Abstract
Background
We aimed to appraise the evidence relating to the measurement properties of unidimensional tools to quantify pain after surgery. Furthermore, we wished to identify the tools used to assess interference of pain with functional recovery.
Methods
Four electronic sources (MEDLINE, Embase, CINAHL, PsycINFO) were searched in August 2020. Two reviewers independently screened articles and assessed risk of bias using the COnsensus-based Standards for the selection of health Measurement INstruments (COSMIN) checklist.
Results
Thirty-one studies with a total of 12 498 participants were included. Most of the studies failed to meet the methodological quality standards required by COSMIN. Studies of unidimensional assessment tools were underpinned by low-quality evidence for reliability (five studies), and responsiveness (seven studies). Convergent validity was the most studied property (13 studies) with moderate to high correlation ranging from 0.5 to 0.9 between unidimensional tools. Interpretability results were available only for the visual analogue scale (seven studies) and numerical rating scale (four studies). Studies on functional assessment tools were scarce; only one study included an ‘Objective Pain Score,’ a tool assessing pain interference with respiratory function, and it had low-quality for convergent validity.
Conclusions
This systematic review challenges the validity and reliability of unidimensional tools in adult patients after surgery. We found no evidence that any one unidimensional tool has superior measurement properties in assessing postoperative pain. In addition, because promoting function is a crucial perioperative goal, psychometric validation studies of functional pain assessment tools are needed to improve pain assessment and management.
Clinical trial registration
PROSPERO CRD42020213495.
Keywords: COnsensus-based Standards for the selection of health Measurement INstruments (COSMIN), functional pain assessment tool, pain scores, postoperative pain, tool utility, unidimensional pain assessment
Editor's key points.
-
•
Well validated assessment tools are essential for measuring postoperative pain intensity and impact
-
•
This systematic review shows that despite many tools available, evidence regarding their validity or reliability is scarce.
-
•
After surgery, the Visual Analogue Scale (VAS) showed the highest error rate in general and was the least preferred compared to the 0-10 Numerical Rating Scale (NRS).
-
•
Importantly statistically significant changes in VAS or NRS do not necessarily indicate clinically important changes, and NRS cut-off points used by healthcare professionals to determine acute pain severity do not always reflect patients' desire for analgesics.
Patients experience acute pain after surgery as a result of tissue damage and inflammation at the operation site.1, 2, 3 Careful assessment of pain using a valid and reliable tool4 is the first step towards a rational choice of analgesic therapy,5 which is essential for ensuring patient comfort, mobility, and satisfaction and reducing healthcare costs.6 The most commonly used tools for the assessment of postoperative pain are unidimensional and assess only pain intensity.4 These include the visual analogue scale (VAS),7 numerical rating scale (NRS),8 verbal rating scale (VRS),9 sometimes referred to as the verbal descriptor scale (VDS),10 and faces pain scales (FPS).11 They are quick to administer and do not encroach on the time required for usual care.12
Despite their extensive use, the reliance on these unidimensional tools as the sole approach to measuring pain is currently insufficient as the cut-off points commonly used by healthcare providers do not reflect the patient's desire for additional analgesics.13,14 Furthermore, patients have reported difficulties in describing the complexity of their pain experience by a single numerical value, descriptive words, or as a mark on a line.12 Striving to lower pain intensity scores to zero as suggested by the ‘Pain as the 5th Vital Sign’ campaign has not improved pain outcomes,15, 16, 17 and resulted in increased opioid analgesic use in the post-anaesthesia care unit (PACU).17 Furthermore, Vila and colleagues18 highlighted the potential hazard associated with a pain score-based treatment algorithm in increasing the prevalence of sedation-related side-effects by more than twofold. Treating pain as the fifth vital sign has been abandoned now as it may have contributed to the current US opioid epidemic.19,20
Restoration of function by allowing the patient to breathe, cough, ambulate, and turn in bed is important for postoperative pain relief.21,22 Therefore, assessing the functional impact of pain, which includes patient-centred objective assessment by a healthcare provider who judges if the pain prevents the patient from performing activities that help accelerate recovery, could be an appropriate alternative to achieve better pain assessment.23 Hence, options to treat pain will be used to maximise functional capacity, rather than striving to reduce the patient's postoperative pain score to below a specified numerical value.4,20
Despite being used widely, the validity, reliability, and utility of unidimensional pain assessment tools for postoperative patients have not been reviewed systematically. The aim of this systematic review was to appraise the available evidence concerning the measurement properties of different unidimensional and functional pain assessment tools when used to assess postoperative pain in hospitalised adults.
Methods
We performed this systematic review according to COnsensus-based Standards for the selection of health Measurement INstruments (COSMIN) (http://www.cosmin.nl/) guidelines, and reported it according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement guidelines.24
Search strategy
We performed a systematic search of the MEDLINE, Embase, PsycINFO (all via OVID) and CINAHL (via EBSCOhost) databases from their inception to August 2020. Our search strategy consisted of four search concepts: (1) measurement properties or outcome terms, (2) pain assessment tool terms, (3) acute postoperative pain, and (4) limits (English language or English translation, human adults ≥18 yr old). We combined the first three using the Boolean operator AND, which works as a conjunction to narrow the search to include our specific three search concepts resulting in more focused results. This was then combined with the result string of the fourth concept to limit the results. We performed these steps separately for each pain assessment tool. We carried out backward citation tracking as well by checking the reference lists from eligible studies. The comprehensive search strategy used is provided in Supplementary material, Appendix S1.
Inclusion criteria
We included any of the following pain measurement tools to assess acute pain in hospitalised adult patients from all surgical specialties: unidimensional pain assessment tools (including the numerical pain rating scale, VRS, VAS, faces scales [Wong-Baker FACES, Faces Pain Scale – Revised]), and functional pain assessment tools included any tool that helps assess acute pain based on its interference with functional activity, including walking, breathing, turning in bed, and coughing. Included functional pain assessment tools could be used objectively by the clinician or when self-reported by patients.
We included instrument validation or instrument evaluation types of studies. Any studies that included at least one or more of the instruments to evaluate postoperative pain and assessed at least one of the nine measurement properties identified by COSMIN taxonomy: internal consistency, test–retest reliability, measurement error, content validity, structural validity, construct validity, hypothesis testing, cross-cultural validity, criterion validity, and responsiveness were considered (Appendix S2). In addition, we included any study that evaluated any of the specified additional outcomes of the tools, including feasibility, interpretability, and desire for analgesia.
Exclusion criteria
We excluded abstracts, editorials, reviews, and studies that included paediatric or adolescent populations, or sedated, mechanically ventilated and critically ill patients.
Selection of articles
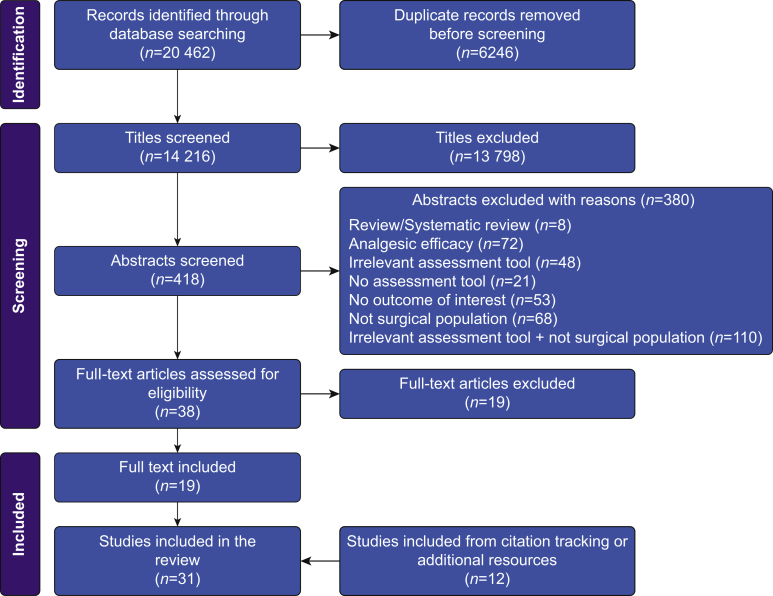
After our database search, we collated and uploaded all identified citations to EndNote X9 (Clarivate Analytics, Philadelphia, PA, USA) and removed duplicates. The identified studies were uploaded to Rayyan QCRI online software.25 Two reviewers (RMB and AI) independently applied the inclusion criteria to the titles, then to relevant abstracts. Afterwards, we thoroughly examined potentially eligible full texts for inclusion. We documented the full search results in the PRISMA flow diagram (Fig. 1). Excluded studies and the reasons for their exclusion are provided in Appendix S3.
