Abstract
Allogeneic islet transplant offers a minimally invasive option for β cell replacement in the treatment of type 1 diabetes (T1D). The CIT consortium trial of purified human pancreatic islets (PHPI) in patients with T1D after kidney transplant (CIT06), a National Institutes of Health–sponsored phase 3, prospective, open-label, single-arm pivotal trial of PHPI, was conducted in 24 patients with impaired awareness of hypoglycemia while receiving intensive insulin therapy. PHPI were manufactured using standardized processes. PHPI transplantation was effective with 62.5% of patients achieving the primary endpoint of freedom from severe hypoglycemic events and HbA1c ≤ 6.5% or reduced by ≥ 1 percentage point at 1 year posttransplant. Median HbA1c declined from 8.1% before to 6.0% at 1 year and 6.3% at 2 and 3 years following transplant (P < .001 for all vs baseline), with related improvements in hypoglycemia awareness and glucose variability. The improved metabolic control was associated with better health-related and diabetes-related quality of life. The procedure was safe and kidney allograft function remained stable after 3 years. These results add to evidence establishing allogeneic islet transplant as a safe and effective treatment for patients with T1D and unstable glucose control despite intensive insulin treatment, supporting the indication for PHPI in the post–renal transplant setting.
Keywords: basic (laboratory) research/science, clinical research/practice, diabetes, diabetes: type 1, islet transplantation
1 ∣. INTRODUCTION
The National Institutes of Health (NIH)-sponsored Clinical Islet Transplantation (CIT) Consortium reported a phase 3 pivotal trial of purified human pancreatic islets (PHPI) for allogeneic pancreatic islet transplant in patients with type 1 diabetes (T1D) with normal renal function complicated by severe hypoglycemia (Protocol CIT07).1 The study used a standardized manufacturing protocol of PHPI 2 and stringently selected patients with impaired awareness of hypoglycemia (IAH) experiencing severe hypoglycemic events (SHEs). The results provide convincing evidence of the safety and efficacy of PHPI transplant for patients with T1D and problematic hypoglycemia3; 88% of patients achieved the primary endpoint of on-target glycemic control (HbA1c < 7.0%) in the absence of SHE during the first year posttransplant.1
Herein we report a parallel phase 3 pivotal trial of PHPI transplant conducted by the CIT Consortium in patients with T1D after kidney transplant (Protocol CIT06). Islet after kidney (IAK) transplant represents a separate indication for islet transplant because of its unique risk: benefit considerations due to a preexisting requirement for immunosuppression to prevent kidney transplant rejection. In islet alone transplant, the major risks are related to de novo exposure to induction and maintenance immunosuppression, whereas in IAK transplant the immunosuppressive risk is limited to induction therapy.
CIT06 corroborates the results of CIT07 regarding the safety and efficacy of PHPI transplant and extends the results to kidney transplant recipients, who were followed for 3 years after transplant. CIT06 PHPI recipients experienced marked improvement in glycemic control, freedom from SHEs, resolution of IAH, and clear improvements in quality of life (QOL), without detriment to kidney graft function. Since kidney transplant recipients have reduced glomerular filtration rates (GFR), preservation of kidney function is of utmost importance. Collectively, these results provide further evidence of the value of allogeneic islet transplant for treatment of patients with T1D inadequately controlled with intensive insulin treatment.
2 ∣. RESEARCH DESIGN AND METHODS
2.1 ∣. Study oversight
Because islet transplants are regulated by the FDA as a drug, this product was evaluated in a phase 3 trial under a US Investigational New Drug (IND) application and a Drug Master File for PHPI in compliance with the Declaration of Helsinki and in accordance with the principles of Good Clinical Practice for Trials on Medicinal Products, as described in the US CFR 21 CFR Parts 45, 50, 56, and 312, and the International Conference on Harmonization “Guidance for Industry: E6 Good Clinical Practice: Consolidated Guidance” dated April 1996. Clinical and manufacturing protocols, endpoints, and the statistical analysis plan were developed by the CIT Consortium with guidance from the Food and Drug Administration (FDA). The study was approved by local institutional review boards and was overseen by an independent National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)-sponsored Data Safety Monitoring Board. SAEs were reviewed by site physicians, the Data Coordinating Center at the University of Iowa, and the NIDDK and National Institute of Allergy and Infectious Diseases (NIAID) medical monitors, who made the final determination of seriousness and attribution. All authors confirm the completeness and accuracy of the data and fidelity to the study protocol.
2.2 ∣. Study design and outcome measures
This pivotal phase 3, prospective, open-label, single-arm study involving subjects with T1D who had previously received a kidney transplant was conducted at 10 centers in North America. The single-arm trial design derived from a lack of feasibility to conduct a randomized controlled study comparing islet transplant with intensive insulin treatment in subjects with T1D and a kidney transplant, the extremely low likelihood that individuals meeting the study entry criteria could spontaneously achieve the primary endpoint (defined later), and the FDA guidance that a single-arm islet transplant trial would be an acceptable license-enabling study.4,5 The primary endpoint was achieving an HbA1c level of ≤6.5% (48 mmol/mol), the glycemic goal recommended by the American College of Endocrinology,6,7 at day 365, or a reduction in HbA1c of at least 1 point from baseline to day 365, and freedom from SHEs from day 28 to day 365 after the initial islet transplant. This endpoint was chosen to reflect the effectiveness of this therapy specifically for treating IAH and associated SHEs. Key secondary endpoints included achieving an HbA1c level of <7.0% (53 mmol/mol), the target recommended by the American Diabetes Association,8 at day 365 and freedom from SHEs from day 28 to day 365, individual components of the composite endpoints, and insulin independence (see Appendix S2). Other efficacy outcomes included assessment of IAH (Clarke score9), hypoglycemia severity (HYPO score10,11), glycemic lability (lability index [LI]10,11), mean amplitude of glycemic excursions (MAGE12), and 72-h glucose profiles by use of a continuous glucose monitoring (CGM) system (iPro® CGMS; Medtronic Minimed11). Safety outcomes included the incidence of serious adverse events (SAEs) related to the islet transplant procedure or immunosuppression, the incidence of de novo anti-HLA antibodies, and kidney function by estimated glomerular filtration rate (eGFR). All aspects of the study design are consistent with the FDA guidance document Considerations for Allogeneic Pancreatic Islet Cell Products.4
The first subject consented on April 8, 2010. All subjects reached the primary endpoint by November 17, 2014, and the secondary endpoints by July 5, 2017.
2.3 ∣. Health-related quality of life, functional health status, and health utility surveys
CIT06 incorporated 4 HRQOL surveys SF-36, Diabetes Distress Score (DDS), Hypoglycemia Fear Score (HFS), and European Quality of Life (EuroQol)13-24 every 3 months before islet transplant, at days 75, 180, 365, 730 and 1095 after the initial islet transplant, and at days 75, 180, 365, 730, and 1095 after the final islet transplant.
2.4 ∣. Recipient selection
Inclusion criteria included age 18-68 years at the time of enrollment, T1D for ≥5 years, stable kidney transplant, absent stimulated C-peptide, IAH as determined by Clarke score,9 and a history of SHEs25 in the prior 12 months (Appendix S3) despite medical care provided by an endocrinologist or diabetologist (see Appendix S2). Among those transplanted, 8 subjects had used continuous subcutaneous insulin infusion (CSII) pretransplant, and 1 subject had used CGM. Each patient's diabetes specialist confirmed that the patient was unable to achieve glycemic control without hypoglycemic episodes, even when the HbA1c level was allowed to rise above 7.5% (58.5 mmol/mol). In the year preceding transplant, each subject experienced ≥1 SHEs (see Appendix S4). Alternatively, when not meeting the hypoglycemia criterion, a subject could meet inclusion criteria if his or her HbA1c was ≥7.5% after having received ≥12 months of prospectively followed intensive insulin therapy30 (see Appendix S2). However, all subjects met the hypoglycemia criterion. Exclusion criteria included body mass index (BMI) >30 kg/m2, weight >90 kg, insulin requirement > 1.0 units/kg/day or < 15 units/day, calculated GFR ≤ 40 mL/min/1.73 m2, history of panel reactive anti-HLA antibodies by Luminex single antigen assay > 50% or presence of any anti-HLA antibodies against the kidney donor, and significant co-morbid conditions.
2.5 ∣. Donor selection, islet manufacture, and islet transplant
PHPI were manufactured at 10 manufacturing facilities, each associated with that clinical site. The CIT-defined manufacturing process used a common master production batch record, including standardized lot release criteria, process controls, test methods,2 and organ donor acceptance criteria. Pancreata from deceased donors 15-65 years of age were processed within 12 hours of retrieval. Donor exclusion criteria included history of diabetes, HbA1c > 6% (42 mmol/mol), and donation after cardiac death.
Each PHPI lot (dose), containing > 5000 islet equivalents (IEQ)/ kg for the first dose and ≥ 4000 IEQ/kg for subsequent doses (if any), was prepared from a single pancreas2 and was transplanted by portal vein infusion. Access to the portal vein was achieved percutaneously or by minilaparotomy (30 and 9 infusions, respectively). Subjects who were not insulin independent at 30 days after the first or second dose were eligible for a subsequent infusion until 8 months after the initial transplant. This left a 4-month posttransplant interval for stabilization before assessment of the primary endpoint.
