Abstract
Extracellular cysteine proteinases, referred to as gingipains, are considered important virulence factors for Porphyromonas gingivalis, a bacterium recognized as a major etiologic agent of chronic periodontitis. We investigated the effect of tetracycline and its analogues, doxycycline and minocycline, on the enzymatic activities of gingipains. Tetracyclines at 100 μM totally inhibited the amidolytic activity of arginine-specific gingipains (HRgpA and RgpB). In contrast, inhibition of Kgp was less efficient and required a somewhat higher concentration of the antibiotic to achieve the same effect. Among tetracycline derivatives, the most potent gingipain inhibitor was doxycycline, followed by tetracycline and minocycline. RgpB was inhibited by doxycycline in an uncompetitive and reversible manner with a 50% inhibitory concentration of 3 μM. Significantly, inhibition was unaffected by calcium, excluding the chelating activity of tetracyclines as the mechanism of gingipain inactivation. In contrast, the inhibitory activities of the tetracyclines were reduced by cysteine, a reducing agent, suggesting an interference of the drug at the oxidative region with the catalytic system of the enzyme. Doxycycline, at 10 μM, significantly inhibited the RgpB-mediated production of vascular permeability-enhancing activity from human plasma, thus proving an effective inhibition of gingipain in vivo. These results indicate a new activity of tetracyclines as cysteine proteinase inhibitors and may explain the therapeutic efficiency of these antibiotics in the treatment of periodontitis.
Tetracycline and its analogues minocycline and doxycycline, as protein synthesis inhibitors in prokaryotes, are important antibiotic agents against a broad spectrum of bacteria. Based on their effectiveness in suppressing gram-negative anaerobic periodontopathogenic microorganisms in the subgingival plaque (35), tetracyclines have been used in dentistry as adjuncts to periodontal therapy. From the 1980s, tetracyclines have been found to exert biological effects independent of the antimicrobial activity (15). Such effects include inhibition of matrix metalloproteases (MMPs) (18), nitric oxide synthases (1), and prostaglandin E2 production (40). In particular, the inhibitory effect of tetracyclines on the activity of MMPs (18), which are thought to be involved in the pathological degradation of the periodontal connective tissue (4), may in part explain the high therapeutic potential of these antibiotics in the treatment of periodontitis (20).
Gingipains are major extracellular cysteine proteinases produced by Porphyromonas gingivalis (8), a recognized causative bacterium of adult periodontitis (11, 24, 45, 49), and they are important virulence factors of this established periodontopathogen (22, 36, 46). Two gingipains referred to as HRgpA and RgpB are arginine-specific proteinases and another gingipain, Kgp, is a lysine-specific proteinase (41, 42). The former proteinases activate prekallikrein, leading to very efficient generation of bradykinin, a potent vascular permeability-enhancing (VPE) peptide (26). In addition, they are able to induce blood clotting through activation of the coagulation cascade at several different levels (25, 29, 30). Moreover, thrombin released in this process is a strong proinflammatory mediator (3, 11, 32, 34). These two gingipain-triggered molecular events could be potentially associated with crevicular fluid production at periodontitis sites and development of the inflammatory disease, respectively. At the same time, the ability of gingipains to degrade fibrinogen in human plasma (28), together with their fibrinolytic activity (27), could be involved in the bleeding tendency at periodontitis lesions. Gingipains can also induce secretion of collagenase from gingival fibroblast (9) and activate pro-MMPs (10). Thus, when this is all taken into account it can be anticipated that inhibition of gingipains by antibiotics may significantly potentiate their therapeutic effects in the treatment of periodontitis. A recent report has indicated that treatment of periodontitis patients with minocycline reduced salivary protease activity (2), some of which is likely to be due to the presence of gingipains (31). This finding prompted us to investigate the direct inhibitory activity of tetracycline and its analogues for gingipains.
MATERIALS AND METHODS
Materials
Tetracycline, minocycline, doxycycline, soybean trypsin inhibitor, porcine pancreas trypsin, bovine pancreas chymotrypsin, and papain were obtained from Sigma Chemical Co. (St. Louis, Mo.). Porcine pancreatic elastase and H-d-Phe-Pro-Arg-chloromethylketone (FPR-CK) were from Elastin Products Co., Inc. (Pacific, Mo.), and BACHEM Bioscience Inc. (Philadelphia, Pa.), respectively. Carbobenzoxy-l-pyroglutamyl-glycyl-l-arginine-4-methyl-coumaryl-7-amide (Z-Pyr-Gly-Arg-MCA) (for HRgpA, RgpB, and papain), t-butyloxycarbonyl-l-valyl-l-leucyl-l-lysine (Boc-Val-Leu-Lys)-MCA (for Kgp), succinyl-l-alanyl-l-alanyl-l-prolyl-l-phenylalanine (Suc-Ala-Ala-Pro-Phe)-MCA (for chymotrypsin), Suc-Ala-Pro-Ala-MCA (for elastase), and a standard 7-amino-4-methyl coumarin (AMC) were purchased from the Peptide Institute (Minoh, Japan). Normal human plasma was obtained by centrifugation of a mixture of 9 volumes of freshly drawn blood from healthy volunteers and 1 volume of 3.8% (wt/vol) sodium citrate. Other chemicals were purchased from Wako Pure Chemical Industries Ltd. (Osaka, Japan).
