Abstract
Purpose of Review:
Define early myocardial metabolic changes among patients with obesity and heart failure, and to describe noninvasive imaging methods and their applications for imaging cardiac metabolic remodeling.
Recent Findings:
Metabolic remodeling precedes, triggers, and sustains functional and structural remodeling in the stressed heart. Alterations in cardiac metabolism can be assessed by using a variety of molecular probes. The glucose tracer analogue, 18F-FDG, and the labeled tracer 11C-palmitate are still the most commonly used tracers to assess glucose and fatty acid metabolism, respectively. The development of new tracer analogues and imaging agents, including those targeting the peroxisome proliferator-activated receptor (PPAR), provide new opportunities for imaging metabolic activities at a molecular level. While the use of cardiac magnetic resonance spectroscopy in the clinical setting is limited to the assessment of intramyocardial and epicardial fat, new technical improvements are likely to increase its usage in the setting of heart failure.
Summary:
Noninvasive imaging methods are an effective tool for the serial assessment of alterations in cardiac metabolism, either during disease progression, or in response to treatment.
Keywords: metabolic imaging, myocardial metabolism, heart failure, obesity
Introduction
Clinical obesity is a paradigm of dysregulated systemic metabolism and known risk factor for multiple diseases including insulin resistance and heart failure [1]. Like obesity, heart failure is a major public health problem. The prevalence of both obesity and heart failure increases with age [1]. Therefore, early detection of patients at-risk for the development of heart failure who would benefit from preventive measures becomes imperative [1]. This review focuses on obese individuals as a group at risk for developing heart failure. We discuss imaging modalities assessing the dysregulated metabolic state of the heart in obese individuals, with the aim of detecting metabolic changes that underlie the development of heart failure and identifying potential targets for treatment.
The conceptual framework presents itself as follows. First, the heart converts chemical energy to mechanical energy through the integrated network of intermediary metabolism of energy providing substrates. Some details are shown in Figure 1. In normal physiologic conditions, the main sources of fuel for the heart are fatty acids and glucose [2, 3]. Secondly, the heart is a metabolic omnivore which has the ability to use a variety of energy-providing substrates including triglycerides, long-chain fatty acids (LCFAs), glucose, glycogen, lactate, pyruvate, ketone bodies, and amino acids [2–4]. These energy-providing substrates are broken down into intermediates. These intermediates then enter the Krebs cycle to provide reducing equivalents leading to oxidative phosphorylation of ADP to ATP, or to provide biosynthetic precursors [3]. Energy providing substrates such as glucose, fatty acids, ketone bodies, and branched chain amino acids (BCAAs) also affect cardiac function through their auxiliary roles as myocardial signaling molecules, influencing mitochondrial protein acetylation and mTOR (mammalian target of rapamycin) signaling [2]. Third, while under resting, fasting conditions, the main contributors for ATP production in the heart are fats (70%) and carbohydrates (30%), but the heart has the ability to adapt and alter its energy metabolism in response to different physiologic states. Long-term over-reliance on a single type of substrate underlies cardiac dysfunction in obesity, diabetes, ischemia, and heart failure [2, 3, 5, 6].
Figure 1:
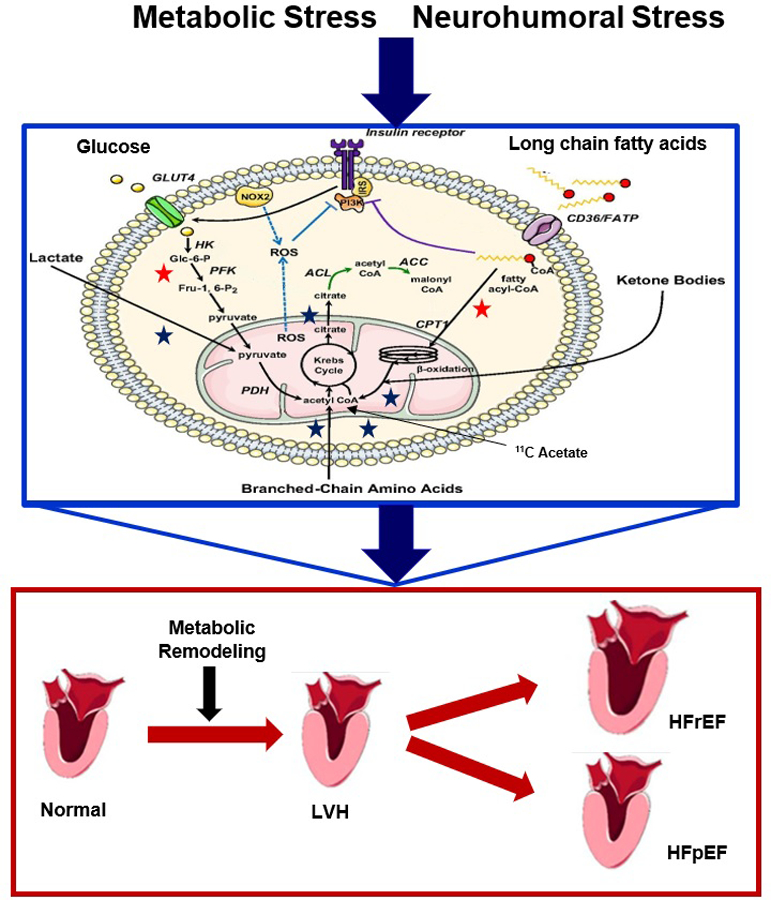
A simplified schematic drawing of molecular pathways for the metabolism of energy-providing substrates in the heart. Metabolic reactions are traced with labeled tracer analogs which are taken into the myocardium and retained, while tracer substrates such as 11C-compounds are taken up, metabolized, and release as 11CO2. For glucose and fatty acid metabolism tracer analogs and/or tracers may be used (red star), while tracers are used for lactate, acetate, amino acids, and ketone body metabolism (blue star). Sustained metabolic or neurohumoral stress leads to altered glucose and fatty acid metabolism. Fatty acid-mediated inhibition of glucose utilization stems from the allosteric effects of metabolites whose concentration rise in response to increased β-oxidation. At the same time, the activity of phosphofructokinase (PFK), which catalyzes the rate-limiting step in glycolysis, is inhibited by citrate. The resulting increase in glucose 6-phosphate (G6P) levels in turn inhibits the activity of hexokinase (HK), and rates of glucose uptake are decreased. Increased flux of glucose through the pyruvate dehydrogenase (PDH) complex inhibits β-oxidation through an increase in cytosolic malonyl-CoA, which acts as an inhibitor of the carnitine palmitoyltransferase 1 (CPT1) reaction (green). The accumulation of fatty acid metabolites such as diacylglycerol, fatty acyl-CoA, and ceramides leads to the activation of novel PKC isoforms and to the inhibitory phosphorylation of insulin receptor substrates (IRSs) (purple). Additionally, the accumulation of G6P activates mTORC1, resulting in increased protein synthesis and ER stress. Consequently, the heart hypertrophies (increased protein synthesis), and eventually fails. As energy demand outstrips energy supply, contractile dysfunction ensues, induced by altered calcium metabolism from oxidative stress and ER stress. Note that metabolic remodeling precedes, triggers, and sustains structural and functional remodeling. Abbreviations used: AMPK: 5′AMP-activated protein kinase. HK: Hexokinase-II. LVH: Left ventricular hypertrophy. HFpEF: Heart Failure with preserved Ejection Fraction. HFrEF: Heart Failure with reduced Ejection Fraction.
