Abstract
Background:
Malaria infection poses a significant risk in pregnancy, yet chemoprophylaxis for pregnant women is limited. A systematic review was conducted to evaluate the incidence of adverse outcomes after atovaquone-proguanil (AP) exposure during pregnancy.
Methods:
Following PRISMA guidelines, the authors searched PubMed, MEDLINE, and the Malaria in Pregnancy Consortium Library to identify relevant literature including infant outcomes after exposure to atovaquone, proguanil, or AP in pregnancy. Two authors independently screened the titles, abstracts, and full texts, and extracted data into an EpiInfo database. Overall proportions and 95% confidence intervals of adverse outcomes were determined by pooling data across studies.
Results:
Of 455 records identified, 16 studies were included: ten AP studies and six proguanil studies. The overall proportions and 95% confidence intervals (CI) of adverse outcomes reported for the 446 women exposed to AP include miscarriage (8.08% CI: 5.07, 12.08%), stillbirth (1.05% CI: 0.03, 5.73%), early neonatal death (0% CI: 0, 7.4%), and congenital anomalies (2.56% CI: 1.28, 4.53%).
Conclusions:
The limited available data suggest that outcomes following AP exposure during pregnancy are similar to expected rates in similar populations. AP may be a promising option for pregnant women, but further data are needed on its safety in pregnancy.
Keywords: Antimalarials, Plasmodium, Chemoprophylaxis, Humans, Female, Infant, Pregnancy, Newborns
1. Introduction
Pregnant women are at increased risk of complications after malaria infection [1]. Non-immune pregnant travelers are particularly susceptible to adverse outcomes following malaria infection, and, if possible, are advised to avoid travel to malaria-endemic regions [2]. When travel cannot be avoided, women are encouraged to adhere to an effective chemoprophylaxis regimen [3].
Many of the antimalarials currently recommended for prophylaxis, such as doxycycline and primaquine, are contraindicated in pregnancy [4,5]. Currently, only two chemoprophylaxis options are recommended for use in pregnancy− chloroquine and mefloquine [6]. Widespread resistance among Plasmodium falciparum parasites to chloroquine has restricted its use to limited geographic areas; thus, mefloquine is often the only available option for chemoprophylaxis. In some areas of Southeast Asia there is resistance to mefloquine, leaving no available safe and effective option for pregnant women [3].
Atovaquone-proguanil (AP, Malarone®) is a drug combination that is recommended for effective malaria prophylaxis and treatment in non-pregnant travelers in regions with resistance to other anti-malarials [7]. Despite its efficacy, limited data on the safety of AP in pregnancy has prevented the drug from being recommended for use during pregnancy [3]. Although historically, proguanil has been used in other countries for the prevention and treatment of malaria in pregnancy, the United States Food and Drug Administration (FDA) cautions against its use in pregnancy due to insufficient controlled studies of its use in pregnant women [8–12]. Unfortunately, proguanil is less effective alone than when used in combination with atovaquone for the treatment of malaria [8]. Atovaquone has been used alone and in combination with azithromycin for the treatment of babesiosis among pregnant women, but due to limited data on its safety it is also not recommended for use in pregnancy by FDA [13,14]. AP is not teratogenic in rats or rabbits at plasma concentrations which correspond to the estimated human exposure during malaria treatment. However, no well-controlled human studies have assessed teratogenicity of AP in pregnant women; thus, the FDA recommends that pregnant women only use AP if the potential benefit outweighs the risk to the fetus [12,15].
Given the need for an effective chemoprophylaxis regimen for pregnant women in areas with chloroquine or mefloquine-resistant malaria, and the dearth of information on the safety of AP use in pregnancy, we conducted a systematic review to assess the risk of adverse pregnancy outcomes or birth defects after exposure to AP at any time point in pregnancy. To our knowledge, no systematic review has reported on pregnancy outcomes after AP exposure, and this report synthesizes the available literature on the topic.
2. Methods
A systematic literature search was performed according to PRISMA guidelines on July 19, 2018 to identify studies in which pregnant women were exposed to atovaquone-proguanil, or to atovaquone or proguanil monotherapy. We searched Pubmed, MEDLINE, and the Malaria in Pregnancy (MiP) Consortium Library, a comprehensive dataset of published and unpublished literature on MiP, with the search terms ((pregnan* OR matern* OR gravid*) AND (malarone OR atovaquone OR proguanil)). Studies were included if they reported pregnancy outcomes after maternal exposure to atovaquone-proguanil, atovaquone, or proguanil in pregnancy. Reviews or studies that lacked primary data were excluded from the analysis, but references of these articles were manually reviewed to identify additional relevant literature. Additionally, studies were excluded if the treatment group did not receive AP, atovaquone, or proguanil (intervention), and if the study did not present adverse infant outcomes (outcomes). Data were extracted by two independent reviewers into Epi Info™ 7 (Atlanta, GA) and then uploaded to SAS v9.3 (Cary, NC) and MS Excel 2016 (Seattle, WA) for comparison and analysis. The analysis was completed with and without single case reports, as it was postulated that case reports may have bias towards reporting adverse outcomes. The proportions and 95% confidence interval of miscarriage, stillbirth, early neonatal death (< 7 days), and congenital anomalies among pregnant women who received AP or proguanil were calculated using Excel and OpenEpi (Atlanta, GA) [16]. Due to the heterogeneity in study designs, a meta-analysis could not be conducted; however, STATA version 14 (Stata-Corp LLC, College Station, TX) was used to calculate odds ratios for the risk of adverse events among randomized clinical trials and generate forest plots.