Proteinase purification.
Kgp, HRgpA, and RgpB were isolated according to the methods described by Pike et al. (41) and Potempa et al. (42). The amount of active enzyme in each purified proteinase was determined by active-site titration using FPR-CK (43). The concentration of active gingipain R was calculated from the amount of inhibitor needed for complete inactivation of the proteinase.
Activation of Rgps.
Each gingipain form was activated with 10 mM cysteine in 0.2 M HEPES buffer (pH 8.0) containing 5 mM CaCl2 at 37°C for 10 min. The activated proteinase (1 μM) was then diluted with 50 mM Tris-HCl (pH 7.4) containing 0.1 M NaCl and 5 mM CaCl2 prior to use.
Proteinase inhibition assays.
Fifty microliters of tetracycline analogue, dissolved in 0.1 M Tris-HCl (pH 7.6) containing 0.15 M NaCl, and an equal volume of a proteinase in the same buffer were mixed in a 96-well microplate and incubated for 5 min at 25°C. Then, 100 μl of an MCA substrate (0.4 mM) in the same buffer was added to the mixture. The residual proteinase activity was measured fluorometrically with a fluorescence spectrophotometer for a 96-well microplate (CytoFluor Series 4000; PerSeptive Biosystems), with fluorescence at 440 ± 20 nm and excitation at 380 ± 20 nm. The velocity of AMC release was calculated by using standard AMC concentrations.
Kinetic analysis of RgpB inhibition by doxycycline
The inhibitory effect of doxycycline on RgpB activity was investigated as a function of substrate concentration ([s], from 2.5 to 40 μM), and the results were plotted as 1/v versus 1/[s], where v is the initial velocity of the substrate cleavage. The values of the Michaelis constant (Km) and the maximum velocity (Vmax) in the Michaelis-Menten equation were determined by using three different plots, [s]0/v versus [s], 1/v versus 1/[s]0 and v versus v/[s]0 (v and [s]0 denote the catalytic rate and the initial substrate concentration, respectively), where the best-fit values were determined by the method of least squares with Taylor expansion, as described by Sakoda and Hiromi (44). The inhibition constant (Ki) of doxycycline in RgpB inhibition was obtained by the equation Vapp = V /(1 + i/Ki), where Vapp and V denote the maximum velocity in the presence or absence of doxycycline, respectively, and i is the doxycycline concentration.
VPE assay.
Normal human plasma (50 μl) supplemented with 1,10-phenanthroline (2 mM) to inhibit kininases was mixed with 25 μl of doxycycline dissolved in 10 mM Tris-HCl (pH 7.4) containing 0.15 M NaCl (TBS), followed by addition of 25 μl of RgpB (40 nM in TBS) 15 s later and incubation in a plastic tube at 25°C for 5 min. The reaction was stopped by adding 400 μl of TBS supplemented with 1,10-phenanthroline (1 mM), soybean trypsin inhibitor (20 μM), leupeptin (10 μM), and FPR-CK (10 μM). Each sample (100 μl) was injected intradermally into the clipped flank of a guinea pig (Albino-Hartley strain; Kyudo Experimental Animals, Kumamoto, Japan) previously anesthetized with an intramuscular injection of ketamine (80 mg/kg of body weight) and having received an intravenous injection of Evans blue dye (2.5% solution in 0.6% saline; 30 mg/kg). The VPE activity of the sample was determined by quantitatively measuring the dye extravasated at injected skin sites, according to the method of Udaka et al. (48). Activity was expressed in terms of mean micrograms of dye released (triplicate assays). Dye leakage at TBS-injected sites was used as a control and the value was subtracted from the value of each sample.
RESULTS
Inhibition of gingipains by tetracycline analogues.