2.6 ∣. Other study treatments
Induction immunosuppression consisted of rabbit antithymocyte globulin (ATG) and etanercept1 for the first transplant, with basiliximab replacing ATG at subsequent transplants and in a single case of suspected sensitivity to ATG. The calcineurin-based maintenance immunosuppression regimen used for the renal transplant was continued after the islet transplant. Up to 10 mg of prednisone was allowed as part of maintenance immunosuppression.
2.7 ∣. HLA typing and HLA antibody assessment and criteria used for eligibility based on sensitization
2.8 ∣. Statistical analysis
Intention-to-treat analysis was used for primary and key secondary endpoints. Failures were imputed for missing outcomes. One-sided tests for whether the true rates are greater than the predetermined minimum rate for efficacy for the primary endpoint (27%), key secondary endpoints (50%), and insulin independence (20%) were used. Complete case analysis was used for other metabolic outcomes. Nonparametric paired (Wilcoxon signed rank) tests were used to compare outcomes at each posttransplant time point with baseline values. To account for multiplicity and preserve the overall false discovery rate of 10%, the Benjamini-Hochberg (BH) method was used separately for the key secondary tests and other metabolic outcomes. The BH thresholds are obtained using all postinitial and postfinal transplant comparisons specified in the statistical analysis plan of the CIT06 final study report. See Appendix S6 for a detailed description of the analysis methods used to obtain the BH thresholds. Results are presented as median IQR, unless otherwise specified.
3 ∣. RESULTS
3.1 ∣. Recipient and donor characteristics
The 24 subject recipients had a median IQR age of 52.7 (29.2-69.6) years at the time of initial islet transplant (performed between April 9, 2010, and July 5, 2014) and included 11 women (46%). Eighteen were white, 1 was Native American or Alaska Native, 1 was African American, and 4 did not report race or ethnicity. The last patient's last visit occurred on July 5, 2017. The median BMI was 24.0 (18.9-30.4) kg/m2, and median duration of T1D was 36.5 (17-55) years. Twenty three subjects met criteria at study entry for receiving IIT and having IAH and ≥1 SHE in the 12 months before study enrollment. One subject met criteria for having IAH and SHE after 2 years on prospectively implemented IIT. IIT was administered using CSII in 8 (33%) and multiple daily injections in the remainder. No subjects were enrolled solely based on persistently elevated HbA1c ≥ 7.5% after ≥12 months of IIT. Baseline autoantibody titers are shown in Table 1; approximately 80% of subjects had autoantibodies directed against insulin and/or glutamic acid decarboxylase-65 (GAD65). Ten subjects (42%) had proliferative retinopathy in one or both eyes. Eleven subjects received a single PHPI infusion, 11 subjects received 2 infusions, and 2 subjects received 3 infusions (Table 1). Donor graft characteristics are presented in Table 1.
TABLE 1.
Recipient (baseline), donor and graft characteristics
| Baseline recipient characteristics |
Observations (n) |
Median (minimum – maximum) or n (%) |
|---|---|---|
| Gender (% male) | 24 | 13 (54.2) |
| Age (y) | 24 | 52.7 (29.2-69.6) |
| Weight (kg) | 24 | 70.9 (48.4-86.5) |
| BMI (kg/m2) | 24 | 24.0 (18.9-30.4) |
| Duration of diabetes (y) | 24 | 36.5 (17.0-55.0) |
| HbA1c (%) | 24 | 8.1 (6.0-12.7) |
| HbA1c (mmol/mol) | 24 | 65 (42-115) |
| Insulin requirement | ||
| Units/d | 24 | 35.9 (18.3-57.8) |
| Units/kg/d | 24 | 0.5 (0.3-0.7) |
| Autoantibodies | ||
| Anti-insulin (% positive) | 24 | 21 (87.5) |
| Anti-GAD65 (% positive) | 24 | 19 (79.2) |
| Anti-ICA512 (% positive) | 24 | 6 (25.0) |
| Clarke score | 24 | 6 (3-7) |
| HYPO score | 16 | 575.5 (60.0-2126.0) |
| SHE 1 y pretransplant (n) | 21 | 6.0 (0.0-30.0) |
| Glycemic LI | 16 | 384.1 (37.6-914.5) |
| MAGE (mg/dL) | 18 | 160.8 (88.2-513.0) |
| eGFR (mL/min/1.73 m2) | 24 | 81.8 (43.0-106.7) |
| Diabetic microvascular complications (left eye) | 24 | |
| Not reported (%) | 2 (8.3) | |
| Not present (%) | 5 (20.8) | |
| Mild/minimal nonproliferative retinopathy (%) | 6 (25.0) | |
| Moderate nonproliferative retinopathy (%) | 2 (8.3) | |
| Severe nonproliferative retinopathy (%) | 1 (4.2) | |
| Proliferative retinopathy (%) | 8 (33.3) | |
| Diabetic microvascular complications (right eye) | 22 | |
| Not reported (%) | 2 (9.1) | |
| Not present (%) | 2 (9.1) | |
| Mild/minimal nonproliferative retinopathy (%) | 6 (27.3) | |
| Moderate nonproliferative retinopathy (%) | 2 (9.1) | |
| Severe nonproliferative retinopathy (%) | 0 (0.0) | |
| Proliferative retinopathy (%) | 10 (45.5) | |
| Donor/pancreas characteristics | ||
| Donor age (y) | 39 | 41.0 (18.0-64.0) |
| Donor BMI (kg/m2) | 39 | 32.1 (20.4-48.0) |
| Donor sex (% male) | 39 | 32 (82.1) |
| Pancreas cold ischemia time (h/per isolation) | 39 | 7.8 (2.4-14.9) |
| PHPI lot characteristics | ||
| Total IEq transplanted (per lot) | 39 | 532 301 (291 908-826 438) |
| Tissue volume (mL/lot) | 39 | 4 (1-15) |
| Total dose/subject | ||
| Total IEq transplanted (per subject) | 24 | 766 357 (367 938-2 075 368) |
| Total IEq/kg transplanted (per subject) | 24 | 11 345 (5168-28 393) |
Abbreviations: BMI, body mass index; eGFR, estimated glomerular filtration rate; GAD, glutamic acid decarboxylase-65; HYPO, hypoglycemia; IEq, islet equivalent; MAGE, mean amplitude of glycemic excursions; PHPI, purified human pancreatic islets; SHE, severe hypoglycemic event.
3.2 ∣. Metabolic control
The primary endpoint of achieving an HbA1c ≤ 6.5% or a reduction in HbA1c of ≥1 point in the absence of experiencing SHEs at day 365 was achieved by 15 subjects (62.5%; P < .001). Fourteen (58.3%; P = .0012) and 11 (45.8%; P = .0369; Figure 1A) subjects also achieved the primary endpoint criteria evaluated at day 730 and day 1095, respectively. Fifteen subjects (62.5%) at day 365, 14 (58.3%) at day 730, and 10 (41.7%) at day 1095 also met the key secondary endpoint of achieving an HbA1c < 7.0% in the absence of experiencing SHEs. No subject was free of SHEs in the year before transplant, and SHEs were eliminated posttransplant in 19 (79.2%) subjects through day 365 (P = .003), 18 (75.0%) at day 730 (P = .011), and 15 (62.5%) at day 1095 (P = .154; Figure 1B). HbA1c, elevated at baseline (8.1% [7.0%-9.3%]), was significantly reduced following transplant (6.0% [5.3%-6.4%] at day 365, P < .001; 6.3% [5.5%-6.7%] at day 730, P = .002; 6.3% [5.5%-6.9%] at day 1095, P < .001; Figure 1C) with 15 (62.5%), 12 (50.0%), and 9 (37.5%) subjects achieving an HbA1c ≤ 6.5% at day 365, day 730, and day 1095, respectively. Insulin requirements at baseline were (0.50 [0.39-0.58] units kg−1 d−1) and decreased dramatically following transplant (0.0 [0.0-0.01] unit kg−1 d−1 at day 365, P < .001; 0.00 [0.0-0.22] unit kg−1 d−1 at day 730, P < .001; 0.00 [0.00-0.26] unit kg−1 d−1 at day 1095, P = .002; Figure 1D) with 9 (37.5%), 7 (29.2%), and 4 (16.7%) subjects meeting criteria for insulin independence at day 365 (P = .036), day 730 (P = .189), and day 1095 (P = .736), respectively.
FIGURE 1.
