Abstract
Background
Fecal metabolomic profiles differ between pediatric inflammatory bowel disease (IBD) patients and controls and may provide new insights in the pathophysiology of IBD. The role of amino acids, however, is not fully elucidated. We aimed to assess fecal amino acid profiles in pediatric IBD.
Methods
In this case-control study, treatment-naïve, newly diagnosed pediatric IBD patients and a non-IBD control group, matched based on sex and age, were included in 2 tertiary centres. Fecal amino acid profiles were assessed using a targeted high-performance liquid chromatography technique. A random forest classifier method was used to develop a prediction model differentiating IBD from controls and predicting IBD phenotype. The association between IBD localization and amino acid concentrations was tested with ordinal regression models.
Results
We included 78 newly diagnosed IBD patients (40 Crohn’s disease [CD], 38 ulcerative colitis [UC]) and 105 controls. Patients with IBD could be differentiated from controls with an accuracy of 82% (sensitivity 63%, specificity 97%). Twenty-nine out of the 42 measured unique amino acids were included in the prediction model. Increased levels of tryptophan, taurine, alanine, ornithine, valine, histidine, and leucine were the most differentiating features. Children with CD and UC could be differentiated from the controls with an accuracy of 80% and 90%, respectively. Inflammatory bowel disease phenotype could not be predicted. Tryptophan, valine, and histidine levels were positively associated with more extended disease in UC patients (P < .05).
Conclusions
Fecal amino acids may enhance understanding of the role of host-microbial interactions in the pathophysiology of IBD and may evolve into biomarkers for pediatric IBD diagnostic and personalized medicine.
Keywords: amino acid analysis, pediatrics, inflammatory bowel disease, metabolomics
Introduction
Inflammatory bowel disease (IBD), including the main phenotypes Crohn’s disease (CD) and ulcerative colitis (UC), is a disorder of the gastrointestinal (GI) tract, characterized by chronic relapsing intestinal inflammation.1 Approximately 25% of patients with IBD present before the age of 20 years,2 and it is forecasted that the largest annual increase in IBD prevalence over the coming decade will be in children.3
Although the exact etiology of IBD remains unclear, a pivotal role of the gut microbiota has been established. Previous studies observed major shifts in gut microbiota composition of patients with IBD, influencing the immune system and metabolic pathways.4–6 There has been a great expansion of microbiota studies, but the field of metabolomics in IBD etiology is relatively new and unexplored. Specifically, fecal metabolomics reports on the interplay between the host, diet, and gut microbiota, complementing sequencing-based microbiota approaches by providing a functional read out of the microbiome.7 Changes in fecal metabolic profiles might evolve into biomarkers, allowing personalized approaches to diagnosis and management of IBD.
A specific interest in the role of amino acids in the etiology and management of IBD has been raised. Amino acids serve as key regulatory factors in cellular and microbial metabolic pathways and have important effects on gut homeostasis.8 Previous studies identified differences in amino acids in the serum,9,10 urine,11 stool,12–14 or mucosa10 of adults with IBD compared with controls. Specifically, patients with IBD showed significantly lower serum levels of the aromatic amino acid tryptophan (TRP) and its metabolites compared with healthy controls, illustrating a potential role as biomarker.15 Several animal studies suggest that TRP and other amino acids could even be a therapeutic target in IBD.8
Only few studies on amino acids in pediatric IBD patients have been performed so far, all characterized by small sample sizes.16–18 Although fecal amino acids seem to be associated with IBD and intestinal inflammation, less is known on its potential to further stratify patients according to disease characteristics such as phenotype or disease localization. In the present study, we aim to assess the potential of fecal amino acids to differentiate de novo IBD from controls, by means of dedicated amino acid analysis (AAA). Secondary aims were to explore differences in amino acid composition between IBD phenotypes and disease localization and determining correlations between inflammatory biomarkers and amino acids levels.
Materials and Methods
Subjects and Methods
This case-control study was performed at the Amsterdam University Medical Centre, locations VUmc and AMC, the Netherlands. Children who were treatment-naïve, were 4-17 years old, and presented with de novo IBD between September 2011 and December 2018 were eligible to participate. Exclusion criteria included proven infectious colitis within the last 3 months prior to inclusion and any use of antibiotics, corticosteroids, or immunosuppressive therapy within the last month prior to inclusion. Included patients undergoing diagnostic endoscopic assessment were asked to collect a fecal sample in a sterile stool container in the week prior to bowel cleansing. The diagnosis of IBD was based on endoscopic, radiologic, and histologic findings according to the revised Porto criteria.19 All pediatric CD and UC patients were classified according to the Paris classification.20 Children with IBD-unclassified (IBD-U) were excluded because the study focused on phenotype-related amino acid profiles. Disease activity at time of diagnosis was assessed by the physician global assessment score (PGA) combined with biomarkers C-reactive protein (CRP), fecal calprotectin (FCP), erythrocyte sedimentation rate (ESR), and albumin. These biomarkers were tested as part of routine diagnostic work-up of IBD, within the interval of maximum 6 weeks before endoscopy.
Controls consisted of 2 subgroups: (1) healthy controls (HC) and (2) controls with GI symptoms. We included individuals as healthy controls from an existing cohort consisting of children age 4-17 years21 matched with the IBD cases by sex and age on a 1:1 ratio. For these HCs, the same exclusion criteria as for the IBD cases were used, extended with GI symptoms fulfilling the Rome IV criteria or functional GI disorders.22 Controls with GI symptoms were patients 4-17 years of age visiting the outpatient clinic of pediatric gastroenterology between October 2016 and December 2018. The exclusion criteria for the controls with GI symptoms were similar to the IBD cases.