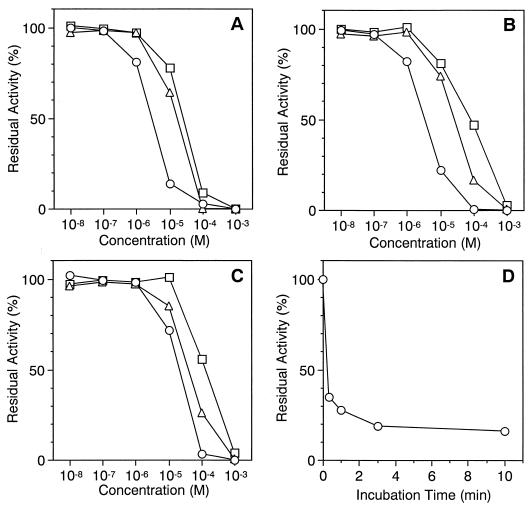
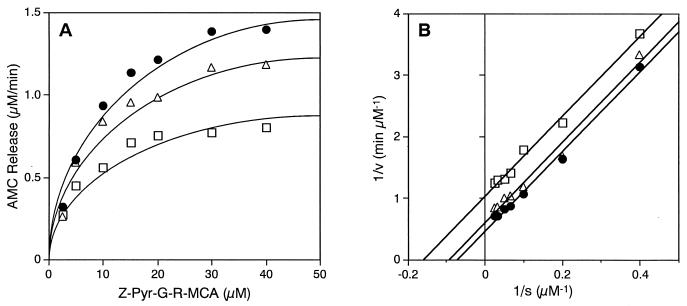
To investigate the inhibitory effect of tetracycline analogues on gingipain activity, RgpB, HRgpA, and Kgp were incubated with various concentrations of tetracycline, minocycline, or doxycycline, and the enzyme residual activity was measured. At a 1 mM concentration all three tetracycline analogues inhibited gingipains completely; however, at the lower micromolar range significantly stronger inhibition was observed for both of the gingipains R (Fig. 1A and B) than for Kgp (Fig. 1C). Among the analogues, doxycycline was the most potent inhibitor, followed by tetracycline and minocycline. Doxycycline inhibited Rgp's activity about 20% at 1 μM, 80% at 10 μM, and completely at 100 μM. In contrast, Kgp retained about 70% activity with 10 μM doxycycline and concentrations of this compound required to inhibit 50% of the activity (IC50) of gingipains R and Kgp were about 3 and 20 μM, respectively. RgpB inhibition by doxycycline was relatively rapid and nearly maximal inhibition was reached after 3 min preincubation (Fig. 1D). To study the mechanism of inhibition, the inhibitory effect of doxycycline on RgpB activity was investigated as a function of substrate concentration and the results were plotted as 1/v versus 1/[s]. The best-fit lines of the plots for the substrate cleavage by RgpB in the presence of doxycycline at 10 or 25 μM were parallel to the best-fit line of the plots in the absence of the antibiotic (Fig. 2), indicating the uncompetitive mechanism of inhibition. The Ki was 54 μM. These results showed that tetracycline and its analogues were potent and uncompetitive gingipain inhibitors. In addition, doxycycline was also an inhibitor of papain, trypsin, chymotrypsin, and elastase, with IC50 of about 30, 50, 70, and 110 μM, respectively (data not shown).
FIG. 1.
Inhibition of gingipains by tetracyclines. (A to C) Fifty microliters of a tetracycline analogue, dissolved in 0.1 M Tris-HCl (pH 7.6) containing 0.15 M NaCl, and an equal volume of a gingipain (2 nM) in the same buffer were mixed in a 96-well microplate and incubated for 5 min at 25°C. Then, 100 μl of 0.4 mM Z-Pyr-Gly-Arg-MCA for RgpB (A) and HRgpA (B) or Boc-Val-Leu-Lys-MCA for Kgp (C) in the same buffer was added to the mixture. ▵, tetracycline; □, minocycline; ○, doxycycline. The concentrations of tetracycline analogues in the initial mixture are shown. (D) Time course of RgpB inhibition by doxycycline. Fifty microliters of doxycycline (20 μM), dissolved in 0.1 M Tris-HCl (pH 7.6) containing 0.15 M NaCl, and an equal volume of RgpB (1 nM) in the same buffer were mixed and incubated at 25°C for various periods, followed by addition of 100 μl of Z-Pyr-Gly-Arg-MCA (0.4 mM). The amount of AMC released by the residual proteinase was measured fluorometrically.
FIG. 2.
Mechanism of RgpB inhibition. Fifty microliters of doxycycline (●, 0 μM; ▵, 80 μM; □, 200 μM), dissolved in 0.1 M Tris-HCl (pH 7.6) containing 0.15 M NaCl, and 100 μl of Z-Pyr-Gly-Arg-MCA in the same buffer were mixed in a 96-well microplate, followed by addition of 50 μl of RgpB (2 nM) in the same buffer. The amount of AMC released by the residual proteinase was measured fluorometrically. (A) AMC release velocity (v) versus Z-Pyr-Gly-Arg-MCA concentration (s). (B) Plot of 1/v versus 1/[s]. The concentrations of Z-Pyr-Gly-Arg-MCA in the final mixture are shown.
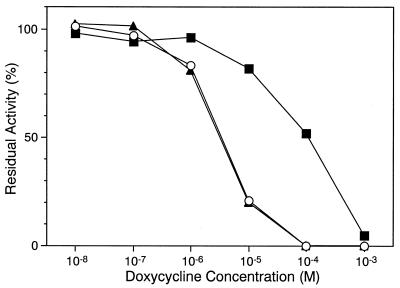
Effect of calcium and cysteine on RgpB inhibition by doxycycline.
Tetracyclines possess chelating activities (37), which are known to be responsible for MMP inhibition (16). To determine whether the gingipain inhibitory activities of the tetracyclines were dependent on their chelating activities, RgpB inhibition by doxycycline was investigated in the presence of calcium. In these experiments it was found that calcium up to 5 mM did not affect the inhibitory potency of doxycycline (Fig. 3).
FIG. 3.
