Abstract
Background
There is controversy regarding optimal use of benzodiazepines during cardiac surgery, and it is unknown whether and to what extent there is variation in practice. We sought to describe benzodiazepine use and sources of variation during cardiac surgeries across patients, clinicians, and institutions.
Methods
We conducted an analysis of adult cardiac surgeries across a multicentre consortium of USA academic and private hospitals from 2014 to 2019. The primary outcome was administration of a benzodiazepine from 2 h before anaesthesia start until anaesthesia end. Institutional-, clinician-, and patient-level variables were analysed via multilevel mixed-effects models.
Results
Of 65 508 patients cared for by 825 anaesthesiology attending clinicians (consultants) at 33 institutions, 58 004 patients (88.5%) received benzodiazepines with a median midazolam-equivalent dose of 4.0 mg (inter-quartile range [IQR], 2.0–6.0 mg). Variation in benzodiazepine dosage administration was 54.7% attributable to institution, 14.7% to primary attending anaesthesiology clinician, and 30.5% to patient factors. The adjusted median odds ratio for two similar patients receiving a benzodiazepine was 2.68 between two randomly selected clinicians and 4.19 between two randomly selected institutions. Factors strongly associated (adjusted odds ratio, <0.75, or >1.25) with significantly decreased likelihoods of benzodiazepine administration included older age (>80 vs ≤50 yr; adjusted odds ratio=0.04; 95% CI, 0.04–0.05), university affiliation (0.08, 0.02–0.35), recent year of surgery (0.42, 0.37–0.49), and low clinician case volume (0.44, 0.25–0.75). Factors strongly associated with significantly increased likelihoods of benzodiazepine administration included cardiopulmonary bypass (2.26, 1.99–2.55), and drug use history (1.29, 1.02–1.65).
Conclusions
Two-thirds of the variation in benzodiazepine administration during cardiac surgery are associated with institutions and attending anaesthesiology clinicians (consultants). These data, showing wide variations in administration, suggest that rigorous research is needed to guide evidence-based and patient-centred benzodiazepine administration.
Keywords: anaesthesia, cardiac procedures, benzodiazepines, cardiovascular surgery, practice patterns, physicians
Editor's key points.
-
•
Benzodiazepines have historically been popular in cardiac surgery, as they are relatively haemodynamically stable, and might decrease the risk of intraoperative awareness with recall.
-
•
In recent years concern has arisen, albeit without evidence, that benzodiazepines might be associated with increased risk of postoperative delirium.
-
•
This study shows that there appears to be arbitrary variability, based largely on institutional culture and clinician bias, regarding the decision to administer benzodiazepines and, when given, the dose chosen for patients undergoing cardiac surgery.
-
•
Where such wide variability exists in clinical practice, it is possible that the choices are inconsequential; however, rigorous research could help to resolve these controversies.
There is a lack of consensus among anaesthesiologists providing care for cardiac surgery patients regarding multiple clinical decisions; benzodiazepine administration is one of them.1 With limited data on the impact of intraoperative benzodiazepines on outcomes after cardiac surgery,1,2 their use is often based on dogma and is hotly debated. Benzodiazepines are used in cardiac surgery for their amnestic effects in the context of a perceived increased risk of intraoperative awareness as has been described in the literaturee1, 2, 3, 4 and practice advisories for cardiac surgical patients.5 However, a potential disadvantage of benzodiazepine use includes the increased risk of delirium as observed in the ICU population.1,6, 7, 8, 9, 10
Perioperative consensus guidelines discourage benzodiazepine administration among older adults,11,12 yet, little is known about benzodiazepine use patterns during cardiac surgery. Currently, there is a paucity of data describing how often benzodiazepines are administered, which patients are more or less likely to receive benzodiazepines, and whether patient, clinician, or institutional factors explain variation in benzodiazepine administration for cardiac surgery. A survey across Canadian academic hospitals found that 89% of cardiac anaesthesiologists routinely used benzodiazepines; yet, a majority believed most cardiac anaesthetics could be performed safely without benzodiazepines.1 Multicentre clinical trials of benzodiazepine administration during cardiac13 and noncardiac14 surgery are currently underway. However, understanding the degree and sources of variation in current practice remains critical to informing practice change, based upon results of such trials.
In this observational study, we assessed perioperative electronic health records (EHRs) of academic and community hospitals in the USA to describe perioperative benzodiazepine administration practice patterns among adult patients undergoing cardiac surgery. We hypothesised that patient, clinician, and institutional factors were independently associated with benzodiazepine use during cardiac surgery, and that a majority of the variation in benzodiazepine use was explained by institution and clinician rather than patient factors.
Methods
Study design
This observational study followed the REporting of studies Conducted using Observational Routinely collected health Data (RECORD) extension of the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines (Supplementary Material 1).15,16 The University of Michigan Institutional Review Board approved this study and waived patient consent (HUM00167369; Ann Arbor, MI, USA). The study proposal was approved by a multicentre peer-review committee on December 2, 2019 before data were accessed.17 Data were obtained from the Multicenter Perioperative Outcomes Group (MPOG) dataset, and data acquisition, quality validation, secure transfer, and processing into pre-computed phenotypes, have been previously described18,19 and used in multiple studies.20,21
Cohort
The study population included adult patients undergoing elective or urgent cardiac surgery with or without cardiopulmonary bypass at participating MPOG academic and community hospitals in the USA from January 1, 2014 to August 1, 2019; data meeting the MPOG perioperative research standard for data quality (Supplementary Material 2) were included. Only the index surgery was included if others occurred within 30 days. Patients with American Society of Anesthesiologists (ASA) physical status 5 or 6, emergent operative status, mechanical circulatory support before or during surgery, or undergoing transplantation, pulmonary thromboendarterectomy, ventricular assist device placement, open aortic procedures, transcatheter procedures, or procedures using circulatory arrest were excluded from our primary analysis. In addition, we excluded cases without invasive arterial catheter monitoring or with a case duration less than 120 min, as these were unlikely to be a complete cardiac case (Fig. 1).18,19
Fig 1.
STROBE-compliant flowchart outlining case totals for each exclusion criteria.15,16 STROBE, Strengthening the Reporting of Observational Studies in Epidemiology; MPOG, Multicenter Perioperative Outcomes Group; VAD, ventricular assist device; IABP, intra-aortic balloon pump.
Primary outcome
The primary outcome was exposure to benzodiazepines in the perioperative period, analysed as a dichotomous variable. Benzodiazepine exposure was defined as the administration of an intravenous, oral, or intranasal dose of midazolam, alprazolam, diazepam, clonazepam, or lorazepam between 2 h before anaesthesia start and anaesthesia end.
Secondary outcomes
We analysed the total perioperative dose of benzodiazepine, standardised as midazolam equivalents in milligrams22, 23, 24, 25, 26, 27 per actual body weight in kilograms (Supplementary Material 3). Histograms were inspected to assess distributions of dosing, and modes were used to establish clinically meaningful ranges for low and high benzodiazepine use by case before data analysis for an a priori secondary analysis.
We also analysed the perioperative timing of benzodiazepine administration during two windows: (1) the pre-induction period, between 2 h before anaesthesia start and entry into the operating room, and (2) the intraoperative period between patient entry into the operating room until anaesthesia end. Among cases using cardiopulmonary bypass, the intraoperative period was further divided into pre-bypass, bypass, and post-bypass periods (Supplementary Material 4).