In the stressed heart, metabolic remodeling precedes, triggers, and sustains functional and structural remodeling (figure 1) [7–9]. The decline in cardiac function, in both obesity and heart failure, is associated with abnormalities in cardiac metabolism, which play a central role in the manifestations of cardiovascular disease and is a contributing factor to the cardiac pathology associated with obesity and heart failure [5, 9–12]. The changes may initially be adaptive before they become maladaptive [13–15]. Novel imaging techniques provide insight to metabolic activity and the myocardial metabolic perturbations that underly cardiovascular disease processes. These imaging techniques expose early indicators of metabolic stress in the heart and may lead to risk stratification of patients most at risk of developing heart failure [3, 10, 16, 17]. More importantly, noninvasive imaging methods are a potential tool for the serial assessment of alterations in cardiac metabolism, either during disease progression or in response to treatment [16, 18]. Currently, there are three main methods used to image myocardial substrate metabolism: single photon emission computed tomography (SPECT), positron emission tomography (PET), and magnetic resonance spectroscopy [3, 16, 19]. We will now first describe the myocardial metabolic changes that occur in both obesity and heart failure, and then discuss opportunities for imaging metabolic remodeling.
Cardiac metabolism in obesity
Cardiac remodeling in patients with obesity is a multi-factorial process involving changes in hemodynamics, neurohormonal signaling, and intermediary metabolism [20]. We have previously proposed that excess nutrients and fuel in patients with obesity activate myocardial cellular mechanisms that cause metabolic adaptations and inflammation, and that insulin resistance protects the heart from fuel overload [13, 21]. Cardiac metabolism is altered in patients with obesity; there is excessive fatty acid oxidation and relatively diminished glucose metabolism [11, 20, 22]. This increase in fatty acid metabolism is linked to insulin resistance and suppresses glucose uptake and oxidation [22]. In the setting of insulin resistance, previous findings from our lab have also demonstrated an inverse relation between plasma free fatty acids levels, and diastolic function [23].
The uptake of triglycerides and of long-chain fatty acids by the heart is highly regulated [6]. When it exceeds beta-oxidation it results in lipotoxicity, which is characterized by an excessive accumulation of intracellular triglycerides and lipids, accompanied by myocardial contractile dysfunction, reduced cardiac efficiency, increased myocardial oxygen consumption, and remodeling [11, 20, 23–25]. Mechanisms promoting metabolic remodeling in obese patients include ectopic cardiac fat deposition, toxic lipid metabolite accumulation, mitochondrial dysfunction, and inflammation [12]. Both, metabolic and cardiovascular changes can already be observed in obese children [26]. While obesity is associated with an unfavorable cardiometabolic profile, weight loss can reverse the metabolic remodeling observed in obesity. Specifically, weight loss decreases total fatty acid utilization and oxidation, and myocardial oxygen consumption, while increasing myocardial glycolysis, myocardial glucose oxidation, and myocardial glucose utilization [11, 12, 27–29]. Additionally, in severely obese patients undergoing bariatric surgery, the effects of restoring a metabolic homeostasis are immediate and include a reversal of insulin resistance and diastolic dysfunction of the left ventricle [30].
Of note, there are different types of cardiac fat. Fat may accumulate around the heart, or as myocellular fat in the heart muscle itself [31]. The latter has also been termed “ectopic” fat. Fat around the heart may be pericardial or epicardial. Pericardial fat is located between the visceral and parietal pericardium, its physiologic role is largely unknown, but it has been proposed to function as structural support, a thermogenic source, or a mechanical scaffolding [12, 32]. In obesity, pericardial fat increases in size which may result in a constrictive, mechanical challenge for the heart. Additionally, pericardial fat is relatively metabolically inactive, and its role as a source of adipokines is unknown and still debated [32]. Epicardial fat is located on the surface of the heart between the outer wall of the myocardium and the visceral layer of pericardium. It is distributed mainly along the atrioventricular and interventricular grooves, and it shares the same microcirculation as the myocardium [12, 32, 33]. The amount of epicardial fat around the heart is associated with obesity, visceral fat, coronary artery disease, and insulin resistance [34, 35]. Epicardial fat is metabolically and hormonally active, releasing adipokines, paracrine factors, and providing energy through the release of free fatty acids [33–36]. Previously, it has been reasoned that epicardial fat delivers substrate directly to the heart and contributes to myocardial steatosis [13, 33]. In contrast to epicardial and pericardial fat, myocardial steatosis, the accumulation of lipid droplets in non-adipocytes within the myocardium, has been associated with left ventricular hypertrophy, structural remodeling, and functional impairment [22]. In obesity, ectopic cardiac fat deposition within and around the heart promotes metabolic alterations, cardiac lipotoxicity, and dysfunction [12, 25].