Fig 1.
PRISMA diagram. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Data extraction
One reviewer (RMB) extracted data from the included full-text articles, with the extraction verified by a second reviewer (AI). The two reviewers resolved any disagreements through discussion, or consultation with other reviewers (RDK, LST, or DNL) when necessary. The extracted data included specific details about the assessment tool used, country, language of scale administration, study design, patient characteristics, surgical procedure, the specific measurement properties assessed, outcomes related to the review question and objectives, and the main statistical analysis.
Assessment of methodology
Two independent reviewers (RMB and AI) critically appraised the methodological quality of studies looking at feasibility and interpretability using a modified version of the Newcastle–Ottawa Scale26 (Appendix S4). For validation studies, we assessed the quality using the COSMIN criteria for methodological quality.27, 28, 29 We included three phases in the assessment of each measurement property. First, we assessed the risk of bias, which pertains to methodological quality in each study: very good, adequate, doubtful, or inadequate quality was assigned to each study. Second, we related the results to a measurement property rated against criteria for ‘sufficient measurement properties’, and the results were classified as sufficient, insufficient, or indeterminate (Appendix S5). Third, we combined the results from each study and graded the quality of evidence for each pain assessment tool. A summary of the scoring criteria and appraisals is provided in (Appendices S6 and S7).
Protocol registration
The protocol was registered (No. CRD42020213495) with the PROSPERO database and can be accessed at https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=213495.
Results
The search identified 14 216 potential studies after removal of duplicates. After reviewing the titles, we excluded 13 798 for irrelevance and another 380 after abstract screening. Of the 38 remaining studies, we excluded 19 after examination of the full texts against the inclusion criteria (Appendix S2). An additional 12 studies were identified through searching the bibliography of eligible studies, so a total of 31 studies2,3,6,13,30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56 (Fig. 1) with 12 498 participants were included. The number of participants in individual studies ranged from 3530 to 3045.31
The distribution of male and female participants in the studies varied, with some studies including only female participants30 or only male participants40 and others not reporting sex distribution.38,50,52,53 The studies matching our inclusion criteria were published between 198252 and 2018,37 and assessed postoperative pain after different types of surgical procedures (Table 1). Nine studies included only cognitively intact patients,6,32,35,38,47,49,51,54,55 whereas two studies included mild cognitively impaired participants.46,56 The remaining 20 studies did not report on cognitive function.2,3,13,30,32, 33, 34, 35, 36,39, 40, 41, 42, 43, 44, 45,48,50,52,53
Table 1.
Characteristics of included studies. BNS, box numerical rating scale; CAS, coloured analogue scale; CCPS, colour circle pain scale; ENT, ear, nose and throat; FPS, face pain scale; ICU, intensive care unit; MPQ, McGill pain questionnaire; M-VRS, modified verbal rating scale with 11 description of pain intensity; NR, not reported; NRS, numerical rating scale; OPS, objective pain score; PCA, patient controlled analgesia; PPI, present pain intensity; PROM/s, patient-reported outcome measures; RWS, red wedge scale; sd, standard deviation; THA, total hip arthroplasty; TKA, total knee arthroplasty; VAS-R, visual analogue scale at rest; VAS-M; visual analogue scale at movement; VDS, verbal descriptor scale; VPS, 11-point verbal scale; VRS∗∗, 4-point verbal rating scale; VRS-5, 5-point verbal rating scale; VRS-P; verbal rating scale for pain relief.
| First author, year (country) | PROM/s | Study design | Surgical procedure | Outcome(s) | High anchor∗ | Main exclusion criteria | Patient characteristics |
|
|---|---|---|---|---|---|---|---|---|
| n (Female%) | Age (yr) Mean (sd) [range] |
|||||||
| Van Dijk, 201513 (The Netherlands) | NRS | Cross-sectional design | Orthopaedic, ENT, gynaecological, cardiothoracic, Others | Ability to detect desire for analgesics | Worst pain imaginable | ICU patients, not proficient in Dutch or English, ambulatory surgery | 1084 (48) | 53 [18–90] |
| Banos, 198934 (Spain) | VAS VRS-5 |
Descriptive correlational design | Abdominal, orthopaedic, gynaecological | Convergent validity | 10 Unbearable pain |
NR | 212 (50) | <30=43 31–50=69 >50=107 |
| Akinpelu, 200230 (Nigeria) | VAS M-VRS BNS |
Cross-sectional design | Caesarean section | Convergent validity | Worst pain Worst imaginable Worst pain |
Complications, illness unconscious | 35 (100) | 31 (5) |
| Briggs, 199935 (UK) | VAS VRS∗∗ |
Secondary analysis of RCT | Orthopaedic | Convergent validity Feasibility |
Number 100 Severe pain at rest and movement |
NR | 417 (45) | 47 (20)∗ 64 (17) |
| Fadaizadeh, 200939 (Iran) | VAS FPS |
Cross-sectional design | General, gynaecological | Convergent validity | 10 Agonised |
History of substance abuse, unconscious | 82 (72) 34 GS 48 GYN |
32 (14) GYN 27 (7) GS 38 (18) |
| Deloach, 199838 (USA) | VAS VPS |
Descriptive correlational design | Various type of surgeries | Convergent validity | Worst imaginable Horrible pain |
NR | NR | NR |
| Pesonen, 200851 (Finland) | VAS VRS-5 RWS FPS-7 |
Descriptive correlational design | Cardiac surgery: elective CABG, valvular repair | Feasibility | Worst possible pain Unbearable pain Worst possible pain Worst possible pain |
Dementia, cognitive impairment | 160 FPS 80 (36) RWS 80 (44) |
73 (5) |
| Aubrun, 200332 (France) | VAS NRS VRS Behavioural scale |
Prospective observational design | Orthopaedic, abdominal, gynaecological, others | Feasibility | Worst imaginable pain Worst imaginable pain Severe NR |
NR | 600 (47) | 51 (17) |
| Myles, 199949 (Australia) | VAS | Clinical study | General, orthopaedic, ENT, faciomaxillary, cardiothoracic | Interpretability | 100 worst pain ever | Severe pain, inability to complete the VAS | 52 (40) | 42 (15) |
| Myles, 200550 (Australia) | VAS | Clinical study | General, orthopaedic, ENT, faciomaxillary, cardiothoracic | Interpretability | 100 worst pain ever | Postoperative delirium Frailty, visual impairment |
22 (NR) | 33 (17) |
| Jensen, 200344 (USA) | VAS VRS-4 VRS-P |
Secondary analysis of RCT | Total knee replacement, hysterectomy, laparotomy | Interpretability | Worst pain Severe pain Complete relief |
NR | 123 (66) | 65 (10) |
| Gerbershagen, 201141 (Germany) | NRS | Comparative study design | Cholecystectomy, thyroidectomy, gastrointestinal, inguinal hernia repair, others | Interpretability | Worst imaginable pain | Repeated surgical, procedures, mechanical ventilation | 444 (44) | 18–20=38 21–30=75 31–40=88 41–50=96 51–60=87 61–70=49 71–80=2 |
| Cepeda, 200336 (USA) | NRS VRS |
Clinical study | Head and neck, thoracic, spinal abdominal, orthopaedic | Interpretability | Worst imaginable Severe pain |
NR | 700 (62) | 50 (15) |
| Jensen, 200245 (USA) | VAS VRS Pain relief |
Secondary analysis of RCT | Total knee replacement, abdominal hysterectomy, laparotomy | Responsiveness | Worst pain Severe pain Complete relief |
NR | 246 (66) | Knee 65 (10) Laparotomy 41 (7.5) |
| Jenkinson, 199543 (UK) | VAS CPI McGill |
RCT | Orthopaedic | Responsiveness | Severe pain | NR | 75 (64) | Male: 41 (13) Female: 43 (12) |
| Aubrun, 200331 (France) | VAS | Clinical study | Orthopaedic, urological, abdominal gynaecological, vascular, thoracic | Interpretability | 100 | Minor pain, delirium, dementia, non-French speaking | 3045 (54) | 50 (18) |
| Sriwatanakul, 198252 (USA) | VAS | Secondary analysis of RCT | NR | Interpretability | Pain as bad as it could be | NR | NR | NR |
| Van Giang, 201555 (Vietnam) | FPS NRS |
Validation study | Orthopaedic | Concurrent validity Responsiveness | The worst possible pain | Hearing impairment Altered mental status |
144 (45) | 37 (13) |
| Van Dijk, 201254 (The Netherlands) | NRS VRS |
Cross-sectional design | General, ENT, orthopaedic, neurosurgical, urological, gynaecological, plastic, vascular, cardiothoracic | Interpretability | 10 Worst pain imaginable |
ICU patients Non-Dutch speaking Cognitive or hearing impairment, inability to use self-report |
2674 (51) | 73 (6) |
| Li, 200747 (China) | VAS NRS-11 VDS FPS |
Prospective clinical study | NR | Convergent validity Scale reliability Responsiveness Feasibility |
10 The most intense imaginable pain 10 The most intense imaginable pain The most intense imaginable pain Worst pain |
