Abstract
The administration of cells as therapeutic agents has emerged as a novel approach to complement the use of small molecule drugs and other biologics for the treatment of numerous conditions. Although the use of cells for structural and/or functional tissue repair and regeneration provides new avenues to address increasingly complex disease processes, it also faces numerous challenges related to efficacy, safety, and translational potential. Recent advances in nanotechnology-driven cell therapies have the potential to overcome many of these issues through precise modulation of cellular behavior. Here we describe several approaches that illustrate the use of different nanotechnologies for the optimization of cell therapies and discuss some of the obstacles that need to be overcome to allow for the widespread implementation of nanotechnology-based cell therapies in regenerative medicine.
Keywords: nanotechnology, stem cell therapy, direct cell reprogramming, regenerative medicine
Graphical Abstract
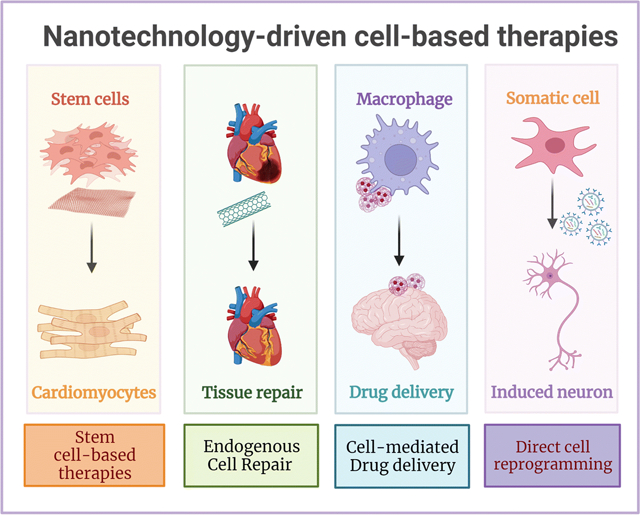
Introduction
The development of modern medicine has been continuously shaped by advances in biomedical sciences and engineering. Historically, progress made in sciences like microbiology, genetics, biochemistry, and pharmacology have greatly advanced the growth of modern therapeutic techniques(1). Contemporary approaches to healthcare are supported by three main therapeutic platforms: small molecule drugs, biological molecules, and medical devices(2). Cell therapy, defined as the use and administration of viable cells as therapeutic agents, has emerged as a new and disruptive therapeutic platform to address unmet medical needs in the treatment of increasingly complex diseases(3). Efficacy and safety are the two main challenges that must be addressed in the development of new therapeutic approaches. Accordingly, the development of cell-based therapies requires extensive investigation to identify safe routes of administration, ensure potency and accumulation in the tissue of interest, optimize the lifetime of therapeutic cells, minimize potential inflammatory and immune responses in target and off-target tissues, and, specifically for stem cell-based therapies, avoid differentiation to undesired cell types or unregulated cell proliferation(4). In most cases, novel cell therapies do not achieve full approval for clinical use due to the lack of safety and/or efficacy. In addition, challenges in the manufacturing and expansion of therapeutic cells can further hinder clinical translation(5).
Nanotechnology-based approaches are a promising strategy for the development of cell therapies with improved safety and efficacy, offering novel alternatives towards clinical implementation. Nanotechnology refers to the understanding and control of matter at the nanometer (nm) scale, and includes the design, construction, and implementation of nanoscale systems in fields such as chemistry, physics, materials science, engineering, and biology(6). The implementation of nanoscale technologies in biomedical sciences has led to the development of novel tools that directly interact with cells (intracellularly or extracellularly) and/or individual components of the cellular microenvironment(7), thus facilitating the modulation of key cellular responses for therapeutic purposes. Given the unique attributes of cells as therapeutic agents, they are suitable to treat multiple disease types, like cancer, metabolic disorders, autoimmune disorders, infections, and tissue degeneration, which has given rise to a diverse number of categories of cell therapies(8). One category in particular, regenerative cell therapies, has received special attention due to its immense potential to reduce tissue degeneration and promote tissue repair for the treatment of multiple and complex disorders (e.g., neurological, cardiovascular, pancreatic, pulmonary, osteological)(9). Consequently, in this concise review we summarize recent progress in the use of nanotechnology for regenerative cell therapies, focusing on the use of stem cells and reprogrammed somatic cells as therapeutic agents. Furthermore, we also discuss key strategies used to improve self-renewal, viability, and the functionality of cellular therapeutics, which is key to achieving the necessary levels of safety and efficacy for clinical translation (Figure 1).
Figure 1.
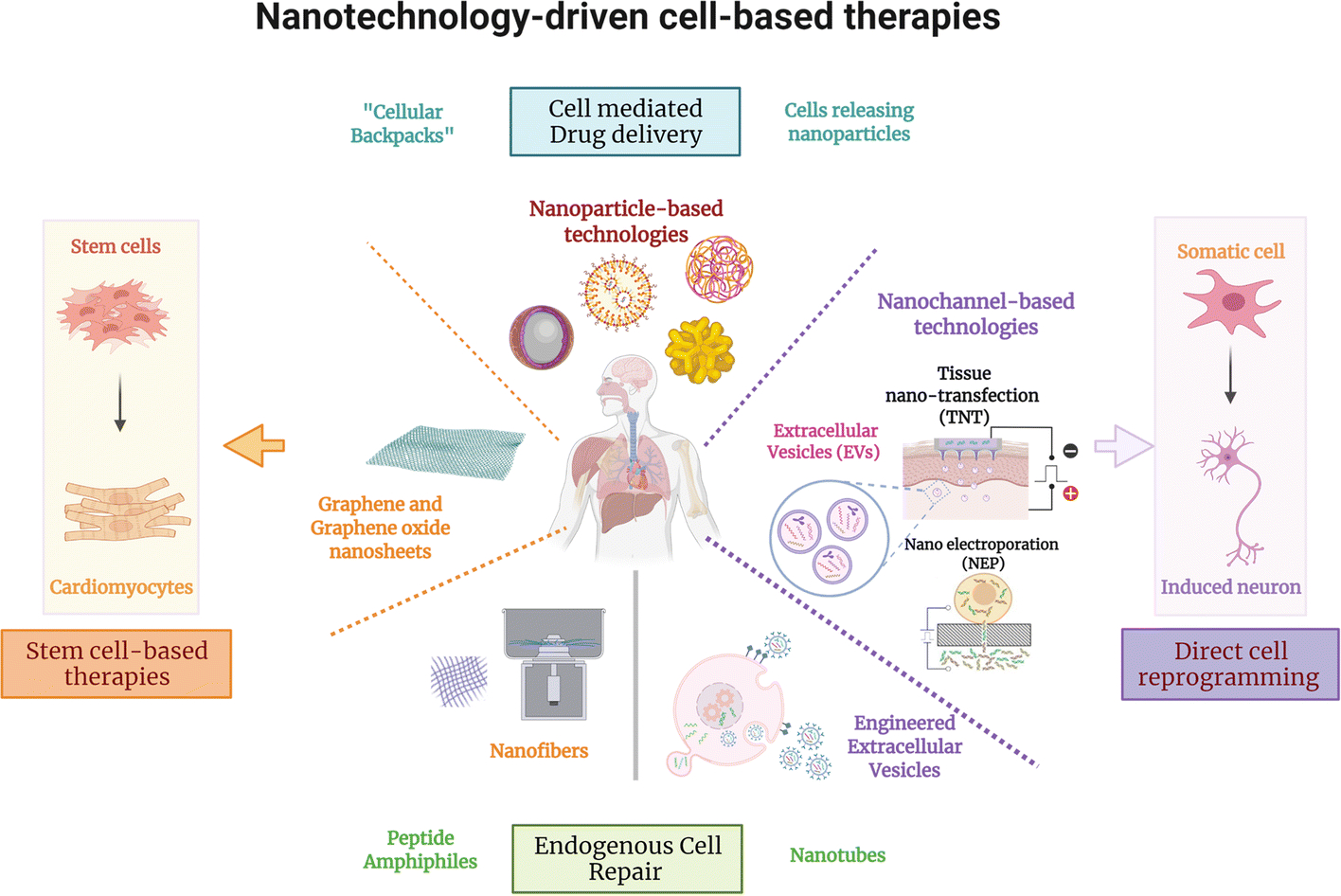
Nanotechnology-based strategies to improve safety and efficacy of stem cell- and direct reprogramming-based cell therapies.
Nanoscale technologies for stem cell-based therapies
The implementation of nanotechnology-based approaches in stem cell research has primarily focused on the improvement of isolation, proliferation, and differentiation protocols for basic biomedical research. However, the regenerative potential of embryonic, tissue-specific, and induced pluripotent stem cells (iPSCs), represents a key advantage for cell-based therapeutics(10). Consequently, numerous nanoscale technologies have been developed and implemented to modulate stem cell behavior for therapeutic applications.
Graphene oxide nanosheets
Graphene Oxide (GO), an oxidized carbon-based monolayer with a hexagonal crystal lattice that has robust mechanical strength and high electrical and thermal conductivities, is widely used in biomedical applications(11). GO has been shown to modulate the self-renewal capacity of embryonic stem (ES) cells(12), a mechanism that is driven by a decrease in integrin-mediated cellular signaling, which regulates cell interactions with different proteins in the extracellular matrix (ECM)(12). This increase in proliferation capacity could be key to developing more effective stem cell expansion strategies for therapeutic purposes. Similarly, GO-based nanomaterials can enhance the differentiation of ES cells into different cell lineages. Yang et al. demonstrated a GO dose-dependent increase in the differentiation of ES cells into Tyrosine Hydroxylase (TH)+ dopaminergic neurons(13), a neuronal subpopulation that is lost during the development of Parkinson’s Disease (PD), and that can be used for cell-based therapies aimed at treating this neurodegenerative condition. Garcia-Alegria et al. showed that GO-coated coverslips increased the generation of hematopoietic cells from mouse and human ES cell-derived hemangioblasts (Figure 2A–E)(14), which could potentially be used for the treatment of leukemias or other hematological disorders like aplastic anemia, or sickle cell disease. In addition to pluripotent ES cells, GO has also been used to improve the differentiation capacity of tissue-specific multipotent stem cells. Park et al., for example, first demonstrated increased differentiation of human neural stem cells (hNSC) into neurons, accompanied by a reduction in the number of GFAP+ glial cells when differentiation was induced on laminin-coated graphene substrates(15), a strategy that aims to enhance the purity of stem cell-derived neurons for therapeutic purposes.
Figure 2. Approaches employing graphene oxide nanosheets and nanofibers to regulate stem cell behavior.