Fecal Sample Collection
Sterile plastic stool containers (Stuhlgefäß 10mL, Greiner Bio-One, Frickenhausen, Germany) and an instruction form for collecting and storing the fecal samples were given to all participants. Participants were requested to collect a fecal sample (around 2 grams per sample) and to store this sample directly in the freezer at home at −20°C. The samples were brought under cooled conditions to the clinic within 1 week after collection at home for storage at −20°C.
Targeted Amino Acid Analysis
A targeted high-performance liquid chromatography (HPLC) technique was used to analyze the fecal samples of IBD patients and controls to study the amino acid concentrations in both groups. To homogenize the samples, around 300mg of feces and 1000 μL of distilled water were mixed by vortex for 1 minute. The homogenized samples were recoded and studied by an independent laboratory scientist who was blinded for the diagnosis.
After freezing the samples at –30°C, freeze drying was performed for 24 hours (Christ Alpha 2–4) in order to minimize the risk of bias due to differences in fecal water content. The residual, around 30 to 50mg depending on the fecal consistency, was mixed with a volume of distilled water maintaining a feces-water ratio of 100 mg:5mL. This blend was then homogenized once more by vortex. For the analysis, 400 μL of the blend was pipetted into a filter and centrifuged for 20 minutes at 14,000 g (Hettig Zentrifugen Mikro 2R). The supernatant was subsequently mixed with an internal standard solution with a 1:1 ratio. Ultimately, this blend was centrifuged for 10 minutes and filtered (Whatman, Buckinghamshire, UK) into compatible containers for the final AAA (Biochrome 30). Amino acids were separated using ion-exchange chromatography and were detected by UV absorbance after postcolumn derivatization with ninhydrin.23
Statistical Analysis
Statistical analyses were performed using the Statistical Package for Social Sciences (SPSS, IBM; v26) and with the statistical software R (version 4.0.3); P < .05 was considered to be statistically significant. For normally distributed data, both means and standard deviations were calculated, whereas medians and interquartile ranges (IQR) were calculated for non-normal distributed data. Demographic and clinical characteristics were compared between groups using the non-parametric Mann–Whitney U test. Categorical variables were compared using the χ2 test.
Missing values in the amino acids data set were classified as missing at random (MAR). Multiple imputation (n=5) was used to replace the missing data. For each imputed data set, we computed the median (and IQR) of the amino acids levels and performed Mann–Whitney U tests to identify amino acids that differed significantly between the IBD cases (CD and UC) and controls, between differences in disease localization, and between IBD patients with and without extraintestinal manifestations (EIMs). The P values were corrected for multiple testing with the Benjamini-Hochberg (BH) procedure. Subsequently, the ranges of the median, IQR, and P values across the 5 data sets were determined. Additionally, for each imputed data set, the association between IBD localization and the levels of amino acids was tested with ordinal regression models. The P values were averaged across the 5 data sets. The Pearson rank correlation coefficient was used to compute correlations between the biomarkers (CRP, ESR, albumin, FCP) and clinical parameters including body mass index (BMI) and age, with levels of amino acids. These correlations were separately determined for CD and UC patients, and the P values were averaged across the 5 data sets.
Random forest
Random forest classifiers (with 500 decision trees) were trained to distinguish IBD from controls and to determine the IBD phenotype based on amino acid profiles.24 Random forest is a machine-learning classification algorithm consisting of a large number of individual decision trees that operate as an ensemble. Each imputed data set was divided into a training set consisting of 2/3 of the participants and a test set consisting of the remaining 1/3 of the participants. Five different random forest classifiers were trained and subsequently tested on all test sets, leading to 25 predictions per test set participant. These predictions were summarized to 1 outcome per participant by majority voting.
Ethical Considerations
The study was approved by the Medical Ethical Review Committee (METc) of VU University Medical Centre under file number 2015.393. All participants gave written informed consent before fecal samples and data were collected.
Results
Patient Population and Baseline Characteristics
We included 78 de novo IBD patients (40 CD and 38 UC) and 105 non-IBD controls, consisting of 84 HC and 21 controls with GI symptoms. The patient characteristics of the 183 included children are depicted in Table 1. Amongst the controls with GI symptoms (n=21), 10 patients underwent endoscopic assessment to exclude IBD based on the following symptoms: a combination of abdominal pain and rectal blood loss (n=5), abdominal pain (n=2), isolated rectal blood loss (n=2), and iron deficiency anemia (n=1). The final diagnosis in the controls with GI symptoms is depicted in Supplementary Table 1. Patients with IBD had a significantly lower BMI compared with controls (P = .027). There were no significant differences between CD and UC patients for BMI, FCP, and albumin. Significant higher levels of CRP (P < .001) and ESR (P = .005) were found in CD patients compared with UC patients. Patients with CD were older at time of diagnosis compared with UC patients (P = .034).
Table 1.