NR | 173 (45) | 45.3 (15) |
| Li, 200946 (China) | FPS NRS IPT |
Descriptive correlational design | Gastrointestinal, orthopaedic, abdominal | Convergent validity Scale reliability Responsiveness Feasibility |
10 10 The most intense imaginable pain |
Did not speak Chinese More than one surgery ASA score of 4 Chronic pain |
180 (68) | 72 (6) |
| Zhou, 201156 (China) | VDS NRS FPS CAS |
Descriptive comparative design | NR | Criterion validity Convergent validity Test–retest reliability Feasibility |
Worst pain | Severe cognitive impairment | 200 (46) | 56 (16) |
| Gagliese, 20056 (Canada) | VAS-H VAS-V NRS VDS MPQ |
Validation study | NR | Feasibility Convergent validity Criterion validity |
10 Worst possible pain 10 Worst pain imaginable Excruciating |
On epidural or regional analgesia, ASA score of >3 Chronic pain, cognitive impairment, opioid or substance abuse |
504 (58) | 53 (15) |
| Tandon, 201653 (India) | OPS NRS |
Descriptive correlational design | Abdominal surgery | Convergent validity | Worst possible pain Inadequate pain relief/pain at rest |
Haemodynamic instability Unable to use a PCA pump |
93 | NR |
| Aziato, 201533 (Ghana) | NRS FPS CCPS |
Two phases: qualitative and psychometric testing | Caesarean section, leg amputation, laminectomy, laparotomy, others | Convergent validity Inter-rater reliability Responsiveness Feasibility |
Worst possible pain Hurts worst |
NR | 150 (77) | <30=44.7 30–39=35 40+=21 |
| Hamzat, 200942 (Ghana) | VAS | Validation study | Various gynaecological procedures | Cross-cultural validity | Worst possible pain | History of psychological or psychiatric disorders | 60 (100) | NR |
| Gagliese, 200340 (Canada) | MPQ PPI VAS-R VAS-M |
Descriptive correlation design | Radical prostatectomy | Convergent validity Responsiveness |
Worst possible pain 5 Excruciating 10 Worst possible 10 Worst possible pain |
Non-English speaker ASA >3 Chronic pain Chronic use of opioids |
200 | Younger patients: 56 (6) Older patients: 67 (3) |
| Myles, 201748 (Australia) | VAS | Observational design | General, orthopaedic, gynaecological, urological, major vascular, cardiac faciomaxillary, others | Test–retest reliability Interpretability |
Very severe pain | Poor English comprehension Drug or alcohol dependence Psychiatric disorder Uncontrolled pain |
219 (68) | 53 (17) |
| Danoff, 201837 (USA) | VAS | Prospective observational design | THA TKA |
Measurement error | Worst possible pain | Preoperative pain Catastrophizing Scale score greater than 30 points | 304 THA (21) TKA (30) |
THA: 60 [20–81] TKA; 63 [46–88] |
| Sloman, 20062 (Israel) | NRS | One group pretest–post-test design | Abdominal, orthopaedic, others | Interpretability | 10 Excruciating | NR | 150 (47) | 47 [14–89] |
| Bodian, 20013 (USA) | VAS McGill |
Clinical study | Intra-abdominal Surgery | Interpretability Desire for analgesics |
Worst pain imaginable | NR | 150 (48) | 49 [37–61] |
The low anchor was “no pain”.
Seven studies were performed in the USA,3,36, 37, 38,44,45,52 three in China,46,47,56 three in Australia,48, 49, 50 and two each in the UK,35,43 the Netherlands,13,54 Ghana,33,42 France,32 and Canada.6,40 One study each was performed in Finland,51 Spain,34 Nigeria,30 Iran,39 India,53 Vietnam,55 Israel,2 and Germany.41 Although all the included studies were reported in English, some of the tools were administered in other languages: Chinese,46,47,56 Twi,33,42 Vietnamese,55 Finnish,51 and both English and Yoruba.30
Using the modified Newcastle–Ottawa Score, the majority of studies looking at feasibility were of medium2,30,32,33,37,39,49,54 or high quality.3,6,13,35,36,41,46, 47, 48,50,51 The methodological quality of three secondary analysis studies that looked at VAS interpretability could not be assessed.44,45,52 The methodological quality for other measurement properties is described under each measurement property section.
The following measurement properties were assessed: measurement error (n=1),37 cross-cultural validity (n=1),42 reliability (n=5),33,46, 47, 48,56 responsiveness (n=7),33,40,43,45, 46, 47,55 and hypothesis testing for construct validity (namely convergent validity; n=13)6,30,33, 34, 35,38, 39, 40,46,47,54, 55, 56 and criterion validity (n=2).6,56 No studies assessed structural validity, internal consistency, or content validity of any pain assessment tool. Interpretability was measured in 11 studies.2,3,31,36,41,44,48, 49, 50,52,54 Two studies included the desire for analgesics as an outcome.3,13 The feasibility of pain assessment tools as an outcome measure was examined in eight studies.6,32,33,35,46,47,51,56
Outcomes for measurement properties
Unidimensional pain assessment tools
Convergent validity
Eight studies6,30,34,35,38, 39, 40,47 reported the convergent validity of the VAS with moderate-to-high correlations between several self-report scales that also measured pain intensity. Similarly, seven studies reported good convergent validity results for VRS,6,34,35,45,47,54,56 and six studies each reported good convergent validity results for NRS6,33,46,47,54,56 and FPS33,39,46,47,55,56 scores (Table 2). The correlations between scores obtained from several unidimensional tools were moderate to high, ranging from 0.5 to 0.9.
Table 2.
Summary of methodological quality of studies using COSMIN risk of bias and measurement properties. COSMIN, COnsensus-based Standards for the selection of health Measurement INstruments; FPS, faces pain scale; LoE, Level of evidence using GRADE approach reported as: High, Moderate, Low, or Very low; NRS, numerical rating scale; OBS, objective pain score; VDS, verbal descriptor scale. Ratings for overall quality reported as sufficient (+), insufficient (–), inconsistent (+/–), indeterminate (?). Empty cells indicate no available results for measurement properties.
| First author, year | Content validity | Structural validity | Internal consistency | Cross-cultural validity | Reliability | Measurement error | Criterion validity | Construct validity/convergent | Responsiveness |
|---|---|---|---|---|---|---|---|---|---|
| VAS Methodological quality assessment (COSMIN risk of bias) | |||||||||
| Banos, 198934 | Adequate | ||||||||
| Akinpelu, 200230 | Doubtful | ||||||||
| Briggs, 199935 | Adequate | ||||||||
| Fadaizadeh, 200939 | Adequate | ||||||||
| DeLoach, 199838 | Doubtful | ||||||||
| Li, 200747 | Inadequate | Adequate | Inadequate | ||||||
| Gagliese, 20056 | Inadequate | Inadequate | |||||||
| Gagliese, 200340 | inadequate | Inadequate | |||||||
| Myles, 201748 | Inadequate | ||||||||
| Jensen, 200245 | Inadequate | ||||||||
| Danoff, 201837 | Adequate | ||||||||
| Hamzat, 200942 | Inadequate | ||||||||
| Rating LoE |
? Very low |
+ Low |
? Moderate |
? Very low |
+ High |
? Low |
|||
| NRS Methodological quality assessment (COSMIN risk of bias) | |||||||||
| Van Dijk, 201254 | Adequate | ||||||||
| Li, 200747 | Inadequate | Adequate | Inadequate | ||||||
| Li, 200946 | Inadequate | Adequate | Inadequate | ||||||
| Zhou, 201156 | Inadequate | Adequate | Adequate | ||||||
| Gagliese, 20056 | Inadequate | Inadequate | |||||||
| Aziato, 201533 | Inadequate | Doubtful | Inadequate | ||||||
|
Rating LoE |
+ Low |
+/– Low |
+ High |
? Low |
|||||
| VDS Methodological quality assessment (COSMIN risk of bias) | |||||||||
| Banos, 198934 | Adequate | ||||||||
| Briggs, 199935 | Adequate | ||||||||
| Van Dijk, 201254 | Adequate | ||||||||
| Li, 200747 | Inadequate | Adequate | |||||||
| Zhou, 201156 | Inadequate | Adequate | Adequate | ||||||
| Gagliese, 20056 | Inadequate | Inadequate | |||||||
| Jensen, 200245 | Inadequate | ||||||||
| Rating LoE |
+ Low |
+/– Low |
+/– High |
? Low |
|||||
| FPS Methodological quality assessment (COSMIN risk of bias) | |||||||||
| Fadaizadeh, 200939 | Adequate | ||||||||
| Van Giang, 201555 | Adequate | Doubtful | |||||||
| Li, 200747 | Inadequate | Adequate | Inadequate | ||||||
| Li, 200946 | Inadequate | Adequate | Inadequate | ||||||
| Zhou, 201156 | Inadequate | Adequate | Adequate | ||||||
| Aziato, 201533 | Inadequate | Doubtful | Inadequate | ||||||
| Rating LoE |
+ Low |
+ Moderate |
+ High |
? Low |
|||||
| OPS Methodological quality assessment (COSMIN risk of bias) | |||||||||
| Tandon, 201653 | Doubtful | ||||||||
| Rating LoE |
+ Very low |
||||||||
Cross-cultural validity
One study42 established the validity of a Twi (Ghanaian) version of the VAS. The pain scores reported by patients using the new instrument correlated significantly with those reported by patients using the original (English) version of the VAS, with the highest correlation on the fifth postoperative day. Because of inadequate quality owing to an extremely serious risk of bias and imprecision, very low quality evidence was reported for cross-cultural validity of the VAS.