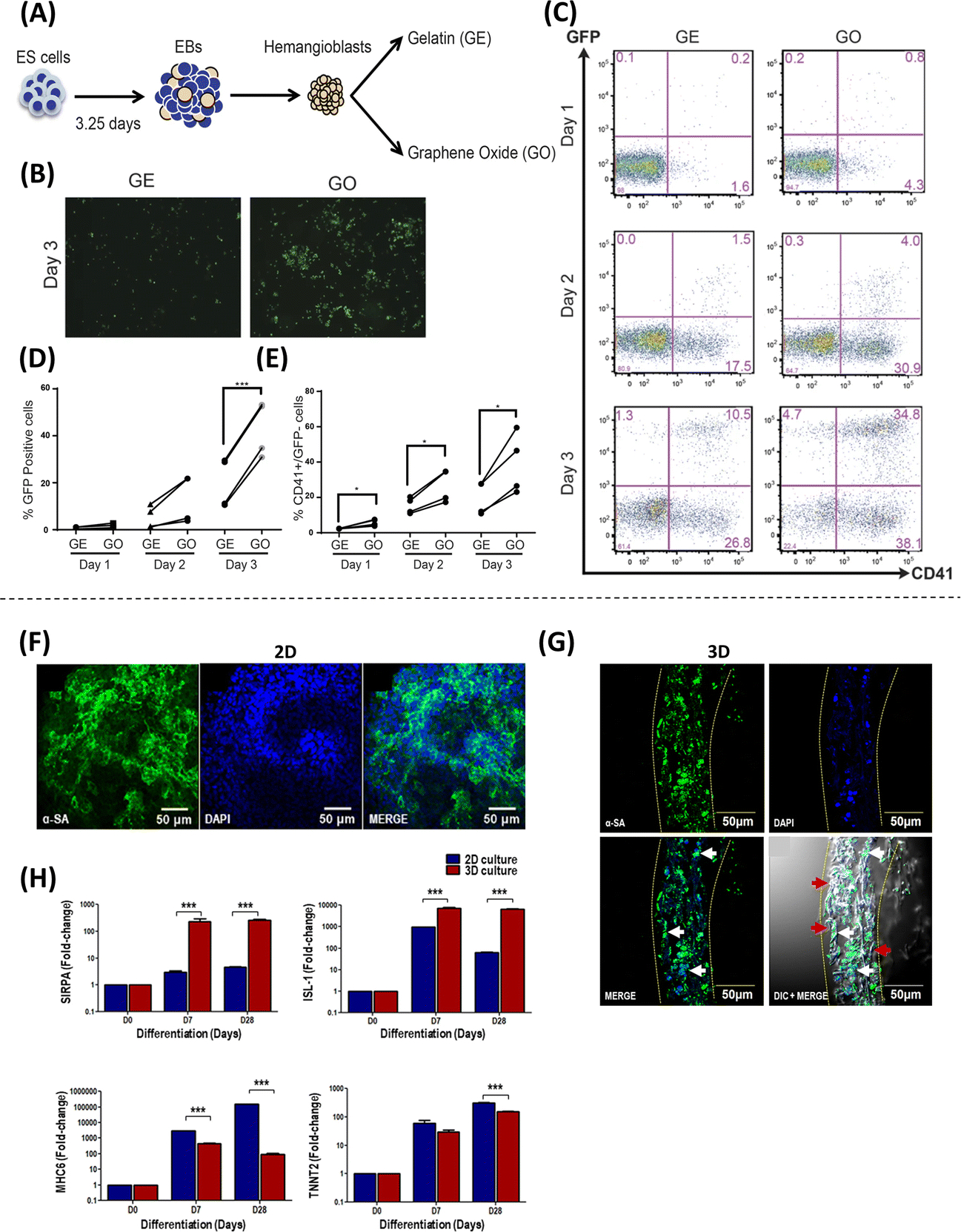
(A) Schematic representation of the experimental design to evaluate the response of hemangioblasts to gelatin (GE) or graphene oxide (GO). Heaemanglioblast cultures on GE/GO showing higher (B and D) GFP signal and (C and E) production of CD41+ / GFP− cells on GO-coated coverslips compared to GE (Adapted from Garcia-Alegria et al., 2016. Ref 14). Expression of sarcomeric alpha-actinin (α-SA), a cardiomyocyte marker, in human iPSCs (hiPSCs) differentiated using gelatin-PCL nanofiber scaffolds in a (F) 2D and (G) 3D culture system. (H) Gene expression of cardiac progenitor and cardiomyocyte markers SIRPA/ISL1, MHC6/TNNT2, respectively during differentiation of hiPSCs into cardiomyocytes in 2D and 3D cultures (Adapted from Sridharan et al., 2021. Ref 27).
Mesenchymal stem-cells (MSCs) have been extensively studied for the repair and regeneration of multiple tissues in preclinical studies and clinical trials(16). Accordingly, multiple studies have explored the effect of GO on MSCs differentiation to multiple lineages (see review from Halim et al.(17)). Remarkably, human MSCs cultured on pre-deposited graphene layers showed preserved viability and increased osteogenic differentiation(18). Furthermore, bone marrow-derived MSCs treated with GO resulted in an acceleration of osteogenic and adipogenic differentiation due to the ability of GO to bind and preconcentrate osteogenic and adipogenic factors near the MSCs, which enhanced the interaction and improved assimilation(19). Insights into the mechanism of action of GO-based nanomaterials could lead to optimized differentiation protocols towards these specific cell types, which could help to advance various therapeutic strategies for musculoskeletal- and adipose tissue-related conditions. A similar preconcentration effect by GO is observed in the differentiation of MSCs toward a chondrogenic lineage, a central mediator in cartilage repair(20). Moreover, graphene seems to influence the differentiation of MSCs to neural lineages. In the case of primary human MSCs obtained from surgically resected adipose tissue, there is increased differentiation towards neurons when cultured on a graphene-based substrate(21). Building on the advances made with GO, new biomaterial formulations have been synthesized by combining GO with a diverse variety of materials for the optimization of MSCs differentiation to neural precursors. For instance, 3D scaffold platforms made from reduced GO and collagen drove significant improvements in the differentiation of MSCs towards neuronal progenitors(22).
Nanofibers
Multiple studies have shown that the biophysical properties of the ECM can modulate the proliferation and differentiation capacity of stem cells(23). This has led to the development of numerous nanofiber-based systems that emulate ECM properties to boost stem cell proliferation and differentiation(24). Particularly, nanofibers made from aliphatic polyesters such as poly-ε-caprolactone (PCL), combined with collagen, improved the differentiation capacity of Wharton’s jelly-derived MSCs into motor neurons(25) and the differentiation of human MSCs into tendon-like tissue(26), which could be key to the development of therapies targeting neuromuscular and musculoskeletal disorders. Additional studies with iPSCs show that gelatin-PCL nanofiber scaffolds supported improved differentiation into cardiomyocytes in a 3D culture system (Figure 2F–H)(27), which could be of relevance to therapies focusing on cardiac tissue repair and regeneration.
In addition to PCL, a wide variety of polymers have been used to develop nanofiber-based platforms for stem cell culture. For example, polyvinylidene fluoride (PVDF)-based nanofibers with a diameter ~200–700 nm that were coated with mussel adhesive protein (MAP) and vitronectin supported increased differentiation of human ES cells into neural progenitors (NPs), along with an improved retention of the differentiation potential of these NPs into neurons, astrocytes, and oligodendrocyte precursors(28), which could be of relevance to cell replacement therapies targeting multiple cellular compartments within the central nervous system. Similarly, studies with iPSCs show enhanced proliferation and increased differentiation into insulin-producing cells when cultured on nanofibers fabricated from Poly-(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV)(29), showing potential for the development of therapies for insulin-dependent diabetes.
Another type of nanomaterial known as peptide amphiphiles (PAs) is also actively being used to enhance stem cell-based therapies. PAs consist of peptides with and hydrophilic head group and at least one hydrophobic tail capable of self-assembly into variety of molecular structures such as nanofibers, tubes, helices, and sheets(30). Scaffolds made of PA nanofibers have been used to enhance the efficiency of stem cell therapy in muscle tissue. In this case, PA-based nanofiber scaffolds containing myogenic precursor cells and growth factors successfully emulated the structure, stiffness, and unidirectional alignment of muscle fibers, which permitted enhanced myoblast alignment and differentiation. Furthermore, the injection of PA-based nanofiber scaffolds loaded with muscle stem cells into the hindlimb of uninjured mice or mice with notexin-induced muscle injury resulted in enhanced engraftment, proliferation and differentiation of therapeutic cells compared to the injection of muscle stem cells alone. These results demonstrate the potential therapeutic value of this method in overcoming deficits of engraftment efficiency associated with standard muscle stem cell delivery approaches(31). PA-based scaffolds made from aligned nanofibers have also been used for neural tissue repair and regeneration, a process that is highly dependent on the orientation and direction of neurons and neurites in order to be effective. The interaction between neural progenitor cells and surface epitopes on the scaffold surface promoted neurite growth along the aligned nanofibers(32). Injection of a co-suspension of PAs and neural progenitor cells into the spinal cord of rats resulted in highly directional neurite outgrowth and migration of dorsal root ganglion cells along the orientation of the nanofibers, which suggests that PA-based nanofiber scaffolds could potentially find applications in the treatment of spinal cord injury(32).
Nanoparticles
Besides the use of nanosheets or nanofibers in stem cell culture, nanoparticles have been widely used in cell therapy approaches as carriers for intracellular delivery of bioactive cargo. For example, mesoporous silica nanoparticles (MSNs) loaded with retinoic acid (RA), a potent neural inducer, were used to increase neuronal differentiation in mouse ES cell cultures compared to direct exposure to RA alone, an effect that appears to be mediated by a more sustained release of RA after the MSNs were internalized by recipient cells(33). Comparable effects were observed when RA was loaded into thermoresponsive nanoparticles made from poly(N-isopropylacrylamide)-co-acrylamide (PNIPAM-co-Am) and used to support neuron-directed differentiation of human iPSCs(34). In addition, magnetically charged nanoparticles can be used to mediate gene transfer into stem cells. In one case, for example, magnetic fields were employed to pre-concentrate magnetic nanoparticles on the membrane of iPSCs and potentiate gene uptake(35). This approach appears to exhibit less toxicity compared to other delivery methods and preserves the differentiation potential of iPSCs.
Beyond in vitro studies, nanoparticles have also been used to support stem cell-based therapies in vivo, specifically in the treatment of brain injury. For example, Poly(b-amino ester) nanoparticles mediated improved transfection efficiencies and cell viability in human neural stem cell (hNSC) cultures. Furthermore, intracranial injection of transfected hNSCs encapsulated in hyaluronic acid hydrogels led to improved neurogenesis in rats subjected to traumatic brain injury(36). Additional in vivo studies have looked at the use of nanoparticles for cell therapies aimed at treating other neurodegenerative conditions. One study looked into harnessing the proliferation and differentiation potential of neural precursors inherently present in the subventricular zone (SVZ) as a therapeutic approach for PD(37). In adult mice, neural precursors in the SVZ use the rostral migratory stream to relocate to the olfactory bulb (OB), where they differentiate into neurons, mature, and integrate into existing neural circuits(38). In a mouse model of PD, treatment with poly(lactic acid-co-glycolic acid) (PLGA) and perfluoro-1,5-crown ether (PFCE) nanoparticles loaded with microRNA-124 (miR-124) led to a significant decrease in motor deficits, as well as an increase in the number of neural precursors migrating toward the OB and into the PD lesions, where increased maturation and circuit integration was observed (Figure 3A–E)(37). miR-124 is a known pro-neurogenic miR that is highly expressed in the SVZ and promotes neurogenesis through the inhibition of Sox9(39). Thus, the use of miR-124 loaded nanoparticles constitutes one of the few examples showing how nanoscale technologies can be used to regulate the induction of tissue-specific progenitor cells as therapeutic agents. Other studies have also investigated the use of nanoparticle-based systems to drive cell therapies for Alzheimer’s Disease (AD). For example, nanoparticles self-assembled from poly(2-hydroxyethyl methacrylate)-RA-poly(carboxybetaine) cell-penetrating peptide (PHEMA-RA-PCB-CPP) polymers were used to simultaneously deliver RA, small interfering RNA (siRNA) targeting Sox9, and traceable superparamagnetic iron oxide nanoparticles (SPIONs) into NSCs that were injected into the hippocampal region of 3xTg-AD mice, which led to a significant improvement in spatial memory(40). The use of SPIONSs allowed for the tracing and identification of therapeutic NSCs in the hippocampal region by magnetic resonance imaging (MRI) (Figure 3F–H)(40). Although nanoparticles have been extensively investigated for applications in gene therapies, the use of nanoparticle-based systems for cell therapies represent a promising alternative approach for tissue repair and regeneration.
