Abstract
BACKGROUND
There are currently no recommendations guiding when best to perform coronary artery calcium (CAC) scanning among young adults to identify those susceptible for developing premature atherosclerosis.
OBJECTIVES
The purpose of this study was to determine the ideal age at which a first CAC scan has the highest utility according to atherosclerotic cardiovascular disease (ASCVD) risk factor profile.
METHODS
We included 22,346 CAC Consortium participants aged 30–50 years who underwent noncontrast computed tomography. Sex-specific equations were derived from multivariable logistic modeling to estimate the expected probability of CAC >0 according to age and the presence of ASCVD risk factors.
RESULTS
Participants were on average 43.5 years of age, 25% were women, and 34% had CAC >0, in whom the median CAC score was 20. Compared with individuals without risk factors, those with diabetes developed CAC 6.4 years earlier on average, whereas smoking, hypertension, dyslipidemia, and a family history of coronary heart disease were individually associated with developing CAC 3.3–4.3 years earlier. Using a testing yield of 25% for detecting CAC >0, the optimal age for a potential first scan would be at 36.8 years (95% CI: 35.5–38.4 years) in men and 50.3 years (95% CI: 48.7–52.1 years) in women with diabetes, and 42.3 years (95% CI: 41.0–43.9 years) in men and 57.6 years (95% CI: 56.0–59.5 years) in women without risk factors.
CONCLUSIONS
Our derived risk equations among health-seeking young adults enriched in ASCVD risk factors inform the expected prevalence of CAC >0 and can be used to determine an appropriate age to initiate clinical CAC testing to identify individuals most susceptible for early/premature atherosclerosis.
Keywords: cardiovascular diseases, coronary artery calcium, multidetector computed tomography, premature atherosclerosis, young adults
Coronary artery calcium (CAC), as measured by noncontrast cardiac computed tomography (CT), is a noninvasive measure of subclinical coronary atherosclerosis that is strongly associated with incident coronary heart disease (CHD) and atherosclerotic cardiovascular disease (ASCVD) (1). Overall, CAC or its absence (CAC = 0), is a robust prognostic indicator for ASCVD-related mortality that is recommended for risk stratification among adults ≥40 years of age at borderline to intermediate risk when there is uncertainty in the initiation of primary prevention pharmacotherapy (2). Recent observations have indicated a use of CAC scoring for clinical decision-making among young adults (age 30–50 years) who have ASCVD risk factors (3–7). However, although the progression of the Agatston CAC score follows an expected exponential growth trajectory, the optimal timing and determinants of initial conversion from CAC = 0 to CAC >0 are unknown (8). Predicting the initial conversion to CAC >0 can help to determine the recommended age for initiating CAC testing in younger adults and identify at-risk individuals several years to decades before the onset of clinical CHD. In particular, such an approach may be valuable to identify young patients who are susceptible for developing early/premature atherosclerosis, allowing for timely initiation of preventive measures.
Currently, ASCVD risk equations help to guide subclinical atherosclerotic disease imaging at middle adult age (9). In this age group, CAC scores of >0 or >100 are commonly used thresholds because CAC <100 represents mild coronary atherosclerotic plaque burden, whereas a CAC score >100 suggests a potential for progression toward very high CAC scores (≥1,000) reflective of secondary prevention risk (10). However, such approaches may have limited value in younger adults because of the strong reliance of ASCVD risk equations on age, and the lower expected CAC burden in young adults. Thus, further studies are required to help guide clinicians and to inform guidelines regarding when to initiate CAC scanning in young adults who may be at-risk for the early or premature manifestation of coronary atherosclerosis and therefore future CHD.
To fill this knowledge gap, we sought to do the following: 1) determine the prevalence, characteristics, and predicted growth rate of premature CAC; and 2) model the probability and initial age of conversion to CAC >0 according to the presence of ASCVD risk factors among younger men and women.
METHODS
STUDY POPULATION.
The CAC Consortium is a multicenter cohort study involving 4 high-volume centers in the United States, Cedars-Sinai Medical Center (Los Angeles, California), PrevaHealth Wellness Diagnostic Center (Columbus, Ohio), Harbor-UCLA Medical Center (Torrance, California), and Minneapolis Heart Institute (Minneapolis, Minnesota). The rationale for the multicenter retrospective cohort study was to assess the association of CAC with long-term, disease-specific mortality, and the study design and methods have been previously described in detail elsewhere (11). In brief, investigators included individuals 18 years of age or older who were free of clinical ASCVD or cardiovascular symptoms at the time of CAC scanning. Participants were referred by a physician to undergo CAC scoring as a part of clinical practice for the purposes of ASCVD risk prediction in the setting of underlying risk factors, an intermediate 10-year ASCVD risk, and/or a significant family history of CHD. A majority of participants reported dyslipidemia and/or family history of CHD as precipitants for undergoing CAC scanning. All study participants provided written informed consent for deidentified clinical research at the respective participating field center, and Institutional Review Board approval for coordinating center actions was by the Johns Hopkins University School of Medicine.
Findings represented in the current analysis represent the baseline CAC Consortium data collection, occurring from 1991–2010. After excluding 43,979 participants ≥50 years of age and 311 patients <30 years of age, there were 22,346 individuals included in the current investigation.
MEASUREMENT OF CAC.
CAC was quantified using noncontrast, ECG-gated cardiac computed tomography (CT) at all participating medical centers according to a standard protocol (11). Both electron beam tomography and multidetector CT were used for imaging, and previous studies have shown no clinically significant differences in CAC measurement between these 2 scanning methods. Calcium scores were computed using the Agatston method, with CAC score groups of 0, 1–100, or ≥100 Agatston units (AU).
EVALUATION OF ASCVD RISK FACTORS.