Cardiac metabolism in heart failure
Obesity is frequently associated with heart failure [23, 37, 38]. It is now well-established that cardiac metabolism is altered in heart failure, and plays an integral part in its development and progression [39–44]. The metabolic remodeling that occurs in heart failure is complex and depends on the type of heart failure, its severity, and its progression, with considerable overlap in the presence of comorbidities such as obesity and type 2 diabetes [40, 41]. In general, heart failure displays a reduction in myocardial mitochondrial oxidative capacity, with metabolic changes resulting in a less efficient transfer of energy from the oxidation of substrates to mechanical work including decreased cardiac work, oxygen consumption, as well as decreased myocardial ATP, and phosphocreatine levels [40, 41]. However, the heart’s preference for oxidation of specific substrates is dependent on the etiology of heart failure; fatty acid oxidation increases in heart failure associated with diabetes and obesity and decreases in heart failure associated with hypertension and ischemia [40]. While glucose uptake and glycolysis are increased in heart failure, there is often a decrease in glucose oxidation [40]. Additionally, amino acid oxidation is decreased, while ketone body oxidation is increased [40]. In patients with heart failure, there is an observed increase in plasma levels of ketone bodies, as well as increases in ketone body metabolism and gene expression for enzymes involved in ketone body oxidation [41, 42]. There are also profound sex-related differences in substrate metabolism in heart failure, such that women with nonischemic heart failure have higher fatty acid uptake and utilization (as well as blood flow) in comparison with men [45]. In short, heart failure is associated with significant changes in cardiac metabolism, and the metabolic remodeling is characterized by a loss of metabolic homeostasis, and reduced substrate selection flexibility and metabolic reserve [40, 41, 43, 44].
Imaging metabolic remodeling
Substrates and metabolites can be traced as they move through the metabolic pathways inside cardiac myocytes [46]. While the cycles involved in each pathway including the Krebs cycle, electron transport chain, and ATP cycle, cannot be imaged, strategic metabolic imaging can obtain snapshots of the flux through selected pathways [46]. Here it is conventional to divide substrate metabolism in the heart into different stages: substrate uptake and metabolism, oxidation, and ATP production - all of which can be traced using positron-labeled tracers [46, 47]. Specific types of tracers are used to assess static and dynamic aspects of myocardial perfusion, substrate uptake and retention, or flux through entire metabolic pathways, and flux through the citric acid cycle [46]. Labeled tracer analogs for glucose and fatty acids are taken up by the heart and retained, while tracer substrates such as 11C-compounds are taken up, metabolized, and released as 11CO2 [46].
The most common methods to image myocardial metabolism are single photon emission computed tomography (SPECT), positron emission tomography (PET) and magnetic resonance spectroscopy (MRS) [3, 10, 16, 17, 48]. Each method has its own set of advantages and limitations which have been discussed extensively in the literature and are beyond the scope of our review [16, 17, 48, 49]. Briefly, SPECT is widely available and has a relatively high-count sensitivity, but SPECT has a low spatial resolution precluding the quantification of cellular metabolic processes [16]. SPECT also uses high levels of ionizing radiation. Newer solid-state cadmium-zinc-telluride SPECT cameras have greater spatial and temporal resolution and offer the possibility of performing the kinetic analyses required to evaluate metabolic processes. Unfortunately, there are very few SPECT radiotracers that have been developed for metabolic analysis. PET uses positron-emitting radionuclides and has quantitative capabilities that allow for the measurement of rates of substrate utilization [10]. It allows for both the accurate quantification of activity in the field of view and its temporal distribution, however, PET systems are also expensive and may also require an onsite cyclotron for the production of radionuclides with short half-lives, such as O-15 and C-11 [17, 48, 49]. Both SPECT and PET utilize radionuclides that produce ionizing radiation. MRS can detect a range of compounds using nuclei such as hydrogen (H-1), carbon (C-13), nitrogen (N-15), sodium (Na-23), and phosphorus (P-31), allowing it to detect several metabolic pathways without the use of radiotracers. In addition, it permits repeated measurements to monitor disease progression [50–52]. However, MRS has an intrinsically low sensitivity, limited spatial resolution, and long image acquisition times [17, 48, 50, 51].
PET/SPECT
SPECT and PET remain the most versatile imaging modalities due to their high sensitivity, depth of penetration, and large number of molecular probes available (Table 1) [53]. As mentioned above, in the failing heart, glucose uptake is most commonly increased while fatty acid oxidation is decreased. Substrate utilization in myocardial metabolism can be examined with a variety of molecular probes [53]. The most commonly used probe to assess glucose metabolism is the glucose positron-emitting tracer analogue 2-deoxy-2-(18F)fluoro-D-glucose (FDG), which is a tracer analog and used to assess glucose uptake and phosphorylation. Glucose metabolism can also be assessed using the PET tracer, 11C-glucose, which has the advantage of being able pass through all of the pathways of glucose metabolism because it is a radiolabeled glucose molecule [53]. In contrast, 11C-acetate is a tracer which is readily oxidized and exponentially decays commensurate with flux through the Krebs cycle [49].
Table 1.
