Abstract
Background:
Side effects associated with use of postoperative narcotics for pain control can delay recovery after abdominally based microsurgical breast reconstruction. The authors evaluated a nonnarcotic pain control regimen in conjunction with bilateral transversus abdominis plane blocks on facilitating early hospital discharge.
Methods:
A retrospective analysis was performed of consecutive patients who underwent breast reconstruction using abdominally based free flaps, with or without being included in a nonnarcotic protocol using intraoperative transversus abdominis plane blockade. During this period, the use of locoregional analgesia evolved from none (control), to continuous bupivacaine infusion transversus abdominis plane and catheters, to single-dose transversus abdominis plane blockade with liposomal bupivacaine solution. Demographic factors, length of stay, inpatient opioid consumption, and complications were reported for all three groups.
Results:
One hundred twenty-eight consecutive patients (182 flaps) were identified. Forty patients (62 flaps) were in the infusion–liposomal bupivacaine group, 48 (66 flaps) were in the single-dose blockade–catheter group, and 40 (54 flaps) were in the control group. The infusion–liposomal bupivacaine patients had a significantly shorter hospital stay compared with the single-dose blockade–catheter group (2.65 ± 0.66 versus 3.52 ± 0.92 days; p < 0.0001) and the control group (2.65 ± 0.66 versus 4.05 ± 1.26 days; p < 0.0001). There was no significant difference in flap loss or major complications among groups.
Conclusions:
When used as part of a nonnarcotic postoperative pain regimen, transversus abdominis plane blocks performed with single injections of liposomal bupivacaine help facilitate early hospital discharge after abdominally based microsurgical breast reconstruction. A trend toward consistent discharge by postoperative day 2 was seen. This could result in significant cost savings for health care systems.
CLINICAL QUESTION/LEVEL OF EVIDENCE:
Therapeutic, III.
Abdominally based microsurgical breast reconstruction has an established record of excellent outcomes.1 However, increased scrutiny of patient experience and treatment costs led us to examine our reconstructive care pathway for opportunities for improvement. We identified postoperative pain management as a target for quality improvement, with the potential for secondary cost reduction through accelerated recovery. Therefore, we instituted an Enhanced Recovery after Surgery protocol using a nonnarcotic pain control regimen in conjunction with two different locoregional anesthesia techniques for all patients undergoing abdominally based microsurgical breast reconstruction.
Because the majority of pain after abdominally based autologous breast reconstruction is derived from the abdominal donor site, the two Enhanced Recovery after Surgery groups both used differing types of transversus abdominis plane blockade as the main form of locoregional analgesia. Transversus abdominis plane blockade involves injecting local anesthetic in the plane between the internal oblique and transversus abdominis muscles containing the tenth thoracic through first lumbar sensory nerves. This results in abdominal wall analgesia, making it ideal after abdominal flap harvest2,3 (Fig. 1).
Fig. 1.
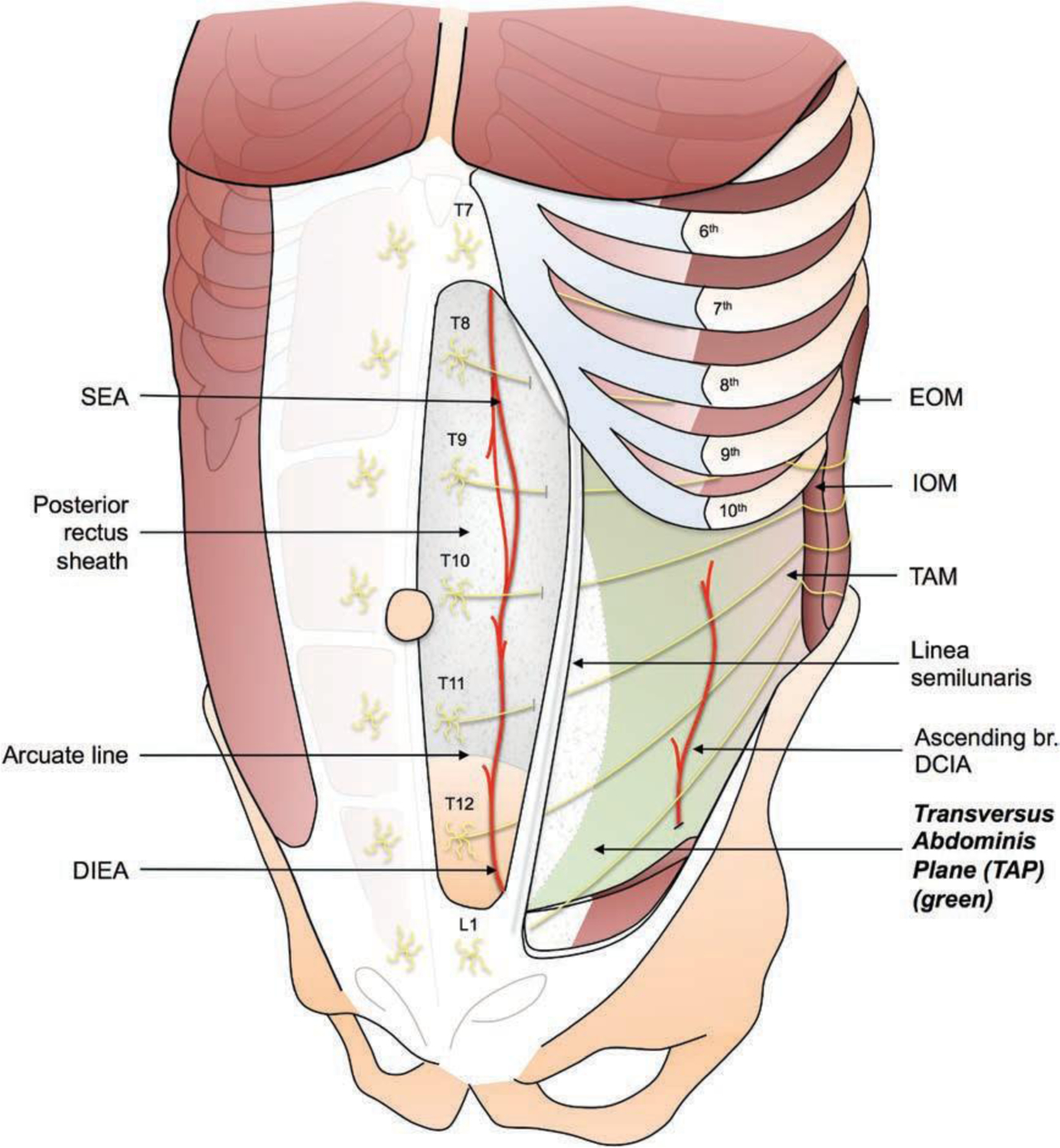
The transversus abdominis plane (TAP). The transversus abdominis plane is located between the internal oblique muscle (IOM) and the transversus abdominis muscle (TAM). The thoracoabdominal nerves T7 to L1 that provide sensibility to the anterior abdominal wall from the xiphoid to the pubis are found within the transversus abdominis plane. SEA, superior epigastric artery; DIEA, deep inferior epigastric artery; EOM, external oblique muscle; DCIA, deep circumflex iliac artery.
Systemic nonnarcotic pain control was implemented to decrease opioid consumption. Although intravenous and oral opioids have been the mainstay of postoperative pain control, they are associated with a number of detrimental effects that can delay recovery and increase hospital length of stay, including nausea, constipation, lethargy, cough suppression, confusion, and orthostatic hypotension.4,5 By reducing these side effects, we sought to hasten resumption of a normal diet and ambulation and to accelerate recovery. We compared the two Enhanced Recovery after Surgery groups with each other and against historic controls for patient outcomes, opioid consumption, and length of stay.