Assessment of ASCVD risk factors occurred during the clinical visit that accompanied CAC testing. Diabetes and hypertension were defined by a previous clinical diagnosis or reported antihypertensive or glucose-lowering medication use. There was no information regarding the differentiation between type 1 vs type 2 diabetes. Dyslipidemia (low-density lipoprotein cholesterol ≥160 mg/dL, hypertriglyceridemia ≥150 mg/dL, and/or low high-density lipoprotein cholesterol <40 mg/dL in men, <50 mg/dL in women) were defined by a previous clinical diagnosis, laboratory results, or use of lipid-lowering therapy. Information on smoking and family history of CHD (first-degree relative with history of CHD at any age) were obtained through self-reported data. The 10-year risk for ASCVD was calculated using the pooled-cohort equations (9), including using the raw equations to extrapolate risk for those <40 years of age.
For the current study sample, 80% of individuals were derived from Cedars-Sinai Medical Center, PrevaHealth Wellness Diagnostic Center, and Minneapolis Heart Institute and had full information on age, sex, race, ASCVD risk factors, and CAC data (11). Multiple imputation was performed on the remaining 20% of participants with limited missing risk factor data who underwent CAC scoring and clinical examination at UCLA-Harbor. Because a higher proportion of younger vs older participants at UCLA-Harbor had missing ASCVD risk factor information, we performed inverse probability weighting on all UCLA-Harbor participants to reduce the impact of multiple imputation and potential systematic error on the current analysis and results. The correlation coefficient for imputed ASCVD scores and directly calculated ASCVD scores was 0.95, indicative of robust agreement.
STATISTICAL ANALYSIS.
Study population characteristics are presented as mean ± SD (standard deviation) for continuous variables, whereas percentages are used for categorical variables. Both the mean and median were used to represent the central tendency of CAC scores. The one-way analysis of variance test and the Kruskal-Wallis equality-of-populations rank test were used to assess differences in normally and non-normally distributed continuous variables, respectively. Differences between categorical variables were evaluated through the chi-square test.
First, we constructed graphs of mean and median CAC among those with CAC >0 as a function of age 0 years. Plaque characteristics, including plaque area (mm2), total CAC volume to Agatston score ratio, and the peak calcium density Agatston weighting factor, were also assessed and graphed as a function of age among persons with CAC >0 to assess the CAC components that contribute to CAC progression over time. Finally, we graphed the growth of CAC over increasing age representing the 85th and 90th percentile in the CAC Consortium (at the top 15% and 10% most likely to have CAC, respectively).
Next, to examine the continuous association of age with the predicted probability of CAC >0, we performed multivariable-adjusted logistic regression separately for men and women with age as the independent variable modeled as a restricted cubic spline. These associations were then graphed and stratified by absence/presence of individual risk factors to demonstrate the age required to observe a 25% probability of CAC >0 (25% testing yield, number needed to scan [NNS] = 4).
For these analyses, we defined the concept of the risk factor-specific “CAC offset period” as how far in advance (in years) an individual with a specific risk factor would develop CAC >0 compared with the same person with no ASCVD risk factors. Specifically, the offset period reflects how much sooner an individual in a higher risk category would develop incident CAC when compared with individuals in the lowest-risk group. The offset period was calculated as the difference between the age of conversion to CAC >0 for an individual with a risk factor(s) and the age of conversion to CAC >0 for an individual without risk factors.
Next, sex-stratified multivariable logistic regression models were constructed, which included all traditional ASCVD risk factors, from which calculators were derived allowing computation of the predicted probability of CAC >0 according to age and traditional risk factor burden separately in men and women. These equations were also used to back-calculate the age at which a prespecified fraction of young men and young women would convert to CAC >0. This approach allowed for the calculation of the recommended age for initiating a CAC scan across several different clinical scenarios. Multiple different prespecified testing yields of 20% (NNS = 5), 25% (NNS = 4), and 33% (NNS = 3) were tested. Example calculations for both the predicted probability of CAC >0 and recommended age for an initial CAC scan based on the risk equations are provided in Supplemental Tables 1 and 2.
Finally, we applied our equations to specific clinical scenarios. Examples of intermediate- and high-risk patient groups were derived based on the strength of association between risk factors and premature CAC in sex-specific multivariable logistic models described in the previous text (Supplemental Table 3). Sex-specific intermediate risk (women: family history of CHD and hypertension, men: smoking and hypertension) and high-risk (diabetes and dyslipidemia in women and men) groups were computed to assess their conversion to CAC >0, and the recommended age for initiation of CAC scanning was calculated as described in the previous text.
The analyses described above model the mean time-to-conversion to CAC >0. Confidence intervals were calculated when presenting the initial age of conversion to CAC >0 by performing calculations corresponding to ± 0.5 on the NNS scale. For example, the lower and upper confidence bounds for a 25% testing yield (NNS = 4) were a 22% testing yield (NNS = 4.5) and a 29% testing yield (NNS = 3.5).
Internal calibration was assessed by plotting observed vs expected probabilities of CAC >0 and computing the area under the curve. All statistical analyses were conducted using STATA 16 (Stata Corp). Statistical significance was defined as a P value <0.05 on a 2-tailed test.
RESULTS
Participants were on average 43.5 years of age, 25% were women, 12.3% were of non-White ethnicity, and there were 7,686 young persons (34.4%) with CAC >0 (Table 1). Among those with CAC >0, the median (Q1, Q3) CAC score was 20 AU (4, 78 AU), and 6,080 persons (79.1%) had prevalent CAC <100. A majority of participants (92.7%) had a low 10-year risk, whereas dyslipidemia (49.6%) and a family history of CHD (49.3%) were the most common ASCVD risk factors. In total, 1 in 5 participants had hypertension (20.1%), whereas a smaller proportion had diabetes (4.0%) or were active smokers (11.0%). Compared with individuals who underwent CAC scans between 1990 and 2000, those undergoing CAC scans from 2001–2010 were more likely to be women and have a family history of CHD, but less likely to have hypertension or diabetes (Supplemental Table 4). Among persons with premature CAC, there were no significant differences in median CAC scores across the study time-period.