Effect of calcium and cysteine on RgpB inhibition by doxycycline. Fifty microliters of doxycycline, dissolved in 0.1 M Tris-HCl (pH 7.6) containing 0.15 M NaCl only (○) or further supplemented with 5 mM NaCl2 (▴) or 10 mM cysteine (▪), and an equal volume of RgpB (2 nM) in the same buffer were mixed in a 96-well microplate and incubated at 25°C for 5 min. Then, 100 μl of Z-Pyr-Gly-Arg-MCA (0.4 mM) in the same buffer was added to the mixture. The amount of AMC released by the residual proteinase was measured fluorometrically.
In addition to divalent cation binding, the lower peripheral part of the tetracycline molecule is essentially an electron-dense region at which oxidative processes occur (33). We have considered that an oxidative state at this region may play a role in gingipain inhibition by tetracyclines. To study this possibility, the inhibitory property of doxycycline against RgpB was examined in the presence of cysteine. Interestingly, this reducing compound profoundly reduced the inhibitory potency of doxycycline (Fig. 3), as indicated by a significant decrease of the IC50 from 3 μM in the absence to more than 100 μM in the presence of 10 mM cysteine. These results suggest that the oxidative state of tetracycline molecules is closely related to the inhibitory activities of these antibiotics for cysteine proteinases.
Inhibition of RgpB production of VPE activity from human plasma by doxycycline.
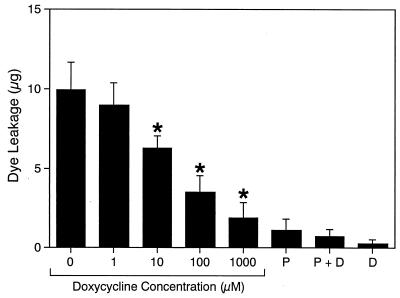
To investigate the possibility that doxycycline could inhibit gingipain activity in vivo, we studied the effect of the drug on the RgpB-dependent production of VPE activity from human plasma. Doxycycline decreased the generation of VPE activity by RgpB in a dose-dependent manner at concentrations above 10 μM and almost completely at 1 mM (Fig. 4). Doxycycline itself and plasma without RgpB treatment exhibited no significant VPE activity. In addition, doxycycline at the concentrations used in these experiments did not affect the VPE activity of bradykinin (data not shown), which is produced by RgpB in plasma. Taken together, these results suggest that doxycline and tetracycline analogues are able to inhibit gingipain activity in vivo.
FIG. 4.
Inhibition by doxycycline of RgpB VPE activity production from human plasma. Normal human plasma (50 μl) supplemented with 1,10-phenanthroline (2 mM) was mixed with 25 μl of doxycycline, followed by addition of 25 μl of RgpB (40 nM) 15 s later and incubation at 25°C for 5 min. The reaction was stopped by adding 400 μl of TBS supplemented with 1,10-phenanthroline (1 mM), soybean trypsin inhibitor (20 μM), leupeptin (10 μM), or FPR-CK (10 μM). The VPE activity of each sample was measured. Activity was expressed in terms of mean micrograms of dye released in triplicate assays. Dye leakage at TBS-injected sites was used as a control and the value was subtracted from the value for each sample. Doxycycline concentrations during incubation are shown. ∗, P < 0.01 for VPE activity of RgpB-treated plasma in the absence of doxycycline. P, plasma alone; P + D, plasma plus doxycycline (1,000 μM); D, doxycycline alone (1,000 μM).
DISCUSSION
To our knowledge, the data presented in this report show for the first time that tetracyclines are potent cysteine proteinase inhibitors. The doxycycline IC50 for RgpB (3 μM) is comparable to that for MMP-13 (2 μM) (21) and lower than that for MMP-8 (30 μM) (18) and MMP-1 (>400 μM) (11). Interestingly, for both types of proteinases doxycycline is the most potent inhibitor of the three tetracycline analogues (18, 20, 47) (Fig. 1A, B, and C). The β-ketone moiety at C-11 and C-12 of the tetracycline rings, which is an electron-dense and reactive region of the molecule, is a Ca2+ and Zn2+ binding site (37) responsible, at least in part, for inhibition of MMP activity (17). Since RgpB inhibition by doxycycline was not affected in the presence of calcium (Fig. 3), the chelating ability of this compound is rather unlikely to be associated with its inhibitory activity. However, the observation that cysteine significantly reduced inhibition of RgpB by doxycycline (Fig. 3) suggests that the electron-dense region of the drug interacts with the proteinase, causing loss of enzymatic activity. Moreover, the fact that doxycycline inhibits both cysteine- and serine-type proteinases of different substrate specificities and mechanisms of catalysis in an uncompetitive manner suggests that the compound binds reversibly through the electron-dense moiety to a proteinase outside the substrate binding site and in this way disturbs the charge-relay system of the enzymes.