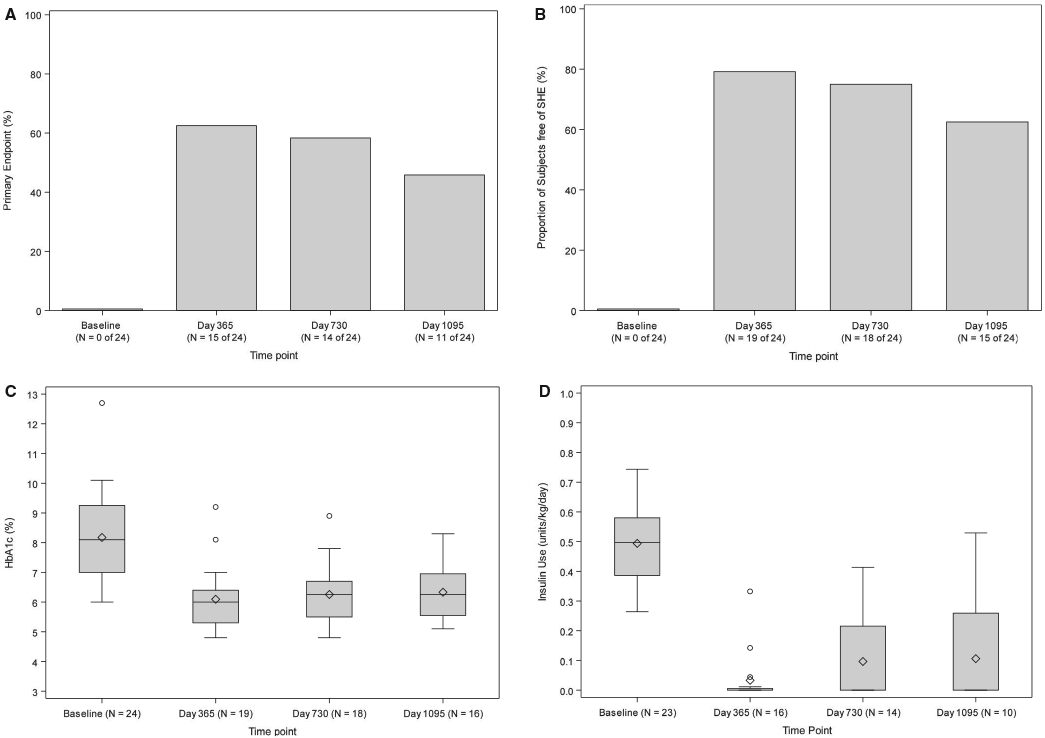
A, primary endpoint (%). An exact 1-sided test for a proportion of ≤.27 vs >0.27 was performed at day 365, day 730, and day 1095. The P values were P = .0003 at day 365, P = .0012 at day 730, and P = .0369 at day 1095. The threshold for statistical significance based on the Benjamini-Hochberg (BH) false discovery rate (FDR) method was 0.0167. B, Free of severe hypoglycemic event (SHE) (%). An exact 1-sided test for proportion ≤0.5 vs >0.5 was performed at day 365, day 730, and day 1095. The P values were P = .0033 at day 365, P = .0113 at day 730, and P = .1537 at day 1095. The threshold for statistical significance based on the BH FDR method was 0.0167. C, HbA1c (%). The Wilcoxon signed rank test for paired outcomes was used to compare the HbA1c levels (%) between baseline and day 365, day 730, and day 1095 following initial purified human pancreatic islets (PHPI) product transplant. The BH method was used to adjust the level of significance in order to preserve the overall false discovery rate of 10%. The upper BH boundary was 0.0794 and the P values for the 2-sided Wilcoxon signed rank tests were P < .0001 for day 365, P = .0016 for day 730, and P = .0002 for day 1095. D, Insulin use (U/kg/d). The Wilcoxon signed rank test for paired outcomes was used to compare insulin use (U/kg/d) at day 365, day 730, and day 1095 following initial PHPI product transplant. The BH method was used to adjust the level of significance in order to preserve the overall false discovery rate of 10%. The upper BH boundary was 0.0794 and the P values for the 2-sided Wilcoxon signed rank tests were P < .0001 for day 365, P = .0001 for day 730, and P = .0020 for day 1095
All subjects exhibited IAH at baseline (Clarke score 6.0 [5.0-6.0]) that was abolished following transplant (0.0 [0.0-0.0] at day 365, day 730, and day 1095, all P < .001; Figure 2A). Hypoglycemia severity, reflected by a markedly elevated HYPO score at baseline (575.5 [370.0-942.5], became negligible following transplant (0.0 [0.0-37.5] at day 365, P = .008; Figure 2B). Glycemic lability was also markedly elevated at baseline (LI 384.1 [302.2-587.4] mmol/L2/h wk−1) and dramatically reduced following transplant (21.4 [14.6-56.0] mmol/L2/h wk−1 at day 365, P = .008; Figure 2C). A similar effect was seen for MAGE at baseline (160.8 [124.5-192.5] mg/dL) and following transplant (59.3 [35.7-82.0] mg/dL at day 365, P = .002; Figure 2D).
FIGURE 2.
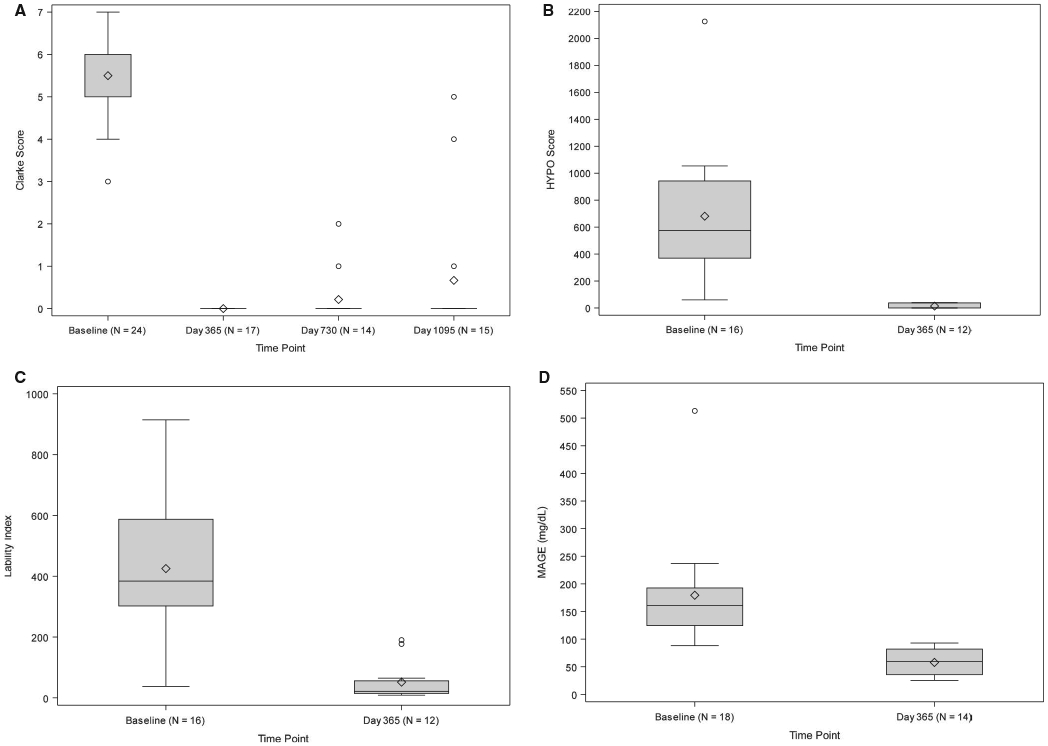
A, Clarke score. The Wilcoxon signed rank Test for paired outcomes was used to compare the median Clarke Survey score between baseline (N = 24) and day 365, day 730, and day 1095 following initial purified human pancreatic islets (PHPI) product transplant. The Benjamini-Hochberg (BH) method was used to adjust the level of significance in order to preserve the overall false discovery rate of 10%. The upper BH boundary was 0.0794 and the P values for the 2-sided Wilcoxon signed rank tests were P < .0001 for day 365, P = .0001 for day 730, and P = .0001 for day 1095. B, hypoglycemia (HYPO) score. The Wilcoxon signed rank test for paired outcomes was used to compare changes in median HYPO score between baseline and day 365 following initial PHPI product transplant. BH method was used to adjust the level of significance to preserve the overall false discovery rate of 10%. The upper BH boundary was 0.0794 and the P value for the 2-sided Wilcoxon signed rank test was .0078. Note: HYPO score results were only collected through day 365. C, Lability index (LI). The Wilcoxon signed rank test for paired outcomes was used to compare the changes in median LI scores between baseline and day 365 following initial PHPI product transplant. The BH method was used to adjust the level of significance to preserve the overall false discovery rate of 10%. The upper BH boundary was 0.0794 and the P value for the 2-sided Wilcoxon signed rank test was .0078. Note: LI results were only collected through day 365. D, Mean amplitude of glycemic excursions (MAGE) score. The Wilcoxon signed rank test for paired outcomes was used to compare changes in MAGE score between baseline and day 365 following initial PHPI product transplant. The BH method was used to adjust the level of significance to preserve the overall false discovery rate of 10%. The upper BH boundary was 0.0794 and the P value for the 2-sided Wilcoxon signed rank test was .0020. Note: MAGE score results were only collected through day 365
3.2.1 ∣. Continuous glucose monitoring
Mean glucose was elevated at baseline (189.0 [147.0-213.0] mg/dL) and decreased substantially following transplant (113.0 [109.0-133.0] at day 365, 130.0 [108.0-145.0] at day 730, and 121.0 [105.5-152.0] mg/dL at day 1095, P ≤ .0625 for all comparisons to baseline (Figure 3A). The threshold for statistical significance based on the false discovery rate (FDR) approach was 0.0794. Glucose SD was also elevated at baseline (72.0 [61.0-80.0] mg/dl) and decreased dramatically following transplant (20.0 [16.0-30.0] at day 365, 21.0 [17.0-31.0] at day 730, and 25.5 [13.5-51.5] mg/dL at day 1095; P ≤ .0625 for all comparisons to baseline; Figure 3B). Time spent within target range (54-180 mg/dL) was 47.85% [27.0%-74.4%] at baseline and increased substantially following transplant (97.2% [88.8%-99.9%] at day 365, 93.8% [83.8%-100.0%] at day 730, and 96.7% [68.5%-99.3%] at day 1095; P ≤ .0625 for all comparisons to baseline; Figure 3C). The increased time in range posttransplant was mainly driven by a reduction in the percentage of time spent with hyperglycemia (>180 mg/dL) from 51.1% (23.4%-72.5%) at baseline to 0.0% (0.0%-5.0%) at day 365, 5.4% (0.0%-16.2%) at day 730, and 2.0% (0.0%-30.0%) at day 1095 (P ≤ .0625 for all comparisons to baseline; Figure 3D). Time spent with clinically important hypoglycemia (<54 mg/dL) was 0.5% (0.0%-2.9%) at baseline, but the posttransplant reduction did not meet statistical significance: (0.0% [0.0%-1.4%] at day 365, 0.0% [0.0%-0.0%] at day 730, and 0.2% [0.0%-2.1%] at day 1095).