TABLE 1.
Characteristics of Young Adults Undergoing Coronary Artery Calcium Testing
| All (N = 22,346) | Women (n = 5,576) | Men (n = 16,770) | P Value | |
|---|---|---|---|---|
| Age, y | 43.5 ± 4.5 | 44.0 ± 4.4 | 43.3 ± 4.6 | <0.001 |
| Race | <0.001 | |||
| White | 87.7 | 85.4 | 88.5 | |
| Black | 2.3 | 3.1 | 2.0 | |
| Hispanic | 3.8 | 4.8 | 3.4 | |
| Asian | 4.3 | 4.9 | 4.1 | |
| Other | 1.9 | 1.8 | 2.0 | |
| CAC prevalence | <0.001 | |||
| CAC 0 | 65.6 | 82.7 | 59.9 | |
| CAC 1–100 | 27.2 | 14.9 | 31.3 | |
| CAC >100 | 7.2 | 2.4 | 8.8 | |
| CAC score,a AU | 95 ± 261 | 84 ± 358 | 97 ± 244 | <0.001 |
| CAC score,a AU | 20 (4, 78) | 14 (3, 55) | 21 (5, 83) | <0.001 |
| 10-year ASCVD risk | <0.001 | |||
| <5% | 92.7 | 97.8 | 91.0 | |
| 5%–7.5% | 4.7 | 1.6 | 5.7 | |
| >7.5% | 2.6 | 0.6 | 3.3 | |
| Hypertension | 20.1 | 18.7 | 20.6 | 0.003 |
| Dyslipidemia | 49.6 | 42.1 | 52.1 | <0.001 |
| Diabetes | 4.0 | 4.2 | 3.9 | 0.21 |
| Current smoking | 11.0 | 11.6 | 10.9 | 0.13 |
| Family history of CHD | 49.3 | 56.0 | 47.0 | <0.001 |
Values are mean ± SD, %, or median (Q1, Q3).
Among persons with CAC >0.
ASCVD = atherosclerotic cardiovascular disease; CAC = coronary artery calcium; CHD = coronary heart disease.
The median and mean CAC scores for young individuals with premature CAC were consistently higher for older vs young individuals, with a steeper curve for men (Figures 1A and 1B). Among individuals in the top 10% and 15% most likely to have premature CAC, the time lag between CAC = 1 to CAC >100 was approximately 10–15 years (Figure 1C). Younger men and women with CAC >0 had a low mean plaque density through 35 years of age, as evidenced by a volume to Agatston ratio >1 (Supplemental Figure 1A). Both predicted coronary plaque area and peak plaque density increased with age among younger persons with CAC >0 (Supplemental Figures 1B and 1C).
FIGURE 1. CAC Score and Estimated Growth Rate Among Young Adults.
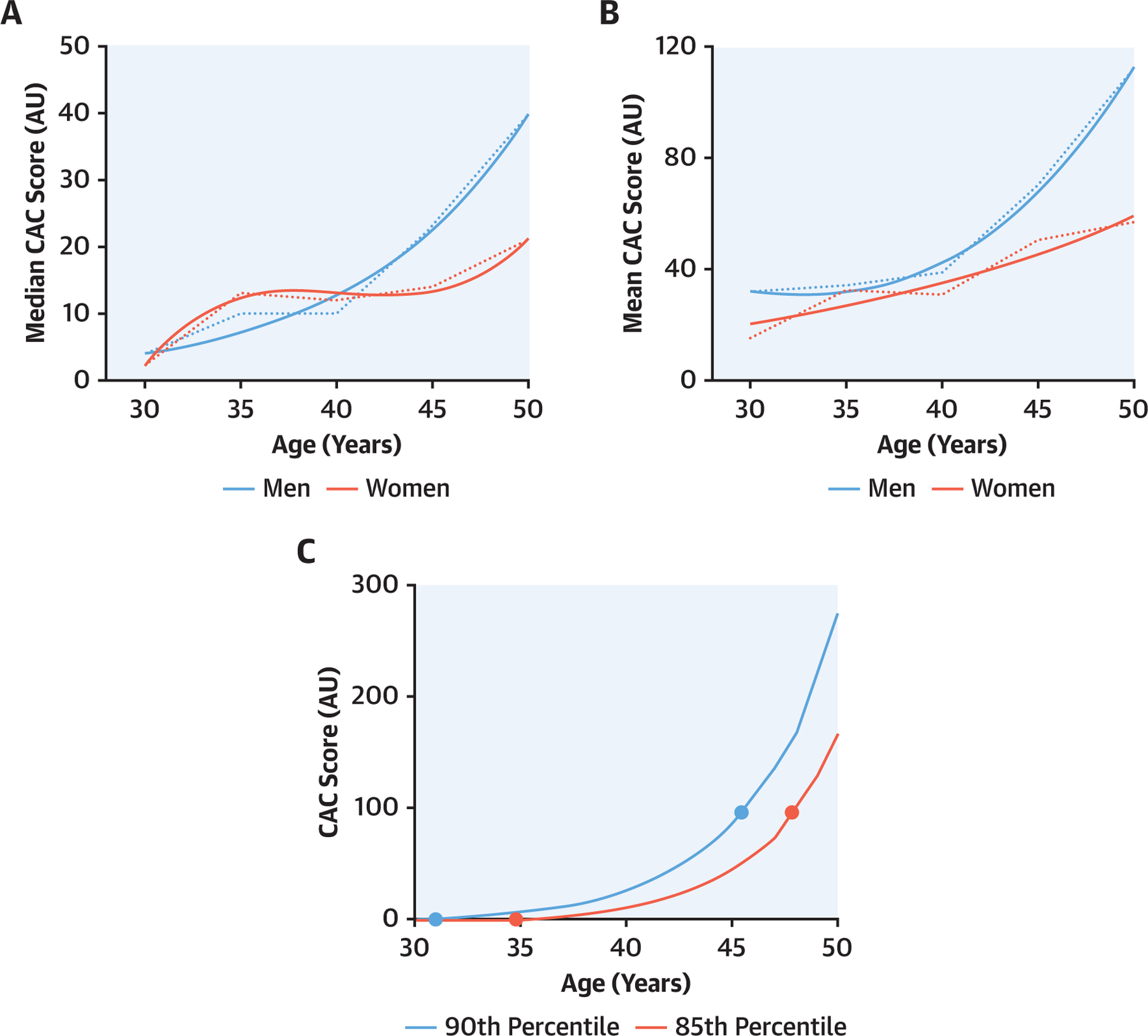
(A) Median coronary artery calcification (CAC) score among young adults with premature CAC. (B) Mean CAC score among young adults with premature CAC. (C) Estimated CAC growth rate among young adults. The estimated mean CAC growth rate was similar across both sexes up to 40 years of age, and the average time from CAC = 1 to CAC > 100 was estimated to be at least 15 years long in duration for men and women. A best fit line was added to fit the most suitable exponential equation to the raw data. AU = Agatston units.