From a physiological point of view, it is interesting that doxycycline significantly inhibited production of RgpB-induced VPE activity from human plasma at concentrations of 10 μM and above (Fig. 4). However, in contrast to the inhibition of RgpB amidolytic activity, inhibition of in vivo activity of this proteinase required about a 10-fold higher doxycycline concentration (Fig. 1A), probably due to the binding of the antibiotic drug (>90%) to plasma proteins (5). Clinical data indicate that administration of minocycline at 150 to 200 mg/day to periodontitis patients shows a positive therapeutic effect (7). This effect is likely because of minocycline enrichment in the periodontal pockets, where P. gingivalis and other periodontopathogens are present, as indicated by the reported 10 to 30 μM concentrations of the antibiotic in the gingival crevicular fluid (7). Similar concentrations in the gingival crevicular fluid are expected after treatment with doxycycline. Indeed, periodontitis patients administered doxycycline orally at 100 mg/day showed considerable improvement of clinical indices. Interestingly, this treatment did not affect the P. gingivalis load at periodontitis sites (14), despite the fact that the MIC of doxycycline for this bacterium is 0.1 μM in vitro (39). This result may indicate that the therapeutic effect of the drug on periodontitis patients is due to its ability to inhibit proteinases, including gingipains and MMPs, rather than to its ability to eradicate P. gingivalis. A higher concentration of the antibiotic could be obtained at the lesion by locally delivered doxycycline (12), increasing the antiproteinase potential at periodontal sites. Unfortunately, high doses of tetracyclines exert side effects such as gastrointestinal disturbance and stimulate the emergence of tetracycline-resistant bacteria. Chemically modified tetracycline analogues, which have a dimethylamino group removed from the C-4 position, are devoid of both antimicrobial activity and side effects (15). Since the dimethylamino group is not involved in the gingipain inhibitory activity of tetracyclines, chemically modified compounds could replace tetracyclines in the treatment of periodontitis, exerting beneficial effects through gingipain inhibition without the side effects associated with the bactericidal activities of this group of antibiotics.
Besides periodontitis, tetracyclines are being used for treatment of skin-blistering diseases, rheumatoid and osteoarthritis, and malignant tumors, and in all cases the beneficial clinical effect is thought to be based on their MMP inhibitory activity (19). The present finding that tetracyclines can inhibit cysteine proteinases, irrespective of the substrate specificity, would extend the therapeutic application of these antibiotics for treatment of diseases associated with abnormal activities of cysteine proteinases, including lysosomal enzymes (cathepsins B, H, K, and L), and caspases. Acute pancreatitis can be considered a disease amenable to treatment with tetracycline, since involvement of cathepsin B was strongly suggested in an experiment using proteinase-deficient animals (23). On the other hand, inhibition of caspase-1 has been shown to slow the progress of pathological changes in a mouse model of Huntington's disease (38), a progressive neurodegenerative disorder resulting in specific neuronal loss and dysfunction in the striatum and cortex. Moreover, minocycline was found to inhibit caspase-1 and caspase-3 gene expression and delay mortality in a transgenic mouse model of Huntington's disease (6). Although the effect of minocycline on that disease appeared to be attributable to inhibition of inducible nitric oxide synthase and caspase gene expression (6), the direct inhibition of caspase enzymatic activity by the drug can also be considered, especially when taking into account the structural similarity between RgpB and caspase-1 (13). Thus, the results presented in this report further support the contention that tetracyclines, due to their relatively low toxicity and various biological activities, may represent new potential therapeutic agents for different diseases. Indeed, in some situations their antibiotic-like activities may be better based on their actions as proteinase inhibitors.
ACKNOWLEDGMENTS
This work was supported by the Japanese Ministry of Education (grant no. 11670219 to T.I.) and by the Committee of Scientific Research (KBN, Warsaw, Poland; grant 6 P04A 047 17 to J.P.).