Baseline characteristics of the cohort.
| CD (n=40) | UC (n=38) | Controls with GI symptoms (n=21) | HC (n=84) | |
|---|---|---|---|---|
| Age, years, median (IQR) | 14.8 (12.4–15.6) | 13.0 (9.6–15.3) | 13.3 (9.5–16.0) | 11.1 (8.9–14.8) |
| Males, n (%) | 20 (50) | 23 (60.5) | 9 (42.9) | 42 (50) |
| Body mass index, kg/m2, median (IQR) | 16.8 (15.6–19.7) | 18.0 (15.0–20.5) | 21.5 (16.9–23.7) | NA |
| Physician global assessment, n (%) | NA | NA | ||
| Quiescent | 0 (0) | 0 (0) | ||
| Mild | 0 (0) | 0 (0) | ||
| Moderate | 8 (20) | 15 (39.5) | ||
| Severe | 32 (80) | 23 (60.5) | ||
| Fecal calprotectin, μg/mg, median (IQR) | 1375 (955–1950) | 1090 (425–1820) | 62 (21–607) | NA |
| CRP, mg/L, median (IQR) | 36 (11–45) | 2.8 (2.5–15.3) | 2.5 (1–6.3) | NA |
| ESR, mm/H, median (IQR) | 40 (20–45) | 20 (8–31) | NA | NA |
| Albumin, g/L, median (IQR) | 36 (29–39) | 37 (33–41) | NA | NA |
| Paris location of CD patients, n (%) | NA | NA | NA | |
| L1: ileal | 4 (10) | |||
| L2: colonic | 11 (27.5) | |||
| L3: ileocolonic | 25 (62.5) | |||
| Upper GI involvement: L4a, L4b | 15 (37.5), 0 (0) | |||
| Paris behaviour of CD patients, n (%) | ||||
| B1: nonstricturing, nonpenetrating | 29 (72.5) | |||
| B2: stricturing | 6 (15) | |||
| B3: penetrating | 5 (12.5) | |||
| B2B3: both penetrating and stricturing | 0 (0) | |||
| P: perianal disease modifier, n (%) | 6 (15) | |||
| Paris location of UC patients, n (%) | NA | NA | NA | |
| E1: ulcerative proctitis | 2 (5.3) | |||
| E2: left sided UC (distal splenic flexure) | 6 (15.8) | |||
| E3: extensive (hepatic flexure distally) | 2 (5.3) | |||
| E4: pancolitis (proximal hepatic flexure) | 28 (73.7) |
Abbreviations: CD, Crohn’s disease; UC, ulcerative colitis; GI, gastrointestinal; HC, healthy control; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate
Classification of Disease and Controls
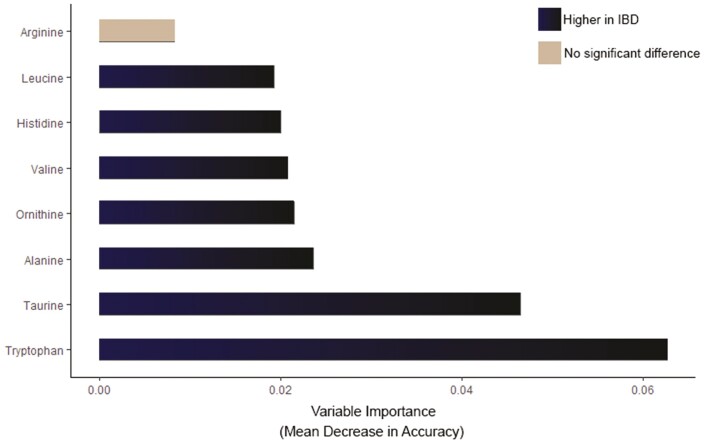
A total of 42 unique amino acids were measured. Twelve amino acids (Supplementary Table 2) with an outflux value below 0.5 were removed from the data set. The outflux of a variable quantifies how well its observed data connect to the missing data on other variables. In addition to these amino acids, sarcosine was also removed due to collinearity issues with other missing amino acids. The missing values were most likely the result of amino acid concentrations below the limit of detection but could also be the result of co-eluting interferences, hampering proper quantification of the amino acid of interest. A random forest classifier was trained on the remaining 29 different amino acids to determine the discriminative power of fecal amino acids to distinguish pediatric IBD from controls. Patients with IBD could be differentiated from controls with an accuracy of 82% (sensitivity 63%, specificity 97%). One false positive and 10 false negative results occurred in the test set containing 61 participants. Increased levels in IBD subjects of TRP, taurine, alanine, ornithine, valine, histidine, and leucine were the most differentiating features in the prediction model. Arginine was also ranked as an important feature in classifying IBD. This feature, however, did not significantly differ (P=.237—0.876) between IBD and controls based on a Mann-Whitney U test. In Figure 1, the random forest variable importance plot is depicted, which expresses how much accuracy the model loses by excluding each amino acid. The median levels (with IQR) of the 29 amino acids and the differences in each amino acid between the IBD cases and controls are depicted in Supplementary Table 3. Correlations between BMI and the most differentiating amino acids (TRP, taurine, alanine, ornithine, valine, histidine, leucine, and arginine) were computed. Body mass index was correlated with histidine and arginine (Pearson r=0.268, P = .034; and Pearson r=0.380, P = .002, respectively). Age was not correlated with levels of the most differentiating amino acids.
Figure 1.
Random forest (RF) variable importance plot in treatment-naïve, newly diagnosed pediatric IBD patients and controls. Mean decrease accuracy is the measure of the performance of the model without each amino acid. A higher value indicates the importance of that amino acid in predicting group (IBD vs controls). The amino acids in dark blue indicate higher median concentrations in IBD. The amino acid in gray did not significantly differ (P ≥ .05) between groups based on a Mann-Whitney U test.