Covariates
Covariates at the patient, clinician, and institutional levels were selected based on availability in MPOG, use in existing cardiac surgery risk scoring systems (e.g. EuroSCORE II),28 or clinical relevance (e.g. potentially impacting a clinician's decision to administer benzodiazepines). Patient-level covariates included patient age (18–50 yr [reference], 51–60, 61–70, 71–80, and over 80 yr), sex (male [reference] and female), race/ethnicity (Non-Hispanic White [reference], Non-Hispanic Black, Hispanic [of any race], and other or multi-racial), BMI (using WHO categories29: underweight [<18.5 kg m−2], normal [18.5–24.9, reference], overweight [25.0–29.9], Class I Obesity [30.0–34.9], Class II Obesity [35.0–39.9], and Class III Obesity [≥40.0]), ASA physical status (ASA 1–3 [reference] and ASA 4), surgical procedure type (coronary artery bypass grafting [CABG] only [reference], valve only, valve and CABG only, and other open cardiac surgical procedures), duration of surgery (in hours), year of procedure (2014 [reference], 2015, 2016, 2017, 2018, and 2019), clinically relevant comorbidities (Enhanced Elixhauser Comorbidities, ICD 9/10 Clinical Modification Algorithm [Supplementary Material 5]30: hypertension, congestive heart failure, arrhythmia, valvular heart disease, pulmonary circulation disorders, diabetes, neurological disorders, renal failure, liver disease, obesity, psychotic disorders, depression, chronic pulmonary disease, history of alcohol use, and history of drug abuse), and first non-artifact mean arterial pressure in the operating room (as defined by consensus guidelines31,32: hypotensive [<70 mm Hg], normotensive [70–107 mm Hg, reference], stage 1 hypertension [108–120 mm Hg], and stage 2 hypertension [>120 mm Hg]). Administration of intraoperative vasopressor, inotropic, and additional induction agents were presented as binary variables, and total bolus doses and maximum infusion rates were reported.
The primary anaesthesiology attending clinician (consultant) was defined as the clinician present during the case for the greatest number of minutes. Annual attending clinician cardiac case volume quintile within our cohort was used as a clinician-level fixed-effect covariate. Institution-level fixed covariates included university affiliation and annual case volume quintile within our cohort.
Data handling
Missing data patterns were assessed for all study variables. Outlier values were rejected and set to missing if outside of the MPOG phenotype valid ranges previously described.18,19 For variables with more than 10% missingness, additional inspection was performed to determine whether data were consistent with missing at random. Institutions were excluded from the analyses if having high levels (>50%) of data missing not at random. Given the low rate of missingness (<20%) after exclusion of three institutions with high missingness across study variables, a complete case approach was used as opposed to multiple imputation.33 Adjudication of a subsample of cases via hand review was performed. The published fill rates for variables in the MPOG approach were 99.9% for some fields and 98.8% for medication administration.18
Statistical analyses
Basic descriptive statistics, histograms, box plots, and scatter plots were used to assess the distribution of independent measures to determine the most appropriate modelling strategies and variable transformations. Tests of differences of means, medians, or proportions were performed using analysis of variance (anova) F-tests, Wilcoxon rank-sum tests, or χ2 tests. Standardised differences >0.2 were considered indicative of a statistically significant imbalanced distribution between groups.34,35
We described the proportion of total variance in benzodiazepine administration attributable to each level (patient, clinician, and institution).36,37 Variance partition estimates were calculated using intraclass correlation coefficients (ICCs) via a generalised linear mixed model approach. The null model considered the nesting of patients within clinicians and clinicians within institutions. The outcome measure considered was a continuous benzodiazepine use measure where outliers had been removed. A post-hoc sensitivity analysis included calculation of ICCs for the binary variable of receipt of a benzodiazepine using the equation: ICC=V/(V+π2/3), where V denotes variance at a given level. As the partition of variance has been shown to have a less intuitive interpretation for binary variables at an epidemiologic level,36 we also reported median odds ratios (ORs) for the binary outcome of receipt of a benzodiazepine. The median OR is defined as the median value of the OR between two randomly selected units at a given level of analysis, calculated by the equation36,37:
| Median odds ratio=e0.95∗√(variance) | (1) |
In the context of this study, a median OR of 2.0 for example at a given level would be interpreted as, for two similar patients the median odds of receiving a benzodiazepine during cardiac surgery would differ by twofold between two randomly selected units at that level (i.e. clinicians or institutions). Multilevel multivariable mixed-effects models were performed with patients nested within clinicians nested within institutions to assess the association between benzodiazepine administration and relevant patient-, clinician-, and institutional-level factors. The multilevel models used random intercepts. Goodness-of-fit statistics considered were the likelihood ratio test and the generalised χ2 test. Estimates were considered statistically significant if P≤0.05. ORs and their 95% confidence interval (CI) were used to quantify the magnitude of effect for each variable. The modelling strategy was the same for secondary outcomes described above as our primary analysis (Supplementary Material 6).
Pre-planned sensitivity analyses
We performed an a priori sensitivity analysis examining cardiac cases that are typically associated with haemodynamic instability such as ASA physical status 5, cases with mechanical circulatory support, use of circulatory arrest, heart transplants, and ascending aortic procedures. Additional methodological details for this sensitivity analysis are given in Supplementary Material 6.
Posthoc sensitivity analyses
We performed a sensitivity analysis using the attending anaesthesiologist present at the case start instead of the primary anaesthesiologist (attending signed in for the most minutes intraoperatively), as most benzodiazepines were administered early in the anaesthetic. We performed this sensitivity analysis using the same methods as our primary analysis (Supplementary Material 6).
Power analysis
Based on a priori sample size estimate of n=63 601 and a prevalence of 89%, this study was powered to estimate the true population-level benzodiazepine use with 1% precision (Supplementary Material 7). Based on simulation studies to estimate multilevel model sample sizes, we would require at least 27 000 cases (30 patients nested within 30 clinicians, nested within 30 institutions) for robust estimates of the standard errors.38 We determined that we would have adequate sample size.
Results
Of 65 508 patients nested within 825 anaesthesiology attending clinicians across 33 institutions, 58 004 patients (88.5%) received benzodiazepines. Among patients receiving benzodiazepines, a median midazolam-equivalent dose of 4.0 mg (inter-quartile range [IQR], 2.0–6.0 mg) was administered; 57 795 patients (99.64%) received midazolam alone, 41 (0.07%) lorazepam alone, and 26 (0.05%) diazepam alone, and 142 (0.24%) received more than one medication. No patients received other less commonly administered benzodiazepines such as oxazepam or temazepam. Bar charts of the midazolam-equivalent total dose and the midazolam-equivalent weight-based dose administered per case are shown in Fig. 2a and b. The most common total doses (2, 4, 5, and 10 mg) matched common vial sizes (or multiples thereof) for intravenous midazolam. Of patients older than 65 yr, 29 824 (84.4%) received benzodiazepines. Compared with those not receiving benzodiazepines, patients receiving benzodiazepines were younger with fewer comorbidities, received care from anaesthesiologists with high cardiac case volumes at non-academic institutions (Table 1), and were less likely to have a fentanyl infusion documented (Supplementary Material 8).
Fig 2.
Bar plots of benzodiazepine total dose by case (a) and total dose per kilogram by case (b) for those receiving benzodiazepines.
Table 1.