Figure 3. Methodologies using nanoparticles to modulate stem cell differentiation.
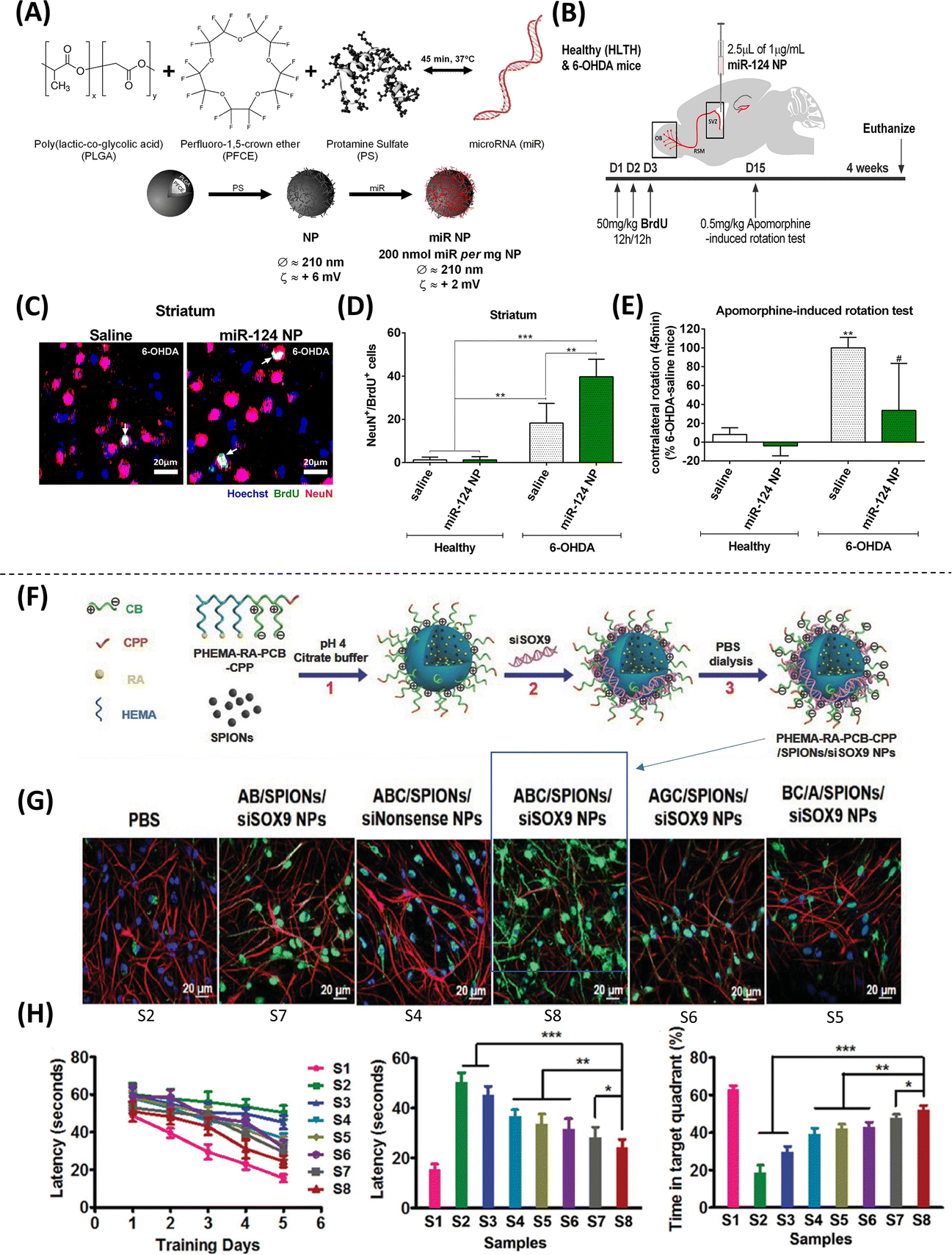
(A) Properties of nanoparticles (NPs) loaded with microRNAs. (B) Experimental setup where mice were subjected to two stereotaxic injections, one in the right striatum to deliver 6-Hydroxydopamine (6-OHDA) to induce a PD phenotype, and another in the right lateral ventricle to deliver miR-124 NPs or saline solution. (C-D) Confocal images of BrdU (proliferation marker, green), Hoechst (nuclear marker, blue), and DCX (mature neuronal marker, red) staining showing an increase in the number of mature neurons (NeuN+/BrdU+ cells) observed in the striatum of mice treated with 6-OHDA and miR-124 NPs compared to healthy controls. (E) Apomorphine-rotation test (behavioral analysis) illustrates a decrease in motor deficits (net contralateral rotations) in mice treated with miR-124 NP (Adapted from Saraiva et al., 2016. Ref 37). (F) Composition of the traceable NPs PHEMA-RA-PCB-CPP/SPIONs/siSOX9 (condensed as ABC/SPIONs/siSOX9 NPs: S8). (G) Immunostaining analysis with MAP-2 (neuronal marker, green), GFAP (glial cell marker, red), and DAPI (nuclear marker, blue), showing higher MAP-2 expression (conversion into neurons) when treated with S8 compared to control. (H) Morris water maze experiments were performed to assess the effect of NP treatment on spatial learning and memory improvement, showing that NSCs treated with S8 NPs could potentially improve cognition and memory (Adapted from Zhang et al., 2016. Ref 40).
Nanofiber-nanoparticle complexes
A few studies have investigated the combined use of nanofibers and nanoparticles to augment the differentiation efficiency of stem cells. Based on previous studies that demonstrated the effect of hydrogels on the stem cell viability and differentiation capacity, along with reports evaluating the effects of SPIONs on stem cell signaling, one study sought to evaluate how SPION-coated gelatin nanofibers embedded in alginate hydrogels altered stem cell viability and differentiation(41). Such composite hydrogels supported improved viability of ecto-mesenchymal stem cells (OE-MSCs) obtained from human olfactory mucosa and led to enhanced differentiation efficiencies into neurons compared to hydrogels without SPIONs(41). In a similar study, collagen type IV nanofibers coated with nucleated gold nanoparticles mediated an improvement of proliferation and differentiation potential of chorion placental-derived MSCs into neural- and cardiac-like cells(42). Likewise, when human adipose stem cells were cultured on gelatin-PCL nanofibers encapsulating both Titanium dioxide and MSNs loaded with metformin, there was an increase in cell viability after prolonged culture, a reduction of specific markers of senescence, and an increase in stemness markers(43). Altogether, these studies suggest that the combination of nanoparticles and nanofibers may improve the delivery of critical cargo while also providing a more suitable microenvironment for cell proliferation, function, and maintenance, under a single platform technology, which could be key to improving the expansion and differentiation efficiencies of stem cells and ultimately help facilitate the clinical translation of stem cell-based therapies.
Nanoscale technologies to potentiate endogenous cell-mediated tissue repair
In addition to supporting therapies based on exogenous/transplanted cells, nanoscale technologies have also been used to drive therapeutic responses via the modulation of endogenous cell responses and phenotypes. For example, PAs have been utilized to mimic specific biological structures and proteins, including the angiogenic signaling protein VEGF. PA nanofilaments containing VEGF-mimetic peptides on their surfaces are capable of phosphorylating VEGF receptors on endothelial cells, leading to an increase in angiogenesis(44). The therapeutic potential of this was demonstrated in vivo in ischemic hind-limbs of mice, in which intramuscular injection of PA nanofilaments with VEGF-mimetic peptides were shown to improve perfusion, motor function, and tissue repair compared to control injections of mimetic peptides alone (Figure 4A–H)(44). Other therapeutic applications of this approach include the repair of spinal cord and peripheral nerves using nanotube scaffolds and nanofibers gels, respectively(45, 46). These nanostructures provide an environment that promotes and guides axon and nerve growth, allowing for the improvement of nerve and motor function in vivo. In addition, nanofibers, nanotubes, and nanoparticles have been used to modify intrinsic cell phenotypes to improve osteogenesis and treat tendon injury. For example, nanotubes loaded with resveratrol were used to create a coating that can be applied to titanium bone implants to reduce the levels of radical oxygen species and inflammation in situ(47). Moreover, PLGA nanofibers have been shown to reduce peritendinous adhesion and guide tendon regeneration in a rat model of Achilles tendon injury(48). The implementation of nanoscale-based technologies to improve endogenous tissue responses has also been used for wound healing applications, where the use of gold nanoparticles was found to significatively accelerate the healing process, increase collagen deposition and angiogenesis, and reduce oxidative stress (49). Related applications include the use of sutures made of biocompatible carbon nanotube fibers to improve myocardial conduction(50), as well as the development of conductive nanofibrous membranes to enhance cardiac function and revascularization after myocardial infarction(Figure 4I–N)(51). The aforementioned studies indicate that besides their use for the delivery of cells as therapeutic agents, nanotechnology-based approaches can also be used to modulate endogenous cell responses as a therapeutic strategy in regenerative medicine.
Figure 4. Approaches used for endogenous cell repair.
