolates ranged from 13 in Ghana (over 2 years) [24] to 17,099 in England and Wales (over 1 year) [45,46] with a median of 1,654. Based on available data from 16 country-level systems, the proportion of all gonorrhoea diagnoses which resulted in an isolate included in surveillance ranged from 1% to 62% (median: 22), although there were differences in how these numbers were reported (Table 1 and Supplementary Table S3 anatomical site, reported cases and data collection, including data on numbers of isolates tested from GASP reports, where available).
Location of laboratories
Susceptibility testing was performed in single, central laboratories for 16 country-level systems and decentralised in 14 with two unspecified. Some European Region GASP participating countries (Euro-GASP) used laboratories in Sweden or the UK [65-68,70]. Within Euro-GASP, eight were centralised and 19 decentralised [70], while for the South East Asia Region GASP four were centralised and two decentralised [72] (Supplementary Table S4: Laboratory procedures used in included surveillance systems).
Antimicrobial susceptibility testing
Sixteen country-level systems used agar dilution, 18 used E-test, and three used both laboratory methods to determine minimum inhibitory concentrations. Five countries reported using disk diffusion. Fifteen country-level systems reported use of a beta lactamase test, while 10 mentioned the use of NG-MAST typing and two reported molecular testing for antimicrobial resistance testing (Supplementary Table S4: Laboratory procedures used in included surveillance systems including data on antimicrobial susceptibility testing methods from GASP reports, where available).
Seventeen country-level systems used Clinical and Laboratory Systems Institute (CLSI) criteria to interpret breakpoints and 10 used EUCAST guidelines, with six using other guidelines or not specifying the criteria used [74,75]. Virtually all country-level systems reported testing for susceptibility to ceftriaxone (n = 27 countries), ciprofloxacin (n = 26), azithromycin (n = 25), and penicillin (n = 22) with smaller proportions for tetracycline (n = 20), spectinomycin (n = 16), cefixime (n = 19) and other drugs such as cefpodoxime (n = 10) (Supplementary Table S4: laboratory procedures).
Discussion
This systematic review of N. gonorrhoeae AMR surveillance systems takes a perspective that is both global and methodological. We have found that in most WHO regions, information on adequate systems was only available from a few countries. We also found major gaps in coverage, comprehensiveness and representativeness as well as wide variation in the methodology. These gaps indicate serious vulnerability in countries' capacity to detect, accurately monitor and respond to N. gonorrhoeae AMR and represents a global health risk in a world connected through travel.
Most striking is the lack of surveillance in low- and middle-income countries with high burden of disease. For example, the WHO African and the Western Pacific Region have the highest gonorrhoea prevalence and incidence [1,76], but we were only able to retrieve information on systems in place for five of 54 WHO African Region countries (Côte d'Ivoire, Ghana, Kenya, South Africa and Zimbabwe) and five of the higher income countries in the WHO Western Pacific Region (Australia, China, Japan, Korea, New Zealand). For all remaining countries, including several with populations greater than 100 million inhabitants, such as Indonesia, Nigeria, Philippines and Vietnam, there were no publications of systematically collected respective data. Also, some countries with very large populations have surveillance systems which report on very small numbers of specimens, e.g. India with a population greater than 1 billion reported only 124 specimens included in surveillance over 4 years [55].
The paucity of surveillance represents a global threat and not just to countries without surveillance. For example, cases of extensively drug-resistant (XDR) gonorrhoea have only been detected in high income countries with surveillance systems and are predominantly associated with travel to countries with limited surveillance [6,10]. Globally, much or indeed most travel for tourism, employment, study and business is likely to occur between countries identified in this review with limited surveillance [77]. As a result, both local transmission and international dissemination of resistance may be entirely undetected.
Where surveillance is not national and comprehensive, many factors may reduce representativeness or the extent to which data collected are reflective of target populations. This has important consequences for whether resistance is detected by surveillance. Ten countries have systems which only sample symptomatic patients who are more likely to be heterosexual men, and less likely to be women who bear the greatest burden of disease and men who have sex with men who have the highest incidence [78]. Resistance patterns may differ by sex and behaviour where gonococcal clones circulating in networks of men who have sex with men are distinct from those found in heterosexual men and women [79]. Many countries only collect specimens from specialist services which may see more patients who been exposed to antibiotics than in primary care [80].
To fill the gaps, WHO has developed standardised gonococcal AMR surveillance protocols, implemented with critical support of GASP regional collaborating centres [76,81], but coverage remains low in many regions. The WHO GASP plays a critically important role in standardising, compiling and presenting data from countries and regions where data would otherwise be unavailable or inaccessible [19]. We searched publications from 2012 to focus our review on currently active surveillance and direct attention to future surveillance needs. It is important to note that many countries with good laboratory capacity were engaged in gonococcal AMR testing before this time. In particular, WHO GASP was established in 1990 and has been collecting and disseminating resistance data and providing standards for laboratory capacity from that time.