Selected characteristics of the overall cohort and distribution across patients receiving vs not receiving benzodiazepines.
| Category | Descriptor | Overall cohort, median/n (IQR/%) (n=65 508) |
Benzodiazepine administered |
Standardised differences | |
|---|---|---|---|---|---|
| Yes, median/n (IQR/%) (n=58 004) |
No, median/n (IQR/%) (n=7504) |
||||
| Patient-level characteristics | |||||
| Anthropometric/demographic data | Age, yr (continuous) | 66 (16) | 65 (16) | 72 (14) | 0.56 |
| Age (categorical) | 0.59 | ||||
| 18–50 | 9160 (14.0) | 8684 (15.0) | 476 (6.3) | ||
| 51–60 | 13 340 (20.4) | 12 472 (21.5) | 868 (11.6) | ||
| 61–70 | 21 541 (32.9) | 19 469 (33.6) | 2072 (27.6) | ||
| 71–80 | 17 230 (26.3) | 14 387 (24.8) | 2843 (37.9) | ||
| >80 | 4237 (6.5) | 2992 (5.2) | 1245 (16.6) | ||
| Height, cm | 172.7 (14.9) | 172.7 (15.2) | 172 (15.3) | 0.11 | |
| Weight, kg | 84.5 (26.0) | 85 (26.3) | 81.8 (25.0) | 0.17 | |
| BMI∗ | 0.13 | ||||
| Underweight | 772 (1.2) | 665 (1.2) | 107 (1.4) | ||
| Normal | 16 016 (24.5) | 13 911 (24) | 2105 (28.1) | ||
| Overweight | 23 676 (36.1) | 20 878 (36.0) | 2798 (37.3) | ||
| Class I Obesity | 14 908 (22.8) | 13 390 (23.1) | 1518 (20.2) | ||
| Class II Obesity | 6535 (10) | 5891 (10.2) | 644 (8.6) | ||
| Class III Obesity | 3601 (5.5) | 3269 (5.6) | 332 (4.4) | ||
| Sex | 0.05 | ||||
| Male | 44 147 (67.4) | 39 245 (67.7) | 4902 (65.3) | ||
| Female | 21 361 (32.6) | 18 759 (32.3) | 2602 (34.7) | ||
| Race/ethnicity | 0.09 | ||||
| Caucasian | 52 528 (80.2) | 46 568 (80.3) | 5960 (79.4) | ||
| Black | 4141 (6.3) | 3614 (6.2) | 527 (7.0) | ||
| Hispanic | 545 (0.8) | 498 (0.9) | 47 (0.6) | ||
| Other or Multiracial | 2579 (3.9) | 2245 (3.9) | 334 (4.5) | ||
| Unknown | 5715 (8.7) | 5079 (8.8) | 636 (8.5) | ||
| Year of surgery | 0.32 | ||||
| 2014 | 8603 (13.1) | 7995 (13.8) | 608 (8.1) | ||
| 2015 | 9982 (15.2) | 9193 (15.9) | 789 (10.5) | ||
| 2016 | 11 518 (17.6) | 10 302 (17.8) | 1216 (16.2) | ||
| 2017 | 13 223 (20.2) | 11 778 (20.3) | 1445 (19.3) | ||
| 2018 | 14 268 (21.8) | 12 158 (21.0) | 2110 (28.1) | ||
| 2019 | 7914 (12.1) | 6578 (11.3) | 1336 (17.8) | ||
| Patient medical history† | Congestive heart failure | 24 572 (37.5) | 21 402 (36.9) | 3170 (42.2) | 0.14 |
| Cardiac arrhythmia | 33 163 (50.6) | 28 471 (49.1) | 4692 (62.5) | 0.33 | |
| Valvular disease | 38 847 (59.3) | 34 246 (59.0) | 4601 (61.3) | 0.10 | |
| Pulmonary circulation disorders | 8711 (13.3) | 7500 (12.9) | 1211 (16.1) | 0.12 | |
| Hypertension | 44 682 (68.2) | 39 164 (67.5) | 5518 (73.5) | 0.20 | |
| Other neurological disorders | 3300 (5.0) | 2785 (4.8) | 515 (6.9) | 0.11 | |
| Chronic pulmonary disease | 13 225 (20.2) | 11 684 (20.1) | 1541 (20.5) | 0.06 | |
| Diabetes mellitus | 13 570 (20.7) | 11 656 (20.1) | 1914 (25.5) | 0.16 | |
| Renal failure | 11 706 (17.9) | 9950 (17.2) | 1756 (23.4) | 0.18 | |
| Liver disease | 2877 (4.4) | 2552 (4.4) | 325 (4.3) | 0.06 | |
| Obesity | 12 997 (19.8) | 11 825 (20.4) | 1172 (15.6) | 0.13 | |
| Alcohol abuse | 535 (0.8) | 496 (0.9) | 39 (0.5) | 0.07 | |
| Drug abuse | 1574 (2.4) | 1461 (2.5) | 113 (1.5) | 0.09 | |
| Psychoses | 308 (0.5) | 266 (0.5) | 42 (0.6) | 0.06 | |
| Depression | 7095 (10.8) | 6244 (10.8) | 851 (11.3) | 0.07 | |
| Preoperative status | ASA physical status | 0.12 | |||
| 1–3 | 18 731 (28.6) | 16 935 (29.2) | 1796 (23.9) | ||
| 4 | 46 777 (71.4) | 41 069 (70.8) | 5708 (76.1) | ||
| Baseline MAP‡ | 0.18 | ||||
| Hypotensive | 4951 (7.6) | 4404 (7.6) | 547 (7.3) | ||
| Normotensive | 47 955 (73.2) | 42 875 (73.9) | 5080 (67.7) | ||
| Stage 1 hypertension | 8620 (13.2) | 7476 (12.9) | 1144 (15.2) | ||
| Stage 2 hypertension | 3982 (6.1) | 3249 (5.6) | 733 (9.8) | ||
| Intraoperative | Procedure type | ||||
| CABG only | 24 674 (37.7) | 21 852 (37.7) | 2822 (37.6) | 0.01 | |
| Valve and CABG only | 7372 (11.3) | 6325 (10.9) | 1047 (14.0) | 0.09 | |
| Valve only | 25 806 (39.4) | 23 048 (39.7) | 2758 (36.8) | 0.06 | |
| Other cardiac¶ | 7653 (11.7) | 6776 (11.7) | 877 (11.7) | 0.01 | |
| Anaesthesia duration (h) | 6.1 (2.2) | 6.1 (2.2) | 6.2 (2.2) | 0.03 | |
| Surgical duration (h) | 4.2 (1.8) | 4.2 (1.8) | 4.3 (1.9) | 0.01 | |
| Cardiopulmonary bypass use documented | 52 583 (80.3) | 47 042 (81.1) | 5541 (73.8) | 0.18 | |
| Provider characteristics | |||||
| Mean provider case volume per year | 0.32 | ||||
| <2 | 298 (0.5) | 261 (0.5) | 37 (0.5) | ||
| 2–9.5 | 2301 (3.5) | 2135 (3.7) | 166 (2.2) | ||
| 9.5–19.5 | 7835 (12.0) | 6800 (11.7) | 1035 (13.8) | ||
| 19.5–37.5 | 14 834 (22.7) | 12 293 (21.2) | 2541 (33.9) | ||
| >37.5 | 40 240 (61.4) | 36 515 (63.0) | 3725 (49.6) | ||
| Institutional characteristics | |||||
| Mean case institutional volume per year | 0.51 | ||||
| <291.5 | 12 067 (18.4) | 10 864 (18.7) | 1203 (16.0) | ||
| 291.5–451.7 | 15 364 (23.5) | 12 692 (21.9) | 2672 (35.6) | ||
| 451.7–704.3 | 11 673 (17.8) | 9971 (17.2) | 1702 (22.7) | ||
| 704.3–2488.5 | 11 473 (17.5) | 11 064 (19.1) | 409 (5.5) | ||
| >2488.5 | 14 931 (22.8) | 13 413 (23.1) | 1518 (20.2) | ||
| University affiliation | 0.50 | ||||
| Non-academic | 9015 (13.8) | 8880 (15.3) | 135 (1.8) | ||
| Academic | 56 493 (86.2) | 49 124 (84.7) | 7369 (98.2) | ||
CABG, coronary artery bypass grafting; IQR, inter-quartile range.