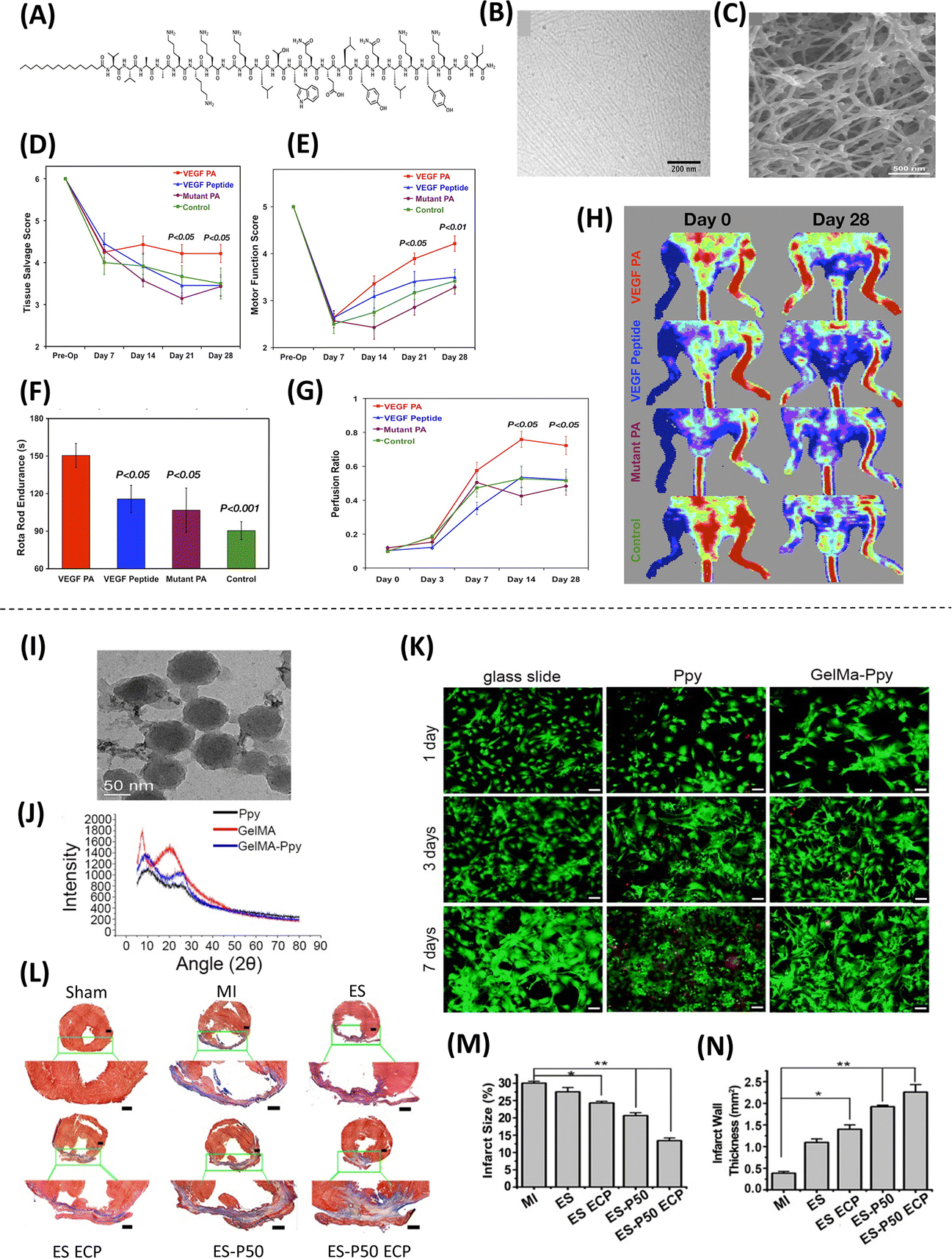
(A) Representation of the chemical structure of the PA intended to mirror VEGF’s activity. (B) Nanofiber structure formed by the VEGF can be seen by cryogenic Transmission electron microscopy (TEM) (C), as well as the interconnected nanofiber gel network, captured by Scanning electron microscopy (SEM). To evaluate the ability of VEGF-mimetic PA as a therapy for ischemic disorders using a murine hind-limb ischemia model, limb salvage and motor function was evaluated, showing (D) an improvement in tissue salvage score (i.e., less necrosis) and (E) a significant effect on active limb motor function in the treatment group compared to the control groups. (F) Functional tests show enhanced walking time preceding failure, and (G-H) Laser Doppler perfusion imaging shows enhanced tissue perfusion ratio in the ischemic hind limb for 28 days following treatment (Adapted from Webber et al., 2011. Ref 44). High concentration of methyl acrylic anhydride-gelatin (GelMA)-Ppy nanoparticles were used to fabricate engineered cardiac patches (ECP). Characterization and analysis of the nanoparticles, showing (I) uniform spherical morphology and size via TEM, and (J) molecular structure via X-ray diffraction (XRD). (K) Live/death staining shows great biocompatibility of the nanoparticles for 7 days without affecting cell growth. (L) Cardiac sections were stained with Masson’s staining for fibrous tissue (blue) and myocardium (red), showing enhanced cardiac function and revascularization for patch-implanted groups, which is also evident in the analysis of (M) the infarct size and (N) the infarct wall thickness (Adapted from He et al., 2018. Ref 51).
Nanoscale technologies for cell-mediated drug delivery
While nanotechnology has commonly been explored for the direct delivery of drugs to target tissues, the use of nanotechnology to facilitate cell-mediated drug delivery has emerged as a promising alternative strategy to the use of more established carrier systems for drug delivery. One example of this is the use of “cellular backpacks”, which are polymeric carriers that are several hundred nm thick, and 7–10 μm in diameter, and can be attached directly to cells(52). These backpacks are unique in their ability to encapsulate therapeutic cargo that travels with the cell carrier, while at the same time avoiding phagocytosis and clearance (52, 53). As such, cellular backpacks have demonstrated therapeutic potential for targeted drug delivery to the brain, mediated by macrophages that can cross the blood brain barrier. This was demonstrated in vivo via injection of macrophages with cellular backpacks containing catalase in a mouse model of brain inflammation(Figure 5A–C)(53). Experiments conducted in vitro using these macrophage-based carriers resulted in reduced neuroinflammatory responses, which further demonstrates the therapeutic potential of this approach. A similar approach explored the use of macrophages to deliver “nanozyme” polycomplexes of catalase and PEI-poly(ethylene glycol) (PEI-PEG)(54). This approach was tested in vivo through tail vein injections of bone marrow-derived macrophages (BMM) containing catalase nanozymes in mice with induced neuroinflammation. Interestingly, nanozyme-loaded BMM’s were observed to cross the BBB, which led to an increase in the accumulation of catalase in target brain tissues compared to mice receiving nanozyme injections alone (Figure 5D–F)(54). Taken together, the use of cellular backpacks and nanozymes for cell-mediated delivery of anti-inflammatory drugs to the brain could prove useful developing treatments for neuroinflammatory diseases such a Parkinson’s disease. Cell-mediated drug delivery has also been investigated for use in drug delivery for deep lung therapy, which is currently limited by the lack of efficient drug delivery methodologies to target tissues. A prominent example of this is the use of chitosan nanoparticles as a drug carriers for delivery mediated by Sertoli cells. In one study, rat Sertoli cells that were preloaded with chitosan nanoparticles containing curcumin were injected into the bloodstream of mice with pulmonary inflammation(55). Subsequent quantification of the distribution of both the nanoparticles and curcumin load indicated that the delivery of these components by Sertoli cells was largely limited to target pulmonary tissues, with significantly less amounts of both components present in other organs such as kidney and liver(55). As such, the use of Sertoli-mediated drug delivery in this regard could be useful for overcoming hurdles associated with targeting pulmonary tissue. Outside of regenerative medicine, the therapeutic use of non-virally transfected myeloid-derived suppressor cells (MDSCs) for extracellular vesicle (EV)-mediated gene delivery has been evaluated in the treatment of tumors in vivo using mice(56). MDSCs, however, are also involved in non-neoplastic conditions such as stroke and Alzheimer’s Disease. Therefore, the implementation of MDSCs for the delivery of therapeutic EVs could also prove useful in regenerative medicine applications.
Figure 5. Methodologies employed for cell-mediated drug delivery.
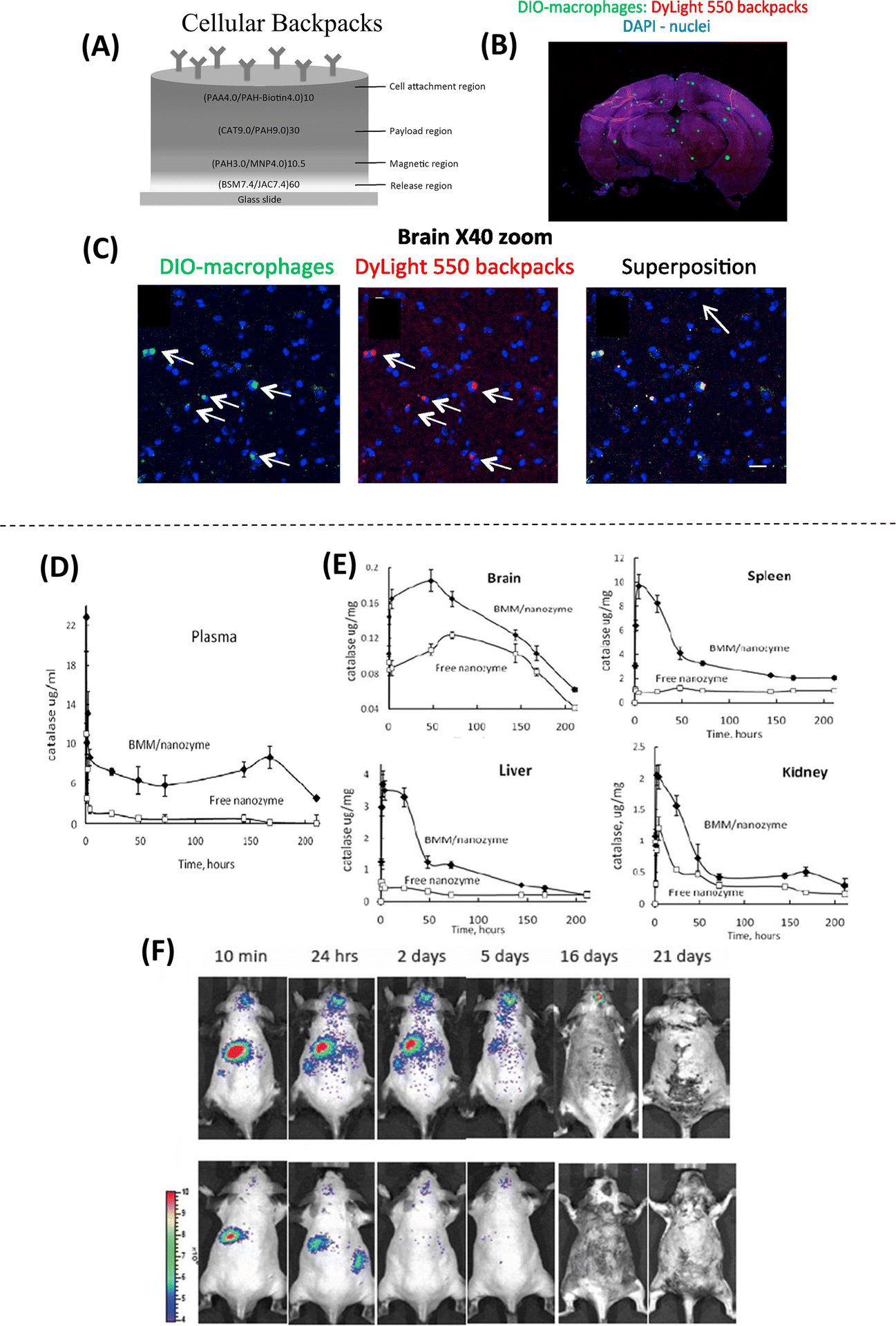
(A) Schematic of the structure of the cellular backpack, loaded with catalase, showing the composition and assembly of different regions from the “release region” to the cell attachment region. (B) Confocal microscopy images of the whole brain after systemic delivery of backpack-carrying macrophages show fluorescently labeled macrophages (green) and backpacks (red). (C) At 40X magnification, co-localization of green and red can be observed, suggesting that the cells facilitated the transport of backpacks to the brain (Adapted from Klyachko et al., 2017. Ref 53). (D) Systemic delivery of bone marrow-derived macrophages (BMM) loaded with a catalase nanozyme in a murine model of brain inflammation shows an increased blood concentration of nanozyme for more than 170 hours after injection. (E) Increased accumulation of catalase is found in all tissues (brain, spleen, liver, and Kidney) when using nanozyme-loaded BMM. Higher accumulation of the nanozyme is found in the spleen and lower accumulation in the brain. (F) Biodistribution of BMM loaded with fluorescently labeled nanozyme, showing targeted drug delivery from peripheral organs to the brain with inflammation for over 16–20 days (top panel) compared to healthy animals (bottom panel) (Adapted from Zhao et al., 2011. Ref 54).