For many countries, comprehensive, national systems involving high quality epidemiological data collection and quality assured laboratory testing are unlikely to be affordable with current levels of resourcing, but remain the gold standard. Countries can nevertheless aim to identify epidemiologically relevant populations at higher risk of STI and therefore more likely to be exposed to antimicrobial resistant N. gonorrhoeae, e.g. sex workers, travellers, men who have sex with men, and ensure that they are appropriately represented and recognised in sampling. Surveillance should include both asymptomatic and symptomatic infection, by identifying people with infection through NAAT testing as well as clinical presentations. Sampling must also incorporate adequate coverage of regions to ensure that geographical diversity can be detected, particularly in regions with increased STI vulnerability. This can be achieved either with decentralised laboratory capacity (with local specimen processing) or specialised transport to a central laboratory.
Barriers to adequate surveillance include lack of funding, prioritisation within national health agendas, human resources, education and training, as well as limited clinical and laboratory infrastructure and the technical complexity of culture-based systems [82]. The current global approach to N. gonorrhoeae AMR surveillance is based on testing specimens for susceptibility using bacterial culture, which is the gold standard but faces major limitations [83]. Firstly, identifying cases for sampling is difficult because most infections in women and men who have sex with men are asymptomatic and much of the world lacks access to the nucleic acid amplifications tests (NAAT) required for screening asymptomatic patients. Secondly, the fastidious nature of N. gonorrhoeae, and its vulnerability to degradation during transport makes culture technically challenging and, in much of the world, restricts surveillance to sites proximal to national reference laboratories [84].
Molecular resistance testing to supplement conventional culture-based methods is a promising approach as specimens can be stored inexpensively and transported and processed centrally [85]. For example, molecular assays in remote parts of the Northern Territory of Australia have produced comparable estimates of resistance compared to culture-based methods [86]. Research into cost-effectiveness of molecular testing in both low- and high-income countries and in different types of clinical settings is required. Because molecular testing can only detect known genetic markers of resistance, it needs to be supported by research to rapidly produce targets for newly identified or emergent resistant strains. Global expansion of molecular testing for Mycobacterium tuberculosis and more recently severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) presents opportunities to address this [87].
Aetiological testing of asymptomatic patients via NAAT testing could be made available in many settings where it is currently not being used by making use of diagnostic systems used for other diseases, such as the GeneXpert platform widely accessible for tuberculosis. Incorporation of NAAT testing has the potential to increase the scope of populations and locations where surveillance can occur. However, even with NAAT testing, the requirement for local gonococcal culture capacity is a substantial obstacle in most regions within the majority of countries, and will require ongoing technical and resource support to implement. Research to determine if other forms of AMR testing, in particular molecular testing, can augment and extend the reach of surveillance is urgently required.
This review has limitations which should be considered in interpreting its findings. Firstly, many countries contributing data which are published in WHO GASP reports may have country level surveillance systems which were not separately published or described and hence not accessible to this review [21]. Secondly, some countries may have published data from surveillance systems but the methods were not sufficiently detailed to meet all inclusion criteria or to determine that they were not one-off studies. As a result, we may have underestimated coverage of surveillance systems in some regions. Thirdly, data from country-level surveillance systems may have been published before 2012 and then not between 2012 and 2020. As a result, systems which are active but have not recently published may not have been included and we may have underestimated coverage of surveillance systems in some regions. However, it should be noted that regular dissemination of data, though not necessarily by publication, is a criterion of a surveillance system.
Conclusions
Our review underscores that, globally, surveillance of gonorrhoea AMR is inadequate. Too few countries have surveillance systems and too few systems are adequate. Without adequate surveillance, countries lack basic information on which to base guidelines to limit treatment failure in the population. Without adequate surveillance, countries’ capacity to detect or respond to resistance when it occurs is absent or limited. However, the capacity-building requirement in many low- and middle-income countries is not to be understated. Simplified and streamlined systems are much more likely to be successfully implemented.
Moving forward, surveillance systems worldwide should be strengthened to effectively monitor and address AMR in N. gonorrhoeae. All countries, will bear the burden of the failure to prevent, detect and respond to N. gonorrhoeae AMR, irrespective of where it arises. Efforts to strengthen surveillance systems should be integrated with antibiotic stewardship initiatives and increasing access to diagnostic technology where it is not currently available.
Statements
Ethical statement: No ethical approval was sought for this systematic review as all data were taken from publicly accessible documents.
Funding statement: This project received funding from the Australian Research Council Industrial Transformation Research Hub to Combat Antimicrobial Resistance (IH190100021).
Supplementary Data
Conflict of interest: None declared.
Authors’ contributions: NM: performed the search, verified the search, collected the data, edited and wrote the manuscript and edited all versions of the manuscript. YZ, PG: performed the search, verified the search, collected the data, wrote earlier versions of the manuscript and edited the manuscript. DL, BD, DW: interpreted the data, contributed to and edited the manuscript. RG, JK: Supervised NM, YZ, PG, led the study, interpreted the data, contributed to and edited the manuscript.