Young men and women in the CAC Consortium but without traditional risk factors were projected to convert to CAC >0 at 42.3 years (95% CI: 41.0–43.9 years) of age and 57.6 years (95% CI: 56.0–59.5 years) of age, respectively. At a given age, the predicted probability of conversion to CAC >0 greatly varied according to sex and individual ASCVD risk factor burden (Figures 2A and 2B). Among individual ASCVD risk factors, diabetes had the most robust association with earlier conversion to CAC >0, with a larger magnitude effect in women (β = 0.71) compared with men (β = 0.63). Beyond diabetes, dyslipidemia strongly increased the risk for premature CAC in women (β = 0.54) and in men (β = 0.34) (Supplemental Table 3).
FIGURE 2. Predicted Probability of CAC >0 According to ASCVD Risk Factors.
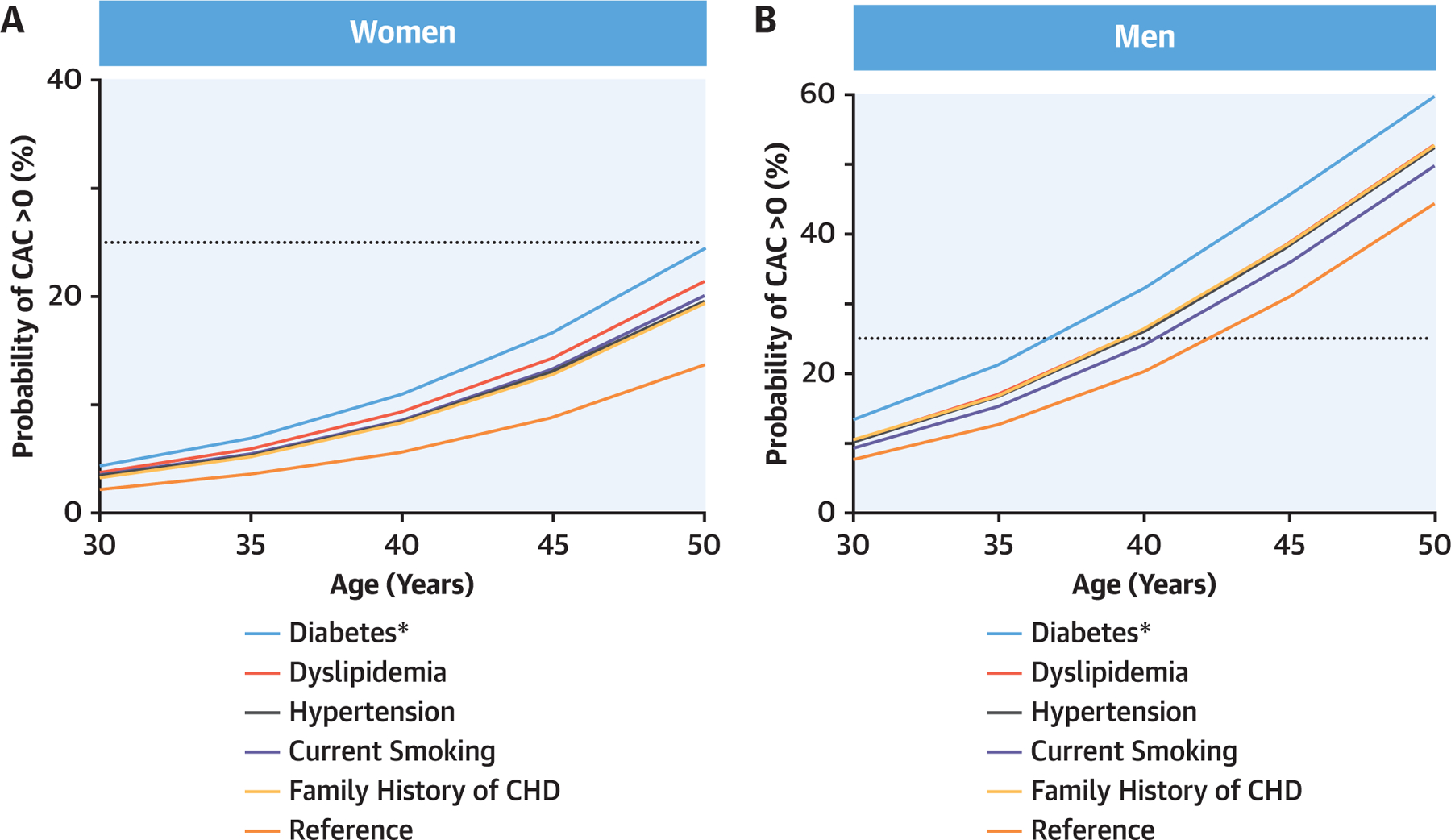
(A) Predicted probability of CAC >0 according to ASCVD risk factors in women. (B) Predicted probability of CAC >0 according to ASCVD risk factors in men. *Among individual ASCVD risk factors, diabetes had the most robust association with earlier conversion to CAC >0, with a larger magnitude effect in women compared with men. ASCVD = atherosclerotic cardiovascular disease; CAC = coronary artery calcification; CHD = coronary heart disease.