Reliability
The VAS showed high scale,46,47 and test–retest reliability48 with an intraclass correlation coefficient of 0.79 (95% confidence interval [CI], 0.49–0.91).48 The NRS demonstrated high test–retest,56 inter-rater,44 and scale reliability.33,46,47,56 VDS demonstrated high scale47 and test–retest reliability.56 Similarly, FPS demonstrated high inter-rater33 and test–retest reliability56 (Table 3). All four scales showed low-quality evidence because of very serious risk of bias.
Table 3.
Reliability of unidimensional pain assessment tools in surgical patients. ∗Average interclass correlation coefficient calculated for 7 days. †No separate result for each scale. ‡Results categorised in 20–44 yr (n=43), 45–59 yr (n=39), 60 yr without cognitive impairment (n=40), ≤60 yr with mild cognitive impairment (n=31). ¶95% confidence interval. FPS, faces pain scale; n, number of patients; NRS, numerical rating scale; PROM/s, patient-reported outcome measures; SD, standard deviation; VAS, visual analogue scale; VDS, verbal descriptor scale.
| First author, year | PROM/s | Pain construct | Reliability |
|||
|---|---|---|---|---|---|---|
| Type | n | Time interval | Interclass correlation coefficient | |||
| Li, 200747 | VAS NRS VDS FPS |
Current, worst, least, average pain on 7 postoperative days | Scale reliability | 173 | Every 24 h | 0.66∗ 0.76∗ 0.72∗ 0.72∗ |
| Li, 200946 | FPS NRS Iowa Pain Thermometer |
Current pain and daily retrospective ratings of worst and least pain | Scale reliability | 180 | Every 24 h | 0.95 to 0.97† |
| Zhou, 201156 | VDS NRS FPS Numeric Box-21 Scale Coloured Analogue Scale |
Recalled pain and postoperative pain | Test–retest reliability | 153 | 24 h | 0.96, 0.88, 0.93, 0.84‡ 0.94, 0.90, 0.91, 0.80‡ 0.93, 0.91, 0.84, 0.80‡ 0.92, 0.91, 0.78, 0.76‡ 0.93, 0.90, 0.88, 0.77¶ |
| Aziato, 201533 | NRS FPS Colour Circle Pain Scale |
No pain – worst possible pain No pain – worst possible pain No pain – unbearable |
Inter-rater reliability | 150 | 5–10 min | 0.92 0.93 0.93 |
| Myles, 201748 | VAS | Pain unchanged or almost the same | Test–retest reliability | 22 | Not reported | 0.79 (0.49–0.91)¶ |
Responsiveness
Seven studies33,40,43,45, 46, 47,55 reported responsiveness results for the four unidimensional pain assessment tools and provided low-quality evidence because of a very serious risk of bias (Table 4). The identified risk of bias was mainly related to the use of inappropriate measures of responsiveness such as effect size and statistical tests used.
Table 4.
Responsiveness results of unidimensional tools. Empty cells indicate data not available or not assessed. ∗P-value is statistically significant at <0.0001. †Knee surgery. ‡Laparotomy. ¶VAS score. §CPI score. ||Time 2 vs time 1. #Time 3 vs time 1. ††Time 4 vs time 1. ‡‡Time 5 vs time. ¶¶Results for younger patient split of the sample at the median age of 62 yr. CCPS, colour circle pain scale; CI, confidence interval; CPI, categorical verbal pain rating scale; FPS, face pain scale; G, group; MPQ, McGill pain questionnaire; PPI, present pain intensity; PROM/s, patient-reported outcome measures; SRM, standardized response mean; VAS, visual analogue scale; VAS-R, visual analogue scale at rest; VAS-M, visual analogue scale at movement; VDS, verbal descriptor scale. Effect size, calculated by taking a mean change of variable and dividing it by standard deviation of that variable.
| First author, year | PROM/s | Time interval | n | Better, same, worse % | Mean difference before and after treatment (95% CI) | Effect size OR SRM (95% CI) | Correlation with changes in other instruments |
|---|---|---|---|---|---|---|---|
| Jensen, 200245 | VAS VDS Relief rating |
Baseline then several times | 123 125 |
10.37,† 20.71‡ 7.17,† 15.09‡ 7.59,† 26,61¶ |
|||
| Jenkinson, 199543 | VAS CPI MPQ |
Baseline then 120 min | 75 | Moderate 2.23,¶ 1.83§ Good 1.91,¶ 3.13§ Complete 1.89,¶ 5§ |
G1;0.99,¶ 1.93§ G2;1.23,¶ 1.82§ G3; 2,¶ 3.29§ G4;1.48,¶ 1.48§ |
CPI 0.67 to VAS | |
| Van Giang, 201555 | FPS NRS |
Every 30 min for 2 h | 144 | –1.17|| –1.59# –1.66†† –1.82‡‡ |
–0.70|| –1.05# –1.20†† –1.31‡‡ |
0.78 | |
| Li, 200747 | VAS NRS VDS FPS |
NR | 28 | 4.3 [2.4]†† 4.2 [2.3]†† 4.5 [2.1]†† 4.3 [1.9]†† |
|||
| Li, 200946 | FPS NRS JPT |
NR | 180 | 14.095|| †† | |||
| Aziato, 201533 | NRS FPS CCPS |
NR | 150 | 2.3 (2.1–2.5)†† 1.5 (1.4–1.6)†† 1.4 (1.3–1.5) †† |
|||
| Gagliese, 200340 | MPQ PPI VAS-R VAS-M |
NR | 200 | 0.31,¶¶ 0.39 0.25,¶¶ 0.26 0.23,¶¶ 0.32 Not reported |
Measurement error
Only one study assessed measurement error of VAS by determining the minimal detectable change (MDC),37 which describes the smallest change outside of inherent measurement error that the VAS can detect. The study showed that the MDC on a 100 mm VAS was 15 mm for total hip arthroplasty and 16 mm for total knee arthroplasty.37 We evaluated the evidence regarding VAS measurement error as moderate quality because we could not determine the minimal important change for VAS in acute pain to compare with MDC and the risk of bias.
Functional pain assessment tool
Only one study examined the ‘Objective Pain Score’, which assesses the interference of pain with respiratory function.53 The study evaluated the correlation between scores obtained from the Objective Pain Score and NRS. Whilst patients rated their pain using a printed NRS, the clinician rated pain using the Objective Pain Score. A linear regression model determined the relationship between NRS and Objective Pain Score, and showed that, for every unit increase in the NRS, the Objective Pain Score decreased by 0.334. The study reported sufficient convergent validity with the NRS, although with low-quality evidence because of risk of bias and imprecision. A summary of findings on all assessed measurement properties is provided (Table 2).
Other outcomes
Interpretability and desire for analgesics
Visual analogue scale
Seven studies31,37,44,48, 49, 50,52 looked at the interpretability of VAS, and one study3 included the desire for analgesics as an outcome. Several studies31,44,52 reported nearly similar cut-off points for VAS, indicating that VAS ratings of 0–5 mm were very likely to be rated as no pain by patients, 6–44 mm were considered mild pain, 45–69 mm were considered moderate pain, and VAS ratings ≥70 mm were suggestive of severe pain.