BMI categorised using WHO categories29: Underweight (<18.5 kg m−2); Normal (18.5–24.9 kg m−2, reference); Overweight (25.0–29.9 kg m−2); Class I Obesity (30.0–34.9 kg m−2); Class II Obesity (35.0–39.9 kg m−2); Class III Obesity (>40.0 kg m−2).
Comorbidities as defined by the Elixhauser Comorbidity Enhanced International Classification of Diseases, Ninth and Tenth Revisions, Clinical Modification Algorithm.30
As defined by the Seventh Report of the Joint National Committee31 and National Heart, Lung, and Blood Institute’s Health Information for the Public32: hypotensive (<70 mm Hg), normotensive (70–107 mm Hg, reference), stage 1 hypertension (108–120 mm Hg), and stage 2 hypertension (>120 mm Hg).
Including Maze procedures, open atrial/ventricular septal defect repairs, cardiac mass excisions, pericardiectomies, isolated open epicardial lead placement, and coronary artery bypass or valve procedures involving pericardiectomy, atrial/ventricular septal defect repair.
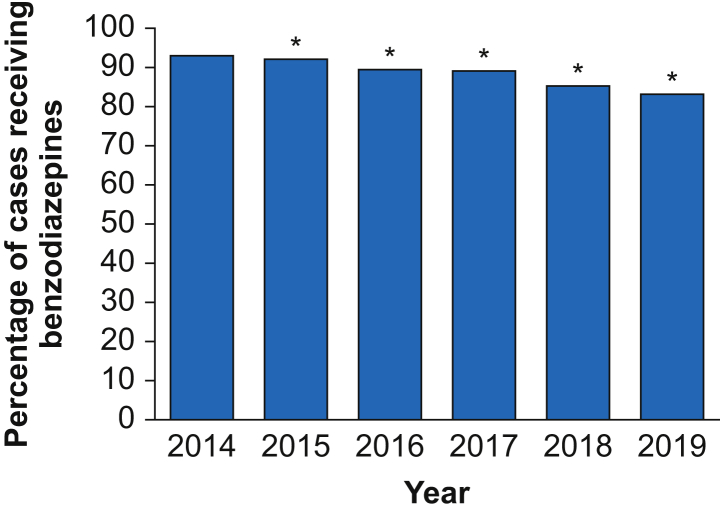
Caterpillar plots illustrating proportions of patients receiving benzodiazepines by anonymised anaesthesiologists and institutions are shown in Fig. 3a and b. Of all anaesthesiologists, 68.5% administered benzodiazepines to more than 90% of their patients, whereas 3.1% administered benzodiazepines to less than half of their patients. Similarly, 57.6% of institutions administered benzodiazepines to more than 90% of their patients, and only one institution administered benzodiazepines to less than half of cardiac surgical patients. Benzodiazepine use decreased over the study period, from 92.9% in 2014 to 83.1% in 2019 (Fig. 4).
Fig 3.
Caterpillar plots of benzodiazepine usage (percent, 95% CI) by clinicians (a; n=816) and institutions (b; n=33). CI, confidence interval.
Fig 4.
Temporal trends in percentage benzodiazepine use by case with 95% CI from 2014 to 2019. ∗Statistically significant difference from 2014. CI, confidence interval.
From the baseline cohort, 5867 (8.9%) patients had missing data, resulting in a complete case analysis cohort of 59 642 patients, 816 clinicians, and 33 institutions for the multilevel model. A missing data analysis is included in Supplementary Material 9, which shows that no additional bias was introduced when removing cases or institutions with large proportions of systemic missingness. Multivariable mixed-effect modelling with random effects of clinician and institution are shown in Table 2. Goodness-of-fit testing indicated the variability in the data was properly modelled without residual over-dispersion (Supplementary Material 10).
Table 2.
Multivariable analysis of patient-, provider-, and institutional-level characteristics and benzodiazepine use.
| Category | Descriptor | OR | 95% CI | P value |
|---|---|---|---|---|
| Patient level | ||||
| Anthropomorphic and demographic data | Age (yr) | |||
| 18–50 | Reference | – | – | |
| 51–60 | 0.67 | (0.58, 0.78) | <0.0001 | |
| 61–70 | 0.37 | (0.32, 0.42) | <0.0001 | |
| 71–80 | 0.14 | (0.12, 0.16) | <0.0001 | |
| >80 | 0.04 | (0.04, 0.05) | <0.0001 | |
| Sex | ||||
| Male | Reference | – | – | |
| Female | 0.95 | (0.89, 1.02) | 0.18 | |
| Race/ethnicity | ||||
| Caucasian | Reference | – | – | |
| Black | 0.80 | (0.70, 0.91) | 0.001 | |
| Hispanic | 0.95 | (0.65, 1.39) | 0.80 | |
| Other or multiracial | 0.85 | (0.74, 1.00) | 0.04 | |
| BMI∗ | ||||
| Underweight | 0.91 | (0.68, 1.20) | 0.49 | |
| Normal | Reference | – | – | |
| Overweight | 1.13 | (1.04, 1.22) | 0.004 | |
| Class I Obesity | 1.23 | (1.11, 1.36) | <0.0001 | |
| Class II Obesity | 1.15 | (1.00, 1.32) | 0.05 | |
| Class III Obesity | 1.02 | (0.85, 1.23) | 0.81 | |
| Preoperative status and comorbidities† | ASA physical status | |||
| 1–3 | Reference | – | – | |
| 4 | 0.87 | (0.80, 0.94) | 0.0005 | |
| Alcohol abuse | 1.14 | (0.78, 1.65) | 0.50 | |
| Cardiac arrhythmia | 0.95 | (0.88, 1.02) | 0.14 | |
| Chronic pulmonary disease | 0.98 | (0.91, 1.06) | 0.65 | |
| Congestive heart failure | 0.81 | (0.75, 0.87) | <0.0001 | |
| Depression | 1.02 | (0.92, 1.12) | 0.74 | |
| Diabetes mellitus | 0.87 | (0.80, 0.94) | 0.0006 | |
| Drug abuse | 1.29 | (1.02, 1.65) | 0.04 | |
| Hypertension | 0.90 | (0.83, 0.98) | 0.02 | |
| Liver disease | 0.89 | (0.76, 1.03) | 0.11 | |
| Obesity | 1.03 | (0.93, 1.15) | 0.55 | |
| Other neurological disorders | 0.75 | (0.66, 0.84) | <0.0001 | |
| Psychoses | 0.81 | (0.54, 1.22) | 0.32 | |
| Pulmonary circulation disorders | 0.85 | (0.78, 0.93) | 0.0005 | |
| Renal failure | 0.83 | (0.77, 0.91) | <0.0001 | |
| Valvular disease | 1.09 | (0.98, 1.20) | 0.10 | |
| Intraoperative data | Baseline MAP‡ | |||
| Hypotensive | 1.00 | (0.88, 1.13) | 0.99 | |
| Normotensive | Reference | – | – | |
| Stage 1 hypertension | 0.91 | (0.83, 1.00) | 0.05 | |
| Stage 2 hypertension | 0.77 | (0.68, 0.86) | <0.0001 | |
| Anaesthesia duration (h) | 1.11 | (1.08, 1.13) | <0.0001 | |
| Cardiopulmonary bypass used | 2.26 | (1.99, 2.55) | <0.0001 | |
| Procedure type | ||||
| CABG only | Reference | – | – | |
| Valve and CABG only | 0.80 | (0.71, 0.91) | 0.0006 | |
| Valve only | 0.94 | (0.85, 1.05) | 0.29 | |
| Other cardiac¶ | 0.81 | (0.72, 0.91) | 0.0004 | |