References
- 1. Newman L, Rowley J, Vander Hoorn S, Wijesooriya NS, Unemo M, Low N, et al. Global estimates of the prevalence and incidence of four curable sexually transmitted infections in 2012 based on systematic review and global reporting. PLoS One. 2015;10(12):e0143304. 10.1371/journal.pone.0143304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yu RX, Yin Y, Wang GQ, Chen SC, Zheng BJ, Dai XQ, et al. Worldwide susceptibility rates of Neisseria gonorrhoeae isolates to cefixime and cefpodoxime: a systematic review and meta-analysis. PLoS One. 2014;9(1):e87849. 10.1371/journal.pone.0087849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lewis DA, Lukehart SA. Antimicrobial resistance in Neisseria gonorrhoeae and Treponema pallidum: evolution, therapeutic challenges and the need to strengthen global surveillance. Sex Transm Infect. 2011;87(Suppl 2):ii39-43. 10.1136/sti.2010.047712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Australasian Sexual Health Alliance (ASHA). Australian STI Management Guidelines: Gonorrhoea. Sydney: ASHA. [Accessed: 13 Sep 2021]. Available from: http://www.sti.guidelines.org.au/sexually-transmissible-infections/gonorrhoea
- 5. Workowski KA, Bolan GA, Centers for Disease Control and Prevention . Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1-137. [PMC free article] [PubMed] [Google Scholar]
- 6. Lahra MM, Martin I, Demczuk W, Jennison AV, Lee KI, Nakayama SI, et al. Cooperative Recognition of Internationally Disseminated Ceftriaxone-Resistant Neisseria gonorrhoeae Strain. Emerg Infect Dis. 2018;24(4). 10.3201/eid2404.171873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Barbee LA. Preparing for an era of untreatable gonorrhea. Curr Opin Infect Dis. 2014;27(3):282-7. 10.1097/QCO.0000000000000058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jennison AV, Whiley D, Lahra MM, Graham RM, Cole MJ, Hughes G, et al. Genetic relatedness of ceftriaxone-resistant and high-level azithromycin resistant Neisseria gonorrhoeae cases, United Kingdom and Australia, February to April 2018. Euro Surveill. 2019;24(8):1900118. 10.2807/1560-7917.ES.2019.24.8.1900118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen SC, Han Y, Yuan LF, Zhu XY, Yin YP. Identification of internationally disseminated ceftriaxone-resistant Neisseria gonorrhoeae strain FC428, China. Emerg Infect Dis. 2019;25(7):1427-9. 10.3201/eid2507.190172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.European Centre for Disease Control and Prevention (ECDC). Rapid Risk Assessment: Extensively drug-resistant (XDR) Neisseria gonorrhoeae in the United Kingdom and Australia – 7 May 2018. Stockholm: ECDC; 2018. Available from: https://www.ecdc.europa.eu/en/publications-data/rapid-risk-assessment-extensively-drug-resistant-xdr-neisseria-gonorrhoeae-united
- 11. Kenyon C, Laumen J, Van Dijck C, De Baetselier I, Abdelatti S, Manoharan-Basil SS, et al. Gonorrhoea treatment combined with population-level general cephalosporin and quinolone consumption may select for Neisseria gonorrhoeae antimicrobial resistance at the levels of NG-MAST genogroup: An ecological study in Europe. J Glob Antimicrob Resist. 2020;23:377-84. 10.1016/j.jgar.2020.10.022 [DOI] [PubMed] [Google Scholar]
- 12. Lahra MM, Ward A, Trembizki E, Hermanson J, Clements E, Lawrence A, et al. Treatment guidelines after an outbreak of azithromycin-resistant Neisseria gonorrhoeae in South Australia. Lancet Infect Dis. 2017;17(2):133-4. 10.1016/S1473-3099(17)30007-5 [DOI] [PubMed] [Google Scholar]
- 13. Trembizki E, Guy R, Donovan B, Kaldor JM, Lahra MM, Whiley DM, GRAND study investigators . Further evidence to support the individualised treatment of gonorrhoea with ciprofloxacin. Lancet Infect Dis. 2016;16(9):1005-6. 10.1016/S1473-3099(16)30271-7 [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization (WHO). Global action plan to control the spread and impact of antimicrobial resistance in Neisseria gonorrohoea 2012. Geneva: WHO; 2012. Available from: https://apps.who.int/iris/handle/10665/44863