3. Results
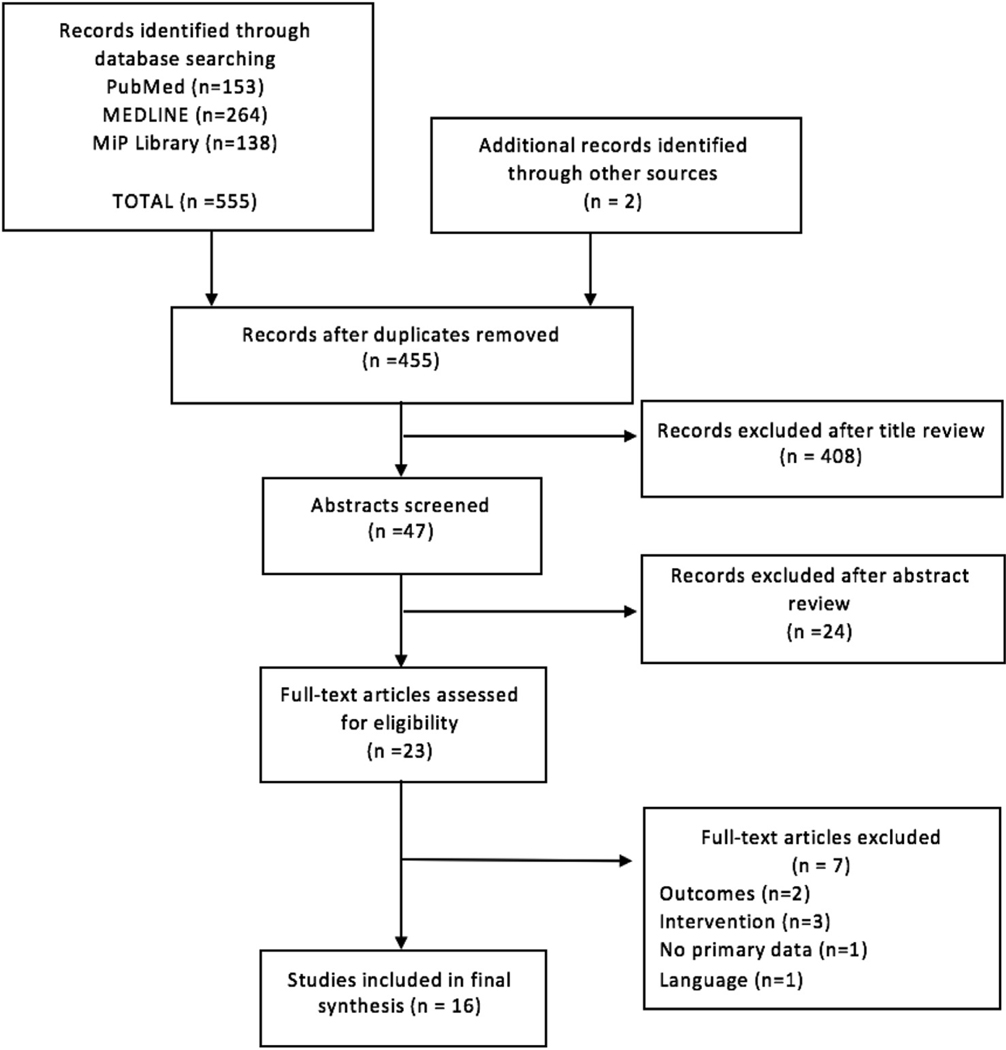
The database search identified 555 records; 455 remained after removal of duplicates (Fig. 1). Following title and abstract screening, the full text of 23 studies was reviewed; 7 were excluded due to a lack of primary data, or intervention/outcome of interest. Of the 16 relevant studies included in the final analysis, there were three cohort studies, five clinical trials, five case series, and three case reports, including 17 discrete populations of women among the included studies. Ten studies, (including 11 discrete populations) reported on infant outcomes following AP exposure in pregnancy, and six studies reported on proguanil (Table 1). There were no studies which reported outcomes after maternal exposure to atovaquone monotherapy. Of the 16 included studies, 56% (9/16) studied populations in endemic areas, and 44% (7/16) reported on traveler groups.