Two studies37,48 determined the interpretability of VAS by identifying the minimal clinically important difference (MCID) defined as the minimal change in score indicating a meaningful change in pain status.57 The use of a combination of distribution- and anchor-based methods resulted in an MCID of 9.9 mm for VAS in assessing several types of surgical procedures.48 In contrast, Danoff and colleagues37 reported higher MCID values for pain improvement in patients undergoing total hip or knee arthroplasty. Pain was improving clinically when the VAS decreased by 19 and 23 mm, respectively.
Bodian and colleagues3 found that the proportion of patients requesting additional analgesia after abdominal surgery increased as VAS increased (4%, 43%, and 80% with VAS scores of 30 mm or less, 31–70 mm, and greater than 70 mm, respectively).
Numerical rating scale
Four studies2,36,41,54 looked at interpretability of the NRS, and one study included desire for analgesics as an outcome.13 Sloman and colleagues2 determined the meaning of changes in NRS in relation to perceived pain relief before and after treatment. Patients who rated their pain relief as ‘minimal’ had, on average, a 35% reduction in NRS. NRS was less sensitive to detect changes from ‘moderate’ to ‘much’ as there was a 67% reduction for those who rated their reduction as ‘moderate’, a 70% decrease for those who rated it is as ‘much’, and a 94% reduction for those assessed their pain reduction as ‘complete’.2
Inconsistent cut-off points between moderate to severe pain were identified for NRS. For example Gerbershagen and colleagues41 determined NRS ≥4 as a cut-point for moderate pain, whereas ‘pain interfering with function’ resulted in a lower cut-off point of NRS ≥3. While using receiver operating characteristic analysis in another study, Van Dijk and colleagues54 found that the sensitivity of NRS to differentiate bearable pain (VRS ≤2) from unbearable pain (VRS >2) reached higher values (94%) for high cut-off point of NRS >5 compared with lower cut-off points of 3 and 4 (sensitivity 72% and 83%), respectively.
In another study, Van Dijk and colleagues13 showed that 19% of patients with NRS scores ranging from 5 to 10 had no desire for additional opioids; meanwhile, 62% reported that they did not want additional opioids because their pain was tolerable. When patients were asked at which score they would request opioids, both the median and the modal pain scores were an NRS of 8.
Feasibility
Eight studies included feasibility of pain assessment tools as an outcome measure.6,32,33,35,46,47,51,56 Error rates were reported as an inability to understand the tool, responses that could not be scored reliably, and lack of responses.6,35,47,51 Some studies reported the most preferred scale or the easiest to complete ones.6,33,46,56 There was a lack of studies that assessed the time required to complete the tool or time taken to train patients or nurses.
For multiple types of surgical procedures and in different populations, VDS or VRS was more successful when compared with other tools. Using VRS in patients aged ≥75 yr after cardiac surgery showed a higher success rate (81%) compared with VAS (60%) and the FPS (44%). These rates varied significantly on all postoperative days (P<0.02).51 The reported reasons for the failure rate, which was identified as failure to understand or express level of pain using the assessment tool, were postoperative confusion, delirium, exhaustion, and an inability to differentiate between facial expressions.51 In a similar way, VRS was more suited for compliance and ease of use after orthopaedic surgery compared with VAS in which 56% of patients included in the study did not understand how to complete VAS and one-third could not perform the assessment using VAS because of visual or hearing impairment.35 Moreover, VAS showed the highest error rate of 12.3% when used in Chinese populations, whereas VRS reported the lowest error rate (0.8%), which was statistically significant (P<0.05).47 Interestingly, 40% of the patients rated NRS as the easiest, most preferred tool for assessment; in contrast, VAS was reported the least preferred.6
From the nurses' perspectives in PACUs, NRS was the most preferred tool in 60% of the included sample.32 Even though the VAS was the recommended tool to be used in the institution where the study was conducted, 50% of the nurses preferred to use either NRS or VRS owing to its complexities making it difficult for patients to understand VAS.32 Three studies reported FPS as the preferred tool among a Chinese population,47 for women,46 middle-aged adults, and older patients without and with mild cognitive impairment, followed by VRS and NRS.56 Likewise, FPS (55%) was preferred to NRS (33%) among a Ghanaian population.33
Discussion
This systematic review presents a comprehensive examination of the measurement properties of unidimensional and functional assessment tools used for adult postoperative patients. The quality of evidence for the measurement properties and utility of the VAS, VDS, NRS, and FPS was suboptimal. Overall, construct validity (convergent validity) was most commonly assessed across measures. Content validity, internal consistency, and structural validity were not assessed as these measures are not designed for single-item scales. The VAS had the greatest number of studies assessing its measurement properties in the postoperative setting, followed by the NRS. Studies on functional pain assessment tools were scarce. Most of the reviewed studies failed to meet the COSMIN methodological standards required. Good-quality studies were found for interpretability and feasibility as assessed by the Newcastle–Ottawa Scale.26
Most of the studies reported sufficient convergent validity of several unidimensional pain assessment tools, indicating that the scales tended to measure score variations in the same direction.58 Similar positive findings of good convergent validity results were reported when these tools were used to assess pain associated with rheumatoid arthritis,59 osteoarthritis,60 and low back pain.61 However, the methodology used to measure convergent validity was limited. Because no gold standard tool exists for assessing pain, most studies assessed the correlation of scores obtained from one unidimensional tool with another, measuring only pain intensity. However, when a multidimensional tool such as the McGill Pain Questionnaire was used as a comparator, studies reported lower correlation scores.6,40,62 This variation may be related to assessor and patient fatigue during the detailed pain assessment.
There was good reliability of pain assessment for all unidimensional tools. However, the quality of evidence was low for all four scales because of serious risk of bias owing to unreported intervals for repeated measures or the use of inappropriate reliability measures by treating ranked NRS, VDS, or FPS scores as a continuous value. Measurement error was only available for VAS; however, the study outcome was indeterminate because we could not determine for VAS in acute pain to compare it with the MDC. When the MDC is smaller than the minimal important change, significant change can be distinguished from measurement error.63
Small, albeit statistically significant changes in VAS do not necessarily indicate clinically important changes to guide the interpretation of studies evaluating analgesic therapies.37 Therefore, obtaining an accurate MCID is crucial.64 Previous studies have shown that the MCID differs by patient population and diagnosis. We identified two studies reporting inconsistent MCID values for the postoperative population.37,48 The MCID tended to be higher in patients who underwent joint arthroplasties than other procedures.48 One explanation might be that patients reporting severe, acute pain need a larger reduction in pain to be clinically meaningful.65
Measures of responsiveness are an important psychometric property to assess the sensitivity of change in pain over time.66 Measures of responsiveness used included effect size, standardized response mean, and scores before and after intervention.33,40,43,44,46,47,55 According to COSMIN methodology, effect size and standardised response mean are inappropriate to assess responsiveness because they measure the size of the change scores rather than their validity. Moreover, the P-value of statistical tests only measures the statistical significance of the change in scores rather than their validity.63
Pain assessment tools help diagnose surgical catastrophes, allow communication between healthcare providers, and are used to assess efficacy of analgesic treatments and allow comparison between therapies. As no agreement exists on how to identify the optimal cut-off point of a unidimensional pain assessment tool, various arbitrarily chosen values are used.41 In general, VAS cut-off points of 30, 70, and 100 mm indicate the upper boundaries of mild, moderate, and severe pain, respectively. However, a recent study conducted found a higher cut-off point between mild and moderate pain of around 55 mm on the VAS, which is greater than the values reported by most earlier studies and physicians' consensus.44,67, 68, 69
NRS cut-off points used by healthcare professionals do not necessarily reflect patients' desire for additional analgesics.13 Previous studies have also found that a high proportion of patients with pain scores >4 did not demand analgesics (28% of patients visiting an emergency department70 and 42% of children after surgery71). Cho and colleagues62 showed that postoperative patients requested an analgesic when their pain was VAS ≥5.5, NRS ≥6, FPS-R ≥6, or VRS ≥2 (moderate or severe pain). This might be influenced by a general refusal for analgesic medicines, or fear of side-effects or addiction, especially with opioids.13,72,73 Cut-off points, although important, are not validated to guide analgesic interventions.