Tracers and Tracer Analogs Used for the Noninvasive Assessment of Myocardial Metabolic Reactions in Vivo
| Radionuclide | Half-life | Compound | Present Use |
|---|---|---|---|
|
SPECT
| |||
| 123I | 13.3 h | IPPA | Fatty acid uptake, oxidation, and storage |
| BMIPP | Fatty acid storage | ||
| 99mTc | 6.0 h | Annexin-V | Apoptosis |
|
PET | |||
| 15O | 2.0 min | 15O2 | Oxygen consumption |
| 11C | 20.4 min | Acetate | Oxygen consumption |
| Palmitate | Fatty acid uptake, oxidation, and storage | ||
| Glucose | Glucose uptake, glycolysis, oxidation, and glycogen turnover | ||
| Lactate | Lactate uptake and oxidation | ||
| KSM-01 | PPARα activation | ||
| Glutamate | Amino acid oxidation | ||
| Aspartate | Amino acid oxidation | ||
| Methionine | Amino acid oxidation | ||
| Acetoacetate | Ketone body metabolism | ||
| β-hydroxybutyrate | Ketone body metabolism | ||
| 18F | 110 min | FDG | Glucose transport and phosphorylation |
| FBEM-Cys40-exedin-4 | Glucagon-like peptide-1 | ||
| FTHA, FTP, FCPHA, and FTO | Fatty acid uptake and oxidation | ||
| F7 | Fatty acid uptake, oxidation and storage | ||
| F3 | PPARγ activation | ||
| Fatty acid uptake, oxidation, and storage | |||
Abbreviations used: BMIPP indicates 123I-β-methyl-iodophenyl pentadecanoic acid; F3, 2-chloro-N-(4-(2-fluoroethyl)phenyl)-5-nitrobenzamide; F7, 15-(4-(2-fluoroethoxy)phenyl)pentadecanoic acid; FCPHA, trans-9(RS)-18F-fluoro-3,4(RS,RS)-methyleneheptadecanoic acid; FDG, fluorodeoxyglucose; FTHA,14-(R,S)-fluoro-6-thiaheptadecanoic acid; FTO, 18-fluoro-4-thia-oleate; FTP, 16-fluoro-4-thia-palmitate; IPPA, p-123I-iodophenylpentadecanoic acid; KSM-01, p-methoxyphenyl ureidothiobutyric derivative, a peroxisomal proliferator-activated receptor agonist; PET, positron emission tomography; PPAR, peroxisome proliferator-activated receptor; and SPECT, single-photon emission computed tomography.
The concept that myocardial metabolic remodeling precedes the onset of left ventricular hypertrophy and heart failure has been corroborated using FDG PET in multiple studies [9, 54–56]. Using FDG PET, the laboratory of Dr. Kundu was able to demonstrate that dysregulated glucose metabolism is a feature of a maladapted failing heart and that metabolic changes precede and trigger contractile dysfunction and left ventricular hypertrophy, in rat and mouse models [9, 54, 56]. Most importantly, targeting the metabolic dysregulation with the antidiabetic drug metformin, reverses the metabolic and structural changes in the heart [57]. In humans, a study using FDG PET, conducted by Hamirani et al., revealed that in hypertensive patients, increased glucose metabolic remodeling is detectable before the development of left ventricular hypertrophy, as evidenced by an increased rate of 18F-FDG uptake [55]. In contrast, patients with left ventricular hypertrophy and diastolic dysfunction exhibited lower glucose uptake rates [55], consistent with the observation of impaired left ventricular relaxation in the presence of high plasma levels of long-chain fatty acids [23].
Traditionally, FDG PET is used to identify perfusion-metabolism mismatch in hibernating myocardium [8]. Hibernation is suspected when a region with reduced contractile activity has absent or reduced perfusion but remains viable, as demonstrated by the uptake and retention of 18F-FDG. Using FDG PET, clinicians are able to differentiate between hibernating (still metabolically active) or scarred (no longer metabolically active) myocardium and use the results to determine if revascularization is needed [58]. Studies have shown PET-guided revascularization significantly improves angina and heart failure symptoms[58–60]. A recent review has also suggested that surgical revascularization should be performed to prevent further damage by protecting the residual viable myocardium from subsequent acute coronary events [61]. In short, determining myocardial viability, through metabolic imaging is strongly favored for the early identification of patients who would benefit from revascularization.
Fatty acid metabolism can also be assessed using PET. As mentioned above, fatty acid metabolism is altered in obese patients. Recent research has examined the use of radiolabeled fatty acid probes for imaging myocardial fatty acid oxidation [62]. Fatty acid analogs such as 18F-fluoro-6-thia-heptadecanoicacid or 11C-palmitate are used to evaluate fatty acid uptake, and metabolism through beta-oxidation and the Krebs cycle [63]. Other 18F-labeled fatty acid analogs include 16-18F-fluoro-4-thia-palmitate, trans-9(RS)-18F-fluoro-3,4(RS,RS) methyleneheptadecanoic acid, and 18-18F-fluoro-4-thia-oleate [53]. While these molecules are easily taken up by cardiac myocytes, they are poor at distinguishing between fatty acid uptake, oxidation, esterification, and storage pathways because their chemical structures do not allow complete metabolism [53]. Currently, 11C-palmitate is the most physiological tracer available and is likely the most optimal tracer for assessing beta oxidation of long-chain fatty acids and mitochondrial function because of its similarity to non-radiolabeled palmitate.
An emerging new opportunity for imaging cardiac metabolism is the development of imaging agents that target peroxisome proliferator-activated receptor (PPAR) [53]. The ligand-activated nuclear receptors, PPAR-α and PPAR-γ, play a significant role in regulating myocardial fat metabolism and free fatty acid concentrations [53]. The PPAR agonists, 11C-KSM1 and 18F3, are radiolabeled biomarkers that target PPAR-α and PPAR-γ, respectively. These were recently developed to assess PPAR activities, and their use is currently under investigation [53]. Imaging the PPAR system would allow for a more detailed evaluation of the factors driving myocardial fatty acid metabolism [10].
Cardiac MRS/MRI
The use of cardiac MRS/MRI (magnetic resonance spectroscopy/imaging) in the clinical setting has been minimal, as it has mainly been limited to research [52]. A review by Dellegrottaglie et al., advocates for the use of cardiac MRS in clinical practice to allow clinicians to better understand changes in cardiac pathophysiology and allow for the initial characterization and monitoring of patients with heart failure [52]. 1H-MRS and 31P-MRS have been the most promising for potential clinical applications because they do not require the use of stable isotope tracers, relying instead on the 1H and 31P isotopes that are the most common forms present in the environment and in vivo. 1H-MRS is used to examine triglyceride content and creatine levels, while 31P-MRS is used to measure energetics, specifically phosphocreatine (PCr) and ATP content [52]. Changes in the PCr/ATP ratio have been proposed as a predictor of cardiac functional status in heart failure and could be used as an imaging biomarker to assess heart failure severity [52]. Additionally, cardiac proton (H+) MRS is used clinically to study the myocardial metabolic effects of nutritional and pharmacological interventions in heart failure patients and those with obesity [52]. However, the technical limitations associated with cardiac MRS, including the poor signal amplitude and limited spatial and temporal resolution, must be addressed if there is to be any future potential use of this imaging application.