Fig. 1.
PRISMA diagram.
Table 1.
Summary and description of 16 studies included after systematic review.
| Publication | Location | Design | Druga | Description | Total Enrolled | Miscarriage (n/N) | Still-birth (n/N) | Early Death (n/N) | Cong. Anomaly (n/N) |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| McGready et al, 2005 [18] | Thailand | Clinical Trial | AP | Women receiving artesunate-atovaquone-proguanil (AAP) in the 2nd and 3rd trimester | 39 | 0/34 | 1/34 | 2/34 | |
| Tan et al, 2018 [43] | USA | Cohort | AP | Questionnaire distributed to women detailing birth outcome after exposure to prophylaxis at any point in pregnancy | 10 | 0/10 | 0/10 | 0/10 | |
| Pasternak et al, 2011 [2] | Denmark | Cohort | AP | Retrospective study using Danish Medical Birth Register to evaluate outcome after AP exposure during 1st trimester | 149 | 2/149 | |||
| Reuvers et al, 2012 [17] | European countries | Case Series | AP | Case series using data from six centers in the European Network of Teratology Information services detailing outcomes after AP use in 1st trimester | 165 | 21/165 | 7/165 | ||
| McGready et al, 2003 [44] | Thailand | Case Series | AP | Women receiving AP at any time in pregnancy | 27 | 0/27 | 0/27 | 0/27 | 0/27 |
| McGready et al, 2003 [45] | Thailand | Case Series | AP | Women receiving artesunate-atovaquone-proguanil (AAP) in 2nd or 3rd trimester of pregnancy | 27 | 0/21 | 0/21 | 0/21 | 0/21 |
| Na-Bangchang et al, 2005 [46] | Zambia Thailand |
Case Series Case series |
AP AP |
Women in 3rd trimester of pregnancy treated with AP after acute, uncomplicated mono-infection with P. falciparum Pregnant women in 3rd trimester treated with AP after acute, uncomplicated mono-infection with P. falciparum |
18 8 |
0/16 0/6 |
|||
| Kaser et al, 2015 [47] | Multiple | Case Report | AP | Single case report of woman from non-endemic country who received AP prophylaxis in 2nd trimester | 1 | 0/1 | 0/1 | 0/1 | |
| de Lima Corvino et al, 2018 [48] | Malawi | Case Report | AP | Single case report of traveler in Malawi with accidental exposure to AP after conception | 1 | 0/1 | 0/1 | 0/1 | |
| Krekora et al, 2017 [49] | Tanzania | Case Report | AP | Single case report of traveler in Tanzania who took AP after conception | 1 | 0/1 | 0/1 | ||
| Mutabingwa et al, 1993 [19] | Tanzania | Clinical Trial | Proguanil | Pregnant women received therapy with proguanil alone (N = 153) or proguanil plus chloroquine (N = 115 at anytime during pregnancy | 268 | 2/214b | 1/214b | ||
| Fleming, A., 1990 [20] | Nigeria | Clinical Trial | Proguanil | Women randomized to receive proguanil before or during 2nd trimester. | 160 | 6/134 | |||
| Kasso et al, 2012 [21] | Nigeria | Clinical Trial | Proguanil | Women at an unknown point in pregnancy randomized to receive proguanil while attending antenatal clinic at the University of Port Harcourt Teaching Hospital | 175 | 0/139 | 0/139 | 0/139 | 0/139 |
| Mutabingwa et al, 2009 [22] | Tanzania | Clinical Trial | Proguanil | Pregnant women randomized to receive chlorproguanil-dapsone | 81 | 1/74 | 5/74 | 13/74 | |
| Phillips-Howard et al, 1998 [50] | Many | Cohort | Proguanil | Questionnaire given to pregnant travelers who took a prophylactic drug during the 1st trimester | 118 | 9/118 | 2/118 | ||
| Bouvier et al, 1997 [51] | Mali | Case Series | Proguanil | Woman received proguanil from onset of pregnancy until delivery | 309 | 6/302 | 26/302 | ||
AP = Atovaquone-proguanil.
At the time the outcome was assessed, there were 124 women who received proguanil alone and 90 who received proguanil plus chloroquine.
Of the 1557 women exposed to atovaquone-proguanil and proguanil in this review, the proportion of pregnancies ending in miscarriage after exposure to AP and proguanil was 8.08% (21/260, CI: 5.07,12.08%) and 2.99% (23/768, CI: 1.91,4.46%), respectively (Table 2). Of the eight AP studies reporting miscarriage data, one study reported 21 cases, and the remaining seven studies reported zero miscarriages amongst a collective sample size of 95 women [17]. Three AP publications did not report miscarriage rates. After AP exposure, the proportion of pregnancies ending in stillbirth was 1.05% (1/95, CI: 0.03, 5.73%) and early neonatal death was 0% (0/48, CI: 0, 7.4%), respectively. The proportion of congenital anomalies after exposure to AP and proguanil monotherapy was 2.56% (11/430, CI: 1.28, 4.53%) and 4.53% (15/331, CI: 2.56, 7.36%), respectively. There were no significant differences in the proportions of adverse events when single case reports were excluded from the analysis.