Previously, postoperative pain assessment and management was focused on providing humanitarian pain relief, which constitutes only one objective to tackle a complex experience, and that was achieved by using unidimensional scores. However, healthcare providers should address pain by several approaches to determine if the pain is tolerable, is hindering recovery, or requires intervention.62
Efforts have been made to encourage use of multidimensional tools to assess postoperative pain. A recent systematic review indicated that the Brief Pain Inventory and the American Pain Society Pain Outcomes Questionnaire – Revised were the two commonly used and studied multidimensional pain assessment tools for patients after surgery, followed by the McGill Pain Questionnaire. These multidimensional tools showed good ratings for some psychometric properties such as internal consistency. However, this recommendation was based on low- to moderate-quality evidence.66 Moreover, these tools involve a detailed assessment that can range from 5 to 30 min,74 hindering routine use for frequent assessment in a busy surgical ward.20 Alternatively, functional pain assessment has been recommended.14,75
However, as no gold standard objective measures exist for pain-related functional capacity in postoperative patients,76 we included objective tools assessing the impact of pain on function. Only one study reported sufficient convergent validity of functional assessment based on pain interference with normal breathing and NRS score.53 The low methodological quality of the study limits the generalisability of the result. Other researchers have tried to incorporate a non-formally validated three-level ‘Functional Activity Score’20 into clinical practice. One study in a Chinese population combining the Functional Activity Score and dynamic NRS found that this allowed nurses to guide and educate patients to better use patient-controlled analgesia to facilitate functional recovery.77 In addition, a pilot study in hospitalised patients validated a four-level scale (no interference, interference with some or most activities, or inability to do any activity).78 It established the convergent validity of this tool compared with NRS and VAS in cognitively intact patients. Patients aged ≥40 yr also preferred a functional assessment scale,78 possibly because functional assessment considered the impact of pain on activity.
The heterogeneity of study designs, including the assessment scales used, surgical procedures, sample sizes, countries in which the studies were conducted, and the languages used, make determining the most feasible assessment tool difficult. However, the VAS showed the highest error rate and was the least preferred in several studies, whereas the VRS showed the lowest error rate. Difficulties comprehending the VAS and linearly quantifying pain resulted in a higher frequency of incomplete responses, especially for older patients.12,13 Therefore, older adults and children who have less abstract thinking ability might prefer a categorical scale such as the VRS for easier use.14 Interestingly, although the FPS is commonly used in paediatric populations, it was also the most preferred tool in the Ghanaian and Chinese adult populations. This might be because of the simplicity of facial expressions, which can quickly reflect pain. Alternatively, cultural aspects may explain why the FPS was preferred.79
Strengths and limitations
The main strength of this review is that it includes the most frequently used unidimensional and functional pain assessment tools. In addition, we put no limits on publication date, enabling us to obtain information on early studies of these tools. To our knowledge, this is the first review to evaluate the validity of these tools, focusing solely on postsurgical populations and applying COSMIN methodology.
Potential limitations include the fact that the search strategy may have excluded grey literature and studies published in languages other than English. However, we tried to limit the effect of language and publication biases by searching the references of included studies. In addition, the clinical diversity and limitations in the methodologies and quality of the included studies, may have reduced the strength of the conclusions.
Conclusions
This systematic review challenges the validity and reliability of unidimensional tools to quantify pain in adult patients after surgery. Despite their extensive use, no evidence clearly suggests that one tool has superior measurement properties in assessing postoperative pain. Therefore, future studies should be prioritised to assess their validity, reliability, measurement error, and responsiveness using COSMIN methodology. Moreover, adequate quality head-to-head comparison studies are required to assess several unidimensional pain assessment tools alongside other tools covering multiple dimensions of the pain experience. In addition, because promoting function is a crucial perioperative goal, psychometric validation studies of functional pain assessment tools are warranted to identify patients who need additional interventions to promote recovery and improve postoperative pain assessment and management.
Authors' contributions
Study design: RMB, AI, DNL, RDK, NAL, LST
Literature search: RMB, AI
Data extraction: RMB, AI
Data analysis: RMB, AI
Data interpretation: RMB, AI, DNL, RDK, NAL, LST
Writing of the manuscript: RMB, AI, DNL, RDK, NAL, LST
Critical review: RMB, AI, DNL, RDK, NAL, LST
Approval of submitted manuscript: RMB, AI, DNL, RDK, NAL, LST
Overall supervision: DNL, RDK
Acknowledgements
The authors acknowledge the contribution of Douglas Grindlay, Information Specialist in Centre of Evidence Based Dermatology, the University of Nottingham for his guidance in building the search strategy.
Handling editor: Nadine Attal
Footnotes
This paper was presented at the Annual Meeting of the Surgical Research Society in March 2021, and a preliminary version has been published in abstract form in Br J Surg 2021; 108: znab282.044. doi 10.1093/bjs/znab282.044.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2021.11.032.
Declarations of interest
The authors declare that they have no conflicts of interest.
Funding
Medical Research Council [MR/K00414X/1]; and Arthritis Research UK [19891]; Saudi Arabian Cultural Bureau and the University of Nottingham (PhD studentship for RMB).
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary material is available at British Journal of Anaesthesia online.
References
- 1.Carr D.B., Goudas L.C. Acute pain. Lancet. 1999;353:2051–2058. doi: 10.1016/S0140-6736(99)03313-9. [DOI] [PubMed] [Google Scholar]
- 2.Sloman R., Wruble A.W., Rosen G., Rom M. Determination of clinically meaningful levels of pain reduction in patients experiencing acute postoperative pain. Pain Manag Nurs. 2006;7:153–158. doi: 10.1016/j.pmn.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 3.Bodian C.A., Freedman G., Hossain S., Eisenkraft J.B., Beilin Y. The visual analog scale for pain. Anesthesiology. 2001;95:1356–1361. doi: 10.1097/00000542-200112000-00013. [DOI] [PubMed] [Google Scholar]
- 4.Breivik H., Borchgrevink P.C., Allen S.M., et al. Assessment of pain. Br J Anaesth. 2008;101:17–24. doi: 10.1093/bja/aen103. [DOI] [PubMed] [Google Scholar]
- 5.Ravaud P., Keita H., Porcher R., Durand-Stocco C., Desmonts J., Mantz J. Randomized clinical trial to assess the effect of an educational programme designed to improve nurses’ assessment and recording of postoperative pain. Br J Surg. 2004;91:692–698. doi: 10.1002/bjs.4506. [DOI] [PubMed] [Google Scholar]
- 6.Gagliese L., Weizblit N., Ellis W., Chan V.W.S. The measurement of postoperative pain: a comparison of intensity scales in younger and older surgical patients. Pain. 2005;117:412–420. doi: 10.1016/j.pain.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 7.Joyce C., Zutshi D., Hrubes V., Mason R. Comparison of fixed interval and visual analogue scales for rating chronic pain. Eur J Clin Pharmacol. 1975;8:415–420. doi: 10.1007/BF00562315. [DOI] [PubMed] [Google Scholar]
- 8.Jensen M.P., Karoly P., Braver S. The measurement of clinical pain intensity: a comparison of six methods. Pain. 1986;27:117–126. doi: 10.1016/0304-3959(86)90228-9. [DOI] [PubMed] [Google Scholar]
- 9.Ohnhaus E.E., Adler R. Methodological problems in the measurement of pain: a comparison between the verbal rating scale and the visual analogue scale. Pain. 1975;1:379–384. doi: 10.1016/0304-3959(75)90075-5. [DOI] [PubMed] [Google Scholar]