PATIENTS AND METHODS
Institutional review board approval was obtained and retrospective analysis performed of the electronic health records of patients who underwent breast reconstruction performed by the senior author (M.L.S.) at a single teaching hospital using abdominally based free flaps between December of 2010 and December of 2015. The study consisted of three consecutive groups of patients. The first group (control) were treated with a standard narcotic-based pain control regimen without locoregional anesthesia; the second group (transversus abdominis plane–catheter group) were treated with a nonnarcotic pain control regimen along with indwelling infusion catheters in the transversus abdominis plane to continuously administer local anesthetic; and the final group (transversus abdominis plane–liposomal bupivacaine group) were treated with the same nonnarcotic pain control regimen but with intraoperative injections of a long-acting local anesthetic. Exclusion criteria were age older than 70 years, history of narcotic drug abuse, allergy to local anesthetics, and underlying chronic pain syndromes. Demographic factors, clinical variables, opioid consumption, length of stay, and complications were recorded and compared. Length of stay was defined as the number of days from patient admission after surgery to the day of hospital discharge.
Operative Technique
The internal mammary or thoracodorsal vessels were used as recipient vessels in all patients. Standard surgical practices, including administration of deep vein thrombosis prophylaxis and perioperative antibiotics, were used. Fluid administration during surgery was kept under 500 cc/hour unless low urine output necessitated additional volume repletion.
Locoregional Analgesia Technique
For both the transversus abdominis plane–catheter and transversus abdominis plane–liposomal bupivacaine groups, transversus abdominis plane blockade was performed intra-operatively after rectus sheath repair and before abdominal skin closure. Under ultrasound guidance (Flex Focus 400exp ultrasound system; BK Ultrasound, Peabody, Mass.), transfascial injections were performed by the operating surgeon using an epidural needle (CONTIPLEX Tuohy Continuous Nerve Block Set; B. Braun Medical, Inc., Bethlehem, Pa.). The fascial plane between the internal oblique and transversus abdominis muscles was identified at the anterior axillary line, midway between the costal margin and iliac crest (Figs. 2 and 3). [See Figure, Supplemental Digital Content 1, which shows ultrasound images that clearly delineates the plane and allows proper delivery of the anesthetic between the internal oblique and transversus abdominis muscles. Sonographic anatomy of the ultrasound-guided transversus abdominis plane (TAP) block. (Left image) Pre-injection songographic image shows an axial view of the layers of the anterolateral abdominal wall. To the left of the image is lateral and to the right is medial. The green line represents the transversus abdominis plane (TAP). (Right image) Post-injection image shows the needle (grey line with two grey arrowheads pointing to it) entering into the TAP. Local anesthetic (LA), represented by the shaded green area between the internal oblique (IO) muscle and the transversus abdominis (TA) muscle, is seen as a lens-shaped space surrounding the tip of the needle. http://links.lww.com/PRS/C233.]
Fig. 2.
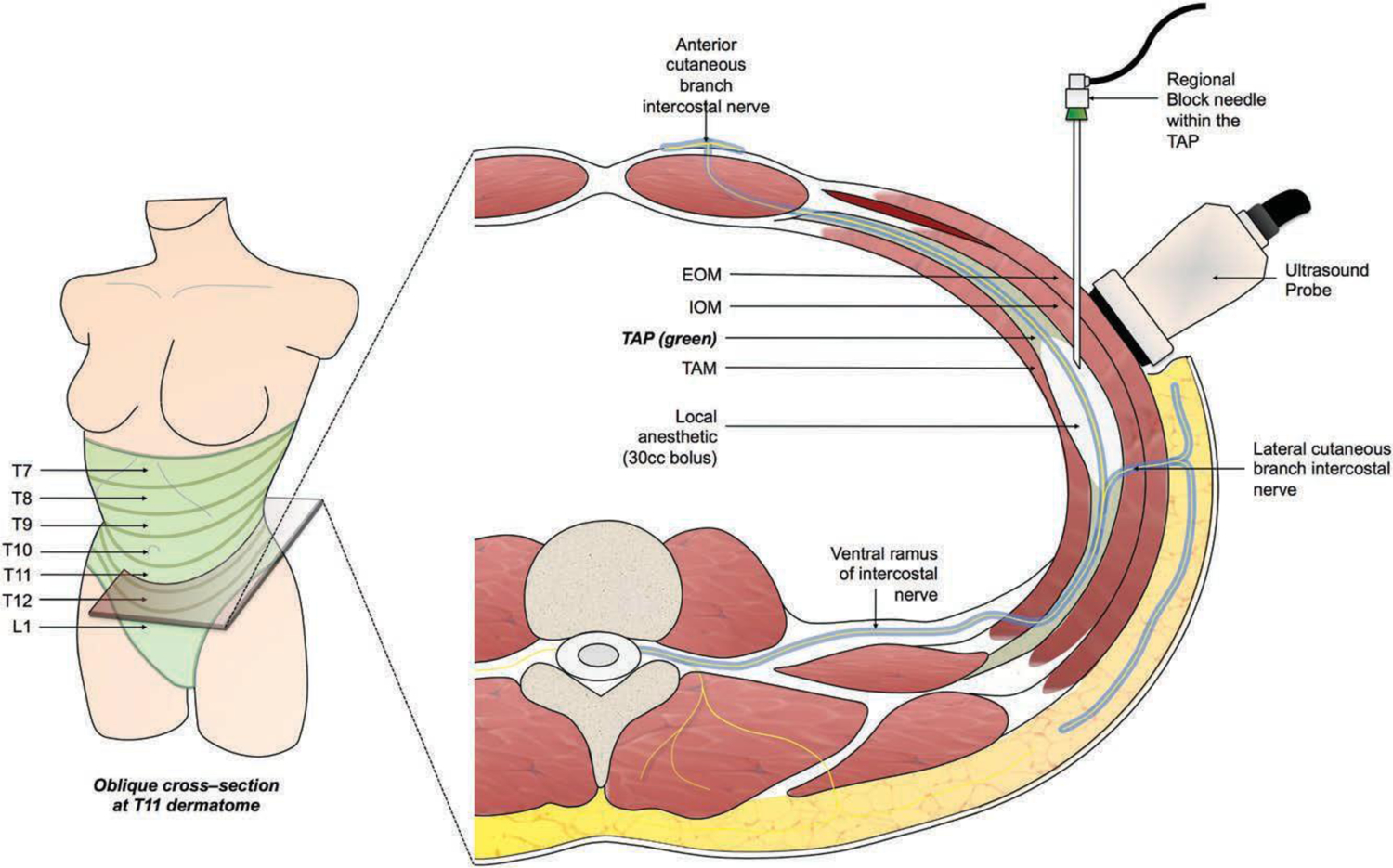
Intraoperative transversus abdominis plane (TAP) blockade. Under ultrasound guidance, a regional block catheter is passed into the transversus abdominis plane at the anterior-axillary line, midway between the costal margin and iliac crest. Thirty cubic centimeters of the liposomal bupivacaine solution is injected per side. An oblique cross-section at the level of T11 is shown (right). EOM, external oblique muscle; IOM, internal oblique muscle; TAM, transversus abdominis muscle.
Fig. 3.
Intraoperative view of transversus abdominis plane block injection.
For the transversus abdominis plane–catheter group, 30 cc of 0.25% bupivacaine was injected bilaterally, and then bilateral epidural catheters were passed transcutaneously into the plane and connected to a 400-cc-capacity external fixed rate pump filled with 0.25% bupivacaine (On-Q pump model P400X4D, Halyard Health Global Head-quarters, Alpharetta, Ga.) with a delivery rate through each catheter of 2 cc/hour.