Table 2.
Summary of proportions of adverse outcomes reported for atovaquone-proguanil and proguanil.
| Summary of adverse outcomes by drug | ||||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Atovaquone-Proguanil | Proguanil | |||||||
|
|
||||||||
| Miscarriage | Stillbirth | Early Death | Congenital Anomaly | Miscarriage | Stillbirth | Early Death | Congenital Anomaly | |
|
| ||||||||
| n/N Percentage (95% CI) | 21/260 8.08 (5.07, 12.08) | 1/95 1.05 (0.03, 5.73) | 0/48 0 (0, 7.4) | 11/430 2.56 (1.28, 4.53) | 23/907 2.53 (1.61, 3.78) | 2/427 0.47 (0.06, 1.68) | 31/515 6.02 (4.13, 8.43) | 15/331 4.53 (2.56, 7.36) |
When considering only results from the five randomized clinical trials (Table 3), only one of which included AP, neither AP nor proguanil, either alone or in other combinations, were associated with a statistically significant increase in congenital anomalies (Fig. 2a), death (Fig. 2b), stillbirth (Fig. 2c.), or miscarriage (Fig. 2d.), though the numbers for all were extremely small.
Table 3.
Summary of outcomes from the five clinical trials included in the review.
| Publication | Location | Intervention (N enrolled) | Comparatora (N enrolled) | Atovaquone-Proguanil OR Proguanil-containing regimen (n/N) | Comparator (n/N) a | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Miscarriage | Still-birth | Early Death | Cong. Anomaly | Miscarriage | Still-birth | Early Death | Cong. Anomaly | ||||
|
| |||||||||||
| McGready et al, 2005 [18] | Thailand | Atovaquone-proguanil (N = 39) | Quinine (N = 42) | 0/34 | 1/34 | NA | 2/34 | 0/38 | 0/38 | NA | 1/38 |
| Mutabingwa et al, 1993 [19] | Tanzania | Proguanil alone (N = 124) or proguanil plus chloroquine (N = 90) | Chloroquine (N = 155) | 2/214 | 1/214 | NA | NA | 0/113 | 0/113 | NA | NA |
| Fleming, A., 1990 [20] | Nigeria | Proguanil (N = 160) | Placebo(N = 40) | 6/134 | NA | NA | NA | 0/32 | NA | NA | NA |
| Kasso et al, 2012 [21] | Nigeria | Proguanil (N = 175) | Sulphadoxine-pyrimethamine (N = 175) | 0/139 | 0/139 | 0/139 | 0/139 | 0/142 | 0/142 | 0/142 | 0/142 |
| Mutabingwa et al, 2009 [22] | Tanzania | Chlorproguanil-Dapsone (N = 81) | Sulphadoxine-Pyrimethamine (N = 28) SP + amodiaquine (N = 80) Artesunate + Amodiaquine (N = 83) |
0/74 | 1/74 | 5/74 | 13/74 | 0/26 0/75 0/79 |
1/26 1/75 4/79 |
1/26 2/75 0/79 |
6/26 14/75 15/79 |
The comparator outcomes correspond to the drug regimen defined in the first comparator column.
Fig. 2.
a. Congenital anomalies. b. Death. c. Stillbirth. d. Miscarriage.
4. Discussion
This review highlights the paucity of available data from the 446 women exposed to AP to evaluate the safety of AP for use in pregnant women. There is only one study which directly compares birth outcomes following in utero atovaquone-proguanil exposure to another antimalarial, specifically, quinine; this study found no significant difference in adverse birth outcomes (5.9% AP vs 2.6% QN, p = 0.599), though the numbers were small [18]. There were four studies comparing outcomes following proguanil exposure to placebo or another antimalarial (chloroquine, sulfadoxine-pyrimethamine (SP), artesunate-amodiaquine, SP-amodiaquine), none of which found a significantly increased risk of miscarriage, stillbirth, or congenital anomaly associated with in utero exposure to proguanil, though the numbers are very small [19–22]. Thus, the limited available data on adverse birth outcomes and birth defects after AP exposure suggests that such outcomes are rare.