FIGURE 3.
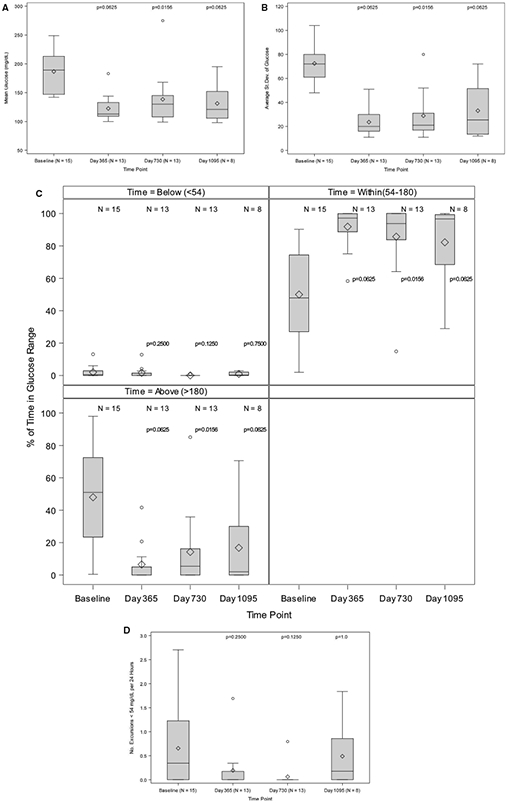
A, Mean glucose levels (mg/dL). The Wilcoxon signed rank test for paired outcomes was used to compare mean continuous glucose monitoring system (CGMS®) glucose (mg/dL) from baseline to day 365, day 730, and day 1095 following initial purified human pancreatic islets (PHPI) product transplant. The Benjamini-Hochberg (BH) method was used to adjust the level of significance to preserve the overall false discovery rate of 10%. The upper BH boundary was 0.0794 and the P values for the 2-sided Wilcoxon signed rank tests were P = .0625 for day 365, P = .0156 for day 730, and P = .0625 for day 1095. B, Average SD of glucose levels (mg/dL). The Wilcoxon signed rank test for paired outcomes was used to compare average SD of glucose levels (mg/dL) from baseline to day 365, day 730 and day 1095 following initial PHPI product transplant. The BH method was used to adjust the level of significance to preserve the overall false discovery rate (FDR) of 10%. The upper BH boundary was 0.0794 and the P values for the 2-sided Wilcoxon signed rank tests were P = .0625 for day 365, P = .0156 for day 730, and P = .0625 for day 1095. C, Percent of time in glucose range. The Wilcoxon signed rank test for paired outcomes was used to compare CGMS® percent excursions for < 54, within (54-180), and >180, from baseline to day 365, day 730, and day 1095 following initial PHPI product transplant. The BH method was used to adjust the level of significance to preserve the overall FDR of 10%. The upper BH boundary was 0.0794 and the P values for the 2-sided Wilcoxon signed rank tests were, respectively, P = .2500, P = .0625 and P = .0625 for day 365; P = .1250, P = .0156, and P = .0156 for day 730; and P = .7500, P = .0625, and P = .0625 for day 1095. D, Glucose level (mg/dL) number of excursions < 54 mg/dL per 24 h. The Wilcoxon signed rank test for paired outcomes was used to compare the median number of hypoglycemic excursions per day from baseline to day 365, day 730, and day 1095 following initial PHPI product transplant. The BH method was used to adjust the level of significance to preserve the overall FDR of 10%. The upper BH boundary was 0.0794 and the P values for the 2-sided Wilcoxon signed rank tests were P = .2500 for day 365, P = .1250 for day 730, and P = 1.0 for day 1095
3.2.2 ∣. Mixed-meal tolerance test
Fasting glucose was elevated at baseline (147.5 [92.5-162.5] mg/dL) and decreased following transplant at day 365 (109.0 [101.0-115.0] mg/dL, P = .036), day 730 (112.0 [99.5-127.0] mg/dL, P = .175), and day 1095 (111.0 [99.00-127.0] mg/dL, P = .334; Figure 4A). At the baseline, the 60-minute glucose was 264.0 (216.5-312.5) mg/dL and decreased following transplant at day 365 (200.0 [151.0-215.0] mg/dL, P = .002), day 730 (166.0 [140.0-238.0] mg/dL, P = .013), and day 1095 (153.0 [116.0-183.0] mg/dL, P = .003; Figure 4A). At baseline, the 90-minute glucose increased further to 308.0 [272.0-380.0] mg/dL, while after transplant, it decreased: at day 365 (155.0 [130.0-208.0] mg/dL, P < .001), day 730 (155.5 [111.0-282.0] mg/dL, P = .002), and day 1095 (164.0 [128.0-218.0] mg/dL, P < .001; Figure 4A). C-peptide was undetectable in all subjects before transplant and was restored following transplant with fasting levels 1.8 (1.6-2.5) at day 365, 1.8 (1.5-2.1) at day 730, and 1.3 (0.8-1.6) ng/mL at day 1095. At 60 minutes postmeal ingestion, C-peptide increased to 5.2 (3.6-7.7) at day 365, 4.4 (2.7-5.1) at day 730, and 4.4 (1.2-8.2) ng/mL at day 1095 (P ≤ .001 for all comparisons vs baseline). Similarly, the 90-minute C-peptide increased to 5.5 (4.1-6.7) at day 365, 4.0 (3.6-6.8) at day 730, and 4.4 (1.3-7.2) ng/mL at day 1095 (P < .001 for all comparisons vs baseline; Figure 4B).
FIGURE 4.
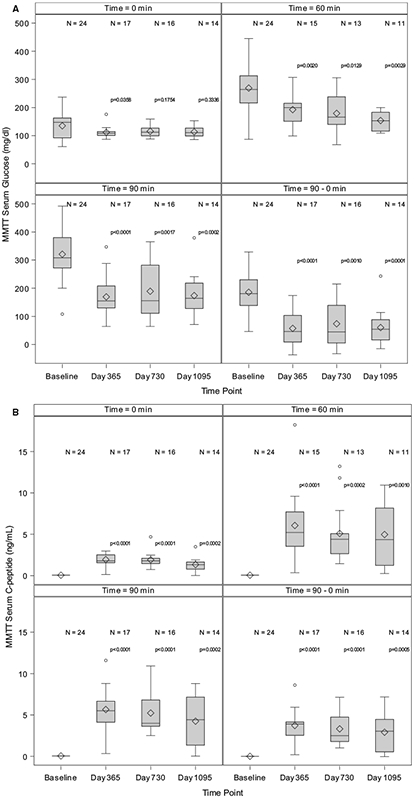
A, Mixed-meal tolerance test (MMTT)-derived serum glucose levels. The Wilcoxon signed rank test for paired outcomes was used to compare glucose measures for basal (0 minutes), 60 minutes, 90 minutes, and the change from 0 minutes to 90 minutes from baseline to day 365, day 730, and day 1095 following initial purified human pancreatic islets (PHPI) product transplant. The Benjamini-Hochberg (BH) method was used to adjust the level of significance to preserve the overall false discovery rate (FDR) of 10%. The upper BH boundary was 0.0794 and the P values for the 2-sided Wilcoxon signed rank tests were, respectively, P = .0358, P = .0020, P < .0001, and P < .0001 for day 365; P = .1754, P = .0129, P = .0017, and P = .0010 for day 730; and P = .3336, P = .0029, P = .0002, and P = .0001 for day 1095. Note: The 60-minute glucose levels (mg/dL) were not required in Protocol CIT06 (CIT consortium trial of PHPI transplant in patients with type 1 diabetes after kidney transplant). B, MMTT-derived serum C-peptide levels. The Wilcoxon signed rank test for paired outcomes was used to compare C-peptide measures for basal (0 minutes), 60 minutes, 90 minutes, and the change from 0 minutes to 90 minutes from baseline to day 365, day 730, and day 1095 following initial PHPI product transplant. The BH method was used to adjust the level of significance to preserve the overall FDR of 10%. The upper BH boundary was 0.0794 and the P values for the 2-sided Wilcoxon signed rank tests were, respectively, P < .0001, P < .0001, P < .0001 and P < .0001 for day 365; P < .0001, P = .0002, P < .0001, and P < .0001 for day 730; and P = .0002, P = .0010, P = .0002, and P = .0005 for day 1095.
