Mechanisms of resistance to immune checkpoint (ICI) therapy in advanced urothelial carcinoma (aUC) are unclear, with efforts to study these hampered by difficulty in obtaining paired pre- and post-therapy biopsies. This article reports results of a study of an amplicon-based next-generation sequencing assay to identify genomic alterations pre- and post-therapy in patients with aUC receiving either ICI therapy or platinum-based chemotherapy.
Keywords: ctDNA, immunotherapy, urothelial cancer, response, resistance
Abstract
Serial evaluation of circulating tumor DNA may allow noninvasive assessment of drivers of resistance to immune checkpoint inhibitors (ICIs) in advanced urothelial cancer (aUC). We used a novel, amplicon-based next-generation sequencing assay to identify genomic alterations (GAs) pre- and post-therapy in 39 patients with aUC receiving ICI and 6 receiving platinum-based chemotherapy (PBC). One or more GA was seen in 95% and 100% of pre- and post-ICI samples, respectively, commonly in TP53 (54% and 54%), TERT (49% and 59%), and BRCA1/BRCA2 (33% and 33%). Clearance of ≥1 GA was seen in 7 of 9 patients responding to ICI, commonly in TP53 (n = 4), PIK3CA (n = 2), and BRCA1/BRCA2 (n = 2). A new GA was seen in 17 of 20 patients progressing on ICI, frequently in BRCA1/BRCA2 (n = 6), PIK3CA (n = 3), and TP53 (n = 3), which seldom emerged in patients receiving PBC. These findings highlight the potential for longitudinal circulating tumor DNA evaluation in tracking response and resistance to therapy.
Mechanisms of resistance to immune checkpoint (ICI) therapy in advanced urothelial carcinoma (aUC) are unclear, with efforts to study these hampered by difficulty in obtaining paired pre- and post-therapy biopsies. This article reports the results of a study of an amplicon-based next-generation sequencing assay to identify genomic alterations pre- and post-therapy in patients with aUC receiving either ICI therapy or platinum-based chemotherapy.
Introduction
Fifteen to twenty percent of patients with advanced urothelial carcinoma (aUC) respond to immune checkpoint inhibitors (ICI), but the majority are primarily refractory to ICI or develop early resistance to therapy.1,2 Mechanisms of resistance to ICI therapy in aUC are unclear, with efforts to study these hampered by difficulty in obtaining paired pre- and post-therapy biopsies.
Circulating tumor (ct) DNA may be detected via targeted next-generation sequencing (NGS) panels and is increasingly being used in aUC.3 Prior studies have shown that >90% of patients with aUC have a genomic alteration (GA) detectable by panel-based ctDNA testing,4,5 and the recent BISCAY trial used ctDNA as a means of biomarker selection.6 Serial ctDNA evaluation offers the ability to track disease status non-invasively and monitor GAs that may correlate with response and resistance to therapy. We evaluated serial plasma collections from patients with aUC receiving ICI and profiled ctDNA using a novel and sensitive amplicon-based NGS assay.
Patients with aUC at our institution who had ≥2 mL of plasma available prior to (“pre”) and either during or after completion of ICI (“post”) were eligible. Paired “pre” and “post” samples underwent ctDNA evaluation with 7-30 ng of DNA using an 80-gene amplicon-based NGS assay including the detection of fusions, (Lucence LiquidHallmark, Supplementary Table S1).7 The primary objective was to identify evolving ctDNA GAs post-ICI and secondarily to explore associations between GAs and radiologic response assessed by investigators per RECIST 1.1.
A total of 39 patients were included. Baseline characteristics are shown in Supplementary Table S2. One or more GAs were detected in ctDNA in 37 (95%) pre-therapy and 39 (100%) post-therapy samples; the median number of unique GAs detected per patient both pre- and post-ICI was 3. The most commonly GAs seen pre- and post-ICI were in TP53 (54% and 54%), TERT (49% and 59%), and BRCA1/BRCA2 (33% and 33%, Table 1). FGFR2/3 variants were seen in 3 patients pre-ICI, while a new FGFR2/3 variant was detected in two patients post-ICI. Microsatellite instability was detected in 1 patient. Across all samples sequenced, a median of 99.8% of reads had coverage >100× (range 82.2-100).