Hyperpolarization allows for a greater magnetic resonance signal by mixing an electron paramagnetic agent with a 13C-containing molecule, in a magnetic field at very low temperatures [51, 64]. This process polarizes the free radicals. The polarized electrons are then transferred to the 13C nucleus by microwave irradiation. When combined with metabolic tracers 1-13C and 2-13C pyruvate, real time imaging of myocardial substrate metabolism was observed [50]. However, a major constraint of the method is the metabolic stress imposed by the high concentrations of hyperpolarized pyruvate that need to be administered. Another study was also able to assess pyruvate metabolism, using 13C hyperpolarized imaging, which demonstrated a strong signal and proved to be feasible for assessing flux through the pyruvate dehydrogenase complex in the myocardium [65]. Additionally, a recent review examined the use of hyperpolarized magnetic resonance to define and further characterize myocardial metabolism in heart failure and demonstrated that it was over 10,000 times more sensitive than conventional MRS [64]. Other technical improvements such as the development of advanced shimming algorithms, radiofrequency pulses, pulse sequences, multichannel detection coils, use of hyperpolarized nuclei, and scanning at higher magnetic field strengths will allow for better clinical applicability [51]. In vivo evaluation of cardiac metabolism, using MRS could provide important and clinically useful information in the setting of heart failure.
Cardiac MRS/MRI is also used to quantify total epicardial adipose tissue (EAT), which is associated with myocardial metabolic alterations [66]. Cardiac MRI allows for the measurement of epicardial fat volume and fat area [67]. Lipids stored in intracellular and extracellular compartments can be distinguished and quantified using cardiac MRS [68]. Together, cardiac MRS/MRI is a useful tool for quantifying myocardial triglyceride content and screening patients for fatty heart [69]. Epicardial fat can also be visualized using echocardiography and CT [35]. Given its affordability, availability, and short testing time, echocardiography of epicardial fat thickness, provides the most useful method for widescale imaging of epicardial fat [67]. Overall, intramyocardial triglyceride imaging will allow researchers to further understand the relationship between obesity, fatty acid metabolism, and myocardial function, as well as differences that exist between different demographics [62].
Future directions
Factors that drive obesity-related changes in myocardial fatty acid metabolism are incompletely understood and imaging fatty acid metabolism provides a tool to explore in detail these mechanisms [62]. In obese patients at risk of developing heart failure to due lipotoxicity, radiolabeled fatty acid probes facilitate the development of targeted therapies that influence myocardial fatty acid uptake and metabolism [25, 62]. Imaging metabolic remodeling in patients with obesity will allow clinicians to detect early subclinical heart disease and improve a patient’s prognosis through early initiation of preventative therapy [46, 66]. This may be especially important for the management of obese children, where significant cardiac remodeling and dysfunction has been shown to begin as young as age eight [70].
PET/SPECT imaging may also develop as a useful tool to risk stratify hypertensive patients for the development of heart failure so that pharmacologic interventions such as beta blockers, metformin, or mTOR inhibitors could be administered to prevent the progression to left ventricular hypertrophy and heart failure [9]. In preclinical studies, metformin normalized myocardial metabolism by modulating the activities of AMP‐activated protein kinase and mammalian target‐of‐rapamycin [57]. This prevented left ventricular hypertrophy, improved cardiac function, and led to reductions in blood pressure and reduced FDG uptake to normal, in spontaneously hypertensive rats [57]. Beta blockers have also been shown to prevent metabolic alterations and subsequent myocardial structural and functional remodeling [56]. In general, the concept of rebalancing substrate metabolism of the heart using pharmacologic interventions may become an effective strategy for heart failure treatment [6]. In addition to pharmacologic treatments, changes in cardiac metabolism of obese patients have been observed by clinicians, using 15O-water and 1-11C-glucose PET imaging, following weight loss interventions [27, 29].
Numerous imaging modalities already exist to characterize different aspects of cardiovascular disease processes. However, metabolism and cardiac function are most often not assessed together, and imaging methods are frequently used in isolation which may not capture the multi-dimensional nature of a disease such as diabetes or the metabolic syndrome [71, 72]. A combined PET/MR stress test to examine cardiac function and metabolism simultaneously has the potential to assist clinicians in identifying subtle metabolic and functional abnormalities in patients with obesity or those at risk of heart failure progression [71, 72]. As the feasibility has now been demonstrated in animal models, future studies are likely to use this imaging modality to evaluate cardiac disease progression in humans [72].
Conclusions
Metabolic remodeling precedes, triggers, and sustains functional and structural remodeling in the stressed heart. Serial assessments of this remodeling by SPECT, PET, or MRS, allows for early detection, monitoring of disease progression, and assessment of the response to treatment. Metabolic imaging methods are powerful tools to provide insight into the metabolic changes that occur in various disease processes and has enhanced the discovery of new treatments that use metabolic modulation to target the detrimental effects of myocardial metabolic remodeling. Future research will now focus on improving current non-invasive imaging modalities, conducting studies which use imaging to monitor disease progression, and determining the effectiveness of early metabolic interventions for the treatment of obese patients at risk of developing heart failure. Specifically, tracking metabolic changes of the heart in the dysregulated states of obesity and diabetes mellitus emerges as a useful tool for choosing the most effective interventions to unload the heart from metabolic stress.