Approximately 1 year after this trial, 2 control subjects with GI symptoms underwent a second endoscopic assessment due to persistent suspicion of IBD following the first endoscopy without macroscopic and histological abnormalities. This second endoscopy revealed colitis, indicated as IBD-U. Fecal AAA was only performed during the first endoscopic assessment. Levels of taurine were high (within the IQR of patients with IBD), whereas levels of TRP, alanine, ornithine, and valine were low (within the IQR of controls). A second random forest classifier was trained on the amino acid data set in which these 2 patients were included in the IBD group. The accuracy remained 82%.
Ulcerative colitis and controls
A random forest classifier was trained to differentiate UC patients from controls. Children with UC could be correctly separated from controls with an accuracy of 90% (sensitivity 80%, specificity 94%). The amino acids that had the greatest impact on the random forest classifier for differentiating UC from controls were increased levels of taurine, TRP, ornithine, and histidine. Sulfo-L-cysteine was the fifth-highest feature but did not significantly differ between UC and controls based on a Mann-Whitney U test. In Figure 2, the variable importance plot is depicted. The accuracy increased to 92% in the data set in which the 2 patients with IBD-U were included in the IBD group. Body mass index was negatively correlated with taurine (Pearson r=−0.357, P = .049) in UC subjects.
Figure 2.
Random forest (RF) variable importance plot, in patients with ulcerative colitis and controls. The amino acids in dark blue indicate higher median concentrations in ulcerative colitis. The amino acid in gray did not significantly differ (P ≥ .05) between groups based on a Mann-Whitney U test.
Crohn’s disease and controls
Crohn’s disease patients could be differentiated from controls with an accuracy of 80% (sensitivity 56%, specificity 91%). In CD subjects, increased levels of TRP, citrulline, alanine, phenylalanine, ornithine, and α-aminobutyric acid were the most differentiating features in the prediction model (Figure 3). The accuracy increased to 82% in the data set in which the 2 patients with IBD-U were included in the IBD group. Body mass index correlated with ornithine and phenylalanine in patients with CD (Pearson r=0.465, P = .007; and Pearson r=0.392, P = .026, respectively).
Figure 3.
Random forest (RF) variable importance plot in patients with Crohn’s disease and controls. The amino acids in dark blue indicate higher median concentrations in Crohn’s disease.
Ulcerative colitis and Crohn’s disease
None of the 29 amino acids were significantly different between patients with UC and CD based on Mann-Whitney U tests (Supplementary Table 3). A random forest classifier for IBD phenotype was trained with an accuracy of 58%, indicating that UC patients could not be correctly separated from CD patients based on their amino acids profiles.
Healthy controls and controls with GI symptoms
None of the 29 amino acids differed significantly between healthy controls and controls with GI symptoms. The median amino acid fecal concentrations in both control groups are depicted in Supplementary Table 4.
Amino Acids Levels and Inflammatory Biomarkers
No significant correlations were observed between levels of amino acids and levels of FCP, albumin, and ESR (Table 2). The C-reactive protein concentrations were significantly positively correlated with concentrations of TRP and ornithine in patients with CD—but not in UC (Table 2).
Table 2.
Correlations between amino acids levels and inflammatory biomarkers.
| Fecal calprotectin | Albumin | CRP | ESR | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Amino acid | Pearson r | P | Pearson r | P | Pearson r | P | Pearson r | P | |
| Ulcerative | Tryptophan | −0.092 | 0.597 | 0.342 | 0.065 | −0.010 | 0.953 | 0.206 | 0.303 |
| colitis | Taurine | 0.022 | 0.898 | 0.252 | 0.180 | 0.098 | 0.559 | 0.192 | 0.338 |
| Histidine | −0.180 | 0.301 | 0.150 | 0.429 | 0.113 | 0.501 | 0.005 | 0.978 | |
| Ornithine | 0.137 | 0.433 | 0.222 | 0.237 | 0.028 | 0.866 | 0.036 | 0.859 | |
| Sulfo-L-cysteine | −0.131 | 0.455 | 0.136 | 0.473 | −0.194 | 0.243 | 0.011 | 0.956 | |
| Crohn’s | Tryptophan | 0.103 | 0.526 | −0.077 | 0.664 | 0.425 | 0.007b | 0.128 | 0.480 |
| disease | Citrulline | 0.033 | 0.839 | 0.143 | 0.420 | 0.183 | 0.266 | 0.203 | 0.258 |
| Ornithine | 0.205 | 0.204 | 0.036 | 0.841 | 0.387 | 0.015a | 0.212 | 0.237 | |
| Alanine | 0.300 | 0.060 | 0.022 | 0.903 | 0.308 | 0.057 | 0.205 | 0.253 | |
| Phenyalanine | 0.194 | 0.229 | 0.075 | 0.674 | 0.268 | 0.099 | 0.234 | 0.190 | |
| α-Aminobutyric acid | 0.068 | 0.678 | −0.092 | 0.619 | 0.143 | 0.399 | 0.140 | 0.435 | |
Correlation is significant at the 0.05 level (2-tailed)
Correlation is significant at the 0.01 level (2-tailed)
Abbreviations: CRP, C-reactive protein, ESR, erythrocyte sedimentation rate
Amino Acids Levels and Disease Localization
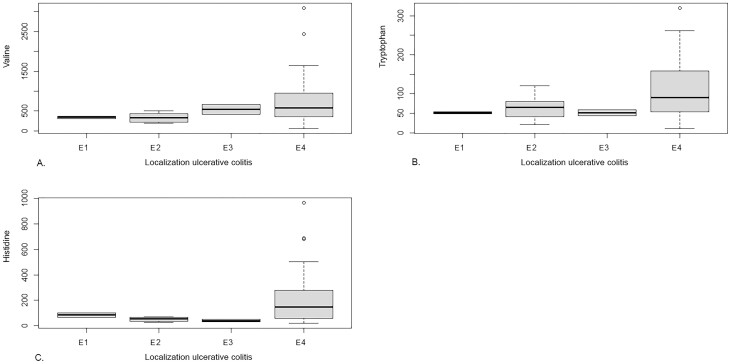
Associations between levels of the most differentiating features for classifying IBD (TRP, taurine, ornithine, histidine, and valine) and disease localization of UC and CD were studied. Levels of TRP, valine, and histidine were positively associated with more extended disease (pancolitis) in UC patients (P < .05), as depicted in Figure 4. No association was found between localization of CD and amino acids levels. Additionally, an association between levels of sulfo-L-cysteine and disease localization in UC patients was tested; no significant association was observed. Levels of citrulline, alanine, phenylalanine, and α-aminobutyric acid were not significantly associated with disease localization in CD patients. Furthermore, we searched for potential differences in all 29 amino acids concentrations between IBD patients with isolated ileal disease (n=4) and colonic disease (n=74), but no differences were observed.