3.2.3 ∣. Quality of life
Following transplant, diabetes distress decreased from baseline (DDS score 2.3 [1.7-3.1]) to day 75 (1.8 [1.7-2.8], P = .002), day 365 (1.5 [1.3-1.9], P = .006), day 730 (1.6 [1.2-1.8], P = .019), and day 1095 (1.7 [1.1-2.4], P = .008; Figure 5A) with no difference in measures seen between those subjects who were and were not insulin independent at day 75 (P = .949), day 365 (P = .218), day 730 (P = .178), and day 1095 (P = .279). Similarly, fear of hypoglycemia was reduced following transplant from baseline (HFS score 2.0 [1.7-2.4]) at day 75 (1.3 [0.96-1.9], P < .001), day 365 (0.74 [0.0-1.74], P = .002), day 730 (0.41 [0.0-1.6], P = .039), and day 1095 (0.6 [0.0-1.7]; P = .047, Figure 5B). There was a trend toward less fear of hypoglycemia in subjects who were insulin independent vs insulin dependent (Figure 5C). The EuroQOL Visual Analog Scale that assesses more general QOL increased significantly by day 365, 79.0 (75.0-82.0, P < .001); however, there was only a trend toward significance at days 730, 80.0 (74.0-85.0, P = .095), and 1095, 78.0 (70.0-85.5, P = .033; Figure 5D).
FIGURE 5.
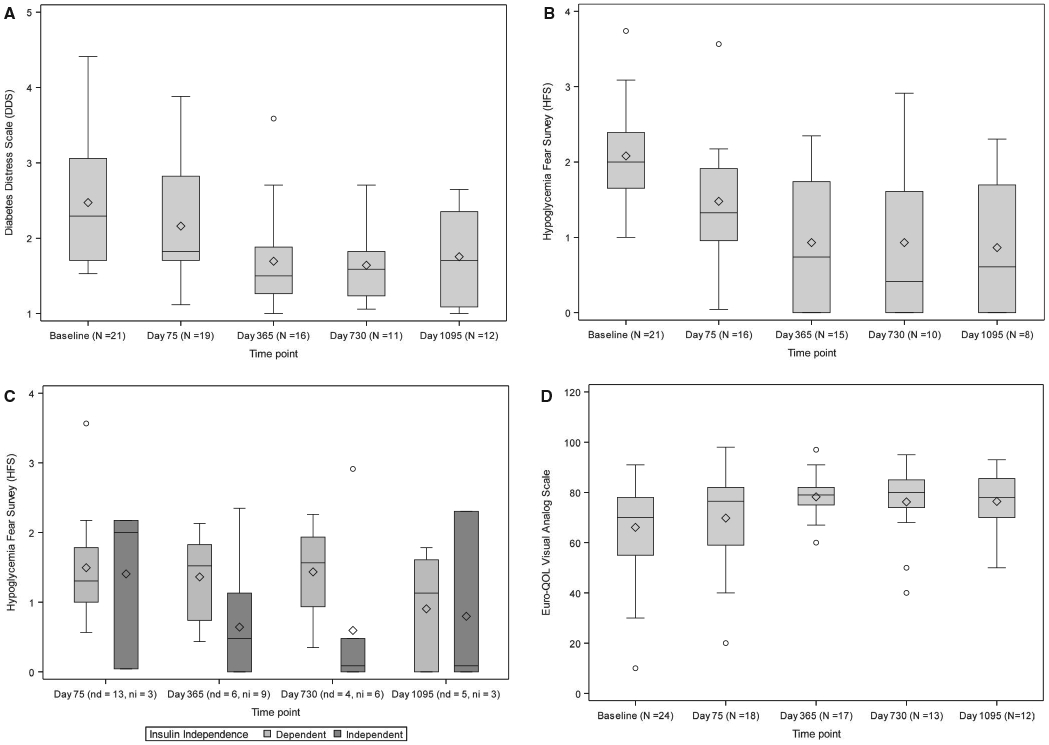
A, Diabetes Distress Scale (DDS) scores. The Wilcoxon signed rank test for paired outcomes was used to compare DDS from baseline and day 75, day 365, day 730, and day 1095 following initial purified human pancreatic islets (PHPI) product transplant. The Benjamini-Hochberg (BH) method was used to adjust the level of significance to preserve the overall false discovery rate (FDR) of 10%. The upper BH boundary was 0.0229 and the P values for the 2-sided Wilcoxon signed rank test were, respectively, P = .0020, P = .0062, P = .0195 and P = .0078. B, Overall Hypoglycemia Fear Survey (HFS) scores. The Wilcoxon signed rank test for paired outcomes was used to compare HFS from baseline to day 75, day 365, day 730, and day 1095 following initial PHPI product transplant. The BH method was used to adjust the level of significance to preserve the overall FDR of 10%. The upper BH boundary was 0.0229 and the P values for the 2-sided Wilcoxon signed rank test were, respectively, P = .0005, P = .0024, P = .0391, and P = .0469. C, Hypoglycemia Fear Survey (HFS) score as a function of insulin independence. The sample sizes are recorded as (nd = number of insulin dependent, ni = number of insulin independent) at day 75, day 365, day 730, and day 1095 following initial PHPI product transplantation. The BH method was used to adjust the level of significance to preserve the overall FDR of 10%. The upper BH boundary was 0.0229. Pairwise Wilcoxon P values comparing HFS scores among those who met insulin independence vs those who did not for day 75, day 365, day 730, and day 1095 were, respectively, P = .7250, P = .0783, P = .0171 and P = 1.0. D, Overall visual analog scale (VAS) Scores. The Wilcoxon signed rank test for paired outcomes was used to compare EuroQOL VAS from baseline to day 75, day 365, day 730, and day 1095 following initial PHPI product transplant. The BH method was used to adjust the level of significance to preserve the overall FDR of 10%. The upper BH boundary was 0.0229 and the P values for the 2-sided Wilcoxon signed rank test were, respectively, P = .1061, P = .0002, P = .0952 and P = .0327
3.2.4 ∣. Relationship of PHPI dose to primary endpoint
Subjects who met the primary endpoint received 14 978 ± 6296 IEQ/kg, which was greater than for those not meeting the primary endpoint (8589 ± 2790 IEQ/kg [P = .009], Table 2). Additionally, more subjects who met the primary endpoint received >1 infusion of PHPI (P = .033).
TABLE 2.
Transplant number and IEQs
| Variable | Number of transplants |
IEq/kg Mean (SD) |
Median | Minimum | Maximum | 95% CI for the mean |
|---|---|---|---|---|---|---|
| Subjects with 1 PHPI transplant only | ||||||
| Only 1 transplant | 11 | 8311 (2732) | 7341 | 5168 | 12 513 | (6475, 10 147) |
| Subjects with 2 PHPI transplants | ||||||
| 1st transplant | 11 | 7422 (2978) | 5733 | 5110 | 13 330 | (5421, 9423) |
| 2nd transplant | 11 | 7035 (1958) | 6590 | 4206 | 10 739 | (5720, 8350) |
| Total | 22 | 7228 (2467) | 6482 | 4206 | 13 330 | (6134, 8322) |
| Subjects with 3 PHPI transplants | ||||||
| First transplant | 2 | 8994 (1349) | 8994 | 8041 | 9948 | (0, 21 113) |
| Second transplant | 2 | 8145 (814) | 8145 | 7570 | 8721 | (830, 15 461) |
| Third transplant | 2 | 8625 (3183) | 8625 | 6374 | 10 876 | (0, 37 225) |
| Total | 6 | 8588 (1633) | 8381 | 6374 | 10 876 | (6874, 10 302) |
Abbreviations: IEq, islet equivalent; PHPI, purified human pancreatic islets.