REFERENCES
- 1.Amin A R, Attur M G, Thakker G D, Patel P D, Vyas P R, Patel R N, Patel I R, Abramson S B. A novel mechanism of action of tetracyclines: effects on nitric oxide synthases. Proc Natl Acad Sci USA. 1996;93:14014–14019. doi: 10.1073/pnas.93.24.14014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atilla A, Balcan M, Biçakçi N, Kazandi A. The effect of non-surgical periodontal and adjunctive minocycline-HCl treatments on the activity of salivary proteases. J Periodontol. 1996;67:1–6. doi: 10.1902/jop.1996.67.1.1. [DOI] [PubMed] [Google Scholar]
- 3.Bar-Shavit R, Kahn A, Fenton II J W, Wilner G D. Monocyte chemotaxis: stimulation by specific exosite region in thrombin. Science. 1983;220:728–730. doi: 10.1126/science.6836310. [DOI] [PubMed] [Google Scholar]
- 4.Birkedal-Hansen H. Role of matrix metalloproteinases in human periodontal diseases. J Periodontol. 1993;64:474–484. doi: 10.1902/jop.1993.64.5s.474. [DOI] [PubMed] [Google Scholar]
- 5.Campistron G, Coulais Y, Caillard C, Mosser J, Pontagnier H, Houin G. Pharmacokinetics and bioavailability of doxycycline in humans. Arzneim Forsch. 1986;36:1705–1707. [PubMed] [Google Scholar]
- 6.Chen M, Ona V O, Li M, Ferrante R J, Fink K B, Zhu S, Bian J, Guo L, Farrell L A, Hersch S M, Hobbs W, Vonsattel J P, Cha J H, Friedlander R M. Minocycline inhibits caspase-1 and caspase-3 expression and delays mortality in a transgenic model of Huntington disease. Nat Med. 2000;6:797–801. doi: 10.1038/77528. [DOI] [PubMed] [Google Scholar]
- 7.Ciancio S G. Clinical experiences with tetracyclines in the treatment of periodontal diseases. Ann N Y Acad Sci. 1994;732:132–139. doi: 10.1111/j.1749-6632.1994.tb24730.x. [DOI] [PubMed] [Google Scholar]
- 8.Curttis M A, Kuramitsu H K, Lanz M, Macrina F L, Nakayama K, Potempa J, Raynolds E C, Aduse-Opoku J. Molecular genetics and nomenclature of proteases of Porphyromonas gingivalis. J Periodont Res. 1999;34:464–472. doi: 10.1111/j.1600-0765.1999.tb02282.x. [DOI] [PubMed] [Google Scholar]
- 9.DeCarlo A A, Grenett H E, Harber G J, Windsor L J, Bodden M K, Birkedal-Hansen B, Birkedal-Hansen H. Induction of matrix metalloproteinases and a collagen-degrading phenotype in fibroblasts and epithelial cells by secreted Porphyromonas gingivalis proteinase. J Periodont Res. 1998;33:408–420. doi: 10.1111/j.1600-0765.1998.tb02337.x. [DOI] [PubMed] [Google Scholar]
- 10.DeCarlo A A, Windsor L J, Bodden M K, Harber G J, Birkedal-Hansen B, Birkedal-Hansen H. Activation and novel processing of matrix metalloproteinases by a thiol-proteinase from the oral anerobe Porphyromonas gingivalis. J Dent Res. 1997;76:1260–1270. doi: 10.1177/00220345970760060501. [DOI] [PubMed] [Google Scholar]
- 11.DeMichele M A A, Moon D G, Fenton II J W, Minnear F L. Thrombin's enzymatic activity increases permeability of endothelial cell monolayers. J Appl Physiol. 1990;69:1599–1606. doi: 10.1152/jappl.1990.69.5.1599. [DOI] [PubMed] [Google Scholar]
- 12.Drisko C H. The use of locally delivered doxycycline in the treatment of periodontitis. Clinical results. J Clin Periodontol. 1998;25:942–952. doi: 10.1111/j.1600-051x.1998.tb02396.x. [DOI] [PubMed] [Google Scholar]
- 13.Eichinger A, Beisel H-G, Jacob U, Huber R, Medrano F-J, Banbula A, Potempa J, Travis J, Bode W. Crystal structure of gingipain R: an Arg-specific bacterial cysteine protease with a caspase-like fold. EMBO J. 1999;18:5453–5462. doi: 10.1093/emboj/18.20.5453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feres M, Haffajee A D, Goncalves C, Allard K A, Som S, Goodson J M, Socransky S S. Systemic doxycycline administration in the treatment of periodontal infections. I. Effect on the subgingival microbiota. J Clin Periodontol. 1999;26:775–783. doi: 10.1111/j.1600-051x.1999.tb02520.x. [DOI] [PubMed] [Google Scholar]
- 15.Golub L M, Lee H-M, Ryan M E, Giannoble W V, Payne J, Sorsa T. Tetracyclines inhibit connective tissue breakdown by multiple non-antimicrobial mechanisms. Adv Dent Res. 1998;12:12–26. doi: 10.1177/08959374980120010501. [DOI] [PubMed] [Google Scholar]
- 16.Golub L M, Lee H-M, Lehrer G, Nemirof A, McNamara T F, Kaplan R, Ramamurthy N S. Minocycline reduces gingival collagenolytic activity during diabetes. Preliminary observations and a proposed new mechanism of action. J Periodont Res. 1983;18:516–526. doi: 10.1111/j.1600-0765.1983.tb00388.x. [DOI] [PubMed] [Google Scholar]