| Year of surgery | ||||
| 2014 | reference | – | – | |
| 2015 | 0.88 | (0.76, 1.01) | 0.06 | |
| 2016 | 0.73 | (0.63, 0.83) | <0.0001 | |
| 2017 | 0.77 | (0.67, 0.89) | 0.0003 | |
| 2018 | 0.55 | (0.47, 0.63) | <0.0001 | |
| 2019 | 0.42 | (0.37, 0.49) | <0.0001 | |
| Provider level | ||||
| Mean case volume per year | ||||
| <2 | 0.44 | (0.25, 0.75) | 0.002 | |
| 2–9.5 | 1.43 | (0.97, 2.12) | 0.09 | |
| 9.5–19.5 | 0.86 | (0.62, 1.20) | 0.36 | |
| 19.5–37.5 | 0.73 | (0.55, 0.99) | 0.04 | |
| >37.5 | Reference | – | – | |
| Institution level | ||||
| Mean case volume per year | ||||
| <291.5 | 1.51 | (0.07, 33.7) | 0.70 | |
| 291.5–451.7 | 1.05 | (0.04, 25.2) | 0.98 | |
| 451.7–704.3 | 1.14 | (0.04, 29.7) | 0.95 | |
| 704.3–2488.5 | 6.5 | (0.17, 250.4) | 0.32 | |
| >2488.5 | Reference | – | – | |
| University affiliation | ||||
| Non-academic | Reference | – | – | |
| Academic | 0.08 | (0.02, 0.35) | 0.0007 | |
CABG, coronary artery bypass grafting; CI, confidence interval; OR, odds ratio.
BMI categorized using WHO categories29: Underweight (<18.5 kg m−2); Normal (18.5–24.9 kg m−2, reference); Overweight (25.0–29.9 kg m−2); Class I Obesity (30.0–34.9 kg m−2); Class II Obesity (35.0–39.9 kg m−2); Class III Obesity (>40.0 kg m−2).
Comorbidities as defined by the Elixhauser Comorbidity Enhanced International Classification of Diseases, Ninth and Tenth Revisions, Clinical Modification Algorithm.30
As defined by the Seventh Report of the Joint National Committee31 and National Heart, Lung, and Blood Institute’s Health Information for the Public32: hypotensive (<70 mm Hg), normotensive (70–107 mm Hg, reference), stage 1 hypertension (108–120 mm Hg), and stage 2 hypertension (>120 mm Hg).
Including Maze procedures, open atrial/ventricular septal defect repairs, cardiac mass excisions, pericardiectomies, isolated open epicardial lead placement, and coronary artery bypass or valve procedures involving pericardiectomy, atrial/ventricular septal defect repair.
Multivariable random effect modelling of institution, clinician, and patient using the null model demonstrated that 54.7% of the variation in benzodiazepine administration was attributable to the institution, 14.7% was attributable to the primary anaesthesia attending, and 30.5% was attributable to patient factors for benzodiazepine use as a continuous variable. For the sensitivity analysis using the binary variable of receipt of any benzodiazepine, 44.4% of the variation was attributable to the institution, 23.0% was attributable to the primary anaesthesia attending, and 32.6% was attributable to patient factors. The median OR derived from the adjusted model for benzodiazepine administration was 4.19 between institutions and 2.68 between clinicians. Put into context, for two similar patients the median odds of receiving a benzodiazepine during cardiac surgery would differ by more than fourfold between two randomly selected institutions, and by nearly threefold between two randomly selected attending anaesthesiologists.
Following multivariable modelling (Table 2), patient-level factors independently associated with a statistically significant decreased likelihood of benzodiazepine administration included older age (>80 vs ≤50 yr; adjusted odds ratio [aOR]=0.04; 95% CI, 0.04–0.05; P<0.0001), recent year of surgery (2019 vs 2014; aOR=0.42; 95% CI, 0.37–0.49; P<0.0001), Non-Hispanic Black race (aOR=0.80; 95% CI, 0.7 0–0.91; P=0.001), other race or multiracial (aOR=0.85; 95% CI, 0.74–1.00; P=0.04), ASA class 4 status (aOR=0.87; 95% CI, 0.80–0.94; P=0.0005), baseline stage 2 hypertension on arrival to the OR (aOR=0.77; 95% CI, 0.68–0.86; P<0.0001), combined valve and CABG surgery (aOR=0.80; 95% CI, 0.71–0.91; P=0.0006) or other cardiac surgery (aOR=0.81; 95% CI, 0.72–0.91; P=0.0004) relative to isolated CABG, and history of congestive heart failure (aOR=0.81; 95% CI, 0.75–0.87; P<0.0001), diabetes (aOR=0.87; 95% CI, 0.80–0.94; P=0.0006), hypertension (aOR=0.90; 95% CI, 0.83–0.98; P=0.02), other neurological disorders (aOR=0.75; 95% CI, 0.66–0.84; P<0.0001), pulmonary circulation disorders (aOR=0.85; 95% CI, 0.78–0.93; P=0.0005), and renal failure (aOR=0.83; 95% CI, 0.77–0.91; P<0.0001). Conversely, factors associated with a statistically significant increased likelihood of benzodiazepine use were history of drug abuse (aOR=1.29; 95% CI, 1.02–1.65; P=0.04), overweight range BMI (aOR=1.13; 95% CI, 1.04–1.22; P=0.004), Class I Obesity (aOR=1.23; 95% CI, 1.11–1.36; P<0.0001), use of cardiopulmonary bypass (aOR=2.26; 95% CI, 1.99–2.55; P<0.0001), and hours of anaesthesia (aOR=1.11; 95% CI, 1.08–1.13; P<0.0001). The lowest attending clinician-level case volume quintile and the second highest case volume quintile were associated with a decreased likelihood of benzodiazepine use (aOR=0.44; 95% CI, 0.25–0.75; P=0.002; and aOR=0.73; 95% CI, 0.55–0.99; P=0.04, respectively) relative to the highest clinician case volume quintile. At the institution level, 27 of 33 hospitals (82%) were university-affiliated, and university affiliation was strongly associated with a decreased odds of benzodiazepine administration (aOR=0.08; 95% CI, 0.02–0.35; P=0.0007).