Compared with individuals without risk factors, younger persons with 1 or more risk factors would convert to CAC >0 at least 3.3 years earlier on average assuming a 25% testing yield (Table 2). Diabetes had the strongest influence on the CAC >0 offset period, because men and women with diabetes would develop incident CAC a respective 5.5 years and 7.3 years earlier on average compared with those without diabetes. In contrast, hypertension, dyslipidemia, current cigarette smokers, and a family history of CHD were each individually associated with developing CAC 3.3–4.3 years earlier. Additive interaction was observed for pairs and clusters of risk factors on the average conversion time to CAC >0. In general, women experienced larger magnitude offset periods for premature CAC compared with men for a given risk factor profile (Supplemental Figure 2).
TABLE 2.
Predicted Age to CAC >0 According to ASCVD Risk Factor Status and a 25% Testing Yield
| Predicted Age to CAC >0, y | Average Years Earlier to CAC >0 | ||
|---|---|---|---|
| Women | Men | All | |
| None | 57.6 (56.0–59.5) | 42.3 (41.0–43.9) | – |
| Family history of CHD | 53.3 (51.8–55.2) | 39.3 (38.0–40.9) | 3.7 |
| Current cigarette smoking | 52.9 (51.3–54.8) | 40.4 (39.0–42.0) | 3.3 |
| Dyslipidemia | 52.1 (50.5–53.9) | 39.3 (37.9–40.9) | 4.3 |
| Hypertension | 53.1 (51.6–55.1) | 39.4 (38.1–41.0) | 3.7 |
| Diabetes | 50.3 (48.7–52.1) | 36.8 (35.5–38.4) | 6.4 |
| Dyslipidemia + family history of CHD | 47.8 (46.2–49.7) | 36.3 (34.9–37.9) | 7.9 |
| Hypertension + family history of CHD | 48.9 (47.3–50.8) | 36.4 (35.1–38.0) | 7.3 |
The upper and lower limits of the parentheses correspond to confidence intervals with ± 0.5 persons on the number needed to scan (NNS) scale. Upper limit: NNS = 3.5 (29% testing yield), lower limit: NNS = 4.5 (22% testing yield).
Abbreviations as in Table 1.
The initial conversion to CAC >0 across optimal and illustrative intermediate- and high-risk groups according to CAC scan testing yield is presented in Figures 3A and 3B. Assuming a 25% conversion to CAC >0 (NNS = 4), women and men with an intermediate risk factor profile would develop incident CAC at 48.9 years (95% CI: 47.3–50.8 years) and 37.5 years (95% CI: 36.1–39.1 years) of age, respectively. For individuals with high risk, CAC >0 conversion would occur at 44.7 years (95% CI: 43.1–46.6 years) in women and 33.8 years (95% CI: 32.4–35.4 years) in men, given the same 25% testing yield. The predicted age of conversion to CAC >0 was earlier in models using lower testing yields (20% and 25%) vs those using a higher testing yield (33%).
FIGURE 3. Predicted Age for First CAC Scan According to RF Profile.

(A) Predicted age for first coronary artery calcification (CAC) scan according to risk factor (RF) profile in women. (B) Predicted age for first CAC scan according to RF profile in men. *Assuming a 25% conversion to CAC >0 (needed to scan [NNS] = 4), women and men with an intermediate risk factor profile would develop incident CAC at 48.5 years and 36.7 years of age, respectively. Error bars indicate the upper and lower limits corresponding to confidence intervals with ± 0.5 persons on the NNS scale. Upper limit: NNS = 3.5 (29% testing yield), lower limit: NNS = 4.5 (22% testing yield).
Overall, there was high concordance between observed and expected results, suggestive of strong internal validation for the developed risk equations (Supplemental Figure 3, Supplemental Table 5). Based on these results, the risk equations were internally well calibrated and demonstrated an expected/observed ratio of nearly 1 for both White women and White men, who contributed a majority of observations (88%).
DISCUSSION
To our knowledge, this is the first study to derive clinical risk equations for the initial conversion from CAC = 0 to CAC ≥1, which can be used to guide the timing of initiating CAC testing in young adults. Among more than 23,000 young adults of predominantly White ethnicity, we found that more than one-third had CAC >0, of whom a majority had CAC scores <50 with low plaque density suggestive of the earliest manifestation of coronary atherosclerosis. Our derived risk equations among health-seeking young adults enriched in ASCVD risk factors inform the expected prevalence of CAC >0 and can be used to determine an appropriate age to initiate CAC testing to identify individuals most susceptible for early/premature atherosclerosis (Central Illustration).
CENTRAL ILLUSTRATION. Probability of Coronary Artery Calcification >0 According to Age and Atherosclerotic Cardiovascular Disease Risk Factor Burden.