- 10.Le Resche L., Burgess J., Dworkin S. Reliability of visual analog and verbal descriptor scales for "objective" measurement of temporomandibular disorder pain. J Dent Res. 1988;67:33–36. doi: 10.1177/00220345880670010601. [DOI] [PubMed] [Google Scholar]
- 11.Wong D.L., Baker C.M. Pain in children: comparison of assessment scales. Pediatr Nurs. 1988;14:9–17. [PubMed] [Google Scholar]
- 12.Coll A.M., Ameen J.R., Mead D. Postoperative pain assessment tools in day surgery: literature review. J Adv Nurs. 2004;46:124–133. doi: 10.1111/j.1365-2648.2003.02972.x. [DOI] [PubMed] [Google Scholar]
- 13.Van Dijk J.F.M., Kappen T.H., Schuurmans M.J., van Wijck A.J.M. The relation between patients’ NRS pain scores and their desire for additional opioids after surgery. Pain Pract. 2015;15:604–609. doi: 10.1111/papr.12217. [DOI] [PubMed] [Google Scholar]
- 14.Pasero C., Quinlan-Colwell A., Rae D., Broglio K., Drew D. American Society for Pain Management Nursing position statement: prescribing and administering opioid doses based solely on pain intensity. Pain Manag Nurs. 2016;17:170–180. doi: 10.1016/j.pmn.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 15.Chou R., Gordon D.B., de Leon-Casasola O.A., et al. Management of postoperative pain: a clinical practice guideline from the American pain society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists' committee on regional anesthesia, executive committee, and administrative council. J Pain. 2016;17:131–157. doi: 10.1016/j.jpain.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 16.Mularski R.A., White-Chu F., Overbay D., Miller L., Asch S.M., Ganzini L. Measuring pain as the 5th vital sign does not improve quality of pain management. J Gen Intern Med. 2006;21:607–612. doi: 10.1111/j.1525-1497.2006.00415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frasco P.E., Sprung J., Trentman T.L. The impact of the Joint Commission for Accreditation of Healthcare Organizations pain initiative on perioperative opiate consumption and recovery room length of stay. Anesth Analg. 2005;100:162–168. doi: 10.1213/01.ANE.0000139354.26208.1C. [DOI] [PubMed] [Google Scholar]
- 18.Vila H., Smith R.A., Augustyniak M.J., et al. The efficacy and safety of pain management before and after implementation of hospital-wide pain management standards: is patient safety compromised by treatment based solely on numerical pain ratings? Anesth Analg. 2005;101:474–480. doi: 10.1213/01.ANE.0000155970.45321.A8. [DOI] [PubMed] [Google Scholar]
- 19.Laycock H.C., Harrop-Griffiths W. Assessing pain: how and why? Anaesthesia. 2021;76:559–562. doi: 10.1111/anae.15407. [DOI] [PubMed] [Google Scholar]
- 20.Levy N., Sturgess J., Mills P. Pain as the fifth vital sign” and dependence on the “numerical pain scale” is being abandoned in the US: why? Br J Anaesth. 2018;120:435–438. doi: 10.1016/j.bja.2017.11.098. [DOI] [PubMed] [Google Scholar]
- 21.Levy N., Mills P., Rockett M. Post-surgical pain management: time for a paradigm shift. Br J Anaesth. 2019;123:e182–e186. doi: 10.1016/j.bja.2019.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kehlet H. Postoperative pain relief—what is the issue? Br J Anaesth. 1994;72:375–378. doi: 10.1093/bja/72.4.375. [DOI] [PubMed] [Google Scholar]
- 23.Van Boekel R.L.M., Vissers K.C.P., van der Sande R., Bronkhorst E., Lerou J.G.C., Steegers M.A.H. Moving beyond pain scores: multidimensional pain assessment is essential for adequate pain management after surgery. PLoS One. 2017;12 doi: 10.1371/journal.pone.0177345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liberati A., Altman D.G., Tetzlaff J., et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1–e34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 25.Ouzzani M., Hammady H., Fedorowicz Z., Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016;5:210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wells G.A., Shea B., O’Connell D., et al. Ottawa Hospital Research Institute; Ottawa, Canada: 2021. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses.http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp Available at. [Google Scholar]
- 27.Prinsen C.A., Mokkink L.B., Bouter L.M., et al. COSMIN guideline for systematic reviews of patient-reported outcome measures. Qual Life Res. 2018;27:1147–1157. doi: 10.1007/s11136-018-1798-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Terwee C.B., Mokkink L.B., Knol D.L., Ostelo R.W., Bouter L.M., de Vet H.C. Rating the methodological quality in systematic reviews of studies on measurement properties: a scoring system for the COSMIN checklist. Qual Life Res. 2012;21:651–657. doi: 10.1007/s11136-011-9960-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mokkink L.B., De Vet H.C.W., Prinsen C.A.C., et al. COSMIN risk of bias checklist for systematic reviews of patient-reported outcome measures. Qual Life Res. 2018;27:1171–1179. doi: 10.1007/s11136-017-1765-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akinpelu A.O., Olowe O.O. Correlative study of 3 pain rating scales among obstetric patients. Afr J Med Med Sci. 2002;31:123–126. [PubMed] [Google Scholar]
- 31.Aubrun F., Langeron O., Quesnel C., Coriat P., Riou B. Relationships between measurement of pain using visual analog score and morphine requirements during postoperative intravenous morphine titration. Anesthesiology. 2003;98:1415–1421. doi: 10.1097/00000542-200306000-00017. [DOI] [PubMed] [Google Scholar]
- 32.Aubrun F., Paqueron X., Langeron O., Coriat P., Riou B. What pain scales do nurses use in the postanaesthesia care unit? Eur J Anaesthesiol. 2003;20:745–749. doi: 10.1017/s0265021503001212. [DOI] [PubMed] [Google Scholar]
- 33.Aziato L., Dedey F., Marfo K., Avoka Asamani J., Clegg-Lamptey J.N.A. Validation of three pain scales among adult postoperative patients in Ghana. BMC Nurs. 2015;14:42. doi: 10.1186/s12912-015-0094-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Banos J.E., Bosch F., Canellas M., Bassols A., Ortega F., Bigorra J. Acceptability of visual analogue scales in the clinical setting: a comparison with verbal rating scales in postoperative pain. Methods Find Exp Clin Pharmacol. 1989;11:123–127. [PubMed] [Google Scholar]
- 35.Briggs M., Closs J.S. A descriptive study of the use of visual analogue scales and verbal rating scales for the assessment of postoperative pain in orthopedic patients. J Pain Symptom Manage. 1999;18:438–446. doi: 10.1016/s0885-3924(99)00092-5. [DOI] [PubMed] [Google Scholar]
- 36.Cepeda M.S., Africano J.M., Polo R., Alcala R., Carr D.B. What decline in pain intensity is meaningful to patients with acute pain? Pain. 2003;105:151–157. doi: 10.1016/s0304-3959(03)00176-3. [DOI] [PubMed] [Google Scholar]
- 37.Danoff J.R., Goel R., Sutton R., Maltenfort M.G., Austin M.S. How much pain is significant? Defining the minimal clinically important difference for the visual analog scale for pain after total joint arthroplasty. J Arthroplasty. 2018;33:S71. doi: 10.1016/j.arth.2018.02.029. –5. e2. [DOI] [PubMed] [Google Scholar]
- 38.Deloach L.J., Higgins M.S., Caplan A.B., Stiff J.L. The visual analog scale in the immediate postoperative period. Anesth Analg. 1998;86:102–106. doi: 10.1097/00000539-199801000-00020. [DOI] [PubMed] [Google Scholar]
- 39.Fadaizadeh L., Emami H., Samii K. Comparison of visual analogue scale and faces rating scale in measuring acute postoperative pain. Arch Iran Med. 2009;12:73–75. [PubMed] [Google Scholar]
- 40.Gagliese L., Katz J. Age differences in postoperative pain are scale dependent: a comparison of measures of pain intensity and quality in younger and older surgical patients. Pain. 2003;103:11–20. doi: 10.1016/s0304-3959(02)00327-5. [DOI] [PubMed] [Google Scholar]
- 41.Gerbershagen H.J., Rothaug J., Kalkman C.J., Meissner W. Determination of moderate-to-severe postoperative pain on the numeric rating scale: a cut-off point analysis applying four different methods. Br J Anaesth. 2011;107:619–626. doi: 10.1093/bja/aer195. [DOI] [PubMed] [Google Scholar]
- 42.Hamzat T., Samir M., Peters G. Development and some psychometric properties of Twi (Ghanaian) version of the visual analogue scale. Afr J Biomed Res. 2009;12:145–148. [Google Scholar]
- 43.Jenkinson C., Carroll D., Egerton M., et al. Comparison of the sensitivity to change of long and short form pain measures. Qual Life Res. 1995;4:353–357. doi: 10.1007/BF01593888. [DOI] [PubMed] [Google Scholar]
- 44.Jensen M.P., Chen C., Brugger A.M. Interpretation of visual analog scale ratings and change scores: a reanalysis of two clinical trials of postoperative pain. J Pain. 2003;4:407–414. doi: 10.1016/s1526-5900(03)00716-8. [DOI] [PubMed] [Google Scholar]