Nanoscale technologies for direct reprogramming-based cell therapies
Direct cell reprogramming refers to the process of inducing a change in cell fate without the need for a pluripotent intermediate state(57). Direct reprogramming has opened up the possibility for the development of patient-specific cell-based therapies(58) that can overcome major limitations of traditional approaches by utilizing more readily-available cell sources (e.g., skin fibroblasts) and bypassing the need for induced pluripotency(59), thus offering significant improvements in safety and efficacy. While traditional approaches to cell reprogramming, such as the use of viral vectors, have produced promising results, biosafety concerns and capsid size restrictions pose significant hurdles to clinical translation. As such, a variety of nanotechnologies, including nanoparticles and nanotransfection methods, have been developed to overcome these limitations.
Nanoparticles in direct cell reprogramming
In recent years, various studies have investigated the use of engineered nanoparticles in direct cell reprogramming applications. Nanoparticles are typically used as carriers of bioactive cargo (e.g., nucleic acids, protein, etc.) responsible for inducing direct nuclear reprogramming. The vast majority of nanoparticle systems used for bioactive cargo delivery are based on polyethylenimine (PEI) polyplexes, which facilitate cellular uptake via endocytosis(60). One study looked into the use of nanocomplexes made from MSNs, PEI, and recombinant proteins of HNF4A and FOXA3 transcription factors, to drive effective conversions of mouse fibroblasts into induced hepatocyte-like cells with reduced toxicity compared to commercially available carrier systems like Lipofectamine 2000, or PEI alone, suggesting that MSN-based nanoparticles may offer a safer alternative for cell reprogramming that is more suitable for therapeutic applications (Figure 6A–B)(61). Another notable application of nanoparticles in direct cell reprogramming is the use of gold nanoparticles for the induction of cardiomyocytes in vitro and in vivo. Nanocomplexes consisting of PEI and gold nanoparticles loaded with Gata4, Mef2c, and Tbx5 (GMT) genes successfully converted fibroblasts into cardiomyocytes in vitro(62). Additionally, successful cardiac reprogramming and improved cardiac function were reported when these nanoparticles were injected into the hearts of mice after myocardial infarction, suggesting potential for future therapeutic applications based on cardiac tissue reprogramming (Figure 6C–F)(62). PEI-miRNA polyplexes encapsulated in PLGA nanospheres have also been used to mediate direct cell reprogramming. These nanospheres were loaded with miR-1 and miR-133, and subsequently used to treat human cardiac fibroblasts in vitro, resulting in improved cellular uptake and induction of adult human cardiomyocyte-like cells compared to PEI and lipofectamine(63). While the use of these nanospheres has not been demonstrated in applications of cell therapy, directly, these results indicate that this technique has great potential for such applications and thus warrants further exploration in vivo. On another front, electromagnetized gold nanoparticles have been shown to augment reprogramming efficiencies following viral transfection of reprogramming factors. In this approach, fibroblasts are transfected with lentiviruses and seeded onto an electromagnetized gold nanoparticle substrate in the presence of an electromagnetic field (EMF). This causes the nanoparticles to become transiently magnetized and enhances the transfer of energy from the EMF to recipient cells, resulting in potentiation of reprogramming efficiency through induction of histone acetyltransferase Brd2 and subsequent acetylation of histone H3K27, which opens up the chromatin and potentiates neuronal gene expression(64). This was demonstrated in vitro using fibroblasts that were transfected with induced dopaminergic reprogramming factor genes Ascl1, Pitx3, Lmx1a and Nurr1 (APLN), where a significant increase in reprogramming efficiency to induced dopaminergic neurons was observed after exposure to an EMF compared to APLN-transfected fibroblasts treated with EMF or gold nanoparticles alone(64). In the same report and similar to the results obtained with stem cells, in vivo direct reprogramming using a PD mouse model injected with gold nanoparticles and lentivirus containing the APLN cocktail, followed by application of an EMF, showed increased reprogramming efficiency, as well significant amelioration of PD(64).
Figure 6. Approaches implementing nanoparticles for the direct reprogramming of somatic cells.
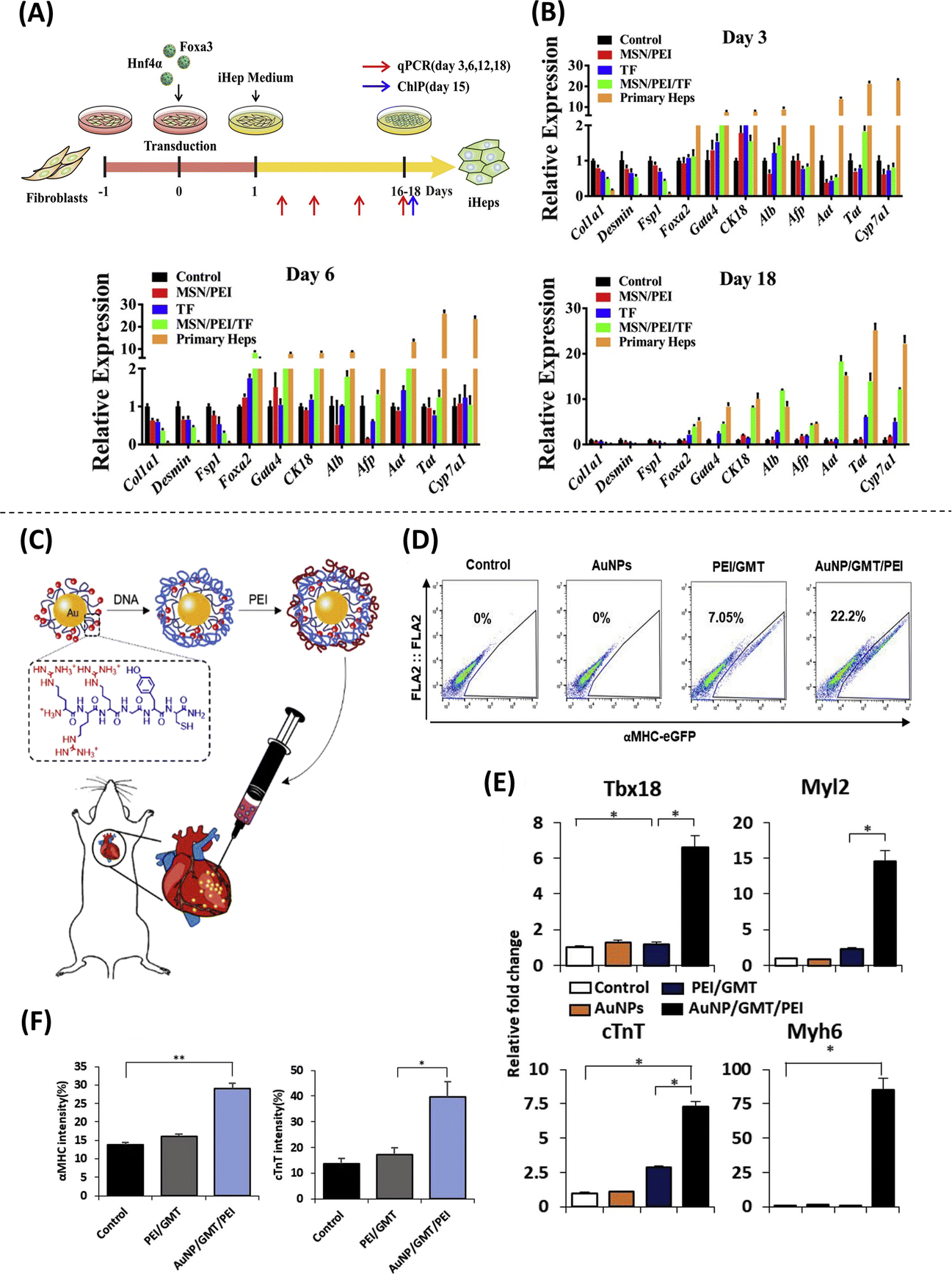
(A) Induced hepatocyte-like cells (iHeps) converted from mouse fibroblasts using MSN/PEI/Transcription factor nanocomplexes. (B) Successful conversion into iHeps by quantitative expression, showing gradual downregulation of Col1a1, Desmin and Fsp1 (fibroblasts genes) and upregulation of hepatocyte genes (rest of the genes) in the treatment group compared to other groups (Adapted from Wang et al., 2020. Ref 61). (C) Schematic illustration of direct injection of PEI/gold nanoparticles loaded with GMT genes (AuNP/GMT/PEI) into the heart of a mouse. (D) Cell cytometry analysis showing efficient reprogramming from mouse embryonic fibroblasts into induced cardiomyocytes after using AuNP/GMT/PEI nanocomplexes by the expression of αMHC. (E) Upregulation of cardiomyocyte genes in the treatment group compared to control via qRT-PCR. (F) immunostaining of injured heart displaying a significant increase in the number of cardiac Troponin T + (cTNT) and α-MHC+ cells relative to controls (Adapted from Chang et al., 2019. Ref 62).
Nanochannel-based transfection in direct cell reprogramming
In addition to nanoparticle-driven reprogramming, nanochannel-based transfection is also being actively explored for direct cell reprogramming applications. Electroporation refers to the use of electric fields to disrupt the permeability of the cell membrane and allow for the transfer of exogenous cargo into the cytosol, including proteins and nucleic acids(65). However, traditional electroporation methods typically yield low transfection efficiencies and suboptimal cell viabilities. Nanochannel-mediated electrotransfection or nanotransfection has emerged as a promising alternative approach to standard bulk electroporation. Recent studies have shown that the implementation of electric fields through nanochannels, in vitro or in vivo, leads to increased transmembrane potential, localized membrane poration, and active electrophoretic delivery of charged cargo (e.g., nucleic acids) into the cells, which collectively translates into improved transfection efficiencies and superior cell viabilities(66–71). In vitro nanotransfection has been used to drive fast and effective direct reprogramming of fibroblasts into induced neurons via the delivery of pro-neurogenic factors Ascl1, Brn2, and Myt1L (ABM), or into induced endothelial cells via delivery of provasculogenic factors Etv2, Fli1, and Foxc2 (EFF)(66, 68, 71). The translational potential of this platform nanotechnology in direct reprogramming applications was recently demonstrated in a study in which EFF-nanotransfected fibroblasts were injected intracranially into mice that had suffered an ischemic stroke, resulting in improved brain vascularization, reduced edema, decreased gliosis, and increased neuronal preservation associated with improved sensorimotor function (Figure 7)(68). Moreover, tissue nanotransfection (TNT) has been demonstrated as a method to electrophoretically deliver molecular cargo directly to tissues for the purposes of direct cell reprogramming in vivo (Figure 8A–J)(66). Notably, the use of TNT to deliver EFF into the skin and peripheral nerves has been shown to mediate direct reprogramming-based regenerative processes that can protect ischemic limbs from necrosis(66) and promote nerve tissue repair following crush injury (Figure 8K–N)(69). Interestingly, nanotransfection-driven reprogramming seems to be partially mediated by extracellular vesicles (EVs), which appear to allow for the propagation of reprogramming cargo delivery beyond the nanotransfected cells(66, 68).
Figure 7. Nanochannel-based technology to modulate the direct reprogramming of fibroblasts into induced endothelial cells as therapeutic agents for stroke.