For the transversus abdominis plane–liposomal bupivacaine group, the injections were performed in a similar fashion, but using a mixture of 20 cc of 1.3% injectable liposomal bupivacaine, 30 cc of 0.25% standard bupivacaine, and 80 cc of normal saline, with 30 cc of this mixture injected on each side. The remaining anesthetic was injected in the lower abdominal incision, the pectoralis major muscle near the anastomotic site, the superficial serratus muscle plane, and the drain sites.
Postoperative Pain Control
Control Group
A patient-controlled analgesia intravenous opioid pump was provided for all patients, with additional opioids provided by nursing on an as-needed basis for breakthrough pain. Patients were switched to as-needed oral opioids, once they were tolerating an oral diet, and then discharged on a similar regimen.
Transversus Abdominis Plane–Catheter and Transversus Abdominis Plane–Liposomal Bupivacaine Groups
The transversus abdominis plane–catheter and transversus abdominis plane–liposomal bupivacaine groups received ketorolac 15 mg intravenously and acetaminophen 1000 mg intravenously at the end of surgery and then every 6 hours postoperatively. Once patients tolerated an oral diet, typically on postoperative day 1, medications were switched to oral delivery using ketorolac 10 mg and acetaminophen 650 mg every 6 hours. Oral opioids were ordered on an as-needed basis to treat breakthrough pain. At discharge, patients received a prescription for ketorolac 10 mg, to take every 6 hours as needed through postoperative day 5, and an oral opioid, in case additional medication was needed after finishing the ketorolac. Patients also took acetaminophen, 650 mg every 6 hours as needed.
Total narcotic use during hospitalization was calculated by converting inpatient opioid intake into intravenous morphine equivalents using standardized calculations. Cumulative postoperative morphine requirements were recorded for each postoperative day and reported as milligrams per day.
Postoperative Monitoring, Activity Protocols, Discharge Criteria, and Follow-Up
For All Patients
While in the surgical intensive care unit or step-down unit, patients were monitored with Doppler checks every hour for 24 hours and then every 2 hours for 24 hours thereafter. When transferred to the standard inpatient floor, they underwent Doppler checks every 4 hours. The Foley catheter was discontinued once patients were able to get out of bed. Discharge criteria included the following: stable vital signs and examination, adequate pain control on oral medication, toleration of a regular diet, and the ability to ambulate independently. Patients followed up in the office 2 to 5 days after discharge for drain removal.
Control Group
Patients were transferred from the operating room directly to the surgical intensive care unit for 2 days for flap monitoring, pain control, and assistance in getting out of bed and ambulating. They were transferred from the surgical intensive care unit to a standard hospital ward for the remainder of their hospital stay. Patients were out of bed to a chair and started on a liquid diet on postoperative day 1 and then ambulated and advanced to a regular diet on postoperative day 2.
Transversus Abdominis Plane–Catheter Group
Patients were initially monitored in the surgical intensive care unit for 2 days, but this soon decreased to 1 day because of better pain control and ability to ambulate and tolerate a regular diet on postoperative day 1. Transversus abdominis plane catheters were left in place for 3 days to maximize pain control during the period of maximal discomfort. On postoperative day 3, the catheters were discontinued and, if discharge criteria were met, the patients discharged to home.
Transversus Abdominis Plane–Liposomal Bupivacaine Group
Patients were monitored in the surgical intensive care unit for 1 day, but this soon changed because patients were universally able to ambulate and tolerate a regular diet on postoperative day 1 and did not require the surgical intensive care unit nursing support. Therefore, instead of going to the surgical intensive care unit, patients went to the postanesthesia care unit for recovery and then were transferred to the step-down unit for flap monitoring.
Postoperative Anticoagulation
Control Group
Patients were given low-molecular-weight dextran 40 at 25 cc/hour intravenously for 24 hours and then switched to aspirin 325 mg orally every 24 hours for 3 weeks. Heparin 5000 units was given subcutaneously every 12 hours for the first 72 hours and patients wore lower leg serial compression devices while in bed for deep vein thrombosis prophylaxis.
Transversus Abdominis Plane–Catheter Group
Patients initially received the same protocol as above, but with the addition of ketorolac as describe under Postoperative Pain Control above. Subsequently, dextran and aspirin were removed from the protocol, and only heparin 5000 U given subcutaneously every 12 hours was administered for two doses, after which patients were ambulating and heparin was discontinued. Patients wore serial compression devices while in bed. Ketorolac was continued for 5 days postoperatively.
Transversus Abdominis Plane–Liposomal Bupivacaine Group
Patients received heparin 5000 U subcutaneously every 12 hours for two doses and ketorolac for 5 days. Patients wore serial compression devices while in bed.
Complications
Complications such as bleeding, infection, delayed healing, and flap loss were evaluated and major complications defined as those that required readmission or reoperation within the 30-day postoperative period.
Statistical Analysis
Continuous variables were reported as means and standard deviations; categorical variables were reported as frequencies. Associations between each study group and primary (i.e., length of stay) and secondary (i.e., inpatient opioid consumption and complication rates) outcomes were assessed and adjusted for confounding variables. Differences between group means were assessed using the Welch analysis of variance, with a value of p < 0.05 being considered significant and the Welch t test used for pairwise comparisons with the Bonferroni correction value of p = 0.016. All analyses were conducted using SAS 9.4 (SAS Institute, Inc., Cary, N.C.).
RESULTS
One hundred twenty-eight consecutive patients were identified as having an abdominally based free flap between December of 2010 and December of 2015, for a total of 182 flaps. Forty patients (54 flaps) were in the control group, 48 (66 flaps) were in the transversus abdominis plane–catheter group, and 40 (62 flaps) were in the transversus abdominis plane–liposomal bupivacaine group. There were no significant differences in baseline characteristics between groups, except for mesh placement and timing of reconstruction (Tables 1 and 2). There was a statistically significant difference in the number of patients with mesh placement between groups but no difference in length of stay between patients with and without mesh. Similarly, there was a significant difference in the number of immediate and delayed reconstructions between groups, but no significant difference in length of stay between immediate and delayed reconstructions. Neither mesh placement nor timing of reconstruction was associated with increased opioid use.
Table 1.
Demographics and Comorbid Conditions
| Controls | TAP-Cath | TAP-LB | p | |
|---|---|---|---|---|
| No. | 40 | 48 | 40 | |
| Mean age, yr | 48.4 ± 7.8 | 50.6 ± 8.8 | 50.2 ± 8.5 | 0.4331 |
| Mean ASA class | 2.1 | 2.1 | 2.0 | 0.3865 |
| Mean BMI, kg/m2 Comorbidities |
28.0 ± 5.5 | 26.2 ± 5.0 | 28.0 ± 5.4 | 0.1847 0.2664 |
| None | 33 | 36 | 26 | |
| 1–3 | 7 | 12 | 13 | |
| >3 | 0 | 0 | 1 |
ASA, American Society of Anesthesiologists; BMI, body mass index; TAP-Cath, transversus abdominis plane–catheter; TAP-LB, transversus abdominis plane–liposomal bupivacaine.
Table 2.