Individual atherosclerotic cardiovascular disease risk factors and risk factor profiles inform the expected prevalence of premature coronary artery calcification and can be used to determine the recommended age to initiate a coronary artery calcification scan in young adults. Overall, our results demonstrated that an initial coronary artery calcification scan can be considered between 30 and 40 years of age in men with at least 1 risk factor and between 40 and 50 years of age in women with diabetes or who have multiple atherosclerotic cardiovascular disease risk factors. ASCVD = atherosclerotic cardiovascular disease; CAC = coronary artery calcification; CHD = coronary heart disease; DLD = dyslipidemia; HTN = hypertension.
Although previous analyses have shown that up-stream risk factors and risk factor profiles inform the expected prevalence of CAC, these studies have not provided specific age recommendations nor created risk calculators that may be used in the clinical setting (3,12,13). For example, Carr et al (3) showed that young adults with a 10-year risk above vs below the sample median had an approximately 4-fold higher prevalence of CAC >0. However, this analysis conducted in the CARDIA (Coronary Artery Risk Development in Young Adults) study did not incorporate the concept of the initial age to CAC >0 conversion when presenting their findings. Likewise, although elevated non–high-density lipoprotein cholesterol in adolescence and young adulthood has also been strongly associated with premature CAC, the actual age at which such individuals develop incident CAC has not been reported or defined (12).
Current guidelines recommend consideration of CAC scoring in selected adults with a 10-year ASCVD risk between 5%–19.9% (14); however, our findings demonstrate a potential utility for CAC scanning among a subgroup of young adults at-risk for CHD who almost all had a 10-year risk <5%. Our results are thus consistent with prior data using carotid ultra-sound to show that there is a moderate prevalence of subclinical atherosclerosis in younger patients deemed as low-risk by traditional ASCVD risk scoring (15). Early CAC testing may be especially important in such at-risk young adult populations, including those with familial hypercholesterolemia, polygenic hypercholesterolemia, and/or those with a family history of premature CHD (16,17). Furthermore, a majority (79%) of participants with premature CAC in our study had a CAC score <50 AU, with a majority of these participants having low attenuation plaque through nearly 40 years of age. The identification of CAC below the 100-AU threshold is useful for early risk factor control. Consistent with the known exponential growth rate of CAC (3) and assuming that individuals stay on the same percentile curve over time, our study suggests that growth from CAC = 1 to CAC >100 occurs over approximately 10–15 years, allowing ample time for the initiation of prevention strategies in the setting of early CAC testing before the onset of clinical ASCVD.
The identified age testing recommendations for a first CAC scan here are similar to a recent study which proposed CAC testing recommendations based on results using a genetic risk score (GRS) leveraging 142 variants associated with CHD among approximately 8,500 participants (17). Here, investigators found that the probability of CAC >0 exceeded 25% among men and women who had a GRS ≥2 standard deviations by 40 years and 50 years of age, respectively, and that the GRS modified the probability of CAC >0 within risk factor subgroups. Our results enrich such findings in the setting of a larger cohort using commonly measured risk factors to identify the conversion to CAC >0, and suggest that the development of a CAC calculator may help guide clinicians regarding the optimal time to scan younger persons. Future CAC calculators derived from both genetic and traditional risk factor burden will be necessary for accurate ASCVD risk assessment to guide primary prevention therapy among younger individuals who are at-risk for future CHD.
The probability of developing CAC >0 among young adults varied across risk factor groups, further demonstrating that the ideal scan time for subclinical atherosclerosis imaging differs according to ASCVD risk factor burden (18–20). Diabetes was most strongly associated with premature CAC among both sexes, as the predicted age of conversion to CAC >0 for men and women with diabetes was 36.8 years and 50.3 years, respectively. These findings build on previous observations, which show that the presence of diabetes confers a 40% reduction in the so called “warranty period” of CAC = 0 (18), and that approximately one-third of younger persons with diabetes and a median age 36 years have identifiable calcified coronary plaque on CT angiography (21). Furthermore, recent analyses have also shown that individuals with diabetes experience a higher ASCVD mortality rate associated with CAC >0 starting from 30 years of age (3,4) and that diabetes and insulin resistance are the strongest risk factors for premature CHD among women (20).
Similar to the ACC/AHA primary prevention and National Lipid Association guidelines, our developed risk equations for premature CAC also recognize individuals with diabetes as a high-risk subgroup. For example, the National Lipid Association 2020 guidelines has suggested that it is reasonable to obtain a CAC scan among persons with diabetes aged 30–39 years when there is uncertainty regarding the initiation of statin therapy (22). The predictive utility of CAC for ASCVD outcomes is similar in types 1 and 2 diabetes (23). However, individuals with type 1 diabetes may develop CAC as young as 17 years of age (24); therefore, further studies in this patient population are required.
In addition to diabetes, other individual risk factors in men and risk factor clustering in both men and women were associated with a high probability of CAC >0 in young adulthood. By 40 years of age, approximately 1 of every 4 men with hypertension, dyslipidemia, smoking, or a family history of CHD would have CAC >0. A 25% probability threshold would similarly be reached for all women aged 50–60 years who had 1 or more risk factors. Furthermore, additive interaction for traditional risk factors was also noted. For example, a woman with dyslipidemia and a family history of CHD would have incident CAC an average 6 years earlier compared with a woman without risk factors given an NNS of 4, consistent with the sum of the individual offset periods for dyslipidemia (3 years) and family history (3 years).