- 15. Wi TE, Ndowa FJ, Ferreyra C, Kelly-Cirino C, Taylor MM, Toskin I, et al. Diagnosing sexually transmitted infections in resource-constrained settings: challenges and ways forward. J Int AIDS Soc. 2019;22(S6) Suppl 6;e25343. 10.1002/jia2.25343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Unemo M, Shafer WM. Antimicrobial resistance in Neisseria gonorrhoeae in the 21st century: past, evolution, and future. Clin Microbiol Rev. 2014;27(3):587-613. 10.1128/CMR.00010-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mohammed H, Ison CA, Obi C, Chisholm S, Cole M, Quaye N, et al. Frequency and correlates of culture-positive infection with Neisseria gonorrhoeae in England: a review of sentinel surveillance data. Sex Transm Infect. 2015;91(4):287-93. 10.1136/sextrans-2014-051756 [DOI] [PubMed] [Google Scholar]
- 18. Unemo M, Golparian D, Eyre DW. Antimicrobial Resistance in Neisseria gonorrhoeae and Treatment of Gonorrhea. Methods Mol Biol. 2019;1997:37-58. 10.1007/978-1-4939-9496-0_3 [DOI] [PubMed] [Google Scholar]
- 19. Wi T, Lahra MM, Ndowa F, Bala M, Dillon JR, Ramon-Pardo P, et al. Antimicrobial resistance in Neisseria gonorrhoeae: Global surveillance and a call for international collaborative action. PLoS Med. 2017;14(7):e1002344. 10.1371/journal.pmed.1002344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Unemo M, Lahra MM, Cole M, Galarza P, Ndowa F, Martin I, et al. World Health Organization Global Gonococcal Antimicrobial Surveillance Program (WHO GASP): review of new data and evidence to inform international collaborative actions and research efforts. Sex Health. 2019;16(5):412-25. 10.1071/SH19023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organization (WHO). WHO guidelines on ethical issues in public health surveillance. Geneva: WHO; 2017. Available from: https://www.who.int/publications/i/item/who-guidelines-on-ethical-issues-in-public-health-surveillance
- 22.World Health Organization (WHO). National antimicrobial resistance surveillance systems and participation in the Global Antimicrobial Resistance Surveillance System (GLASS): core components checklist and questionnaire. Geneva: WHO; 2016. Available from: https://apps.who.int/iris/handle/10665/251552
- 23. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. 10.1371/journal.pmed.1000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Duplessis C, Puplampu N, Nyarko E, Carroll J, Dela H, Mensah A, et al. Gonorrhea surveillance in Ghana, Africa. Mil Med. 2015;180(1):17-22. 10.7205/MILMED-D-13-00418 [DOI] [PubMed] [Google Scholar]
- 25. Omolo MJ, Pole L, Mwangi I, Kimani J, Anzala O, Oloo J, et al. P2.30 Survey of antimicrobial resistance in clinical Neisseria gonorrhoeae isolated over a period of four years in nairobi - kenya. Sex Transm Infect. 2017;93:A81- A82. [Google Scholar]
- 26.Kularatne R, Maseko V, Gumede L, Radebe F, Kufa-Chakezha T. P3.186 Neisseria gonorrhoeae antimicrobial resistance surveillance in Johannesburg, South Africa. Sex Transm Infect. 2017;93(2):A162. 10.1136/sextrans-2017-053264.421 10.1136/sextrans-2017-053264.421 [DOI]
- 27. Kularatne RMV, Gumede L, Radebe F, Kufa-Chakezha T. Neisseria gonorrheae antimicrobial resistance surveillance in Gauteng Province, South Africa. Communicable Diseases Surveillance Bulletin. 2016;14(3):56-64. [Google Scholar]
- 28. Latif AS, Gwanzura L, Machiha A, Ndowa F, Tarupiwa A, Gudza-Mugabe M, et al. Antimicrobial susceptibility in Neisseria gonorrhoeae isolates from five sentinel surveillance sites in Zimbabwe, 2015-2016. Sex Transm Infect. 2018;94(1):62-6. 10.1136/sextrans-2016-053090 [DOI] [PubMed] [Google Scholar]
- 29. Yéo A, Kouamé-Blavo B, Kouamé CE, Ouattara A, Yao AC, Gbedé BD, et al. Establishment of a gonococcal antimicrobial surveillance programme, in accordance with World Health Organization standards, in Côte d’Ivoire, Western Africa, 2014-2017. Sex Transm Dis. 2019;46(3):179-84. 10.1097/OLQ.0000000000000943 [DOI] [PubMed] [Google Scholar]
- 30. Gianecini R, Romero MLM, Oviedo C, Vacchino M, Galarza P, Gonococcal Antimicrobial Susceptibility Surveillance Programme-Argentina (GASSP-AR) Working Group . Emergence and spread of Neisseria gonorrhoeae Isolates with decreased susceptibility to extended-spectrum cephalosporins in Argentina, 2009 to 2013. Sex Transm Dis. 2017;44(6):351-5. 10.1097/OLQ.0000000000000603 [DOI] [PubMed] [Google Scholar]
- 31. Bazzo ML, Golfetto L, Gaspar PC, Pires AF, Ramos MC, Franchini M, et al. First nationwide antimicrobial susceptibility surveillance for Neisseria gonorrhoeae in Brazil, 2015-16. J Antimicrob Chemother. 2018;73(7):1854-61. 10.1093/jac/dky090 [DOI] [PubMed] [Google Scholar]
