Abstract
Circumstantial evidence in human malaria suggests that elimination of parasites by drug treatment meets higher success rates in individuals having some background immunity. In this study, using the rodent malaria model Plasmodium chabaudi, we show that drug-resistant parasites can be cleared by drugs when the host is partially immune.
Malaria due to Plasmodium falciparum is still a major cause of mortality and morbidity in the tropical and subtropical areas of the globe, where around 200 million persons are at constant risk of infection, with some parts of Africa being the worst affected (12). Although antimalarial vaccines are being produced and tested (2, 5), the control of malaria relies heavily on chemotherapy, as many of the available antimalarial drugs are effective, cheap, and easy to distribute. However, in recent years, drug-resistant parasites have emerged and are now widespread. This trend presents a serious challenge to the control of malaria (16, 22) despite our increasing understanding of the genetic and molecular basis of resistance.
In this context, any strategies that maximize the effectiveness of drugs or suboptimal vaccines may lead to significant progress. Among the factors upon which the efficacy of antimalarial chemotherapy is thought to depend (22) is the patient's immune status. This is a subject of some importance because evidence of interactions may influence our use of chemotherapy in areas of drug resistance and our assessment of the value of suboptimal vaccines.
Using the rodent malaria-causing organism Plasmodium chabaudi, which is a good laboratory model for understanding the biology of drug-resistant P. falciparum infections (6), we have studied the relationship between immunity of the host and the capacities of chloroquine and mefloquine to clear resistant parasites. We show that resistant parasites which survive drug treatment in naïve hosts are cleared more efficiently by the same drug dose administered to partially immune hosts.
MATERIALS AND METHODS
Experimental design.
The procedure involved, first, making mice partially immune to a drug-resistant or drug-sensitive clone of P. chabaudi, then reinfecting them with the same clone, and finally treating them with the drugs under investigation. All combinations of three treatments were tested: (i) sensitive or resistant parasite clones, (ii) drug-treated or non-drug-treated parasites, and (iii) immunized or nonimmunized animals. The immunized group was challenged with the parasite homologous to that used for immunization, e.g., a group challenged with sensitive parasites was immunized with sensitive parasites. Two separate experiments were performed: experiment 1 investigated responses of parasites to chloroquine, and experiment 2 investigated responses of parasites to mefloquine.
Parasites and mice.
Three P. chabaudi clones which were either resistant or sensitive to chloroquine or mefloquine (Table 1) were used. They were all derived from a single drug-sensitive parasite clone, AS. This was obtained originally from its natural host in the Central African Republic and subsequently passaged through laboratory mice and mosquitoes (3). A pyrimethamine-resistant clone, ASpyr, was derived from AS following selection with pyrimethamine (21). A stable chloroquine-resistant clone, AS15CQ, was derived from ASpyr by long-term selection with increasing concentrations of chloroquine (14). Finally, a stable mefloquine-resistant clone, AS15MF, was derived from AS15CQ by short-term selection under increasing doses of mefloquine (15). While other mutations may have occurred during their routine maintenance in the laboratory, these clones are considered to be effectively congenic, except for the genes involved in drug resistance.
TABLE 1.
Characteristics of parasite clones used in the experiments
| Expt no. | Parasite clone | Sensitivity to drug | Reference |
|---|---|---|---|
| 1 | ASpyr | Chloroquine sensitive | 21 |
| AS15CQ | Chloroquine resistant | 14 | |
| 2 | AS15CQ | Mefloquine sensitive | 14 |
| AS15MF | Mefloquine resistant | 15 |
The mice used were inbred CBA females aged between 4 and 6 weeks at the time of infection.
Immunization.
In order to induce partial immunity, mice were inoculated intraperitoneally with 104 live parasites. Five or 6 days later, when the infection was becoming patent, parasites were cleared with 200 mg of mefloquine per kg of body weight given orally over a period of 4 days; this was curative for all clones including AS15MF (data not shown). Control naïve mice were inoculated with citrate saline (0.85% NaCl, 1.5% trisodium citrate) and treated with the drug in the same way.
Experimental infection and treatment.