Lastly, a word about the elusive goal of imaging myocardial protein turnover. When Rudolf Schoenheimer first deployed stable isotopes in metabolic research some 80 years ago, he made a fundamental discovery that “not only the fuel but the structural materials are in a steady state of flux.” [73]. Although rates of myocardial protein turnover are considerable, there are currently no imaging methods for assessing this dynamic process. If technically feasible, imaging protein turnover may provide unprecedented new insights into the pathophysiology of heart disease.
Acknowledgments
Work in the authors lab was supported by grants from the US Public Health Service (R01- HL-061483, R01- HL-073162). We thank Drs. Linda Peterson (Washington University St. Louis) and Raymond R. Russell (Alpert School of Medicine, Brown University). We also thank Mrs. Anna Menezes for expert editorial assistance.
Footnotes
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Groenewegen A, Rutten FH, Mosterd A, Hoes AW: Epidemiology of heart failure. Eur. J. Heart Fail 2020, 22(8):1342–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lopaschuk GD, Ussher JR: Evolving concepts of myocardial energy metabolism: more than just fats and carbohydrates. Circ Res 2016, 119(11):1173–1176. [DOI] [PubMed] [Google Scholar]
- 3.Taegtmeyer H, Young ME, Lopaschuk GD, Abel ED, Brunengraber H, Darley-Usmar V, Des Rosiers C, Gerszten R, Glatz JF, Griffin JL: Assessing cardiac metabolism: a scientific statement from the American Heart Association. Circ Res 2016, 118(10):1659–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taegtmeyer H: Carbohydrate interconversions and energy production. Circulation 1985, 72(5 Pt 2):IV1–8. [PubMed] [Google Scholar]
- 5.Schulze PC, Drosatos K, Goldberg IJ: Lipid use and misuse by the heart. Circ Res 2016, 118(11):1736–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Glatz JF, Nabben M, Young ME, Schulze PC, Taegtmeyer H, Luiken JJ: Re-balancing cellular energy substrate metabolism to mend the failing heart. Biochim Biophys Acta Mol Basis Dis 2020, 1866(5):165579. • Overview of myocardial substrate utilization in cardiac dysfunction.
- 7.Taegtmeyer H, Golfman L, Sharma S, Razeghi P, van Arsdall M: Linking gene expression to function: metabolic flexibility in the normal and diseased heart. Ann N Y Acad Sci 2004, 1015(1):202–213. [DOI] [PubMed] [Google Scholar]
- 8.Taegtmeyer H, Dilsizian V: Imaging myocardial metabolism and ischemic memory. Nat. Clin. Pract. Cardiovasc. Med 2008, 5(2): S42–S48. [DOI] [PubMed] [Google Scholar]
- 9.Kundu BK, Zhong M, Sen S, Davogustto G, Keller SR, Taegtmeyer H: Remodeling of glucose metabolism precedes pressure overload-induced left ventricular hypertrophy: review of a hypothesis. Cardiology 2015, 130(4):211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gropler RJ: Recent advances in metabolic imaging. J Nucl Cardiol 2013, 20(6):1147–1172. [DOI] [PubMed] [Google Scholar]
- 11.Rider O, Cox P, Tyler D, Clarke K, Neubauer S: Myocardial substrate metabolism in obesity. Int J Obes 2013, 37(7):972–979. [DOI] [PubMed] [Google Scholar]
- 12. Piché M, Poirier P: Obesity, ectopic fat and cardiac metabolism. Expert review of endocrinology & metabolism 2018, 13(4):213–221. • Examines how metabolic alterations in obesity and ectopic cardiac fat accumulation, translate into cardiac energy metabolism disturbances that may lead to adverse effects on the cardiovascular system.
- 13.Harmancey R, Wilson CR, Taegtmeyer H: Adaptation and maladaptation of the heart in obesity. Hypertension 2008, 52(2):181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taegtmeyer H, McNulty P, Young ME: Adaptation and maladaptation of the heart in diabetes: Part I: general concepts. Circulation 2002, 105(14):1727–1733. [DOI] [PubMed] [Google Scholar]
- 15.Young ME, McNulty P, Taegtmeyer H: Adaptation and maladaptation of the heart in diabetes: Part II: potential mechanisms. Circulation 2002, 105(15):1861–1870. [DOI] [PubMed] [Google Scholar]
- 16.Curley D, Plaza BL, Shah AM, Botnar RM: Molecular imaging of cardiac remodelling after myocardial infarction. Basic Res Cardiol 2018, 113(2):1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gropler RJ, Beanlands RS, Dilsizian V, Lewandowski ED, Villanueva FS, Ziadi MC: Imaging myocardial metabolic remodeling. J Nucl Med 2010, 51 Suppl 1:88S–101S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Osterholt M, Sen S, Dilsizian V, Taegtmeyer H: Targeted metabolic imaging to improve the management of heart disease. JACC: Cardiovascular Imaging 2012, 5(2):214–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gewirtz H, Dilsizian V: Myocardial viability: survival mechanisms and molecular imaging targets in acute and chronic ischemia. Circ Res 2017, 120(7):1197–1212. [DOI] [PubMed] [Google Scholar]
- 20.Alpert MA, Karthikeyan K, Abdullah O, Ghadban R: Obesity and cardiac remodeling in adults: mechanisms and clinical implications. Prog Cardiovasc Dis 2018, 61(2):114–123. [DOI] [PubMed] [Google Scholar]
- 21. Koutroumpakis E, Jozwik B, Aguilar D, Taegtmeyer H: Strategies of unloading the failing heart from metabolic stress. Am J Med 2020, 133(3):290–296. • Summary of current knowledge on the pathophysiology of non-ischemic heart failure in the state of metabolic dysregulation.