Clinical and Surgical Variables
| Controls | TAP-Cath | TAP-LB | p | |
|---|---|---|---|---|
| No. | 40 | 48 | 40 | |
| Clinical variable | ||||
| Chemotherapy | 0.8964 | |||
| No | 33 | 38 | 33 | |
| Yes | 7 | 10 | 7 | |
| Radiotherapy | 0.1129 | |||
| No | 35 | 34 | 28 | |
| Yes | 5 | 14 | 12 | |
| Surgical variables | ||||
| Laterality | 0.1372 | |||
| Unilateral | 26 | 30 | 18 | |
| Bilateral | 14 | 18 | 22 | |
| Timing | 0.0111 | |||
| Immediate | 40 | 41 | 33 | |
| Delayed | 0 | 7 | 7 | |
| Type of abdominal flap | 0.2293 | |||
| Total no. | 54 | 66 | 62 | |
| MSI/II (TRAM) | 1 | 3 | 5 | |
| MSIII (DIEP) | 53 | 62 | 55 | |
| MSIY (SIEA) | 0 | 1 | 2 | |
| Abdominall mesh | 0.0250 | |||
| No | 40 | 47 | 35 | |
| Yes | 0 | 1 | 5 |
MS, muscle-sparing; TRAM, transverse abdominis myocutaneous; DIEP, deep inferior epigastric artery perforator; SIEA, superficial inferior epigastric artery; TAP-Cath, transversus abdominis plane–catheter; TAP-LB, transversus abdominis plane–liposomal bupivacaine.
Length of Stay
Average length of stay for each group is reported in Figure 4 and Table 3. The transversus abdominis plane–liposomal bupivacaine group had a significantly shorter stay compared with the transversus abdominis plane–catheter group (2.65 days versus 3.52 days; p < 0.0001) and the control group (2.65 days versus 4.05 days; p < 0.0001). In addition, when comparing the first 20 patients in the transversus abdominis plane–liposomal bupivacaine group to the following 20 patients, the average stay decreased significantly from 3.00 days versus 2.3 days (p = 0.0006).
Fig. 4.
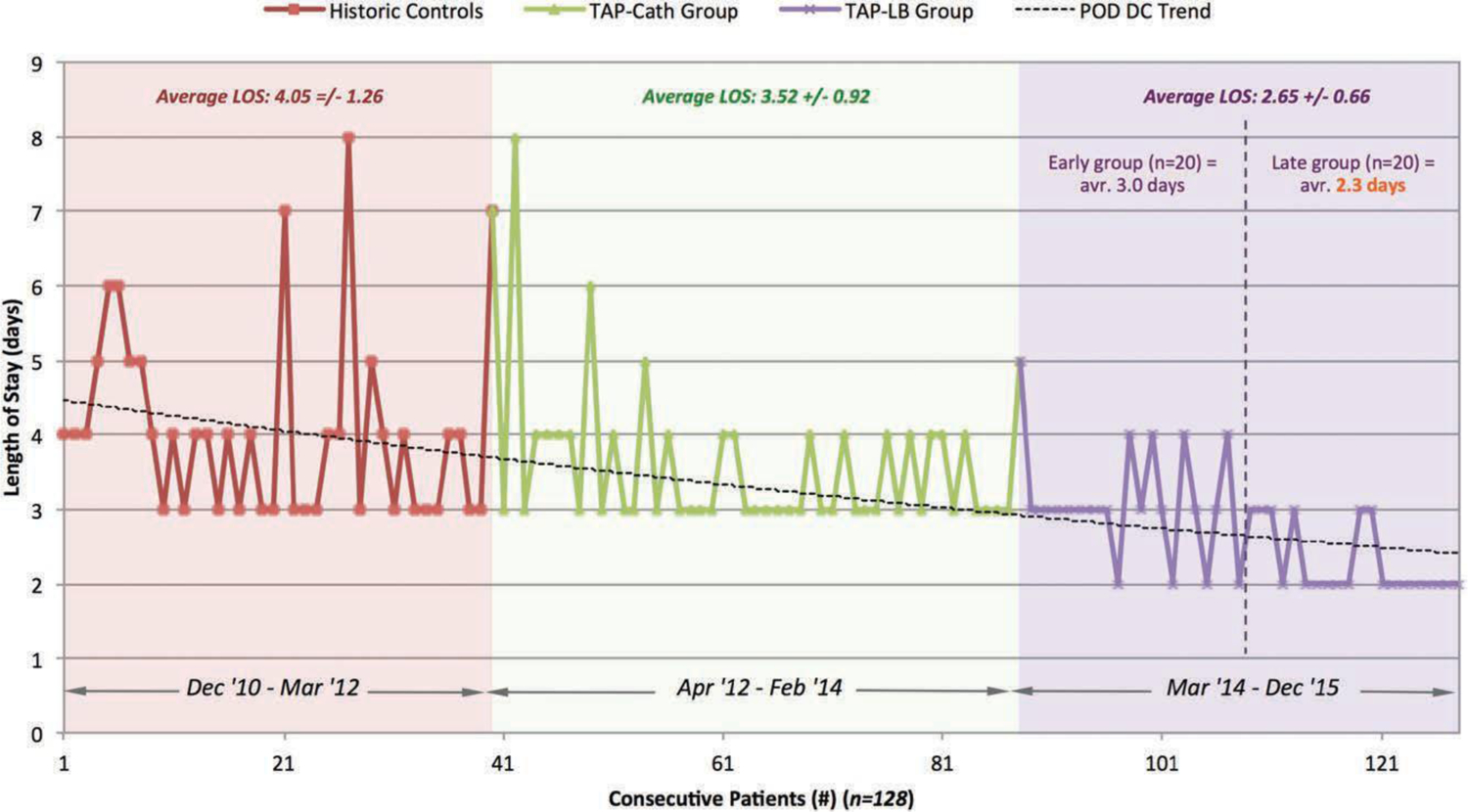
Length of stay following microsurgical abdominally based breast reconstruction (December of 2010 through December of 2015) relative to changing locoregional analgesia methods. LOS, length of stay; TAP-Cath, transversus abdominis plane–catheter; TAP-LB, transversus abdominis plane–liposomal bupivacaine; POD DC, postoperative day discharged.
Table 3.
Average Length of Stay
| No. | LOS (days) | p * | |
|---|---|---|---|
| Group | |||
| Controls | 40 | 4.05 ± 1.26 | |
| TAP-Cath | 48 | 3.52 ± 0.92 | |
| TAP-LB | 40 | 2.65 ± 0.66 | |
| Global† | <0.0001 | ||
| TAP-Cath vs. controls‡ | 0.0234 | ||
| TAP-LB vs. controls | <0.0001 | ||
| TAP-LB vs. TAP-Cath | <0.0001 |
LOS, length of stay; TAP-Cath, transversus abdominis plane–catheter; TAP-LB, transversus abdominis plane–liposomal bupivacaine.
Models were adjusted for timing of surgery.
Determined using the Welch analysis of variance.
Pairwise comparisons were performed using the Welch t test with the Bonferroni correction value of p = 0.016.
Use of Opioids
The average opioid use per postoperative day is shown in Figure 5 and Table 4. The transversus abdominis plane–liposomal bupivacaine group used significantly less opioids compared with the control group for the first 3 postoperative days. After postoperative day 3, the transversus abdominis plane–liposomal bupivacaine group demonstrated a trend toward lower average daily opioid use. However, this did not reach statistical significance. During the first 2 postoperative days, the transversus abdominis plane–liposomal bupivacaine group had significantly less opioid use compared with the transversus abdominis plane–catheter group.
Fig. 5.