Each experiment consisted of eight treatment groups (Table 2). Two to 3 weeks after immunization, all mice were challenged intraperitoneally with 106 live parasites on day zero. (It was assumed that all residual mefloquine used in the immunization procedure had been eliminated at this point, since mefloquine has a short half-life in mice, of approximately 18 h [16].) Three hours later an oral dose (5 mg/kg) of chloroquine (experiment 1), of mefloquine (experiment 2), or of diluent (untreated groups) was administered. This dose was repeated every 24 h for 6 or 4 consecutive days for experiment 1 or 2, respectively. Parasitemias were monitored by microscopic examination of Giemsa-stained thin blood smears every 2 days from day 5 or 6 for up to 30 days. Counts of parasites were made in approximately 5,000 red blood cells to obtain the percentage of parasitemias.
TABLE 2.
Treatment groups
| Expt no. | Immunizing parasite | Challenge parasite | Group no. | Drug treatment |
|---|---|---|---|---|
| 1 | None | ASpyr | 1 | None |
| 5 | Chloroquine | |||
| None | AS15CQ | 2 | None | |
| 6 | Chloroquine | |||
| ASpyr | ASpyr | 3 | None | |
| 7 | Chloroquine | |||
| AS15CQ | AS15CQ | 4 | None | |
| 8 | Chloroquine | |||
| 2 | None | AS15CQ | 1 | None |
| 5 | Mefloquine | |||
| None | AS15MF | 2 | None | |
| 6 | Mefloquine | |||
| AS15CQ | AS15CQ | 3 | None | |
| 7 | Mefloquine | |||
| AS15MF | AS15MF | 4 | None | |
| 8 | Mefloquine |
Statistical methods.
For statistical evaluation of the effects of drug treatment and immunity upon the growth of resistant and sensitive clones, the log of the area under the parasitemia curve from days 0 to 12 postinfection was calculated for each mouse. Analyses of variance were performed on these data using models that included main effects for parasite clone (resistant versus sensitive), immunity (naïve versus partially immune), and drug treatment (untreated versus drug treated). Their two-way interactions were included in the model where significant (at a P level of <0.05). Within each experiment, analyses were performed separately for drug-treated and untreated groups because these groups had different residual variances.
RESULTS
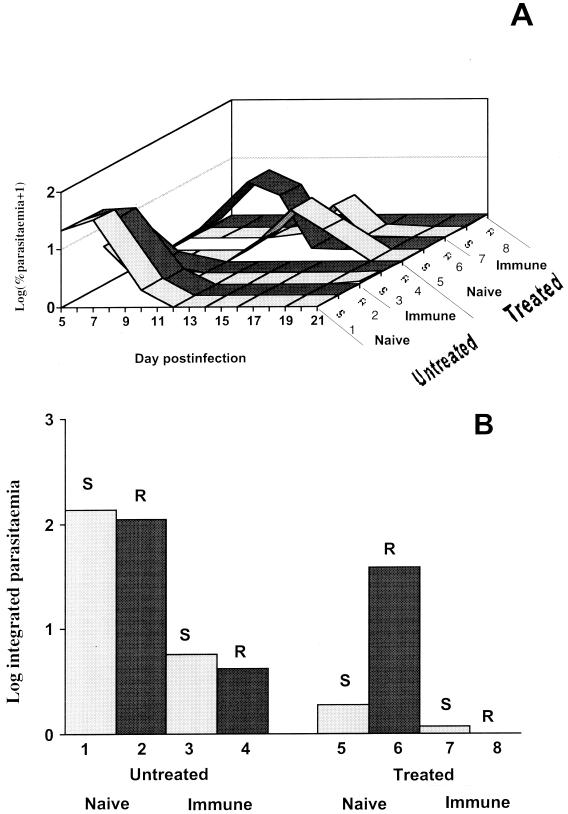
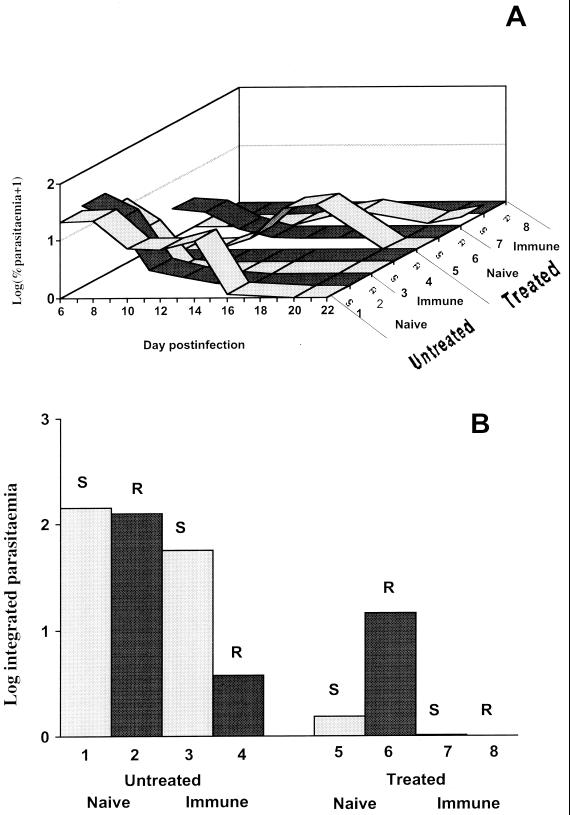
Results for experiments 1 and 2 are shown in Fig. 1 and 2, respectively. Figures 1A and 2A show the log parasitemia profiles for each group of mice from day 5 or 6 postinfection to day 21 or 22. Figures 1B and 2B show indexes of the total number of parasites produced when drugs were present in the bloodstream (log of the area under the parasitemia curve, from days 0 to 12). The results from experiments 1 and 2 (treatments with chloroquine and mefloquine, respectively) were similar and are therefore described together below.