- 22.Sletten AC, Peterson LR, Schaffer JE: Manifestations and mechanisms of myocardial lipotoxicity in obesity. J Intern Med 2018, 284(5):478–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leichman JG, Aguilar D, King TM, Vlada A, Reyes M, Taegtmeyer H: Association of plasma free fatty acids and left ventricular diastolic function in patients with clinically severe obesity–. Am J Clin Nutr 2006, 84(2):336–341. [DOI] [PubMed] [Google Scholar]
- 24.Mahajan R, Lau DH, Sanders P: Impact of obesity on cardiac metabolism, fibrosis, and function. Trends Cardiovasc Med 2015, 25(2):119–126. [DOI] [PubMed] [Google Scholar]
- 25.Sharma S, Adrogue JV, Golfman L, Uray I, Lemm J, Youker K, Noon GP, Frazier O, Taegtmeyer H: Intramyocardial lipid accumulation in the failing human heart resembles the lipotoxic rat heart. The FASEB Journal 2004, 18(14):1692–1700. [DOI] [PubMed] [Google Scholar]
- 26.Corica D, Oreto L, Pepe G, Calabrò MP, Longobardo L, Morabito L, Pajno GB, Alibrandi A, Aversa T, Wasniewska M: Precocious preclinical cardiovascular sonographic markers in metabolically healthy and unhealthy childhood obesity. Frontiers in endocrinology 2020, 11:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Madigan MJ Jr, Racette SB, Coggan AR, Stein RI, McCue LM, Gropler RJ, Peterson LR: Weight loss affects intramyocardial glucose metabolism in obese humans. Circ. Cardiovasc. Imaging 2019, 12(8):e009241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kosmala W, Sanders P, Marwick TH: Subclinical myocardial impairment in metabolic diseases. JACC Cardiovasc. Imaging 2017, 10(6):692–703. [DOI] [PubMed] [Google Scholar]
- 29.Lin CH, Kurup S, Herrero P, Schechtman KB, Eagon JC, Klein S, Dávila‐Román VG, Stein RI, Dorn‐II GW, Gropler RJ: Myocardial oxygen consumption change predicts left ventricular relaxation improvement in obese humans after weight loss. Obesity 2011, 19(9):1804–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leichman JG, Wilson EB, Scarborough T, Aguilar D, Miller CC III, Yu S, Algahim MF, Reyes M, Moody FG, Taegtmeyer H: Dramatic reversal of derangements in muscle metabolism and left ventricular function after bariatric surgery. Am J Med 2008, 121(11):966–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thomas SY, Harmancey R, Taegtmeyer H: Fat around the heart. JACC Cardiovasc. Imaging 2010, 3(7):786–787. [DOI] [PubMed] [Google Scholar]
- 32.Iacobellis G: Epicardial and Pericardial Fat: Close, but Very Different. Obesity 2009, 17(4):626. [DOI] [PubMed] [Google Scholar]
- 33.Ferrara D, Montecucco F, Dallegri F, Carbone F: Impact of different ectopic fat depots on cardiovascular and metabolic diseases. J Cell Physiol 2019, 234(12):21630–21641. [DOI] [PubMed] [Google Scholar]
- 34.Rabkin S: Epicardial fat: properties, function and relationship to obesity. Obesity reviews 2007, 8(3):253–261. [DOI] [PubMed] [Google Scholar]
- 35.Iacobellis G: Local and systemic effects of the multifaceted epicardial adipose tissue depot. Nat. Rev. Endocrinol 2015, 11(6):363–371. [DOI] [PubMed] [Google Scholar]
- 36.Iacobellis G, Corradi D, Sharma AM: Epicardial adipose tissue: anatomic, biomolecular and clinical relationships with the heart. Nature clinical practice Cardiovascular medicine 2005, 2(10):536–543. [DOI] [PubMed] [Google Scholar]
- 37.Algahim MF, Lux TR, Leichman JG, Boyer AF, Miller CC III, Laing ST, Wilson EB, Scarborough T, Yu S, Snyder B: Progressive regression of left ventricular hypertrophy two years after bariatric surgery. Am J Med 2010, 123(6):549–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Algahim MF, Sen S, Taegtmeyer H: Bariatric surgery to unload the stressed heart: a metabolic hypothesis. American Journal of Physiology-Heart and Circulatory Physiology 2012, 302(8):H1539–H1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tuunanen H, Knuuti J: Metabolic remodelling in human heart failure. Cardiovasc Res 2011, 90(2):251–257. [DOI] [PubMed] [Google Scholar]
- 40.Lopaschuk GD, Karwi QG, Tian R, Wende AR, Abel ED: Cardiac energy metabolism in heart failure. Circ Res 2021, 128(10):1487–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gibb AA, Hill BG: Metabolic coordination of physiological and pathological cardiac remodeling. Circ Res 2018, 123(1):107–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kolwicz SC Jr, Airhart S, Tian R: Ketones step to the plate: a game changer for metabolic remodeling in heart failure? Circulation 2016, 133(8):689–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fragasso G: Deranged Cardiac Metabolism and the Pathogenesis of Heart Failure. Card Fail Rev 2016, 2(1):8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kadkhodayan A, Coggan AR, Peterson LR: A “PET” area of interest: myocardial metabolism in human systolic heart failure. Heart Fail Rev 2013, 18(5):567–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kadkhodayan A, Lin CH, Coggan AR, Kisrieva-Ware Z, Schechtman KB, Novak E, Joseph SM, Dávila-Román VG, Gropler RJ, Dence C: Sex affects myocardial blood flow and fatty acid substrate metabolism in humans with nonischemic heart failure. J Nucl Cardiol 2017, 24(4):1226–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taegtmeyer H: Tracing cardiac metabolism in vivo: one substrate at a time. J Nucl Med 2010, 51 Suppl 1:80S–87S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Olson RE: Myocardial metabolism in congestive heart failure. J Chronic Dis 1959, 9(5):442–464. [DOI] [PubMed] [Google Scholar]
- 48.Herrero P, Gropler RJ: Imaging of myocardial metabolism. J Nucl Cardiol 2005, 12(3):345–358. [DOI] [PubMed] [Google Scholar]
- 49.Peterson LR, Gropler RJ: Radionuclide imaging of myocardial metabolism. Circ. Cardiovasc. Imaging 2010, 3(2):211–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rider OJ, Tyler DJ: Clinical implications of cardiac hyperpolarized magnetic resonance imaging. J Cardiovasc Magn Reson 2013, 15(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van Ewijk PA, Schrauwen‐Hinderling VB, Bekkers SC, Glatz JF, Wildberger JE, Kooi ME: MRS: a noninvasive window into cardiac metabolism. NMR Biomed 2015, 28(7):747–766. [DOI] [PubMed] [Google Scholar]
- 52. Dellegrottaglie S, Scatteia A, Pascale CE, Renga F, Perrone-Filardi P: Evaluation of Cardiac Metabolism by Magnetic Resonance Spectroscopy in Heart Failure. Heart Fail Clin 2019, 15(3):421–433. • Comprehensive overview of cardiac magnetic resonance spectroscopy use to assess metabolic changes in heart failure.