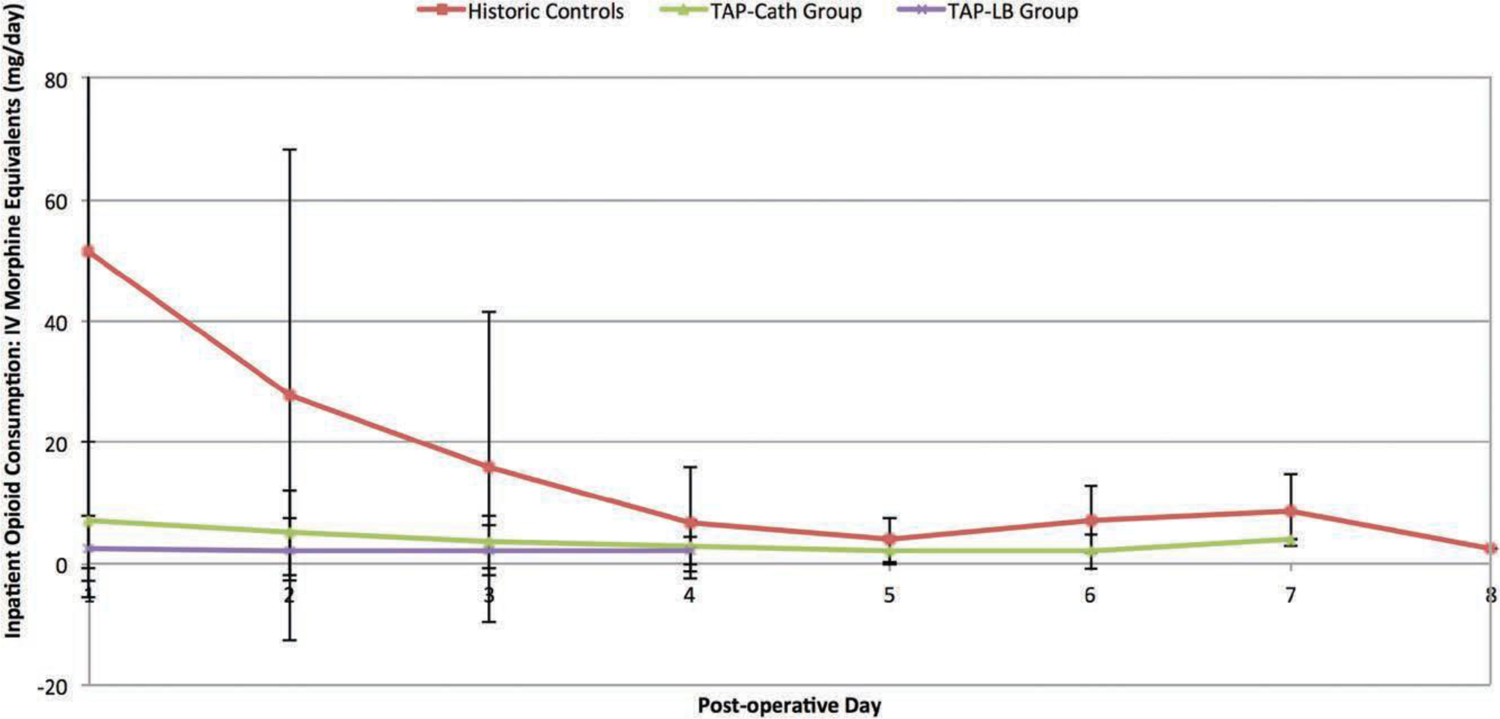
Inpatient opioid consumption: intravenous morphine equivalents (mg/day) following microsurgical abdominally based breast reconstruction relative to changing locoregional analgesia delivery methods.
Table 4.
Average Inpatient Opioid Consumption per Hospital Day*
| No. | POD 0–1 | POD 2 | POD 3 | POD 4 | POD 5 | POD 6 | POD 7 | POD 8 | |
|---|---|---|---|---|---|---|---|---|---|
| Group | |||||||||
| Controls, mg | 40 | 52.86 ± 52.0 | 29.9 ± 42.53 | 16.0 ± 26.83 | 7.11 ± 10.27 | 4.13 ± 3.93 | 8.50 ± 5.74 | 12.00 ± 2.83 | 0 ± 0.00 |
| TAP-Cath, mg | 48 | 7.25 ± 12.82 | 5.17 ± 7.05 | 3.60 ± 4.35 | 2.95 ± 4.24 | 2.00 ± 2.31 | 2.00 ± 2.83 | 4.00 ± 0.00 | — |
| TAP-LB, mg | 40 | 1.88 ±3.97 | 2.12 ± 4.95 | 2.23 ± 4.12 | 2.00 ± 2.31 | — | — | — | — |
| Global p† | <0.0001 | <0.0001 | 0.0181 | 0.1998 | 0.2685 | 0.1391 | — | — | |
| TAP-Cath vs. controls‡ | <0.0001 | 0.0026 | 0.0146 | — | — | — | — | — | |
| TAP-LB vs. controls‡ | <0.0001 | 0.0009 | 0.0075 | — | — | — | — | — | |
| TAP-LB vs.TAP-Cath‡ | 0.0081 | 0.0270 | 0.2157 | — | — | — | — | — |
POD, postoperative day; TAP-Cath, transversus abdominis plane-catheter; TAP-LB, transversus abdominis plane-liposomal bupivacaine.
Intravenous morphine equivalents (in milligrams).
Determined using the Welch analysis of variance using a significance level of 0.05.
Pairwise comparisons were performed using the Welch t test with a Bonferroni correction of 0.016.
Complications
The overall rate of flap loss was 2.2 percent, without any significant difference between the groups. The overall complication rate was 8.7 percent. There were no significant differences in complication rates between the groups, although those in the transversus abdominis plane–catheter group had a higher rate of transfusions (p = 0.0084) (Table 5). There were no complications related to the administration of transversus abdominis plane blocks.
Table 5.
Major Complications
| Controls | TAP-Cath | TAP-LB | p | |
|---|---|---|---|---|
| No. | 40 | 48 | 40 | |
| Total major* | 2 | 6 | 3 | 0.4585 |
| 30-day readmission | 0 | 3 | 1 | 0.3886 |
| Return to OR† | 2 | 7 | 3 | 0.7145 |
| Flap loss (rate) | 0 | 2 | 2 | 0.8550 |
| Transfusions | 1 | 11 | 1 | 0.0084 |
| Other‡ | 2 | 5 | 1 | 0.3061 |
TAP-Cath, transversus abdominis plane–catheter; TAP-LB, transversus abdominis plane–liposomal bupivacaine; OR, operating room.
Total number of patients experiencing major complications.
May include multiple return trips to the operating room for a single patient.
Other complications include hematoma, surgical-site infections, and wound dehiscence.
DISCUSSION
Health care reform and reimbursement models are focused on improving quality and reducing costs. Currently, hospital reimbursement for mastectomy patients averages less than 2 inpatient days. One of the most important determinants of health care costs is length of stay, with 32.2 percent of health care costs being spent on inpatient care alone.6,7 As bundled payment models grow in popularity, health care providers have been incentivized to develop Enhanced Recovery after Surgery protocols to safely reduce length of stay while maintaining patient satisfaction and surgical quality.8,9 These protocols have gained traction in other specialties such as colorectal surgery, but are relatively new in reconstructive microsurgery.
One concern with early discharge after microsurgical breast reconstruction is that flap compromise may occur after the patient is discharged and an opportunity at salvage will be missed. Many institutions feel that postoperative day 4 is the most appropriate day for discharge, as this gives adequate time for close flap observation and medical management.1 However, multiple studies have shown that both take-back and salvage rates for free flaps decrease significantly beyond 48 hours from surgery.10–20 Our take-back rate for vascular compromise during the study period was 4.7 percent, comparable to the flap thrombosis rates of 2.4 to 9 percent reported in the literature.20–24 Of these, 67 percent (n = 4) of the take-backs occurred during the initial hospitalization, and the two flaps that were salvaged were taken back by postoperative day 2. All four flaps taken back after postoperative day 2 were lost. Two of these patients lost their flaps after discharge to home.