FIG. 1.
Results of experiments of P. chabaudi infections with resistant parasites (R) or sensitive parasites (S) in naïve and immunized mice following treatment (Treated) or no treatment (Untreated) with chloroquine. Numbers 1 to 8 represent the experimental groups shown in Table 2. (A) Parasitemias (log10 transformed) from days 5 to 21 postinfection. (B) Total parasitemias (log10 transformed) integrated over days 0 to 12 postinfection.
FIG. 2.
Results of experiments of P. chabaudi infections as for Fig. 1, except that the drug used was mefloquine.
Untreated mice, days 0 to 12 postinfection.
As expected, parasitemias in untreated partially immune mice were much lower than in untreated naïve mice during the first 12 days of infection (P < 0.001 and P < 0.01 for experiments 1 and 2, respectively), showing that partial immunity was successful in reducing parasite growth in the absence of drugs (Fig. 1B and 2B). Drug-resistant parasites showed slightly lower total parasitemias than did drug-sensitive clones, but these differences were not significant in either experiment (P = 0.10 and P = 0.19). There was no significant interaction between clone and immunity in untreated mice (P = 0.35 and P = 0.47).
Treated mice, days 0 to 12 postinfection.
In nonimmune mice, the drug-resistant clones produced much higher total parasitemias under drug treatment than did drug-sensitive clones (P < 0.001 for both experiments), as expected (Fig. 1B and 2B). However, in partially immune mice, the parasitemias of the drug-resistant clones under the same drug treatment were much reduced and similar to those of the drug-sensitive clones (P = 0.37 and P = 0.96) (Fig. 1B and 2B). The resistant clones also produced significantly lower parasitemias in immune mice than in naïve animals (P < 0.001 in both experiments).
While partial immunity reduced the growth of the drug-resistant clone in the absence of drugs, there was a further reduction when both drugs and partial immunity were present in both experiments (P < 0.05 and P < 0.001).
After day 12 postinfection.
In untreated mice, peak parasitemias occurred within 8 days and were usually cleared by day 12 postinfection. After drug treatment, however, some experimental groups showed recrudescence of parasites (Fig. 1A and 2A). These recrudescences were most pronounced with sensitive parasites which had not reached high parasitemias prior to day 12. We do not understand why sensitive parasites recrudesced slightly in immune mice under treatment, whereas resistant clones did not. A likely possibility is that prior immunity requires boosting by the presence of significant parasite numbers early in the challenging infection in order to be effective and that poor growth of sensitive clones under drug treatment is insufficient to restimulate this immune response. Resistant parasites showed either no recrudescence (immune mice) or typically small (and delayed in experiment 2) recrudescences in naïve mice.
DISCUSSION
The results of our experiments, specifically the observation that partial immunity can render drug-resistant parasites sensitive, indicate that the interaction between drugs and immunity reported previously (1, 13, 18, 22) also applies to drug-resistant parasites.
It has long been suggested that partially immune patients (e.g., those individuals exposed to malaria since birth) respond better to chemotherapy than nonimmune individuals (22, 23). There is clinical evidence from field studies, albeit circumstantial, that appears to support this view (7, 17, 19, 20, 22, 23, 24). In addition, experimental animal models also appear to support these observations (4, 8, 10), thus suggesting that immunity increases drug efficacy. However, our present study appears to be the first to show that drug-resistant parasites may behave as sensitive ones in the presence of partial immunity.