Regarding timing of benzodiazepine administration within each case, 29% were administered solely preoperatively (pre-induction), 61% solely intraoperatively, and 10% during both windows. Among patients undergoing cardiopulmonary bypass, 58% received benzodiazepines pre-bypass, 29% while on bypass, and 27% post-bypass (non-mutually exclusive).
For the secondary analysis, we described patients who received low- vs high-dose benzodiazepines, defined as <0.05 vs ≥0.05mg kg−1, respectively. Among those who received benzodiazepines, the adjusted median OR was 3.07 between clinicians and 6.91 between institutions for receiving low- vs high-dose benzodiazepines. Factors strongly associated with low-dose relative to high-dose benzodiazepine administration were older age, recent year of surgery, Non-Hispanic Black race, and university affiliation. History of alcohol abuse, history of drug abuse, use of cardiopulmonary bypass, and longer anaesthesia duration were strongly associated with a lower likelihood of receiving low- vs high-dose benzodiazepines (Supplementary Material 11). The results of the a priori sensitivity analysis on the cohort with a higher potential for haemodynamic instability, and results of the post-hoc sensitivity analysis using the starting anaesthesiology attending clinician instead of primary attending clinician, were similar to the primary analysis (Supplementary Material 12–15).
Discussion
Similar to prior studies of anaesthesiology practice patterns,37,39,40 we observed substantial variation in benzodiazepine administration across clinicians and institutions: two-thirds of the variation in benzodiazepine administration was explained by the institution or clinician, with more than half of the variation explained by the institution alone. This variation in practice attributable to the clinician or institutional levels is complex and may represent institutional protocols, cultural dogma, clinician training, dynamic – rather than static – clinician behaviours, or individualised care based on unmeasured patient factors, which therefore appear to be random.37,41 Nonetheless, the identification of institutional variation in practice patterns in studies such as this, emphasises the importance of multicentre, rather than single-centre, studies of practices, which potentially differ dramatically between institutions or clinicians.
The wide variation we observed may reflect contradictory recommendations presented in best practice guidelines, or that benzodiazepine administration is nuanced and individualised after weighing competing patient factors, such as the risk of intraoperative awareness vs the risk of postoperative delirium, and a global benchmark may not be appropriate. Notably, a recent systematic review of 60 anaesthesia evidence-based guidelines found that only 16% of recommendations were based on level 1 evidence, and more than half were based on case reports or expert consensus.42,43 The ASA's 2006 intraoperative awareness practice guidelines support administration of benzodiazepines to avoid intraoperative awareness.5 Meanwhile, the 2015 American Geriatrics Society best practice statement for postoperative delirium in older adults11 and the 2018 ASA Brain Health Initiative Guidelines44 recommend avoiding benzodiazepines in patients older than 65 yr. In addition, the Society of Critical Care Medicine 2018 Prevention and Management of Pain, Agitation/Sedation, Delirium, Immobility, and Sleep Disruption in Adult Patients in the ICU12 advised against use of benzodiazepines in the ICU population citing that use of benzodiazepines is a key modifiable risk factor associated with delirium, and specifically suggests use of non-benzodiazepines for sedation after cardiac surgery.12 Despite these guidelines, and consistent with our findings, a recent study in the noncardiac population found that 65% of patients older than 50 yr received benzodiazepines.45
Although benzodiazepine use is common in this cohort, practice variations congruent with these guidelines are apparent. Consistent with a reported increased risk of intraoperative awareness among surgeries using cardiopulmonary bypass,3, 4, 5 patients undergoing surgery with cardiopulmonary bypass use were twice as likely to receive a benzodiazepine. Next, as patient age increased, the frequency of receiving a benzodiazepine decreased, from 94.8% of patients aged 18–50 yr to only 70.6% of patients older than 80 yr (Table 1). In addition, over time, benzodiazepine use declined from 92.9% in 2014 to 83.1% in 2019 (Fig. 4). These patterns likely represent the limitations of translating conflicting guidelines in the setting of the operating room and the ICU.
Large perioperative prospective trials linking practice patterns to outcomes are needed to provide evidence to support decisions clinicians make daily.46,47 In the absence of evidence from large, multicentre trials regarding benzodiazepine administration, practice will be experience- rather than evidence-based.2 More evidence is needed to inform whether this practice variation is irrelevant, or a target for practice improvement. Large multicentre trials are underway currently which have the opportunity to provide outcomes-based evidence for practice.13,14
Strengths and limitations
There are several limitations to this retrospective observational study. First, data greater than 2 h before the case were not reliably available. Patients may have received benzodiazepines greater than 2 h preoperatively as part of anxiolysis or sedation for a minor procedure (e.g. arterial line placement). However, because benzodiazepines are controlled substances, the documentation of the primary outcome in the perioperative record is highly reliable within the studied time window.
Next, as this retrospective study involved routinely collected EHR data from multiple institutions, variations in documentation patterns likely existed, although they were mitigated by using pre-computed MPOG phenotypes,18,19 artifact reduction algorithms,19 and adjudication of a subsample of cases via hand review. Unfortunately, high-quality patient-level data for cardiac performance measures or home benzodiazepine use were not available and therefore may downwardly bias the patient-attributable fraction. Although we used data from 33 MPOG hospitals, most were university-affiliated, and we were likely unable to completely capture clinician or institutional factors that contribute to the decision to administer benzodiazepines. Clinician demographic or licensure data were not available for analysis. At the institutional level, to maintain institutional anonymity, granular regional descriptors were not analysed. In addition, institutions outside of the USA were not included.
Finally, this study did not evaluate outcomes of benzodiazepine administration as granular, cardiac surgery-specific variables needed for accurate risk adjustment were not available in our dataset. Prospective studies are needed to best assess the impact of intraoperative benzodiazepine use on outcomes.
Conclusions
Institution and clinician factors, rather than patient factors, explain two-thirds of the variation in benzodiazepine administration during cardiac surgery. These data may serve as a model for understanding cardiac anaesthesiology practice variation and underscoring the opportunity for and providing clinical context for the results of high-quality randomised trials to investigate optimal intraoperative benzodiazepine dosing strategies for improved outcomes.
Authors' contributions
Study design/planning: all authors
Data collection: AMJ, GM, SK, MRM
Data analysis and interpretation: all authors
Writing first draft of manuscript: AMJ, GM, SK, MRM
Revising manuscript and review before submission: all authors
Acknowledgements
The authors gratefully acknowledge Robert Coleman, Department of Anesthesiology, University of Michigan Health System, Ann Arbor, MI, USA, for his contributions in data acquisition and electronic search query programming for this project. We also acknowledge Rachel Hurwitz for her assistance in data presentation. The authors recognise the following members of the MPOG Perioperative Clinical Research Committee: Bhiken I. Naik, Departments of Anesthesiology and Neurological Surgery, University of Virginia, Charlottesville, VA, USA; Mark Neuman, Department of Anesthesiology, University of Pennsylvania, Philadelphia, PA, USA; Robert E. Freundlich, Department of Anesthesiology, Vanderbilt University Medical Center, Nashville, TN, USA; Robert B. Schonberger MD, MHS, Associate Professor, Department of Anesthesiology, Yale School of Medicine, New Haven, CT, USA; Patrick J. McCormick, Department of Anesthesiology & Critical Care Medicine, Memorial Sloan Kettering Cancer Center, New York, NY, USA; Nathan L. Pace, Department of Anesthesiology, University of Utah, Salt Lake City, UT, USA; Jochen D. Muehlschlegel, Department of Anesthesiology, Perioperative and Pain Medicine, Brigham and Women's Hospital, Harvard Medical School, Boston, MA, USA; Germaine Cuff, Department of Anesthesiology, Perioperative Care and Pain Medicine, NYU Langone Health, New York, NY, USA; Wilton A. van Klei, Department of Anesthesiology, UMC Utrecht, Utrecht, Netherlands; Lee-lynn Chen, Department of Anesthesia and Perioperative Care, University of California San Francisco, San Francisco, CA, USA; and Karen B. Domino, Department of Anesthesiology and Pain Medicine, University of Washington, Seattle, WA, USA.