We identified high doses of narcotics used to provide postoperative pain control as a major obstacle to achieving early hospital discharge. Narcotic use is associated with nausea, constipation, orthostatic hypotension, drowsiness, confusion, and dizziness, which can delay recovery. Achieving early nonnarcotic pain control hastens ambulation and resumption of a regular diet. We therefore focused on reducing reliance on narcotics by using intraoperative locoregional anesthesia and postoperative nonnarcotic pain medications. Current treatment modalities following breast reconstruction include narcotic analgesia, nonnarcotic analgesia, local anesthesia, regional anesthesia, muscle relaxants, and gamma-amino-butyric acid analogues.4 The consensus is that a multimodal approach leads to better outcomes with improved postoperative pain control and decreased mortality and morbidity after major surgical procedures.25–30
Several studies have shown that Enhanced Recovery after Surgery protocols safely facilitate early hospital discharge following breast reconstruction with abdominally based free flaps.8,31,32 The use of transversus abdominis plane blockade for abdominally based free flap breast reconstruction has evolved over time. A single-dose intraoperative ultrasound-guided transversus abdominis plane block for abdominally based free flaps was first described by Hivelin et al. in 2011.33 The authors conducted a prospective study of 30 consecutive women undergoing immediate deep inferior epigastric artery perforator (DIEP) flap breast reconstruction after modified radical mastectomy. The last 15 patients received bilateral transversus abdominis plane blocks with 1.5 mg/kg ropivacaine on each side immediately after flap harvesting and had significantly reduced postoperative morphine requirements for the first 24 (32.0 mg versus 40.2 mg) and 48 hours (51.7 mg versus 60.5 mg). Similarly, Wheble et al. demonstrated lower use of morphine (15.4 mg versus 71.4 mg), shorter length of stay (4.75 days versus 7.00 days), and fewer episodes of perioperative nausea and vomiting in patients who received intraoperative transversus abdominis plane blockade with 0.25% Chirocaine (Levobupivacaine Hydrochloride; AbbVie, Berkshire, United Kingdom) while undergoing abdominally based free flap breast reconstruction.34
Transversus abdominis plane blocks using nonliposomal forms of local anesthetic can produce clinically useful analgesia for only 12 to 24 hours.35,36 To provide analgesia of longer duration, we began using a continuous postoperative infusion of bupivacaine through catheters placed within the transversus abdominis plane as described previously by Zhong et al.37–39 To maximize the benefit of the catheters, they were left in place until postoperative day 3, at which point the catheters were removed and, if all other criteria were met, the patient was discharged to home. Using this technique, average length of stay was significantly decreased from the historic controls but did not decrease below 3 days because of the reluctance to send patients home with the cumbersome external pump.
Most recently, transversus abdominis plane blockade using single-dose liposomal bupivacaine (Exparel; Pacira Pharmaceuticals, Inc., Parsippany, N.J.) has demonstrated similar benefits of providing long-term analgesia without the need for infusion catheters and cumbersome external pain pumps. Liposomal bupivacaine uses DepoFoam (Pacira), a lipid-based delivery system that provides prolonged, sustained release of local anesthetic over 96 hours and has been shown to provide benefit up to 72 hours postoperatively.40–42 The introduction of singe-dose liposomal bupivacaine to our practice in early 2014 allowed us to provide long-term analgesia to the abdominal donor site without the need for transversus abdominis plane–catheters and cumbersome external infusion pumps. In addition, this removed the barrier preventing patients from being discharged before catheter removal on postoperative day 3 while still providing the benefit of long-term locoregional analgesia. Given the ease of delivery, it was also given locally in the breast region and at the drain sites to further suppress postoperative pain.
In a recent study by Batdorf et al., use of an Enhanced Recovery after Surgery protocol enabled the authors to reduce length of stay after abdominally based microsurgical breast reconstruction from 5.5 days to 3.9 days. Similar to the current study, they used transversus abdominis plane blocks with liposomal bupivacaine and a nonnarcotic postoperative pain control regimen.31 However, the study was criticized because the Enhanced Recovery after Surgery group had significantly more DIEP flaps versus free transverse abdominis myocutaneous flaps when compared to the control group, which was felt to be a confounding variable when looking at length of stay.43,44
In our study, there was no significant difference in DIEP versus free transverse abdominis myocutaneous versus superficial inferior epigastric artery flaps between study groups, and we were able to reduce our average length of stay significantly from 4.05 days in the control group to 3.52 days in the transversus abdominis plane–catheter group and to 2.65 days in the transversus abdominis plane–liposomal bupivacaine group. With each change in the Enhanced Recovery after Surgery protocol, there was a lag phase in reducing the length of stay, as the changes in discharge timing were driven by improvements in speed of patient recovery. The shortened recuperative time led to changes in location of recovery from surgical intensive care unit to step-down unit and timing of ambulation and advancement of diet. This in turn allowed a reduction in time to discharge. In fact, when we compared the first 20 transversus abdominis plane–liposomal bupivacaine patients to the last 20 patients, the average length of stay dropped from 3.0 days to 2.3 days (Fig. 3). Similar to other authors, we found decreased opioid consumption in the first 3 postoperative days in the transversus abdominis plane–catheter group compared with the control group and a further decrease in opioid use in the transversus abdominis plane–liposomal bupivacaine group compared with the transversus abdominis plane–catheter group, perhaps related to the addition of locoregional analgesia in the breast region. Overall, we found that patients in the transversus abdominis plane–liposomal bupivacaine group not only were discharged earlier, but also had subjectively less pain at follow-up and often did not take any narcotics after discharge.
The transversus abdominis plane–catheter group had significantly more transfusions compared with the other groups. There was an increased number of anticoagulant medications given to patients in this group before adjustments were made to the postoperative anticoagulation protocol; however, we could not identify a causal relationship to the increased transfusion requirement.
Hivelin et al. reported that the additional operative time associated with transversus abdominis plane blocks, from drawing the landmarks and preparing the probe to completing the injections, averaged 21.3 minutes.33 Currently, it takes us 5 minutes to perform bilateral transversus abdominis plane injections, and we are able to perform the injections concomitantly with other portions of the procedure, such as flap inset.34
Risks associated with transversus abdominis plane block include intravascular injection and intraperitoneal injury, which can be minimized by using ultrasound guidance.33,34,45,46 Using this technique, none of our patients experienced any complications associated with this procedure. Use of liposomal bupivacaine for nerve blocks is still considered an off-label use. Surgeons should be familiar with the prescribing information and warnings; in particular, that mixing of lidocaine with liposomal bupivacaine can result in rapid release of bupivacaine and inadvertent overdosage.
Study Limitations
As a retrospective study based on a single surgeon’s experience, there are inherent biases in the study design. Increasing surgeon experience can affect the length and outcome of procedures over time; however, the senior surgeon in this study had been in practice over a decade and had performed hundreds of abdominally based free flap breast reconstructions before starting this initiative. Another potential bias affecting length of stay is the surgeon’s comfort with reducing duration of inpatient flap monitoring. We reviewed the literature on microvascular flap reconstruction, which revealed that inpatient monitoring beyond 48 hours provided rapidly diminishing returns in terms of flap salvage.11–20,47 Also, the literature suggests that increased length of stay increases the risk of nosocomial infections,48,49 which further supported an enhanced recovery protocol.