Figure 4.
A, Box Whisker plot for levels of valine associated with ulcerative colitis localization, according to the Paris classification (E1: ulcerative proctitis, E2: left sided UC, E3: extensive UC, E4: pancolitis). Ordinal regression model showed a positively significant (P < .05) association between levels of valine and more extended disease in UC patients. B, Box Whisker plot, illustrating a positively significant (P < .05) association between levels of tryptophan and more extended disease (pancolitis) in UC patients. C, Box Whisker plot, illustrating a positively significant (P < .05) association between levels of histidine and more extended disease (pancolitis) in UC patients.
Amino Acids Levels and Extraintestinal Manifestations
A post hoc analysis was conducted to study whether amino acids levels were different in IBD patients with and without EIMs. In total, 1 or multiple EIMs were reported in 6 of 78 patients (7.7%) at time of diagnosis, including involvement of joints (n=4), skin (n=2), or liver (n=1). Children with EIMs had higher concentrations of arginine (median 140 vs 12-17, P = .020-0.033) and proline (median 524 vs 144, P = .018). Adjusted P values were larger than the false discovery rate of 5%. However, note that due to the large imbalance between the number of patients with and without EIMs, the statistical power of the test is severely reduced.
Discussion
In this study, we assessed the potential of fecal amino acids to differentiate de novo IBD from controls. Fecal AAA allowed for the differentiation of patients with IBD from controls with an accuracy of 82%, based on 29 unique amino acids. The amino acids with the highest impact on the prediction model were increased levels of TRP, taurine, alanine, ornithine, valine, histidine, and leucine.
Fecal AAA could detect de novo IBD with a sensitivity of 63% and a high specificity of 97% (accuracy 82%). Currently, inflammatory markers including FCP, CRP, ESR, and albumin are used in the diagnostic workup of IBD, which are highly sensitive but hampered by low specificities.25 Assessment of these inflammatory markers substantiated with fecal AAA might have the potential to increase the accuracy for detection of pediatric IBD. To evaluate this, however, future studies consisting of an intention-to-diagnose cohort and using a prediction model including both the inflammatory markers and fecal amino acids are needed.
Tryptophan was the most differentiating amino acid for classifying IBD in our cohort. Tryptophan and its metabolites have previously been associated with IBD. Reduced TRP serum levels in adults with IBD vs control subjects were detected by previous studies.10,15 Tryptophan is an essential amino acid for the intestinal mucosal cells and is considered to play a role in mucosal inflammation, epithelial barrier, and energy homeostasis of the host. There are 3 major metabolic pathways of TRP comprising (1) kynurenine (KYN) pathway; (2) indole pathway via intestinal microbiota; and (3) 5-hydroxytryptamine pathway. These pathways activate the release of ligands of aryl hydrocarbon receptor and cytokines, and influencing the function of many immune cells upon inflammation.26,27 The study by Nikolaus et al,15 observed an altered activity of the KYN pathway and an association between the composition of fecal microbiota and serum levels of TRP in patients with IBD. These findings indicate that increased catabolism of TRP or a reduced microbial diversity can contribute to differences in TRP levels between patients with IBD and controls. These metabolic pathways of TRP and its association with fecal microbiota have been poorly studied in children with IBD. However, several previous studies assessed the TRP serum and fecal levels, showing reduced TRP serum levels17 and increased TRP fecal levels in pediatric IBD patients compared with controls.16,17,28–30 Malabsorption and protein loss through colonic leakage are considered to play an important role in causing these TRP level differences.31
Increased taurine levels were found to be strongly predictive for classifying IBD. Specifically, taurine was ranked as the most differentiating feature in UC. Elevated taurine fecal levels have been previously observed in patients with UC.12,17,32 Taurine is a bile acid component modulated by commensal bacteria, which plays multiple roles in regulation of immune and metabolic processes.33 In the neutrophil cell, taurine has anti-inflammatory and antioxidant functions.34 Primary bile acids are conjugated with glycine or taurine before excretion via biliary ducts. In the gut lumen, bile acids are deconjugated by the microbiota. Elevated taurine fecal levels might therefore be caused by a reduced capacity of the gut microbiota to deconjugate and desulfate bile acids in patients with IBD.4,30,35