- 45.Jensen M.P., Chen C., Brugger A.M. Postsurgical pain outcome assessment. Pain. 2002;99:101–109. doi: 10.1016/s0304-3959(02)00063-5. [DOI] [PubMed] [Google Scholar]
- 46.Li L., Herr K., Chen P. Postoperative pain assessment with three intensity scales in Chinese elders. J Nurs Scholarsh. 2009;41:241–249. doi: 10.1111/j.1547-5069.2009.01280.x. [DOI] [PubMed] [Google Scholar]
- 47.Li L., Liu X., Herr K. Postoperative pain intensity assessment: a comparison of four scales in Chinese adults. Pain Med. 2007;8:223–234. doi: 10.1111/j.1526-4637.2007.00296.x. [DOI] [PubMed] [Google Scholar]
- 48.Myles P., Myles D., Galagher W., et al. Measuring acute postoperative pain using the visual analog scale: the minimal clinically important difference and patient acceptable symptom state. Br J Anaesth. 2017;118:424–429. doi: 10.1093/bja/aew466. [DOI] [PubMed] [Google Scholar]
- 49.Myles P.S., Troedel S., Boquest M., Reeves M. The pain visual analog scale: is it linear or nonlinear? Anesth Analg. 1999;89:1517–1520. doi: 10.1097/00000539-199912000-00038. [DOI] [PubMed] [Google Scholar]
- 50.Myles P.S., Urquhart N. The linearity of the visual analogue scale in patients with severe acute pain. Anaesth Intensive Care. 2005;33:54–58. doi: 10.1177/0310057X0503300108. [DOI] [PubMed] [Google Scholar]
- 51.Pesonen A., Suojaranta-Ylinen R., Tarkkila P., Rosenberg P.H. Applicability of tools to assess pain in elderly patients after cardiac surgery. Acta Anaesthesiol Scand. 2008;52:267–273. doi: 10.1111/j.1399-6576.2007.01480.x. [DOI] [PubMed] [Google Scholar]
- 52.Sriwatanakul K., Kelvie W., Lasagna L., Calimlim J.F., Weis O.F., Mehta G. Studies with different types of visual analog scales for measurement of pain. Clin Pharmacol Ther. 1983;34:234–239. doi: 10.1038/clpt.1983.159. [DOI] [PubMed] [Google Scholar]
- 53.Tandon M., Singh A., Saluja V., Dhankhar M., Pandey C.K., Jain P. Validation of a new “objective pain score” Vs. “numeric rating scale” for the evaluation of acute pain: a comparative study. Anesth Pain Med. 2016;6 doi: 10.5812/aapm.32101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Van Dijk J.F., Kappen T.H., van Wijck A.J., Kalkman C.J., Schuurmans M.J. The diagnostic value of the numeric pain rating scale in older postoperative patients. J Clin Nurs. 2012;21:3018–3024. doi: 10.1111/j.1365-2702.2012.04288.x. [DOI] [PubMed] [Google Scholar]
- 55.Van Giang N., Chiu H.-Y., Thai D.H., Kuo S.-Y., Tsai P.-S. Validity, sensitivity, and responsiveness of the 11-face faces pain scale to postoperative pain in adult orthopedic surgery patients. Pain Manag Nurs. 2015;16:678–684. doi: 10.1016/j.pmn.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 56.Zhou Y., Petpichetchian W., Kitrungrote L. Psychometric properties of pain intensity scales comparing among postoperative adult patients, elderly patients without and with mild cognitive impairment in China. Int J Nurs Stud. 2011;48:449–457. doi: 10.1016/j.ijnurstu.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 57.Farrar J.T., Young J.P., Jr., LaMoreaux L., Werth J.L., Poole R.M. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94:149–158. doi: 10.1016/S0304-3959(01)00349-9. [DOI] [PubMed] [Google Scholar]
- 58.Hjermstad M.J., Fayers P.M., Haugen D.F., et al. Studies comparing numerical rating scales, verbal rating scales, and visual analogue scales for assessment of pain intensity in adults: a systematic literature review. J Pain Symptom Manage. 2011;41:1073–1093. doi: 10.1016/j.jpainsymman.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 59.Sendlbeck M., Araujo E.G., Schett G., Englbrecht M. Psychometric properties of three single-item pain scales in patients with rheumatoid arthritis seen during routine clinical care: a comparative perspective on construct validity, reproducibility and internal responsiveness. RMD Open. 2015;1 doi: 10.1136/rmdopen-2015-000140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alghadir A.H., Anwer S., Iqbal A., Iqbal Z.A. Test-retest reliability, validity, and minimum detectable change of visual analog, numerical rating, and verbal rating scales for measurement of osteoarthritic knee pain. J Pain Res. 2018;11:851–856. doi: 10.2147/JPR.S158847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chiarotto A., Maxwell L.J., Ostelo R.W., Boers M., Tugwell P., Terwee C.B. Measurement properties of visual analogue scale, numeric rating scale, and pain severity subscale of the brief pain inventory in patients with low back pain: a systematic review. J Pain. 2019;20:245–263. doi: 10.1016/j.jpain.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 62.Cho S., Kim Y.J., Lee M., Woo J.H., Lee H.J. Cut-off points between pain intensities of the postoperative pain using receiver operating characteristic (ROC) curves. BMC Anesthesiol. 2021;21:29. doi: 10.1186/s12871-021-01245-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.De Vet H.C., Terwee C.B., Mokkink L.B., Knol D.L. Cambridge University Press; Cambridge: 2011. Measurement in medicine: a practical guide. [Google Scholar]
- 64.Wells G., Beaton D., Shea B., et al. Minimal clinically important differences: review of methods. J Rheumatol. 2001;28:406–412. [PubMed] [Google Scholar]
- 65.Tubach F., Ravaud P., Baron G., et al. Evaluation of clinically relevant states in patient reported outcomes in knee and hip osteoarthritis: the patient acceptable symptom state. Ann Rheum Dis. 2005;64:34–37. doi: 10.1136/ard.2004.023028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lapkin S., Ellwood L., Diwan A., Fernandez R. Reliability, validity, and responsiveness of multidimensional pain assessment tools used in postoperative adult patients: a systematic review of measurement properties. JBI Evid Synth. 2021;19:284–307. doi: 10.11124/JBISRIR-D-19-00407. [DOI] [PubMed] [Google Scholar]
- 67.Boonstra A.M., Preuper H.R.S., Balk G.A., Stewart R.E. Cut-off points for mild, moderate, and severe pain on the visual analogue scale for pain in patients with chronic musculoskeletal pain. Pain. 2014;155:2545–2550. doi: 10.1016/j.pain.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 68.Serlin R.C., Mendoza T.R., Nakamura Y., Edwards K.R., Cleeland C.S. When is cancer pain mild, moderate or severe? Grading pain severity by its interference with function. Pain. 1995;61:277–284. doi: 10.1016/0304-3959(94)00178-H. [DOI] [PubMed] [Google Scholar]
- 69.Zelman D.C., Hoffman D.L., Seifeldin R., Dukes E.M. Development of a metric for a day of manageable pain control: derivation of pain severity cut-points for low back pain and osteoarthritis. Pain. 2003;106:35–42. doi: 10.1016/s0304-3959(03)00274-4. [DOI] [PubMed] [Google Scholar]
- 70.Blumstein H.A., Moore D. Visual analog pain scores do not define desire for analgesia in patients with acute pain. Acad Emerg Med. 2003;10:211–214. doi: 10.1111/j.1553-2712.2003.tb01992.x. [DOI] [PubMed] [Google Scholar]
- 71.Voepel-Lewis T., Burke C.N., Jeffreys N., Malviya S., Tait A.R. Do 0–10 numeric rating scores translate into clinically meaningful pain measures for children? Anesth Analg. 2011;112:415–421. doi: 10.1213/ANE.0b013e318203f495. [DOI] [PubMed] [Google Scholar]
- 72.Gan T., Lubarsky D., Flood E., et al. Patient preferences for acute pain treatment. Br J Anaesth. 2004;92:681–688. doi: 10.1093/bja/aeh123. [DOI] [PubMed] [Google Scholar]
- 73.Miaskowski C. The impact of age on a patient's perception of pain and ways it can be managed. Pain Manag Nurs. 2000;1:2–7. doi: 10.1053/jpmn.2000.9760. [DOI] [PubMed] [Google Scholar]
- 74.Wilkie D.J., Savedra M.C., Holzemer W.L., Tesler M.D., Paul S.M. Use of the McGill Pain Questionnaire to measure pain: a meta-analysis. Nurs Res. 1990;39:36–41. [PubMed] [Google Scholar]
- 75.Levy N., Quinlan J., El-Boghdadly K., et al. An international multidisciplinary consensus statement on the prevention of opioid-related harm in adult surgical patients. Anaesthesia. 2021;76:520–536. doi: 10.1111/anae.15262. [DOI] [PubMed] [Google Scholar]
- 76.White P.F., Kehlet H. Improving postoperative pain management: what are the unresolved issues? Anesthesiology. 2010;112:220–225. doi: 10.1097/ALN.0b013e3181c6316e. [DOI] [PubMed] [Google Scholar]
- 77.Tong Y.G., Konstantatos A.H., Yan C., Ling C. Improving pain management through addition of the functional activity score. Aust J Adv Nurs. 2018;35:52–60. [Google Scholar]
- 78.Halm M., Bailey C., St Pierre J., et al. Pilot evaluation of a functional pain assessment scale. Clin Nurse Spec. 2019;33:12–21. doi: 10.1097/NUR.0000000000000416. [DOI] [PubMed] [Google Scholar]
- 79.Pasero C., McCaffery M. Mosby; St. Louis, MO: 2010. Pain assessment and pharmacologic management. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.