- 17.Golub L M, Ramamurthy N S, McNamara T F, Greenwald R A, Rifkin B R. Tetracyclines inhibit connective tissue breakdown: new therapeutic implication for an old family of drugs. Crit Rev Oral Biol Med. 1991;2:297–322. doi: 10.1177/10454411910020030201. [DOI] [PubMed] [Google Scholar]
- 18.Golub L M, Sorsa T, Lee H-M, Ciancio S, Sorbi D, Ramamurthy N S, Grubber B, Salo T, Konttinen Y T. Doxycycline inhibits neutrophil (PMN)-type matrix metalloproteinases in human adult periodontitis gingiva. J Clin Perodontol. 1995;22:100–109. doi: 10.1111/j.1600-051x.1995.tb00120.x. [DOI] [PubMed] [Google Scholar]
- 19.Golub L M, Suomalainen K, Sorsa T. Host modulation with tetracyclines and their chemically modified analogues. Curr Opin Dent. 1992;2:80–90. [PubMed] [Google Scholar]
- 20.Golub L M, Wolff M, Roberts S, Lee H-M, Leung M, Payonk G S. Treating periodontal diseases by blocking tissue-destructive enzymes. J Am Dent Assoc. 1994;125:163–171. doi: 10.14219/jada.archive.1994.0261. [DOI] [PubMed] [Google Scholar]
- 21.Greenwald R A, Golub L M, Ramamurthy N S, Chowdhury M, Moak S A, Sorsa T. In vitro sensitivity of the three mammalian collagenases to tetracycline inhibition: relationship to bone and cartilage degradation. Bone. 1998;22:33–38. doi: 10.1016/s8756-3282(97)00221-4. [DOI] [PubMed] [Google Scholar]
- 22.Grenier D, Mayrand D. Selected characteristics of pathogenic and nonpathogenic strains of Bacteroides gingivalis. J Clin Microbiol. 1987;25:738–740. doi: 10.1128/jcm.25.4.738-740.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Halangk W, Lerch M M, Brandt-Nedelev B, Roth W, Ruthenbuerger M, Reinheckel T, Domschke W, Lippert H, Peters C, Deussing J. Role of cathepsin B in intracellular trypsinogen activation and the onset of acute pancreatitis. J Clin Investig. 2000;106:773–781. doi: 10.1172/JCI9411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holt S C, Ebersole J, Fenton J, Brunsvold M, Kornmann K S. Implantation of Bacteroides gingivalis in nonhuman primates initiates progression of periodontitis. Science. 1987;239:55–57. doi: 10.1126/science.3336774. [DOI] [PubMed] [Google Scholar]
- 25.Imamura T, Banbula A, Pereira P J B, Travis J, Potempa J. Activation of human prothrombin by arginine-specific cysteine proteinases (gingipains R) from Porphyromonas gingivalis. J Biol Chem. 2001;275:18984–18991. doi: 10.1074/jbc.M006760200. [DOI] [PubMed] [Google Scholar]
- 26.Imamura T, Pike R N, Potempa J, Travis J. Pathogenesis of periodontitis: a major arginine-specific cysteine proteinase from Porphyromonas gingivalis induces vascular permeability enhancement through activation of the kallikrein/kinin pathway. J Clin Investig. 1994;93:361–367. doi: 10.1172/JCI117330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Imamura T, Potempa J, Travis J. Comparison of pathogenic properties between two types of arginine-specific cysteine proteinase (gingipains-R) from Porphyromonas gingivalis. Microb Pathog. 2000;29:155–163. doi: 10.1006/mpat.2000.0380. [DOI] [PubMed] [Google Scholar]
- 28.Imamura T, Potempa J, Pike R N, Moore J N, Barton M H, Travis J. Effect of free and vesicle-bound cysteine proteinases of Porphyromonas gingivalis on plasma clot formation: implications for bleeding tendency at periodontitis sites. Infect Immun. 1995;63:4877–4882. doi: 10.1128/iai.63.12.4877-4882.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Imamura T, Potempa J, Tanase S, Travis J. Activation of blood coagulation factor X by arginine-specific cysteine proteinases (gingipain-Rs) from Porphyromonas gingivalis. J Biol Chem. 1997;272:16062–16067. doi: 10.1074/jbc.272.25.16062. [DOI] [PubMed] [Google Scholar]
- 30.Imamura T, Tanase S, Hamamoto T, Potempa J, Travis J. Activation of blood coagulation factor IX by gingipains R, arginine-specific cysteine proteinases from Porphyromonas gingivalis. Biochem J. 2001;353:325–331. doi: 10.1042/0264-6021:3530325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ingman T, Sorsa T, Konttinen Y T, Liede K, Saari H, Lindy O, Suomalainen K. Salivary collagenase, elastase- and trypsin-like proteases as biochemical markers of periodontal tissue destruction in adult and localized juvenile periodontitis. Oral Microbiol Immunol. 1993;8:298–305. doi: 10.1111/j.1399-302x.1993.tb00578.x. [DOI] [PubMed] [Google Scholar]
- 32.Jones A, Geczy C L. Thrombin and factor Xa enhance the production of interleukin-1. Immunology. 1990;71:236–241. [PMC free article] [PubMed] [Google Scholar]
- 33.Kruk I, Lichszeld K, Michalska K, Nizinkiewicz K, Wronska J. The extra-weak chemiluminescence generated during oxidation of some tetracycline antibiotics. 1. Auto-oxidation. J Photochem Photobiol B. 1992;14:329–343. doi: 10.1016/1011-1344(92)85112-8. [DOI] [PubMed] [Google Scholar]