Reported rates of miscarriage range from 11 to 22% among women who know that they are pregnant, regardless of malaria endemicity [23–26]. Rates of stillbirth range from 1.5 to 10.6% in malaria endemic areas, but are lower, around 1% or less, in non-endemic countries such as the US and Europe [26–29]. It is well established that the rates of miscarriage are highest early in pregnancy and then fall after 16 weeks [23]. A study in a rural area of Kenya with high malaria and HIV prevalence followed women from conception to delivery and found a probability of miscarriage of 18.9% by 28 weeks gestation [23]. The overall miscarriage rate was similarly high (20%) amongst refugee women followed weekly on the malaria endemic Thai-Burmese border [26]. The incidence of miscarriage was higher in women who had a single episode of malaria in their first trimester (34%) than women unaffected by malaria in the same region (19%) [26]. The miscarriage rate of 8.08% found in this review is therefore well within the expected rate in similar epidemiological settings. Furthermore, all of the miscarriages found in this review were from one study evaluating miscarriage following 1st trimester exposures among European travelers, and no data on outcomes of unexposed pregnancies nor pregnancies exposed to other antimalarials were reported, so no comparisons could be made within this population [17].
Pregnant women with malaria are at increased risk of stillbirth; it is estimated that antenatal P. falciparum malaria infection may cause 12–20% of stillbirths in Africa [30]. Rates of stillbirths in malaria endemic areas of sub-Saharan Africa range from 1.5% to 10.6% and 0.9%–6.9% in primigravid and multigravid women, respectively, while much lower rates (1.2–2.6%) have been reported in Southeast Asia [27,31]. Average stillbirth rates in southern Asia and sub-Saharan Africa are reported to be 2.55% and 2.87%, respectively [32]. The available data suggests that there does not appear to be an increased risk of stillbirth following exposure to malarone.
Neonates in developing countries are exceptionally vulnerable in their first seven days of life as 73% of neonatal deaths occur during the first week of life [33]. WHO reported an aggregate neonatal mortality rate of 27.2 and 22.6 deaths per 1000 live births in Sub-Saharan Africa and Southeast Asia, respectively [34]. Early neonatal mortality rates at the Shoklo Malaria Research unit on the Thailand-Myanmar border were reported to be 6.6 per 1000 live births [35]. Among 27 women from this population exposed to AP in pregnancy, there were no reported early neonatal deaths. Given no reported early neonatal deaths in a relatively small sample size, the statistical rule of three (3/n estimates the upper end of the 95% confidence interval when there are zero events in a small sample size) was used to estimate the upper bounds of probability of early neonatal deaths in the population at 6.25%, which falls within the expected rate [36]. Thus, there does not appear to be an increased risk of early neonatal death following in utero exposure to malarone.
The incidence of congenital anomalies varies world-wide, and due to the absence of birth defect registries in many parts of the developing world, it is difficult to ascertain incidence [37]. Approximately 7% of all live births result in a congenital anomaly, though reported rates range from 30.3 per 1000 live births in the U.S. to 73.5 per 1000 births in Nigeria [38–40]. Studies from Thailand and Uganda found the incidence rate for major anomalies to be 26.12 and 20.3 per 1000 live births, respectively [41,42]. Due to our small sample size, the confidence interval around our proportion is large; however, it is well within the range of expected rates.
Though intentional exposure to AP is not recommended in pregnancy, inadvertent exposure may occur when a woman is on prophylaxis prior to conception. A retrospective cohort study among women physicians and employees of the Centers for Disease Control and Prevention found that unintentional exposure to AP occurred in 1.2% of women [43]. This highlights the importance of developing better registries to capture the outcomes of inadvertent exposures, as well as the need for additional research on the safety of AP in pregnancy. There were numerous limitations to this review. First, the small pooled sample size limits the strength of the evidence. Second, there was not enough consistency in study designs, and only four of the studies were randomized clinical trials, precluding a meta-analysis or comparison to other drugs. Third, due to the paucity of data, single case reports and case series were included in the analysis, despite a likely bias towards reporting adverse outcomes. However, the results were not significantly different when the case reports were excluded. Finally, there was limited geographic variation in the analysis as most of the AP studies were from the Thai-Burmese border.
5. Conclusion
The limited available data suggests that the rates of adverse events after AP exposure during pregnancy are not higher than the expected rates in similar populations, suggesting that AP may be a promising option for malaria prophylaxis in pregnant women. There is a pressing need for studies to evaluate the safety of AP in pregnancy. Though this literature review may not be sufficient to support interventional studies, additional cohort studies from existing data sources should be conducted to quantify the impact of exposure of AP on maternal and fetal outcomes.
Acknowledgments
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Abbreviations
- AP
Atovaquone-proguanil
- CI
Confidence interval
- MiP
Malaria in Pregnancy
- FDA
United States Food and Drug Administration
Footnotes
Disclaimer
The views expressed in this report are those of the authors and do not necessarily represent the official position of the U.S. Centers for Disease Control and Prevention.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.tmaid.2019.01.008.