How might the interaction between drug resistance and immunity be mediated? First, the effects observed in this study may result from a direct effect of immunity on parasite numbers. The combination of drugs and immunity may be sufficient to limit parasite population growth to virtually zero, whereas drugs or immunity alone may be insufficient to keep growth in check when the parasite is equipped with a drug resistance mechanism. Second, it is possible that there is a direct interaction between the parasite's drug resistance apparatus and the host's immune clearance mechanisms or the parasite's response to these.
The findings reported here may have important implications for vaccine development and antimalarial drug use policy. Our results suggest that suboptimal vaccines may have value when combined with antimalarial chemotherapy to clear resistant parasites and thus to control disease levels, a factor that is especially relevant in areas where the human population is only semi-immune to malaria. In addition, such vaccines may be particularly advantageous for the protection of nonimmune visitors to areas where drug-resistant parasites are prevalent. However, our results also suggest that parasites assessed to be drug resistant on the basis of genotyping or in vitro testing may prove to be drug sensitive in patients with some level of immunity. Thus, the clinical responses of such patients may be more effective than those predicted on the basis of parasite typing. In contrast, in areas of malaria endemicity where partial immunity is widespread (such as sub-Saharan Africa), assessment of drug failure rates in vivo may lead to underestimation of the prevalence of drug-resistant parasites; this may encourage a misplaced confidence in antimalarial treatment among nonimmune visitors. Finally, the combination of immunity and drugs will strongly influence the rates of recrudescence following drug treatment, subclinical infections, and transmission. As these are key factors that determine the rate of spread of drug resistance (9, 11), they need to be taken into account when managing drug resistance.
ACKNOWLEDGMENTS
We are grateful to Richard Fawcett and Ronnie Mooney for technical assistance and to Alex Rowe and Alison Creasey for critical reading of the manuscript.
Pedro Cravo was supported by CMDT and PRAXIS XXI (ref. BD/13824/97) of Portugal, Margaret J. Mackinnon was supported by the Leverhulme Trust and the University of Edinburgh, and the remaining authors and their work were funded by the Medical Research Council of Great Britain (Programme Grant to David Walliker [ref. G8009302R]). Animal procedures were conducted under license, following the United Kingdom Animals Scientific Procedures.
REFERENCES
- 1.Awasthi A, Mehrotra S, Bhakuni V, Dutta G P, Levy H B, Maheshwari R K. Poly ICLC enhances the antimalarial activity of chloroquine against multidrug-resistant Plasmodium yoelii nigeriensis in mice. J Interferon Cytokine Res. 1997;17:419–423. doi: 10.1089/jir.1997.17.419. [DOI] [PubMed] [Google Scholar]
- 2.Ballou W R, Kester K E, Stoute J A, Heppner D G. Malaria vaccines: triumphs or tribulations? Parassitologia. 1999;41:403–408. [PubMed] [Google Scholar]
- 3.Beale G H, Carter R, Walliker D. Genetics. In: Killick-Kendrick R, Peters W, editors. Rodent malaria. London, United Kingdom: Academic Press; 1978. pp. 213–245. [Google Scholar]
- 4.Bjorkman A. Interactions between chemotherapy and immunity to malaria. Prog Allergy. 1988;41:331–356. doi: 10.1159/000318627. [DOI] [PubMed] [Google Scholar]
- 5.Brown G V. Progress in the development of malaria vaccines: context and constraints. Parassitologia. 1999;41:429–432. [PubMed] [Google Scholar]
- 6.Carlton J-M, Hayton K, Cravo P, Walliker D. Of mice and malaria mutants: unravelling the genetics of drug resistance using rodent malaria models. Trends Parasitol. 2001;17:236–242. doi: 10.1016/s1471-4922(01)01899-2. [DOI] [PubMed] [Google Scholar]