3.2.5 ∣. Sensitization to HLA
All 24 subjects had negative calculated PRAs (cPRAs) pretransplant. However, in 1 subject (subject 22, Table 3) who initially had negative cPRAs, anti-HLA antibodies developed while on the waiting list with the pretransplant cPRA increasing to 96%. This result was not available at the time of islet transplant, and the subject received a PHPI product in the presence of shared kidney and islet donor specific antibody (DSA). This islet graft failed by day 178 after initial transplant due to presumed rejection. The other subject (subject 1) with initially negative cPRA who subsequently developed pretransplant cPRA had a positive posttransplant cPRA only at day 1218 after initial transplant (Table 4). Of the 24 subjects, only 6 subjects developed posttransplant cPRAs (Tables 3 and 4). Two subjects (subject 2 and subject 20) developed de novo anti-HLA antibodies with transient positive cPRAs that were not donor directed or associated with islet graft failure; 2 subjects (subject 6 and subject 7) developed de novo anti-HLA antibodies and positive cPRAs without detectable islet DSA but had islet graft failure; and 2 subjects (subject 8 and subject 10) developed de novo anti-HLA antibodies with positive cPRAs with islet DSA and had islet graft failure. One subject (subject 8) had a weakly reactive DSA (MFI 700-960) present pretransplant but below the predetermined threshold for positivity; the subject received a PHPI product containing this antigen specificity and experienced a rapid increase in preexisting islet DSA associated with subsequent islet graft failure by day 55 after initial transplant. One subject (subject 23) had weak renal HLA class II DSA pretransplant and developed posttransplant weak HLA class I DSA to the kidney and first islet donor (all DSA were below the predetermined threshold for positivity) (Tables 3 and 4); this patient's renal function was stable (>60 mL/min/1.7 m2) during follow-up (day 2191 after initial islet transplant) but had islet graft failure. Another subject (subject 24) had weak renal DSA posttransplant but maintained stable renal function (>60 mL/min/1.7 m2) during follow-up (day 772 after initial islet transplant) without islet graft failure.
TABLE 3.
Pretransplant cPRA, HLA antibody, and DSA
| Subject | Baseline maximum cPRA |
Anti-HLA Ab |
Renal DSA |
Islet DSA |
|---|---|---|---|---|
| 1a | 7 | Yes | No | No |
| 2 | 0 | No | No | No |
| 6 | 0 | No | No | No |
| 7 | 0 | No | No | No |
| 8b | 0 | Weak | No | Weak |
| 10 | 0 | No | No | No |
| 12 | 0 | No | No | No |
| 20 | 0 | No | No | No |
| 22 | 96 | Yes | Yes | Yes |
| 23c | 0 | No | Weak | No |
| 24 | 0 | No | No | No |
Weak HLA class I reactivity (A23-2100 MFI).
Weak donor specific antibody (DSA) to islet HLA class II specificity (DQ7; mean fluorescence intensity (MFI) in the range of 700-960) was present pretransplant which was below the predetermined threshold for positivity.
Weak DSA to kidney HLA class II specificity (DQ7,8,9; MFI 500-1300) which was below the predetermined threshold for positivity.
TABLE 4.
Posttransplant cPRA, HLA antibody, and DSA
| Subject | Posttransplant maximum cPRA |
Anti-HLA Ab persistent de novo |
Renal DSA persistent de novo |
Islet DSA persistent de novo |
Islet graft failure |
|---|---|---|---|---|---|
| 1 | 7 | No | No | No | No |
| 2 | 5 | Yes | No | No | No |
| 6 | 46 | Yes | No | No | Yes |
| 7 | 26 | Yes | No | No | Yes |
| 8 | 56 | Yes | No | Yes | Yes |
| 10 | 75 | Yes | No | Yes | Yes |
| 12 | 0 | No | No | No | No |
| 20 | 8 | Yes | No | No | No |
| 22a | 96 | No | No | No | Yes |
| 23b | 0 | Weak | Weak | Weak | Yes |
| 24c | 0 | Weak | Weak | No | No |
Abbreviations: DSA, donor specific antibody; cPRA, calculated PRA; MFI, mean fluorescence intensity; PHPI, purified human pancreatic islets.
Anti-HLA antibodies developed while the subject was on the waiting list with the pretransplant cPRA increasing to 96%. This result was not available at the time of islet transplantation, and the subject received a PHPI product in the presence of a shared kidney and islet DSA.
Weak HLA class I DSA (HLA-A2, 600-800 MFI) developed posttransplant to the kidney and first islet donor. In addition, weak DSA were directed to the kidney and second islet cell donor (DQ7,8,9, 200-1000 MFI). These antibodies were below the predetermined threshold for positivity.
Possible weak renal DSA developed posttransplant to HLA-DQ5,6 (MFI value in the 200-1800) which was below the predetermined threshold for positivity.
3.2.6 ∣. Kidney function
Kidney function assessed by median eGFR decreased modestly from 82 (56-86) mL/min/1.73 m2 at baseline (n = 24) to 70 [52-83] mL/min/1.73 m2 at day 75 (n = 21) (P < .001), and then returned towards baseline at 73 (58-92) mL/min/1.73 m2 by day 365 (n = 19) (P = .568) after initial transplant, and increased further to 76 (63-88) mL/ min/1.73 m2 (P = .268) and 78 (66-88) mL/min/1.73 m2 (P = .583) at days 730 (n = 14) and 1095 (n = 14), respectively, after the final transplant (see Appendix S16). No patients experienced renal allograft rejection.
3.2.7 ∣. Adverse events
The 24 subject recipients in CIT06 experienced 22 SAEs from induction immunosuppression initiation through day 365 posttransplant and 24 additional SAEs through day 1095 after final transplant (Appendix S17). One SAE involved hypoglycemia occurring 48 minutes after completing initial PHPI infusion; postinfusion hypoglycemia is a known post–islet transplant risk. Another subject experienced an inpatient hypoglycemia SAE 2 days following an initial PHPI infusion while still receiving intravenous insulin. Two subjects experienced neutropenia, and 3 subjects had nausea or vomiting (1 associated with an episode of acute kidney injury) that met criteria as SAEs and were attributed to immunosuppression. One subject was hospitalized for worsening hyperglycemia related to acute islet transplant failure 2 weeks after receiving an initial PHPI infusion. One subject was hospitalized for pneumonia possibly related to immunosuppression. The remaining SAEs reported were unrelated to either the PHPI infusion or immunosuppression. There were no procedure-related bleeding events. One subject experienced left portal branch vein thrombosis immediately following a second PHPI infusion that resolved with 6 months of anticoagulation therapy. Eight subjects experienced 24 additional SAEs after day 365 following the initial transplant through the end of CIT06 follow-up. Seven of these SAEs occurred in 5 subjects and were attributed to immunosuppressive medications: these included 4 urinary tract infections and 3 upper respiratory infections.
4 ∣. DISCUSSION
The safety and efficacy of PHPI transplant were explored in patients with T1D who previously received a kidney transplant. These study subjects required chronic immunosuppression for maintaining their kidney allografts; thus, PHPI transplant did not require additional maintenance immunosuppression. The results of CIT06, like those of CIT07, find PHPI to be safe and effective at achieving on-target glycemic control in the absence of SHEs and better disease-specific QOL scores. Importantly, long-term function of the kidney allograft was not compromised by this intervention as shown by stable eGFR to 3 years after the final islet transplant. Five subjects rejected transplanted islets 55, 73, 178, 184, and 201 days after islet infusion; 2 subjects withdrew consent (day 141 and day 246); and 2 subjects did not meet the primary endpoint at day 365 (Appendix S12). One subject was lost to follow-up at day 849 and 1 subject withdrew consent at day 1174 after transplant (Appendix S6). Also noteworthy is that differences in glycemic control, absence of hypoglycemia, recovery of hypoglycemia awareness, and QOL were durable and evident at 3 years despite the small number of patients enrolled and that 3 of 24 patients terminated participation early in the study course and the loss of other evaluable patients by year 3.
In the current study, the endpoint of HbA1c ≤ 6.5% and freedom from SHEs were achieved in 14 of 24 (58%) subjects by intention-to-treat analysis (an additional subject met the primary endpoint by experiencing a reduction in HbA1c of ≥1 point and freedom from SHEs. Thus, a total of 15 of 24 subjects [62.5%]) were successes. This rate of success was somewhat less than that seen in the CIT07 trial, in which 81% of subjects met the stringent 6.5% HbA1c endpoint. This is potentially a consequence of the poorer starting glycemic control of CIT06 vs CIT07 patients (HbA1c values of 8.1% vs 7.2%, respectively), and the use of maintenance prednisone in CIT06.
PHPI transplant in kidney transplant recipients proved highly effective at improving and maintaining metabolic control. Median HbA1c28 was reduced from baseline levels of 8.1% to 6.0% at 1 year and to 6.3% at both 2 and 3 years following the initial transplant. CIT07 reached HbA1c levels of 5.6% and 5.8% at 1 and 2 years. The higher HbA1c levels seen in CIT06 may reflect higher initial HbA1c levels and the allowance of low-dose glucocorticoid as part of the maintenance immunosuppression. Significant improvements were also evident in other measures of glycemic control including fasting glucose, glucose tolerance during the mixed-meal tolerance test (MMTT), mean glucose and time in range assessed by CGM system, and glucose variability assessed by the glycemic lability index, MAGE, and CGMS glucose SD. Such marked glycemic control stabilization has not been previously observed when using real-time CGM in patients with T1D complicated by IAH and experiencing SHEs26,27 who were treated conventionally with insulin. Emerging diabetes technologies such as next-generation insulin pumps with predictive low-glucose management technology and closed-loop systems with automated insulin or insulin and glucagon delivery have only recently been tested in patients with T1D having IAH and experiencing SHEs28,29 or following kidney transplant; future studies are needed to determine how these interventions compare with islet transplant.