References
- [1].Desai M, ter Kuile FO, Nosten F, McGready R, Asamoa K, Brabin B, et al. Epidemiology and burden of malaria in pregnancy. Lancet Infect Dis 2007;7:93–104. 10.1016/S1473-3099(07)70021-X. [DOI] [PubMed] [Google Scholar]
- [2].Pasternak B, Hviid A. Atovaquone-proguanil use in early pregnancy and the risk of birth defects. Arch Intern Med 2011;171:259–60. 10.1001/archinternmed.2010.521. [DOI] [PubMed] [Google Scholar]
- [3].Pregnant Travelers - Chapter 8 – 2018 Yellow Book | Travelers’ Health | CDC n.d. https://wwwnc.cdc.gov/travel/yellowbook/2018/advising-travelers-with-specific-needs/pregnant-travelers (accessed July 23, 2018).
- [4].Tan KR, Magill AJ, Parise ME, Arguin PM. Centers for Disease Control and Prevention. Doxycycline for malaria chemoprophylaxis and treatment: report from the CDC expert meeting on malaria chemoprophylaxis. Am J Trop Med Hyg 2011;84:517–31. 10.4269/ajtmh.2011.10-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hill DR, Baird JK, Parise ME, Lewis LS, Ryan ET, Magill AJ. Primaquine: report from CDC expert meeting on malaria chemoprophylaxis I. Am J Trop Med Hyg 2006;75:402–15. [PubMed] [Google Scholar]
- [6].CDC. CDC - malaria - travelers - choosing a drug to prevent malaria. 2018. https://www.cdc.gov/malaria/travelers/drugs.html, Accessed date: 25 July 2018.
- [7].Looareesuwan S, Viravan C, Webster HK, Kyle DE, Hutchinson DB, Canfield CJ. Clinical studies of atovaquone, alone or in combination with other antimalarial drugs, for treatment of acute uncomplicated malaria in Thailand. Am J Trop Med Hyg 1996;54:62–6. [DOI] [PubMed] [Google Scholar]
- [8].Roggelin L, Cramer JP. Malaria prevention in the pregnant traveller: a review. Trav Med Infect Dis 2014;12:229–36. 10.1016/j.tmaid.2014.04.007. [DOI] [PubMed] [Google Scholar]
- [9].Boggild AK, Parise ME, Lewis LS, Kain KC. Atovaquone-proguanil: report from the CDC expert meeting on malaria chemoprophylaxis (II). Am J Trop Med Hyg 2007;76:208–23. [PubMed] [Google Scholar]
- [10].Ward SA, Sevene EJP, Hastings IM, Nosten F, McGready R. Antimalarial drugs and pregnancy: safety, pharmacokinetics, and pharmacovigilance. Lancet Infect Dis 2007;7:136–44. 10.1016/S1473-3099(07)70025-7. [DOI] [PubMed] [Google Scholar]
- [11].Eriksson B, Björkman A, Keisu M. How safe is proguanil? A post-marketing investigation of side-effects. Scand J Infect Dis 1991;23:489–93. 10.3109/00365549109075098. [DOI] [PubMed] [Google Scholar]
- [12].FDA. Malarone Prescribing Information n.d. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/021078s022lbl.pdf (accessed July 30, 2018).
- [13].Petersen E. Toxoplasmosis. Semin Fetal Neonatal Med 2007;12:214–23. 10.1016/j.siny.2007.01.011. [DOI] [PubMed] [Google Scholar]
- [14].CDC. CDC - babesiosis - resources for health professionals. 2018. https://www.cdc.gov/parasites/babesiosis/health_professionals/index.html, Accessed date: 23 July 2018.
- [15].GlaxoSmithKline. Product information, Malarone (atovaquone and proguanil hydrochloride). https://gsksource.com/pharma/content/gsk/source/us/en/brands/malarone.html (accessed July 23, 2018).