Handling editor: Michael Avidan
Footnotes
Abstract and oral presentation at the American Society of Anesthesiology Virtual 2020 Meeting. Presented virtually on October 5, 2020. Oral presentation at the Multicenter Perioperative Outcomes Group Annual Retreat in San Diego, CA, USA, on October 8, 2021.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2021.11.040.
Declarations of interest
MRM has received a research grant from the US National Institutes of Health – National Heart, Lung, and Blood Institute [K01HL141701]. AMJ has been funded by a US National Institutes of Health T32 Grant, National Institute of General Medical Sciences [T32GM103730] and has received research support paid to the University of Michigan and unrelated to this present work, from Becton, Dickinson and Company.
Funding
US National Institutes of Health, National Heart, Lung, and Blood Institute (K01HL141701 to MRM); US National Institutes of Health T32 Grant, National Institute of General Medical Sciences (T32GM103730 to AMJ). Additional support from the Department of Anesthesiology, University of Michigan Medical School (Ann Arbor, MI, USA); departmental and institutional resources at each contributing site in the Multicenter Perioperative Outcomes Group. In addition, partial funding to support underlying electronic health record data collection into the Multicenter Perioperative Outcomes Group registry was provided by Blue Cross Blue Shield of Michigan/Blue Care Network as part of the Blue Cross Blue Shield of Michigan/Blue Care Network Value Partnerships program. Although Blue Cross Blue Shield of Michigan/Blue Care Network and Multicenter Perioperative Outcomes Group work collaboratively, the opinions, beliefs and viewpoints expressed by the authors do not necessarily reflect the opinions, beliefs, and viewpoints of Blue Cross Blue Shield of Michigan/Blue Care Network or any of its employees.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Spence J., Belley-Côté E., Devereaux P.J., et al. Benzodiazepine administration during adult cardiac surgery: a survey of current practice among Canadian anesthesiologists working in academic centres. Can J Anaesth. 2018;65:263–271. doi: 10.1007/s12630-017-1047-1. [DOI] [PubMed] [Google Scholar]
- 2.Spence J., Belley-Côté E., Lee S.F., et al. The role of randomized cluster crossover trials for comparative effectiveness testing in anesthesia: design of the Benzodiazepine-Free Cardiac Anesthesia for Reduction in Postoperative Delirium (B-Free) trial. Can J Anaesth. 2018;65:813–821. doi: 10.1007/s12630-018-1130-2. [DOI] [PubMed] [Google Scholar]
- 3.Sebel P.S., Bowdle T.A., Ghoneim M.M., et al. The incidence of awareness during anesthesia: a multicenter United States study. Anesth Analg. 2004;99:833–839. doi: 10.1213/01.ANE.0000130261.90896.6C. table of contents. [DOI] [PubMed] [Google Scholar]
- 4.Orser B.A., Mazer C.D., Baker A.J. Awareness during anesthesia. CMAJ. 2008;178:185–188. doi: 10.1503/cmaj.071761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Society of Anesthesiologists Task Force on Intraoperative Awareness Practice advisory for intraoperative awareness and brain function monitoring: a report by the American Society of Anesthesiologists task force on intraoperative awareness. Anesthesiology. 2006;104:847–864. doi: 10.1097/00000542-200604000-00031. [DOI] [PubMed] [Google Scholar]
- 6.Kassie G.M., Nguyen T.A., Kalisch Ellett L.M., Pratt N.L., Roughead E.E. Preoperative medication use and postoperative delirium: a systematic review. BMC Geriatr. 2017;17:298. doi: 10.1186/s12877-017-0695-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saczynski J.S., Marcantonio E.R., Quach L., et al. Cognitive trajectories after postoperative delirium. N Engl J Med. 2012;367:30–39. doi: 10.1056/NEJMoa1112923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riker R.R., Shehabi Y., Bokesch P.M., et al. SEDCOM (Safety and Efficacy of Dexmedetomidine Compared with Midazolam) Study Group. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA. 2009;301:489–499. doi: 10.1001/jama.2009.56. [DOI] [PubMed] [Google Scholar]
- 9.Pandharipande P.P., Pun B.T., Herr D.L., et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA. 2007;298:2644–2653. doi: 10.1001/jama.298.22.2644. [DOI] [PubMed] [Google Scholar]
- 10.Pandharipande P., Shintani A., Peterson J., et al. Lorazepam is an independent risk factor for transitioning to delirium in intensive care unit patients. Anesthesiology. 2006;104:21–26. doi: 10.1097/00000542-200601000-00005. [DOI] [PubMed] [Google Scholar]
- 11.American Geriatrics society expert panel on postoperative delirium in older adults: postoperative delirium in older adults: best practice statement from the American Geriatrics society. J Am Coll Surg. 2015;220:136–148.e1. doi: 10.1016/j.jamcollsurg.2014.10.019. [DOI] [PubMed] [Google Scholar]
- 12.Devlin J.W., Skrobik Y., Gélinas C., et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46:e825–e873. doi: 10.1097/CCM.0000000000003299. [DOI] [PubMed] [Google Scholar]
- 13.Spence J., Belley-Côté E., Jacobsohn E., et al. Restricted versus liberal intraoperative benzodiazepine use in cardiac anaesthesia for reducing delirium (B-Free Pilot): a pilot, multicentre, randomised, cluster crossover trial. Br J Anaesth. 2020;125:38–46. doi: 10.1016/j.bja.2020.03.030. [DOI] [PubMed] [Google Scholar]
- 14.Kowark A., Rossaint R., Keszei A.P., et al. Impact ofPReOperative Midazolam on OuTcome of Elderly patients (I-PROMOTE): study protocol for a multicentre randomised controlled trial. Trials. 2019;20:430. doi: 10.1186/s13063-019-3512-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Von Elm E., Altman D.G., Egger M., et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 16.Benchimol E.I., Smeeth L., Guttmann A., et al. RECORD Working Committee The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) statement. PLoS Med. 2015;12 doi: 10.1371/journal.pmed.1001885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Multicenter Perioperative Outcomes Group (MPOG): Write a Research Proposal 2019. Available from https://mpog.org/write-a-research-proposal/.
- 18.Colquhoun D.A., Shanks A.M., Kapeles S.R., et al. Considerations for Integration of perioperative electronic health records across institutions for research and quality improvement: the approach taken by the Multicenter Perioperative Outcomes Group. Anesth Analg. 2020;130:1133–1146. doi: 10.1213/ANE.0000000000004489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MPOG Phenotype Browser. Available from https://phenotypes.mpog.org/.