In a previous case-control study consisting of 30 children with IBD and 15 healthy children,16 increased levels of TRP, histidine, phenylalanine, leucine, tyrosine, and valine in stool were found to be strong predictors for discrimination of de novo IBD from controls using the same technique, which is largely in line with the present study. Elevated levels of ornithine and alanine were also observed in that study; however, these differences did not reach statistical significance, which might be explained by their small sample size of 45 pediatric patients.16 Taurine levels were not assessed. In a study by Kolho et al on metabolomics in pediatric IBD (69 patients with IBD and 29 controls),17 increased fecal levels of TRP, glycine, citrulline, serine, ornithine, and—to a lesser extent—alanine and phenylalanine were observed as differentiating features in both UC and CD patients compared with intention-to-diagnose controls. Levels of threonine, histidine, and taurine were only elevated in patients with UC. In the present study, these amino acids were also significantly increased in CD based on Mann-Whitney U tests. However, comparable with the findings of Kolho et al, histidine and taurine were ranked as the most differentiating features in UC but not in CD. In that study, only a subset of patients donated fecal samples (23 IBD patients and 14 controls) compared with the 183 collected samples (78 IBD and 105 controls) in the present study. Another study by Diederen et al29 found higher fecal concentrations of 17 out of 20 unique amino acids detected in pediatric patients with CD (n=43) compared with healthy controls (n=18) using the same technique. These 17 amino acids were also significantly elevated in the patients with CD in the present study. In contrast to our results, glutamic acid and taurine were not elevated in CD in that study. A study by Ni et al comprising 90 children with CD and 26 HCs36 observed that the majority of the fecal metabolites belonging to the class “amino acids and their derivates” were significantly associated with CD. There was a positive correlation between fecal amino acid (and their derivates) concentrations and the abundance of proteobacteria: a microbial signature of a dysbiotic gut microbiota. It was suggested that increased use of nitrogen for amino acid biosynthesis in proteobacteria contributes to microbiota shifts (dysbiosis) and CD.
More studies on fecal amino acid profiles were performed in adults with IBD,12–14 showing elevated amino acid fecal levels in IBD vs controls, which is consistent with our findings. A study by Marchesi et al13 detected a selection of amino acids (leucine, isoleucine, valine, alanine, lysine, asparagine, aspartic acid, tyrosine, and glutamate) in fecal extracts of adults with IBD (n=20) vs healthy individuals (n=13) by using nuclear magnetic resonance (NMR) spectroscopy. Levels of alanine, isoleucine, leucine, lysine, and valine were elevated in CD, whereas levels of glutamate and lysine were elevated in UC compared with controls. It was hypothesized that elevated fecal amino acid concentrations were due to malabsorption from the intestines or by concomitant protein losing enteropathy, driven by inflammation. In contrast to our treatment-naïve patients with IBD, all the adults with IBD were actively treated with prednisolone and 5-aminosalicylic at time of sampling, possibly influencing the outcomes.
All 23 amino acids that differed significantly between patients with IBD and controls were characterized by high fecal concentrations in IBD. This might be caused by malabsorption in the intestine, systematic loss of amino acids due to colonic leakage by the inflamed mucosal tissue, alterations in metabolic pathways, or shifts in microbial composition.31 The finding that levels of TRP, valine, and histidine were positively associated with more extended disease in patients with UC supports the hypothesis of colonic leakage of amino acids, resulting in high fecal amino acid concentrations. Because the amino acid transporters are mainly located in the small intestines, malabsorption would be a less plausible explanation for the association between higher fecal amino acids levels and more extended UC localization.37 Recently, a study by Galipeau et al,38 showed increased fecal proteolytic and elastase activity before UC onset, associated with changes in gut microbiota. Specifically, elastase activity directly correlated with Bacteroides vulgatus, which is a known proteolytic taxa. Consequently, the high concentrations of fecal amino acids in the patients with UC in our cohort might also be related to increased proteolytic and elastase activity rather than protein-losing enteropathy.
To the best of our knowledge, we report on the largest pediatric cohort so far regarding fecal AAA in newly diagnosed, treatment-naïve IBD. A strength of this study is that we included de novo, treatment-naïve, pediatric IBD patients who collected fecal samples before bowel preparation, circumventing the certain risk of bias by medication and colonic lavage on outcome. A second strength is that we used an HPLC technique for amino acid detection, which is highly sensitive compared with the commonly used NMR spectroscopy in previous fecal metabolomics studies.39 A limitation of this study is that we did not use questionnaires on dietary intake and stool consistency of the participants at the time of sampling, possibly influencing the differences in amino acids fecal levels. However, to minimize the risk of bias due to differences in fecal water content, the samples were freeze dried. A second limitation is that we were unable to calculate the classifier of FCP vs the classifier of AAA because only half of the controls with GI symptoms underwent endoscopic examination, and FCP was not assessed in the diagnostic workup in a small proportion of controls with GI symptoms. Finally, we did not include an independent validation cohort to confirm our findings. Future studies on the amino acid metabolism that take into account diet and the gut microbiota are therefore warranted for a better understanding of the role of amino acids in the disease pathogeneses of pediatric IBD.