- 32. Martin I, Sawatzky P, Liu G, Allen V, Lefebvre B, Hoang L, et al. Decline in decreased cephalosporin susceptibility and increase in azithromycin resistance in Neisseria gonorrhoeae, Canada. Emerg Infect Dis. 2016;22(1):65-7. 10.3201/eid2201.151247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Martin I, Sawatzky P, Allen V, Lefebvre B, Hoang L, Naidu P, et al. Multidrug-resistant and extensively drug-resistant Neisseria gonorrhoeae in Canada, 2012-2016. Can Commun Dis Rep. 2019;45(2-3):45-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kirkcaldy RD, Harvey A, Papp JR, Del Rio C, Soge OO, Holmes KK, et al. Neisseria gonorrhoeae antimicrobial susceptibility surveillance - The Gonococcal Isolate Surveillance Project, 27 sites, United States, 2014. MMWR Surveill Summ. 2016;65(7):1-19. 10.15585/mmwr.ss6507a1 [DOI] [PubMed] [Google Scholar]
- 35. Mann LMMPH, Kirkcaldy RDMD, Papp JRP, Torrone EAPM. Susceptibility of Neisseria gonorrhoeae to Gentamicin-Gonococcal Isolate Surveillance Project, 2015-2016. Sex Transm Dis. 2018;45(2):96-8. 10.1097/OLQ.0000000000000693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.United States Centers for Disease Control and Prevention (US CDC). Gonococcal Isolate Surveillance Program Protocol. Atlanta: CDC; 2016. Available from: https://stacks.cdc.gov/view/cdc/40081
- 37. Kersh EN, Pham CD, Papp JR, Myers R, Steece R, Kubin G, et al. Expanding U.S. Laboratory Capacity for Neisseria gonorrhoeae Antimicrobial Susceptibility Testing and Whole-Genome Sequencing through the CDC’s Antibiotic Resistance Laboratory Network. J Clin Microbiol. 2020;58(4):e01461-19. 10.1128/JCM.01461-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.United States Centers for Disease Control and Prevention (US CDC). Gonococcal Isolate Surveillance Project (GISP) and Enhanced GISP (eGISP). Atlanta: US CDC; 2020. Available from: https://www.cdc.gov/std/gisp/GISP_eGISP_Protocol_January_2020.pdf
- 39. Gratrix J, Kamruzzaman A, Martin I, Smyczek P, Read R, Bertholet L, et al. Surveillance for antimicrobial resistance in gonorrhea: The Alberta Model, 2012−2016. Antibiotics (Basel). 2018;7(3):E63. 10.3390/antibiotics7030063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hançali A, Ndowa F, Bellaji B, Bennani A, Kettani A, Charof R, et al. Antimicrobial resistance monitoring in Neisseria gonorrhoeae and strategic use of funds from the Global Fund to set up a systematic Moroccan gonococcal antimicrobial surveillance programme. Sex Transm Infect. 2013;89(Suppl 4):iv24-7. 10.1136/sextrans-2013-051166 [DOI] [PubMed] [Google Scholar]
- 41. Stary A, Heller-Vitouch C, Binder M, Geusau A, Stary G, Rappersberger K, et al. Gonococcal infections in Austria: a long-term observation of prevalence and resistance profiles from 1999 to 2014. J Dtsch Dermatol Ges. 2015;13(11):1136-45. 10.1111/ddg.12816 [DOI] [PubMed] [Google Scholar]
- 42. Lebedzeu F, Golparian D, Titov L, Pankratava N, Glazkova S, Shimanskaya I, et al. Antimicrobial susceptibility/resistance and NG-MAST characterisation of Neisseria gonorrhoeae in Belarus, Eastern Europe, 2010-2013. BMC Infect Dis. 2015;15(1):29. 10.1186/s12879-015-0755-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Clifton S, Bolt H, Mohammed H, Town K, Furegato M, Cole M, et al. Prevalence of and factors associated with MDR Neisseria gonorrhoeae in England and Wales between 2004 and 2015: analysis of annual cross-sectional surveillance surveys. J Antimicrob Chemother. 2018;73(4):923-32. 10.1093/jac/dkx520 [DOI] [PubMed] [Google Scholar]
- 44.United Kingdom Health Security Agency. Gonococcal resistance to antimicrobials surveillance programme report. Data on trends in antimicrobial resistance and decreased susceptibility in gonococcal infection in England and Wales are provided by GRASP. London: gov.uk; 2013. Available from: https://www.gov.uk/government/publications/gonococcal-resistance-to-antimicrobials-surveillance-programme-grasp-report
- 45.United Kingdom Health Security Agency. Antimicrobial resistance in Neisseria gonorrhoeae in England and Wales: Key findings from the Gonococcal Resistance to Antimicrobials Surveillance Programme (GRASP 2018). London; gov.uk; 2019. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1033882/GRASP_2020_Report.pdf