Our results should also be considered in the context of whole organ pancreas transplant, a therapy for TID patients undergoing renal transplant that reliably restores euglycemia. However, pancreas alone transplant after a renal transplant entails a major operation that carries greater procedural risks than islet transplant that may offset its potential for lifesaving benefit.30 Perhaps this, together with the improvements in competing therapies (exogeneous insulin delivery devices, and islet transplant), explains the marked decline in activity of pancreas alone transplants over the past 15 years from a peak of > 600 cases in 2004 to < 150 in 2019. The current trial demonstrates that islet after kidney transplant can provide an alternative to isolated whole organ pancreas transplant that is safe and effective.
An important correlate of successfully achieving the primary endpoint was the number of islet infusions and the administered islet mass. On average, those achieving the primary endpoint received 2 separate infusions comprising nearly 14 978 IEq/kg vs 1 infusion totaling about 8589 IEq/kg in those who did not achieve the primary endpoint (P = .009). Similarly, achieving insulin independence was associated with a greater number of islet infusions and receiving a greater islet mass, with successful subjects averaging 2 infusions and 16 117 IEq/kg vs 1 infusion and 10462 IEq/kg in subjects not gaining insulin independence (P = .009).
Subjects also experienced resolution of SHEs, the primary indication for transplant in these patients. Each transplanted subject experienced SHEs in the year before transplant; during the first year posttransplant, 19 of 24 (79%) were free of SHEs (P = .003). Of the remaining 5 subjects, 3 experienced ≥1 SHE each in the first year posttransplant. Two of these 3 subjects had experienced prior islet graft failure. Two additional subjects withdrew early and were not assessed for SHEs for the complete first year posttransplant. This SHE reduction was associated with parallel improvements in other metrics of hypoglycemia awareness (Clarke score) and severity (HYPO score), which in CIT07 was associated with recovery of hypoglycemia counterregulation.31 Freedom from SHEs was not as tightly linked as the degree of metabolic control to the delivered islet mass, with even partially functioning grafts ameliorating hypoglycemia, consistent with prior reports.1,32,33
HRQOL also significantly improved after PHPI transplant. Both the DDS and the HFS scores improved significantly over the 3 years after PHPI transplant. These improvements in HRQOL are similar to the results observed in CIT07.1
Successful kidney transplant confers lifesaving benefit in T1D patients with end-stage diabetic nephropathy. Thus, an essential tenet in IAK transplant is avoiding harm to the kidney allograft and fostering its long-term function. Theoretically, the longer-term islet transplant should help avoid recurrent diabetic nephropathy posttransplant by achieving better glycemic control; on the other hand, immune sensitization, due to exposure to potentially multiple islet donors and alterations in immunosuppression, could theoretically disrupt stable allograft function. In CIT06, we observed an initial modest decline in GFR at day 75 compared with baseline; however, renal function recovered to near baseline over the subsequent 3 years following initial PHPI transplant. In contrast, CIT07 subjects were new to maintenance immunosuppression and demonstrated a decline in GFR by 12 mL/min/1.73 m2 at 1 year and 20 mL/min/1.73 m2 at 2 years, presumably attributable to initiating calcineurin inhibitor–based immunosuppression.33,34 Importantly, no kidney graft rejection episodes were reported after PHPI transplant in CIT06 and no new anti-donor antibodies to the kidney donor were identified. However, 2 subjects (8.3%) developed new anti-donor islet antibodies posttransplant, indicating immune sensitization. One of these subjects (subject 8, Tables 3 and 4) had low-level islet donor-specific antibody pretransplant that increased posttransplant and was associated with early graft failure at day 55. The current threshold for declining pancreata when low-level islet DSAs exist may need to be reevaluated in light of these results. The rate of de novo sensitization was low in both CIT06 (12.5% over 3 years) and CIT07 (8.5% over 2 years).
4.1 ∣. Review of safety and SAEs
Amongst the 24 CIT06 subjects, there were 22 SAEs from induction immunosuppression initiation through day 365 post initial islet transplant and 24 additional SAEs occurring though day 1095 following final islet transplant. Thirteen SAEs were related or possibly related to immunosuppression treatment. No procedure-related bleeding events were associated with the 39 PHPI infusions. All SAEs resolved without permanent sequalae.
4.2 ∣. Conclusion
This phase 3 pivotal trial in subjects with T1D post kidney transplant shows that PHPI transplant improves glycemic control, ameliorates problematic hypoglycemia, and significantly improves HRQOL. PHPI transplant was safe, without evidence of impairment in kidney allograft function. Islet transplant thus offers patients with T1D post kidney transplant the option of a safe and minimally invasive procedure when problematic hypoglycemia or poor glycemic control persists despite optimized medical therapy.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the study participants and the referring endocrinologists and diabetologists. We also thank the following industry sponsors for contributing their products to the trial: LifeScan Inc/Johnson & Johnson Co.
In Memoriam: During the 16-year period of CIT activity, we lost 2 beloved colleagues who were integral to the success of the consortium. William (Bill) Clarke, PhD, died in October 2017 after a remarkable 50-year career in the field of biostatistics. He co-founded the Clinical Trials Statistical and Data Management Center (CTSDMC) and was the Principal Investigator of the Clinical Islet Transplantation Consortium Data Coordinating Center. Evidence of his expertise and dedication can be seen in every analysis, report, and manuscript produced by the consortium through the present day. Kathryn Chaloner, PhD, died in October 2014. Kathryn was a distinguished statistician, leader, and colleague, and she authored the Bayesian data analysis that formed the backbone of the CIT consortium, allowing CIT investigators to submit data from all centers participating in the consortium to support Biologic License Applications to the FDA. Her creative, innovative spirit continues in the ongoing research endeavors of investigators who participated in the CIT consortium. ClinicalTrials.gov number, NCT00434811.
Funding information
Supported by grants from the National Institute of Allergy and Infectious Diseases and the National Institute for Diabetes and Digestive and Kidney Diseases to the following institutions: Emory University (U01AI089317), Northwestern University (U01AI089316), University of Alberta, Edmonton (U01AI065191), University of California San Francisco (U01DK085531), University of Illinois, Chicago (5U01DK070431), University of Iowa (U01DK070431), University of Miami (U01DK070460), University of Minnesota (U01AI065193), University of Pennsylvania (U01DK070430), and Uppsala University (U01AI065192). In addition, the study was supported by the following GCRC and CTSA awards to the following institutions: Emory University (UL1TR000454), Northwestern University (UL1RR025741 and UL1TR000150), University of California San Francisco (UL1TR000004), University of Illinois, Chicago (UL1TR000050), University of Miami (UL1TR000460), University of Minnesota, (M01-RR000400 and UL1TR000114), and University of Pennsylvania (M01-RR00040 and UL1TR000003).
Abbreviations:
- ATG
antithymocyte globulin
- BH
Benjamini-Hochberg
- BMI
body mass index
- CGM
continuous glucose monitoring
- CGMS
continuous glucose monitoring system
- CIT
clinical islet transplant
- CIT06
CIT consortium trial of PHPI transplant in patients with type 1 diabetes after kidney transplant
- CIT07
CIT consortium trial of PHPI in type I diabetic patients with normal renal function and severe hypoglycemia
- cPRA
calculated PRA
- CSII
continuous subcutaneous insulin infusion
- DDS
Diabetes Distress Scale
- DSA
donor specific antibody
- eGFR
estimated glomerular filtration rate
- FDA
US Food and Drug Administration
- FDR
false discovery rate
- GAD65
glutamic acid decarboxylase-65
- GFR
glomerular filtration rate
- HFS
Hypoglycemic Fear Survey
- HRQOL
health-related quality of life
- HYPO
hypoglycemia
- IAH
impaired awareness of hypoglycemia
- IAK
islet after kidney
- IEq
islet equivalent
- IIT
intensive insulin therapy, defined as intensive insulin therapy with target HbA1c levels of ≤ 6.5%
- IND
investigational new drug
- LI
lability index
- MAGE
mean amplitude of glycemic excursions
- MFI
mean fluorescence intensity
- MID
minimally important difference
- MMTT
mixed-meal tolerance test
- NIAID
National Institute of Allergy and Infectious Diseases
- NIDDK
National Institute of Diabetes and Digestive and Kidney Diseases
- NIH
National Institutes of Health
- PHPI
purified human pancreatic islets
- QOL
quality of life
- SAB
single antigen bead assay
- SAE
serious adverse event
- SF-36
Short Form-36 health survey
- SHE
severe hypoglycemic event
- T1D
type 1 diabetes
- VAS
visual analog scale
Footnotes
DISCLOSURE
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. The following authors reported potential dualities of interest (relationships with a company whose products or services are related to the subject matter of the manuscript): Dr Kamoun: Omixon: Scientific Advisory Board; Luminex Corporation: Transplant Diagnostic Advisory Board. Dr Rickels: Xeris Pharmaceuticals (research support); Sernova Corporation (consultant); Semma Therapeutics (consultant). Dr Markmann: eGenesis and Qihan Bio, Scientific Advisory Board. Dr Senior: Novo Nordisk (grant support to institution); ViaCyte (grant support to institution). Dr Ricordi: co-inventor on patents related to islet isolation processing aspects that are in part used for current islet cell product manufacturing (does not receive any royalty or financial benefit from these patents or from islet cell processing activities). Dr Oberholzer: Siglion Therapeutics (Consultant); BetaO2 (Consultant): CellTrans, Inc (President). Dr Alejandro: eGenesis (Consultant), Eli Lilly (Consultant), Vertex Pharmaceuticals (Consultant). Dr Bellin: ViaCyte Inc. (research support), Dexcom (research support), Insulet (Data Safety Monitoring Board membership). Dr Shapiro: ViaCyte Inc. (Consultant). BJH: Sanofi (Consultant), Novartis (Consultant), Dompé (Consultant).