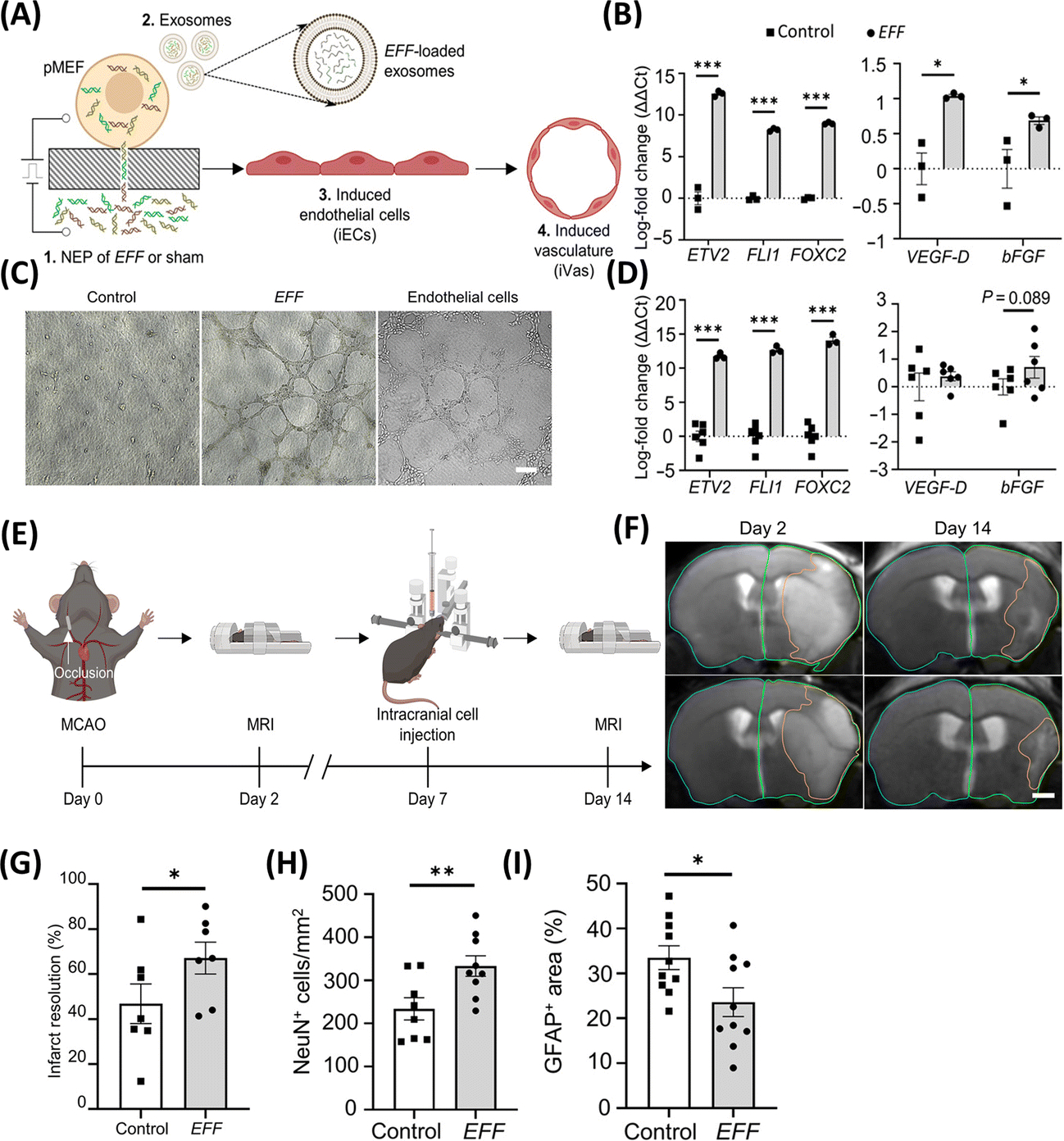
(A) Schematic diagram illustrating the nanotransfection with EFF, release of pro-vasculogenic/angiogenic EVs (e.g., exosomes), and reprogramming of fibroblasts into induced endothelial cells (iECs) that subsequently mediate the formation of induced vasculature (iVas). (B) Upregulation of genes in nanotransfected cells and (C) loaded in released exosomes. (D) In vitro tube formation assay in the EFF group. (E) Schematic diagram of middle cerebral artery occlusion, intracranial injection, and MRIs. (F, G) T2-weighted MR images post-stroke show that intracranial injection of EFF-nanotransfected cells led to significantly improved infarct resolution compared to control in mice that exceeded 17% weight loss. EFF-nanotransfected cells injected in mice show (H) superior neuronal cellularity (NeuN) and (I) reduced astroglial scar formation (GFAP) (Adapted from Lemmerman et al., 2021. Ref 68).
Figure 8. Methodologies employing nanochannel-based technologies to regulate the direct reprogramming of fibroblasts into neurons in vivo and promote nerve tissue repair.
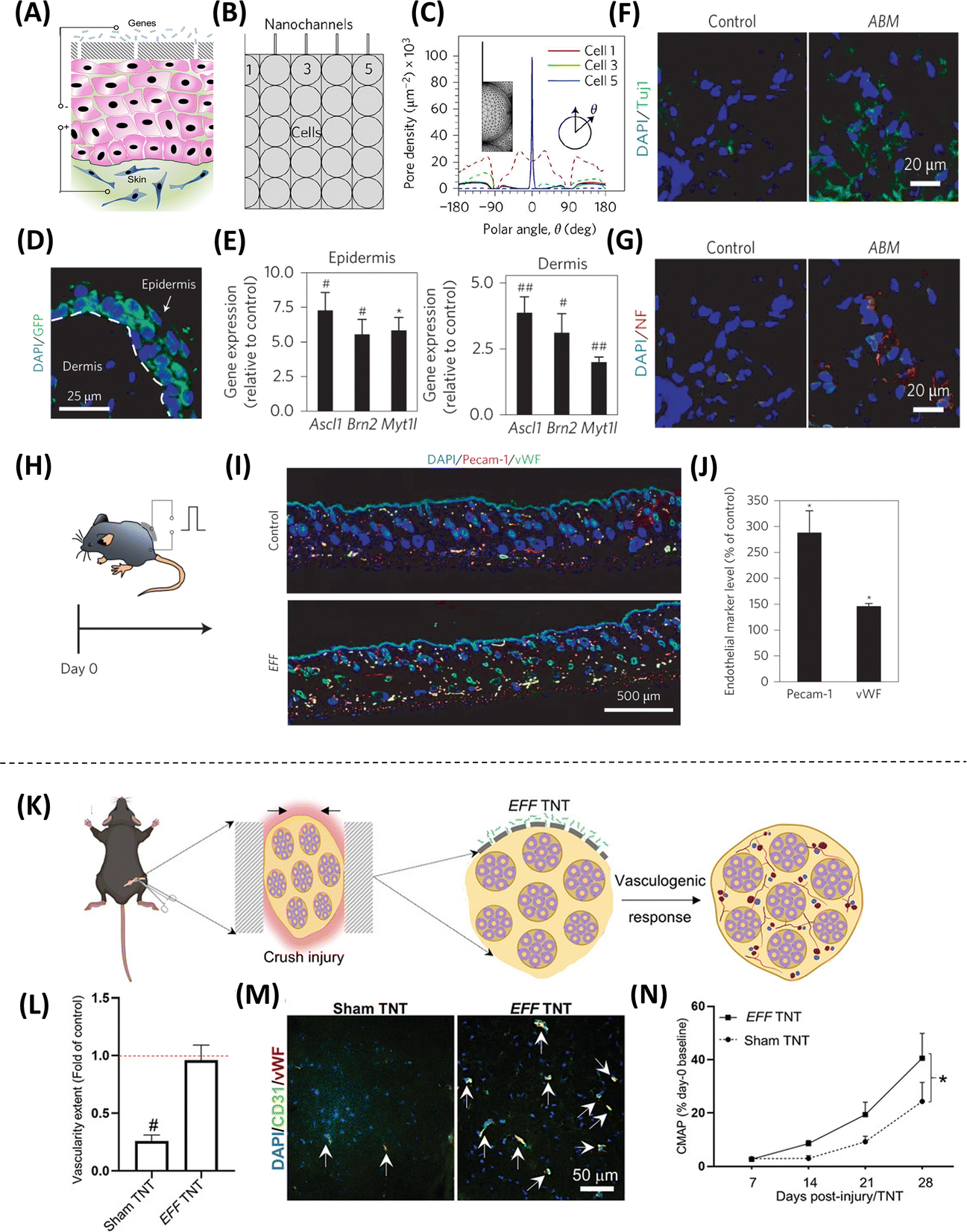
(A) Schematic representation of the TNT procedure on the skin, where an electric field is applied through the electrodes to create nanopores in the membranes of exposed cells and drive cargo into the skin-cells via electrophoresis. (B) The outermost cell layer is in direct contact with the Nanochannels. (C) Simulations show focused (solid) compared to widespread (dashed) poration in TNT vs. Bulk electroporation (BEP). (D) Mouse skin showing successful gene delivery and expression via confocal imaging. (E) Epidermis and dermis analyses showing gene expression using Laser capture microdissection (LCM) and qRT–PCR. Immunostaining results display increased (F) TUJ1 and (G) neurofilament (NF) expression after TNT-based delivery of ABM. (H) TNT with EFF on the skin of mice led to (I-J) increased iVAS (Pecam-1, vWF) at day 7 (Adapted from Gallego-Perez et al., 2017. Ref 66). (K) Delivery of EFF using TNT in a crushed nerve tissue model, which leads to (L-M) increased vascularity, as well as (N) accelerated recovery of nerve function, which was assessed using compound muscle action potential (CMAP) measurements (Adapted from Moore et al., 2020. Ref 69).
Engineered EVs in direct cell reprogramming
The observation that nanotransfection can result in the release of engineered EVs (eEVs) loaded with customizable reprogramming cargo led to a series of experiments probing the role of eEVs in the modulation of direct reprogramming and tissue reparative processes (66, 72). For example, eEVs derived from skin cells nanotransfected with ABM reprogramming factors were shown to successfully convert fibroblasts into induced neurons, in vitro, as well as improve stroke recovery in mice in vivo (66). Similarly, eEVs derived from skin cells nanotransfected with EFF were shown to successfully convert fibroblasts into induced endothelial cells, as well as increase tissue perfusion in mice with critical limb ischemia(66). These observations demonstrating the potential applications of eEVs in direct reprogramming-based cell therapies were recently corroborated in a separate report, in which Foxf1-loaded EVs were used to reprogram nucleus pulposus cells in vitro and in vivo, which could potentially lead to the development of novel therapies for intervertebral disc degeneration(73).
Conclusions
Cell-based therapies have emerged as a promising alternative strategy for the treatment of a wide variety of conditions. The implementation of various nanoscale technologies in stem cell research, including nanofibers, graphene nanosheets, and nanoparticles, among others, has been shown to support enhanced safety and efficacy during stem cell propagation and differentiation processes. In addition, nanoscale platforms such as nanoparticles, nanospheres, nanotransfection, and eEVs, among others, could potentially provide effective ways to modulate cellular plasticity for therapeutic purposes both in vitro and in vivo. Collectively, nanoscale technologies have the potential to address key hurdles in the development of regenerative cell therapies and ultimately facilitate the clinical translation of such therapies. However, there are still several roadblocks hampering translation of different cell therapies. One of the biggest hurdles is scale-up to obtain an adequate number of functional therapeutic cells(74). Scale-up requirements in turn significantly increase the manufacturing costs and complicate the procurement of homogeneous populations of therapeutic cells for safe use in patients(74–76). Additional aspects to be considered include the need for more readily available and healthy cell sources. As such, allogenic cell sources may offer an advantage over autologous cell sources. However, allogenic cells run the risk of eliciting adverse immune responses in some cases, thus requiring the use of systemic immunosuppressants that could lead to secondary complications. Nevertheless, recent studies have shown that nanoscale technologies could also be used to reduce immune rejection of allogenic cell grafts(77, 78). Moreover, in addition to the technologies surveyed in this mini-review focused on stem cell- and direct reprogramming-based cell therapies, other emergent nanotechnologies for improved non-viral gene delivery ex vivo(79–81), and/or targeted delivery of genes/therapeutics to specific cell and tissue niches, in vivo(82–84), have the potential to continue to revolutionize the field of cell therapies in regenerative medicine by enabling safer and more effective approaches to deploy living therapeutics to damaged organs and tissues. Future efforts, however, should continue to strive towards clinical trials to assess not only the safety but also efficacy of cell therapies and their enabling technologies.