- 46.United Kingdom Health Security Agency. Surveillance of antimicrobial resistance in Neisseria gonorrhoeae Key findings from the Gonococcal Resistance to Antimicrobials Surveillance Programme (GRASP). London; gov.uk; 2016. Available from: https://www.gov.uk/government/publications/gonococcal-resistance-to-antimicrobials-surveillance-programme-grasp-protocol/gonococcal-resistance-to-antimicrobials-surveillance-programme-grasp-protocol
- 47.Scottish Bacterial Sexually Transmitted Infections Reference Laboratory (SBSTIRL). SBSTIRL User Manual 2015. Edinburgh: Health Protection Scotland and NHS Lothian 2015. Available from: https://www.uslegalforms.com/form-library/329054-scottish-bacterial-sexually-transmitted-infections-reference-laboratory-sbstirl-user-manual-2015
- 48. La Ruche G, Goubard A, Bercot B, Cambau E, Semaille C, Sednaoui P. Gonococcal infections and emergence of gonococcal decreased susceptibility to cephalosporins in France, 2001 to 2012. Euro Surveill. 2014;19(34):28. 10.2807/1560-7917.ES2014.19.34.20885 [DOI] [PubMed] [Google Scholar]
- 49. Buder S, Dudareva S, Jansen K, Loenenbach A, Nikisins S, Sailer A, et al. Antimicrobial resistance of Neisseria gonorrhoeae in Germany: low levels of cephalosporin resistance, but high azithromycin resistance. BMC Infect Dis. 2018;18(1):44. 10.1186/s12879-018-2944-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Stefanelli P, Vescio MF, Landini MP, Dal Conte I, Matteelli A, Cristaudo A, et al. Time trend analysis (2009-2016) of antimicrobial susceptibility in Neisseria gonorrhoeae isolated in Italy following the introduction of the combined antimicrobial therapy. PLoS One. 2017;12(12):e0189484. 10.1371/journal.pone.0189484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hofstraat SH, Götz HM, van Dam AP, van der Sande MA, van Benthem BH. Trends and determinants of antimicrobial susceptibility of Neisseria gonorrhoeae in the Netherlands, 2007 to 2015. Euro Surveill. 2018;23(36). 10.2807/1560-7917.ES.2018.23.36.1700565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kubanov A, Solomka V, Plakhova X, Chestkov A, Petrova N, Shaskolskiy B, et al. Summary and Trends of the Russian Gonococcal Antimicrobial Surveillance Programme, 2005 to 2016. J Clin Microbiol. 2019;57(6):e02024-18. 10.1128/JCM.02024-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kovari H, de Melo Oliveira MD, Hauser P, Läuchli S, Meyer J, Weber R, et al. Decreased susceptibility of Neisseria gonorrhoeae isolates from Switzerland to Cefixime and Ceftriaxone: antimicrobial susceptibility data from 1990 and 2000 to 2012. BMC Infect Dis. 2013;13(1):603. 10.1186/1471-2334-13-603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shepherd J, Wallace L, McHugh M, Cullen B, Cameron R, Goldberg D. Gonococcal antibiotic surveillance in Scotland (GASS): prevalence, patterns and trends in 2018. Edinburgh: Health Protection Scotland; 2019. Available from: https://hps.scot.nhs.uk/web-resources-container/gonococcal-antibiotic-surveillance-in-scotland-gass-prevalence-patterns-and-trends-in-2018 [Google Scholar]
- 55. Kulkarni SV, Bala M, Muqeeth SA, Sasikala G, Nirmalkar AP, Thorat R, et al. Antibiotic susceptibility pattern of Neisseria gonorrhoeae strains isolated from five cities in India during 2013-2016. J Med Microbiol. 2018;67(1):22-8. 10.1099/jmm.0.000662 [DOI] [PubMed] [Google Scholar]
- 56. Malla S, Dumre SP, Shakya G, Kansakar P, Rai B, Hossain A, et al. The challenges and successes of implementing a sustainable antimicrobial resistance surveillance programme in Nepal. BMC Public Health. 2014;14(269):269. 10.1186/1471-2458-14-269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sirivongrangson P, Girdthep N, Sukwicha W, Buasakul P, Tongtoyai J, Weston E, et al. The first year of the global Enhanced Gonococcal Antimicrobial Surveillance Programme (EGASP) in Bangkok, Thailand, 2015-2016. PLoS One. 2018;13(11):e0206419. 10.1371/journal.pone.0206419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yin YP, Han Y, Dai XQ, Zheng HP, Chen SC, Zhu BY, et al. Susceptibility of Neisseria gonorrhoeae to azithromycin and ceftriaxone in China: A retrospective study of national surveillance data from 2013 to 2016. PLoS Med. 2018;15(2):e1002499. 10.1371/journal.pmed.1002499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lo JY, Ho KM, Lo AC. Surveillance of gonococcal antimicrobial susceptibility resulting in early detection of emerging resistance. J Antimicrob Chemother. 2012;67(6):1422-6. 10.1093/jac/dks036 [DOI] [PubMed] [Google Scholar]