Table 1.
Common genomic alterations present at baseline (pre-ICI) and during or completion of ICI therapy (post-ICI).
| Gene | Pre-ICI, n (%) | Post-ICI, n (%) |
|---|---|---|
| TP53 | 21 (54) | 21 (54) |
| TERT | 19 (49) | 23 (59) |
| BRCA1/BRCA2 | 13 (33) | 13 (33) |
| CCND1/CCND2/CDKN2A/CDK6 | 6 (15) | 4 (10) |
| RAS | 5 (13) | 4 (10) |
| PIK3CA | 5 (13) | 5 (13) |
| EGFR | 3 (8) | 4 (10) |
| FGFR2/3 | 3 (8) | 5 (13) |
| ERBB2 | 1 (3) | 1 (3) |
| Rb | 1 (3) | 3 (8) |
| ALK | 1 (3) | 2 (5) |
Abbreviation: ICI, immune checkpoint inhibitor.
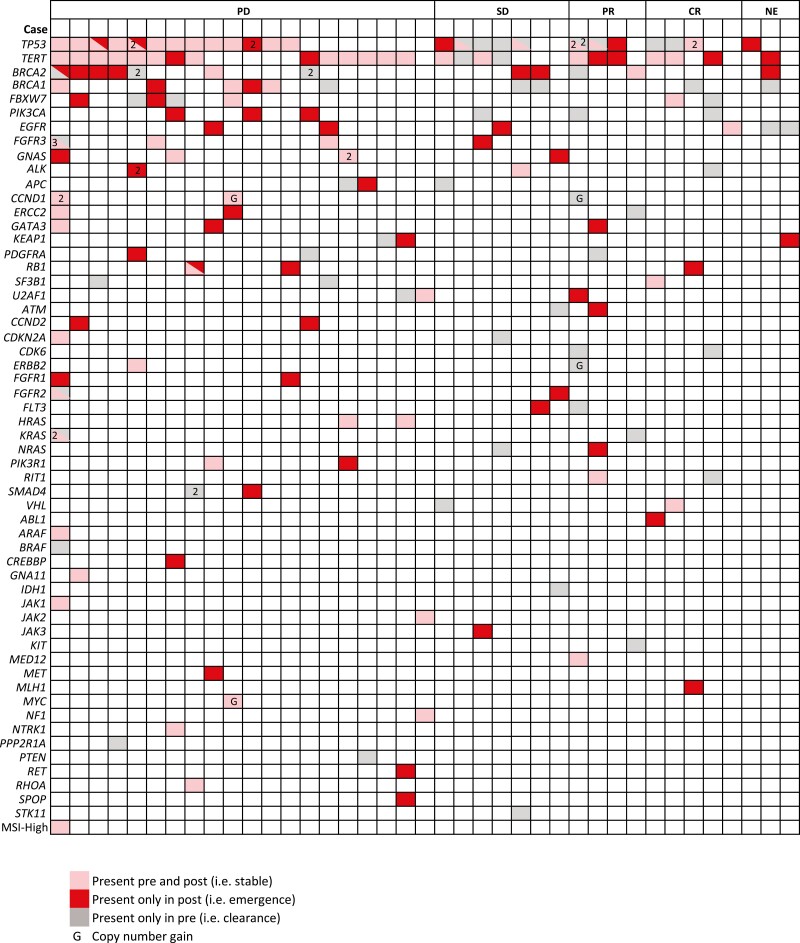
At the time of the “post” sample, amongst 36 evaluable patients, 9 (25%) had a complete or partial response, 7 (19%) had stable disease, and 20 (56%) had progressive disease (PD) by radiologic assessment. Figure 1 shows the spectrum of GAs detected in ctDNA pre- and post-ICI, including GAs that were stable, disappeared, or emerged during or after ICI therapy. Among the 9 patients responding to ICI, 7 (78%) demonstrated clearance of one or more GAs by ctDNA, most commonly in TP53 (n = 4), PIK3CA (n = 2), and BRCA1/BRCA2 (n = 2). Patients in whom clearance of TP53 variants was seen during ICI therapy had a higher likelihood of response compared to those in whom TP53 variants remained or emerged during therapy (50% vs. 12.5%, χ2 = 4, P = .046). Of the 20 patients with PD, 17 (85%) showed emergence of a new GA, most commonly in BRCA1/BRCA2 (n = 6), CCND2/Rb (n = 4), TP53 (n = 3), and PIK3CA (n = 3). No responses were seen in patients in whom a BRCA1/BRCA2 (n = 9) or PIK3CA (n = 3) variant emerged during therapy, while none of the 3 patients with a baseline FGFR2/3 variant responded to ICI.