- 20.Sun E., Mello M.M., Rishel C.A., et al. Multicenter Perioperative Outcomes Group (MPOG). Association of overlapping surgery with perioperative outcomes. JAMA. 2019;321:762–772. doi: 10.1001/jama.2019.0711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kheterpal S., Vaughn M.T., Dubovoy T.Z., et al. Sugammadex versus neostigmine for reversal of neuromuscular blockade and postoperative pulmonary complications (STRONGER): a multicenter matched cohort analysis. Anesthesiology. 2020;132:1371–1381. doi: 10.1097/ALN.0000000000003256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walbergh E.J., Wills R.J., Eckhert J. Plasma concentrations of midazolam in children following intranasal administration. Anesthesiology. 1991;74:233–235. doi: 10.1097/00000542-199102000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Payne K., Mattheyse F.J., Liebenberg D., Dawes T. The pharmacokinetics of midazolam in paediatric patients. Eur J Clin Pharmacol. 1989;37:267–272. doi: 10.1007/BF00679782. [DOI] [PubMed] [Google Scholar]
- 24.Wright S.W., Chudnofsky C.R., Dronen S.C., et al. Comparison of midazolam and diazepam for conscious sedation in the emergency department. Ann Emerg Med. 1993;22:201–205. doi: 10.1016/s0196-0644(05)80203-3. [DOI] [PubMed] [Google Scholar]
- 25.Ochs H.R., Otten H., Greenblatt D.J., Dengler H.J. Diazepam absorption. Dig Dis Sci. 1982;27:225–230. doi: 10.1007/BF01296920. [DOI] [PubMed] [Google Scholar]
- 26.Chouinard G., Lefko-Singh K., Teboul E. Metabolism of anxiolytics and hypnotics: benzodiazepines, buspirone, zoplicone, and zolpidem. Cell Mol Neurobiol. 1999;19:533–552. doi: 10.1023/A:1006943009192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barr J., Zomorodi K., Bertaccini E.J., Shafer S.L., Geller E. A double-blind, randomized comparison of i.v. lorazepam versus midazolam for sedation of ICU patients via a pharmacologic model. Anesthesiology. 2001;95:286–298. doi: 10.1097/00000542-200108000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Nashef S.A.M., Roques F., Sharples L.D., et al. EuroSCORE II. Eur J Cardiothorac Surg. 2012;41:734–744. doi: 10.1093/ejcts/ezs043. discussion 744–5. [DOI] [PubMed] [Google Scholar]
- 29.World Health Organization Body Mass Index – BMI. Available from https://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi.
- 30.Li B., Evans D., Faris P., Dean S., Quan H. Risk adjustment performance of Charlson and Elixhauser comorbidities in ICD-9 and ICD-10 administrative databases. BMC Health Serv Res. 2008;8:12. doi: 10.1186/1472-6963-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chobanian A.V., Bakris G.L., Black H.R., et al. National heart, Lung, and Blood Institute Joint national committee on prevention, detection, evaluation, and treatment of high Blood pressure, national high Blood pressure education program coordinating committee: the seventh report of the Joint national committee on prevention, detection, evaluation, and treatment of high Blood pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 32.NIH National Heart, Lung and Blood Institute Health Topics: Low Blood Pressure. Available from: https://www.nhlbi.nih.gov/health-topics/low-blood-pressure. Accessed October 12, 2020.
- 33.Graham J.W. Missing data analysis: making it work in the real world. Annu Rev Psychol. 2009;60:549–576. doi: 10.1146/annurev.psych.58.110405.085530. [DOI] [PubMed] [Google Scholar]
- 34.Cohen J. Lawrence Earlbaum Associates; Hillside, NJ: 1998. Statistical power analysis for the behavioural Sciences. [Google Scholar]
- 35.McKenzie K., Martin L., Ouellette-Kuntz H. Needles in the haystack: using open-text fields to identify persons with intellectual and developmental disabilities in administrative home care data. Res Dev Disabil. 2017;69:85–95. doi: 10.1016/j.ridd.2017.07.019. [DOI] [PubMed] [Google Scholar]
- 36.Merlo J., Chaix B., Ohlsson H., et al. A brief conceptual tutorial of multilevel analysis in social epidemiology: using measures of clustering in multilevel logistic regression to investigate contextual phenomena. J Epidemiol Community Health. 2006;60:290–297. doi: 10.1136/jech.2004.029454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McIsaac D.I., Wijeysundera D.N., Bryson G.L., Huang A., McCartney C.J.L., van Walraven C. Hospital-, anesthesiologist-, and patient-level variation in primary anesthesia type for hip fracture surgery: a population-based cross-sectional analysis. Anesthesiology. 2018;129:1121–1131. doi: 10.1097/ALN.0000000000002453. [DOI] [PubMed] [Google Scholar]
- 38.Maas C.J.M., Hox J.J. Sufficient sample sizes for multilevel modeling. Methodology. 2005;1:86–92. [Google Scholar]
- 39.Wijeysundera D.N., Austin P.C., Beattie W.S., Hux J.E., Laupacis A. Variation in the practice of preoperative medical consultation for major elective noncardiac surgery: a population-based study. Anesthesiology. 2012;116:25–34. doi: 10.1097/ALN.0b013e31823cfc03. [DOI] [PubMed] [Google Scholar]
- 40.MacKay E.J., Groeneveld P.W., Fleisher L.A., et al. Practice pattern variation in the use of transesophageal echocardiography for open valve cardiac surgery. J Cardiothorac Vasc Anesth. 2019;33:118–133. doi: 10.1053/j.jvca.2018.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sessler D.I. Implications of practice variability. Anesthesiology. 2020;132:606–608. doi: 10.1097/ALN.0000000000003162. [DOI] [PubMed] [Google Scholar]
- 42.Laserna A., Rubinger D.A., Barahona-Correa J.E., et al. Levels of evidence supporting the North American and European perioperative care guidelines for anesthesiologists between 2010 and 2020: a systematic review. Anesthesiology. 2021;135:31–56. doi: 10.1097/ALN.0000000000003808. [DOI] [PubMed] [Google Scholar]
- 43.Neuman M.D., Apfelbaum J.L. Clinical practice guidelines in anesthesiology: adjusting our expectations. Anesthesiology. 2021;135:9–11. doi: 10.1097/ALN.0000000000003809. [DOI] [PubMed] [Google Scholar]
- 44.Berger M., Schenning K.J., Brown C.H., 4th, et al. Best practices for postoperative brain health: recommendations from the fifth international perioperative neurotoxicity working group. Anesth Analg. 2018;127:1406–1413. doi: 10.1213/ANE.0000000000003841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lei V.J., Navathe A.S., Seki S.M., Neuman M.D. Perioperative benzodiazepine administration among older surgical patients. Br J Anaesth. 2021;127:e69–e71. doi: 10.1016/j.bja.2021.05.016. [DOI] [PubMed] [Google Scholar]
- 46.Myles P.S. Why we need large randomized studies in anaesthesia. Br J Anaesth. 1999;83:833–834. doi: 10.1093/bja/83.6.833. [DOI] [PubMed] [Google Scholar]
- 47.Devereaux P.J., Chan M.T.V., Eisenach J., Schricker T., Sessler D.I. The need for large clinical studies in perioperative medicine. Anesthesiology. 2012;116:1169–1175. doi: 10.1097/ALN.0b013e31825037bc. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.