- [16].Dean A, Sullivan K, Soe M. OpenEpi: Open Source Epidemiologic Statistics for Public Health. https://www.openepi.com/Menu/OE_Menu.htm (accessed July 23, 2018). [Google Scholar]
- [17].Reuvers N, Vial T, Schaefer C, Elefant E, Santis M, Malm H, et al. Pregnancy outcome after first trimester exposure to malarone (Atovaquone-Proguanil): a prospective case-series. Birth Defects Res A Clin Mol Teratol 2012;94:329. 329. [Google Scholar]
- [18].McGready R, Ashley EA, Moo E, Cho T, Barends M, Hutagalung R, et al. A randomized comparison of artesunate-atovaquone-proguanil versus quinine in treatment for uncomplicated falciparum malaria during pregnancy. J Infect Dis 2005;192:846–53. 10.1086/432551. [DOI] [PubMed] [Google Scholar]
- [19].Mutabingwa TK, Malle LN, de Geus A, Oosting J. Malaria chemosuppression in pregnancy. II. Its effect on maternal haemoglobin levels, placental malaria and birth weight. Trop Geogr Med 1993;45:49–55. [PubMed] [Google Scholar]
- [20].Fleming AF, Ghatoura GB, Harrison KA, Briggs ND, Dunn DT. The prevention of anaemia in pregnancy in primigravidae in the Guinea savanna of Nigeria. Ann Trop Med Parasitol 1986;80:211–33. [DOI] [PubMed] [Google Scholar]
- [21].Kasso T, Jeremiah I, John CT. Proguanil versus sulphadoxine-pyrimethamine for malaria chemoprophylaxis in pregnancy: a randomised controlled trial. J Clin Trials 2012;2:122. 10.4172/2167-0870.1000122. [DOI] [Google Scholar]
- [22].Mutabingwa TK, Muze K, Ord R, Briceno M, Greenwood BM, Drakeley C, et al. Randomized trial of artesunate+amodiaquine, sulfadoxine-pyrimethamine+amodiaquine, chlorproguanal-dapsone and SP for malaria in pregnancy in Tanzania. PLoS One 2009;4:e5138. 10.1371/journal.pone.0005138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Dellicour S, Aol G, Ouma P, Yan N, Bigogo G, Hamel MJ, et al. Weekly miscarriage rates in a community-based prospective cohort study in rural western Kenya. BMJ Open 2016;6:e011088 10.1136/bmjopen-2016-011088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ammon Avalos L, Galindo C, Li DK. A systematic review to calculate background miscarriage rates using life table analysis. Birth Defects Res Part A Clin Mol Teratol 2012;94:417–23. 10.1002/bdra.23014. [DOI] [PubMed] [Google Scholar]
- [25].Bardos J, Hercz D, Friedenthal J, Missmer SA, Williams Z. A national survey on public perceptions of miscarriage. Obstet Gynecol 2015;125(6):1313–20. 10.1097/AOG.0000000000000859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].McGready R, Lee S, Wiladphaingern J, Ashley E, Rijken M, Boel M, et al. Adverse effects of falciparum and vivax malaria and the safety of antimalarial treatment in early pregnancy: a population-based study. Lancet Infect Dis 2012;12:388–96. 10.1016/S1473-3099(11)70339-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Mutabingwa TK. Malaria and pregnancy: epidemiology, pathophysiology and control options. Acta Trop 1994;57:239–54. 10.1016/0001-706X(94)90070-1. [DOI] [PubMed] [Google Scholar]
- [28].American College of Obstetricians and Gynecologists. ACOG practice bulletin. Management of recurrent pregnancy loss. Number 24, February 2001. (Replaces technical bulletin number 212, september 1995). American College of obstetricians and gynecologists. Int J Gynaecol Obstet Off Organ Int Fed Gynaecol Obstet 2002;78:179–90. [DOI] [PubMed] [Google Scholar]
- [29].Zeitlin J, Mortensen L, Cuttini M, Lack N, Nijhuis J, Haidinger G, et al. Declines in stillbirth and neonatal mortality rates in Europe between 2004 and 2010: results from the Euro-Peristat project. J Epidemiol Community Health 2016;70:609–15. 10.1136/jech-2015-207013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Moore KA, Simpson JA, Scoullar MJL, McGready R, Fowkes FJI. Quantification of the association between malaria in pregnancy and stillbirth: a systematic review and meta-analysis. Lancet Glob Health 2017;5:e1101–12. 10.1016/S2214-109X(17)30340-6. [DOI] [PubMed] [Google Scholar]
- [31].Blencowe H, Cousens S, Jassir FB, Say L, Chou D, Mathers C, et al. National, regional, and worldwide estimates of stillbirth rates in 2015, with trends from 2000: a systematic analysis. Lancet Glob Health 2016;4:e98–108. 10.1016/S2214-109X(15)00275-2. [DOI] [PubMed] [Google Scholar]
- [32].Taylor SM, Kuile FO ter. Stillbirths: the hidden burden of malaria in pregnancy. Lancet Glob Health 2017;5. 10.1016/S2214-109X(17)30378-9. e1052–3. [DOI] [PubMed] [Google Scholar]
- [33].UNICEF. Commiting to child survival: a promise renewed progress report 2014. 2014. http://files.unicef.org/publications/files/APR_2014_web_15Sept14.pdf, Accessed date: 1 August 2018.