- 53.Shirani J, Singh A, Agrawal S, Dilsizian V: Cardiac molecular imaging to track left ventricular remodeling in heart failure. J Nucl Cardiol 2017, 24(2):574–590. [DOI] [PubMed] [Google Scholar]
- 54. Li J, Kemp BA, Howell NL, Massey J, Mińczuk K, Huang Q, Chordia MD, Roy RJ, Patrie JT, Davogustto GE: Metabolic changes in spontaneously hypertensive rat hearts precede cardiac dysfunction and left ventricular hypertrophy. J. Am. Heart Assoc 2019, 8(4):e010926. •• In the stressed heart, metabolic remodeling precedes, triggers, and sustains functional and structural remodeling.
- 55.Hamirani YS, Kundu BK, Zhong M, McBride A, Li Y, Davogustto GE, Taegtmeyer H, Bourque JM: Noninvasive Detection of Early Metabolic Left Ventricular Remodeling in Systemic Hypertension. Cardiology 2016, 133(3):157–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhong M, Alonso CE, Taegtmeyer H, Kundu BK: Quantitative PET imaging detects early metabolic remodeling in a mouse model of pressure-overload left ventricular hypertrophy in vivo. J Nucl Med 2013, 54(4):609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Li J, Minćzuk K, Massey JC, Howell NL, Roy RJ, Paul S, Patrie JT, Kramer CM, Epstein FH, Carey RM: Metformin improves cardiac metabolism and function, and prevents left ventricular hypertrophy in spontaneously hypertensive rats. J. Am. Heart Assoc 2020, 9(7):e015154. •• Metformin ameliorates cardiac metabolic abnormalities that develop in response to chronic pressure overload and thereby lessen hypertension induced LVH, even in patients without diabetes.
- 58.Khalaf S, Chamsi-Pasha M, Al-Mallah MH: Assessment of myocardial viability by PET. Curr Opin Cardiol 2019, 34(5):466–472. [DOI] [PubMed] [Google Scholar]
- 59.Kloner RA: Stunned and hibernating myocardium: where are we nearly 4 decades later? J. Am. Heart Assoc 2020, 9(3):e015502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ryan MJ, Perera D: Identifying and managing hibernating myocardium: what’s new and what remains unknown? Curr. Heart Fail. Rep 2018, 15(4):214–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Panza JA, Chrzanowski L, Bonow RO: Myocardial Viability Assessment Before Surgical Revascularization in Ischemic Cardiomyopathy: JACC Review Topic of the Week. J Am Coll Cardiol 2021, 78(10):1068–1077. [DOI] [PubMed] [Google Scholar]
- 62.Mather KJ, DeGrado TR: Imaging of myocardial fatty acid oxidation. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids 2016, 1861(10):1535–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ylä-Herttuala E, Saraste A, Knuuti J, Liimatainen T, Ylä-Herttuala S: Molecular imaging to monitor left ventricular remodeling in heart failure. Curr. Cardiovasc. Imaging Rep 2019, 12(4):1–13.31186826 [Google Scholar]
- 64. Apps A, Lau J, Peterzan M, Neubauer S, Tyler D, Rider O: Hyperpolarised magnetic resonance for in vivo real-time metabolic imaging. Heart 2018, 104(18):1484–1491. • Opportunities and limitations of a novel approach to assess cardiac metabolism.
- 65.Cunningham CH, Lau JY, Chen AP, Geraghty BJ, Perks WJ, Roifman I, Wright GA, Connelly KA: Hyperpolarized 13C metabolic MRI of the human heart: initial experience. Circ Res 2016, 119(11):1177–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ng AC, Delgado V, Borlaug BA, Bax JJ: Diabesity: the combined burden of obesity and diabetes on heart disease and the role of imaging. Nat. Rev. Cardiol 2021, 18(4):291–304. [DOI] [PubMed] [Google Scholar]
- 67.Davidovich D, Gastaldelli A, Sicari R: Imaging cardiac fat. Eur. Heart J. Cardiovasc. Imaging 2013, 14(7):625–630. [DOI] [PubMed] [Google Scholar]
- 68.McGavock JM, Victor RG, Unger RH, Szczepaniak LS: Adiposity of the heart*, revisited. Ann Intern Med 2006, 144(7):517–524. [DOI] [PubMed] [Google Scholar]
- 69.Szczepaniak LS, Victor RG, Orci L, Unger RH: Forgotten but not gone: the rediscovery of fatty heart, the most common unrecognized disease in America. Circ Res 2007, 101(8):759–767. [DOI] [PubMed] [Google Scholar]
- 70.Jing L, Binkley CM, Suever JD, Umasankar N, Haggerty CM, Rich J, Nevius CD, Wehner GJ, Hamlet SM, Powell DK: Cardiac remodeling and dysfunction in childhood obesity: a cardiovascular magnetic resonance study. J Cardiovasc Magn Reson 2016, 18(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Peterson LR, Gropler RJ: Metabolic and molecular imaging of the diabetic cardiomyopathy. Circ Res 2020, 126(11):1628–1645. • Comprehensive review on non-invasive metabolic imaging methods in patients with diabetes.
- 72.Barton GP, Vildberg L, Goss K, Aggarwal N, Eldridge M, McMillan AB: Simultaneous determination of dynamic cardiac metabolism and function using PET/MRI. J Nucl Cardiol 2019, 26(6):1946–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schoenheimer R: The dynamic state of body constituents. The dynamic state of body constituents 1946.