Figure 1.
Spectrum of genomic alterations detected by ctDNA pre- and post-immune checkpoint inhibitor therapy, stratified by response to therapy (each column represents an individual patient and numbers indicate the total number of variants for a given gene in an individual patient). ctDNA, circulating tumor DNA.
We also evaluated 6 patients with aUC who received first-line platinum-based chemotherapy (PBC) and had paired pre- and post-therapy samples (Supplementary Tables S3 and S4). Overall, ctDNA was detected in all 12 samples (100%); emergence of a BRCA1 variant was seen in one patient while emergence of a TP53 or PIK3CA variant was not seen.
Several findings from our study are noteworthy. First, the 80-gene Lucence LiquidHallmark assay exhibited excellent sensitivity and detected at least one GA in the vast majority (≥95%) of patients with UC utilizing a small amount of plasma (~2 mL). The frequency and spectrum of GAs in our study are similar to prior work4,5 and confirm the utility of this assay in detecting GAs via ctDNA in UC.
Our findings provide insight into the genomic evolution of UC during ICI therapy and its relationship with response to therapy. Clearance of GAs in oncogenic drivers such as TP53, BRCA1/2, and PIK3CA was noted in the majority of patients who were responding to ICI therapy, while emergence of new variants in these genes was noted in most patients who were progressing on ICI. This suggests that tracking ctDNA during therapy may provide a dynamic evaluation of response and complement radiologic assessment. Furthermore, we noted emergence of a new FGFR2/3 variant during therapy in 2 patients, suggesting that serial testing for these alterations may be needed during a patient’s disease course given the availability of a biomarker-directed therapy, erdafitinib, in this population.8
Recent work has shown that the presence of ctDNA can identify those with minimal residual disease in resected UC who benefit from adjuvant atezolizumab and that clearance of ctDNA during adjuvant and neoadjuvant ICI is associated with better outcomes.9 Our results build upon this to show that ctDNA changes are associated with response and resistance to ICI in the metastatic setting. Furthermore, we used a tumor-agnostic and sensitive ctDNA platform—rather than a tumor-informed bespoke gene panel9—which may be more easily applied in routine clinical practice.
Our results provide a rationale for a possible therapeutic combination of ICI with PARP, CKD4/6, and PIK3CA/Akt inhibition in aUC since patients with disease progression on ICI demonstrated frequent emergence of GAs in these pathways. Furthermore, new GAs in these pathways generally did not emerge in the small comparator cohort of patients receiving first-line PBC, suggesting that these may specifically be involved in mediating resistance to ICI.10
In summary, this longitudinal evaluation of ctDNA in paired pre and post-ICI therapy samples from patients with aUC using a sensitive amplicon-based NGS platform provides insights into GAs associated with response and resistance to ICIs. While these findings are hypothesis-generating and require validation and evaluation in other settings (chemotherapy, antibody-drug conjugates), noninvasive serial evaluation of ctDNA may assist in monitoring response to therapy and guide the development of rational therapeutic combinations with ICI.
Supplementary Material
Funding
Lucence LiquidHallmark assay performed free of charge by Lucence Diagnostics.