Although there was no significant difference in rate of flap loss between groups, there was a slight increase in flap loss in the Enhanced Recovery after Surgery groups compared with the control group, which might have reached significance if a larger sample size were studied. This raises the question, “What is the acceptable cost to salvage one additional flap?” Extending inpatient monitoring past postoperative day 2 would only be expected to achieve an additional 0.1 to 0.5 percent reduction in risk of flap loss. Depending on how long monitoring is extended, this could amount to millions of dollars in added cost to salvage a single flap.18,47,50
The grouping of consecutive patients, instead of randomization, may also incur a selection bias. Although patient-reported pain scores during inpatient stay were reviewed for the study, the data were too incomplete to allow for useful analysis, so supplemental opioid consumption was used as a surrogate indicator for adequacy of pain control in the Enhanced Recovery after Surgery groups. Certain aspects of our treatment protocol evolved during the study period, and even though these were driven by the acceleration in patient recovery with the Enhanced Recovery after Surgery protocols, they may have impacted our primary and secondary endpoints. Use of liposomal bupivacaine in the breast region and the drain sites in the transversus abdominis plane–liposomal bupivacaine group may have further suppressed pain and may have accounted for some component of the opioid reduction compared with the transversus abdominis plane–catheter group. However, breast pain usually does not result in hospital stay longer than 2 days, as evidenced by the fact that most patients undergoing implant-based reconstruction are able to leave on postoperative day 1 or 2.51 Therefore, it probably was not the major factor facilitating early discharge. Another factor to be considered is that early discharge from the hospital might result in an increased number of outpatient visits. Although this was not noted to be an issue during the study, it was not specifically measured as a study outcome. Despite these limitations, this study demonstrates that, by using a multimodal Enhanced Recovery after Surgery protocol that incorporates transversus abdominis plane blocks with liposomal bupivacaine, it is possible to consistently and safely discharge patients undergoing microvascular abdominally based breast reconstruction on postoperative day 2.
CONCLUSIONS
Transversus abdominis plane blockade with liposomal bupivacaine, in conjunction with a multimodal nonnarcotic approach to postoperative pain control, is effective in achieving early patient mobilization, decreased inpatient opioid consumption, and reduced hospital stay following microsurgical abdominally based breast reconstruction, without increasing readmissions or complications. This approach enables patients to consistently leave the hospital on postoperative day 2, which represents a significant reduction in length of stay compared with other studies. The ability to achieve pain control without the use of narcotics eliminates many untoward side effects that can delay recovery. These promising results warrant further examination of Enhanced Recovery after Surgery protocols and long-acting locoregional blocks in microsurgical abdominally based breast reconstruction.
Supplementary Material
Footnotes
Presented at the New York Regional Society of Plastic Surgeons, 2016 Annual Residents Night, in New York, New York, March 14, 2016; the 33rd Annual Meeting of the Northeastern Society of Plastic Surgeons, in Baltimore, Maryland, October 14 through 16, 2016; and The Meeting 2016: Annual Meeting of the American Society of Plastic Surgeons, in Los Angeles, California, September 23 through 27, 2016.
REFERENCES
- 1.Nelson JA, Cleveland E, Sieber B, Rohrbach JI, Serletti JM, Kanchwala S. Breast reconstruction modality outcome study: A comparison of expander/implants and free flaps in select patients. Plast Reconstr Surg. 2013;131:928–934. [DOI] [PubMed] [Google Scholar]
- 2.Momoh AO, Hilliard PE, Chung KC. Regional and neuraxial analgesia for plastic surgery: Surgeon’s and anesthesiologist’s perspectives. Plast Reconstr Surg. 2014;134(Suppl 2):58S–68S. [DOI] [PubMed] [Google Scholar]
- 3.Tran TM, Ivanusic JJ, Hebbard P, Barrington MJ. Determination of spread of injectate after ultrasound-guided transversus abdominis plane block: A cadaveric study. Br J Anaesth. 2009;102:123–127. [DOI] [PubMed] [Google Scholar]
- 4.Pizzi LT, Toner R, Foley K, et al. Relationship between potential opioid-related adverse effects and hospital length of stay in patients receiving opioids after orthopedic surgery. Pharmacotherapy 2012;32:502–514. [DOI] [PubMed] [Google Scholar]
- 5.Oderda GM, Said Q, Evans RS, et al. Opioid-related adverse drug events in surgical hospitalizations: Impact on costs and length of stay. Ann Pharmacother. 2007;41:400–406. [DOI] [PubMed] [Google Scholar]
- 6.Shah A, Rowlands M, Krishnan N, Patel A, Ott-Young A. Thoracic intercostal nerve blocks reduce opioid consumption and length of stay in patients undergoing implant-based breast reconstruction. Plast Reconstr Surg. 2015;136:584e–591e. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Medicare and Medicaid Services. National health expenditures 2015 highlights. Available at: https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/downloads/high-lights.pdf. Accessed December 26, 2016.
- 8.Davidge K, Armstrong KA, Brown M, et al. Shifting autologous breast reconstruction into an ambulatory setting: Patient-reported quality of recovery. Plast Reconstr Surg. 2015;136:657–665. [DOI] [PubMed] [Google Scholar]
- 9.Hussey PS, Sorbero ME, Mehrotra A, Liu H, Damberg CL. Episode-based performance measurement and payment: Making it a reality. Health Aff (Millwood) 2009;28:1406–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nelson JA, Kim EM, Eftekhari K, et al. Late venous thrombosis in free flap breast reconstruction: Strategies for salvage after this real entity. Plast Reconstr Surg. 2012;129:8e–15e. [DOI] [PubMed] [Google Scholar]
- 11.Yang X, Li S, Wu K, et al. Surgical exploration of 71 free flaps in crisis following head and neck reconstruction. Int J Oral Maxillofac Surg. 2016;45:153–157. [DOI] [PubMed] [Google Scholar]
- 12.Nahabedian MY, Momen B, Manson PN. Factors associated with anastomotic failure after microvascular reconstruction of the breast. Plast Reconstr Surg. 2004;114:74–82. [DOI] [PubMed] [Google Scholar]
- 13.Chen KT, Mardini S, Chuang DC, et al. Timing of presentation of the first signs of vascular compromise dictates the salvage outcome of free flap transfers. Plast Reconstr Surg. 2007;120:187–195. [DOI] [PubMed] [Google Scholar]
- 14.Bui DT, Cordeiro PG, Hu QY, Disa JJ, Pusic A, Mehrara BJ. Free flap reexploration: Indications, treatment, and outcomes in 1193 free flaps. Plast Reconstr Surg. 2007;119:2092–2100. [DOI] [PubMed] [Google Scholar]
- 15.Yu P, Chang DW, Miller MJ, Reece G, Robb GL. Analysis of 49 cases of flap compromise in 1310 free flaps for head and neck reconstruction. Head Neck 2009;31:45–51. [DOI] [PubMed] [Google Scholar]
- 16.Selber JC, Angel Soto-Miranda M, Liu J, Robb G. The survival curve: Factors impacting the outcome of free flap take-backs. Plast Reconstr Surg. 2012;130:105–113. [DOI] [PubMed] [Google Scholar]
- 17.Yang Q, Ren ZH, Chickooree D, et al. The effect of early detection of anterolateral thigh free flap crisis on the salvage success rate, based on 10 years of experience and 1072 flaps. Int J Oral Maxillofac Surg. 2014;43:1059–1063. [DOI] [PubMed] [Google Scholar]