As noted in our analysis, the gap in age between recommended CAC screening in men and women was large (~15 years); however, the utilization of a 25% detection threshold for the NNS for CAC >0 is arbitrary. Given that women have worse ASCVD outcomes compared with men for a given CAC burden (25), it may be reasonable to screen young women who are at risk for CHD at a higher NNS threshold. However, the utility of early CAC testing in women of child-bearing age who have diabetes and/or significant ASCVD risk factor burden requires further assessment in followup studies. Overall, CAC = 0 does not guarantee zero atherosclerosis (26), and future studies that incorporate several different subclinical atherosclerosis measures and imaging modalities are therefore required.
Strengths of the current study include the measurement of CAC among more than 20,000 young men and women with data on plaque density (27,28). We conducted a derivation of a novel clinical CAC calculator for predicting the initial conversion to CAC >0 based on traditional ASCVD risk factors. Previous studies involving CAC measurement in younger adults have been somewhat limited beyond associative statistics (4). Our analyses provide relevant risk equations for clinicians and future guideline makers that are flexible according to the testing yield (NNS). Thus, clinical CAC testing recommendations for younger adults will have to strike a balance across pre-scan probabilities as well as the patient-provider values and goals.
STUDY LIMITATIONS.
Limitations of the analysis include a low proportion of non-White participants and a relatively high proportion of participants with a family history of CHD compared with the general population. Given that the study participants were all clinically referred for CAC testing, the CAC Consortium includes a sample of predominantly health-seeking participants who are enriched in ASCVD risk factors, and likely other risk-enhancing factors. Therefore, CAC Consortium participants do not represent a general population-based sample, but rather represent a general cardiology referral population or individuals who seek out medical care out of concern for their future ASCVD risk. Future studies that incorporate a larger proportion of African American and other minority populations are undoubtedly necessary to inform CAC screening recommendations in young adults of diverse ethnicities.
Although our findings reflect the associations of ASCVD risk factors with the predicted prevalence of premature CAC, individuals in the current study did not have a second CAC scan for true modeling of longitudinal CAC incidence. Accordingly, our findings are thus better interpreted as the fraction of a population that will develop CAC as a function of age as opposed to the probability that an individual patient will develop CAC. Future prospective studies with multiple CAC scans that begin in young adulthood and in more ethnically diverse populations will be required for reproducibility. Furthermore, there was an overall low proportion of individuals with certain risk factors, including diabetes, and risk factors were in part self-reported. For example, we were unable to differentiate between different severity thresholds of dyslipidemia (low-density lipoprotein cholesterol ≥130 mg/dL vs ≥160 mg/dL vs ≥190 mg/dL), controlled vs uncontrolled hypertension, diabetes duration, and/or type 1 vs type 2 diabetes. Moreover, because of the risk of introducing a bias toward a lower-risk sample after excluding the small proportion of participants on statin therapy (7%), we elected to retain persons on statin therapy in the analyses, although information on statin intensity and duration was not systematically collected in the CAC Consortium.
CONCLUSIONS
Approximately one-third of younger persons with an overall low predicted 10-year ASCVD risk had premature CAC. Compared with a person without risk factors, an individual with diabetes would develop premature CAC 6.4 years earlier on average, whereas this age offset is projected to be smaller and similar for smoking, hypertension, dyslipidemia, and family history (3.3–4.3 years). These results suggest that risk factor profiles inform the expected prevalence of premature CAC and can be used to determine the recommended age to initiate an appropriate CAC scan in young adults to identify those who are susceptible for developing premature atherosclerosis.
Supplementary Material
PERSPECTIVES.
COMPETENCY IN PATIENT CARE AND PROCEDURAL OUTCOMES:
Approximately one-third of young adults at-risk for coronary heart disease have premature coronary artery calcification (CAC). Based on sex-specific multivariable logistic modeling for a 25% likelihood of detecting CAC, the optimal age for a first scan is approximately 37 years for men and 50 years for women with diabetes, and 42 years for men and 58 years for women without risk factors for premature atherosclerosis.
TRANSLATIONAL OUTLOOK:
Additional risk calculators derived from more diverse populations and genetic data are necessary to guide primary preventive therapy among young individuals at risk of atherosclerosis.
ACKNOWLEDGMENTS
The authors thank the other investigators, the staff, and the participants of the CAC Consortium for their valuable contributions.
FUNDING SUPPORT AND AUTHOR DISCLOSURES
This project was supported in part by a research grant from the National Institutes of Health-National Heart, Lung, and Blood Institute (L30 HL110027). Dr Blaha has received grants from the National Institutes of Health, U.S. Food and Drug Administration, American Heart Association, Amgen, and Aetna Foundation; and has received honoraria from Amgen, Sanofi, Regeneron, Novartis, Novo Nordisk, Bayer, Akcea, 89Bio, Zogenix, Tricida, and Gilead. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
ABBREVIATIONS AND ACRONYMS
- ASCVD
atherosclerotic cardiovascular disease
- AU
Agatston units
- CAC
coronary artery calcification
- CHD
coronary heart disease
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
APPENDIX For supplemental tables and figures, please see the online version of this paper.