Conflict of Interest
Arvind Ravi: Tyra Biosciences, Hala Solutions (C/A); Charlene Mantia: Bristol Myers Squibb (RF); Bradley A. McGregor: Bayer, Astellas, AstraZeneca, Seattle Genetics, Eisai, Calithera, Exelixis, Nektar, Pfizer, Janssen, Genentech, EMD Serono (C/A), Bristol Myers Squibb, Calithera, Exelixis, Seattle Genetics (RF—inst); Michelle Pek, Yukti Choudhury, Min-Han Tan: Lucence (E, OI); Guru P. Sonpavde: BMS, Genentech, EMD Serono, Merck, Sanofi, Seattle Genetics/Astellas, AstraZeneca, Exelixis, Janssen, Bicycle Therapeutics, Pfizer, Immunomedics/Gilead, Scholar Rock, G1 Therapeutics, Eli Lilly/Loxo Oncology (C/A), Sanofi, AstraZeneca, Immunomedics/Gilead, QED, Predicine, BMS (RF—inst), BMS, Bavarian Nordic, Seattle Genetics, QED, G1 Therapeutics (Other—steering committee, unpaid), AstraZeneca, EMD Serono, Debiopharm (Other—steering committee, paid), Mereo (Other—Data safety monitoring committee), BMS, AstraZeneca (Other—travel), Physicians Education Resource (PER), Onclive, Research to Practice, Medscape (all educational), Cancer Network, Masters Lecture Series (MLS) (Other—speaking fees). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board.
Author Contributions
Conception/design: P.R., G.P.S., M.-H.T. Provision of study material/patients: G.P.S. Collection and/or assembly of data: P.R., A.R., I.B.R., D.F., C.C., M.P., Y.C. Data analysis and interpretation: P.R., A.R., I.B.R., M.P., Y.C. Manuscript writing: P.R. Final approval of manuscript: All authors.
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Bellmunt J, de Wit R, Vaughn DJ, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376:1015-1026. 10.1056/NEJMoa1613683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Powles T, Duran I, van der Heijden MS, et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2018;391:748-757. 10.1016/S0140-6736(17)33297-X [DOI] [PubMed] [Google Scholar]
- 3. Green EA, Li R, Albiges L, et al. Clinical utility of cell-free and circulating tumor DNA in kidney and bladder cancer: a critical review of current literature. Eur Urol Oncol. 2021. [DOI] [PubMed] [Google Scholar]
- 4. Agarwal N, Pal SK, Hahn AW, et al. Characterization of metastatic urothelial carcinoma via comprehensive genomic profiling of circulating tumor DNA. Cancer. 2018;124:2115-2124. 10.1002/cncr.31314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Grivas P, Lalani AA, Pond GR, et al. Circulating tumor DNA alterations in advanced urothelial carcinoma and association with clinical outcomes: a pilot study. Eur Urol Oncol. 2019;3:695-699. [DOI] [PubMed] [Google Scholar]
- 6. Powles T, Carroll D, Chowdhury S, et al. An adaptive, biomarker-directed platform study of durvalumab in combination with targeted therapies in advanced urothelial cancer. Nat Med. 2021;27:793-801. 10.1038/s41591-021-01317-6. [DOI] [PubMed] [Google Scholar]
- 7. Poh J, Ngeow KC, Pek M, et al. Comprehensive molecular profiling of advanced cancers in a real-world setting using an ultrasensitive amplicon-based next-generation sequencing (NGS) liquid biopsy assay. J Clin Oncol. 2021;39(suppl 15; abstr 3062). [Google Scholar]
- 8. Loriot Y, Necchi A, Park SH, et al. Erdafitinib in locally advanced or metastatic urothelial carcinoma. N Engl J Med. 2019;381:338-348. 10.1056/NEJMoa1817323 [DOI] [PubMed] [Google Scholar]
- 9. Powles T, Assaf ZJ, Davarpanah N, et al. ctDNA guiding adjuvant immunotherapy in urothelial carcinoma. Nature. 2021;595:432-437. 10.1038/s41586-021-03642-9 [DOI] [PubMed] [Google Scholar]
- 10. Fares CM, Van Allen EM, Drake CG, Allison JP, Hu-Lieskovan S.. Mechanisms of resistance to immune checkpoint blockade: why does checkpoint inhibitor immunotherapy not work for all patients? Am Soc Clin Oncol Educ Book. 2019;39:147-164. 10.1200/EDBK_240837 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.