- 18.Chang EI, Carlsen BT, Festekjian JH, Da Lio AL, Crisera CA. Salvage rates of compromised free flap breast reconstruction after recurrent thrombosis. Ann Plast Surg. 2013;71:68–71. [DOI] [PubMed] [Google Scholar]
- 19.Brown JS, Devine JC, Magennis P, Sillifant P, Rogers SN, Vaughan ED. Factors that influence the outcome of salvage in free tissue transfer. Br J Oral Maxillofac Surg. 2003;41:16–20. [DOI] [PubMed] [Google Scholar]
- 20.Kroll SS, Schusterman MA, Reece GP, et al. Timing of pedicle thrombosis and flap loss after free-tissue transfer. Plast Reconstr Surg. 1996;98:1230–1233. [DOI] [PubMed] [Google Scholar]
- 21.Khouri RK, Cooley BC, Kunselman AR, et al. A prospective study of microvascular free-flap surgery and outcome. Plast Reconstr Surg. 1998;102:711–721. [DOI] [PubMed] [Google Scholar]
- 22.Vijan SS, Tran VN. Microvascular breast reconstruction pedicle thrombosis: How long can we wait? Microsurgery 2007;27:544–547. [DOI] [PubMed] [Google Scholar]
- 23.Trussler AP, Watson JP, Crisera CA. Late free-flap salvage with catheter-directed thrombolysis. Microsurgery 2008;28:217–222. [DOI] [PubMed] [Google Scholar]
- 24.Mirzabeigi MN, Wang T, Kovach SJ, Taylor JA, Serletti JM, Wu LC. Free flap take-back following postoperative microvascular compromise: Predicting salvage versus failure. Plast Reconstr Surg. 2012;130:579–589. [DOI] [PubMed] [Google Scholar]
- 25.Rosero EB, Joshi GP. Preemptive, preventive, multimodal analgesia: What do they really mean? Plast Reconstr Surg. 2014;134(Suppl 2):85S–93S. [DOI] [PubMed] [Google Scholar]
- 26.Woolf CJ; American College of Physicians; American Physiological Society. Pain: Moving from symptom control toward mechanism-specific pharmacologic management. Ann Intern Med. 2004;140:441–451. [DOI] [PubMed] [Google Scholar]
- 27.Kehlet H, Dahl JB. The value of “multimodal” or “balanced analgesia” in postoperative pain treatment. Anesth Analg. 1993;77:1048–1056. [DOI] [PubMed] [Google Scholar]
- 28.Kehlet H, Werner M, Perkins F. Balanced analgesia: What is it and what are its advantages in postoperative pain? Drugs 1999;58:793–797. [DOI] [PubMed] [Google Scholar]
- 29.Lavand’homme P, De Kock M. The use of intraoperative epidural or spinal analgesia modulates postoperative hyperalgesia and reduces residual pain after major abdominal surgery. Acta Anaesthesiol Belg. 2006;57:373–379. [PubMed] [Google Scholar]
- 30.Farragher RA, Laffey JG. Postoperative pain management after cesarean section. In: Sorten G, Carr DB, Harmon D, Puig MM, Browne, eds. Postoperative Pain Management: An Evidence-Based Guide to Practice. Philadelphia: Saunders Elsevier; 2006:225–238. [Google Scholar]
- 31.Batdorf NJ, Lemaine V, Lovely JK, et al. Enhanced recovery after surgery in microvascular breast reconstruction. J Plast Reconstr Aesthet Surg. 2015;68:395–402. [DOI] [PubMed] [Google Scholar]
- 32.Rosero EB, Joshi GP. Preemptive, preventive, multimodal analgesia: What do they really mean? Plast Reconstr Surg. 2014;134(Suppl 2):85S–93S. [DOI] [PubMed] [Google Scholar]
- 33.Hivelin M, Wyniecki A, Plaud B, Marty J, Lantieri L. Ultrasound-guided bilateral transversus abdominis plane block for postoperative analgesia after breast reconstruction by DIEP flap. Plast Reconstr Surg. 2011;128:44–55. [DOI] [PubMed] [Google Scholar]
- 34.Wheble GA, Tan EK, Turner M, Durrant CA, Heppell S. Surgeon-administered, intra-operative transversus abdominis plane block in autologous breast reconstruction: A UK hospital experience. J Plast Reconstr Aesthet Surg. 2013;66:1665–1670. [DOI] [PubMed] [Google Scholar]
- 35.Carney J, McDonnell JG, Ochana A, Bhinder R, Laffey JG. The transversus abdominis plane block provides effective postoperative analgesia in patients undergoing total abdominal hysterectomy. Anesth Analg. 2008;107:2056–2060. [DOI] [PubMed] [Google Scholar]
- 36.Jankovic Z Transversus abdominis plane block: The Holy Grail of anaesthesia for (lower) abdominal surgery. Period Biol. 2009;111:203–208. [Google Scholar]
- 37.Zhong T, Wong KW, Cheng H, et al. Transversus abdominis plane (TAP) catheters inserted under direct vision in the donor site following free DIEP and MS-TRAM breast reconstruction: A prospective cohort study of 45 patients. J Plast Reconstr Aesthet Surg. 2013;66:329–336. [DOI] [PubMed] [Google Scholar]
- 38.Zhong T, Ojha M, Bagher S, et al. Transversus abdominis plane block reduces morphine consumption in the early postoperative period following microsurgical abdominal tissue breast reconstruction: A double-blind, placebo-controlled, randomized trial. Plast Reconstr Surg. 2014;134:870–878. [DOI] [PubMed] [Google Scholar]
- 39.Giordano S, Veräjänkorva E, Koskivuo I, Suominen E. Effectiveness of local anaesthetic pain catheters for abdominal donor site analgesia in patients undergoing free lower abdominal flap breast reconstruction: A meta-analysis of comparative studies. J Plast Surg Hand Surg. 2013;47:428–433. [DOI] [PubMed] [Google Scholar]
- 40.Gorfine SR, Onel E, Patou G, Krivokapic ZV. Bupivacaine extended-release liposome injection for prolonged postsurgical analgesia in patients undergoing hemorrhoidectomy: A multicenter, randomized, double-blind, placebo-controlled trial. Dis Colon Rectum 2011;54:1552–1559. [DOI] [PubMed] [Google Scholar]
- 41.Golf M, Daniels SE, Onel E. A phase 3, randomized, placebo-controlled trial of DepoFoam bupivacaine (extended-release bupivacaine local analgesic) in bunionectomy. Adv Ther. 2011;28:776–788. [DOI] [PubMed] [Google Scholar]
- 42.Dasta J, Ramamoorthy S, Patou G, Sinatra R. Bupivacaine liposome injectable suspension compared with bupivacaine HCl for the reduction of opioid burden in the postsurgical setting. Curr Med Res Opin. 2012;28:1609–1615. [DOI] [PubMed] [Google Scholar]
- 43.Varadhan KK, Lobo DN, Ljungqvist O. Enhanced recovery after surgery: The future of improving surgical care. Crit Care Clin. 2010;26:527–547, x. [DOI] [PubMed] [Google Scholar]
- 44.Al Omran Y, Anwar MO. Re: ‘Enhanced recovery after surgery in microvascular breast reconstruction’. J Plast Reconstr Aesthet Surg. 2015;68:743–744. [DOI] [PubMed] [Google Scholar]
- 45.Farooq M, Carey M. A case of liver trauma with a blunt regional anesthesia needle while performing transversus abdominis plane block. Reg Anesth Pain Med. 2008;33:274–275. [DOI] [PubMed] [Google Scholar]
- 46.Lancaster P, Chadwick M. Liver trauma secondary to ultrasound-guided transversus abdominis plane block. Br J Anaesth. 2010;104:509–510. [DOI] [PubMed] [Google Scholar]
- 47.Henderson PW, Fernandez JG, Cemal Y, et al. Successful salvage of late anastomotic thrombosis after free tissue transfer. J Reconstr Microsurg. 2016;32:316–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dulworth S, Pyenson B. Healthcare-associated infections and length of hospital stay in the Medicare population. Am J Med Qual. 2004;19:121–127. [DOI] [PubMed] [Google Scholar]
- 49.Hassan M, Tuckman H, Patrick R, et al. Hospital length of stay and probability of acquiring infection. Int J Pharm Healthc Mark. 2010;4:324–338. [Google Scholar]
- 50.Ellison A Average cost per inpatient stay across 50 states. Available at: http://www.beckershospitalreview.com/finance/average-cost-per-inpatient-day-across-50-states-2016.html. Accessed December 26, 2016.
- 51.Wilson AJ, Mirzabeigi MN, Serletti JM. Putting it all together: Managing pain in autologous and implant-based breast reconstruction. Plast Reconstr Surg. 2014;134(Suppl 2):120S–125S. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