- 7.Draper C C, Brubaker G, Geser A, Kilimali V A, Wernsdorfer W H. Serial studies on the evolution of chloroquine resistance in an area of East Africa receiving intermittent malaria chemosuppression. Bull W H O. 1985;63:109–118. [PMC free article] [PubMed] [Google Scholar]
- 8.Golenser J, Verhave J P, de Valk J, Heeren J, Meuwissen J H. Studies on the role of antibodies against sporozoites in Plasmodium berghei malaria. Isr J Med Sci. 1978;14:606–610. [PubMed] [Google Scholar]
- 9.Hastings I M. A model for the origins and spread of drug-resistant malaria. Parasitology. 1997;115:133–141. doi: 10.1017/s0031182097001261. [DOI] [PubMed] [Google Scholar]
- 10.Lwin M, Targett G A, Doenhoff M J. Reduced efficacy of chemotherapy of Plasmodium chabaudi in T cell-deprived mice. Trans R Soc Trop Med Hyg. 1987;81:899–902. doi: 10.1016/0035-9203(87)90343-9. [DOI] [PubMed] [Google Scholar]
- 11.Mackinnon M J, Hastings I M. The evolution of multiple drug resistance in malaria parasites. Trans R Soc Trop Med Hyg. 1998;92:188–195. doi: 10.1016/s0035-9203(98)90745-3. [DOI] [PubMed] [Google Scholar]
- 12.Marsh K. Malaria disaster in Africa. Lancet. 1998;352:924. doi: 10.1016/S0140-6736(05)61510-3. [DOI] [PubMed] [Google Scholar]
- 13.Mohan K, Sam H H, Stevenson M M. Therapy with a combination of low doses of interleukin-12 and chloroquine completely cures blood-stage malaria, prevents severe anemia, and induces immunity to reinfection. Infect Immun. 1999;67:513–519. doi: 10.1128/iai.67.2.513-519.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Padua R A. Plasmodium chabaudi: genetics of resistance to chloroquine. Exp Parasitol. 1981;52:419–426. doi: 10.1016/0014-4894(81)90101-6. [DOI] [PubMed] [Google Scholar]
- 15.Padua R A, Walliker D. Mefloquine resistance in Plasmodium chabaudi. Trans R Soc Trop Med Hyg. 1978;72:643. [Google Scholar]
- 16.Peters W. Chemotherapy and drug resistance in malaria. 2nd ed. London, United Kingdom: Academic Press; 1987. [Google Scholar]
- 17.Price R N, Nosten F, Luxemburger C, van Vugt M, Phaipun L, Chongsuphajaisiddhi T, White N J. Artesunate/mefloquine treatment of multi-drug resistant falciparum malaria. Trans R Soc Trop Med Hyg. 1997;91:574–577. doi: 10.1016/s0035-9203(97)90032-8. [DOI] [PubMed] [Google Scholar]
- 18.Siddiqui W A, Kans S C, Kramer K, Case S, Palmer K, Niblack J F. Use of a synthetic adjuvant in an effective vaccination of monkeys against malaria. Nature. 1981;289:64–66. doi: 10.1038/289064a0. [DOI] [PubMed] [Google Scholar]
- 19.ter Kuile F O, Luxemburger C, Nosten F, Thwai K L, Chongsuphajaisiddhi T, White N J. Predictors of mefloquine treatment failure: a prospective study of 1590 patients with uncomplicated falciparum malaria. Trans R Soc Trop Med Hyg. 1995;89:660–664. doi: 10.1016/0035-9203(95)90435-2. [DOI] [PubMed] [Google Scholar]
- 20.Tin F, Nyunt-Hlaing Comparative drug trial of a sulfadoxine/pyrimethamine and a sulfalene/pyrimethamine combination against Plasmodium falciparum infections in semi-immune populations of Burma. Southeast Asian J Trop Med Public Health. 1984;15:238–248. [PubMed] [Google Scholar]
- 21.Walliker D, Carter R, Sanderson A. Genetic studies on Plasmodium chabaudi: recombination between enzyme markers. Parasitology. 1975;66:309–320. doi: 10.1017/s0031182000048824. [DOI] [PubMed] [Google Scholar]
- 22.White N J. Why is it that antimalarial drug treatments do not always work? Ann Trop Med Parasitol. 1998;92:449–458. doi: 10.1080/00034989859429. [DOI] [PubMed] [Google Scholar]
- 23.White N J, Nosten F, Looareesuwan S, Watkins W M, Marsh K, Snow R W, Kokwaro G, Ouma J, Hien T T, Molyneux M E, Taylor T E, Newbold C I, Ruebush T K, Danis M, Greenwood B M, Anderson R M, Olliaro P. Averting a malaria disaster. Lancet. 1999;353:1965–1967. doi: 10.1016/s0140-6736(98)07367-x. [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization. Chemotherapy of malaria and resistance to antimalarials. WHO Tech Rep Ser. 1973;529:1–21. [PubMed] [Google Scholar]