REFERENCES
- 1.Budoff MJ, Young R, Burke G, et al. Ten-year association of coronary artery calcium with atherosclerotic cardiovascular disease (ASCVD) events: the Multi-Ethnic Study of Atherosclerosis (MESA). Eur Heart J. 2018;39:2401–2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sc S, Sperling L, Virani SS, Yeboah J. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:e285–e350. [DOI] [PubMed] [Google Scholar]
- 3.Carr JJ, Jacobs DR, Terry JG, et al. Association of coronary artery calcium in adults aged 32 to 46 years with incident coronary heart disease and death. JAMA Cardiol. 2017;2:391–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miedema MD, Dardari ZA, Nasir K, et al. Association of coronary artery calcium with long-term, cause-specific mortality among young adults. JAMA Netw Open. 2019;2:e197440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Osei AD, Uddin SMI, Dzaye O, et al. Predictors of coronary artery calcium among 20–30-year-olds: The Coronary Artery Calcium Consortium. Atherosclerosis. 2020;301:65–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bild DE, Folsom AR, Lowe LP, et al. Prevalence and correlates of coronary calcification in black and white young adults: The Coronary Artery Risk Development In Young Adults (CARDIA) Study. Arterioscler Thromb Vasc Biol. 2001;21:852–857. [DOI] [PubMed] [Google Scholar]
- 7.Mahoney LT, Burns TL, Stanford W, et al. Coronary risk factors measured in childhood and young adult life are associated with coronary artery calcification in young adults: The Muscatine study. J Am Coll Cardiol. 1996;27:277–284. [DOI] [PubMed] [Google Scholar]
- 8.Blaha MJ. Predicting the age of conversion to CAC >0: a role for polygenic risk scores? J Am Coll Cardiol Img. 2021;14(7):1407–1409. 10.1016/j.jcmg.2020.12.007 [DOI] [PubMed] [Google Scholar]
- 9.Goff DC, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014. Jul 1;63(25 Pt B):2935–2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peng AW, Dardari Z, Blumenthal RS, et al. Very high coronary artery calcium (CAC {greater than or equal to} 1000) and association with CVD events, non-CVD outcomes, and mortality: results from the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation. 2021;143:1571–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blaha MJ, Whelton SP, Al Rifai M, et al. Rationale and design of the coronary artery calcium consortium: A multicenter cohort study. J Cardiovasc Comput Tomogr. 2017;11:54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Armstrong MK, Fraser BJ, Hartiala O, et al. Association of non-high-density lipoprotein cholesterol measured in adolescence, young adulthood, and mid-adulthood with coronary artery calcification measured in mid-adulthood. JAMA Cardiol. 2021;6(6):661–668. 10.1001/jamacardio.2020.7238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saad M, Pothineni NV, Thomas J, et al. Coronary artery calcium scoring in young adults: evidence and challenges. Curr Cardiol Rep. 2018;20:10. [DOI] [PubMed] [Google Scholar]
- 14.Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol. J Am Coll Cardiol. 2019;73(24):e285–e350. [DOI] [PubMed] [Google Scholar]
- 15.Mortensen MB, Fuster V, Muntendam P, et al. A simple disease-guided approach to personalize ACC/AHA-recommended statin allocation in elderly people: the BioImage Study. J Am Coll Cardiol. 2016;68:881–889. [DOI] [PubMed] [Google Scholar]
- 16.Singh A, Gupta A, Collins BL, et al. Familial hypercholesterolemia among young adults with myocardial infarction. J Am Coll Cardiol. 2019;73:2439–2450. [DOI] [PubMed] [Google Scholar]
- 17.Severance LM, Carter H, Contijoch FJ, Mcveigh ER. Targeted coronary artery calcium screening in high-risk younger individuals using consumer genetic screening results. J Am Coll Cardiol Img. 2021;14(7):1398–1406. 10.1016/j.jcmg.2020.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dzaye O, Dardari ZA, Cainzos-Achirica M, et al. Warranty period of a calcium score of zero: comprehensive analysis from the Multiethnic Study of Atherosclerosis. J Am Coll Cardiol Img. 2020;14:990–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dzaye O, Dardari ZA, Cainzos-Achirica M, et al. Incidence of new coronary calcification: time to conversion from CAC = 0. J Am Coll Cardiol. 2020;75:1610–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ridker PM, Glynn RJ, Mora S. Association of lipid, inflammatory, and metabolic biomarkers with age at onset for incident coronary heart disease in women. JAMA Cardiol. 2021;6:437–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nezarat N, Budoff MJ, Luo Y, et al. Presence, characteristics, and volumes of coronary plaque determined by computed tomography angiography in young type 2 diabetes mellitus. Am J Cardiol. 2017;119:1566–1571. [DOI] [PubMed] [Google Scholar]
- 22.Orringer CE, Blaha MJ, Blankstein R, et al. The National Lipid Association scientific statement on coronary artery calcium scoring to guide preventive strategies for ASCVD risk reduction. J Clin Lipidol. 2020;15:33–60. [DOI] [PubMed] [Google Scholar]
- 23.Budoff M, Backlund JYC, Bluemke DA, et al. The association of coronary artery calcification with subsequent incidence of cardiovascular disease in type 1 diabetes: the DCCT/EDIC Trials. J Am Coll Cardiol Img. 2019;12:1341–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Starkman HS, Cable G, Hala V, Hecht H, Donnelly CM. Delineation of prevalence and risk factors for early coronary artery disease by electron beam computed tomography in young adults with type I diabetes. Diabetes Care. 2003;26:433–436. [DOI] [PubMed] [Google Scholar]
- 25.Shaw LJ, Min JK, Nasir K, et al. Sex differences in calcified plaque and long-term cardiovascular mortality: observations from the CAC Consortium. Eur Heart J. 2018;39:3727–3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mehta A, Rigdon J, Tattersall MC, et al. Association of carotid artery plaque with cardiovascular events and incident coronary artery calcium in individuals with absent coronary calcification: the MESA. Circ Cardiovasc Imaging. 2021;14:e011701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cainzos-Achirica M, Agrawal T. Implications of the plaque density paradox for risk assessment in 2020. J Am Coll Cardiol Img. 2021;14:243–245. [DOI] [PubMed] [Google Scholar]
- 28.Criqui MH, Knox JB, Denenberg JO, et al. Coronary artery calcium volume and density: potential interactions and overall predictive value: the Multi-Ethnic Study of Atherosclerosis. J Am Coll Cardiol Img. 2017;10:845–854. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