Conclusions
In conclusion, in this pediatric IBD case-control study, fecal amino acids were shown to differentiate de novo IBD from controls with an accuracy of 82% based on a set of amino acids, including increased levels of TRP as the most differentiating amino acid. We observed that pediatric IBD influences fecal amino acids, which may enhance understanding of the pathophysiology. Future studies are warranted to investigate whether fecal amino acids are associated with the gut microbiota composition and to investigate the potential of fecal AAA as biomarker for monitoring disease activity and predicting treatment response.
Supplementary Material
Glossary
Abbreviations
- AAA
amino acid analysis
- BH
Benjamini-Hochberg
- BMI
body mass index
- CD
Crohn’s disease
- CRP
C-reactive protein
- EIM
extraintestinal manifestations
- ESR
erythrocyte sedimentation rate
- FCP
fecal calprotectin
- GI
gastrointestinal
- HC
healthy controls
- HPLC
high-performance liquid chromatography
- IBD
inflammatory bowel disease
- IBD-U
inflammatory bowel disease unclassified
- IQR
interquartile ranges
- KYN
kynurenine
- MAR
missing at random
- NMR
nuclear magnetic resonance
- PGA
physician global assessment score
- TRP
tryptophan
- UC
ulcerative colitis
Author Contributions
J.J. coordinated and supervised data collection, carried out the statistical analyses, drafted the initial version of this article, and reviewed and revised this article. I.A. collected data and reviewed this article. N.dB. and T.dM. conceptualized and designed this study, coordinated and supervised data collection, and critically reviewed this article for important intellectual content. E.S., A.B., and E.J. contributed to the analysis and interpretation of data and critically reviewed this article for important intellectual content. J.C. carried out the statistical analyses and critically reviewed this article for important intellectual content. J.vL. and M.B. critically reviewed this article for important intellectual content. All authors approved the final version of the article, including the authorship list.
Funding
This work was supported by the “Right on Time” grant with number WO 19 – 25, from The Dutch Digestive Foundation (MLDS).
Conflicts of Interest
N.dB. has served as consultant and principal investigator for TEVA Pharma BV and Takeda. J.vL. reports consulting, travel, and/or speaker fees and research support from AbbVie, Janssen, Nestlé Health Science, and Novalac. J.J., E.S., I.A., A.B., E.J., J.C., M.B., and T.dM. have nothing to declare.
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material.
References
- 1. Neurath MF, Travis SP.. Mucosal healing in inflammatory bowel diseases: a systematic review. Gut. 2012;61:1619–1635. [DOI] [PubMed] [Google Scholar]
- 2. Rosen MJ, Dhawan A, Saeed SA.. Inflammatory bowel disease in children and adolescents. JAMA Pediatr. 2015;169:1053–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Coward S, Clement F, Benchimol EI, et al. Past and future burden of inflammatory bowel diseases based on modeling of population-based data. Gastroenterology. 2019;156:1345–1353.e4. [DOI] [PubMed] [Google Scholar]
- 4. Lloyd-Price J, Arze C, Ananthakrishnan AN, et al. ; IBDMDB Investigators . Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature. 2019;569:655–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Imhann F, Vich Vila A, Bonder MJ, et al. Interplay of host genetics and gut microbiota underlying the onset and clinical presentation of inflammatory bowel disease. Gut. 2018;67:108–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gevers D, Kugathasan S, Denson LA, et al. The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe. 2014;15:382–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zierer J, Jackson MA, Kastenmüller G, et al. The fecal metabolome as a functional readout of the gut microbiome. Nat Genet. 2018;50:790–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sugihara K, Morhardt TL, Kamada N.. The role of dietary nutrients in inflammatory bowel disease. Front Immunol. 2018;9:3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Scoville EA, Allaman MM, Brown CT, et al. Alterations in lipid, amino acid, and energy metabolism distinguish Crohn’s disease from ulcerative colitis and control subjects by serum metabolomic profiling. Metabolomics. 2018;14:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ooi M, Nishiumi S, Yoshie T, et al. GC/MS-based profiling of amino acids and TCA cycle-related molecules in ulcerative colitis. Inflamm Res. 2011;60:831–840. [DOI] [PubMed] [Google Scholar]
- 11. Piestansky J, Olesova D, Galba J, et al. Profiling of amino acids in urine samples of patients suffering from inflammatory bowel disease by capillary electrophoresis-mass spectrometry. Molecules. 2019;24. doi: 10.3390/molecules24183345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bjerrum JT, Wang Y, Hao F, et al. Metabonomics of human fecal extracts characterize ulcerative colitis, Crohn’s disease and healthy individuals. Metabolomics. 2015;11:122–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Marchesi JR, Holmes E, Khan F, et al. Rapid and noninvasive metabonomic characterization of inflammatory bowel disease. J Proteome Res. 2007;6:546–551. [DOI] [PubMed] [Google Scholar]
- 14. Jansson J, Willing B, Lucio M, et al. Metabolomics reveals metabolic biomarkers of Crohn’s disease. Plos One. 2009;4:e6386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nikolaus S, Schulte B, Al-Massad N, et al. Increased tryptophan metabolism is associated with activity of inflammatory bowel diseases. Gastroenterology. 2017;153:1504–1516.e2. [DOI] [PubMed] [Google Scholar]