Acknowledgements:
Some illustrations were created using biorender.com
Funding Statement:
Funding for this work was partly provided by a New Innovator Award DP2EB028110 (NIBIB/NIH), DP1DK126199 (NIDDK/NIH), and the Lisa Dean Moseley Foundation.
Footnotes
Conflict of Interest Statement:
The authors have no conflicts of interest to declare.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Wang M-L. The Modern Pharmaceutical Industry: History, Current Position and Challenges. Global Health Partnerships: The Pharmaceutical Industry and BRICA. London: Palgrave Macmillan UK; 2009. p. 33–80. [Google Scholar]
- 2.Mason C, Brindley DA, Culme-Seymour EJ, Davie NL. Cell therapy industry: billion dollar global business with unlimited potential. Regen Med. 2011;6(3):265–72. [DOI] [PubMed] [Google Scholar]
- 3.Fischbach MA, Bluestone JA, Lim WA. Cell-based therapeutics: the next pillar of medicine. Sci Transl Med. 2013;5(179):179ps7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Au P, Hursh DA, Lim A, Moos MC Jr., Oh SS, Schneider BS, et al. FDA oversight of cell therapy clinical trials. Sci Transl Med. 2012;4(149):149fs31. [DOI] [PubMed] [Google Scholar]
- 5.Towards advanced cell therapies. Nature Biomedical Engineering. 2018;2(6):339–40. [DOI] [PubMed] [Google Scholar]
- 6.Science N,. TC. National nanotechnology initiative, research and development leading to a revolution in technology and industry. A supplement to the President’s FY 2006 budget. 2005.
- 7.Heath JR. Nanotechnologies for biomedical science and translational medicine. Proc Natl Acad Sci U S A. 2015;112(47):14436–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mount NM, Ward SJ, Kefalas P, Hyllner J. Cell-based therapy technology classifications and translational challenges. Philos Trans R Soc Lond B Biol Sci. 2015;370(1680):20150017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanchez A, Schimmang T, Garcia-Sancho J. Cell and tissue therapy in regenerative medicine. Adv Exp Med Biol. 2012;741:89–102. [DOI] [PubMed] [Google Scholar]
- 10.Yamanaka S Pluripotent Stem Cell-Based Cell Therapy-Promise and Challenges. Cell Stem Cell. 2020;27(4):523–31. [DOI] [PubMed] [Google Scholar]
- 11.Dreyer DR, Park S, Bielawski CW, Ruoff RS. The chemistry of graphene oxide. Chem Soc Rev. 2010;39(1):228–40. [DOI] [PubMed] [Google Scholar]
- 12.Jing G, Li K, Sun F, Niu J, Zhu R, Qian Y, et al. Layer-Number-Dependent Effects of Graphene Oxide on the Pluripotency of Mouse Embryonic Stem Cells Through the Regulation of the Interaction Between the Extracellular Matrix and Integrins. Int J Nanomedicine. 2021;16:3819–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang D, Li T, Xu M, Gao F, Yang J, Yang Z, et al. Graphene oxide promotes the differentiation of mouse embryonic stem cells to dopamine neurons. Nanomedicine (Lond). 2014;9(16):2445–55. [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Alegria E, Iliut M, Stefanska M, Silva C, Heeg S, Kimber SJ, et al. Graphene Oxide promotes embryonic stem cell differentiation to haematopoietic lineage. Sci Rep. 2016;6:25917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park SY, Park J, Sim SH, Sung MG, Kim KS, Hong BH, et al. Enhanced differentiation of human neural stem cells into neurons on graphene. Adv Mater. 2011;23(36):H263–7. [DOI] [PubMed] [Google Scholar]
- 16.Galipeau J, Sensebe L. Mesenchymal Stromal Cells: Clinical Challenges and Therapeutic Opportunities. Cell Stem Cell. 2018;22(6):824–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Halim A, Luo Q, Ju Y, Song G. A Mini Review Focused on the Recent Applications of Graphene Oxide in Stem Cell Growth and Differentiation. Nanomaterials (Basel). 2018;8(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nayak TR, Andersen H, Makam VS, Khaw C, Bae S, Xu X, et al. Graphene for controlled and accelerated osteogenic differentiation of human mesenchymal stem cells. ACS Nano. 2011;5(6):4670–8. [DOI] [PubMed] [Google Scholar]
- 19.Lee WC, Lim CH, Shi H, Tang LA, Wang Y, Lim CT, et al. Origin of enhanced stem cell growth and differentiation on graphene and graphene oxide. ACS Nano. 2011;5(9):7334–41. [DOI] [PubMed] [Google Scholar]
- 20.Lee WC, Lim CH, Kenry, Su C, Loh KP, Lim CT. Cell-assembled graphene biocomposite for enhanced chondrogenic differentiation. Small. 2015;11(8):963–9. [DOI] [PubMed] [Google Scholar]
- 21.Kim J, Park S, Kim YJ, Jeon CS, Lim KT, Seonwoo H, et al. Monolayer Graphene-Directed Growth and Neuronal Differentiation of Mesenchymal Stem Cells. J Biomed Nanotechnol. 2015;11(11):2024–33. [DOI] [PubMed] [Google Scholar]
- 22.Guo W, Wang S, Yu X, Qiu J, Li J, Tang W, et al. Construction of a 3D rGO-collagen hybrid scaffold for enhancement of the neural differentiation of mesenchymal stem cells. Nanoscale. 2016;8(4):1897–904. [DOI] [PubMed] [Google Scholar]
- 23.Vining KH, Mooney DJ. Mechanical forces direct stem cell behaviour in development and regeneration. Nat Rev Mol Cell Biol. 2017;18(12):728–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kenry Lim CT. Nanofiber technology: current status and emerging developments. Progress in Polymer Science. 2017;70:1–17. [Google Scholar]
- 25.Bagher Z, Azami M, Ebrahimi-Barough S, Mirzadeh H, Solouk A, Soleimani M, et al. Differentiation of Wharton’s Jelly-Derived Mesenchymal Stem Cells into Motor Neuron-Like Cells on Three-Dimensional Collagen-Grafted Nanofibers. Mol Neurobiol. 2016;53(4):2397–408. [DOI] [PubMed] [Google Scholar]
- 26.Sankar D, Mony U, Rangasamy J. Combinatorial effect of plasma treatment, fiber alignment and fiber scale of poly (epsilon-caprolactone)/collagen multiscale fibers in inducing tenogenesis in non-tenogenic media. Mater Sci Eng C Mater Biol Appl. 2021;127:112206. [DOI] [PubMed] [Google Scholar]
- 27.Sridharan D, Palaniappan A, Blackstone BN, Dougherty JA, Kumar N, Seshagiri PB, et al. In situ differentiation of human-induced pluripotent stem cells into functional cardiomyocytes on a coaxial PCL-gelatin nanofibrous scaffold. Mater Sci Eng C Mater Biol Appl. 2021;118:111354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jeon BM, Yeon GB, Goo HG, Lee KE, Kim DS. PVDF Nanofiber Scaffold Coated with a Vitronectin Peptide Facilitates the Neural Differentiation of Human Embryonic Stem Cells. Dev Reprod. 2020;24(2):135–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abazari MF, Zare Karizi S, Hajati-Birgani N, Norouzi S, Khazeni Z, Hashemi J, et al. PHBV nanofibers promotes insulin-producing cells differentiation of human induced pluripotent stem cells. Gene. 2021;768:145333. [DOI] [PubMed] [Google Scholar]
- 30.Cui H, Cheetham AG, Pashuck ET, Stupp SI. Amino Acid Sequence in Constitutionally Isomeric Tetrapeptide Amphiphiles Dictates Architecture of One-Dimensional Nanostructures. Journal of the American Chemical Society. 2014;136(35):12461–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sleep E, Cosgrove BD, McClendon MT, Preslar AT, Chen CH, Sangji MH, et al. Injectable biomimetic liquid crystalline scaffolds enhance muscle stem cell transplantation. Proceedings of the National Academy of Sciences. 2017;114(38):E7919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berns EJ, Sur S, Pan L, Goldberger JE, Suresh S, Zhang S, et al. Aligned neurite outgrowth and directed cell migration in self-assembled monodomain gels. Biomaterials. 2014;35(1):185–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park SJ, Kim S, Kim SY, Jeon NL, Song JM, Won C, et al. Highly Efficient and Rapid Neural Differentiation of Mouse Embryonic Stem Cells Based on Retinoic Acid Encapsulated Porous Nanoparticle. ACS Appl Mater Interfaces. 2017;9(40):34634–40. [DOI] [PubMed] [Google Scholar]
- 34.Seo HI, Cho AN, Jang J, Kim DW, Cho SW, Chung BG. Thermo-responsive polymeric nanoparticles for enhancing neuronal differentiation of human induced pluripotent stem cells. Nanomedicine. 2015;11(7):1861–9. [DOI] [PubMed] [Google Scholar]
- 35.Yamoah MA, Moshref M, Sharma J, Chen WC, Ledford HA, Lee JH, et al. Highly efficient transfection of human induced pluripotent stem cells using magnetic nanoparticles. Int J Nanomedicine. 2018;13:6073–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li X, Tzeng SY, Liu X, Tammia M, Cheng YH, Rolfe A, et al. Nanoparticle-mediated transcriptional modification enhances neuronal differentiation of human neural stem cells following transplantation in rat brain. Biomaterials. 2016;84:157–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saraiva C, Paiva J, Santos T, Ferreira L, Bernardino L. MicroRNA-124 loaded nanoparticles enhance brain repair in Parkinson’s disease. J Control Release. 2016;235:291–305. [DOI] [PubMed] [Google Scholar]
- 38.Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132(4):645–60. [DOI] [PubMed] [Google Scholar]
- 39.Cheng LC, Pastrana E, Tavazoie M, Doetsch F. miR-124 regulates adult neurogenesis in the subventricular zone stem cell niche. Nat Neurosci. 2009;12(4):399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang R, Li Y, Hu B, Lu Z, Zhang J, Zhang X. Traceable Nanoparticle Delivery of Small Interfering RNA and Retinoic Acid with Temporally Release Ability to Control Neural Stem Cell Differentiation for Alzheimer’s Disease Therapy. Adv Mater. 2016;28(30):6345–52. [DOI] [PubMed] [Google Scholar]
- 41.Karimi S, Bagher Z, Najmoddin N, Simorgh S, Pezeshki-Modaress M. Alginate-magnetic short nanofibers 3D composite hydrogel enhances the encapsulated human olfactory mucosa stem cells bioactivity for potential nerve regeneration application. Int J Biol Macromol. 2021;167:796–806. [DOI] [PubMed] [Google Scholar]
- 42.Orza A, Soritau O, Olenic L, Diudea M, Florea A, Rus Ciuca D, et al. Electrically conductive gold-coated collagen nanofibers for placental-derived mesenchymal stem cells enhanced differentiation and proliferation. ACS Nano. 2011;5(6):4490–503. [DOI] [PubMed] [Google Scholar]
- 43.Pourpirali R, Mahmoudnezhad A, Oroojalian F, Zarghami N, Pilehvar Y. Prolonged proliferation and delayed senescence of the adipose-derived stem cells grown on the electrospun composite nanofiber coencapsulated with TiO2 nanoparticles and metformin-loaded mesoporous silica nanoparticles. Int J Pharm. 2021;604:120733. [DOI] [PubMed] [Google Scholar]