- [34].WHO | Global Strategy for Women’s, Children’s and Adolescents’ Health (2016–2030). WHO; n.d. http://apps.who.int/gho/data/node.gswcah (accessed August 1, 2018). [Google Scholar]
- [35].Janet S, Carrara VI, Simpson JA, Thin NWW, Say WW, Paw NTM, et al. Early neonatal mortality and neurological outcomes of neonatal resuscitation in a resource-limited setting on the Thailand-Myanmar border: a descriptive study. PLoS One 2018;13:e0190419 10.1371/journal.pone.0190419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Jovanovic BD, Levy PS. A look at the rule of three. Am Statistician 1997;51:137–9. 10.2307/2685405. [DOI] [Google Scholar]
- [37].Mashuda F, Zuechner A, Chalya PL, Kidenya BR, Manyama M. Pattern and factors associated with congenital anomalies among young infants admitted at Bugando medical centre, Mwanza, Tanzania. BMC Res Notes 2014;7:195. 10.1186/1756-0500-7-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Christianson A, Howson CP, Modell B. Global report on birth defects. March of Dimes 2006:76. https://www.marchofdimes.org/global-report-on-birth-defects-the-hidden-toll-of-dying-and-disabled-children-full-report.pdf (accessed August 1, 2018). [Google Scholar]
- [39].CDC. Data and statistics | birth defects | NCBDDD | CDC. Cent Dis Control Prev; 2018. https://www.cdc.gov/ncbddd/birthdefects/data.html, Accessed date: 30 July 2018. [Google Scholar]
- [40].Howson CP, Christianson A, Modell B. Controlling Birth Defects: Reducing the Hidden Toll of Dying and Disabled Children in Low-Income Countries 2008. https://preparingforlife.net/media/uploads/file/bb3b77_256418c8c9604a9386cff8c63031f377.pdf (accessed July 30, 2018). [Google Scholar]
- [41].Pangkanon S, Sawasdivorn S, Kuptanon C, Chotigeat U, Vandepitte W. Establishing of national birth defects registry in Thailand. J Med Assoc Thail Chotmaihet Thangphaet 2014;97(Suppl 6):S182–8. [PubMed] [Google Scholar]
- [42].Ndibazza J, Lule S, Nampijja M, Mpairwe H, Oduru G, Kiggundu M, et al. A description of congenital anomalies among infants in Entebbe, Uganda. Birt Defects Res A Clin Mol Teratol 2011;91:857–61. 10.1002/bdra.20838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Tan KR, Fairley JK, Wang M, Gutman JR. A survey on outcomes of accidental atovaquone-proguanil exposure in pregnancy. Malar J 2018;17:198. 10.1186/s12936-018-2352-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].McGready R, Keo NK, Villegas L, White NJ, Looareesuwan S, Nosten F. Artesunate-atovaquone-proguanil rescue treatment of multidrug-resistant Plasmodium falciparum malaria in pregnancy: a preliminary report. Trans R Soc Trop Med Hyg 2003;97:592–4. [DOI] [PubMed] [Google Scholar]
- [45].McGready R, Stepniewska K, Edstein MD, Cho T, Gilveray G, Looareesuwan S, et al. The pharmacokinetics of atovaquone and proguanil in pregnant women with acute falciparum malaria. Eur J Clin Pharmacol 2003;59:545–52. 10.1007/s00228-003-0652-9. [DOI] [PubMed] [Google Scholar]
- [46].Na-Bangchang K, Manyando C, Ruengweerayut R, Kioy D, Mulenga M, Miller GB, et al. The pharmacokinetics and pharmacodynamics of atovaquone and proguanil for the treatment of uncomplicated falciparum malaria in third-trimester pregnant women. Eur J Clin Pharmacol 2005;61:573–82. 10.1007/s00228-005-0969-7. [DOI] [PubMed] [Google Scholar]
- [47].Kaser AK, Arguin PM, Chiodini PL, Smith V, Delmont J, Jimenez BC, et al. Imported malaria in pregnant women: a retrospective pooled analysis. Trav Med Infect Dis 2015;13:300–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].de Lima Corvino DF, Chandorkar AA, Mora Carpio AL, Climaco A. When epidemiology is the clue to a positive outcome: a case of malaria during pregnancy. Am J Case Rep 2018;19:128–32. 10.12659/AJCR.905543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Krekora M, Zych-Krekora K, Slodki M, Grzesiak M, Respondek-Liberska M. The course of pregnancy and delivery in a patient with malaria. Ginekol Pol 2017;88:574–5. 10.5603/GP.a2017.0103. [DOI] [PubMed] [Google Scholar]
- [50].Phillips-Howard PA, Steffen R, Kerr L, Vanhauwere B, Schildknecht J, Fuchs E, et al. Safety of mefloquine and other antimalarial agents in the first trimester of pregnancy. J Trav Med 1998;5:121–6. [DOI] [PubMed] [Google Scholar]
- [51].Bouvier P, Breslow N, Doumbo O, Robert CF, Picquet M, Mauris A, et al. Seasonality, malaria, and impact of prophylaxis in a West African village. II. Effect on birthweight. Am J Trop Med Hyg 1997;56:384–9. [DOI] [PubMed] [Google Scholar]