- 16. Bosch S, Struys EA, van Gaal N, et al. Fecal amino acid analysis can discriminate de novo treatment-naïve pediatric inflammatory bowel disease from controls. J Pediatr Gastroenterol Nutr. 2018;66:773–778. [DOI] [PubMed] [Google Scholar]
- 17. Kolho KL, Pessia A, Jaakkola T, et al. Faecal and serum metabolomics in paediatric inflammatory bowel disease. J Crohns Colitis. 2017;11:321–334. [DOI] [PubMed] [Google Scholar]
- 18. Bosch S, El Manouni El Hassani S, Brizzio Brentar M, et al. Fecal amino acid profiles exceed accuracy of serum amino acids in diagnosing pediatric inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2020;71:371–375. [DOI] [PubMed] [Google Scholar]
- 19. Levine A, Koletzko S, Turner D, et al. ; European Society of Pediatric Gastroenterology, Hepatology, and Nutrition . ESPGHAN revised porto criteria for the diagnosis of inflammatory bowel disease in children and adolescents. J Pediatr Gastroenterol Nutr. 2014;58:795–806. [DOI] [PubMed] [Google Scholar]
- 20. Levine A, Griffiths A, Markowitz J, et al. Pediatric modification of the Montreal classification for inflammatory bowel disease: the Paris classification. Inflamm Bowel Dis. 2011;17:1314–1321. [DOI] [PubMed] [Google Scholar]
- 21. de Meij TG, Budding AE, de Groot EF, et al. Composition and stability of intestinal microbiota of healthy children within a Dutch population. Faseb J. 2016;30:1512–1522. [DOI] [PubMed] [Google Scholar]
- 22. Thapar N, Benninga MA, Crowell MD, et al. Paediatric functional abdominal pain disorders. Nat Rev Dis Primers. 2020;6:89. [DOI] [PubMed] [Google Scholar]
- 23. Spackman DH, Stein WH, Moore S.. Automatic recording apparatus for use in chromatography of amino acids. Anal Chem. 1958;30:1190–1206. [PubMed] [Google Scholar]
- 24. Breiman L. Random forests. Mach Learn. 2001;45:5–32. [Google Scholar]
- 25. Henderson P, Anderson NH, Wilson DC.. The diagnostic accuracy of fecal calprotectin during the investigation of suspected pediatric inflammatory bowel disease: a systematic review and meta-analysis. Am J Gastroenterol. 2014;109:637–645. [DOI] [PubMed] [Google Scholar]
- 26. Li X, Zhang ZH, Zabed HM, et al. An insight into the roles of dietary tryptophan and its metabolites in intestinal inflammation and inflammatory bowel disease. Mol Nutr Food Res. 2020:e2000461. doi: 10.1002/mnfr.202000461. [DOI] [PubMed] [Google Scholar]
- 27. Lamas B, Natividad JM, Sokol H.. Aryl hydrocarbon receptor and intestinal immunity. Mucosal Immunol. 2018;11:1024–1038. [DOI] [PubMed] [Google Scholar]
- 28. Alghamdi A, Gerasimidis K, Blackburn G, et al. Untargeted metabolomics of extracts from faecal samples demonstrates distinct differences between paediatric Crohn’s disease patients and healthy controls but no significant changes resulting from exclusive enteral nutrition treatment. Metabolites. 2018;8. doi: 10.3390/metabo8040082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Diederen K, Li JV, Donachie GE, et al. Exclusive enteral nutrition mediates gut microbial and metabolic changes that are associated with remission in children with Crohn’s disease. Sci Rep. 2020;10:18879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jacobs JP, Goudarzi M, Singh N, et al. A disease-associated microbial and metabolomics state in relatives of pediatric inflammatory bowel disease patients. Cell Mol Gastroenterol Hepatol. 2016;2:750–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bosch S, de Meij TGJ, de Boer NK.. Altered tryptophan levels in patients with inflammatory bowel disease owing to colonic leakage, metabolism, or malabsorption? Gastroenterology. 2018;154:1855–1856. [DOI] [PubMed] [Google Scholar]
- 32. Le Gall G, Noor SO, Ridgway K, et al. Metabolomics of fecal extracts detects altered metabolic activity of gut microbiota in ulcerative colitis and irritable bowel syndrome. J Proteome Res. 2011;10:4208–4218. [DOI] [PubMed] [Google Scholar]
- 33. Brestoff JR, Artis D.. Commensal bacteria at the interface of host metabolism and the immune system. Nat Immunol. 2013;14:676–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schaffer S, Kim HW.. Effects and mechanisms of taurine as a therapeutic agent. Biomol Ther (Seoul). 2018;26:225–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Duboc H, Rajca S, Rainteau D, et al. Connecting dysbiosis, bile-acid dysmetabolism and gut inflammation in inflammatory bowel diseases. Gut. 2013;62:531–539. [DOI] [PubMed] [Google Scholar]
- 36. Ni J, Shen TD, Chen EZ, et al. A role for bacterial urease in gut dysbiosis and Crohn’s disease. Sci Transl Med. 2017;9. doi: 10.1126/scitranslmed.aah6888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Terada T, Shimada Y, Pan X, et al. Expression profiles of various transporters for oligopeptides, amino acids and organic ions along the human digestive tract. Biochem Pharmacol. 2005;70:1756–1763. [DOI] [PubMed] [Google Scholar]
- 38. Galipeau HJ, Caminero A, Turpin W, et al. ; CCC Genetics, Environmental, Microbial Project Research Consortium . Novel fecal biomarkers that precede clinical diagnosis of ulcerative colitis. Gastroenterology. 2021;160:1532–1545. [DOI] [PubMed] [Google Scholar]
- 39. Zhang A, Sun H, Wang P, et al. Modern analytical techniques in metabolomics analysis. Analyst. 2012;137:293–300. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material.