- 44.Webber MJ, Tongers J, Newcomb CJ, Marquardt K-T, Bauersachs J, Losordo DW, et al. Supramolecular nanostructures that mimic VEGF as a strategy for ischemic tissue repair. Proceedings of the National Academy of Sciences. 2011;108(33):13438–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Usmani S, Franceschi Biagioni A, Medelin M, Scaini D, Casani R, Aurand ER, et al. Functional rewiring across spinal injuries via biomimetic nanofiber scaffolds. Proc Natl Acad Sci U S A. 2020;117(41):25212–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu X, He L, Li W, Li H, Wong WM, Ramakrishna S, et al. Functional self-assembling peptide nanofiber hydrogel for peripheral nerve regeneration. Regen Biomater. 2017;4(1):21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang R, Yan Y, Wu Z, Wei Y, Song H, Zhu L, et al. Resveratrol-loaded titania nanotube coatings promote osteogenesis and inhibit inflammation through reducing the reactive oxygen species production via regulation of NF-κB signaling pathway. Mater Sci Eng C Mater Biol Appl. 2021;131:112513. [DOI] [PubMed] [Google Scholar]
- 48.Uyanik O, Pekkoc-Uyanik KC, Findik S, Avci A, Altuntas Z. Prevention of peritendinous adhesions with electrospun poly (lactic acid-co-glycolic acid) (PLGA) bioabsorbable nanofiber: An experimental study. Colloids Surf B Biointerfaces. 2022;209(Pt 2):112181. [DOI] [PubMed] [Google Scholar]
- 49.Kim JE, Lee J, Jang M, Kwak MH, Go J, Kho EK, et al. Accelerated healing of cutaneous wounds using phytochemically stabilized gold nanoparticle deposited hydrocolloid membranes. Biomater Sci. 2015;3(3):509–19. [DOI] [PubMed] [Google Scholar]
- 50.McCauley MD, Vitale F, Yan JS, Young CC, Greet B, Orecchioni M, et al. In Vivo Restoration of Myocardial Conduction With Carbon Nanotube Fibers. Circ Arrhythm Electrophysiol. 2019;12(8):e007256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.He Y, Ye G, Song C, Li C, Xiong W, Yu L, et al. Mussel-inspired conductive nanofibrous membranes repair myocardial infarction by enhancing cardiac function and revascularization. Theranostics. 2018;8(18):5159–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Doshi N, Swiston AJ, Gilbert JB, Alcaraz ML, Cohen RE, Rubner MF, et al. Cell-Based Drug Delivery Devices Using Phagocytosis-Resistant Backpacks. Advanced Materials. 2011;23(12):H105–H9. [DOI] [PubMed] [Google Scholar]
- 53.Klyachko NL, Polak R, Haney MJ, Zhao Y, Gomes Neto RJ, Hill MC, et al. Macrophages with cellular backpacks for targeted drug delivery to the brain. Biomaterials. 2017;140:79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao Y, Haney MJ, Mahajan V, Reiner BC, Dunaevsky A, Mosley RL, et al. Active Targeted Macrophage-mediated Delivery of Catalase to Affected Brain Regions in Models of Parkinson’s Disease. J Nanomed Nanotechnol. 2011;S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kumar A, Glaum M, El-Badri N, Mohapatra S, Haller E, Park S, et al. Initial observations of cell-mediated drug delivery to the deep lung. Cell Transplant. 2011;20(5):609–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Duarte-Sanmiguel S, Panic A, Dodd DJ, Salazar-Puerta A, Moore JT, Lawrence WR, et al. In Situ Deployment of Engineered Extracellular Vesicles into the Tumor Niche via Myeloid-Derived Suppressor Cells. Adv Healthc Mater. 2021:e2101619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang H, Yang Y, Liu J, Qian L. Direct cell reprogramming: approaches, mechanisms and progress. Nat Rev Mol Cell Biol. 2021;22(6):410–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vierbuchen T, Wernig M. Direct lineage conversions: unnatural but useful? Nat Biotechnol. 2011;29(10):892–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ben-David U, Benvenisty N. The tumorigenicity of human embryonic and induced pluripotent stem cells. Nat Rev Cancer. 2011;11(4):268–77. [DOI] [PubMed] [Google Scholar]
- 60.Evans CW, Fitzgerald M, Clemons TD, House MJ, Padman BS, Shaw JA, et al. Multimodal Analysis of PEI-Mediated Endocytosis of Nanoparticles in Neural Cells. ACS Nano. 2011;5(11):8640–8. [DOI] [PubMed] [Google Scholar]
- 61.Wang M, Yu J, Cai L, Yang X. Direct reprogramming of mouse fibroblasts into hepatocyte-like cells by polyethyleneimine-modified nanoparticles through epigenetic activation of hepatic transcription factors. Materials Today Chemistry. 2020;17:100281. [Google Scholar]
- 62.Chang Y, Lee E, Kim J, Kwon Y-W, Kwon Y, Kim J. Efficient in vivo direct conversion of fibroblasts into cardiomyocytes using a nanoparticle-based gene carrier. Biomaterials. 2019;192:500–9. [DOI] [PubMed] [Google Scholar]
- 63.Muniyandi P, Palaninathan V, Mizuki T, Maekawa T, Hanajiri T, Mohamed MS. Poly(lactic-co-glycolic acid)/Polyethylenimine Nanocarriers for Direct Genetic Reprogramming of MicroRNA Targeting Cardiac Fibroblasts. ACS Applied Nano Materials. 2020;3(3):2491–505. [Google Scholar]
- 64.Yoo J, Lee E, Kim HY, Youn D-h, Jung J, Kim H, et al. Electromagnetized gold nanoparticles mediate direct lineage reprogramming into induced dopamine neurons in vivo for Parkinson’s disease therapy. Nature Nanotechnology. 2017;12(10):1006–14. [DOI] [PubMed] [Google Scholar]
- 65.Yarmush ML, Golberg A, Sersa G, Kotnik T, Miklavcic D. Electroporation-based technologies for medicine: principles, applications, and challenges. Annu Rev Biomed Eng. 2014;16:295–320. [DOI] [PubMed] [Google Scholar]
- 66.Gallego-Perez D, Pal D, Ghatak S, Malkoc V, Higuita-Castro N, Gnyawali S, et al. Topical tissue nanotransfection mediates non-viral stroma reprogramming and rescue. Nat Nanotechnol. 2017;12(10):974–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhao X, Huang X, Wang X, Wu Y, Eisfeld A-K, Schwind S, et al. Nanochannel Electroporation as a Platform for Living Cell Interrogation in Acute Myeloid Leukemia. Advanced Science. 2015;2(12):1500111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lemmerman LR, Balch MHH, Moore JT, Alzate-Correa D, Rincon-Benavides MA, Salazar-Puerta A, et al. Nanotransfection-based vasculogenic cell reprogramming drives functional recovery in a mouse model of ischemic stroke. Science advances. 2021;7(12):eabd4735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Moore JT, Wier CG, Lemmerman LR, Ortega-Pineda L, Dodd DJ, Lawrence WR, et al. Nanochannel-Based Poration Drives Benign and Effective Nonviral Gene Delivery to Peripheral Nerve Tissue. Advanced Biosystems. 2020;4(11):2000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Boukany PE, Morss A, Liao WC, Henslee B, Jung H, Zhang X, et al. Nanochannel electroporation delivers precise amounts of biomolecules into living cells. Nat Nanotechnol. 2011;6(11):747–54. [DOI] [PubMed] [Google Scholar]
- 71.Gallego-Perez D, Otero JJ, Czeisler C, Ma J, Ortiz C, Gygli P, et al. Deterministic transfection drives efficient nonviral reprogramming and uncovers reprogramming barriers. Nanomedicine. 2016;12(2):399–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ortega-Pineda L, Sunyecz A, Salazar-Puerta AI, Rincon-Benavides MA, Alzate-Correa D, Anaparthi AL, et al. Designer Extracellular Vesicles Modulate Pro-Neuronal Cell Responses and Improve Intracranial Retention. Advanced Healthcare Materials.n/a(n/a):2100805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tang S, Salazar Puerta A, Richards J, Khan S, Hoyland J, Gallego-Perez D, et al. Non-viral reprogramming of human nucleus pulposus cells with FOXF1 via extracellular vesicle delivery: An In Vitro and In Vivo study. European Cells and Materials. 2021;41:90–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pigeau GM, Csaszar E, Dulgar-Tulloch A. Commercial Scale Manufacturing of Allogeneic Cell Therapy. Frontiers in Medicine. 2018;5(233). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nogueira DES, Cabral JMS, Rodrigues CAV. Single-Use Bioreactors for Human Pluripotent and Adult Stem Cells: Towards Regenerative Medicine Applications. Bioengineering. 2021;8(5):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sah J Challenges of Stem Cell Therapy in Developing Country. Journal of Stem Cell Research & Therapeutics. 2016;1:1–3. [Google Scholar]
- 77.Bryant J, Hlavaty KA, Zhang X, Yap W-T, Zhang L, Shea LD, et al. Nanoparticle delivery of donor antigens for transplant tolerance in allogeneic islet transplantation. Biomaterials. 2014;35(31):8887–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wilson JT, Chaikof EL. Challenges and emerging technologies in the immunoisolation of cells and tissues. Advanced drug delivery reviews. 2008;60(2):124–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cao Y, Ma E, Cestellos-Blanco S, Zhang B, Qiu R, Su Y, et al. Nontoxic nanopore electroporation for effective intracellular delivery of biological macromolecules. Proceedings of the National Academy of Sciences. 2019;116(16):7899–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xie X, Xu AM, Leal-Ortiz S, Cao Y, Garner CC, Melosh NA. Nanostraw–Electroporation System for Highly Efficient Intracellular Delivery and Transfection. ACS Nano. 2013;7(5):4351–8. [DOI] [PubMed] [Google Scholar]
- 81.Vasdekis AE, Scott EA, O’Neil CP, Psaltis D, Hubbell JA. Precision Intracellular Delivery Based on Optofluidic Polymersome Rupture. ACS Nano. 2012;6(9):7850–7. [DOI] [PubMed] [Google Scholar]
- 82.Joo J, Kwon EJ, Kang J, Skalak M, Anglin EJ, Mann AP, et al. Porous silicon–graphene oxide core–shell nanoparticles for targeted delivery of siRNA to the injured brain. Nanoscale Horizons. 2016;1(5):407–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kwon EJ, Lasiene J, Jacobson BE, Park I-K, Horner PJ, Pun SH. Targeted nonviral delivery vehicles to neural progenitor cells in the mouse subventricular zone. Biomaterials. 2010;31(8):2417–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Moyer TJ, Kassam HA, Bahnson ESM, Morgan CE, Tantakitti F, Chew TL, et al. Shape-Dependent Targeting of Injured Blood Vessels by Peptide Amphiphile Supramolecular Nanostructures. Small. 2015;11(23):2750–5. [DOI] [PMC free article] [PubMed] [Google Scholar]