- 60. Yasuda M, Hatazaki K, Ito S, Kitanohara M, Yoh M, Kojima M, et al. Antimicrobial Susceptibility of Neisseria gonorrhoeae in Japan from 2000 to 2015. Sex Transm Dis. 2017;44(3):149-53. 10.1097/OLQ.0000000000000556 [DOI] [PubMed] [Google Scholar]
- 61. Lee H, Unemo M, Kim HJ, Seo Y, Lee K, Chong Y. Emergence of decreased susceptibility and resistance to extended-spectrum cephalosporins in Neisseria gonorrhoeae in Korea. J Antimicrob Chemother. 2015;70(9):2536-42. 10.1093/jac/dkv146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Institute for Environmental Science and Research Limited. Antimicrobial resistance and molecular epidemiology of Neisseria gonorrhoeae in New Zealand, 2014-15. Porirua: New Zealand Ministry of Health; 2015. Available from: https://surv.esr.cri.nz/PDF_surveillance/Antimicrobial/Gono/Ngonosurveyfinalreport2015.pdf
- 63.Institute for Environmental Science and Research Limited. Sexually Transmitted Infections in New Zealand: Annual Surveillance Report 2014. Porirua: New Zealand Ministry of Health; 2015. Available from: https://surv.esr.cri.nz/PDF_surveillance/STISurvRpt/2014/FINAL2014AnnualSTIReport.pdf
- 64. Lahra MM, Shoushtari M, George CRR, Armstrong BH, Hogan TR, National Neisseria Network, Australia . Australian Gonococcal Surveillance Programme Annual Report, 2019. Commun Dis Intell (2018). 2020;44:44. 10.33321/cdi.2020.44.58 [DOI] [PubMed] [Google Scholar]
- 65.European Centre for Disease Control and Prevention (ECDC). Response plan to control and manage the threat of multi-drug resistant gonorrhoea in Europe. Stockholm: ECDC; 2012. Available: https://www.ecdc.europa.eu/en/publications-data/response-plan-control-and-manage-threat-multidrug-resistant-gonorrhoea-europe
- 66.European Centre for Disease Control and Prevention (ECDC). Gonococcal antimicrobial susceptibility surveillance in Europe, 2015. Stockholm: ECDC; 2017. Available from: https://www.ecdc.europa.eu/en/publications-data/gonococcal-antimicrobial-susceptibility-surveillance-europe-2015
- 67. Cole MJ, Spiteri G, Jacobsson S, Woodford N, Tripodo F, Amato-Gauci AJ, et al. Overall Low Extended-Spectrum Cephalosporin Resistance but high Azithromycin Resistance in Neisseria gonorrhoeae in 24 European Countries, 2015. BMC Infect Dis. 2017;17(1):617. 10.1186/s12879-017-2707-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Spiteri G, Cole M, Unemo M, Hoffmann S, Ison C, van de Laar M. The European Gonococcal Antimicrobial Surveillance Programme (Euro-GASP)--a sentinel approach in the European Union (EU)/European Economic Area (EEA). Sex Transm Infect. 2013;89(Suppl 4):iv16-8. 10.1136/sextrans-2013-051117 [DOI] [PubMed] [Google Scholar]
- 69. Day MJ, Spiteri G, Jacobsson S, Woodford N, Amato-Gauci AJ, Cole MJ, et al. Stably high azithromycin resistance and decreasing ceftriaxone susceptibility in Neisseria gonorrhoeae in 25 European countries, 2016. BMC Infect Dis. 2018;18(1):609. 10.1186/s12879-018-3528-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.European Centre for Disease Prevention and Control (ECDC). Gonococcal antimicrobial susceptibility surveillance in Europe-Results summary 2018. Stockholm ECDC; 2020. Available from: https://www.ecdc.europa.eu/en/publications-data/gonococcal-antimicrobial-susceptibility-surveillance-europe-2018
- 71. Dillon J-AR, Trecker MA, Thakur SD, Gonococcal Antimicrobial Surveillance Program Network in Latin America and Caribbean 1990-2011 . Two decades of the gonococcal antimicrobial surveillance program in South America and the Caribbean: challenges and opportunities. Sex Transm Infect. 2013;89(Suppl 4):iv36-41. 10.1136/sextrans-2012-050905 [DOI] [PubMed] [Google Scholar]
- 72. Bala M, Kakran M, Singh V, Sood S, Ramesh V, Members of WHO GASP SEAR Network . Monitoring antimicrobial resistance in Neisseria gonorrhoeae in selected countries of the WHO South-East Asia Region between 2009 and 2012: a retrospective analysis. Sex Transm Infect. 2013;89(Suppl 4):iv28-35. 10.1136/sextrans-2012-050904 [DOI] [PubMed] [Google Scholar]
- 73. Lahra MM, Lo YR, Whiley DM. Gonococcal antimicrobial resistance in the Western Pacific Region. Sex Transm Infect. 2013;89(Suppl 4):iv19-23. 10.1136/sextrans-2012-050906 [DOI] [PubMed] [Google Scholar]