No other potential dualities of interest relative to this manuscript were reported.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
Clinical Islet Transplantation Consortium (see Supplemental Appendix S1).
DATA AVAILABILITY STATEMENT
NIAID/ImmPort: The data that support the findings of this study are openly available in in ImmPort (https://www.immport.org/shared/study/SDY1331) http://doi.org/10.21430/M33GZ8UR05, reference number SDY1331. NIDDK: Data openly available in a public repository that does not issue DOIs.
REFERENCES
- 1.Hering BJ, Clarke WR, Bridges ND, et al. Phase 3 trial of transplantation of human islets in type 1 diabetes complicated by severe hypoglycemia. Diabetes Care. 2016;39:1230–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ricordi C, Goldstein JS, Balamurugan AN, et al. National Institutes of Health-sponsored Clinical Islet Transplantation Consortium phase 3 trial: manufacture of a complex cellular product at eight processing facilities. Diabetes. 2016;65:3418–3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choudhary P, Rickels MR, Senior PA, et al. Evidence-informed clinical practice recommendations for treatment of type 1 diabetes complicated by problematic hypoglycemia. Diabetes Care. 2015;38:1016–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.US Food and Drug Administration. Guidance for industry: considerations for allogeneic pancreatic islet cell products (article online). 2009; Available from https://www.fda.gov/media/77497/download. Accessed 20 October 2015 [Google Scholar]
- 5.Tiwari JL, Schneider B, Barton F, Anderson SA. Islet cell transplantation in type 1 diabetes: an analysis of efficacy outcomes and considerations for trial designs. Am J Transplant. 2012;12(7):1898–1907. [DOI] [PubMed] [Google Scholar]
- 6.Handelsman Y, Bloomgarden ZT, Grunberger G, et al. American Association of Clinical Endocrinologists and American College of Endocrinology – Clinical practice guidelines for developing a diabetes mellitus comprehensive care plan. Endocrine Practice. 2015;21(suppl 1):1–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.American College of of Endocrinology consensus statement on guidelines for glycemic control. Endocrine Practice. 2002;8(suppl 1):5–11.11939753 [Google Scholar]
- 8.American Diabetes Association. Position statement: 5. Glycemic Targets. Diabetes Care. 2016;39(suppl 1):S39–46. [DOI] [PubMed] [Google Scholar]
- 9.Clarke WL, Cox DJ, Gonder-Frederick LA, Julian D, Schlundt D, Polonsky W. Reduced awareness of hypoglycemia in adults with IDDM: a prospective study of hypoglycemic frequency and associated symptoms. Diabetes Care. 1995;18:517–522. [DOI] [PubMed] [Google Scholar]
- 10.Ryan EA, Shandro T, Green K, et al. Assessment of the severity of hypoglycemia and glycemic lability in type 1 diabetic subjects undergoing islet transplantation. Diabetes. 2004;53:955–962. [DOI] [PubMed] [Google Scholar]
- 11.Senior PA, Bellin MD, Alejandro R, et al. Clinical Islet Transplant Consortium. Consistency of quantitative scores of hypoglycemia severity and glycemic lability and comparison with continuous glucose monitoring system measures in long-standing type 1 diabetes. Diabetes Technol Ther. 2015;17:235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Service FJ, Molnar GD, Rosevear JW, Ackerman E, Gatewood LC, Taylor WF. Mean amplitude of glycemic excursions, a measure of diabetic instability. Diabetes. 1970;19:644–655. [DOI] [PubMed] [Google Scholar]
- 13.Foster ED, Bridges ND, Feurer ID, Eggerman TL, Hunsicker LG, Alejandro R. Clinical Islet Transplantation Consortium. Improved health-related quality of life in a phase 3 islet transplantation trial in type 1 diabetes complicated by severe hypoglycemia. Diabetes Care. 2018;41:1001–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Polonsky WH, Fisher L, Earles J, et al. Assessing psychosocial distress in diabetes: development of the diabetes distress scale. Diabetes Care. 2005;28:626–631. [DOI] [PubMed] [Google Scholar]
- 15.Fisher L, Hessler DM, Polonsky WH, Mullan J. When is diabetes distress clinically meaningful?: establishing cut points for the diabetes distress scale. Diabetes Care. 2012;35:259–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cox DJ, Irvine A, Gonder-Frederick L, Nowacek G, Butterfield J. Fear of Hypoglycemia: quantification, validation, and utilization. Diabetes Care. 1987;10:617–621. [DOI] [PubMed] [Google Scholar]
- 17.Irvine AA, Cox D, Gonder-Frederick L. Fear of hypoglycemia: relationship to physical and psychological symptoms in patients with insulin-dependent diabetes mellitus. Health Psychol. 1992;11:135–138. [DOI] [PubMed] [Google Scholar]
- 18.Jacobson AM, de Groot M, Samson JA. The evaluation of two measures of quality of life in patients with type i and type ii diabetes. Diabetes Care. 1994;17:267–274. [DOI] [PubMed] [Google Scholar]
- 19.Ahroni JH, Boyko EJ. Responsiveness of the SF-36 among veterans with diabetes mellitus. J Diabetes Complications. 2000;14:31–39. [DOI] [PubMed] [Google Scholar]
- 20.Bjorner JB, Lyng Wolden M, Gundgaard J, Miller KA. Benchmarks for interpretation of score differences on the SF-36 health survey for patients with diabetes. Value Health. 2013;16:993–1000. [DOI] [PubMed] [Google Scholar]
- 21.EuroQoL Group. EuroQoL – a new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199–208. [DOI] [PubMed] [Google Scholar]
- 22.Hart HE, Bilo HJ, Redekop WK, Stolk RP, Assink JH, Meyboom-de JB. Quality of life of patients with type I diabetes mellitus. Qual Life Res. 2003;12:1089–1097. [DOI] [PubMed] [Google Scholar]
- 23.Janssen MF, Lubetkin EI, Sekhobo JP, Pickard AS. The use of the EQ-5D preference-based health status measure in adults with type 2 diabetes mellitus. Diabet Med. 2011;28:395–413. [DOI] [PubMed] [Google Scholar]
- 24.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003;41:582–592. [DOI] [PubMed] [Google Scholar]
- 25.Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care. 2013;26:1384–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.The Diabetes Control and Complications Trial Research Group. The effective treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Eng IJ Med. 1993;329:977–986. [DOI] [PubMed] [Google Scholar]
- 27.Rickels MR, Peleckis AJ, Dalton-Bakes C, et al. Continuous glucose monitoring for hypoglycemia avoidance and glucose counterregulation in long-standing type 1 diabetes. J Clin Endocrinol Metab. 2018;103:105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rickels MR. Hypoglycemia-associated autonomic failure, counter-regulatory responses, and therapeutic options in type 1 diabetes. Ann N Y Acad Sci. 2019;1454:68–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rickels MR, Peleckis AJ, Markmann E, et al. Long-term improvement in glucose control and counterregulation by islet transplantation for type 1 diabetes. J Clin Endocrinol Metab. 2016;101:4421–4430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Venstrom JM, McBride MA, Rother KI, Hirshberg B, Orchard TJ, Harlan DM. Survival after pancreas transplantation in patients with diabetes and preserved kidney function. JAMA. 2003;290(21):2817–2823. [DOI] [PubMed] [Google Scholar]
- 31.Nijhoff MF, Engelse MA, Dubbeld J, et al. Glycemic stability through islet-after-kidney transplantation using an Alemtuzumab-based induction regimen and long-term triple-maintenance immunosuppression. Am J Transplant. 2016;16:246–253. [DOI] [PubMed] [Google Scholar]
- 32.Vantyghem MC, Raverdy V, Balavoine AS, et al. Continuous glucose monitoring after islet transplantation in type 1 diabetes: an excellent graft function (β-score greater than 7) Is required to abrogate hyperglycemia, whereas a minimal function is necessary to suppress severe hypoglycemia (β-score greater than 3). J Clin Endocrinol Metab. 2012;97:E2078–E2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naesens M, Kuypers DR, Sarwal M. Calcineurin inhibitor nephrotoxicity. Clin J Am Soc Nephrol. 2009;4:481–508. [DOI] [PubMed] [Google Scholar]
- 34.Paty BW, Koh A, Senior P. Pancreas and islet transplantation. Can J Diabetes. 2013;37:S94–96. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
NIAID/ImmPort: The data that support the findings of this study are openly available in in ImmPort (https://www.immport.org/shared/study/SDY1331) http://doi.org/10.21430/M33GZ8UR05, reference number SDY1331. NIDDK: Data openly available in a public repository that does not issue DOIs.