- 34.Lerner U H, Gustafson G T. Blood coagulation and bone metabolism: some characteristics of the bone resorptive effect of thrombin in mouse calvarial bones in vitro. Biochim Biophys Acta. 1988;964:309–318. doi: 10.1016/0304-4165(88)90031-1. [DOI] [PubMed] [Google Scholar]
- 35.Lindhe J, Liljenberg B, Adielsson B. Effect of long-term tetracycline therapy on human periodontal disease. J Clin Periodontol. 1983;10:590–601. doi: 10.1111/j.1600-051x.1983.tb01297.x. [DOI] [PubMed] [Google Scholar]
- 36.Marsh P D, McKee A S, McDermid A S, Dowsett A B. Ultrastructure and enzyme activities of a virulent and an avirulent variant of Bacteroides gingivalis W50. FEMS Microbiol Lett. 1989;59:181–185. doi: 10.1016/0378-1097(89)90482-5. [DOI] [PubMed] [Google Scholar]
- 37.Newman E C, Frank C W. Circular dichroism spectra of tetracycline complexes with Mg2+ and Ca2+ J Pharm Sci. 1976;65:1728–1732. doi: 10.1002/jps.2600651209. [DOI] [PubMed] [Google Scholar]
- 38.Ona V O, Li M, Vonsattel J P, Andrew L J, Khan S Q, Chung W M, Frey A S, Menon A S, Li X J, Stieg P E, Yuan J, Penney J B, Young A B, Cha J H, Friedlander R M. Inhibition of caspase-1 slows disease progression in a mouse model of Huntington's disease. Nature. 1999;399:263–267. doi: 10.1038/20446. [DOI] [PubMed] [Google Scholar]
- 39.Pajukanta R, Asikainen S, Forsblom B, Saarela M, Jousimies-Somer H. β-Lactamase production and in vitro antimicrobial susceptibility of Porphyromonas gingivalis. FEMS Immunol Med Microbiol. 1993;6:241–244. doi: 10.1111/j.1574-695X.1993.tb00334.x. [DOI] [PubMed] [Google Scholar]
- 40.Patel R N, Attur M G, Dave M N, Patel I V, Stuchin S A, Abramson S B, Amin A R. A novel mechanism of action of chemically modified tetracyclines: inhibition of COX-2-mediated prostaglandin E2 production. J Immunol. 1999;163:3459–3467. [PubMed] [Google Scholar]
- 41.Pike R, McGraw W, Potempa J, Travis J. Lysine- and arginine-specific proteinases from Porphyromonas gingivalis: isolation and evidence for the existence of complexes with hemagglutinins. J Biol Chem. 1994;269:406–411. [PubMed] [Google Scholar]
- 42.Potempa J, Mikolajczyk-Pawlinska J, Brassell D, Nelson D, Thøgersen I B, Enghild J J, Travis J. Comparative properties of two cysteine proteinases (gingipain Rs), the products of two related but individual genes of Porphyromonas gingivalis. J Biol Chem. 1998;273:21648–21657. doi: 10.1074/jbc.273.34.21648. [DOI] [PubMed] [Google Scholar]
- 43.Potempa J, Pike R, Travis J. Titration and mapping of the active site of cysteine proteinases from Porphyromonas gingivalis (gingipains) using peptidyl chloromethanes. Biol Chem. 1997;378:223–230. doi: 10.1515/bchm.1997.378.3-4.223. [DOI] [PubMed] [Google Scholar]
- 44.Sakoda M, Hiromi K. Determination of the best-fit values of kinetic parameters of the Michaelis-Menten equation by the method of least squares of the Taylor expansion. J Biochem. 1976;80:547–555. doi: 10.1093/oxfordjournals.jbchem.a131310. [DOI] [PubMed] [Google Scholar]
- 45.Slots J. Importance of black-pigmented Bacteroides in human periodontal disease. In: Genco R J, Mergenhagen S E, editors. Host-parasite interactions in periodontal diseases. Washington, D.C.: American Society for Microbiology; 1982. pp. 27–45. [Google Scholar]
- 46.Smalley J W, Birss A J, Kay H M, McKee A S, Marsh P D. The distribution of trypsin-like enzyme activity in the cultures of a virulent and an avirulent strain of Bacteroides gingivalis W50. Oral Microbiol Immunol. 1989;4:178–181. doi: 10.1111/j.1399-302x.1989.tb00249.x. [DOI] [PubMed] [Google Scholar]
- 47.Sorsa T, Ding Y, Salo T, Lauhio A, Teronen O, Ingman T, Ohtani H, Andoh N, Takeha S, Konttinen Y T. Effect of tetracyclines on neutrophil, gingival and salivary collagenases: a functional and western blot assessment with special references to their cellular sources in periodontal diseases. Ann N Y Acad Sci. 1994;732:112–131. doi: 10.1111/j.1749-6632.1994.tb24729.x. [DOI] [PubMed] [Google Scholar]
- 48.Udaka K, Takeuchi Y, Movat H Z. Simple method for quantitation of enhanced vascular permeability. Proc Soc Exp Biol Med. 1970;133:1384–1387. doi: 10.3181/00379727-133-34695. [DOI] [PubMed] [Google Scholar]
- 49.Zambon J J. Microbiology of periodontal disease. In: Genco R J, Goldman H M, Cohen D W, editors. Contemporary periodontics. C.V. St. Louis, Mo: Mosby; 1990. pp. 147–160. [Google Scholar]