- 74. Leclercq R, Cantón R, Brown DF, Giske CG, Heisig P, MacGowan AP, et al. EUCAST expert rules in antimicrobial susceptibility testing. Clin Microbiol Infect. 2013;19(2):141-60. 10.1111/j.1469-0691.2011.03703.x [DOI] [PubMed] [Google Scholar]
- 75. Humphries R, Bobenchik AM, Hindler JA, Schuetz AN. Overview of changes to the clinical and laboratory standards institute performance standards for antimicrobial susceptibility testing, M100, 31st Edition. J Clin Microbiol. 2021;59(12):e0021321. 10.1128/JCM.00213-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Taylor MM, Korenromp E, Wi T. Pathways and progress to enhanced global sexually transmitted infection surveillance. PLoS Med. 2017;14(6):e1002328. 10.1371/journal.pmed.1002328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dwyer L. Trends underpinning global tourism in the coming decade. Global tourism: Routledge; 2012. p. 542-58. [Google Scholar]
- 78. Mohammed H, Ison CA, Obi C, Chisholm S, Cole M, Quaye N, et al. Frequency and correlates of culture-positive infection with Neisseria gonorrhoeae in England: a review of sentinel surveillance data. Sex Transm Infect. 2015;91(4):287-93. 10.1136/sextrans-2014-051756 [DOI] [PubMed] [Google Scholar]
- 79. Buckley C, Forde BM, Trembizki E, Lahra MM, Beatson SA, Whiley DM. Use of whole genome sequencing to investigate an increase in Neisseria gonorrhoeae infection among women in urban areas of Australia. Sci Rep. 2018;8(1):1503. 10.1038/s41598-018-20015-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Kirkcaldy RD, Schlanger K, Papp JR, Torrone EA. Considerations for Strengthening Surveillance of Neisseria gonorrhoeae Antimicrobial Resistance and Interpreting Surveillance Data. Sex Transm Dis. 2017;44(3):154-6. 10.1097/OLQ.0000000000000584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.World Health Organization (WHO). Gonococcal antimicrobial resistance in the Western Pacific Region [fact sheet]. Geneva: WHO; 2017. Available from: https://iris.wpro.who.int/handle/10665.1/13688
- 82. Wilson ML, Fleming KA, Kuti MA, Looi LM, Lago N, Ru K. Access to pathology and laboratory medicine services: a crucial gap. Lancet. 2018;391(10133):1927-38. 10.1016/S0140-6736(18)30458-6 [DOI] [PubMed] [Google Scholar]
- 83.World Health Organization (WHO). Manual for the laboratory identification and antimicrobial susceptibility testing of bacterial pathogens of public health importance in the developing world: Haemophilus influenzae, Neisseria meningitidis, Streptococcus pneumoniae, Neisseria gonorrhoea, Salmonella serotype Typhi, Shigella, and Vibrio cholerae. Geneva: WHO; 2003. Available from: https://apps.who.int/iris/handle/10665/68554
- 84. Unemo M, Seifert HS, Hook EW, 3rd, Hawkes S, Ndowa F, Dillon JR. Gonorrhoea. Nat Rev Dis Primers. 2019;5(1):79. 10.1038/s41572-019-0128-6 [DOI] [PubMed] [Google Scholar]
- 85. Goire N, Lahra MM, Chen M, Donovan B, Fairley CK, Guy R, et al. Molecular approaches to enhance surveillance of gonococcal antimicrobial resistance. Nat Rev Microbiol. 2014;12(3):223-9. 10.1038/nrmicro3217 [DOI] [PubMed] [Google Scholar]
- 86. Whiley DM, Trembizki E, Buckley C, Freeman K, Baird RW, Beaman M, et al. Molecular Antimicrobial Resistance Surveillance for Neisseria gonorrhoeae, Northern Territory, Australia. Emerg Infect Dis. 2017;23(9):1478-85. 10.3201/eid2309.170427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Scott C, Walusimbi S, Kirenga B, Joloba M, Winters M, Abdunoor N, et al. Evaluation of Automated Molecular Testing Rollout for Tuberculosis Diagnosis Using Routinely Collected Surveillance Data - Uganda, 2012-2015. MMWR Morb Mortal Wkly Rep. 2017;66(12):339-42. 10.15585/mmwr.mm6612a6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Wi T, Lahra MM, Ndowa F, Bala M, Dillon JR, Ramon-Pardo P, et al. Antimicrobial resistance in Neisseria gonorrhoeae: Global surveillance and a call for international collaborative action. PLoS Med. 2017;14(7):e1002344. 10.1371/journal.pmed.1002344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.World Health Organisation (WHO). Antimicrobial Resistance Global Surveillance Report. Geneva: WHO; 2014. Available from: https://apps.who.int/iris/handle/10665/112642
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.